Abstract
Metacognition is the ability to think about thinking. Although monitoring and controlling one’s knowledge is a key feature of human cognition, its evolutionary origins are debated. In the current study, we examined whether rhesus monkeys (Macaca mulatta; N = 120) could make metacognitive inferences in a one-shot decision. Each monkey experienced one of four conditions, observing a human appearing to hide a food reward in an apparatus consisting of either one or two tubes. The monkeys tended to search the correct location when they observed this baiting event, but engaged in information seeking—by peering into a center location where they could check both potential hiding spots—if their view had been occluded and information seeking was possible. The monkeys only occasionally approached the center when information seeking was not possible. These results show that monkeys spontaneously use information about their own knowledge states to solve naturalistic foraging problems, and thus provide the first evidence that nonhumans exhibit information-seeking responses in situations with which they have no prior experience.
Keywords: comparative psychology, theory of mind, monitoring, metacognition, open data, open materials
Metacognition encompasses a set of cognitive processes allowing individuals to think about thinking (Flavell, 1979). Humans recognize their own uncertainty when they do not know some piece of information, and take appropriate action to remedy the situation if needed. Such metacognitive awareness underpins a wide variety of human cognitive functions, including memory (Nelson & Narens, 1990; Schwartz, 1994), numerical judgments (Vo, Li, Kornell, Pouget, & Cantlon, 2014), pedagogical learning (Kelemen, Winningham, & Weaver, 2007), social cognition (Flavell, 1999; Frith, 2012), and self-awareness (Koriat, 2007). The breadth of domains influenced by metacognition suggests that this representational capacity is a fundamental component of the human mind. Work on human development further highlights that young infants also engage in relatively spontaneous monitoring of their own knowledge (Goupil, Romand-Monnier, & Kouider, 2016). Whether such humanlike metacognition is seen in other species is currently a matter of debate (Carruthers, 2008; Kornell, 2009; Smith, Beran, Cosuchman, & Coutinho, 2008; Terrace & Son, 2009), and some researchers have proposed that metacognition is a uniquely human trait (Carruthers, 2008; Frith, 2012; Metcalfe & Shimamura, 1994).
Do other species spontaneously recognize their own knowledge states, as humans do? Foundational work on this question has used perceptual judgment and memory tasks to test whether animals recognize when they do not know something. In these tasks, animals are trained to perform a psychophysical discrimination or to make a memory judgment—but then periodically receive an ambiguous set of stimuli to test whether they would use an opt-out response when faced with a difficult choice. This work has found that many species—including rhesus monkeys (Castro & Wasserman, 2013; Hampton, 2001; Kornell, Son, & Terrace, 2007; Shields, Smith, & Washburn, 1997; Smith, Shields, Allendoerfer, & Washburn, 1998; Smith, Shields, Schull, & Washburn, 1997), dolphins (Smith et al., 1995), and rats (Foote & Crystal, 2012)—will opt out when faced with uncertain or difficult decisions.
However, some researchers have argued that these sorts of responses may stem from simpler learning processes rather than the sort of metacognitive reasoning about knowledge states that humans exhibit (Carruthers, 2008; Jozefowiez, Staddon, & Cerutti, 2009; Le Pelley, 2012). These uncertainty-monitoring tasks involve extensive training and reward history, and the opt-out response may become reinforced over time because the animals lose rewards or receive time-outs for incorrect choices. As ambiguous stimuli are inherently yoked to task difficulty, animals may also choose to opt out to avoid the aversive qualities of those cues. Perspectives from computational neuroscience further indicate that calculations of decision confidence in such contexts can involve relatively simple operations (Kepecs & Mainen, 2012; Kepecs, Uchida, Zariwala, & Mainen, 2008). Indeed, recent evidence has shown that invertebrates (e.g., bees) correctly make opt-out responses when faced with challenging perceptual discriminations (Perry & Barron, 2013), suggesting that nonhumans may succeed at uncertainty-monitoring tasks via psychological mechanisms different from those used by humans reasoning about their own knowledge or ignorance.
Other researchers have tested animal metacognition in situations that harness animals’ natural information-seeking responses without such extensive training or experience. In the first such study to use this approach (Call & Carpenter, 2001), chimpanzees, orangutans, and children knew that food had been placed in one of two opaque tubes but did not necessarily know which one. In fact, both apes and humans chose the correct container if they had seen which one was baited, but if they had not seen the specific location of the food, they first reoriented by crouching down to look inside the containers before making a choice. Current evidence shows that all four great ape species exhibit such information-seeking responses when they lack relevant knowledge (Beran, Smith, & Perdue, 2013; Call, 2010; Call & Carpenter, 2001; Marsh & MacDonald, 2012). However, there appears to be a phylogenetic divide between the more robust information-seeking responses of great apes and those of other animals. For example, capuchins exhibit such looking responses regardless of whether they are knowledgeable or ignorant (Basile, Hampton, Suomi, & Murray, 2009; Paukner, Anderson, & Fujita, 2006), and dogs and rats do not show such looking responses at all (Bräuer, Call, & Tomasello, 2004; McMahon, Macpherson, & Roberts, 2010; Roberts, McMillan, Musoline, & Cole, 2012). A study with rhesus monkeys showed that they were more likely to engage in information seeking when they lacked knowledge than when they had seen the baited location—but these looking responses appeared only after the monkeys were given extensive training with the task and had initial experiences encouraging them to look inside the tubes (Hampton, Zivin, & Murray, 2004).
These findings are puzzling given evidence that, like apes, rhesus monkeys can think about knowledge states in other contexts. In humans, metacognitive capacities are thought to be conceptually interrelated with the ability to think about the knowledge states of other individuals (e.g., Carruthers, 2008; Flavell, 1999; Frith, 2012; Kuhn, 2000). Past research has shown that both apes and rhesus macaques predict that other individuals will act in accordance with their knowledge (Hare, 2011; Kaminski, Call, & Tomasello, 2008; Marticorena, Ruiz, Mukerji, Goddu, & Santos, 2011; Martin & Santos, 2014). For example, rhesus monkeys preferentially attempt to steal food from a human competitor who lacks knowledge about their approach behavior compared with a competitor who has perceptual access to their approach (Flombaum & Santos, 2005; Santos, Nissen, & Ferrugia, 2006)—much as chimpanzees do (Hare, Call, & Tomasello, 2006; Melis, Call, & Tomasello, 2006). If thinking about one’s own knowledge state and thinking about others’ knowledge states recruit shared cognitive capacities, species that can represent other individuals’ knowledge states should also show other metacognitive capacities that involve thinking about their own knowledge states. Comparative work is therefore uniquely positioned to assess whether the capacities for thinking about one’s own knowledge and for thinking about others’ knowledge can emerge independently, by examining the phylogenetic coherence of these cognitive abilities across species (see Santos & Rosati, 2015).
In the current study, we examined whether rhesus monkeys could spontaneously use information about their own knowledge, with the largest sample of nonhumans tested to date (N = 120). Previous work has shown that members of this species are able to act on others’ knowledge states when faced with one-shot competitive problems that emulate the foraging interactions these monkeys routinely face in their social lives (Flombaum & Santos, 2005; Santos et al., 2006). We therefore used a one-shot foraging task to examine rhesus monkeys’ metacognition. Subjects observed a human appearing to bait an apparatus with food and could either directly approach the location that had been baited or peer into a center location that provided visual access to both possible food locations. Note that our task is the first to test nonhuman metacognition in the absence of any prior experimental experiences with the task: Each monkey completed only a single trial and was therefore naive to the novel affordances of our setup. The monkeys had to infer the functionality of using the central vantage point to determine the food’s location—a strategy that would reflect humanlike reasoning about their own ignorance.
Method
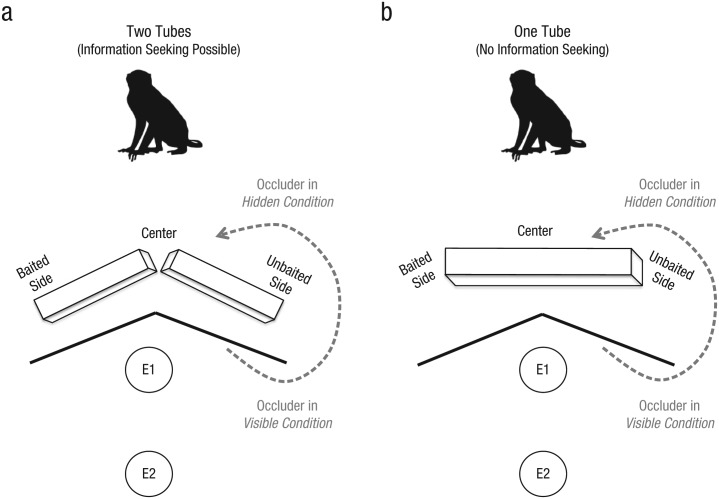
Rhesus monkeys (Macaca mulatta) completed a single trial in which they could approach an apparatus to search for hidden food. In the two-tubes condition, they saw two tubes placed in a V formation, and an experimenter appeared to bait one of the distal side openings with food (see Fig. 1a). We examined whether monkeys who lacked knowledge about the food’s location would spontaneously recognize their ignorance and remedy it by engaging in information seeking. We predicted that the monkeys would preferentially look in the correct side if they saw where the food had been placed (visible-baiting condition; see Video S1 in the Supplemental Material available online), but would instead approach the center to peer into both tubes at the same time (and thus potentially gain information about the food’s location) if they lacked this knowledge (hidden-baiting condition; see Video S2 in the Supplemental Material). We also examined whether center looks reflected true information seeking by using a one-tube condition, in which such searches did not provide any new perceptual information. Specifically, a different set of monkeys saw the experimenter appear to bait one side of a single tube without any center opening through which to see the sides (see Fig. 1b; see Videos S3 and S4 in the Supplemental Material). We predicted that these monkeys would be less likely than those in the two-tubes condition to approach the center, because they could not gain any information from doing so. We again included both hidden- and visible-baiting conditions in this one-tube condition, which allowed us to examine whether the monkeys exhibited a general pattern of exploratory searching whenever they did not know the food’s location (as argued by Carruthers, 2008), or flexibly showed this response when it could generate new useful information in the two-tubes condition. Finally, we examined the monkeys’ decision latencies to test our prediction that engaging metacognitive processes would result in slower reaction times than would searching at known locations of food.
Fig. 1.
Setup for the study. In the two-tubes condition (a), the monkeys could approach the center location to peer into both tubes from the same vantage point; such information seeking was not possible in the one-tube condition (b). In both situations, the monkeys either saw the baiting event (visible baiting; side counterbalanced across subjects) or did not (hidden baiting) because Experimenter 1 (E1) first moved the occluder to block their view. Experimenter 2 (E2) filmed the trial from a farther distance.
Subjects
We tested 30 monkeys in each of four conditions, selecting a sample size similar to that in prior research conducted with the same monkey population at Cayo Santiago using methods in which monkeys’ responses involved approaching different objects or locations (e.g., Flombaum & Santos, 2005; Santos et al., 2006). The final sample of 120 monkeys included 50 females and 70 males, with a mean age of 9.7 years (range: 1.9–25.6 years; the sex and age distributions were similar across the four conditions). The Cayo Santiago population in Puerto Rico consists of more than 1,200 individually identifiable monkeys living in natural social groups (Rawlins & Kessler, 1987). The monkeys tested in this study were naive to the apparatus. No monkeys in the population at the time of our study had previously participated in studies in which they were required to retrieve objects from inside opaque tubes or look inside tubes to identify the location of food, although some had participated in studies in which they could approach platforms or boxes that contained food.
Some monkeys that were approached for testing did not produce responses that could be scored. The most common reason was that there was interference by other monkeys (n = 42; see Video S5 in the Supplemental Material); for example, sometimes the target was displaced by another (often higher-ranking) monkey and was unable to approach the apparatus, or another monkey who did not properly observe the experimenter’s demonstration approached the apparatus (typically from the side) before the target subject approached from its appropriately centered position. In other cases, the monkey left the testing area (e.g., walked away into a bush) without clearly searching in the apparatus at all before the preset 1-min cutoff we timed with a stopwatch (n = 19; see Video S6 in the Supplemental Material), perhaps because of the presence or activity of other monkeys in the vicinity (e.g., the monkey may have heard a fight). In still other cases, the monkey simply did not approach the apparatus within the 1-min cutoff (n = 15; see Video S7 in the Supplemental Material), which could have been because of disinterest or because of unrelated reasons, such as the presence of a higher-ranking monkey making the target monkey afraid to approach. Finally, in one case, the monkey ran to the apparatus, grabbed it, and pulled it into a tree. These monkeys did not produce scorable responses in this task and thus were not part of the final sample of 120 monkeys included in analyses. Note that the completion rate in this study was similar to the completion rates in past work using similar approach measures in this free-ranging population (e.g., Flombaum & Santos, 2005; Santos et al., 2006).
Procedure
Each monkey completed one trial. First, two experimenters approached a calmly sitting monkey. Experimenter 1, the actor, knelt approximately 2 m away from the monkey; Experimenter 2 stood about 2 m further away and filmed the testing area with a handheld camera.
At the start of the trial, Experimenter 1 placed a black folding occluder (made of poster board; each wing 30 in. wide by 20 in. tall) in front of her (so that it blocked her shoulders and arms from the monkey’s perspective). In the two-tubes condition, she placed two large white tubes (20 in. long, 4 in. wide, 4 in. tall; made of poster board) on the ground in front of the occluder. She then picked up the tubes so their open ends were oriented toward the monkey, and tapped them together so that the monkey attended and could observe that they were empty. Next, she placed the tubes on the ground in a V formation, with the point oriented toward the monkey (the bottoms of the tubes attached to a small piece of poster board with Velcro, to ensure that they were always positioned in the same orientation). Consequently, the monkey could approach the tubes and peer into both from the center opening, where the tubes met. In the one-tube condition, the experimenter placed one white tube (30 in. long) on the ground in front of her, picked it up so that its open end was oriented toward the monkey, and then tapped the tube with her other hand so the monkey would attend and see that it was empty. She then placed the tube on the ground with its open ends perpendicular to the monkey. In both conditions, the distal sides of the tubes were 30 in. apart, so the distance costs were equivalent between conditions; this distance was designed to be wide enough to pose a small travel cost if the monkey approached the wrong location, but still enable the experimenter to manipulate the apparatus.
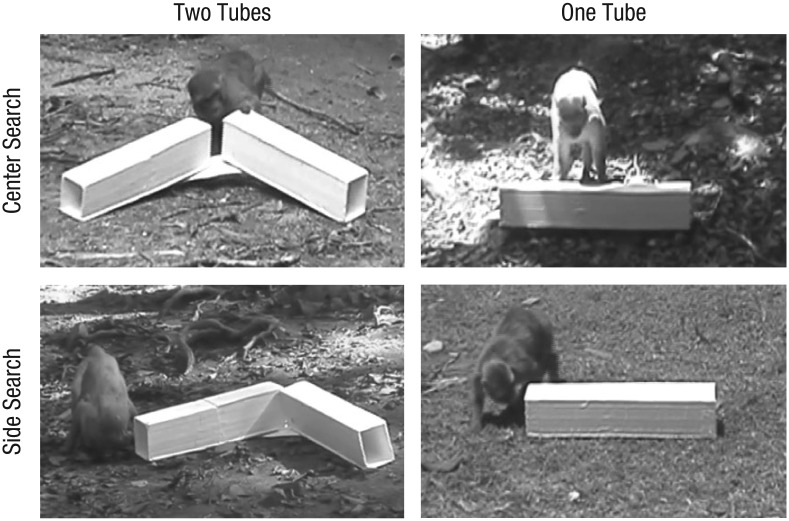
In the visible-baiting conditions, Experimenter 1 left the occluder in its initial location behind the tube or tubes, so that the monkey could see the apparatus during the baiting process. She held up a food reward (a plastic cherry), and as the monkey was watching, she moved her hand to one of the distal tube ends (left or right, counterbalanced across subjects), using the matching hand to make this movement. She appeared to place the fruit inside that specific end by reaching inside and joggling her hand for 3 s (in fact, she used a typical fake-baiting procedure in which she removed the fruit in her palm surreptitiously). In the hidden-baiting conditions, the procedure was similar except that the experimenter moved the occluder in front of the tube or tubes before the baiting event. After holding up the fruit (using either the left or the right hand, counterbalanced across subjects), she moved it downward and joggled her hand for 3 s, but the occluder blocked her body movements from the monkey’s perspective. In all conditions, the experimenter then picked up the occluder and walked back to Experimenter 2 (in a straight line, to avoid biasing the monkey toward one side), so that the monkey would feel comfortable approaching the tube or tubes (see Fig. 2 for examples of the monkeys’ responses).
Fig. 2.
Screen captures of search responses. In the two-tubes condition (left column) and one-tube condition (right column), monkeys could either look in the center (top row) or check a distal side (bottom row).
Coding and data analysis
All sessions were videotaped and coded from video by two independent coders. Each video was clipped to start when Experimenter 1 stood up to walk toward Experimenter 2. The new clips were then given random identification labels so that the coders could score the monkeys’ responses blind to knowledge condition (hidden or visible baiting) and baiting location (left or right); whether there was one or two tubes present was inherently visible in the videos. The coders scored the video clips for several measures assessing how the monkeys approached and searched for the food.
First, the coders scored each monkey’s first look: whether the monkey first searched at the left side, right side, or center location. A response was counted as a center look if the monkey looked into the two tubes from the center position or walked down the midline and stared behind or under the center of the tube or tubes (as the monkeys could not actually look inside the tubes from the center position in the one-tube setup, we scored approaching the center and looking down behind or under the tubes as a center approach in both conditions in order to keep the coding consistent across conditions). Second, the coders scored each subject’s reaction time (i.e., the time from the beginning of the clip, when the experimenter left the apparatus, until the first look), so that we could examine if engaging metacognitive processes would result in slower reaction times than would searching at known locations of food. Third, when a monkey in the two-tubes condition initially searched at the center, the coders scored whether the monkey checked both sides by visibly looking into both tubes from the center (such a response was not possible in the one-tube condition). The two coders showed perfect agreement for the location of the first search (κ = 1.0); their scored reaction times for this search were highly correlated (r = .99), and their agreement for whether the monkey checked both sides was excellent (κ = .87; agreement on 69 of 72 trials with center searches).
Results
We first examined the monkeys’ propensity to approach the center location first (see Fig. 3). In the hidden-baiting, two-tubes condition, 27 of the 30 monkeys approached and looked at the center location; this number was significantly above chance (there were three potential responses: center, correct side, or incorrect side), χ2(2, N = 30) = 43.40, p < .001. In contrast, in the visible-baiting, two-tubes condition, only 13 of the 30 monkeys looked in the center; 17 approached the correct side, and this number was above chance, χ2(2, N = 30) = 15.80, p < .001. Moreover, the monkeys were much less likely to approach the center location when there was no opening to look through: Only 8 of the 30 monkeys in the hidden-baiting, one-tube condition did so (12 approached the correct side), χ2(2, N = 30) = 0.80, p = .67, and no monkeys in the visible-baiting, one-tube condition did so (28 approached the correct side), χ2(2, N = 30) = 48.80, p < .001. Thus, in the one-tube condition, in which it was not possible to gain any additional knowledge by approaching the center, the majority of the monkeys approached one of the side openings, regardless of whether they had seen the baiting. Note that this was the case even though the experimenter’s actions were identical in the one-tube and the two-tubes conditions, which suggests that the monkeys did not actually infer that she had placed the food in the center of the array if they could not see the baiting. Thus, the center searches in the two-tubes condition likely reflected actual information seeking.
Fig. 3.
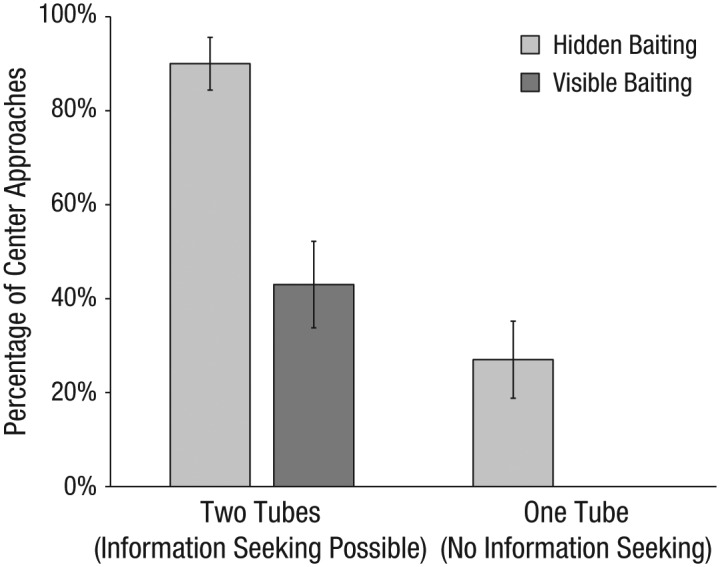
Percentage of monkeys that approached the center location first in the four conditions. Error bars indicate ±1 SE.
To compare the monkeys’ propensity to look in the center in the four conditions, we performed logistic regression in R (R Core Team, 2015). We used the glm function to fit binomial models with a logit link function, and compared model fit using likelihood ratio tests. We first fitted a basic model with sex (as a factor) and age (as a covariate) to account for individual subjects’ characteristics. To assess the importance of viewing the baiting event, we then included knowledge condition (hidden or visible baiting) as a predictor. This improved model fit, χ2(1) = 18.21, p < .001, which indicated that the monkeys were more likely to approach the center if they had not seen the baiting. In the third model, we included information-seeking condition (two tubes or one tube) to account for the effect of whether it was possible to gain new knowledge by searching at the center. We found that this model had better fit than the second model, χ2(1) = 50.0, p < .001: The monkeys were more likely to approach the center when information seeking was possible (in the two-tubes condition) than when it was not (in the one-tube condition). In addition, this model revealed that, across conditions, males searched at the center more often than females did (see Table 1 for parameters from this best-fitting model). In summary, our regression results show that the monkeys were more likely to search at the center if they lacked knowledge about the baiting location (in the hidden conditions, in which their view had been obstructed) and if information seeking was actually possible (in the two-tubes condition, in which they could check inside both tubes).
Table 1.
Parameters From the Best-Fitting Model Predicting Propensity to Search in the Center Location
| Predictor | Estimate | SE | z | p |
|---|---|---|---|---|
| Age (covariate) | 0.037 | 0.048 | 0.773 | .44 |
| Sex (reference: female) | 1.226 | 0.567 | 2.162 | < .05 |
| Knowledge condition (reference: visible baiting) | 3.016 | 0.709 | 4.255 | < .001 |
| Information-seeking condition (reference: two tubes) | −3.842 | 0.739 | −5.198 | < .001 |
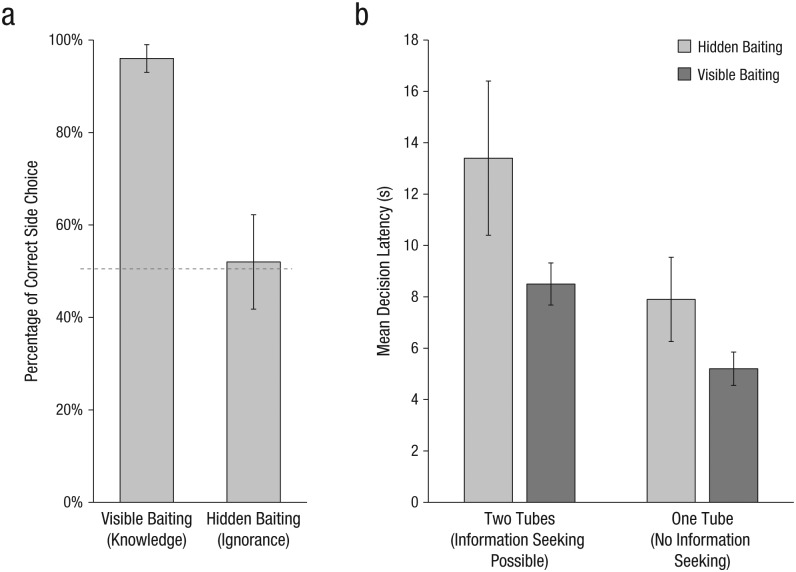
We performed several checks of these results. First, we examined whether the monkeys’ approaches to the side locations reflected true knowledge about the baiting event (see Fig. 4a). Of the monkeys that approached a side in the visible-baiting conditions, 96% chose the correct location (binomial test, n = 47, p < .001). In contrast, in the hidden conditions, only 52% of the monkeys that approached a side approached the correct side, which was not different from chance (n = 25, p = 1.00). These results indicate that the monkeys did indeed recall the location if they saw the baiting event, but were not able to infer the correct location using some other cue (e.g., the hand the experimenter used or her general body movements) if their view was obscured by the barrier.
Fig. 4.
Percentage of correct side choices and decision latencies. The graph in (a) shows the percentage of subjects (among those who chose to approach a side) that approached the correct side in the two knowledge conditions; the dashed line indicates chance performance. The graph in (b) presents the mean reaction time in each of the four conditions. Error bars indicate ±1 SE.
Second, we checked whether the monkeys actually tried to look into both tubes when they approached the center in the two-tubes condition (see Video S2 for an example of this response). In fact, the majority of monkeys that looked in the center (74% in the hidden-baiting condition, 62% in the visible-baiting condition) were scored as visibly checking both tubes when they peered inside, and these percentages did not differ between the visible- and hidden-baiting conditions, Pearson χ2(1, N = 40) = 0.66, p > .4. This further supports the interpretation of center searches as information-seeking responses.
Finally, we examined the monkeys’ decision latencies, as we predicted that engaging metacognitive processes would result in slower reaction times. We first looked at average response latencies in a univariate analysis of variance with knowledge condition (hidden or visible baiting) and information-seeking condition (two tubes or one tube) as between-subjects factors (see Fig. 4b). In fact, the monkeys were slower to search when the baiting event was hidden, and they did not know where the food had been placed (M = 11.0 s, SE = 1.6), than when it was visible (M = 6.6 s, SE = 0.9), F(1, 116) = 5.93, p < .05, ηp2 = .049. They were also slower to search when information seeking was possible (two-tubes condition: M = 10.7 s, SE = 1.8) than when it was not (one-tube condition: M = 6.9, SE = 0.6), F(1, 116) = 4.45, p < .05, ηp2 = .037. There was no interaction between knowledge condition and information-seeking condition. That is, the monkeys took longer to make a decision if they lacked knowledge or if approaching the center to engage in information seeking was an option.
We then further broke down the decision latencies to see if they were related to the monkeys’ actual choice patterns. We first compared reaction times when monkeys in the two-tubes condition approached the correct (baited) side and when they approached the center (i.e., excluding those few individuals that made clear errors, such as by approaching the incorrect side). In fact, the monkeys’ decision latencies were longer when they looked at the center location (M = 12.0 s, SE = 2.1) than when they looked at the correct side (M = 4.7 s, SE = 0.5), t(55) = 3.30, p < .005, Cohen’s d = 0.64, and latencies to search at the center did not differ between the two knowledge conditions (hidden vs. visible baiting), t(38) = −0.04, p > .97, Cohen’s d = 0.01. That is, monkeys that decided to approach the center and seek information showed slower latencies than those that approached the correct side, regardless of whether they had seen the baiting. This pattern of results suggests that information seeking—by looking in the center opening—was driven by similar cognitive processes among monkeys who had and had not previously seen the baiting.
We then examined response latencies in the one-tube condition, in which information seeking was not possible. In this condition, monkeys that did not see the baiting showed similar response latencies when they approached a side (M = 8.3 s, SE = 0.7) and when they approached the center (M = 9.3 s, SE = 2.6), t(28) = 0.52, p > .6, Cohen’s d = 0.22. Moreover, the monkeys in the one-tube condition were faster to approach a side if they had seen the baiting (M = 4.9 s, SE = 0.6) than if they had not, t(48) = 3.66, p = .001, Cohen’s d = 1.04. Thus, when the monkeys did not know the location of the food and could not seek out more information, they showed similar slow responses regardless of whether they ultimately decided to approach the center or a side. Overall, these results for decision latency indicate that the monkeys were slower to make a choice when faced with situations that should engage more metacognitive monitoring: when they did not know the location of the food because their view of the baiting event was obscured, as well as when information seeking was possible because there was a center opening to look through.
Discussion
Taken together, these results provide strong evidence that monkeys are sensitive to their own knowledge state and exhibit spontaneous information-seeking responses when they are ignorant. In particular, the monkeys checked the center more often when they did not know the location of food than when they did—but only occasionally approached the center when there was no opening to look through. In contrast to previous work examining similar information-seeking responses (Basile et al., 2009; Beran et al., 2013; Call, 2010; Call & Carpenter, 2001; Hampton et al., 2004; Paukner et al., 2006), our study involved only a single trial. Our results therefore cannot be explained by prior experience or learning in the task and provide the first evidence that any nonhuman species shows information-seeking responses in a one-shot situation. Our results suggest that monkeys may engage metacognitive processes in a humanlike fashion, effortlessly recognizing their own knowledge states and automatically seeking out new information.
Together with previous work (Flombaum & Santos, 2005; Marticorena et al., 2011; Santos et al., 2006), our results suggest that monkeys can think about knowledge states in others as well as in themselves. These results stand in contrast to previous evidence that macaques make information-seeking responses only following explicit training (Hampton et al., 2004)—as well as broader evidence that monkeys show less sensitive information-seeking responses compared with apes (Basile et al., 2009; Paukner et al., 2006). One possible explanation for this discrepancy is that monkeys exhibit more robust metacognitive abilities when faced with more naturalistic situations that emulate typical foraging problems. Similarly, it has been proposed that primates often show particularly robust theory-of-mind skills in competitive foraging contexts that are ecologically valid (Hare, 2001). Overall, this set of results suggests that rhesus macaques—like apes—can represent both their own and others’ knowledge states, a pattern of evolutionary coherence that supports the hypothesis that metacognition and theory of mind share underlying cognitive mechanisms.
In our study, the monkeys exhibited slower decision latencies in situations predicted to engage metacognitive processes: when the monkeys lacked information about the food’s location or when information seeking was generally possible. Previous work showed that apes were more likely to engage in information seeking to locate hidden food when they experienced a delay after viewing a baiting event, but before they could make a response (Call, 2010; Call & Carpenter, 2001). One explanation for this finding is that subjects forgot the baited location during the delay; alternatively, they may have double-checked their memory of the baited location when there was a delay. It is possible that similar processes drove some of the monkeys’ responses in our study. However, it is also important to note that the current results reflect the intrinsic response latencies of the decision makers, rather than effects of an externally imposed delay.
Finally, we found that monkeys that were presented with information-seeking opportunities were willing to pay a small energetic cost (i.e., to peer into the tubes at the center location)—even when they did not need to do so. Indeed, more than a third of the monkeys that directly saw the baiting event in the two-tubes condition nonetheless peered into the center first. Given that the vast majority of the monkeys approached the correct side in the visible-baiting, one-tube condition, in which such information seeking was not possible, it is unlikely that these center searches in the visible-baiting, two-tubes condition represented a memory failure. One possibility is that the monkeys checked the center because the costs of doing so were generally very low, given that the center location was slightly closer to their starting position than the correct end of the tube was. In line with this view, previous work has indicated that primates will engage in information seeking when it is unnecessary—but are less likely to do so as the costs associated with information seeking increase (Call, 2010; Hampton et al., 2004). Further research could test this possibility in our task by manipulating the accessibility of the vantage point that provides visible access to the potentially baited locations.
An alternative explanation is that rhesus monkeys value metacognitive information seeking in and of itself, and are intrinsically motivated to seek information. In humans, the set of cognitive, emotional, and motivational processes reflecting a desire to learn what is unknown is called curiosity (Loewenstein, 1994). People attach inherent value to acquiring information (Gottlieb, Oudeye, Lopes, & Baranes, 2013) and are willing to give up time and resources to acquire it (Kang et al., 2009). Although there is some evidence that other animals engage in costly information seeking in computer-based tasks (Kidd & Hayden, 2015), it is currently unclear if these responses stem from humanlike metacognitive awareness of ignorance. Our results hint at the possibility that other primates may also be intrinsically motivated to remedy uncertainty, and future research could explicitly examine how they trade off between information and the costs of acquiring it. Disentangling whether nonhumans do share these motivational components is a critical next step in illuminating the evolution of human metacognition.
Supplementary Material
Acknowledgments
We thank Alyssa Arre and Samantha Monier for assistance with data collection and coding; Steve Worthington at Harvard’s Institute for Quantitative Social Science for advice on statistical analyses; and Angelina Ruiz Lambides, Nahiri Rivera Barreto, and Giselle Caraballo Cruz for support at Cayo Santiago. This research was approved by the Institutional Animal Care and Use Committee of Cayo Santiago and by Yale University.
Footnotes
Action Editor: Leaf Van Boven served as action editor for this article.
Declaration of Conflicting Interests: The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
Funding: This research was supported by the National Institute of Mental Health (R01MH096875) and by National Center for Research Resources Grant CM-5-P40RR003640-13, awarded to the Caribbean Primate Research Center and the University of Puerto Rico–Medical Sciences Campus. L. R. Santos was supported by a James S. McDonnell Foundation Award (220020242) and by Yale University.
Supplemental Material: Additional supporting information can be found at http://pss.sagepub.com/content/by/supplemental-data
Open Practices:


All data and materials have been made available via Dryad and can be accessed at http://dx.doi.org/10.5061/dryad.5t0b8. The complete Open Practices Disclosure for this article can be found at http://pss.sagepub.com/content/by/supplemental-data. This article has received badges for Open Data and Open Materials. More information about the Open Practices badges can be found at https://osf.io/tvyxz/wiki/1.%20View%20the%20Badges/ and http://pss.sagepub.com/content/25/1/3.full.
References
- Basile B. M., Hampton R. R., Suomi S. J., Murray E. A. (2009). An assessment of memory awareness in tufted capuchin monkeys (Cebus apella). Animal Cognition, 12, 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran M. J., Smith J. D., Perdue B. M. (2013). Language-trained chimpanzees (Pan troglodytes) name what they have seen but look first at what they have not seen. Psychological Science, 24, 660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräuer J., Call J., Tomasello M. (2004). Visual perspective taking in dogs (Canis familiaris) in the presence of barriers. Applied Animal Behaviour Science, 88, 299–317. [Google Scholar]
- Call J. (2010). Do apes know that they could be wrong? Animal Cognition, 13, 689–700. [DOI] [PubMed] [Google Scholar]
- Call J., Carpenter M. (2001). Do apes and children know what they have seen? Animal Cognition, 3, 207–220. [Google Scholar]
- Carruthers P. (2008). Meta-cognition in animals: A skeptical look. Mind & Language, 23, 58–89. [Google Scholar]
- Castro L., Wasserman E. A. (2013). Information-seeking behavior: Exploring metacognitive control in pigeons. Animal Cognition, 16, 241–254. [DOI] [PubMed] [Google Scholar]
- Flavell J. H. (1979). Metacognition and cognitive monitoring: A new area of cognitive–developmental inquiry. American Psychologist, 34, 906–911. [Google Scholar]
- Flavell J. H. (1999). Cognitive development: Children’s knowledge about the mind. Annual Review of Psychology, 50, 21–45. [DOI] [PubMed] [Google Scholar]
- Flombaum J. I., Santos S. (2005). Rhesus monkeys attribute perceptions to others. Current Biology, 15, 447–452. [DOI] [PubMed] [Google Scholar]
- Foote A. L., Crystal J. D. (2012). “Play it Again”: A new method for testing metacognition in animals. Animal Cognition, 15, 187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith C. D. (2012). The role of metacognition in human social interactions. Philosophical Transactions of the Royal Society B: Biological Sciences, 367, 2213–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb J., Oudeye P. Y., Lopes M., Baranes A. (2013). Information-seeking, curiosity, and attention: Computational and neural mechanisms. Trends in Cognitive Sciences, 17, 585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goupil L., Romand-Monnier M., Kouider S. (2016). Infants ask for help when they know they don’t know. Proceedings of the National Academy of Sciences, USA, 113, 3492–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton R. R. (2001). Rhesus monkeys know when they remember. Proceedings of the National Academy of Sciences, USA, 98, 5359–5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton R. R., Zivin A., Murray E. A. (2004). Rhesus monkeys (Macaca mulatta) discriminate between knowing and not knowing and collect information as needed before acting. Animal Cognition, 7, 239–246. [DOI] [PubMed] [Google Scholar]
- Hare B. (2001). Can competitive paradigms increase the validity of experiments on primate social cognition? Animal Cognition, 4, 269–280. [DOI] [PubMed] [Google Scholar]
- Hare B. (2011). From hominoid to hominid mind: What changed and why? Annual Review of Anthropology, 40, 293–309. [Google Scholar]
- Hare B., Call J., Tomasello M. (2006). Chimpanzees deceive a human competitor by hiding. Cognition, 101, 495–514. [DOI] [PubMed] [Google Scholar]
- Jozefowiez J., Staddon J. E. R., Cerutti D. T. (2009). Metacognition in animals: How do we know that they know? Comparative Cognition & Behavior Reviews, 4, 29–39. [Google Scholar]
- Kaminski J., Call J., Tomasello M. (2008). Chimpanzees know what others know, but not what they believe. Cognition, 109, 224–234. [DOI] [PubMed] [Google Scholar]
- Kang M. J., Hsu M., Krajbich I. M., Loewenstein G., McClure S. M., Wang J. T., Camerer C. F. (2009). The wick in the candle of learning: Epistemic curiosity activates reward circuitry and enhances memory. Psychological Science, 20, 963–973. [DOI] [PubMed] [Google Scholar]
- Kelemen W. L., Winningham R. G., Weaver C. A. (2007). Repeated testing sessions and scholastic aptitude in college students’ metacognitive accuracy. European Journal of Cognitive Psychology, 19, 689–717. [Google Scholar]
- Kepecs A., Mainen Z. F. (2012). A computational framework for the study of confidence in humans and animals. Philosophical Transactions of the Royal Society B: Biological Sciences, 367, 1322–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepecs A., Uchida U., Zariwala H. A., Mainen Z. F. (2008). Neural correlates, computation and behavioural impact of decision confidence. Nature, 455, 227–231. [DOI] [PubMed] [Google Scholar]
- Kidd C., Hayden B. Y. (2015). The psychology and neuroscience of curiosity. Neuron, 88, 449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koriat A. (2007). Metacognition and consciousness. In Zelazo P. D., Thompson E. (Eds.), The Cambridge handbook of consciousness (pp. 289–325). Cambridge, England: Cambridge University Press. [Google Scholar]
- Kornell N. (2009). Metacognition in humans and animals. Current Directions in Psychological Science, 18, 11–15. [Google Scholar]
- Kornell N., Son L. K., Terrace H. S. (2007). Transfer of metacognitive skills and hint seeking in monkeys. Psychological Science, 18, 64–71. [DOI] [PubMed] [Google Scholar]
- Kuhn D. (2000). Metacognitive development. Current Directions in Psychological Science, 9, 178–181. [Google Scholar]
- Le Pelley M. E. (2012). Metacognitive monkeys or associative animals? Simple reinforcement learning explains uncertainty in nonhuman animals. Journal of Experimental Psychology: Learning, Memory, and Cognition, 38, 686–708. [DOI] [PubMed] [Google Scholar]
- Loewenstein G. (1994). The psychology of curiosity: A review and reinterpretation. Psychological Bulletin, 116, 75–98. [Google Scholar]
- Marsh H. L., MacDonald S. E. (2012). Orangutans (Pongo abelii) “play the odds”: Information-seeking strategies in relation to cost, risk, and benefit. Journal of Comparative Psychology, 126, 263–278. [DOI] [PubMed] [Google Scholar]
- Marticorena D. C., Ruiz A. M., Mukerji C., Goddu A., Santos L. R. (2011). Monkeys represent others’ knowledge but not their beliefs. Developmental Science, 14, 1406–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A., Santos L. R. (2014). The origins of belief representation: Monkeys fail to automatically represent others’ beliefs. Cognition, 130, 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon S., Macpherson K., Roberts W. A. (2010). Dogs choose a human informant: Metacognition in canines. Behavioural Processes, 85, 293–298. [DOI] [PubMed] [Google Scholar]
- Melis A., Call J., Tomasello M. (2006). Chimpanzees (Pan troglodytes) conceal visual and auditory information from others. Animal Behaviour, 120, 154–162. [DOI] [PubMed] [Google Scholar]
- Metcalfe J., Shimamura A. P. (1994). Metacognition: Knowing about knowing. Cambridge, MA: MIT Press. [Google Scholar]
- Nelson T. O., Narens L. (1990). Metamemory: A theoretical framework and new findings. In Bower G. H. (Ed.), The psychology of learning and motivation (Vol. 26, pp. 125–173). San Diego, CA: Academic Press. [Google Scholar]
- Paukner A., Anderson J. R., Fujita K. (2006). Redundant food searches by capuchin monkeys (Cebus apella): A failure of metacognition? Animal Cognition, 9, 110–117. [DOI] [PubMed] [Google Scholar]
- Perry C. J., Barron A. B. (2013). Honey bees selectively avoid difficult choices. Proceedings of the National Academy of Sciences, USA, 110, 19155–19159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2015). R (Version 3.2.3) [Computer software]. Retrieved from https://www.R-project.org/
- Rawlins R. C., Kessler M. J. (Eds.). (1987). The Cayo Santiago macaques: History, behavior and biology. Albany: State University of New York Press. [Google Scholar]
- Roberts W. A., McMillan N., Musoline E., Cole M. (2012). Information seeking in animals: Metacognition? Comparative Cognition & Behavior Reviews, 7, 85–109. [Google Scholar]
- Santos L. R., Nissen A. G., Ferrugia J. A. (2006). Rhesus monkeys, Macaca mulatta, know what others can and cannot hear. Animal Behaviour, 71, 1175–1181. [Google Scholar]
- Santos L. R., Rosati A. G. (2015). The evolutionary roots of human decision making. Annual Review of Psychology, 66, 321–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz B. L. (1994). Sources of information in metamemory: Judgments of learning and feelings of knowing. Psychonomic Bulletin & Review, 1, 357–375. [DOI] [PubMed] [Google Scholar]
- Shields W., Smith J., Washburn D. (1997). Uncertain responses by humans and rhesus monkeys (Macaca mulatta) in a psychophysical same-different task. Journal of Experimental Psychology: General, 126, 147–164. [DOI] [PubMed] [Google Scholar]
- Smith J. D., Beran M. J., Cosuchman J. J., Coutinho V. C. (2008). The comparative study of metacognition: Sharper paradigms, safer inferences. Psychonomic Bulletin & Review, 15, 679–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. D., Schull J., Strote J., McGee K., Egnor R., Erb L. (1995). The uncertain response in the bottlenosed dolphin (Tursiops truncatus). Journal of Experimental Psychology: General, 124, 391–408. [DOI] [PubMed] [Google Scholar]
- Smith J. D., Shields W. E., Allendoerfer K. R., Washburn W. A. (1998). Memory monitoring by animals and humans. Journal of Experimental Psychology: General, 127, 227–250. [DOI] [PubMed] [Google Scholar]
- Smith J. D., Shields W. E., Schull J., Washburn D. A. (1997). The uncertain response in humans and animals. Cognition, 62, 75–97. [DOI] [PubMed] [Google Scholar]
- Terrace H. S., Son L. K. (2009). Comparative metacognition. Current Opinion in Neurobiology, 19, 67–74. [DOI] [PubMed] [Google Scholar]
- Vo V. A., Li R., Kornell N., Pouget A., Cantlon J. F. (2014). Young children bet on their numerical skills: Metacognition in the numerical domain. Psychological Science, 25, 1712–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.