Abstract
Background:
We tested the hypothesis that nebulized budesonide would improve lung mechanics and oxygenation in patients with early acute lung injury (ALI) and/or acute respiratory distress syndrome (ARDS) during protective mechanical ventilation strategy without adversely affecting systemic hemodynamics.
Methods:
Patients with ALI/ARDS were included and assigned into two groups; budesonide group (30 cases) in whom 1 mg–2 ml budesonide suspension was nebulized through the endotracheal tube and control group (30 cases) in whom 2 ml saline (placebo) were nebulized instead of budesonide. This regimen was repeated every 12 h for three successive days alongside with constant ventilator settings in both groups. Hemodynamics, airway pressures, and PaO2/FiO2 were measured throughout the study period (72 h) with either nebulized budesonide or saline. Furthermore, tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-6 (IL-6) were analyzed serologically as markers of inflammation at pre- and post-nebulization sessions.
Results:
We found a significant difference between the two groups regarding PaO2/FiO2 (P = 0.023), peak (P = 0.021), and plateau (P = 0.032) airway pressures. Furthermore, TNF-α, IL-1β, and IL-6 were significantly reduced after budesonide nebulizations. No significant difference was found between the two groups regarding hemodynamic variables.
Conclusion:
Nebulized budesonide improved oxygenation, peak, and plateau airway pressures and significantly reduced inflammatory markers (TNF-α, IL-1β and IL-6) without affecting hemodynamics.
Trial Registry:
Australian New Zealand Clinical Trial Registry (ANZCTR) at the number: ACTRN12615000373572.
Key words: Acute lung injury, inflammatory markers, nebulized budesonide
Introduction
Acute lung injury/acute respiratory distress syndrome (ALI/ARDS) is a critical illness characterized by increased permeability of alveolo-capillary barrier. Furthermore, fluid clearance is reduced, resulting in congestion, atelectasis, and alveolar flooding which can impair gas exchange and respiratory mechanics.[1,2] The hallmark of ALI/ARDS on the cellular level is increased pulmonary capillary permeability and fluid leakage into the pulmonary parenchyma due to the release of neutrophils, cytokines, and other inflammatory mediators[3] in response to injury or infection.
Considering the fact that inflammation is the cornerstone in pathogenesis of ALI/ARDS, inhaled corticosteroids, which are much less likely to produce systemic adverse events than systemic corticosteroids,[4] are the most potent and consistently effective anti-inflammatory agents available and they have been used successfully in a variety of animal models of ALI.[5] Furthermore, there is a valuable sufficient evidence to support a preliminary clinical trial of inhaled corticosteroids in patients at high risk of ARDS as well as with early and/or late ARDS using markers of inflammation as a primary outcome.[6]
Budesonide is a locally-acting glucocorticoid typically administered through nebulization and has a powerful anti-inflammatory action, reduces airway edema, inhibits airway remodeling and is widely used in treating chronic obstructive pulmonary disease, bronchial asthma and many other pulmonary disorders.[7] In the last years, perioperative use of nebulized budesonide in selected patients has led to satisfactory pulmonary protective effects.[8] The aim of this study is to assess the efficacy of nebulized budesonide in improving respiratory mechanics, reducing markers of inflammation (tumor necrosis factor-alpha [TNF-α], interleukin-1 beta [IL-1β] and interleukin-6 [IL-6]) and improving patient's oxygenation in patients with ALI/ARDS.
Methods
This randomized, double-blind, placebo-controlled study was carried out in Qena University Hospital's Intensive Care Unit (ICU) in the period from January 2014 to January 2016. Written informed consent was taken from every patient's relatives or caregivers.
Ethical approval for this study was provided by the Ethical Committee of Qena University Hospitals, Qena, Egypt (Chairperson Prof. Ahmad Abolyosr) on January 3, 2014.
The study was registered in the Australian New Zealand Clinical Trials Registry (ANZCTR) and the allocated number is: ACTRN12615000373572. The web address of the trial is http://www.ANZCTR.org.au/ACTRN12615000373572.aspx.
Assigning patients and ventilator settings
We enrolled 60 patients who were either admitted directly to the ICU or referred to the ICU from chest diseases department. The study interventions began within 6–12 h of ALI/ARDS confirmation of diagnosis. Patients were randomly assigned to one of two groups according to a computer-generated randomization table. Group A (n = 30) patients received nebulized budesonide and Group B (n = 30) patients received nebulized saline solution as a placebo. Enrollment and assigning patients to each group were done by a physician different from the one doing nebulization of the drug. Patients should fulfill the criteria of the ALI/ARDS according to the 2012 Berlin definition of ALI/ARDS[9] to be included in the study [Table 1]. Exclusion criteria were refusal of consent (by relatives), age younger than 18 years or older than 65 years, chronic obstructive pulmonary disease, restrictive respiratory insufficiency, pneumonia, increased intracranial pressure, bronchopleural fistula, the persistence of unstable postsurgical hemodynamics despite appropriate supportive therapy, liver cell failure (Child-Pugh Class B or C), end-stage chronic renal failure on hemodialysis, acute myocardial infarction, and neuromuscular disease.
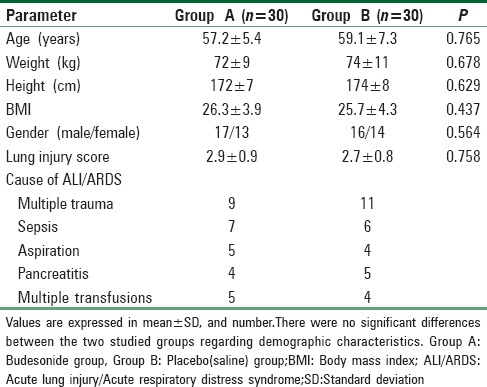
Table 1.
Patient's characteristics
On admission, plain chest X-ray films to the patients were taken for confirmation of diagnosis and follow-up. All patients had a radial arterial line for pressure monitoring and arterial blood sampling. Arterial blood samples were taken for blood gas analysis (using ABL800 blood gas analyzer) and calculating the PaO2/FiO2 to assess the degree of severity of ALI/ARDS. Patients were anesthetized by propofol 2% 1–2 mg/kg intravenous (IV) and intubated after relaxation with atracurium 0.4–0.6 mg/kg IV followed by propofol infusion (5–50 µg/kg/min) through a syringe pump or midazolam (0.02–0.1 mg/kg/min) infused intravenously in 50 ml crystalloid solution. Daily interruption of continuous sedative infusion was considered at a fixed time every morning to allow for accurate neurological assessment. Atracurium was continuously infused as (0.3–0.6 mg/kg/h) for relaxation and proper measurement of respiratory mechanics. Patients were ventilated with Nellcor Puritan Bennett 840 ventilator system (USA). Ventilator was set on volume-controlled mode with a tidal volume of 6 ml/kg (predicted body weight) and respiratory rate that kept carbon dioxide tension between 35 and 45 mmHg. The fraction of inspired oxygen (FiO2) was set at 1.0 during the study period. Inspiration: Expiration time ratio was set as 1:2. Positive end-expiratory pressure (PEEP) was set either 5 cmH2O above the lower inflection point or 10 cmH2O in the absence of the lower inflection point (protective mechanical ventilation strategy).[10]
This was a 3-day study in which every patient's baseline measurements obtained before budesonide (or placebo) nebulization were compared with measurements obtained 1 h after nebulization in both groups. Ventilator settings including mode of ventilation (volume controlled ventilation), FiO2 (100%), tidal volume (6 ml/predicted body weight) and PEEP (10 cmH2O) were not changed during the study.
At each study period, pulmonary mechanics were assessed by recording the peak and plateau airway pressures from the screen of Binette respirometer during mechanical ventilation. The PaO2/FiO2 ratio was calculated from the measured PaO2 and the inspired oxygen concentration. Vital signs measurements including mean arterial pressure (MAP), heart rate (HR), and arterial oxygen saturation by pulse oximetry were monitored regularly every 30 min.
IL-1β, IL-6 and TNF-α were analyzed at baseline (prenebulization) and 72 h after nebulization in both groups.
Nebulized budesonide
Prepared as 1 mg ampoule enveloped in aluminum foil (2 ml suspension available as Pulmicort Respules®)[11] to be used in a pressurized nebulizer (not used in ultrasonic nebulizers) the ampoule was gently shaked and then squeezed into the nebulizer. Nebulization was performed using specific ventilator nebulizer (Aeroneb Pro-Aeroneb professional nebulizer system Aerogen (Ireland) Ltd., Galway, Ireland, SN: AP-1107867) with an oxygen flow of 8 L/min. Nebulization of either budesonide (2 ml, 1 mg concentration) in the active group (or saline [2 ml] in the control group) was connected after the Y-connection into the endotracheal tube every 12 h at a fixed time for three successive days. Before each nebulization, recruitment maneuver is done by increasing peak airway so as to get a plateau pressure of 30 cmH2O for 30 s. The nebulization lasted 15 min. for each session and was performed twice daily (at 9 a.m. and 9 p.m.). The anesthetist who was doing nebulization was blinded about the nature of nebulized drug. Furthermore, nebulization was stopped if hemodynamic instability occurred (HR or MAP >or <20% of the prenebulization recorded).
Cytokine assays
To determine blood concentration of IL-1β, IL-6 and TNF-α, 3 ml blood were withdrawn by venipuncture from each patient during the first 6–12 h after diagnosis. The blood samples were taken on plain vacutainer, centrifuged at 1000 rpm for 10 min, and serum is separated and aliquoted. Three aliquotes for each sample were kept in the freezer at −80°C till used for IL-1β, IL-6, and TNF-α assays.
The quantitative determination of IL-1β, IL-6 and TNF-α in serum were done by Diaclone ELISA kits, France. The procedures were solid phase sandwich ELISA which recognizes both natural and recombinant human IL-1β, IL-6 and TNF-α. The sensitivity of the Diaclone ELISA kits was 6.5 pg/ml, 0.81 pg/ml, and <8 pg/ml and the expected values were <6.5 pg/ml, 0.81–4.72 pg/ml, and <8 pg/ml for IL-1β, IL-6 and TNF-α, respectively.
Medical management of patients was set according to the protocols of the ICU of Qena University Hospital for every case as needed and according to the cause of ALI/ARDS.
Sample size
Based on previously published data, we assumed confidence level of 95% to detect 15% difference in the PO2/FiO2 ratio: The sample size of 27 cases per group was calculated (GraphPad InStat 3).[12] To compensate for possible dropouts, it was decided to include 30 patients in each group.
Statistical analysis
Statistical analysis was performed using the SPSS program version 20. Continuous data are expressed as mean ± standard deviation for normally distributed data or median (interquartile range) for nonnormally distributed data. Normally distributed data were compared using unpaired t-test, and nonnormally distributed data using the rank sum test. Baseline patient's clinical characteristics in the two groups were compared using Chi-square test. The value of P < 0.05 was considered statistically significant.
Results
This study was conducted on 60 adult patients admitted to the ICU of Qena University Hospital either directly or referred from chest department. The cause of admission is shown in Table 1. The patient's characteristics were comparable in the two groups with no significant differences [Table 1].
There were no significant differences in mean HR and mean blood pressure between the two studied groups and when compared to prenebulization data [Table 2].
Table 2.
Cardio-respiratory and ventilator parameters
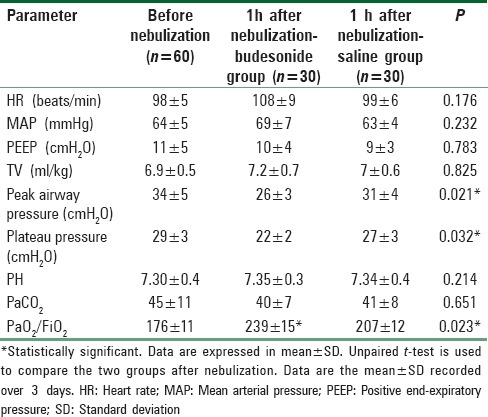
Ventilator settings parameters were comparable in the two groups as no significant differences were recorded [Table 2].
Nebulized budesonide administration had led to significant decrease in peak (P = 0.021) and plateau (P = 0.032) airway pressures when compared with the other placebo group [Table 2].
Oxygenation index (PaO2/FiO2) showed improvement in budesonide group (239 ± 15) compared with the placebo group (207 ± 12) with statistically significant difference (P = 0.023) [Table 2].
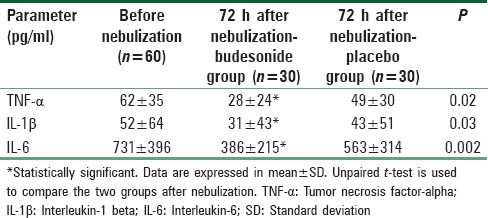
Regarding serum concentration of cytokines, budesonide group showed statistically significant decrease in the levels of TNF-α, IL-1β, and IL-6 compared with placebo group (TNF-α 28 ± 24 vs. 49 ± 30, IL-1β 31 ± 43 vs. 43 ± 51, and IL-6 386 ± 215 vs. 563 ± 314, respectively; P < 0.05) [Table 3].
Table 3.
Level of serum inflammatory cytokines (tumor necrosis factor-α, interleukin-1b and interleukin-6)
Discussion
The results of this study showed that budesonide nebulization significantly improved peak and plateau airway pressures and oxygenation in the form of increased PaO2/FiO2 ratio together with significant anti-inflammatory effect manifested by reducing the values of inflammatory cytokines (IL-1β, IL-6 and TNF-α) in patients with early ALI/ARDS.
Budesonide is an inhaled glucocorticoid which inhibits a variety of inflammatory cells, reduces the production of inflammatory mediators and consequently has a significant anti-inflammatory effect. It also induces vasoconstriction, inhibits mucosal edema, reduces cell exudation, and prevents airway remodeling.[13] Compared to systemic glucocorticoids, budesonide nebulization has the following advantages; high concentration primarily in the lungs, high hepatic clearance, and high glucocorticoid receptor affinity. During nebulization, the nebulizer unit breaks the liquid into micro-particles, which are directly inhaled into the lower respiratory tract and rapidly absorbed by the pulmonary mucosa, thus increasing the local drug concentration.
Studies in asthma have shown that inhaled steroids with an extra-fine particle size (around 1 µm) easily reaches the distal airways[14] where they have a greater effect on lung mechanics and symptoms than conventionally sized particles (around 5 µm), which are preferentially deposited in larger airways. Modern nebulizers are now capable of producing particles of this optimal size.[15]
Because inflammation occurring in ARDS is predominantly on the alveolar side of the interstitial space,[16] it is reasonable to expect that the terminal airways and the alveoli would be more accessible to inhaled rather than intravenous drugs.
This pharmacologic and pharmacokinetic characteristics of budesonide make it a valuable adjuvant for patients with ALI/ARDS on mechanical ventilation as the disease, in its early stages, is characterized by the presence of the acute phase response which is a series of complex neuro-immunologic reactions that lead to release of cytokines and other immunologically activated proteins in an attempt to restore homeostasis.[17] These inflammatory mediators play a cardinal role in the pathogenesis of ARDS and also responsible for the extra-pulmonary organ failure.[18,19]
Accordingly, nebulized budesonide can obtund the sequelae of the acute phase response and reduces alveolar inflammation. In addition, it inhibits airway inflammation, alleviates edema, inhibits remodeling and promotes suctioning which maintains pulmonary function during mechanical ventilation. At the same time, it reduces the adverse events associated with systemic corticosteroid administration such as increased blood glucose level, hypothalamic-pituitary-adrenal axis suppression, bone demineralization, perforated peptic ulcer, and altered immunity.[20,21,22] So, it can act as a pulmonary protective agent during mechanical ventilation for ALI/ARDS patients.
Ju et al. in a randomized controlled study evaluated the effect of preoperative budesonide nebulization on inflammation in thoracotomy patients receiving single lung ventilation and revealed that preoperative budesonide nebulization reduced the peak airway pressure and plateau pressure, increased pulmonary compliance, inhibited inflammation and finally improved pulmonary function.[23]
There is only one study of inhaled steroids for the prophylaxis of ARDS in humans.[24] Sixty-three patients inhaled 880 µg fluticasone every 12 h for 12 weeks from the start of chemotherapeutic regimen known to cause pulmonary toxicity. Compared with 45 historical controls, delayed pulmonary toxicity syndrome was reduced from 73% to 35% (P = 0.0003). The severity of illness was less than in ARDS, but this, at least, suggests inhaled steroids may protect against a pulmonary insult.
Jansson et al. reported leucocyte/neutrophil elevation in bronchoalveolar lavage (BAL) fluid and edema in both lungs after intrathecal lipo-polysaccharide administration of 0.5 mg/ml/kg in the 4th h in a murine model of ARDS. Pretreatment with nebulized budesonide completely prevented pulmonary injury and inflammation with reduced levels of TNF-α, IL-1β and IL-6 in the BAL fluid, compared with the control group, after lipopolysaccharide at a low dose; its partial, but notable, effect on the administration of lipopolysaccharide at a high dose was reported.[5]
In another study, pigs treated with inhaled budesonide immediately before or 30 min after inhalation of chlorine gas had improved clinical indices of lung injury (such as hemorrhagic edema fluid in the ventilator tubing, PaO2, lung compliance, and pulmonary wet-dry weight ratio).[25]
Limitations
There are several potential limitations in this study. First, larger prospective controlled studies are necessary to ensure definite conclusions about the value of nebulized budesonide in ALI/ARDS patients. Second, the shunt fraction should have studied to evaluate the direct effect of the drug. Third, a longer duration of therapy together with longer surveillance may be required to evaluate the effect of the drug on ventilator-free days and mortality.
Conclusion
This study showed reduced inflammatory cytokine mediators, improved respiratory mechanics and oxygenation index (PaO2/FiO2) without adversely affecting hemodynamics due to early use of nebulized budesonide combined with the protective ventilation strategy during the management of ALI/ARDS patients.
Financial support and sponsorship
South Valley University and Qena University Hospital, Qena, Egypt.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Tomashefski JF., Jr Pulmonary pathology of acute respiratory distress syndrome. Clin Chest Med. 2000;21:435–66. doi: 10.1016/s0272-5231(05)70158-1. [DOI] [PubMed] [Google Scholar]
- 2.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–49. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 3.Bauer TT, Montón C, Torres A, Cabello H, Fillela X, Maldonado A, et al. Comparison of systemic cytokine levels in patients with acute respiratory distress syndrome, severe pneumonia, and controls. Thorax. 2000;55:46–52. doi: 10.1136/thorax.55.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maltais F, Ostinelli J, Bourbeau J, Tonnel AB, Jacquemet N, Haddon J, et al. Comparison of nebulized budesonide and oral prednisolone with placebo in the treatment of acute exacerbations of chronic obstructive pulmonary disease: A randomized controlled trial. Am J Respir Crit Care Med. 2002;165:698–703. doi: 10.1164/ajrccm.165.5.2109093. [DOI] [PubMed] [Google Scholar]
- 5.Jansson AH, Eriksson C, Wang X. Effects of budesonide and N-acetylcysteine on acute lung hyperinflation, inflammation and injury in rats. Vascul Pharmacol. 2005;43:101–11. doi: 10.1016/j.vph.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Reade MC, Milbrandt EB. Is there evidence to support a phase II trial of inhaled corticosteroids in the treatment of incipient and persistent ARDS? Crit Care Resusc. 2007;9:276–85. [PubMed] [Google Scholar]
- 7.Hernando R, Drobnic ME, Cruz MJ, Ferrer A, Suñé P, Montoro JB, et al. Budesonide efficacy and safety in patients with bronchiectasis not due to cystic fibrosis. Int J Clin Pharm. 2012;34:644–50. doi: 10.1007/s11096-012-9659-6. [DOI] [PubMed] [Google Scholar]
- 8.Cheng AB, Shang XJ. Airway protective effect of budesonide suspension for patients undergoing general anesthesia. J Pract Med. 2013;29:675–6. [Google Scholar]
- 9.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: The Berlin definition. JAMA. 2012;307:2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 10.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 11.AstraZeneca. Pulmicort Respule s® (Budesonide inhalation suspension), Product of Sweden. Wilington, DE 19850: AstraZeneca; 2007. [Google Scholar]
- 12.GraphPad Software. QuikCalcs Online Calculators for Scientists, Assigns Subjects to Groups. Available from: http://www.graphpad.com/quickcalcs/randomize2 .
- 13.Fang Q, Schulte NA, Kim H, Kobayashi T, Wang X, Miller-Larsson A, et al. Effect of budesonide on fibroblast-mediated collagen gel contraction and degradation. J Inflamm Res. 2013;6:25–33. doi: 10.2147/JIR.S35136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin RJ. Therapeutic significance of distal airway inflammation in asthma. J Allergy Clin Immunol. 2002;109(2 Suppl):S447–60. doi: 10.1067/mai.2002.121409. [DOI] [PubMed] [Google Scholar]
- 15.Fink JB, Dhand R. Aerosol therapy in mechanically ventilated patients: Recent advances and new techniques. Semin Respir Crit Care Med. 2000;21:183–201. doi: 10.1055/s-2000-9854. [DOI] [PubMed] [Google Scholar]
- 16.de Nucci G, Moncada S. Inhaled corticosteroids for respiratory distress? Lancet. 1985;2:1061. doi: 10.1016/s0140-6736(85)90922-5. [DOI] [PubMed] [Google Scholar]
- 17.Lee WL, Downey GP. Neutrophil activation and acute lung injury. Curr Opin Crit Care. 2001;7:1–7. doi: 10.1097/00075198-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202:145–56. doi: 10.1002/path.1491. [DOI] [PubMed] [Google Scholar]
- 19.Park WY, Goodman RB, Steinberg KP, Ruzinski JT, Radella F, 2nd, Park DR, et al. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1896–903. doi: 10.1164/ajrccm.164.10.2104013. [DOI] [PubMed] [Google Scholar]
- 20.Chung KF, Caramori G, Adcock IM. Inhaled corticosteroids as combination therapy with beta-adrenergic agonists in airways disease: Present and future. Eur J Clin Pharmacol. 2009;65:853–71. doi: 10.1007/s00228-009-0682-z. [DOI] [PubMed] [Google Scholar]
- 21.Fahim A, Faruqi S, Wright CE, Kastelik JA, Morice AH. Comparison of the effect of high-dose inhaled budesonide and fluticasone on adrenal function in patients with severe chronic obstructive pulmonary disease. Ann Thorac Med. 2012;7:140–4. doi: 10.4103/1817-1737.98846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silverman M, Sheffer AL, Díaz PV, Lindberg B. Safety and tolerability of inhaled budesonide in children in the steroid treatment as regular therapy in early asthma (START) trial. Pediatr Allergy Immunol. 2006;17(Suppl 17):14–20. doi: 10.1111/j.1600-5562.2006.00380.x. [DOI] [PubMed] [Google Scholar]
- 23.Ju YN, Gao H, Huang Z, Niu FF, Li Fu, Xia Pei Song, et al. Effect of inhaled budesonide before operation on inflammatory responses to one-lung ventilation in patients undergoing thoracotomy. Chin J Anesthesiol. 2013;33:714–7. [Google Scholar]
- 24.McGaughey DS, Nikcevich DA, Long GD, Vredenburgh JJ, Rizzieri D, Smith CA, et al. Inhaled steroids as prophylaxis for delayed pulmonary toxicity syndrome in breast cancer patients undergoing high-dose chemotherapy and autologous stem cell transplantation. Biol Blood Marrow Transplant. 2001;7:274–8. doi: 10.1053/bbmt.2001.v7.pm11400949. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Winskog C, Edston E, Walther SM. Inhaled and intravenous corticosteroids both attenuate chlorine gas-induced lung injury in pigs. Acta Anaesthesiol Scand. 2005;49:183–90. doi: 10.1111/j.1399-6576.2004.00563.x. [DOI] [PubMed] [Google Scholar]


