Abstract
Three in vitro dose calibration curves for biodosimetry such as dicentric chromosome assay, fluorescence in situ hybridization (FISH) assay for translocation, and micronuclei (MNs) in binucleated cell assay were established after exposure to ionizing radiation. Peripheral blood lymphocyte samples obtained from healthy donors were irradiated with 60Co source at a dose rate of 0.5 Gy/min to doses of 0.1–6 Gy. The results from three in vitro dose calibration curves for biodosimetry were analyzed to understand the relationship among biodosimetry assay techniques. Our comparison demonstrates that there is a very strong positive correlation among the dicentric assay, FISH, and MNs analysis, and these three biodosimetry assays strongly support the in vitro dose reconstruction and the emergency preparedness of public or occupational radiation overexposure.
Keywords: Dicentric assay, fluorescence in situ hybridization, ionizing radiation, micronucleus assay, translocations
Introduction
Dose estimations of individuals occupationally exposed to ionizing radiation are currently carried out by the evaluation of personal dosimeters, for example, film badges, or by activity monitoring of the surroundings and subsequent calculations of the exposure. In the case of uncontrolled exposures, for example, accidental whole- or partial-body exposures, the exposed doses can be overestimated than the annual dose limit. In such situations, personal dosimeter readings are often imprecise or not available at all and the retrospective estimation of the dose will be necessary. Detailed knowledge of the doses received by individual provides the basis for follow-up examination and their further medical treatment. This will substantially reduce the possibility of further consequences. Dicentric chromosome (DC) assay, in biological dosimetry by the determination of the rate of unstable chromosome aberrations in peripheral blood lymphocytes, is commonly considered as “gold standard technique” of radiation when blood samples can be collected in 2 months after the exposure and are adopted at all reference biodosimetry laboratories.[1,2,3,4] Cytokinesis-block micronucleus (CBMN) assay is an additional assay used for radiation biodosimetry.[4,5] Recent reports from various laboratories have demonstrated its potential applicability for dose assessment. CBMN assay is now being considered as a very attractive tool for triage biodosimetry because of its advantages such as simplicity of scoring, accuracy, and most importantly, the easiness of automation using microscopy and flow cytometry.[5,6,7,8,9,10,11,12] Fluorescence in situ hybridization (FISH) is commonly used for retrospective dose estimations containing acute and chronic exposures. Consequently, this assay is being considered as the most suitable one for detecting occupational exposures.[3,4] The information obtained from these techniques may help to perform triage in radiation/nuclear mass casualty events (i.e., explosion of a nuclear power plant and terrorist attack with dirty bomb). In such an event, it is important that biological dosimetry laboratories are capable to respond adequately using cytogenetic assays in a triage mode, speeding up the analysis (i.e., with computer-assisted microscopy), and by networking with other laboratories. Therefore, there is an urgent need for updating existing knowledge, by producing documents/technical reports/manuals, by standardization of techniques, and by building of networks and initiating joint projects. Here, we report a linear-quadratic model of dose-response curve for three techniques such as DC assay in metaphases, FISH in metaphases, and micronuclei (MNs) assay in binucleated cells, and the Pearson's correlation analysis was performed to understand the correlation among the results from three biodosimetry techniques.
Materials and Methods
Dicentric chromosome assay
Human peripheral blood was obtained from three apparently healthy donors (32-, 33-, and 39-year-old males) under the Institutional Ethics Review Board procedures. Blood from each donor was placed in heparinized Vacutainer tubes (Becton Dickinson, USA). Whole blood was aliquoted into nine separate tubes (one as a control and the others for acute single exposure to doses of 0.1, 0.25, 0.5, 0.75, 1, 2, 4, and 6 Gy gamma rays (60Co source-Atomic Energy of Canada, Ltd., Ontario, Canada) at a dose rate of 0.5 Gy/min. The procedures for lymphocyte culture were followed according to the description in the International Atomic Energy Agency technical report 405.[4] In brief, the exposed cells were cultured in 10 ml of Roswell Park Memorial Institute (RPMI) 1640 supplemented with 20% fetal bovine serum (FBS), 100 U/ml penicillin and 10 µg/ml streptomycin, and 30 µl/ml phytohemagglutinin (PHA) and kept in an incubator at 37°C in a 5% CO2 humidified atmosphere for 48 h. To block the mitotic process of the cells at the metaphase stage, colcemid was added for the last 4 h of culture at a final concentration of 0.1 mg/ml. The harvested cells were treated with hypotonic 0.075 M KCl for 20 min and fixed with Carnoy's fixative (3:1 = methanol: acetic acid glacial) 3 times. Two slides for each sample were prepared, encoded, and then stained with 4% Giemsa and mounted. Mitotic cells on the slide were captured under Metafer System (MetaSystems, Germany). Scoring was done by a single scorer in complete metaphases. Tricentrics and tetracentrics were considered as two and three dicentric equivalents, respectively, and ring was skipped for dose-response curve.
Fluorescence in situ hybridization
Two slides were prepared for each sample, encoded, and then FISH assay was carried out under the manufacture's manual with mixture of chromosome probes #1, #4, and #8 with fluorescein isothiocyanate and count stained with 4',6-diamidino-2-phenylindole. The stained mitotic cells on the slide were captured under Metafer System. Scoring was done by a single scorer in complete metaphases.
Micronuclei assay in binucleated cells
Human peripheral blood was obtained from normal three healthy donors (composed of 41-, 44-, 50-year-old females) under the Institutional Ethics Review Board procedures. The heparinized whole blood was divided into seven containers - one group as a control and the others for single exposure to 0, 0.1, 0.25, 0.5, 0.75, 1, and 2 Gy gamma at a dose rate of 0.5 Gy/min. Exposed cells were cultured in 10 ml of RPMI 1640 supplemented with 20% FBS, 30 µl/ml PHA, 100 U/ml penicillin and 100 µg/ml streptomycin and kept in an incubator at 37°C in a 5% CO2 humidified atmosphere for 43 h. Cytochalasin B was added for the last 29 h of culture at a final concentration of 3 ug/ml to block the cytoplasmic division. The harvested cells were treated with hypotonic 0.075 M KCl for 3 min and fixed with Carnoy's fixative (3:1 = methanol: acetic acid glacial) 3 times. Two slides were prepared for each sample, encoded, and then stained with 4% Giemsa and mounted. Binucleated cells on the slide were captured under Metafer System. A cell was considered as aberrant if it had over one micronucleus from each binucleated cell. Scoring was done by a single scorer.
Statistics
Statistical analysis of the data for linear-quadratic model of dose-response curve of three techniques was performed using Dose Estimate software version 4.1. To verify dose-response relationship, we performed Pearson's correlation analysis between sets of the results of biodosimetry assays. Pearson's correlation coefficient measures linear correlation between the pairwise DC, FISH, and MN. The correlation coefficient value 1 means strong positive linear relation, 0 means no correlation, and −1 means total negative correlation. P value for each correlation coefficient is given to decide its significance between sets of biodosimetry assays.
Results and Discussion
Dicentric chromosome assay
The results of the frequencies of chromosome aberrations in mitotic cells after irradiation by 0, 0.1, 0.25, 0.5, 0.75, 1, 2, 4, and 6 Gy gamma rays as a single dose are presented in Table 1 and Figure 1. As shown in Table 1, there is a significant difference between nonirradiated and irradiated group. The dose-response curve was fitted by Dose Estimate software version 4.1 as shown in Figure 1. The DC yield was fitted with a linear-quadratic model as the following equation: Y = c + bD + aD,2 where Y is the yield of dicentric frequency, D is absorbed dose in Gy, a is the corresponding quadratic coefficient, b is the linear coefficient, and c is the background frequency. Dose-response curve of DC fitted using a linear quadratic equation ML_Linear-Quadratic_Fit_Yield = 0.0049 (±0.0020) +0.0159 (±0.0073) × D + 0.0234 (±0.0033) × D2 is showed in Figure 1 with the 95% lower confidence limit and upper confidence limit. Weighted Chi-squared is 516.7000, degrees of freedom is 6, and P value for goodness of fit is 0.0000. P values for coefficients (z-test): P_A is 0.0463, P_alpha 0.0727, and P_beta is 0.0004. Correlation coefficient, r is 0.9985. Based on the resulting coefficients, the dose estimation was done by input, the number of aberrations observed and cells scored.
Table 1.
Distribution and frequencies of dicentric chromosome aberrations in human peripheral blood lymphocytes following irradiation with 60Co (γ-rays) at a dose rate of 0.5 Gy/min to doses of 0.1 to 6 Gy
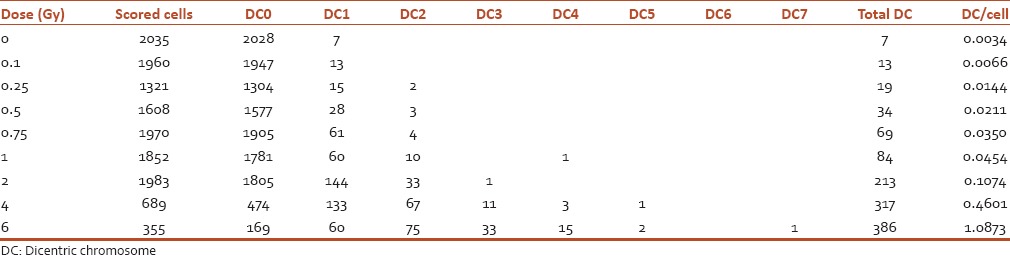
Figure 1.
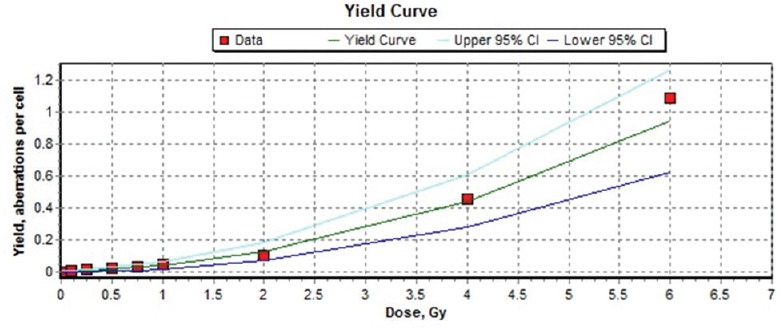
Dose-response curve for dicentric chromosomes in human peripheral blood lymphocytes following irradiation with 60Co γ-rays to doses of 0.1 to 2 Gy at a dose rate of 0.5 Gy/min. The curve was generated by Dose Estimate software version 4.1
Chromosome translocation (fluorescence in situ hybridization) assay
Frequencies of chromosome translocations in mitotic cells after irradiation with 0, 0.1, 0.25, 0.5, 0.75, 1, 2, 4, and 6 Gy gamma rays are shown in Table 2 and Figure 2. There was a statistically significant difference between the nonirradiated and irradiated group. Dose-response curve of chromosome translocations fitted using a linear quadratic equation ML_Linear-Quadratic_Fit_Yield = 0.0012 (±0.0004) + 0.0109 (±0.0028) × D + 0.0418 (±0.0013) × D2 is showed in Figure 2 with the 95% lower confidence limit and upper confidence limit. Weighted Chi-squared is 118.8000, degrees of freedom is 6, and P value for goodness of fit is 0.0000. P values for coefficients (z-test): P_A is 0.0375, P_alpha is 0.0084, and P_beta is 0.0000. Correlation coefficient, r is 0.9998. Based on the resulting coefficients, the dose estimation was done by computing the number of aberrations observed and cells scored.
Table 2.
Distribution and frequencies of chromosome translocations (Ch #1, 4, and 8, fluorescent in situ hybridization) in human peripheral blood lymphocytes following irradiation with 60Co (γ-rays) at a dose rate of 0.5 Gy/min to doses of 0.1-6 Gy
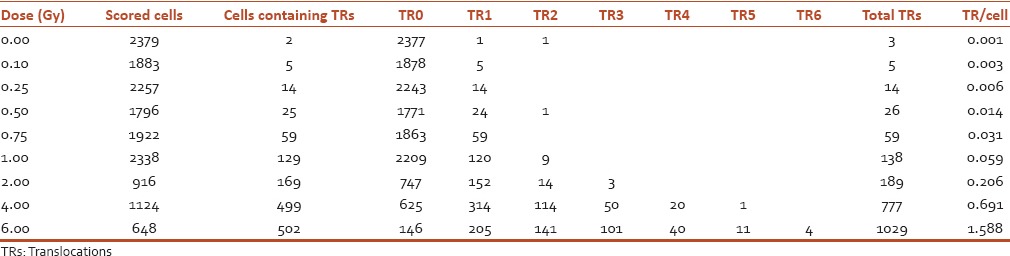
Figure 2.
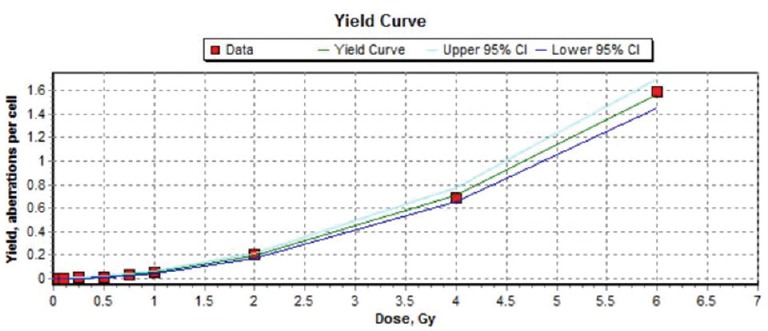
Dose-response curve for chromosome translocations (Ch #1, 4, and 8) in human peripheral blood lymphocytes following irradiation with 60Co γ-rays to doses of 0.1 to 2 Gy at a dose rate of 0.5 Gy/min. The curve was generated by Dose Estimate software version 4.1
Micronuclei assay in binucleated cells
The results of the frequencies of MNs in binucleated cell after irradiation by 0, 0.1, 0.25, 0.5, 0.75, 1, and 2 Gy gamma rays as a single dose are shown in Table 3 and Figure 3. Data from Table 3 show significant difference among nonirradiated and irradiated group. Dose-response curve of MNs in binucleated cells was fitted using a linear quadratic equation ML_Linear-Quadratic_Fit_Yield = 0.0367 (±0.0056) + 0.0937 (±0.0226) × D + 0.0445 (±0.0136) × D2 is showed in Figure 3 with the 95% lower confidence limit and upper confidence limit. Weighted Chi-squared is 16.4400, degrees of freedom is 4, and P value for goodness of fit is 0.5012. P values for coefficients (z-test): P_A is 0.0027, P_alpha is 0.0144, P_beta is 0.0307. Correlation coefficient, r is 0.9968. Based on the resulting coefficients, the dose estimation was done by input, the number of aberrations observed and cells scored.
Table 3.
Distribution and frequencies of micronuclei in binucleated cell in human peripheral blood lymphocytes following irradiation with 60Co (γ-rays) at a dose rate of 0.5 Gy/min to doses of 0.1-2 Gy
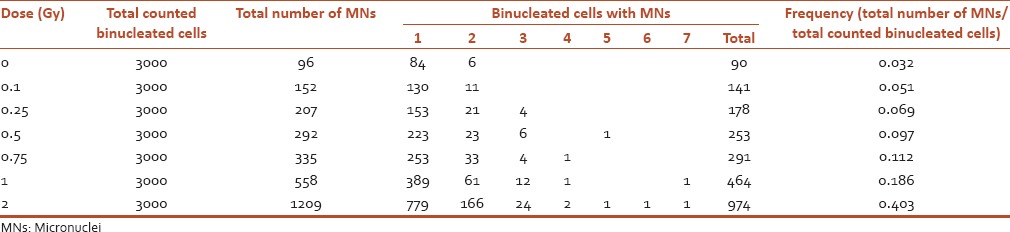
Figure 3.
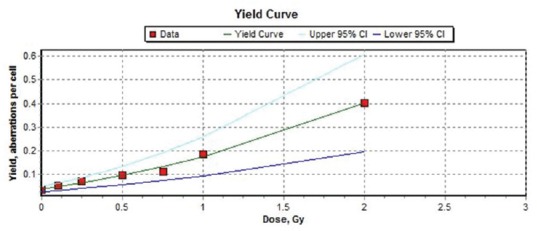
Dose-response curve for micronuclei in binucleated cell (micronuclei) in human peripheral blood lymphocytes following irradiation with 60Co γ-rays to doses of 0.1 to 2 Gy at a dose rate of 0.5 Gy/min. The curve was generated by Dose Estimate software version 4.1
Comparison of the biodosimetry techniques following radiation exposure
Table 4 shows the significant Pearson's correlation coefficient r and P value. There appears to be a very strong positive correlation between the DC and translocation_FISH (TR_FISH) techniques (r = 0.9920, P < 0.0001) [Table 4 and Figure 4a]. The scatter plot suggests a relationship between the DC and TR_FISH biodosimetry techniques, with larger values of DC tending to be associated with larger values of TR_FISH. The DC and MN techniques have strong positive correlation (r = 0.99440, P < 0.0001) [Table 4 and Figure 4b]. The scatter plot suggests a relationship between the DC and MN biodosimetry techniques, with larger values of DC tending to be associated with larger values of MN. The TR_FISH and MN techniques have strong positive correlation (r = 0.99011, P < 0.0001) [Table 4 and Figure 4c]. The scatter plot suggests a relationship between the TR_FISH and MN biodosimetry techniques, with larger values of TR_FISH tending to be associated with larger values of MN.
Table 4.
Pearson correlation analysis between sets of biodosimetry techniques
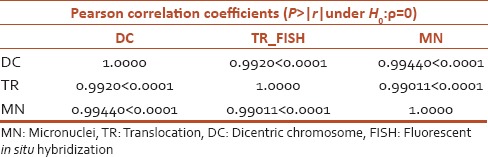
Figure 4.
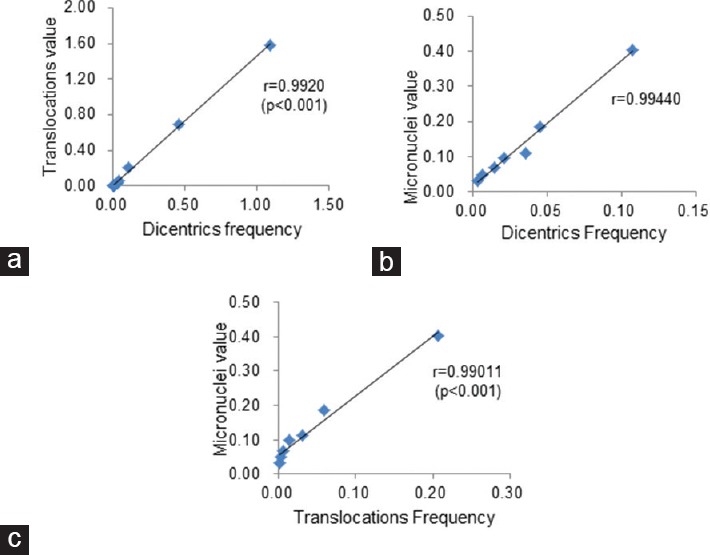
Scatter plot of the results from the DC and the FISH biodosimetry techniques (a), the DC and the MN biodosimetry technique (b), and the TR_FISH and the MN biodosimetry technique (c). DC: Dicentric chromosome, TR_FISH: Translocation_fluorescence in situ hybridization, MN: Micronuclei in binucleated cells
Conclusion
Our data showed that the DC and FISH, the DC and MN, the FISH and MN biodosimetry techniques were linearly correlated very strongly [Table 4 and Figure 4].
Financial support and sponsorship
This work has been carried out under the IAEA-CRP (E35008) contract number 17085 and was supported by the National Research Foundation of Korea (NRF) grant (NRF-2014M2B2B1071171) funded by the Korean government (MSIP).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Liu Q, Cao J, Liu Y, Lü YM, Qin B, Jiang B, et al. Follow-up study by chromosome aberration analysis and micronucleus assays in victims accidentally exposed to 60Co radiation. Health Phys. 2010;98:885–8. doi: 10.1097/HP.0b013e3181c4b9c1. [DOI] [PubMed] [Google Scholar]
- 2.Romm H, Wilkins RC, Coleman CN, Lillis-Hearne PK, Pellmar TC, Livingston GK, et al. Biological dosimetry by the triage dicentric chromosome assay: Potential implications for treatment of acute radiation syndrome in radiological mass casualties. Radiat Res. 2011;175:397–404. doi: 10.1667/RR2321.1. [DOI] [PubMed] [Google Scholar]
- 3.International Atomic Energy Agency. Cytogenetic Dosimetry: Applications in Preparedness for and Response to Radiation Emergencies. Vienna: International Atomic Energy Agency; 2011. [Google Scholar]
- 4.IAEA (International Atomic Energy Agency). Cytogenetic Analysis for Radiation Dose Assessment. IAEA Technical Report Series, No. 405. Vienna, Austria: International Atomic Energy Agency; 2001. [Google Scholar]
- 5.Fenech M, Morley AA. Kinetochore detection in micronuclei: An alternative method for measuring chromosome loss. Mutagenesis. 1989;4:98–104. doi: 10.1093/mutage/4.2.98. [DOI] [PubMed] [Google Scholar]
- 6.Das B, Karuppasamy CV. Spontaneous frequency of micronuclei among the newborns from high level natural radiation areas of Kerala in the Southwest Coast of India. Int J Radiat Biol. 2009;85:272–80. doi: 10.1080/09553000902751462. [DOI] [PubMed] [Google Scholar]
- 7.Demidenko E, Williams BB, Swartz HM. Radiation dose prediction using data on time to emesis in the case of nuclear terrorism. Radiat Res. 2009;171:310–9. doi: 10.1667/RR1552.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almassy Z, Kanyar B, Koteles GJ. Frequency of micronuclei in X-irradiated human lymphocytes. Int J Radiat Biol. 1986;49:719–23. [Google Scholar]
- 9.Savage JR. A comment on the quantitative relationship between micronuclei and chromosomal aberrations. Mutat Res. 1988;207:33–6. doi: 10.1016/0165-7992(88)90008-5. [DOI] [PubMed] [Google Scholar]
- 10.Go YJ, Shin JH, Jeong KS, Park SJ, Lee MH, Kwak DM, et al. Dose estimation with the calibration of dose-response curve of micronucleus in human peripheral lymphocytes induced by 50MeV proton beams. Iran J Radiat Res. 2011;8:231–6. [Google Scholar]
- 11.Selvan GT, Bhavani M, Vijayalakshmi J, Paul Solomon FD, Chaudhury NK, Venkatachalam P. Delayed mitogenic stimulation decreases DNA damage assessed by micronucleus assay in human peripheral blood lymphocytes after (60) co irradiation. Dose Response. 2014;12:498–508. doi: 10.2203/dose-response.13-060.Selvan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willems P, August L, Slabbert J, Romm H, Oestreicher U, Thierens H, et al. Automated micronucleus (MN) scoring for population triage in case of large scale radiation events. Int J Radiat Biol. 2010;86:2–11. doi: 10.3109/09553000903264481. [DOI] [PubMed] [Google Scholar]


