Neuromyelitis optica spectrum disorder (NMOSD) is a rare relapsing autoimmune demyelinating disease of the CNS that predominantly affects the optic nerves and the spinal cord.1 Because of the severity and poor recovery of attacks, aggressive immunosuppressive agents are used early in the clinical course to reduce relapse frequency. Apart from classical immunosuppressant agents, biologicals such as rituximab, eculizumab, or tocilizumab have been used for relapse prevention.2 Based on retrospective data, 17–53% of patients have break-through relapses under the most commonly used treatments azathioprine, mycophenolate mofetil, or rituximab.3
While established in myeloproliferative diseases, the JAK/STAT (Janus kinase/signal transducer and activator of transcription) signaling cascade has emerged as a new target for the treatment of autoimmune diseases. JAK inhibitors have proven to be effective in rheumatic diseases.4 Ruxolitinib is an orally available and potent selective inhibitor of the Janus kinases JAK1 and JAK2.
Here, we report the case of a patient with highly active neuromyelitis optica who continued to accumulate neurologic disability while receiving numerous immunosuppressive treatments and was ultimately treated with ruxolitinib.
Classification of evidence.
This provides class IV evidence. It is a single observational study without controls.
Case presentation.
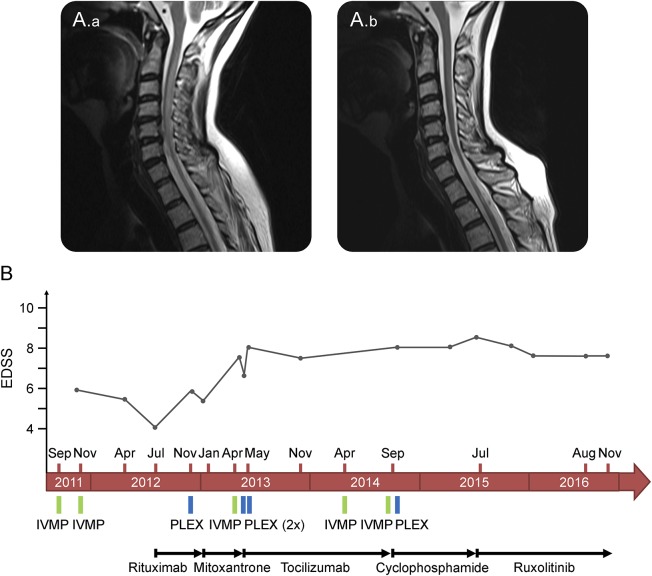
A 55-year-old female patient with extensive myelitis was diagnosed in 2011 with neuromyelitis optica based on clinical presentation, MRI findings, and the presence of antibodies directed against aquaporin-4. The initial manifestation of the disease with extensive spinal cord lesions (C6-T2) was treated with repetitive high-dose IV steroids. Remission was incomplete resulting in paraparesis, and treatment with rituximab was started. Four months later, she experienced a relapse with extensive myelitis (T4-T12), resulting in worsening of the paraparesis. Plasma exchange led to marginal clinical improvement. She then received 1 cycle of mitoxantrone (12 mg/m2). Two months later, she presented with another relapse resulting in paraplegia and severe urinary incontinence. Again, high-dose steroid therapy and plasma exchange only led to minor clinical improvement. Tocilizumab was initiated in a dose regimen of 8 mg/kg body weight. After 14 months of stable disease, paraparesis worsened again and MRI showed new spinal contrast-enhancing lesions. Repeated steroid pulses and plasma exchange could not restore ambulation. In September 2014, immunosuppressive treatment with cyclophosphamide (750 mg/m2 every 4 weeks) was started. In July 2015, the patient relapsed with severe tetraparesis with only some preserved motor function in the left arm. MRI of the spinal cord revealed extensive longitudinal T2 lesions (C1-C7 and T12-L1) with contrast enhancement. Treatment with ruxolitinib was initiated in an initial dose regimen of 10 mg twice daily. Motor function of the left arm improved significantly. Two months later, the dose of ruxolitinib was increased to 15 mg twice daily. In November 2015, the patient was admitted to hospital due to infection of a stage IV decubitus ulcer, which was treated with ciprofloxacine, surgical debridement, and wound closure with a myocutaneous flap. In January 2016, the patient was readmitted to hospital with infection of the myocutaneous flap requiring anti-infective and surgical treatment. Ruxolitinib was paused for 4 weeks, then restarted in a dose regimen of 5 mg twice daily and increased to 10 mg twice daily after 3 months. Follow-up MRI of the spinal cord in January 2016 showed regression of the T2 lesion with no detectable contrast enhancement (figure). Her clinical condition has now been stabilized for 16 months. MRI of the spinal cord in August 2016 showed no sign of inflammatory activity.
Figure. MRI and clinical course.
(A) Sagittal T2-weighted turbo spin echo MRI sequences show longitudinally extensive hyperintense intramedullary lesion expanding from cervical level C1 to C7 in July 2015 (A.a) and regression of the lesion (and progression of spinal cord atrophy) 6 months after the initiation of treatment with ruxolitinib in January 2016 (A.b). (B) Graphic demonstration of clinical course and therapeutic interventions. EDSS = Expanded Disability Status Scale; IVMP = intravenous methylprednisolone; PLEX = plasma exchange.
Discussion.
Our report provides evidence to suggest that therapy with JAK inhibitors may be a feasible, orally available immunosuppressive therapy in patients with NMOSD otherwise unresponsive to established treatment approaches.
A number of studies have supported the role of multiple cytokines that signal through JAK1 or JAK2 in the pathogenesis of NMOSD (e.g., interleukin [IL]-6 and IL-10).5 The concept of using ruxolitinib as an off-label pharmacotherapy for NMOSD was based on the idea that a broad cytokine inhibition through JAK/STAT signaling pathway blockade instead of a specific inhibition of single immunologic pathways by an antibody treatment might be a more efficient escalation strategy in treatment-refractory NMOSD.
An increased rate of infections, among them severe infections such as tuberculosis, fungal infection, and Pneumocystis jirovecii pneumonia, has been observed in clinical trials with JAK inhibitors.4 Our case shows that despite such complications, clinical stabilization of highly active NMOSD might be obtained. Because of the oral dosing of ruxolitinib with short-term drug turnover, JAK inhibitors might offer an attractive treatment concept for refractory NMOSD. However, we are aware that despite having achieved the longest remission period so far, patient's lack of relapses may be due to natural remission of the disease.
Footnotes
Author contributions: Sibylle C. Hodecker: drafting/revising the manuscript; acquisition of data; and analysis and interpretation of data. Jan-Patrick Stellmann, Sina C. Rosenkranz, Kim Young, Brigitte Holst, Manuel A. Friese, and Christoph Heesen: drafting/revising the manuscript.
Study funding: No targeted funding
Disclosure: S.C. Hodecker received travel funding from Genzyme. J.-P. Stellman received travel funding and/or speaker honoraria from Genzyme, Biogen, Novartis and received research support from Merck Serono. S.C. Rosenkranz received travel funding from Biogen. K. Young and B. Holst report no disclosures. M.A. Friese received research support from the German Research Foundation and the German Ministry of Education and Research. C. Heesen received speaker honoraria and research grants from Biogen, Genzyme, Novartis, Merck, and Roche; is on the editorial board of International Journal of MS Care; and received research support from German Ministry of Research and Hertie Foundation. Go to Neurology.org/nn for full disclosure forms. The Article Processing Charge was paid by the authors.
References
- 1.Wingerchuk DM, Banwell B, Bennett JL, et al. . International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015;85:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papadopoulos MC, Bennett JL, Verkman AS. Treatment of neuromyelitis optica: state-of-the-art and emerging therapies. Nat Rev Neurol 2014;10:493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mealy MA, Wingerchuk DM, Palace J, Greenberg BM, Levy M. Comparison of relapse and treatment failure rates among patients with neuromyelitis optica: multicenter study of treatment efficacy. JAMA Neurol 2014;71:324–330. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz DM, Bonelli M, Gadina M, O'Shea JJ. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat Rev Rheumatol 2016;12:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kothur K, Wienholt L, Brilot F, Dale RC. CSF cytokines/chemokines as biomarkers in neuroinflammatory CNS disorders: a systematic review. Cytokine 2016;77:227–237. [DOI] [PubMed] [Google Scholar]