Summary
The distinction of hepatocellular adenoma from well-differentiated hepatocellular carcinoma (HCC) arising in noncirrhotic liver can be challenging, particularly when tumors histologically resembling hepatocellular adenoma occur in unusual clinical settings such as in a man or an older woman or show focal atypical morphologic features. In this study, we examine the morphologic, immunohistochemical, and cytogenetic features of hepatocellular adenoma–like neoplasms occurring in men, women 50 years or older or younger than 15 years, and/or those with focal atypia (small cell change, pseudogland formation, and/or nuclear atypia), designated atypical hepatocellular neoplasms, where the distinction of hepatocellular adenoma versus HCC could not be clearly established. Immunohistochemistry was performed for β-catenin, glutamine synthetase, and serum amyloid A in 31 hepatocellular adenomas, 20 well-differentiated HCCs, and 40 atypical hepatocellular neoplasms. Chromosomal gains/losses had previously been determined in 37 cases using comparative genomic hybridization or fluorescence in situ hybridization. β-Catenin activation was observed in 35% of atypical hepatocellular neoplasms compared with 10% of typical hepatocellular adenomas (P < .05) and 55% of well-differentiated HCCs (P = .14). Cytogenetic changes typically observed in HCC were present in all atypical hepatocellular neoplasms with β-catenin activation. β-Catenin activation in atypical hepatocellular neoplasms was also associated with atypical morphologic features. Follow-up data were limited, but adverse outcome was observed in 2 atypical hepatocellular neoplasms with β-catenin activation (1 recurrence, 1 metastasis); transition to areas of HCC was observed in 1 case. The similarity in morphologic and cytogenetic features of β-catenin–activated hepatocellular adenoma–like tumors and HCC suggests that the former tumors represent an extremely well-differentiated variant of HCC. Published by Elsevier Inc.
Keywords: Hepatocellular adenoma, β-Catenin, Glutamine synthetase, Atypical morphology, Chromosomal abnormalities
1. Introduction
Hepatocellular carcinoma (HCC) is a malignant liver tumor with a substantial risk of recurrence, metastasis, and death, representing the third leading cause of cancer-related mortality worldwide [1,2]. In contrast, hepatocellular adenomas (HCAs) are benign liver tumors that are generally treated with simple resection because of the risk of rupture and associated HCC, but conservative management, such as observation, may be considered for small HCAs [3,4]. It can be challenging to distinguish well-differentiated HCC (WD-HCC) arising in a noncirrhotic liver from HCA, especially when limited tissue from needle biopsies is available. Typical histologic findings of HCC such as macrotrabecular or pseudoglandular architecture, small cell change, mitotic activity, vascular invasion, and fragmentation or loss of reticulin network may not be present in WD-HCC, whereas focal cytologic and/or architectural atypia can be seen in HCA. Because HCA usually occurs in young women, the diagnostic difficulties can be compounded when tumors histologically resembling HCA occur in unusual clinical settings, such as in a man or an older woman.
Cytogenetic studies have shown characteristic chromosomal abnormalities in HCC, such as gains at chromosomal regions 8q, 1q, 20q, 7q, Xq, 5p, and 17q, and/or losses at 16q, 17p, 4q, 8p, 1p, 13q, and 16p, whereas HCA only rarely shows chromosomal abnormalities [5–12]. A subset of HCA-like tumors that occur in men or older women shows cytogenetic changes typical of HCC. It has been suggested that these tumors may represent extremely well-differentiated forms of HCC [12].
HCAs are classified into 4 categories based on molecular and/or immunohistochemical features originally defined by Bioulac-Sage et al [13] and currently recognized by the World Health Organization: hepatocyte nuclear factor 1α (HNF1α) inactivated, β-catenin activated, inflammatory, and unclassified [13–15]. HNF1α-inactivated HCAs are characterized by HNF1α mutation and immunohistochemical loss of fatty acid–binding protein. These tumors show prominent steatosis, lack cytologically atypical features, occur in women, and have a low risk of HCC [13,14]. Inflammatory HCAs (IHCAs) are characterized by immunohistochemical expression of serum amyloid A (SAA) protein and can show mutations in genes encoding components of the interleukin-6 signaling pathway [13,14,16]. These tumors demonstrate inflammation, telangiectasia (dilated sinusoids), and ductular reaction. β-Catenin–activated HCAs are characterized by β-catenin mutation accompanied by immunohistochemical expression of β-catenin in the nuclei and/or diffuse expression of glutamine synthetase (GS) in the cytoplasm [13,14]. These tumors often occur in males, show cytologically atypical features, and are often associated with HCC at diagnosis or follow-up.
We have earlier described the high risk for HCC in HCA-like tumors that occur in men, older women, or those with atypical cytologic characteristics [12]. These tumors were designated as atypical hepatocellular neoplasms (AHNs) and show early cytogenetic changes of HCC, such as gains of chromosomes 1 and 8. AHNs described in our previous study and β-catenin–activated HCAs share a variety of features including frequent occurrence in males, atypical cytologic features, and association with HCC at diagnosis or on follow-up. In this study, we examined the morphologic, immunohistochemical, and cytogenetic features of HCA-like neoplasms with atypical features (designated AHNs, where the distinction of HCA versus HCC could not be clearly established). These findings were compared with typical HCA and WD-HCC.
2. Materials and methods
2.1. Case selection
Cases were selected from the pathology files of the University of California, San Francisco, and included 31 HCAs (19 core needle biopsies and 12 resections), 20 WD-HCCs (10 core needle biopsies and 10 resections), and 40 AHNs (23 core needle biopsies and 17 resections). Two HCA biopsies were from separate lesions in the same patient; otherwise, none of the cases were from the same patient. There was no significant fibrosis or history of chronic liver diseases such as viral hepatitis, autoimmune hepatitis, or biliary disease in any HCA, AHN, or HCC. Slides were reviewed to confirm the diagnosis in each case. Clinicopathologic information including age and sex were obtained from the pathology report. The designation of AHN was applied in 2 situations:
Atypical age/sex: tumors that resembled HCA morphologically but occurred in men (any age) or women 50 years or older or younger than 15 years.
Atypical morphology: tumors that resembled HCA morphologically but had focal cytologic or architectural atypia, involving less than 5% of the tumor, which comprised small cell change, pseudogland formation, and/or nuclear atypia.
The study was approved by the institutional review board of the University of California, San Francisco.
2.2. Morphologic and immunohistochemical evaluation
Hematoxylin and eosin (H&E) and reticulin stains performed on formalin-fixed, paraffin-embedded tissue sections were reviewed for each case. For immunohistochemical staining, formalin-fixed, paraffin-embedded tissue sections on Superfrost Plus slides were heated at 65°C to 70°C for 60 minutes, deparaffinized and hydrated to distilled water, and placed in citrate buffer (pH 6.0) in a Pascal pressure cooker (Dako, Carpinteria, CA, USA), according to the manufacturer's instructions. Slides were washed in running distilled water and then incubated with blocking solution (3% hydrogen peroxide, 0.05% Tween-20, 0.1% sodium azide in calcium-magnesium–free phosphate-buffered saline [PBS]) for 10 minutes. After 2 washes in deionized water, slides were held in 0.05% Tween-20 in PBS until they were loaded on the immunostainer (Dako). Slides were incubated in the following monoclonal primary antibodies for 30 minutes at room temperature: β-catenin (clone 14 used at 1:200 dilution; BD Biosciences, San Jose, CA), serum amyloid A (SAA; clone mc1 used at 1:50 dilution; Dako), GS (clone Mab302 used at 1:250 dilution; Chemicon/Millipore, Billerica, MA). After washing with 0.2% Tween-20 in PBS, slides were incubated with the ENVISION+ system (Dako) for 15 minutes (β-catenin and GS) or 30 minutes (SAA). The slides were washed again with 0.2% Tween-20 in PBS, incubated for 10 minutes at room temperature in diaminobenzidine (DAB+) liquid (Dako), and washed in water. Slides were counterstained with hematoxylin for 10 seconds, washed in tap water, incubated in PBS for 30 seconds, washed in tap water, dehydrated in graded ethanols, cleared in xylene, and coverslipped.
H&E-stained sections were evaluated for inflammation (small lymphocytes in sinusoids or in stroma surrounding vessels), telangiectasia (sinusoidal dilatation), and atypia (small cell change, pseudogland formation, and/or nuclear atypia). SAA staining was scored as positive (diffuse moderate to strong cytoplasmic staining) or negative. β-catenin staining was scored as positive (any nuclear staining) or negative (cytoplasmic and/or membrane staining). GS staining was scored as diffuse positive (moderate to strong cytoplasmic staining in >50% of tumor cells) or negative (perivascular staining, patchy parenchymal or absent/weak staining). The diffuse positive cases were further subdivided into diffuse complete (>90% tumor cells positive) and intermediate (50%–90% tumor cells positive). Cases with any nuclear β-catenin staining or diffuse positive GS staining were considered β-catenin activated [13].
2.3. Chromosomal abnormalities
Comparative genomic hybridization (CGH) and/or fluorescence in situ hybridization (FISH) analysis had been performed previously on a subset of these tumors, as described [12,17]. Tumors were considered to have chromosomal abnormalities if chromosomal changes typical of HCC were present [5–11]. These included gains at 8q, 1q, 7q, or 20q and losses at 16q, 4q, 8p, 13q, or 17p on CGH or gains of chromosomes 1, 8, and/or c-myc by FISH.
2.4. Statistics
The differences in morphologic characteristics, immunohistochemical profiles, and chromosomal abnormalities were compared using the χ2 test. A P value of less than .05 was considered significant.
3. Results
3.1. Morphologic evaluation
Of the 40 AHN cases, atypical morphologic features were seen in 28 cases (70%) (Table 1; Figs. 1–4). The atypical features included small cell change (23 cases), pseudoacinar architecture (2 cases), and nuclear atypia (8 cases). The other AHN cases did not show atypical morphologic features but were classified as AHN because of male sex (n = 4) and/or age 50 years or greater (n = 9; 1 man, 8 women). By definition, all HCAs occurred in women and did not show atypical morphologic features (Fig. 5). There was no loss or fragmentation of the reticulin network in any HCA or AHN. There was no significant difference in biopsies versus resection among the 3 groups (P = .74).
Table 1.
Morphologic, immunohistochemical, and cytogenetic features of HCAs and AHNs
| HCA, % (n) |
AHN, % (n) |
P | |
|---|---|---|---|
| Age ≥50 y | 0 (0/28) | 40 (14/35) | .00015* |
| Male sex | 0 (0/28) | 29 (10/35) | .0020* |
| Steatosis | 45 (14/31) | 55 (22/40) | .41 |
| Inflammation | 48 (15/31) | 35 (14/40) | .26 |
| Telangiectasia | 45 (14/31) | 33 (13/40) | .28 |
| Ductular reaction | 16 (5/31) | 8 (3/40) | .25 |
| Atypical morphologic features |
0 (0/31) | 70 (28/40) | <.00001* |
| β-Catenin activated | 10 (3/30) | 35 (14/40) | .016* |
| SAA positive | 62 (18/29) | 56 (20/36) | .60 |
| Chromosomal abnormalities |
0 (0/9) | 59 (10/17) | .0034* |
NOTE. Age, sex, and atypical morphologic features are part of the definition of AHNs, so it is expected that these P values would be significant.
P < .05 was considered statistically significant.
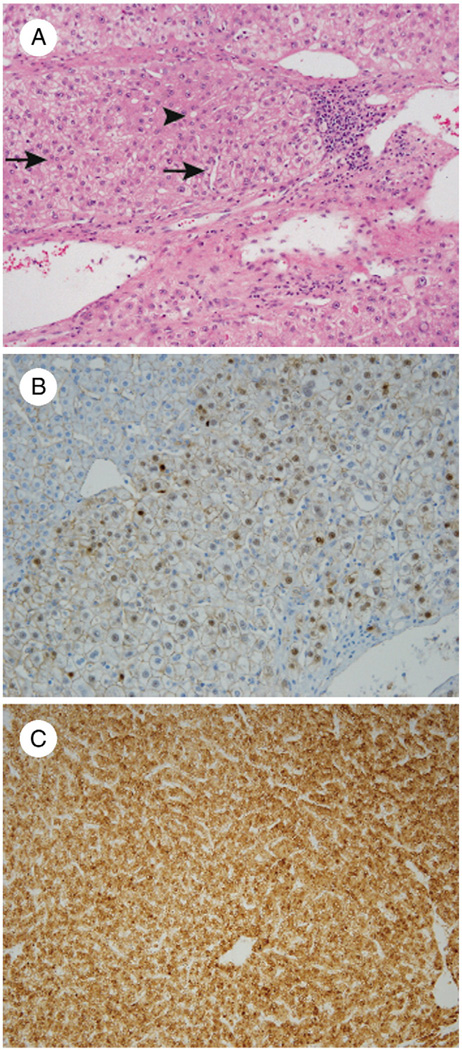
Fig. 1.
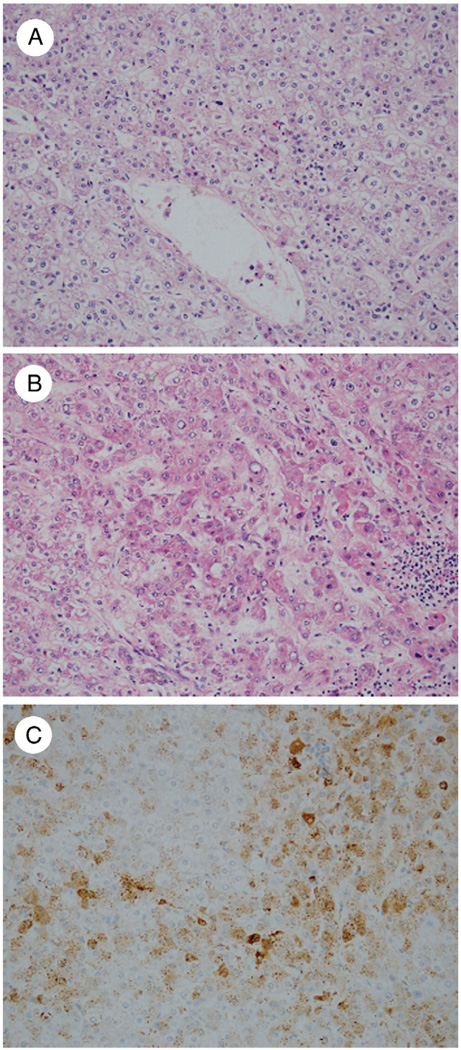
A, AHN in a 27-year-old woman with focal pseudoacinar architecture (arrowhead) and small cell change featuring small-sized cells with high cell density and nuclei that are nearly touching each other (arrows) (H&E, ×200). Immunohistochemistry shows nuclear staining with β-catenin (B: ×200) and a diffuse complete pattern of staining with GS (C: 100×).
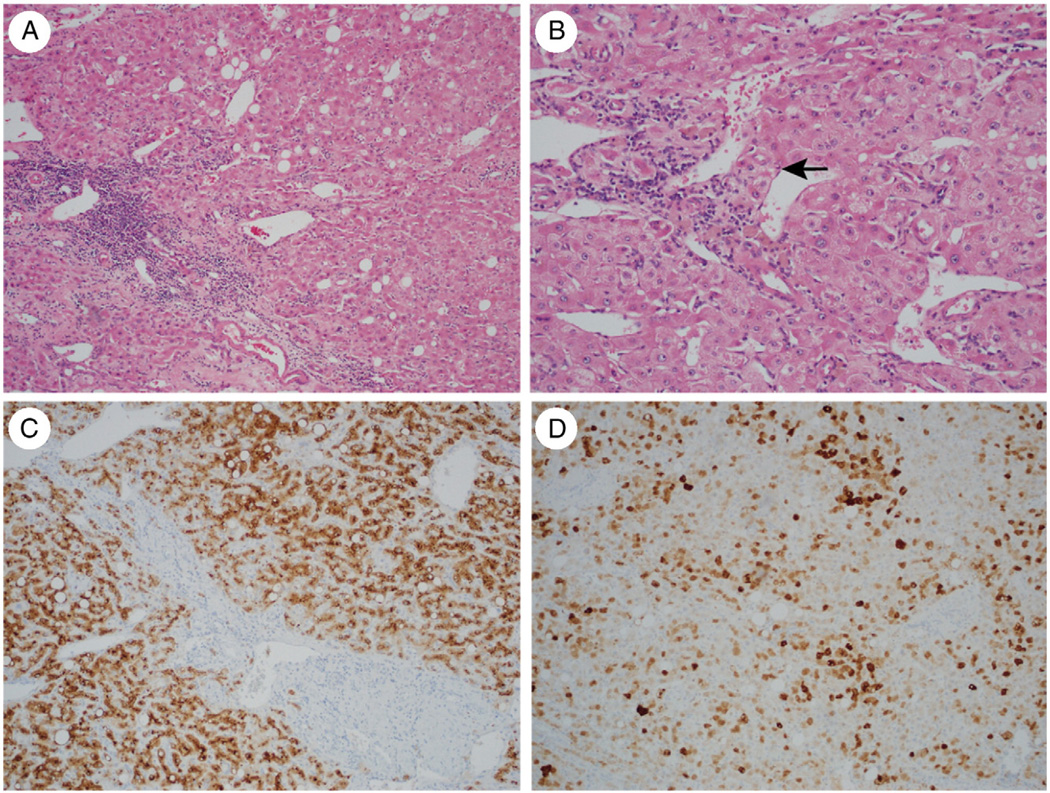
Fig. 4.
A, Atypical IHCA in a 55-year-old woman showing fibrous areas with thick-walled arterioles and prominent inflammation (H&E, ×100). B, Focal areas show small cell change and thick cell plates (arrow) (H&E, ×200). C, Immunohistochemistry for SAA protein is strongly positive (×200). D, GS shows a diffuse intermediate pattern of staining (×100).
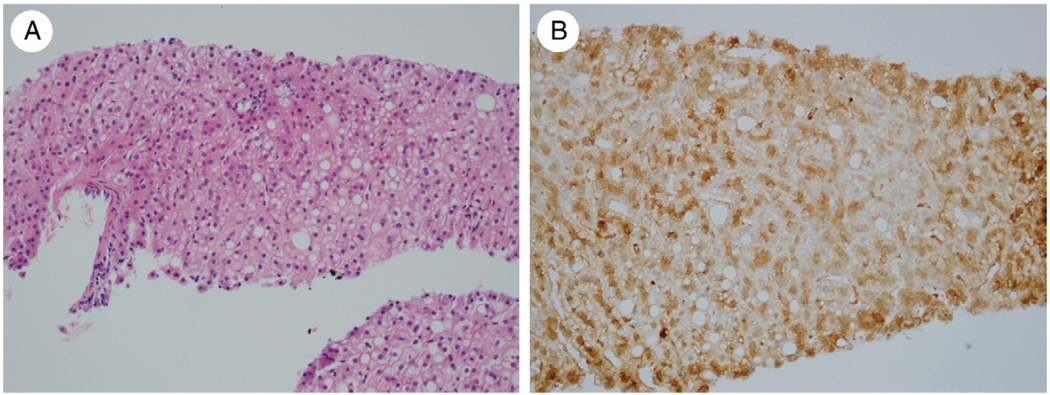
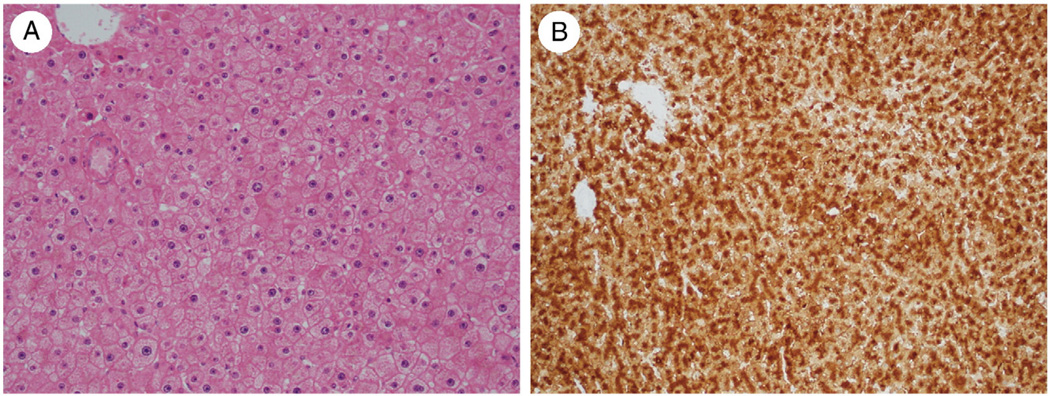
Fig. 5.
A, HCA in a 43-year-old woman with no morphologic atypia (H&E, ×200). B, Immunohistochemistry for GS shows a diffuse intermediate pattern of staining (×200).
3.2. SAA immunohistochemistry
SAA positivity was observed in 18 (62%) of 29 HCAs and 20 (56%) of 36 AHNs (Table 1). These tumors often exhibited telangiectasia (dilated sinusoids), inflammation, and/or ductular reaction and were classified as IHCAs (typical IHCA for HCA and atypical IHCA for AHN) (Table 2; Figs. 4 and 6). SAA reactivity was seen in 4 (21%) of 19 HCCs (Table 3).
Table 2.
Morphologic, immunohistochemical, and cytogenetic features of typical and atypical IHCAs, other HCAs, and other AHNs
| Typical IHCA (SAA+), % (n) |
Other HCA (SAA−), % (n) |
P | Atypical IHCA (SAA+), % (n) |
Other AHN (SAA−), % (n) |
P | |
|---|---|---|---|---|---|---|
| Age ≥50 y | 0 (0/18) | 0 (0/9) | NA | 47 (8/17) | 20 (3/15) | .11 |
| Male sex | 0 (0/18) | 0 (0/9) | NA | 47 (8/17) | 13 (2/15) | .04* |
| Steatosis | 39 (7/18) | 55 (6/11) | .41 | 60 (12/20) | 50 (8/16) | .55 |
| Inflammation | 56 (10/18) | 45 (5/11) | .60 | 50 (10/20) | 19 (3/16) | .05 |
| Telangiectasia | 61 (11/18) | 27 (3/11) | .077 | 50 (10/20) | 13 (2/16) | .02* |
| Ductular reaction | 22 (4/18) | 9 (1/11) | .36 | 10 (2/20) | 0 (0/16) | .19 |
| Atypical morphologic features | 0 (0/18) | 0 (0/11) | NA | 55 (11/20) | 94 (15/16) | .01* |
| β-Catenin activation | 17 (3/18) | 0 (0/11) | .15 | 35 (7/20) | 38 (6/16) | .88 |
| Chromosomal abnormalities | 0 (0/5) | 0 (0/3) | NA | 70 (7/10) | 43 (3/7) | .26 |
Abbreviation: NA, not applicable.
P < .05 was considered statistically significant.
Fig. 6.
IHCA with no morphologic atypia (A: H&E, ×100) and strong staining for SAA protein (B: ×100). C, Immunohistochemistry for GS shows a diffuse complete pattern of staining (×200).
Table 3.
Morphologic, immunohistochemical, and cytogenetic features of AHNs and WD-HCCs
| AHN, % (n) |
WD-HCC, % (n) |
P | |
|---|---|---|---|
| Age ≥50 y | 40 (14/35) | 71 (10/14) | .047* |
| Male sex | 29 (10/35) | 50 (7/14) | .15 |
| Atypical morphologic features |
70 (28/40) | 100 (20/20) | .0062* |
| β-Catenin activated | 35 (14/40) | 55 (11/20) | .14 |
| SAA positive | 56 (20/36) | 21 (4/19) | .014* |
| Chromosomal abnormalities typical of HCC |
59 (10/17) | 55 (6/11) | .82 |
P < .05 was considered statistically significant.
Atypical morphologic features were seen in 11 (55%) of 20 atypical IHCAs and included small cell change (9 cases), pseudoacinar architecture (2 cases), and nuclear atypia (3 cases) (Table 2; Fig. 4). Eight (47%) of 17 atypical IHCAs occurred in men. All typical IHCAs occurred in women and did not show atypical morphologic features.
3.3. β-Catenin and GS immunohistochemistry
Fourteen (35%) of 40 AHNs showed β-catenin activation, defined by nuclear β-catenin staining and/or diffuse positive GS staining (Table 1; Figs. 1–4). This was similar to the findings in HCC, where 11 (55%) of 20 cases showed β-catenin activation (P = .14; Table 3), but significantly higher than what was seen in HCA, where β-catenin activation was seen in 3 (10%) of 30 cases (P <.05; Table 1). All cases with nuclear β-catenin staining showed diffuse GS staining. In 5 cases of AHN, 5 cases of HCC, and 3 cases of HA, there was no nuclear β-catenin staining, but cases were scored as abnormal because of diffuse positive GS staining. Most cases with diffuse positive GS staining showed a diffuse complete positive pattern, with GS staining in more than 90% of tumor cells. In 2 HAs (both SAA positive) and 4 AHNs (3 SAA positive and 1 SAA negative), GS showed a diffuse intermediate positive staining with staining in 50% to 90% of tumor cells (Figs. 2, 4, and 5). None of these 6 tumors showed nuclear β-catenin positivity. Among AHNs with diffuse intermediate positive GS staining, 75% (3/4) showed atypical morphologic features and 100% (2/2) showed chromosomal abnormalities (P = .82 and P = .21 compared with AHNs with diffuse complete positive GS staining).
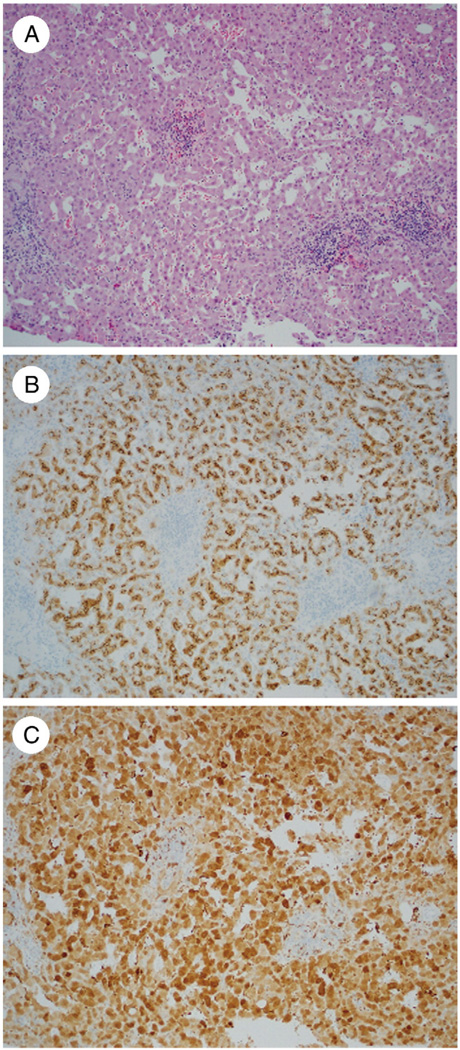
Fig. 2.
A, AHN in a 72-year-old woman with no morphologic atypia in most of the tumor (H&E, ×200). B, Focal areas show small cell change and cytologic atypia (×200). C, Immunohistochemistry for GS shows a diffuse intermediate pattern of staining (×200).
Among AHNs, 13 (93%) of 14 tumors with β-catenin activation showed atypical morphologic features compared with 15 (58%) of 26 AHNs without β-catenin activation (P < .05; Table 4). There was no significant difference in age, sex, the presence of inflammation, and the presence of telangiectasia between tumors with or without β-catenin activation.
Table 4.
Morphologic, immunohistochemical, and cytogenetic features of HCA and AHNs with β-catenin activation
| HCA, β-catenin activated, % (n) |
HCA, β-catenin normal, % (n) |
P | AHN, β-catenin activated, % (n) |
AHN, β-catenin normal, % (n) |
P | |
|---|---|---|---|---|---|---|
| Age ≥50 y | 0 (0/3) | 0 (0/24) | NA | 30 (3/10) | 44 (11/25) | .45 |
| Male sex | 0 (0/3) | 0 (0/24) | NA | 20 (2/10) | 32 (8/25) | .48 |
| Steatosis | 67 (2/3) | 41 (11/27) | .39 | 36 (5/14) | 65 (17/26) | .07 |
| Inflammation | 67 (2/3) | 48 (13/27) | .54 | 36 (5/14) | 35 (9/26) | .94 |
| Telangiectasia | 67 (2/3) | 44 (12/27) | .46 | 29 (4/14) | 35 (9/26) | .70 |
| Ductular reaction | 0 (0/3) | 19 (5/27) | .41 | 0 (0/14) | 12 (3/26) | .19 |
| Atypical morphologic features | 0 (0/3) | 0 (0/27) | NA | 93 (13/14) | 58 (15/26) | .02* |
| Chromosomal abnormalities | 0 (0/0) | 0 (0/9) | NA | 100 (8/8) | 22 (2/9) | .001* |
Abbreviation: NA, not applicable.
P < .05 was considered statistically significant.
Among AHNs, tumors categorized as atypical IHCA (SAA positive) were significantly less likely (11/20; 55%) to show atypical morphologic features compared with SAA-negative AHNs (15/16; 94%) (P <.05; Table 2).
3.4. Chromosomal abnormalities and clinical follow-up
None of the 9 HCAs for which CGH or FISH data were available showed chromosomal abnormalities typical of HCC (Table 1). Chromosomal abnormalities typically seen in HCC were observed in 10 (59%) AHNs and 6 (55%) HCCs (Table 3). The abnormalities in AHNs were gains of chromosome 1 only (2 cases), chromosome 7 only (1 case), c-myc only (1 case), chromosomes 1 and 8 (2 cases), and chromosomes 1, 7, and 8 (4 cases). These chromosomal abnormalities were significantly more common in AHNs compared with HCAs (10/17 versus 0/9, P < .005). In WD-HCCs, these chromosomal abnormalities were seen in 6 (55%) of 11 cases, which was similar to AHNs (P = .82). Of AHNs with chromosomal abnormalities, 8 (80%) of 10 had β-catenin activation and 7 (78%) of 9 had SAA reactivity. Of AHNs without chromosomal abnormalities, none (0/7) had β-catenin activation and 3 (60%) of 5 had SAA reactivity. Of HCCs with chromosomal abnormalities, 4 (67%) of 6 had β-catenin activation and none (0/6) had SAA reactivity. Of HCCs without chromosomal abnormalities, 3 (60%) of 5 had β-catenin activation and 1 (20%) of 5 had SAA reactivity.
Among AHNs, chromosomal abnormalities were significantly more common in tumors with β-catenin activation (8/8 cases; 100%) than in tumors without β-catenin activation (2/9 cases; 22%) (P < .005).
Adverse outcomes were observed in 2 β-catenin–activated AHNs (1 recurrence and 1 metastasis), 1 of which was also SAA positive. Transition to areas of HCC was observed in another tumor with β-catenin activation. Adverse outcome (recurrence) was observed in 1 AHN (SAA positive) without β-catenin activation.
Chromosomal abnormalities were seen in AHNs with focal atypical morphologic changes as well as AHNs classified based on age/sex (Table 5). AHNs with focal atypical morphologic changes had less steatosis, often lacked features of IHCA, and were more likely to show β-catenin activation.
Table 5.
Comparison of morphologic, immunohistochemical, and cytogenetic features of 2 groups of AHNs defined by morphology and by age/sex
| AHN (morphology), % (n) |
AHN (age/sex), % (n) |
P | |
|---|---|---|---|
| Male sex | 26 (6/23) | 33 (4/12) | .65 |
| Steatosis | 43 (12/28) | 83 (10/12) | .02* |
| Inflammation | 25 (7/28) | 58 (7/12) | .04* |
| Telangiectasia | 25 (7/28) | 50 (6/12) | .12 |
| Ductular reaction | 0 (0/28) | 25 (3/12) | .006* |
| β-Catenin activation | 45 (13/28) | 8 (1/12) | .02* |
| SAA positive | 42 (11/26) | 90 (9/10) | .01* |
| Chromosomal abnormalities | 67 (8/12) | 40 (2/5) | .31 |
P < .05 was considered statistically significant.
4. Discussion
The field of HCA has dramatically changed in the last few years with the new classification proposed by Bioulac-Sage et al [13] and Zucman-Rossi et al [14] based on molecular and immunohistochemical features. The classification was updated by the World Health Organization, and 4 categories are now recognized: HNF1α inactivated, β-catenin activated, inflammatory, and unclassified [15]. Our study focused on β-catenin activated and IHCAs with emphasis on correlation of these subtypes with age, sex, cytologic abnormalities, association with HCC, and cytogenetic changes.
The β-catenin pathway is often aberrantly activated in HCC, usually due to stabilizing somatic mutations in the β-catenin gene [18,19]. HCCs with activating β-catenin mutation show nuclear accumulation of β-catenin and upregulation of target genes including GLUL (coding GS) and GPR49 [20,21]. In our study, HCA-like neoplasms that occurred in men (any age) and women 50 years or older, as well as tumors with focal cytologic abnormalities insufficient for an unequivocal diagnosis of HCC, were classified as AHNs. β-catenin activation was observed in 35% of AHNs compared with 10% of typical HCAs and 55% of WD-HCCs.
The French studies that proposed the immunohistochemical HCA classification reported β-catenin activation in 13% to 19% of HCAs [13,14]. Their results showed a significant correlation of β-catenin activation with male sex, cytologic abnormalities, pseudoglandular architecture, and association with HCC. Apart from the current study and these French studies, there are limited data in the literature regarding β-catenin activation in HCA or HCA-like neoplasms with atypical features. In one study, β-catenin mutation was observed in 3 (30%) HCAs, 2 of which occurred in males [22]. Another study showed that 3 (11%) HCAs with atypical features or coexistent HCC were β-catenin activated [23]. In another series reported by Torbenson et al [24], β-catenin nuclear staining was observed in 7 of 15 cases of HCA in premenopausal women. However, 2 cases showed rare positivity, and none of the cases showed mutations in exon 3 of the β-catenin gene. The reason for the discrepancy between the results of Torbenson et al and more recent studies (including the current study and the French studies) is not clear. Factors such as fixation and antigen retrieval may play a role, but because GS data are not available in the study of Torbenson et al, it cannot be directly compared with our or the French study results for which virtually all cases of β-catenin activation were associated with diffuse GS expression.
We have earlier shown that cytogenetic changes typical of HCC are seen in AHN but not in typical HCA [12]. The relationship of cytogenetic changes with β-catenin activation in HCA or AHN has not been previously examined. Our study showed that cytogenetic changes typically observed in HCC were present in all AHNs with β-catenin activation. AHNs with β-catenin activation were associated with male sex, cytologic atypia, and association with HCC at presentation or follow-up, similar to the findings in the French studies [13,14]. The frequent association of β-catenin activation with HCC, both at morphologic and at cytogenetic levels, strongly raises the possibility that these tumors represent an extremely well-differentiated variant of HCC. Although the follow-up in our study was limited, the presence of recurrence or metastasis in 2 AHNs with β-catenin activation further lends credence to this possibility. Given the frequent presence of cytologic atypia, association with HCC, and cytogenetic changes similar to HCC, these tumors should not be diagnosed as HCA based on liver biopsy alone. The term AHN can be used with a recommendation for tumor resection. Even in the original French study, most β-catenin–activated tumors had been diagnosed as borderline between HCA and HCC, but were considered as HCA for the purpose of the study [14].
All cases with nuclear β-catenin staining showed diffuse GS staining, confirming the association between β-catenin pathway activation and GS immunoreactivity that has been previously reported [20]. In 10% of HCAs and 13% of AHNs, diffuse GS staining was present, but there was no nuclear staining with β-catenin. Because more than half of the cases were biopsies, it is possible that some cases with diffuse GS staining and no nuclear staining with β-catenin may have revealed nuclear β-catenin in an unsampled area of the lesion. Other possibilities include low level of nuclear β-catenin that is not demonstrable by immunohistochemistry or mutations in other components of the Wnt signaling pathway. This phenomenon has been reported in 15% of β-catenin–activated HCAs, in which β-catenin mutation was not accompanied by nuclear β-catenin staining, but diffuse GS staining was present [13]. In most cases of β-catenin–activated tumors, GS positivity involves virtually all the tumor cells. In the French study, GS staining was not diffuse in some β-catenin–activated tumors but showed a heterogeneous pattern of staining with positivity in more than 50% of cells. The number of cases with heterogeneous and homogeneous patterns was not mentioned [13]. In our study, this pattern of GS staining was categorized as diffuse intermediate and was observed in 2 typical HCAs and 4 AHNs. The biological significance of the heterogeneous versus the diffuse GS pattern is not clearly understood. There was no significant difference in terms of atypical morphologic features and chromosomal abnormalities in tumors with diffuse complete or diffuse intermediate GS staining. However, none of the cases with intermediate GS staining showed nuclear β-catenin. We did not perform β-catenin mutation analysis in our study, which can be helpful to understand the discrepancy between expression of β-catenin and GS on immunohistochemistry, as well as in explaining the heterogeneous pattern of GS staining. None of our cases showed anastomosing areas of “map-like” GS staining characteristic of focal nodular hyperplasia [25].
In our series, 62%of typical HCAs and 56% of AHNs were classified as typical and atypical IHCA, respectively, based on SAA expression. All 3 typical HCAs with β-catenin activation were IHCAs and were characterized by diffuse GS expression without nuclear β-catenin. None of these showed chromosomal abnormalities typical of HCC. On the other hand, 35% of atypical IHCAs showed β-catenin activation and 70% showed cytogenetic changes typical of HCC. Hence, IHCAs occurring in atypical clinical settings (women ≥50 years old and men) or showing atypical morphologic features can show β-catenin activation and/or chromosomal abnormalities, and a subset of these tumors probably represent WD-HCC.
A limitation of the study is that more than half of the cases were biopsies, and it can be argued that some HCAs and/or AHNs may have showed features of HCC on resection. However, there was no difference in biopsy versus resection in each of the 3 groups, and the overall results were similar when resection specimens alone were considered (details not shown). Another limitation is that follow-up data were not available for most cases. Nonetheless, the presence of atypical morphologic features, HCC-like chromosomal abnormalities, and association with HCC at presentation or follow-up in β-catenin–activated adenoma-like neoplasms suggests that these tumors represent extremely WD-HCC. A small proportion of IHCAs also show β-catenin activation. Regardless of the terminology used to categorize such tumors, their distinct clinical, morphologic, immunohistochemical, cytogenetic, and prognostic features warrant categorization separately from other subtypes of HCA without β-catenin abnormalities, which may have a lower or no significant risk of malignant behavior based on currently available data.
Fig. 3.
A, AHN in a 57-year-old woman with no morphologic atypia (H&E, ×400). B, Immunohistochemistry for GS shows a diffuse complete pattern of staining (×200).
Acknowledgments
Funding sources: This work was supported by the Pathology Core of the UCSF Liver Center (P30 DK026743). Kimberley Evason is a Robert Black Fellow of the Damon Runyon Cancer Research Foundation (DRG-#109-10).
Footnotes
Disclosure/Conflict of interest: The authors have no conflicts of interest to declare.
References
- 1.Lin CL, Kao JH. Optimal management of hepatocellular carcinoma: challenges and opportunities. J Gastroenterol Hepatol. 2010;25:1336–1338. doi: 10.1111/j.1440-1746.2010.06373.x. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–67. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 3.Reddy KR, Kligerman S, Levi J, et al. Benign and solid tumors of the liver: relationship to sex, age, size of tumors, and outcome. Am Surg. 2001;67:173–178. [PubMed] [Google Scholar]
- 4.Terkivatan T, de Wilt JH, de Man RA, et al. Indications and long-term outcome of treatment for benign hepatic tumors: a critical appraisal. Arch Surg. 2001;136:1033–1038. doi: 10.1001/archsurg.136.9.1033. [DOI] [PubMed] [Google Scholar]
- 5.Guan XY, Fang Y, Sham JS, et al. Recurrent chromosome alterations in hepatocellular carcinoma detected by comparative genomic hybridization. Genes Chromosomes Cancer. 2000;29:110–116. [PubMed] [Google Scholar]
- 6.Zondervan PE, Wink J, Alers JC, et al. Molecular cytogenetic evaluation of virus-associated and non-viral hepatocellular carcinoma: analysis of 26 carcinomas and 12 concurrent dysplasias. J Pathol. 2000;192:207–215. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH690>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 7.Balsara BR, Pei J, De Rienzo A, et al. Human hepatocellular carcinoma is characterized by a highly consistent pattern of genomic imbalances, including frequent loss of 16q23.1-24.1. Genes Chromosomes Cancer. 2001;30:245–253. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1083>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 8.Laurent-Puig P, Legoix P, Bluteau O, et al. Genetic alterations associated with hepatocellular carcinomas define distinct pathways of hepatocarcinogenesis. Gastroenterology. 2001;120:1763–1773. doi: 10.1053/gast.2001.24798. [DOI] [PubMed] [Google Scholar]
- 9.Patil MA, Gutgemann I, Zhang J, et al. Array-based comparative genomic hybridization reveals recurrent chromosomal aberrations and Jab1 as a potential target for 8q gain in hepatocellular carcinoma. Carcinogenesis. 2005;26:2050–2057. doi: 10.1093/carcin/bgi178. [DOI] [PubMed] [Google Scholar]
- 10.Wilkens L, Bredt M, Flemming P, Becker T, Klempnauer J, Kreipe HH. Differentiation of liver cell adenomas from well-differentiated hepatocellular carcinomas by comparative genomic hybridization. J Pathol. 2001;193:476–482. doi: 10.1002/path.825. [DOI] [PubMed] [Google Scholar]
- 11.Wong N, Lai P, Lee SW, et al. Assessment of genetic changes in hepatocellular carcinoma by comparative genomic hybridization analysis: relationship to disease stage, tumor size, and cirrhosis. Am J Pathol. 1999;154:37–43. doi: 10.1016/S0002-9440(10)65248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kakar S, Chen X, Ho C, et al. Chromosomal abnormalities determined by comparative genomic hybridization are helpful in the diagnosis of atypical hepatocellular neoplasms. Histopathology. 2009;55:197–205. doi: 10.1111/j.1365-2559.2009.03343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bioulac-Sage P, Rebouissou S, Thomas C, et al. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology. 2007;46:740–748. doi: 10.1002/hep.21743. [DOI] [PubMed] [Google Scholar]
- 14.Zucman-Rossi J, Jeannot E, Nhieu JT, et al. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006;43:515–524. doi: 10.1002/hep.21068. [DOI] [PubMed] [Google Scholar]
- 15.Bosman FT, Carneiro F, Hruban RH, Theise ND. 4. Lyon, France: International Agency for Research on Cancer; 2010. WHO classification of tumours of the digestive system. revised ed. [Google Scholar]
- 16.Rebouissou S, Amessou M, Couchy G, et al. Frequent in-frame somatic deletions activate gp130 in inflammatory hepatocellular tumours. Nature. 2009;457:200–204. doi: 10.1038/nature07475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evason K, Grenert J, Ferrell L, Kakar S. Immunohistochemical classification of hepatic adenomas and atypical hepatocellular neoplasms: correlation with clinicopathologic characteristics and chromosomal abnormalities. Modern Pathol. 2010;23(Suppl. 1):345A. [Google Scholar]
- 18.de La Coste A, Romagnolo B, Billuart P, et al. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci USA. 1998;95:8847–8851. doi: 10.1073/pnas.95.15.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson MD, Monga SP. WNT/beta-catenin signaling in liver health and disease. Hepatology. 2007;45:1298–1305. doi: 10.1002/hep.21651. [DOI] [PubMed] [Google Scholar]
- 20.Zucman-Rossi J, Benhamouche S, Godard C, et al. Differential effects of inactivated Axin1 and activated beta-catenin mutations in human hepatocellular carcinomas. Oncogene. 2007;26:774–780. doi: 10.1038/sj.onc.1209824. [DOI] [PubMed] [Google Scholar]
- 21.Cui J, Zhou XD, Liu YK, Tang ZY, Zile MH. Abnormal beta-catenin gene expression with invasiveness of primary hepatocellular carcinoma in China. World J Gastroenterol. 2001;7:542–546. doi: 10.3748/wjg.v7.i4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen YW, Jeng YM, Yeh SH, Chen PJ. P53 gene and Wnt signaling in benign neoplasms: beta-catenin mutations in hepatic adenoma but not in focal nodular hyperplasia. Hepatology. 2002;36:927–935. doi: 10.1053/jhep.2002.36126. [DOI] [PubMed] [Google Scholar]
- 23.Van der Borght S, Libbrecht L, Katoonizadeh A, et al. Nuclear beta-catenin staining and absence of steatosis are indicators of hepatocellular adenomas with an increased risk of malignancy. Histopathology. 2007;51:855–856. doi: 10.1111/j.1365-2559.2007.02862.x. [DOI] [PubMed] [Google Scholar]
- 24.Torbenson M, Lee JH, Choti M, et al. Hepatic adenomas: analysis of sex steroid receptor status and the Wnt signaling pathway. Mod Pathol. 2002;15:189–196. doi: 10.1038/modpathol.3880514. [DOI] [PubMed] [Google Scholar]
- 25.Bioulac-Sage P, Laumonier H, Rullier A, et al. Over-expression of glutamine synthetase in focal nodular hyperplasia: a novel easy diagnostic tool in surgical pathology. Liver Int. 2009;29:459–465. doi: 10.1111/j.1478-3231.2008.01849.x. [DOI] [PubMed] [Google Scholar]