Abstract
MHC, especially HLA-DR3 and HLA-DR2, is one of the most important genetic susceptibility regions for systemic lupus erythematosus. Human studies to understand the role of specific HLA alleles in disease pathogenesis have been hampered by the presence of strong linkage disequilibrium in this region. To overcome this, we produced transgenic mice expressing HLA-DR3 (DRβ1*0301) and devoid of endogenous class II (both I-A and I-E genes, AE0) on a lupus-prone NZM2328 background (NZM2328.DR3+AE0). Both NZM2328 and NZM2328.DR3+AE0 mice developed anti-dsDNA and glomerulonephritis, but anti-dsDNA titers were higher in the latter. Although kidney histological scores were similar in NZM2328 and NZM2328.DR3+AE0 mice (7.2 ± 4.3 and 8.6 ± 5.7, respectively, p = 0.48), the onset of severe proteinuria occurred earlier in NZM2328.DR3+AE0 mice compared with NZM2328 mice (median, 5 and 9 mo respectively, p < 0.001). Periarterial lymphoid aggregates, classic wire loop lesions, and occasional crescents were seen only in kidneys from NZM2328.DR3+AE0 mice. Interestingly, NZM2328.DR3+AE0 mice, but not NZM2328 mice, spontaneously developed anti-Smith (Sm) Abs. The anti-Sm Abs were seen in NZM2328.DR3+AE0 mice that were completely devoid of endogenous class II (AE−/−) but not in mice homozygous (AE+/+) or heterozygous (AE+/−) for endogenous MHC class II. It appears that only HLA-DR3 molecules can preferentially select SmD-reactive CD4+ T cells for generation of the spontaneous anti-Sm immune response. Thus, our mouse model unravels a critical role for HLA-DR3 in generating an autoimmune response to SmD and lupus nephritis in the NZM2328 background.
Systemic lupus erythematosus (SLE) is a heterogeneous disease with a strong genetic component and is characterized by the production of autoantibodies against spliceosomal, nucleosomal, and other autoantigens. The association of MHC class II haplotypes with lupus has been known for a long time and was recently confirmed in several large-scale genome-wide scans (1–4). Haplotypes containing HLA-DRB1*1501/DQB1*0602 (DR2/DQ6), DRB1*0801/DQB1*0402 (DR8/DQ4), and DRB1*0301/DQB1*0201 (DR3/DQ2) alleles were significantly associated with SLE (3). The association was even stronger for autoantibody subsets. Graham et al. (5) showed that 60% of individuals (SLE patients and unaffected relatives) who were heterozygotes for DR2/DR3 had an Ab to at least one extractable nuclear Ag (i.e., Ro, La, Smith [Sm], ribosomal-P, and ribonucleoprotein [RNP]) compared with 29% of individuals without the risk allele. Furthermore, the DR3/DR3 genotype showed a strong association with anti-Sm Ab and a trend toward association with anti-dsDNA.
NZM2328 is a well-characterized recombinant inbred strain derived from extended intercrosses involving NZB/NZW F1 progenitors. NZM2328 mice develop antinuclear and anti-dsDNA Abs and acute and chronic glomerulonephritis. As in human lupus, there is a distinct gender bias, and only female mice develop chronic glomerulonephritis (6). Several lupus-susceptibility genetic loci have been identified in this strain by extensive backcross analysis (6, 7). Therefore, NZM2328 is a well-characterized, widely used spontaneous mouse model of lupus. However, despite the presence of high expression of IFN-α, anti-Sm and other anti-small nuclear RNP (snRNP) Abs have not been demonstrated in NZM2328 mice (8).
A precise understanding of the roles of specific HLA-DR genes in lupus pathogenesis has not been possible because of the strong linkage disequilibrium in this region. To overcome this difficulty and to specifically investigate the role of HLA-DR3 in lupus, we generated HLA-DR3 (DRβ1*0301)-transgenic mice devoid of endogenous mouse class II (both I-A and I-E genes, AE0) on a NZM2328 background (NZM2328.DR3+AE0). The CD4+ T cell selection and MHC class II–restricted immune responses in these mice are solely dependent on HLA-DR3. The inclusion of AE° in the NZM2328 DR3–transgenic line is to circumvent the influence of the endogenous mouse MHC class II I-A and I-E genes that play important roles in susceptibility to murine lupus (9). Using this mouse model, we demonstrate for the first time, to our knowledge, the extent to which HLA-DR3 molecules contribute to pathogenesis of lupus and influence autoantibody profiles in the NZM2328 background.
Materials and Methods
Mice
HLA-DRB1*0301 (DR3).Aβ0 mice were generated by mating HLA-DR3 mice, a kind gift from Dr. Gunter Hämmerling (German Cancer Research Center, Heidelberg, Germany), with class II I-A− (Aβ0) mice, as described previously (10, 11). DRB1*0301Aβ0 mice were mated with MHCIIΔ/Δ mice lacking both I-A and I-E (hereby denoted as AE0 or AE−/−) genes, which were a kind gift from Drs. C. Benoist and D. Mathias (Harvard Medical School and Brigham and Women’s Hospital, Boston, Massachusetts), to generate HLA-DR3+AE0 (DR3+AE0) mice (12). NZM2328. DR3+AE0 mice were generated by successively backcrossing HLA-DR3+AE0 mice with NZM2328 mice for five generations and then intercrossing. Backcrossing beyond five generations led to increased mortality and infertility. During breeding of HLA-DR3+AE0 mice with NZM2328 mice, as a result of segregation of endogenous mouse MHC class II (AE), we got AE+/− (heterozygous for endogenous class II) mice and AE+/+ mice at the expected Mendelian ratios. Microsatellite data showed that N6 generation of NZM2328.DR3+AE0 mice had 98% NZM2328 background genes. The data presented in this article refer to N6 generation. Expression and segregation of HLA-DR3 during breeding were confirmed by PCR analysis of tail DNA and by flow cytometry using anti–HLA-DR (L227 mAb; ATCC HB-96) on PBMCs. We used mAb anti–mouse I-Ab (BD Pharmingen; clone AF6-120.1; cat. no. 553551), which cross-reacts with I-Au of NZM2328 mice. DR3+AE0 (mixed background of B10, B57BL/6, and 129) and NZM2328 mice were used as controls in the experiments. Mice were examined fortnightly for the development of proteinuria, and sera were collected monthly. All mice were housed in the specific pathogen–free colony at the Mayo Clinic and were cared for per established institutional protocols. The study protocol was approved by the Mayo Clinic Institutional Animal Use and Care Committee.
Assessment of kidney disease and histopathology
Urine protein was estimated using Albustix (Bayer) fortnightly. Mice with urine protein ≥ 3+ (300 mg/dl) confirmed 1 wk apart were considered to have severe proteinuria. Mice with severe proteinuria were sacrificed, and kidneys were collected for histopathology, electron microscopy, and immunofluorescence, as described previously (13). Kidneys, spleens, lungs, hearts, and livers were collected from all mice. Tissues were collected in 10% neutral-buffered saline for routine histopathology and in optimal cutting compound (Tissue-Tek; Sakura Finetek) and snap-frozen at −80°C for immunofluorescence studies.
Kidneys were evaluated by an expert renal pathologist (J.P.G.) for mesangial expansion, endocapillary proliferation, glomerular deposits, extra capillary proliferation, interstitial infiltrates, and tubular atrophy and scored from 0 to 3 (0, <10%; 1, 11–25%; 2, 26–50%, and 3, >51%). The percentages refer to the number of glomeruli that exhibited the changes for each of the listed items. At least 10 glomeruli were assessed per section. A total kidney histological score (KHS) was calculated as the sum of all described items (14).
Direct immunofluorescence
Acetone-fixed cryostat sections (5 µm) were blocked for 30 min with 5% BSA in PBS and incubated for 1 h with either FITC-conjugated goat IgG anti-mouse C3 (ICN/Cappel, Aurora, OH) in PBS (pH 7.2) or PE-conjugated goat anti-mouse IgG containing 5% BSA. Stained sections were washed three times in PBS and examined using an Olympus AX70 research microscope (Olympus America, Center Valley, PA). Images were acquired using an Olympus DP70 camera.
Electron microscopy
Kidney tissue was fixed in Trump’s fixative (1% glutaraldehyde and 4% formaldehyde in 0.1 M phosphate buffer [pH 7.2]) and rinsed three times for 30 min in 0.1 M phosphate buffer (pH 7.2), followed by a 1-h postfix in phosphate-buffered 1% OsO4. Tissue was stained en bloc with 2% uranyl acetate for 30 min at 60°C, dehydrated in progressive concentrations of ethanol and 100% propylene oxide, and embedded in Spurr’s resin (15). Tissue was rinsed with three washes of distilled water between steps. Thin (90-nm) sections were cut, placed on 200-mesh copper grids, and stained with lead citrate; micrographs were taken on a JEOL 1200 EXII operating at 60 kV.
Autoantibodies
Anti-nuclear Abs were determined using Hep2 cells (Bio-Rad Kallestad ANA Screen), as described previously (13). Briefly, sera diluted 1:100 in PBS containing 1% BSA were incubated with fixed Hep-2 cells at room temperature for 1 h. Bound Abs were detected with FITC-coupled goat anti-mouse IgG (Accurate Chemical and Scientific, Westbury, NY). Slides were washed thrice with PBS between each step. Stained slides were examined under a fluorescent microscope. Anti-Sm, anti-dsDNA, and anti-RNP Abs were measured using commercial mouse ELISA kits, per the manufacturer’s protocol (α diagnostics, San Antonio, TX), at 1:100 dilutions. Assays were performed in duplicates.
Western blot
Lysates from WEHI 7.1 cells were run on a 15% SDS-PAGE gel and transferred overnight to nitrocellulose membrane at 4°C. Membrane was blocked with 5% milk and 0.05% Tween for 1 h, and sera samples (1:100 dilution) in 5% milk containing PBS–Tween (PBST) were added. These were incubated for 2 h at room temperature and washed three times with PBST. Peroxide-labeled goat anti-mouse IgG was added at 1:3000 dilution for 1 h, and samples were washed six times with PBST. Blots were developed using a chemiluminescent substrate (Thermo Scientific, Rockford, IL).
Whole-cell lysate immunoprecipitation
This was performed according to the protocol described earlier (16). Briefly, WEHI 7.1 cells were grown in complete RPMI 1640. Washed cells (1 × 106/ml) were resuspended in methionine-free complete RPMI 1640 medium, and 0.5 µg methionine/ml and 100 µCi [35S]methionine/ml were added. After 15 h of incubation, cells were lysed using a Dounce homogenizer. Lysate was spun at 12,000 × g for 5 min. The supernatant was mixed with 5 µl individual mouse serum, and immunoprecipitation was performed as described earlier (16).
Analysis of cell surface staining by flow cytometry
Spleens and thymi were collected in PBS, and cells were obtained by mechanical disruption. Cell suspensions were treated with lysis buffer (0.15 M NH4Cl, 1 mM NaHCO3, 0.1 mM EDTA) for 5 min at room temperature. Cells were washed and resuspended in FACS buffer for flow cytometry analysis. The following Abs from BD Biosciences were used for flow cytometry: CD4-GK1.5, CD8-53-6.7, CD19-1D3, B220-RA3-6B2, Mac-1–M 1/70, and isotype control. Abs to stain various TCR Vβ regions were also obtained from BD Biosciences. Foxp3+ T cells were enumerated using an intracellular staining kit from eBioscience (San Diego, CA). Flow cytometry was performed on a FACScan (BD Biosciences) and analyzed using CellQuest software. Mononuclear cells were gated based on forward and side scatter profiles. Subsequent gating was based on staining with the indicated Abs.
Statistics
The median titer of autoantibodies in different strains was tested prior hoc using the nonparametric Kruskal–Wallis test, followed by post hoc comparisons using the Mann–Whitney rank sum test; the p value was adjusted for the number of paired comparisons. Proteinuria analysis was performed with the log-rank and χ2 tests using GraphPad Prism (version 3.0a; San Diego, CA). A p value < 0.05 was considered significant.
Results
Characterization of NZM2328.DR3+AE0 mice
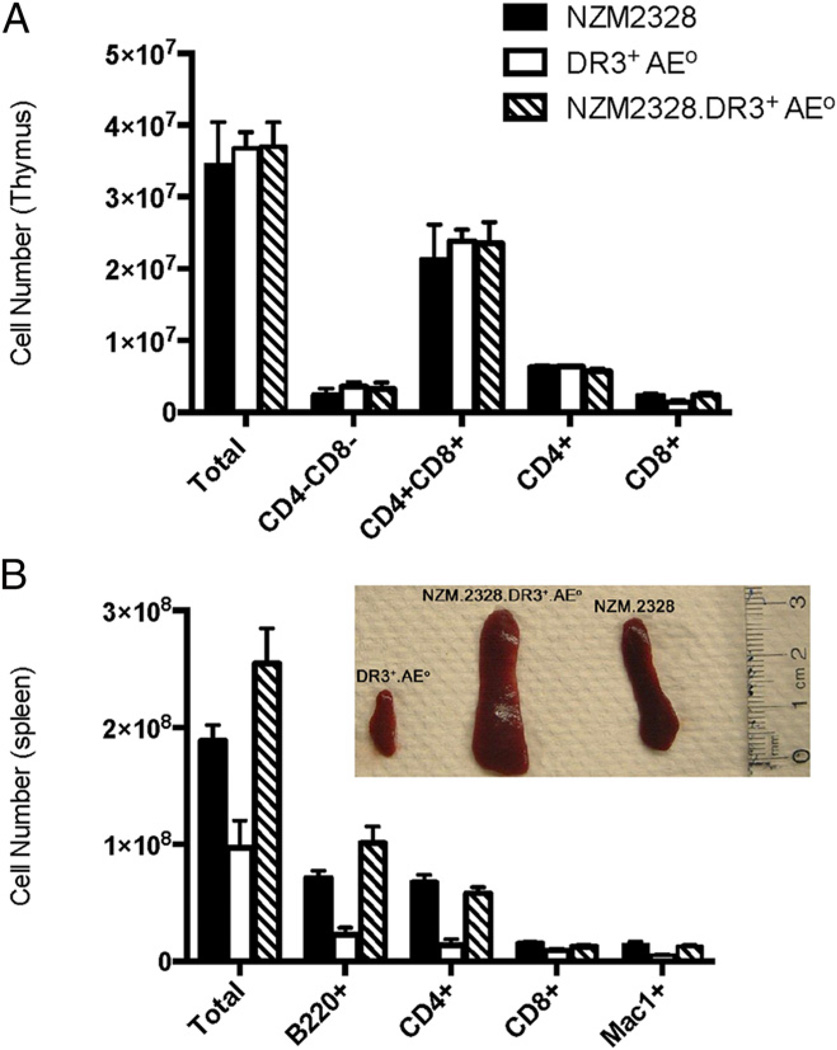
The total numbers of thymocytes (mean ± SD) were similar in NZM2328 (3.44 ± 2.2 × 107), DR3+AE0 (mixed background of B10, C57BL/6, and 129) (3.67 ± 0.5 × 107), and NZM2328. DR3+AE0 (3.69 ± 1.3 × 107) mice (p = 0.159, Fig. 1A). Thymocyte subsets (i.e., CD4−CD8−, CD4+CD8+, CD4+, and CD8+ cells) were also comparable in these three groups (Fig. 1A). The Vβ repertoire of CD4+ and CD8+ T cells in NZM2328, DR3+AE0 and NZM2328.DR3+AE0 mice were not significantly different (Supplemental Fig. 1). The absolute numbers (mean ± SD) of CD4+CD25+ Foxp3+ cells were also similar in NZM2328 mice (1.5 ± 0.2 × 104), DR3+AE0 mice (3.9 ± 1.3 × 104), and NZM2328.DR3+AE0 mice (4.6 ± 1.6 × 104) (p = 0.16, n = 2/group). Supplemental Fig. 2 shows the expression of DR3 on B220+ cells of NZM2328.DR3+AE0 mice. The mean (± SD) total cell number in spleen was increased in NZM2328 (18.9 ± 5.2 × 107) and NZM 2328.DR3+AE0 (25.4 ± 9.5 × 107) mice compared with DR3+AE0 mice (9.7 ± 4.7 × 107) (p = 0.028, Fig. 1B). As shown in Fig. 1B, B220+, CD4+ T, and Mac1+ cells were increased in NZM2328 mice (7.1 ± 2.2 × 107, 6.7 ± 2.5 × 107, and 1.4 ± 0.9 × 107, respectively) and NZM 2328.DR3+AE0 mice (10.1 ± 4.4 × 107, 5.8 ± 1.6 × 107, and 1.2 ± 0.3 × 107, respectively) compared with DR3+AE0 mice (2.3 ± 1.1 × 107, 1.4 ± 0.9 × 107, and 0.5 ± 0.2 × 107, respectively) (p = 0.002, 0.004, and 0.007, respectively), whereas the numbers of CD8+ T cells in spleen were similar in all groups (p = 0.05). Both NZM2328 and NZM 2328. DR3+AE0 mice had splenomegaly (inset, Fig. 1B).
FIGURE 1.
Transgenic expression of HLA-DR3 adequately supports thymocyte and splenocyte development. (A) The total numbers of thymocytes (mean ± SD) were similar among 3–4-mo-old NZM2328 (n = 15), DR3+AE0 (n = 4), and NZM2328.DR3+AE0 (n = 16) mice (p = 0.159). The absolute cell numbers among these groups for the CD4−CD8− (p = 0.21), CD4+CD8+ (p = 0.11), CD4+ (p = 0.10), and CD8+ (p = 0.50) subsets were also comparable. Data represent mean of three experiments. (B) Total number of spleen cells in mice at 3–4 mo of age are increased in NZM2328. DR3+AE0 and NZM2328 mice versus DR3+AE0 mice (p = 0.03). Inset shows massive splenomegaly that was seen in NZM2328.DR3+AE0 and NZM2328 mice; representative photograph is from 8-mo-old mice.
Development of glomerulonephritis and proteinuria in NZM2328.DR3+AE0 and NZM2328 mice
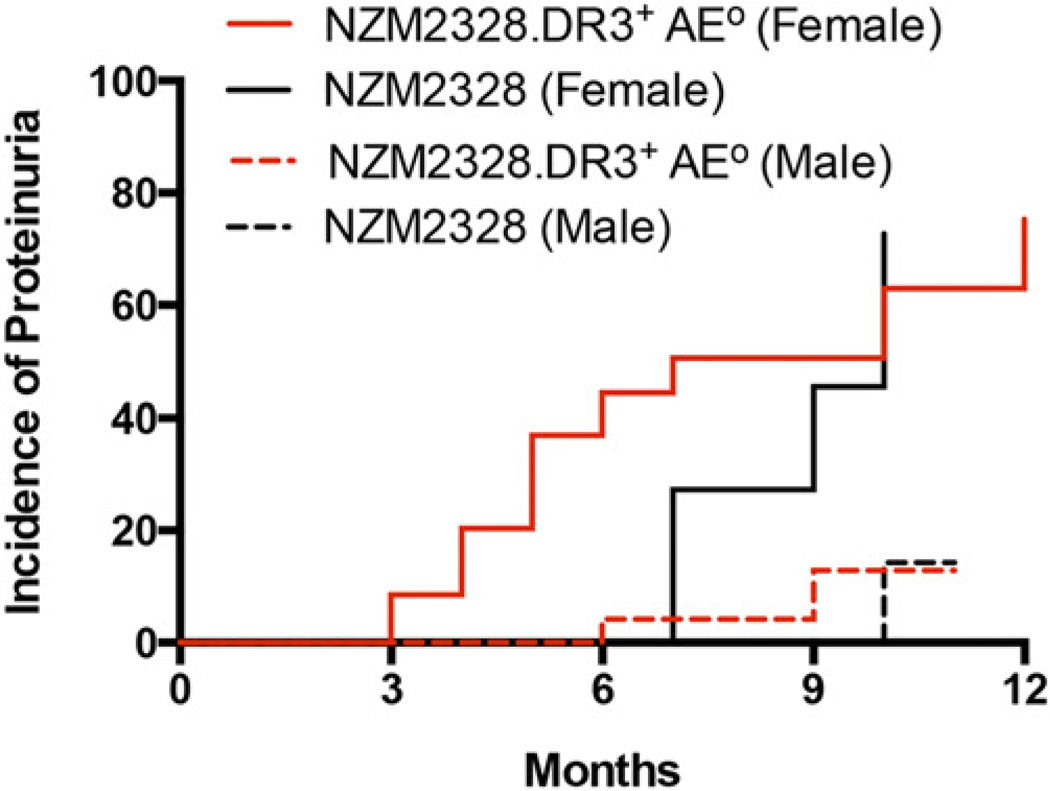
Fig. 2 shows the cumulative incidence of severe proteinuria in NZM2328 and NZM2328.DR3+AE0 mice. No mice in the DR3+AE0 group developed proteinuria. A total of 75% of female NZM2328.DR3+AE0 mice and 72% of female NZM2328 mice developed proteinuria at 12 mo (p = 0.28). Severe proteinuria was infrequent in male NZM2328.DR3+AE0 and NZM2328 mice (13 and 14%, respectively, p = 0.63). The onset of proteinuria in female mice was earlier in NZM2328.DR3+AE0 mice (median, 5 mo; range; 3–11 mo) compared with NZM2328 mice (median, 9 mo; range: 7–10 mo, p < 0.001).
FIGURE 2.
Female NZM2328.DR3+AE0 mice develop proteinuria earlier than NZM2328 mice. Severe proteinuria (>3+) was determined using Albustix. The cumulative incidence of proteinuria in female NZM2328 mice (n = 12) was similar to that in female NZM2328.DR3+AE0 mice (n = 35) (72 and 75%, respectively, p = 0.28, log-rank, Mantel–Cox test). The cumulative incidence was 14% in male NZM2328 mice (n = 18) and 13% in male NZM2328.DR3+AE0 mice (n = 30). The onset of proteinuria was earlier in female NZM2328.DR3+AE0 mice compared with female NZM2328 mice (median, 5 mo versus 9 mo, p < 0.001).
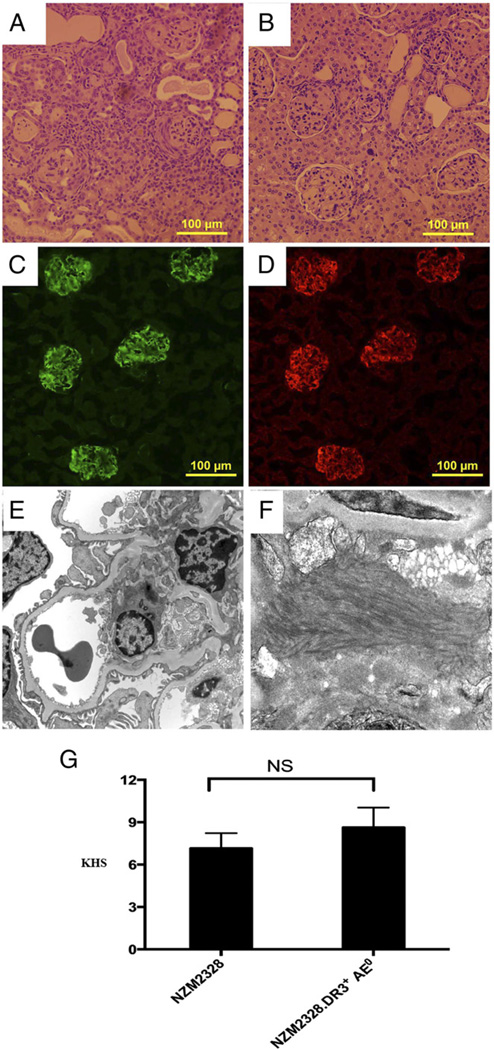
Kidneys from NZM2328.DR3+AE0 mice (Fig. 3A) showed mesangial expansion, glomerular deposits, extracapillary proliferation, interstitial infiltrates, and tubular atrophy dilatation with casts. These changes were less prominent in NZM2328 mice (Fig. 3B). Only NZM2328.DR3+AE0 mice showed perivascular or medullary lymphoid aggregates (4/16), crescents (3/16), necrotizing vasculitis (1/16), and classic wire loop lesions (1/16). Immunofluorescence showed deposition of complement C3 and total IgG in NZM2328.DR3+AE0 mice (Fig. 3C, 3D). Similar IgG and C3 deposits were noted in the kidneys of NZM2328 mice but not in DR3+AE0 mice (data not shown). Electron microscopy of a 5-moold female NZM2328.DR3+AE0 mouse showed immune complex deposits, predominantly within mesangial regions (Fig. 3E, 3F). The peripheral capillary loops were patent, and basement membranes appeared to be of normal thickness. There was very mild and segmental effacement of visceral epithelial cell foot processes. The mean (± SD) KHS for was 7.2 ± 4.3 for NZM2328 mice and 8.6 ± 5.7 for NZM2328.DR3+AE0 mice (p = 0.48) (Fig. 3G). Individual components of the KHS are shown in Supplemental Table I. Lungs showed perivascular lymphoid infiltrates only in 6 of 16 NZM2328.DR3+AE0 mice. No morphologic abnormalities were noted in liver, heart, or skeletal muscle.
FIGURE 3.
NZM2328.DR3+AE0 mice develop severe glomerulonephritis characteristic of lupus. Kidney histopathology (H&E stain) from 5-mo-old female NZM2328.DR3+AE0 (A) and NZM2328 (B) mice show mesangial expansion, mononuclear tubulointerstitial infiltrates, and enlarged and sclerosed glomeruli. Direct immunofluorescence shows deposition of complement C3 (C) and IgG (D) in glomeruli of NZM2328.DR3+AE0 mice. (E) Electron microscopy photomicrograph from 5-mo-old NZM2328.DR3+AE0 mouse showing low-power view of capillary loops and mesangium (original magnification ×2900). The peripheral capillary loops are patent and basement membranes appear to be of normal thickness. There is very mild and segmental effacement of visceral epithelial cell foot processes and deposition of immune complexes in the mesangium. (F) A higher-power electron microscopy view (magnification ×9600) of the immune complex deposits in the mesangial region with fibrillary structures, which are often seen in human lupus nephritis. (G) KHS in NZM2328 mice (n = 16) and NZM2328.DR3+AE0 mice (n = 16) was calculated as described in Materials and Methods. The mean (± SD) KHS for NZM2328 mice was 7.2 ± 4.3, which was similar to the score for NZM2328.DR3+AE0 mice (8.6 ± 5.7, p = 0.48).
Autoantibody profile
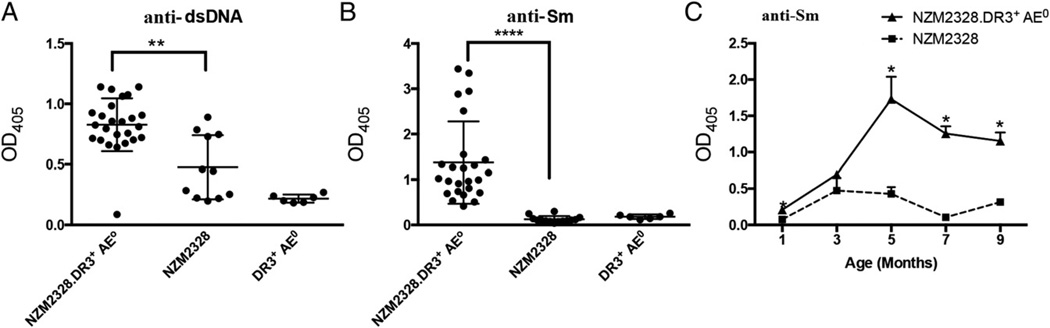
Female NZM2328 and NZM2328.DR3+AE0 mice developed anti-dsDNA Abs; the level of anti-dsDNA was significantly higher in NZM2328.DR3+AE0 mice (p = 0.02, Fig. 4A). Female NZM2328. DR3+AE0 mice developed Abs to Sm, whereas other groups did not (Fig. 4B, NZM2328.DR3+AE0 versus NZM2328, p < 0.0001; NZM2328.DR3+AE0 versus DR3+AE0, p < 0.0001). In this cohort of 25 female NZM2328.DR3+AE0 mice, the levels of anti-SmD Abs, by ELISA, varied from an OD405 ~0.5 to ~3.5 at 1:100 dilution, with the majority of sera showing values between ~0.5 and ~1.5. The anti-Sm Abs appeared at 3 mo of age in NZM2328. DR3+AE0 mice and peaked at 5 mo (Fig. 4C); they were still present when mice were sacrificed or at 9 mo of age.
FIGURE 4.
NZM2328.DR3+AE0 mice develop high titers of anti-dsDNA and anti-Sm Abs. (A and B) Sera from 5-mo-old mice were tested for reactivity against dsDNA and SmD. No reactivity against dsDNA or SmD was seen in NZM2328.DR3+AE0 mice (n = 6). Both NZM2328.DR3+AE0 mice (n = 25) and NZM2328 mice (n = 11) developed anti-dsDNA; however, the titers were higher in NZM2328.DR3+AE0 mice (p = 0.02). (B) Interestingly, NZM2328. DR3+AE0 mice developed Abs to Sm, whereas other groups did not (p < 0.0001, NZM2328.DR3+AE0 versus NZM2328 mice; p < 0.0001, NZM2328. DR3+AE0 versus DR3+AE0 mice). (C) Autoantibody titers to anti-Sm were compared between NZM2328.DR3+AE0 and NZM2328 mice (n = 4–10 mice) at various time points. In NZM2328.DR3+AE0 mice, anti-Sm Abs began appearing at 3 mo of age, peaked at 5 mo of age, and remained detectable up to 9 mo of age. The asterisks denote statistical significance.
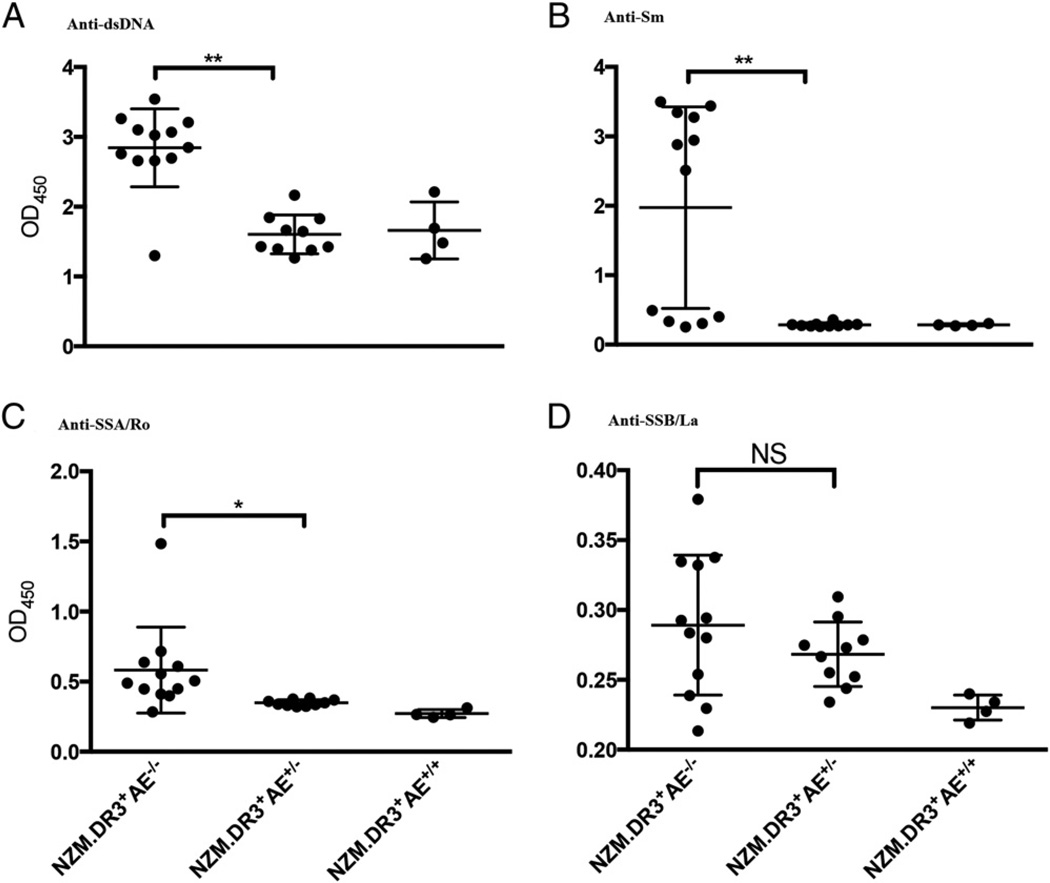
Serum samples were collected from three cohorts of 5-mo-old female mice (NZM2328.DR3+AE−/−, NZM2328.DR3+AE+/−, and NZM2328.DR3+AE+/+) and were assayed for Abs to dsDNA, Sm, SSA/Ro, and SSB/La (Fig. 5). Although all three cohorts made anti-dsDNA Abs, higher levels of anti-dsDNA Abs were detected in NZM2328.DR3+AE−/− mice (Fig. 5A). With regard to anti-Sm Abs, NZM2328.DR3+AE+/− and NZM2328.DR3+AE+/+ mice did not develop significant amounts of anti-Sm Abs, whereas anti-SM Abs were readily detected in 7 of 12 NZM2328.DR3+AE−/− mice (Fig. 5B). In this particular cohort, nonproducers of anti-SmD Abs were seen, in marked contrast with the cohort of mice assayed in Fig. 4. NZM2328.DR3+AE−/− mice also developed low levels of anti-SSA/Ro Abs (mean OD ± SD, 0.58 ± 0.29), which were significantly elevated compared with those in NZM2328.DR3+AE+/− mice (p < 0.05). Low reactivity of anti-SSB/La was observed in both NZM2328.DR3+AE−/− and NZM2328.DR3+AE+/− mice; no statistically significant difference was detected between these two groups. No statistical analysis was done involving the NZM2328.DR3+AE+/+ mice in Fig. 5, because of the small sample assayed. Nevertheless, these mice do not tend to make Abs to SmD, SSA/Ro, or SSB/La.
FIGURE 5.
Anti-dsDNA, anti-Sm, anti-SSA/Ro, and anti-SSB/La Abs in different groups of mice. Sera from different genotypes of NZM2328.DR3+AE−/− (n = 12), NZM2328.DR3+AE+/− (n = 10), and NZM2328.DR3+AE+/+ (n = 4) mice were tested by ELISA for anti-dsDNA (A), anti-Sm (B), anti-SSA/Ro (C), and anti-SSB/La (D) Abs. Higher levels of anti-dsDNA Abs were seen in NZM2328.DR3+AE−/− mice in comparison with NZM2328.DR3+AE+/− mice (p = 0.003). Anti-Sm Abs were seen only in NZM2328.DR3+AE−/− mice (7/12 mice). NZM2328.DR3+AE−/− mice also developed anti-SSA/Ro Abs, which were significantly elevated compared with NZM2328. DR3+AE+/− mice (p < 0.05). Both NZM2328.DR3+AE−/− and NZM2328. DR3+AE+/− mice made low level of anti-SSB. Only anti-dsDNA Abs were seen in the NZM2328.DR3+AE+/+ group. Statistical comparison with other groups was not performed because of the small number of mice in this group. The asterisks denote statistical significance.
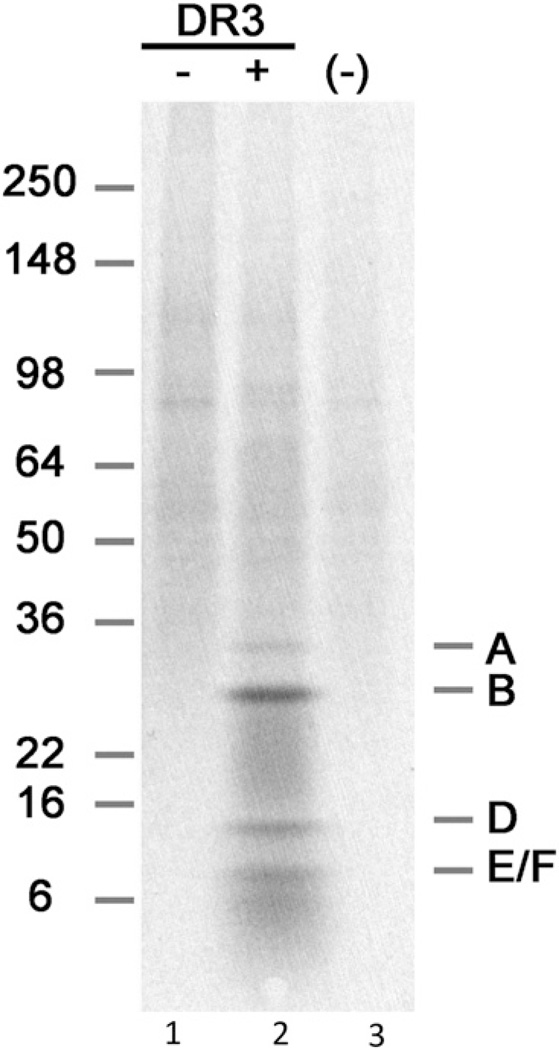
To demonstrate the anti-Sm specificity, pooled sera from NZM2328.DR3+AE0 mice were shown to be able to immunoprecipitate A, SmB, SmD, and E/F proteins of the snRNP particle using [35S]methionine-labeled WEHI 7.1 cell lysate (Fig. 6, lane 2), whereas no reactivity was seen in pooled sera from age-matched NZM2328 mice (Fig. 6, lane 1).
FIGURE 6.
Immunoprecipitation of 35S-labeled snRNP proteins. WEHI 7.1 cells were incubated with [35S]methionine for 15 h, and the lysate was mixed with 5 µl of pooled sera from each group. Lane 1 is a sample from a 9-mo-old female NZM2328 mouse, lane 2 is a sample from a 9-mo-old female NZM2328.DR3+AE0 mouse, and lane 3 is negative control (saline).
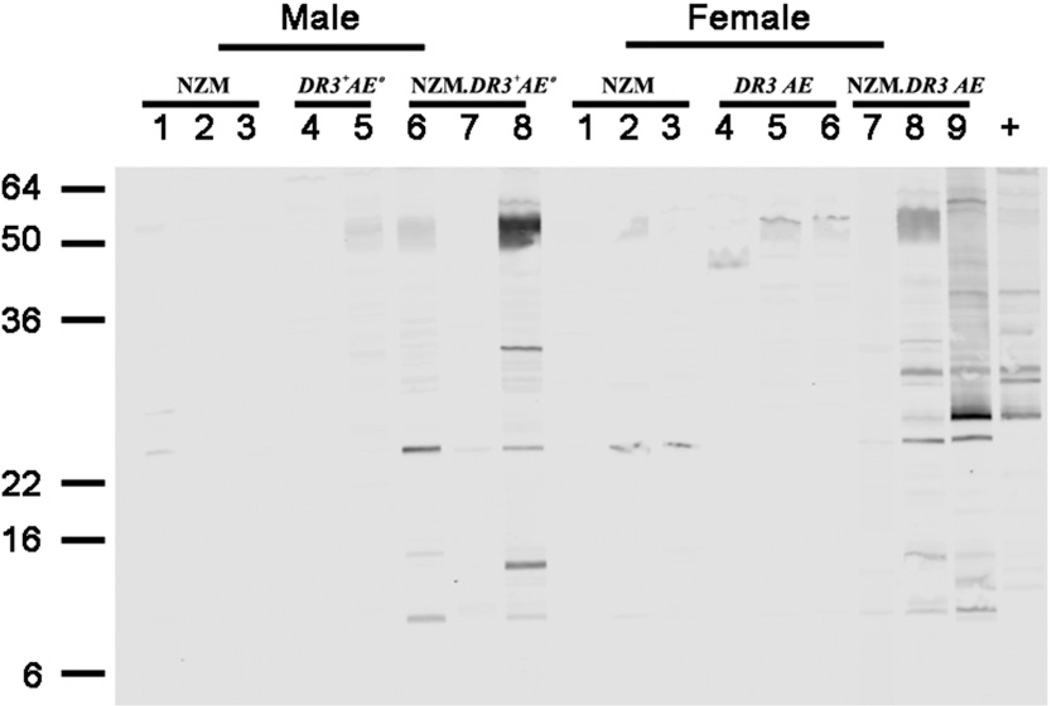
By Western blot analysis, greater autoantibody diversification was seen in the sera of NZM2328.DR3+AE0 mice (Fig. 7). Sera from 5-mo-old male and female NZM2328.DR3+AE0 mice reacted against various cellular constituents, as shown by the strong bands, whereas weak reactivity was seen using age-matched sera from NZM2328 and DR3+AE0 mice. Western blot data showed that autoantibody diversification in NZM2328.DR3+AE0 mice increased with age and was more pronounced in female mice compared with male mice (Supplemental Fig. 3).
FIGURE 7.
NZM2328.DR3+AE0 mice develop a broader spectrum of autoantibodies. Sera from 5-moold mice were diluted 1:100, and their reactivities were tested in Western blots using WEHI 7.1 cell extract. No reactivity and few faint bands were seen in sera from male and female NZM2328 mice, respectively. One of two and two of three male and female DR3+AE0 mice, respectively, showed a few faint bands. In contrast, strong reactivity against various cellular constituents was seen in two of three male and female NZM2328. DR3+AE0 mice. Serum from an MRL/lpr mouse was used as positive control.
Discussion
Human genetic studies showed a significant association of HLA-DR3 with lupus in various ethnicities (1, 2, 5, 17–24). Graham et al. (5) showed a strong risk for SLE in patients with the HLA-DR2 or HLA-DR3 haplotype. The transmitted/nontransmitted ratio was highest for DR3/DR3 and DR2/DR3 genotypes, at 2.1 and 2.3, respectively, compared with 1.4 for the DR3/DRX haplotype [“X” is any non-DR2 or non-DR3 haplotype] and 1.3 for the DR2/DRX haplotype, indicating a dose effect of DR3. Interestingly, HLA-DR3 homozygotes showed strong association with anti-Sm, but not with Ro and La, and a trend toward association with anti-dsDNA. A family-based SNP association study from the U.K. also showed an independent association and dominant inheritance model for HLA-DRB1*0301 (odds ratio [OR] = 2.3) (25). A larger study of white subjects that analyzed single nucleotide polymorphisms in MHC genes showed several independent association signals within the MHC region, especially with DRB1*0301 (OR = 2.21, p = 2.5 × 10−12) (22). When MHC haplotypes were compared between cases and controls, the haplotype containing the HLA-DRB1*0301 allele (~70% of the DRB1*0301 haplotypes) showed a strong association with SLE (15% transmitted versus 6% nontransmitted haplotypes, OR = 2.63, p = 8.32 × 10−15) and the DRB1*1501 allele (9% transmitted versus 6% nontransmitted, OR = 1.54, p = 0.0025). Thus, HLA-DR, especially HLA-DR3, is significantly associated with SLE.
Mechanistically, HLA-DR3 was believed to contribute to lupus pathogenesis through autoantibody generation; however, an independent effect of HLA-DR3 without autoantibody association is possible (26). Several studies also showed a higher risk for lupus nephritis in HLA-DR3+ SLE patients (27–29). Due to linkage disequilibrium and other confounding reasons, a specific role for HLA-DR3 in the pathogenesis of lupus and lupus nephritis, as well as the generation of certain autoantibodies, particularly anti-Sm, has been difficult to prove by human genetic-association studies. Therefore, we used HLA class II–transgenic mice to resolve some of these issues.
Using a panel of HLA-DR– and HLA-DQ–transgenic mice that do not spontaneously develop lupus, we showed previously that HLA-DR3 plays a dominant role in determining the quantitative and qualitative aspects of the immune response to SmD following immunization with recombinant SmD (30). HLA-DR3+Aβ0 mice had the highest titers of anti-SmD Abs, recognized the most numbers of SmD epitopes, and had the most diverse autoantibody specificities when tested by Western blot, with epitope spreading to SmB, A-RNP, and C-RNP. In addition, only HLA-DR3+Aβ0 mice developed anti-dsDNA Abs that were partially cross-reactive with anti-Sm Abs. These studies verified the role of HLA-DR3 in shaping the immune response to lupus-associated autoantigens following active immunizations. Therefore, to specifically address its role in spontaneous lupus, we developed HLA-DR3+AE0 mice on a lupus-prone NZM2328 background.
The HLA-DR3 transgene was fully functional and selected a broad repertoire of T cells in the NZM2328 background, as in the nonautoimmune background. NZM2328.DR3+AE0 mice developed a high incidence and early onset of proteinuria, supporting the association of HLA-DR3 with lupus. Our study also reiterates the importance of non-MHC genes in the etiopathogenesis of lupus, because HLA-DR3 mice on a B6 background do not spontaneously develop lupus. Thus, both MHC and non-MHC genes are required for the occurrence of lupus. The unique aspect of NZM2328.DR3+AE−/− mice was the spontaneous development of high levels of anti-Sm Abs and anti-dsDNA Abs, weakly positive anti-SSA/Ro and anti-SSB/La, and an earlier onset of nephritis. The development of anti-Sm and anti-SSA/Ro was dependent on the presence of human transgene HLA-DR3, because only mice that were completely devoid of their own class II developed anti-Sm Abs. HLA-DR3 mice that expressed endogenous class II either in the homozygous state (NZM2328.DR3+AE+/+) or the heterozygous state (NZM2328.DR3+AE+/−) did not develop anti-Sm, anti-SSA/Ro Abs. We speculate that this unique observation in our model suggests that the HLA-DR3 molecule may mediate the positive selection of SmD-reactive CD4+ T cells in the thymus in the absence of endogenous mouse class II, whereas the presence of endogenous mouse class II may shape an alternate T cell repertoire that does not support the generation of a spontaneous anti-Sm immune response. This could also be due to the expression of chimeric human–mouse MHC class II molecules, which could potentially alter the T cell repertoire. The mice that expressed both DR3 and endogenous class II molecules did not develop proteinuria (data not shown), suggesting the presence of an altered T cell repertoire in these mice. It is of interest to note that both NZM2328.DR3+AE−/− and NZM2328.DR3+AE+/− mice make low levels of anti-SSB/La, suggesting some overlap of the T cell repertoire in these two congenic lines.
It is evident from Fig. 5 that some of the female NZM2328. DR3+AE−/− mice did not make anti-Sm Abs. This finding is similar to the situation in MRL/lpr mice, the only other strain of lupus prone-mice that makes anti-Sm Abs. Extensive investigation of MRL/lpr mice by Eisenberg et al. (31) led to the conclusion that there is stochastic control of anti-Sm Ab production in these mice. It should be noted that this article was published in 1987, and the conclusion is based on negative results. It is more likely that an environmental factor, such as a microbiome difference between these mice, is responsible for the observation that only some NZM2328.DR3+AE−/− mice make anti-Sm Abs.
The role of anti-Sm Abs in the pathogenesis of lupus nephritis was suggested in experimental models (32, 33). Injection of key-hole limpet hemocyanin–coupled SmD peptides into NZB/NZW mice accelerated the onset of nephritis and anti-dsDNA titers (32). Although anti-Sm autoantibodies have not been demonstrated in NZB/NZW mice, SmD183–119 was shown to stimulate T cells isolated from nonimmunized young mice, and T cells from older mice can be stimulated to produce anti-dsDNA Abs and to enhance their cellular production of inflammatory cytokines (33). These investigators concluded that SmD183–119-reactive T cells play an important role in the generation of anti-dsDNA Abs and indirectly contribute to the pathogenesis of lupus nephritis. Anti-Sm Abs in the presence of anti-dsDNA Abs were detected in 92.9% of humans with lupus nephritis (34). The presence of anti-Sm Abs, together with other autoantibodies in unique patterns, was found predominantly in lupus nephritis patients with African ancestry (35, 36). It appears that anti-Sm Abs have significant predicative value for lupus nephritis. In addition, Mannik et al. (37) eluted anti-Sm Abs from postmortem kidneys of lupus patients, suggesting the direct participation of anti-Sm Abs in renal damage in lupus.
The exact role of anti-Sm Abs in lupus nephritis remains to be elucidated. Recently Bruschi et al. (38, 39) eluted Abs from 20 renal biopsy samples from patients with lupus nephritis. By the recent developed proteomic technique, they identified 12 targeted podocyte molecules. They found that podocyte Ags α-enolase and annexin A1 were the most targeted Ags (38). Six of the 20 renal eluates had Abs to α-enolase without Abs to dsDNA. Four of the 20 eluates had Abs to annexin A1 without anti-dsDNA Abs. These investigators concluded that anti-dsDNA Abs may not be the initiating Abs in lupus nephritis. Anti-dsDNA and anti-C1q Abs play an amplification role in the pathogenesis of lupus nephritis (39). From these studies, it is tempting to speculate that anti-Sm Abs may also play a role in amplifying renal damage and enhancing the anti-dsDNA response. The earlier onset of proteinuria and higher renal pathology scores in NZM2328.DR3+AE−/− mice can then be speculated to be due to the presence of high titers of anti-Sm Abs.
In conclusion, our spontaneous lupus mouse model supports an important role for HLA-DRB1*0301 in generating an immune response to anti-Sm and other RNPs and influencing the severity of lupus nephritis in the NZM2328 lupus background. Our model supports the participation of HLA-DRB1*0301 in disease pathogenesis; however, comparison with transgenic mice bearing disease-predisposing DRB1*1501, DQA1*0602, or HLA-DQB1*0201 or disease-protective HLA-DR7, as well as other lupus-prone murine models, may provide additional information regarding the role of the HLA-D region in the pathogenesis of SLE, in general, and the pathogenesis of lupus nephritis, in particular.
Supplementary Material
Acknowledgments
We thank Brittny Murphy for statistical support and Chad R. Clark for laboratory assistance.
This work was supported by National Institute of Arthritis and Musculoskeletal Diseases Grants K23-AR057815, R01-AR047988, R01-AR049449, and R01-AR30752. V.R.C. is supported by National Institute of Arthritis and Musculoskeletal Diseases Grant K23 AR-057815 and the John M. Nasseff Sr. Research Award in Rheumatology honoring Dr. Harvinder Luthra, Mayo Foundation.
Abbreviations used in this article
- KHS
kidney histological score
- OR
odds ratio
- PBST
PBS–Tween
- RNP
ribonucleoprotein
- SLE
systemic lupus erythematosus
- Sm
Smith
- snRNP
small nuclear RNP
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Reveille JD, Moulds JM, Ahn C, Friedman AW, Baethge B, Roseman J, Straaton KV, Alarcón GS. Systemic lupus erythematosus in three ethnic groups: I. The effects of HLA class II, C4, and CR1 alleles, socioeconomic factors, and ethnicity at disease onset. LUMINA Study Group. Lupus in minority populations, nature versus nurture. Arthritis Rheum. 1998;41:1161–1172. doi: 10.1002/1529-0131(199807)41:7<1161::AID-ART4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 2.Gladman DD, Urowitz MB, Darlington GA. Disease expression and class II HLA antigens in systemic lupus erythematosus. Lupus. 1999;8:466–470. doi: 10.1177/096120339900800610. [DOI] [PubMed] [Google Scholar]
- 3.Graham RR, Ortmann WA, Langefeld CD, Jawaheer D, Selby SA, Rodine PR, Baechler EC, Rohlf KE, Shark KB, Espe KJ, et al. Visualizing human leukocyte antigen class II risk haplotypes in human systemic lupus erythematosus. Am. J. Hum. Genet. 2002;71:543–553. doi: 10.1086/342290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harley JB, Alarcón-Riquelme ME, Criswell LA, Jacob CO, Kimberly RP, Moser KL, Tsao BP, Vyse TJ, Langefeld CD. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat. Genet. 2008;40:204–210. doi: 10.1038/ng.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham RR, Ortmann W, Rodine P, Espe K, Langefeld C, Lange E, Williams A, Beck S, Kyogoku C, Moser K, et al. Specific combinations of HLA-DR2 and DR3 class II haplotypes contribute graded risk for disease susceptibility and autoantibodies in human SLE. Eur. J. Hum. Genet. 2007;15:823–830. doi: 10.1038/sj.ejhg.5201827. [DOI] [PubMed] [Google Scholar]
- 6.Waters ST, Fu SM, Gaskin F, Deshmukh US, Sung SS, Kannapell CC, Tung KS, McEwen SB, McDuffie M. NZM2328: a new mouse model of systemic lupus erythematosus with unique genetic susceptibility loci. Clin. Immunol. 2001;100:372–383. doi: 10.1006/clim.2001.5079. [DOI] [PubMed] [Google Scholar]
- 7.Waters ST, McDuffie M, Bagavant H, Deshmukh US, Gaskin F, Jiang C, Tung KS, Fu SM. Breaking tolerance to double stranded DNA, nucleosome, and other nuclear antigens is not required for the pathogenesis of lupus glomerulonephritis. J. Exp. Med. 2004;199:255–264. doi: 10.1084/jem.20031519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sim DL, Bagavant H, Scindia YM, Ge Y, Gaskin F, Fu SM, Deshmukh US. Genetic complementation results in augmented autoantibody responses to lupus-associated antigens. J. Immunol. 2009;183:3505–3511. doi: 10.4049/jimmunol.0901207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirose S, Zhang D, Nozawa S, Nishimura H, Shirai T. The E-linked subregion of the major histocompatibility complex down-regulates autoimmunity in NZB × NZW F1 mice. Immunogenetics. 1994;40:150–153. doi: 10.1007/BF00188179. [DOI] [PubMed] [Google Scholar]
- 10.Kong YC, Lomo LC, Motte RW, Giraldo AA, Baisch J, Strauss G, Hämmerling GJ, David CS. HLA-DRB1 polymorphism determines susceptibility to autoimmune thyroiditis in transgenic mice: definitive association with HLA-DRB1*0301 (DR3) gene. J. Exp. Med. 1996;184:1167–1172. doi: 10.1084/jem.184.3.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strauss G, Vignali DA, Schönrich G, Hämmerling GJ. Negative and positive selection by HLA-DR3(DRw17) molecules in transgenic mice. Immunogenetics. 1994;40:104–108. [PubMed] [Google Scholar]
- 12.Madsen L, Labrecque N, Engberg J, Dierich A, Svejgaard A, Benoist C, Mathis D, Fugger L. Mice lacking all conventional MHC class II genes. Proc. Natl. Acad. Sci. USA. 1999;96:10338–10343. doi: 10.1073/pnas.96.18.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chowdhary VR, Grande JP, Luthra HS, David CS. Characterization of haemorrhagic pulmonary capillaritis: another manifestation of Pristane-induced lupus. Rheumatology (Oxford) 2007;46:1405–1410. doi: 10.1093/rheumatology/kem117. [DOI] [PubMed] [Google Scholar]
- 14.Alperovich G, Rama I, Lloberas N, Franquesa M, Poveda R, Gomà M, Herrero-Fresneda I, Cruzado JM, Bolaños N, Carrera M, et al. New immunosuppresor strategies in the treatment of murine lupus nephritis. Lupus. 2007;16:18–24. doi: 10.1177/0961203306073136. [DOI] [PubMed] [Google Scholar]
- 15.Spurr AR. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- 16.Fisher DE, Reeves WH, Conner GE, Blobel G, Kunkel HG. Pulse labeling of small nuclear ribonucleoproteins in vivo reveals distinct patterns of antigen recognition by human autoimmune antibodies. Proc. Natl. Acad. Sci. USA. 1984;81:3185–3189. doi: 10.1073/pnas.81.10.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gómez-Reino JJ, Martínez-Laso J, Vicario JL, Paz-Artal E, Aragón A, Martín-Villa JM, De Juan MD, Pérez-Aciego P, Arnaiz-Villena A. Immunogenetics of systemic lupus erythematosus in Spanish patients: differential HLA markers. Immunobiology. 1991;182:465–471. doi: 10.1016/s0171-2985(11)80210-x. [DOI] [PubMed] [Google Scholar]
- 18.Galeazzi M, Sebastiani GD, Morozzi G, Carcassi C, Ferrara GB, Scorza R, Cervera R, de Ramon Garrido E, Fernandez-Nebro A, Houssiau F, et al. European Concertee Action on the immunogenetics of SLE. HLA class II DNA typing in a large series of European patients with systemic lupus erythematosus: correlations with clinical and autoantibody subsets. Medicine (Baltimore) 2002;81:169–178. doi: 10.1097/00005792-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Bekker-Mendez C, Yamamoto-Furusho JK, Vargas-Alarcón G, Ize-Ludlow D, Alcocer-Varela J, Granados J. Haplotype distribution of class II MHC genes in Mexican patients with systemic lupus erythematosus. Scand. J. Rheumatol. 1998;27:373–376. doi: 10.1080/03009749850154410. [DOI] [PubMed] [Google Scholar]
- 20.Martín-Villa JM, Martínez-Laso J, Moreno-Pelayo MA, Castro-Panete MJ, Martínez-Quiles N, Alvarez M, de Juan MD, Gómez-Reino JJ, Arnaiz-Villena A. Differential contribution of HLA-DR, DQ, and TAP2 alleles to systemic lupus erythematosus susceptibility in Spanish patients: role of TAP2*01 alleles in Ro autoantibody production. Ann. Rheum. Dis. 1998;57:214–219. doi: 10.1136/ard.57.4.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shankarkumar U, Ghosh K, Badakere SS, Mohanty D. HLA-DRB1*03 and DQB1*0302 associations in a subset of patients severely affected with systemic lupus erythematosus from western India. Ann. Rheum. Dis. 2003;62:92–93. doi: 10.1136/ard.62.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barcellos LF, May SL, Ramsay PP, Quach HL, Lane JA, Nititham J, Noble JA, Taylor KE, Quach DL, Chung SA, et al. High-density SNP screening of the major histocompatibility complex in systemic lupus erythematosus demonstrates strong evidence for independent susceptibility regions. PLoS Genet. 2009;5:e1000696. doi: 10.1371/journal.pgen.1000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harley JB, Sestak AL, Willis LG, Fu SM, Hansen JA, Reichlin M. A model for disease heterogeneity in systemic lupus erythematosus. Relationships between histocompatibility antigens, autoantibodies, and lymphopenia or renal disease. Arthritis Rheum. 1989;32:826–836. [PubMed] [Google Scholar]
- 24.Schur PH, Marcus-Bagley D, Awdeh Z, Yunis EJ, Alper CA. The effect of ethnicity on major histocompatibility complex complement allotypes and extended haplotypes in patients with systemic lupus erythematosus. Arthritis Rheum. 1990;33:985–992. doi: 10.1002/art.1780330710. [DOI] [PubMed] [Google Scholar]
- 25.Fernando MM, Stevens CR, Sabeti PC, Walsh EC, McWhinnie AJ, Shah A, Green T, Rioux JD, Vyse TJ. Identification of two independent risk factors for lupus within the MHC in United Kingdom families. PLoS Genet. 2007;3:e192. doi: 10.1371/journal.pgen.0030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris DL, Fernando MM, Taylor KE, Chung SA, Nititham J, Alarcón-Riquelme ME, Barcellos LF, Behrens TW, Cotsapas C, Gaffney PM, et al. MHC associations with clinical and autoantibody manifestations in European SLE. Genes Immun. 2014;15:210–217. doi: 10.1038/gene.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niu Z, Zhang P, Tong Y. Value of HLA-DR genotype in systemic lupus erythematosus and lupus nephritis: a meta-analysis. Int. J. Rheum. Dis. 2015;18:17–28. doi: 10.1111/1756-185X.12528. [DOI] [PubMed] [Google Scholar]
- 28.Chung SA, Brown EE, Williams AH, Ramos PS, Berthier CC, Bhangale T, Alarcon-Riquelme ME, Behrens TW, Criswell LA, Graham DC, et al. Lupus nephritis susceptibility loci in women with systemic lupus erythematosus. J. Am. Soc. Nephrol. 2014;25:2859–2870. doi: 10.1681/ASN.2013050446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor KE, Chung SA, Graham RR, Ortmann WA, Lee AT, Langefeld CD, Jacob CO, Kamboh MI, Alarcón-Riquelme ME, Tsao BP, et al. Risk alleles for systemic lupus erythematosus in a large case-control collection and associations with clinical subphenotypes. PLoS Genet. 2011;7:e1001311. doi: 10.1371/journal.pgen.1001311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang C, Deshmukh US, Gaskin F, Bagavant H, Hanson J, David CS, Fu SM. Differential responses to Smith D autoantigen by mice with HLA-DR and HLA-DQ transgenes: dominant responses by HLA-DR3 transgenic mice with diversification of autoantibodies to small nuclear ribonucleoprotein, double-stranded DNA, and nuclear antigens. J. Immunol. 2010;184:1085–1091. doi: 10.4049/jimmunol.0902670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eisenberg RA, Craven SY, Warren RW, Cohen PL. Stochastic control of anti-Sm autoantibodies in MRL/Mp-lpr/lpr mice. J. Clin. Invest. 1987;80:691–697. doi: 10.1172/JCI113123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riemekasten G, Kawald A, Weiss C, Meine A, Marell J, Klein R, Hocher B, Meisel C, Hausdorf G, Manz R, et al. Strong acceleration of murine lupus by injection of the SmD1(83-119) peptide. Arthritis Rheum. 2001;44:2435–2445. doi: 10.1002/1529-0131(200110)44:10<2435::aid-art408>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 33.Riemekasten G, Langnickel D, Ebling FM, Karpouzas G, Kalsi J, Herberth G, Tsao BP, Henklein P, Langer S, Burmester GR, et al. Identification and characterization of SmD183-119-reactive T cells that provide T cell help for pathogenic anti-double-stranded DNA antibodies. Arthritis Rheum. 2003;48:475–485. doi: 10.1002/art.10762. [DOI] [PubMed] [Google Scholar]
- 34.Janwityanuchit S, Verasertniyom O, Vanichapuntu M, Vatanasuk M. Anti-Sm: its predictive value in systemic lupus erythematosus. Clin. Rheumatol. 1993;12:350–353. doi: 10.1007/BF02231577. [DOI] [PubMed] [Google Scholar]
- 35.McCarty GA, Harley JB, Reichlin M. A distinctive autoantibody profile in black female patients with lupus nephritis. Arthritis Rheum. 1993;36:1560–1565. doi: 10.1002/art.1780361110. [DOI] [PubMed] [Google Scholar]
- 36.Alba P, Bento L, Cuadrado MJ, Karim Y, Tungekar MF, Abbs I, Khamashta MA, D’Cruz D, Hughes GR. Anti-dsDNA, anti-Sm antibodies, and the lupus anticoagulant: significant factors associated with lupus nephritis. Ann. Rheum. Dis. 2003;62:556–560. doi: 10.1136/ard.62.6.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mannik M, Merrill CE, Stamps LD, Wener MH. Multiple autoantibodies form the glomerular immune deposits in patients with systemic lupus erythematosus. J. Rheumatol. 2003;30:1495–1504. [PubMed] [Google Scholar]
- 38.Bruschi M, Sinico RA, Moroni G, Pratesi F, Migliorini P, Galetti M, Murtas C, Tincani A, Madaio M, Radice A, et al. Glomerular autoimmune multicomponents of human lupus nephritis in vivo: α-enolase and annexin AI. J. Am. Soc. Nephrol. 2014;25:2483–2498. doi: 10.1681/ASN.2013090987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruschi M, Galetti M, Sinico RA, Moroni G, Bonanni A, Radice A, Tincani A, Pratesi F, Migliorini P, Murtas C, et al. Glomerular autoimmune muticomponents of human lupus nephritis in vivo (2): planted antigens. J. Am. Soc. Nephrol. 2015;26:1905–1924. doi: 10.1681/ASN.2014050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.