Abstract
Vitiligo is an autoimmune disease of the skin that leads to life-altering depigmentation and remains difficult to treat. However, clinical observations and translational studies over 30-40 years have led to the development of an insightful working model of disease pathogenesis: Genetic risk spanning both immune and melanocyte functions is pushed over a threshold by known and suspected environmental factors to initiate autoimmune T cell-mediated killing of melanocytes. While under cellular stress, melanocytes appear to signal innate immunity to activate T cells. Once the autoimmune T cell response is established, the IFN-γ-STAT1-CXCL10 signaling axis becomes the primary inflammatory pathway driving both progression and maintenance of vitiligo. This pathway is a tempting target for both existing and developing pharmaceuticals, but further detailing how melanocytes signal their own demise may also lead to new therapeutic targets. Research in vitiligo may be the future key to understand the pathogenesis of organ-specific autoimmunity, as vitiligo is common, reversible, progresses over the life of the individual, has been relatively well-defined, and is quite easy to study using translational and clinical approaches. What is revealed in these studies can lead to innovative treatments and also help elucidate the principles that underlie similar organ-specific autoimmune diseases, especially in cases where the target organ is less accessible.
Introduction
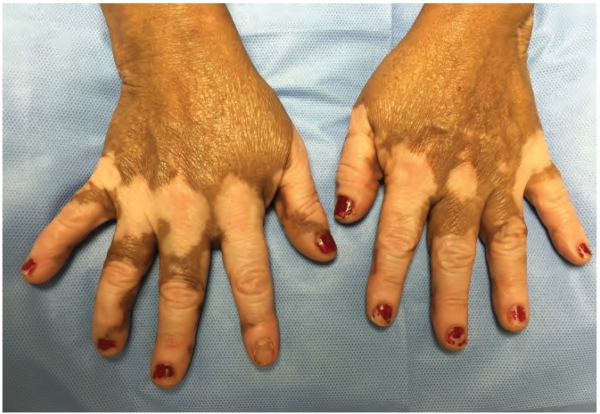
Vitiligo is a common, but under-recognized, autoimmune disease of the skin in which melanocytes are specifically targeted and destroyed by skin-infiltrating, autoreactive CD8+ T cells. Patients develop patchy areas of depigmented skin (Figure 1) that become disfiguring, and thus negatively impact their quality of life (1-3). Approximately 1% of the global population is affected and the disease is reversible via melanocyte regeneration; however, the needs of this population remain unmet as current medical therapies are only moderately effective in reversing depigmentation. This is partly due to the fact that current treatments for vitiligo broadly dampen the immune response and do not specifically target the cells or pathways that are principally responsible for melanocyte destruction (2,3).
Figure 1.
“Disfiguring white patches characteristic of vitiligo.”
To improve the treatment of vitiligo, researchers have studied the disease for over thirty years, advancing our understanding of its pathogenesis. Much of this work has been done directly on patients and their tissues, and thus has led to an advanced understanding of this organ-specific autoimmune disease within the human context. While the mechanism of disease initiation is not yet fully defined, a confluence of genetics and cellular stress most likely provide the fuel and spark, respectively (4). The subsequent CD8+ T cell response is then responsible for the destruction of melanocytes in the skin. Recent insights gleaned from basic and translational studies indicate that vitiligo could be more effectively treated by specifically targeting the pathways that allow CD8+ T cells to initiate and continue their attack on melanocytes (5).
The Genetics Underlying Vitiligo
It is clear that vitiligo is an autoimmune disease, as many of the risk alleles that are associated with vitiligo are immune-specific genes, and vitiligo appears more frequently in family members of patients with autoimmune diseases, including type 1 diabetes, pernicious anemia, and autoimmune thyroiditis (6-9). Similar to other autoimmune diseases, dysregulation of antigen presentation is implicated as several HLA-haplotypes confer risk to developing disease: SNPs in risk alleles HLA-A*02 (10), HLA-DRB1, and HLA-DQA1 (11) lead to increased expression of MHC, which can allow for enhanced immune activation (10,11). NLRP1 is also identified to play a role, possibly through increased IL-1β processing (12); however, whether this is limited to disease initiation or also affects progression is unclear. Other immune genes involved in T cell signaling include PTPN22, TSLP, CCR6, IL2RA, UBASH3A, FOXP3 and GZMB (7), implicating both innate and adaptive responses, as well as regulatory T cells.
Melanocyte-specific genes are implicated in disease pathogenesis as well. TYR encodes tyrosinase, the enzyme that performs the rate-limiting step in melanogenesis (pigment production), and modifications of this enzyme are also associated with anti-melanoma tumor surveillance (13,14). OCA2, involved in melanosomal transport of tyrosine, as well as MC1R, a hormone receptor that promotes melanogenesis, are also implicated (15). Xbp1 (X-box binding protein 1) also confers risk of vitiligo and is more highly expressed in lesional skin (16), suggesting Xbp1 regulation could be involved in maintaining homeostasis in melanocyte surveillance (4). It is a key mediator of the unfolded protein response (UPR), which responds to endoplasmic reticulum (ER) stress (17), but also has additional roles in antigen loading and regulation of dendritic cell (DC) functions (18). Other autoimmune diseases are associated with hypomorphic variants in XBP1 that contribute risk and lead to ER stress and autoinflammation (19).
Intrinsic Stress in Vitiligo
There is a growing body of literature that suggests a role for cellular stress in the development of autoimmunity, including vitiligo (4,20). Vitiligo patients exhibit signs of stress in the skin. Reactive oxygen species (ROS), principally H202, are elevated (21), and when compared to primary melanocytes generated from an unaffected individual, melanocytes from a vitiligo patient exhibit slower growth, demanding catalase supplementation as well as a host of growth factors (22-25). Melanocytes from vitiligo patients have a dilated ER (22), a tell-tale sign of ER stress, and intracellular staining of equally aged cell lines suggests there is dysregulation of protein expression (26), a precipitating factor that can initiate ER stress.
We have previously discussed the hypothesis that melanocytes could be uniquely prone to stress because of their functional and environmental niche in the skin (4,20). Melanogenesis requires the coordination of several energy-intensive processes within the melanocyte that result in generation of ROS. This includes the production of large amounts of protein components of the melanin pathway, melanin synthesis, and orchestrating organelle movement in the cell for pigment distribution. In addition, the redox reactions involved in chemical transformation of tyrosine to melanin directly yield potentially harmful byproducts, including H202. Moreover, the melanocyte’s physical position in the skin allows it to be subjected to UV light, another source that can generate ROS (20,27).
Extrinsic Insults Damage Melanocytes and Can Initiate Vitiligo
Additional in vitro experiments in which the melanocytes were exogenously stressed using reagents that generate ROS resulted in the death of melanocytes from vitiligo patients at far lower exposure levels compared to primary cells derived from healthy donors (26,28). This would suggest that melanocytes from vitiligo patients possess a specific defect that increases their susceptibility to stress. These data have also been paired with relevant clinical observations that vitiligo has a strong environmental component. While there is increased risk to develop vitiligo in families with vitiligo or other organ-specific autoimmune diseases, studies in monozygotic twins reveal only 23% concordance of disease between them (8), suggesting that while genes confer a significant risk for the development of vitiligo, a large proportion is independent of genes, and thus likely incorporates environmental factors as well (20). Importantly, many of these factors and their mechanisms of action in vitiligo have been described. The chemical monobenzyl ether of hydroquinone (MBEH) was first shown to cause vitiligo in tannery workers who developed depigmentation after wearing rubber gloves containing the phenol, and a prescription cream containing the phenol can now be used to electively exacerbate disease in patients aiming for an even skin tone without pigmentation (29,30).
Investigation into the mechanism of action of MBEH has revealed that it works directly upon tyrosinase-positive, pigment-producing cells initiating several events: melanogenesis is inhibited, ROS are produced, the melanocyte undergoes ER stress, the UPR is activated, autophagy pathways are initiated, and exosomes are released (31). Others argue that vitiligo-inducing phenols act via tyrosinase related-protein 1 (TRP1), rather than directly through TYR, although both implicate melanocyte-specific function in toxicity (32). In these experiments, phenols such as 4-tertiary butyl phenol (4-TBP) and MBEH were used in vitro to initiate these cellular responses, and revealed that activation of stress could elicit an immune response, as the stressed melanocytes produced inflammatory cytokines IL-6 and IL-8 (33). Phenol-induced stress of melanocytes leads to the activation of DC and enhanced activation of T cells in co-culture experiments, possibly through the release of HSP70i, a pro-inflammatory signal (31,34,35).
More recently, van den Boorn and colleagues discovered that MBEH-induced haptenization of melanocyte antigens and phenol-induced stress creates an inflammatory environment within the skin that leads to the recruitment of natural killer (NK) cells and the formation of NK memory, which is then capable of continuing autoimmune attack on even remote, unexposed skin (36). Stressed melanocytes may release cell-specific antigens via exosome secretion (31) combined with NK mediated killing of melanocytes to promote DC activation and antigen presentation, which contributes to priming of a T cell response. While some details are still missing, the potential for stressed melanocytes to initiate the autoimmune attack in vitiligo is clear.
CD8+ T Cells Play a Central Role in Depigmentation: Killing Melanocytes
Earlier work detailing the events and pathogenesis in vitiligo demonstrate that CD8+ T cells play a critical role in the destruction of melanocytes. First, patchy infiltrates of T cells were found to localize near melanocytes, the cells responsible for skin pigmentation (37,38). Analysis of vitiligo patient blood using melanocyte antigen-specific tetramers revealed that vitiligo patients have higher frequencies of melanocyte-specific CD8+ T cells in the blood compared to healthy controls, and that these frequencies correlate with total skin involvement (39). The reactivity of these T cells was subsequently tested and found to be capable of killing both melanoma as well as melanocytes derived from the same T cell donors. These T cells possessed a skin homing phenotype, including surface expression of cutaneous lymphocyte antigen (39,40).
Autoreactive T cells kill melanocytes in experiments in which T cells isolated from perilesional vitiligo skin are co-incubated with autologous uninvolved skin. Labeled T cells isolated from vitiligo lesions infiltrated the normal skin, migrated to the epidermal-dermal junction, and were found in close association with dying melanocytes. Depletion of CD8+ T cells prevented melanocyte destruction, whereas enrichment for these cells enhanced it (41), supporting the key role of CD8+ T cells in vitiligo. Our laboratory and others have then expanded on this knowledge by testing melanocyte-reactive T cells in mouse models, revealing that IFN-γ plays a central role in disease pathogenesis (42-45).
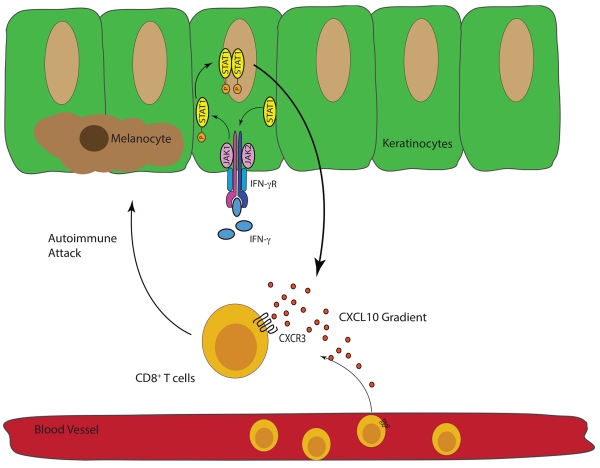
We have continued to dissect the role of IFN-γ and IFN-γ-dependent genes in our mouse model and in human tissues. We discovered that the IFN-γ-derived chemokine CXCL10 is essential for driving vitiligo pathogenesis through the recruitment of autoreactive CD8+ T cells to the epidermis, and autoreactive T cells depend upon the chemokine receptor of CXCL10, CXCR3, to home to the skin to kill melanocytes. Moreover, we found that melanocyte-specific CD8+ T cells in vitiligo patients express higher levels of CXCR3, paralleling our findings in the mouse (46). We also found that the IFN-γ receptor and STAT1 are critical for the development of skin depigmentation as well (in press and unpublished results). IFN-γ signals through the IFN-γ receptor, which recruits Janus Kinases (JAKs) to transduce the signal. JAKs phosphorylate STAT1, a transcription factor that then translocates to the nucleus to induce transcription of IFN-γ-inducible genes, including CXCL10 (Figure 2)(47). Keratinocytes are the principal cellular source of CXCL10 in the epidermis during disease progression (in press). Intriguingly, blocking CXCL10 in our mouse model can both prevent vitiligo as well as restore pigmentation in mice with established disease (46), providing support for targeting this pathway as a treatment strategy.
Figure 2.
“The IFN-γ-STAT1-CXCL10 Axis Drives Melanocyte Destruction.”
IFNγ signals through the IFN-γ receptor (IFN-γR), which then requires JAK1 and JAK2 to phosphorylate the transcription factor STAT-1. Phosphorylated STAT1 homodimerizes and then translocates into the nucleus where IFN-γ-dependent genes, including CXCL9 and CXCL10, are transcribed(47). Autoreactive CXCR3-expressing CD8+ T cells follow these ligands to the skin where they kill melanocytes(46).
The importance of this pathway has been supported by translational studies of other groups (48-50) and by clinical studies in which the drugs that target this signaling axis were tested as treatments of vitiligo patients (51,52). Wang and colleagues tracked the levels of serum CXCL9 and CXCL10 in a cohort of patients and found that CXCL10 correlated with disease severity and inversely with treatment response (50), underpinning its role in human disease pathogenesis. Inhibitors of JAK/STAT signaling, tofacitinib and ruxolitinib, have shown promising results in the repigmentation of two patients with vitiligo (51,52), further validating the importance of this signaling pathway in vitiligo pathogenesis. Moreover, HMG-CoA reductase inhibitors have been shown to inhibit IFN-γ-dependent STAT1 signaling in vitro (53), suggesting statins could be used to inhibit the IFN-γ-STAT1-CXCL10 axis. One patient repigmented shortly after receiving high-dose simvastatin (54), and simvastatin both prevented depigmentation and promoted repigmentation in our mouse model, although its exact mechanism remains undetermined (55). However a small, randomized, placebo-controlled trial did not confirm efficacy in vitiligo patients [in press]. This may be a consequence of potential toxicity and consequent limited dosing of simvastatin in humans, which is not a limitation in mice.
The Roles of Other Cell Types Involved Are Poorly Defined
Additional cell types may also play a role in active disease, but their roles remain less defined than CD8+ T cells. NK, CD11b+CD11c+ cells, and macrophages infiltrate vitiligo lesions (37,38,56,57). Transcriptome and flow analysis of human skin revealed an infiltration of NK cells in lesional and non-lesional skin (56) which, in light of the recent discovery of NK-mediated killing of stressed melanocytes (36), could allow for a larger role in pathogenesis than previously thought. CD11b+CD11c+ cells appear in human and murine vitiligo lesional skin (57) and macrophages are also present in lesional skin (36,37), although the exact roles of these cells remain undefined. Stressed melanocytes release HSP70i (34) as well as exosomes of unknown content (31); it is possible that exosomes contain HSP70i as well as other potential danger associated molecular patterns (DAMPs) that activate nearby cell types (4,20,36).
T regulatory cells (Tregs) are important in controlling inflammation, including many different autoimmune diseases (58). Human studies report dysfunctional Treg responses in vitiligo patients compared to controls, but have not led to a consensus as to where the defect lies, in Treg number, skin homing capacity, or function (59-65). A recent study found that human Tregs control melanocyte-specific, CD8+ T cell responses through induction of CTLA4 in vitro, and that melanocyte-specific, CD8+ T cells exhibit an unregulated immunophenotype in vitiligo patients compared to controls (66). Mouse studies implicate an important role for Tregs as well (44,45), and future translational studies directly in the skin may better reveal their functional mechanism of regulation during disease evolution and maintenance of tolerance.
Resident cells of the skin also have emerging, but as yet undefined, roles in vitiligo. Much of the previous work has focused on melanocytes as a precipitating source of stress, DAMPs, and antigen, but the roles of neighboring cell types, like keratinocytes and Langerhans cells, are emerging. In the recent model of chemical induced vitiligo, Langerhans cells were dispensable (36), but this does not preclude additional roles during active vitiligo. Langerhans cells and dermal dendritic cells have distinct, sometimes antagonistic roles in regulating and promoting immune responses (67-69). Keratinocytes, which make up the majority of the epidermis, are hypothesized to play an important role in promoting T cell recruitment and inflammation in psoriasis (70). A recent study from our lab suggests keratinocytes and their ability to make CXCL10 are required for disease progression (in press). It remains unknown if keratinocytes play a similar role during initiation or in detecting melanocyte stress.
Summary: Realizing a Complete Model of Vitiligo Pathogenesis through Translational Research
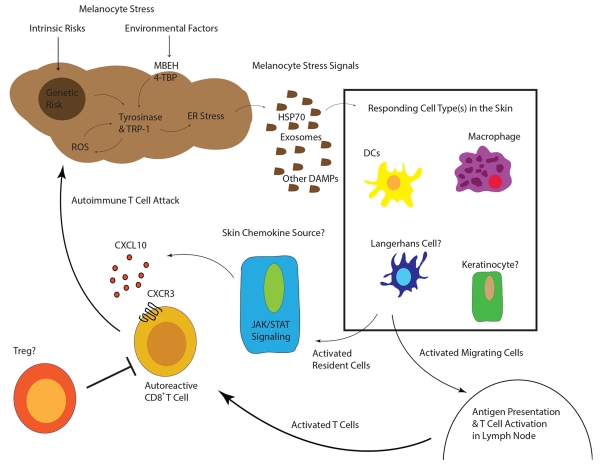
In summary, the past three decades have uncovered many mechanistic details in vitiligo pathogenesis. Genetic studies of vitiligo patients reveal that it is principally an autoimmune disease, although melanocytes may ignite the entire process after crossing a certain threshold level of stress. ROS or ER stress may activate nearby innate myeloid or stromal skin populations, which in turn recruit T cells that kill melanocytes (Figure 3). Perhaps the most important details to be worked out revolve around how melanocytes signal stress in vivo, how it is interpreted by the immune system, and how this leads to disruption of immune tolerance. The mechanisms of T cell-mediated melanocyte killing are more clearly defined: CXCR3-expressing melanocyte-specific CD8+ T cells follow the chemokine CXCL10 to infiltrate the skin and kill melanocytes (46). Targeting aspects of this pathway may lead to better treatments, but more can still be worked out to fully detail what directs these T cells once they infiltrate the skin. What are the roles of the additional infiltrating cell types previously reported? How do melanocytes communicate cellular stress to the immune system? Can the IFN-γ-STAT1-CXCL10 and cellular stress pathways be effectively targeted to halt vitiligo?
Figure 3.
“Working Model of Vitiligo Pathogenesis”
Inherited genetic risk (HLA (11)(10), XBP1 (16), TYR (13), OCA2 (15), M1CR1 (15)) and environmental insults (MBEH and 4-TBP) induce a state of melanocyte stress, exemplified by ER stress. Stressed melanocytes signal to local innate and resident skin cell types via exosomes containing antigen and DAMPs, soluble HSP70, and/or other factors (30-34). Responding cell types are activated by these signals and some may migrate to the draining lymph nodes where they activate T cells. Other responding cells in the skin secrete chemokines to recruit autoreactive T cells, which are directly responsible for killing melanocytes. In active disease, one or more cell types may respond to IFN-γ and secrete CXCL10 to recruit T cells to the skin where melanocytes reside.
Directly testing human skin may be the answer to some of these questions. Mouse models and cell-based systems have helped to identify these pathways, and many translational studies were first initiated in mice or cell-based systems; however, the earliest observations of intrinsic stress, chemical induced vitiligo, and T cell-mediated melanocyte killing came from studying human skin. Vitiligo, in contrast to other autoimmune diseases, is uniquely amenable to translational research, as the target organ, the skin, is easily accessible. It is a disease that progresses over many years, allowing researchers the time to assess these tissues, under both current and novel therapies as well as exacerbating factors such as MBEH. Through such translational efforts, a complete working model of vitiligo pathogenesis can be realized and new therapeutic avenues can be explored, such that the needs of this patient population are finally met. In addition, complex interactions that balance autoimmune inflammation with mechanisms of immune tolerance can be more clearly defined. As such, we suspect that this work will directly inform that of other organ-specific autoimmune diseases, potentially supporting human vitiligo as a relevant model for diseases that are more difficult to study in humans, such as type 1 diabetes and multiple sclerosis.
Highlights.
Genetic risk and environmental factors contribute to cellular stress in melanocytes
Activation of innate pathways is a direct result of melanocyte stress
The IFNγ-STAT1-CXCL10 signaling axis drives vitiligo progression
Translational research in vitiligo is a powerful tool to understand autoimmunity
Acknowledgements
JEH is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the NIH, under award number AR061473, and a research grant from the Dermatology Foundation Stiefel Scholar Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Picardo M, Dell’Anna ML, Ezzedine K, Hamzavi I, Harris JE, Parsad D, Taieb A. Vitiligo. Nature Reviews Disease Primers. 2015;1:15011. doi: 10.1038/nrdp.2015.11. [DOI] [PubMed] [Google Scholar]
- 2.Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. Lancet. 2015;386(9988):74–84. doi: 10.1016/S0140-6736(14)60763-7. [DOI] [PubMed] [Google Scholar]
- 3.Ezzedine K, Sheth V, Rodrigues M, Eleftheriadou V, Harris JE, Hamzavi IH, Pandya AG, Vitiligo Working Group Vitiligo is not a cosmetic disease. Journal of the American Academy of Dermatology. 2015;73(5):883–5. doi: 10.1016/j.jaad.2015.07.039. [DOI] [PubMed] [Google Scholar]
- 4.Richmond JM, Frisoli ML, Harris JE. Innate immune mechanisms in vitiligo: Danger from within. Current Opinion in Immunology. 2013;25(6):676–82. doi: 10.1016/j.coi.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rashighi M, Harris JE. Interfering with the IFN-gamma/CXCL10 pathway to develop new targeted treatments for vitiligo. Annals of Translational Medicine. 2015;3(21):343. doi: 10.3978/j.issn.2305-5839.2015.11.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunliffe WJ, Hall R, Newell DJ, Stevenson CJ. Vitiligo, thyroid disease and autoimmunity. British Journal of Dermatology. 1968;80(3):135–9. doi: 10.1111/j.1365-2133.1968.tb12282.x. [DOI] [PubMed] [Google Scholar]
- 7.Spritz RA. Modern vitiligo genetics sheds new light on an ancient disease. J Dermatol. 2013;40(5):310–8. doi: 10.1111/1346-8138.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alkhateeb A, Fain PR, Thody A, Bennett DC, Spritz RA. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigement Cell & Melanoma Research. 2003;16(3):208–14. doi: 10.1034/j.1600-0749.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- 9.Gill L, Zarbo A, Isedeh P, Jacobsen G, Lim HW, Hamzavi I. Comorbid autoimmune diseases in patients with vitiligo: A cross-sectional study. Journal of the American Academy of Dermatology. 2016;74(2):295–302. doi: 10.1016/j.jaad.2015.08.063. [DOI] [PubMed] [Google Scholar]
- 10**.Hayashi M, Jin Y, Yorgov D, Santorico SA, Hagman J, Ferrara TM, Jones KL, Cavalli G, Dinarello CA, Spritz RA. Autoimmune vitiligo is associated with gain-of- function by a transcriptional regulator that elevates expression of HLA-A * 02 : 01 in vivo. PNAS. 2016;113(5):1357–62. doi: 10.1073/pnas.1525001113. **This vitiligo functional genomics study found that the HLA-A*02:01 risk allele is associated with enhanced expression of class I HLA on the cell surface, allowing for increased presentation of melanocyte specific antigens. These findings underscore the contribution of this HLA risk allele to developing tissue-specific autoimmunity.
- 11**.Cavalli G, Hayashi M, Jin Y, Yorgov D, Santorico SA, Holcomb C, Rastrou M, Erlich H, Tengesdal IW, Dagna L, et al. MHC class II super-enhancer increases surface expression of HLA-DR and HLA-DQ and affects cytokine production in autoimmune vitiligo. PNAS. 2016;113(5):1363–8. doi: 10.1073/pnas.1523482113. ** Similar to the above study, this vitiligo functional genomics study found that the HLA-DR and HLA-DQ risk alleles are associated with enhanced expression of class II HLA on the cell surface due to alteration of the MHC class II super-enhancer. PBMCs with enhanced HLA expression were more responsive to inflammatory triggers, thus supporting the hypothesis that genetic risk promotes loss of immune tolerance through enhanced inflammation.
- 12.Levandowski CB, Mallioux CM, Ferrara TM, Gowan K, Ben S, Jin Y, McFann KK, Holland PJ, Fain PR, Dinarello CA, Spritz RA. NLRP1 haplotypes associated with vitiligo and autoimmunity increase interleukin-1beta processing via the NLRP1 inflammasome. PNAS. 2013;110(8):2952–6. doi: 10.1073/pnas.1222808110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spritz RA. The genetics of generalized vitiligo: autoimmune pathways and an inverse relationship with malignant melanoma. Genome Med. 2010;2(10):78. doi: 10.1186/gm199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamshchikov GV, Mullins DW, Ogino T, Thompson L, Presley J, Galavotti H, Aquila W, Deacon D, Patterson JW, Engelhard VH, et al. Sequential immune escape and shifting of T Cell responses in a long-term survivor of melanoma. Journal of Immunology. 2005;174:6863–71. doi: 10.4049/jimmunol.174.11.6863. [DOI] [PubMed] [Google Scholar]
- 15.Jin Y, Birlea SA, Fain PR, Ferrara TM, Ben S, Riccardi SL, Cole JB, Gowan K, Holland PJ, Bennett DC, et al. Genome-wide association analyses identify 13 new susceptibility loci for generalized vitiligo. Nature Genetics. 2012;44(6):676–80. doi: 10.1038/ng.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren Y, Yang S, Xu S, Gao M, Huang W, Gao T, Huang W, Gao T, Fang Q, Quan C, Zhang C, Sun L, et al. Genetic variation of promoter sequence modulates XBP1 expression and genetic risk for vitiligo. PLoS Genetics. 2009;5(6):e1000523. doi: 10.1371/journal.pgen.1000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee AH, Iwakoshi NN, Glimcher LH. Xbp-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Molecular and Cellular Biology. 2003;23(21):7448–59. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cubillos-Ruiz JR, Silberman PC, Rutkowski MR, Chopra S, Perales-Puchalt A, Song M, Zhang S, Bettigole SE, Gupta D, Holcomb K, et al. ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell. 2015;161(7):1527–38. doi: 10.1016/j.cell.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, Nieuwenhuis EE, Higgins DE, Schreiber S, Glimcher LH, Blumberg RS. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134(5):743–56. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris JE. Cellular stress and innate inflammation in organ-specific autoimmunity: lessons learned from vitiligo. Immunological Reviews. 2015;269(1):11–25. doi: 10.1111/imr.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schallreuter KU, Moore J, Wood JM, Beazley WD, Gaze DC, Tobin DJ, Marshal HS, Panske A, Panzig E, Hibberts NA. In vivo and in vitro evidence for hydrogen peroxide (H2O2) accumulation in the epidermis of patients with vitililigo and its successful removal by a UVB-activated pseudocatalase. Journal of Investigative Dermatology Symposium Proceedings. 1999;4(1):91–6. doi: 10.1038/sj.jidsp.5640189. [DOI] [PubMed] [Google Scholar]
- 22.Boissy RE, Liu Y, Medrano EE, Nordlund JJ. Structural aberration of the rough endoplasmic reticulum and melanosome compartmentalization in long-term cultures of melanocytes. Journal of Investigative Dermatology. 1991;97(3):395–404. doi: 10.1111/1523-1747.ep12480976. [DOI] [PubMed] [Google Scholar]
- 23.Puri N, Mojamdar M, Ramajah A. In vitro growth characteristics of melanocytes obtained from adult normal and vitiligo subjects. Journal of Investigative Dermatology. 1987;88(4):434–8. doi: 10.1111/1523-1747.ep12469795. [DOI] [PubMed] [Google Scholar]
- 24.Puri N, Mojamdar M, Ramajah A. Growth defects of melanocytes in culture from vitiligo subjects are spontaneously corrected in vivo in repigmenting subjects and can be partially corrected by the addition of fibroblast-derived growth factors in vitro. Archives of Dermatological Research. 1989;281(3):178–84. doi: 10.1007/BF00456389. [DOI] [PubMed] [Google Scholar]
- 25.Medrano EE, Nordlund JJ. Successful culture of adult human melanocytes obtained from normal and vitiligo donors. Journal of Investigative Dermatology. 1990;95(4):441–5. [PubMed] [Google Scholar]
- 26.Jimbow K, Chen H, Park JS, Thomas PD. Increased sensitivity of melanocytes to oxidative stress and abnormal expression of tyrosinase-related protein in vitiligo. British Journal of Dermatology. 2001;144(1):55–65. doi: 10.1046/j.1365-2133.2001.03952.x. [DOI] [PubMed] [Google Scholar]
- 27.Meyskens FL, Farmer P, Fruehauf JP. Redox regulation in human melanocytes and melanoma. Pigment cell & Melanoma Research. 2001;14(3):148–54. doi: 10.1034/j.1600-0749.2001.140303.x. [DOI] [PubMed] [Google Scholar]
- 28.Maresca V, Roccella M, Roccella F, Camera E, Del Porto G, Passi S, Grammatico P, Picardo M. Increased sensitivity to peroxidative agents as a possible pathogenic factor of melanocyte damage in vitiligo. Journal of Investigative Dermatology. 1997;109(3):310–3. doi: 10.1111/1523-1747.ep12335801. [DOI] [PubMed] [Google Scholar]
- 29.Oliver EA, Schwartz L, Warren L. Occupational Leukoderma. JAMA. 1937;113:927–8. [Google Scholar]
- 30.Mosher DB, Parrish JA, Fitzpatrick TB. Monobenzylether of hydroquinone: a retrospective study of treatment of 18 vitiligo patients and review of the literature. British Journal of Dermatology. 1977;97(6):669–79. doi: 10.1111/j.1365-2133.1977.tb14275.x. [DOI] [PubMed] [Google Scholar]
- 31.van den Boorn JG, Picavet DI, van Swieten PF, van Veen HA, Konijnenberg D, van Veelen PA, van Capel T, Jong EC, Reits EA, Drijfhout JW, et al. Skin-depigmenting agent monobenzone induces potent T-cell autoimmunity toward pigmented cells by tyrosinase haptenation and melanosome autophagy. The Journal of Investigative Dermatology. 2011;131(6):1240–51. doi: 10.1038/jid.2011.16. [DOI] [PubMed] [Google Scholar]
- 32.Yang F, Sarangarajan R, Le Poole IC, Medrano EE, Boissy RE. The cytotoxicity and apoptosis induced by 4-tertiary butylphenol in human melanocytes are independent of tyrosinase activity. Journal of Investigative Dermatology. 2000;114(1):157–64. doi: 10.1046/j.1523-1747.2000.00836.x. [DOI] [PubMed] [Google Scholar]
- 33.Toosi S, Orlow SJ, Manga P. Vitiligo-inducing phenols activate the unfolded protein response in melanocytes resulting in upregulation of IL6 and IL8. Journal of Investigative Dermatology. 2012;132(11):2601–9. doi: 10.1038/jid.2012.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kroll TM, Bommiasamy H, Boissy RE, Hernandez C, Nickoloff BJ, Mestril R, Le Poole IC. 4-Tertiary butyl phenol exposure sensitizes human melanocytes to dendritic cell-mediated killing: relevance to vitiligo. The Journal of Investigative Dermatology. 2005;124(4):798–806. doi: 10.1111/j.0022-202X.2005.23653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosenson JA, Flood K, Klarquist J, Eby JM, Koshoffer A, Boissy RE, Overbeck A, Tung RC, Le Poole IC. Preferential secretion of inducible HSP70 by vitiligo melanocytes under stress. Pigment Cell & Melanoma Research. 2014;27(2):209–20. doi: 10.1111/pcmr.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36*.van den Boorn JG, Jakobs C, Hagen C, Penn M, Luiten RM, Melief CJ, Tuting T, Garbi N, Hartmann G, Hornung V. Inflammasome-dependent induction of adaptive NK memory. Immunity. 2016;44(6):1406–21. doi: 10.1016/j.immuni.2016.05.008. * This study describes the activation of an NK memory population that targets and kills melanocytes exposed to the inducing phenol MBEH. These cells were dependent on the prior activation of macrophages and the NLRP3 inflammasome. It addresses how cellular stress precipitates organ-specific autoimmunity through activation of the innate immune system.
- 37.Le Poole IC, van den Wijngaard RM, Westerhof W, Das PK. Presence of T cells and macrophages in inflammatory vitiligo skin parallels melanocyte disappearance. The American Journal of Pathology. 1996;148(4):1219–1228. [PMC free article] [PubMed] [Google Scholar]
- 38.van den Wijngaard R, Wankowicz-Kalinska A, Le Poole C, Tigges B, Westerhof W, Das P. Local immune response in skin of generalized vitiligo patients. Lab Invest. 80(8):1299–309. doi: 10.1038/labinvest.3780138. [DOI] [PubMed] [Google Scholar]
- 39.Ogg GS, Dunbar PR, Romero P, Chen J, Cerundolo V. High Frequency of Skin-Homing Melanocyte-Specific Cytotoxic T Lymphocytes in Vitiligo. The Journal of Experimental Medicine. 1998;188(6):1203–8. doi: 10.1084/jem.188.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wankowicz-Kalinska A, van den Wijngaard RM, Tigges BJ, Westerhof W, Ogg GS, Cerundolo V, Storkus WJ, Das PK. Immunopolarization of CD4+ and CD8+ T Cells to Type-1-Like is Associated with Melanocyte Loss in Human Vitiligo. Lab Invest. 2003;83(5):683–95. doi: 10.1097/01.lab.0000069521.42488.1b. [DOI] [PubMed] [Google Scholar]
- 41.van den Boorn JG, Konijnenberg D, Dellemijn TA, van der Veen JP, Bos JD, Melief CJ, Vyth-Dreese FA, Luiten RM. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. The Journal of Investigative Dermatology. 2009;129(9):2220–32. doi: 10.1038/jid.2009.32. [DOI] [PubMed] [Google Scholar]
- 42.Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan CC, Klebanoff CA, Overwijk WW, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. Journal of Immunology. 2005;174(5):2591–601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harris JE, Harris TH, Weninger W, Wherry EJ, Hunter CA, Turka LA. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-y for autoreactive CD8+ T cell accumulation in the skin. Journal of Investigative Dermatology. 2012;132(7):1869–76. doi: 10.1038/jid.2011.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chatterjee S, Eby JM, Al-Khami AA, Soloshchenko M, Kang HK, Kaur N, Naga OS, Murali A, Nishimura MI, Le Poole IC, Mehrotra S. A quantitative increase in regulatory T cells controls development of vitiligo. Journal of Investigative Dermatology. 2014;134(5):1285–94. doi: 10.1038/jid.2013.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gregg RK, Nichols L, Chen Y, Lu B, Engelhard VH. Mechanisms of spatial and temporal development of autoimmune vitiligo in tyrosinase-specific TCR transgenic mice. Journal of Immunology. 2010;184(4):1909–17. doi: 10.4049/jimmunol.0902778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46**.Rashighi M, Agarwal P, Richmond JM, Harris TH, Dresser K, Su M-W, Zhou Y, Deng A, Hunter CA, Luster AD, Harris JE. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Science Translational Medicine. 2014;6(223):223ra23. doi: 10.1126/scitranslmed.3007811. ** This study identified elevated levels of CXCL10 message in the skin and protein in the blood of vitiligo patients, demonstrated that CXCL10 was the key chemokine ligand required for melanocyte destruction in a mouse model, and showed epidermal repigmentation in mice following treatment with a CXCL10 blocking antibody. It was the first to provide proof of concept that targeting the IFNy-STAT1-CXCL10 axis could be used to treat vitiligo.
- 47.Aaronson DS, Horvath CM. A road map for those who don’t know JAK-STAT. Science. 2002;296(5573):1653–5. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- 48.Bertolotti A, Boniface K, Vergier B, Mossalayi D, Taieb A, Ezzedine K, Seneschal J. Type I interferon signature in the initiation of the immune response in vitiligo. Pigment Cell & Melanoma Research. 2014;27(3):398–407. doi: 10.1111/pcmr.12219. [DOI] [PubMed] [Google Scholar]
- 49.Regazzetti C, Joly F, Marty C, Rivier M, Mehul B, Reiniche P, Mounier C, Rival Y, Piwnica D, Cavalié M, et al. Transcriptional analysis of vitiligo skin reveals the alteration of WNT pathway: A promising target for repigmenting vitiligo patients. Journal of Investigative Dermatology. 2015;135(12):3105–14. doi: 10.1038/jid.2015.335. [DOI] [PubMed] [Google Scholar]
- 50.Wang XX, Wang QQ, Wu JQ, Jiang M, Chen L, Zhang CF, Xiang LH. Increased expression of CXCR3 and its ligands in patients with vitiligo and CXCL10 as a potential clinical marker for vitiligo. British Journal of Dermatology. 2016;174(6):1318–26. doi: 10.1111/bjd.14416. [DOI] [PubMed] [Google Scholar]
- 51.Craiglow BG, King BA. Tofacitinib citrate for the treatment of vitiligo: a pathogenesis-directed therapy. JAMA Dermatology. 2015;151(10):1110–2. doi: 10.1001/jamadermatol.2015.1520. [DOI] [PubMed] [Google Scholar]
- 52*.Harris JE, Rashighi M, Nguyen N, Jabbari A, Uleiro G, Clynes R, Christiano AM, Mackay-Wiggan J. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA) Journal of the American Academy of Dermatology. 2016;74(2):370–1. doi: 10.1016/j.jaad.2015.09.073. * In this report, a vitiligo patient taking the Jak-inhibitor ruxolitinib rapidly repigmented, and this was associated with a decline in serum CXCL10 levels. Together with (51), this study supports the hypothesis that targeting the IFNy-STAT1-CXCL10 axis will be useful in the treatment of vitiligo and underscores the importance of this axis in disease pathogenesis.
- 53.Zhao Y, Gartner U, Smith FJ, Mclean WH. Statins downregulate K6a promoter activity: A possible therapeutic avenue for pachyonychia congenita. Journal of Investigative Dermatology. 2011;131(5):1045–52. doi: 10.1038/jid.2011.41. [DOI] [PubMed] [Google Scholar]
- 54.Noël M, Gagné C, Bergeron J, Jobin J, Poirier P. Positive pleiotropic effects of HMG-CoA reductase inhibitor on vitiligo. Lipids in Health and Disease. 2004;3:7. doi: 10.1186/1476-511X-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agarwal P, Rashighi M, Essien KI, Richmond JM, Randall L, Pazoki-Toroudi H, Hunter CA, Harris JE. Simvastatin prevents and reverses depigmentation in a mouse model of vitiligo. J Invest Dermatol. 2015;135(4):1080–8. doi: 10.1038/jid.2014.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu R, Broady R, Huang Y, Wang Y, Yu J, Gao M, Levings M, Wei S, Zhang S, Xu A, et al. Transcriptome analysis reveals markers of aberrantly activated innate immunity in vitiligo lesional and non-lesional Skin. PloS one. 2012;7(12):es1040. doi: 10.1371/journal.pone.0051040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mosenson JA, Zloza A, Nieland JD, Garrett-Mayer E, Eby JM, Huelsmann EJ, Kumar P, Denman CJ, Lacek AT, Kohlhapp FJ, et al. Mutant HSP70 reverses autoimmune depigmentation in vitiligo. Science Translational Medicine. 2013;5(174):174ra28. doi: 10.1126/scitranslmed.3005127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nie J, Li YY, Zheng SG, Tsun A, Li B. FOXP3+ Treg cells and its gender bias in autoimmune diseases. Frontiers in Immunology. 2015;6(493) doi: 10.3389/fimmu.2015.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tembhre MK, Parihar AS, Sharma VK, Sharma A, Chattopadhyay P, Gupta S. Alteration in regulatory T cells and programmed cell death 1-expressing regulatory T cells in active generalized vitiligo and their clinical correlation. British Journal of Dermatology. 2015;172(4):940–50. doi: 10.1111/bjd.13511. [DOI] [PubMed] [Google Scholar]
- 60.Tembhre MK, Sharma VK, Sharma A, Chattopadhyay P, Gupta S. T helper and regulatory T cell cytokine profile in active, stable and narrow band ultraviolet B treated generalized vitiligo. Clinica Chimica Acta. 2013;424:27–32. doi: 10.1016/j.cca.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 61.Dwivedi M, Laddha NC, Arora P, Marfatia YS, Begum R. Decreased regulatory T-cells and CD4+/CD8+ ratio correlate with disease onset and progression in patients with generalized vitiligo. Pigment Cell & Melanoma Research. 2013;26(4):586–91. doi: 10.1111/pcmr.12105. [DOI] [PubMed] [Google Scholar]
- 62.Lili Y, Yi W, Ji Y, Yue S, Weimin S, Ming L. Global activation of CD8+ cytotoxic T lymphocytes correlates with an impairment in regulatory T cells in patients with generalized vitiligo. PloS One. 2012;7(5):e37513. doi: 10.1371/journal.pone.0037513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou L, Li K, Shi YL, Hamzavi I, Gao TW, Henderson M, Huggins RH, Agbai O, Mahmoud B, Mi X, et al. Systemic analyses of immunophenotypes of peripheral T cells in non-segmental vitiligo: implication of defective natural killer T cells. Pigment Cell & Melanoma Research. 2012;25(5):602–11. doi: 10.1111/j.1755-148X.2012.01019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tu CX, Jin WW, Lin M, Wang ZH, Man MQ. Levels of TGF-β1 in serum and culture supernatants of CD4+CD25+ T cells from patients with non-segmental vitiligo. Archives of Dermatological Research. 2011;303(9):685–9. doi: 10.1007/s00403-011-1154-8. [DOI] [PubMed] [Google Scholar]
- 65.Klarquist J, Denman CJ, Hernandez C, Wainwright DA, Strickland FM, Overbeck A, Mehrotra S, Nishimura MI, Le Poole IC. Reduced skin homing by functional Treg in vitiligo. Pigment Cell & Melanoma Research. 2010;23(2):276–86. doi: 10.1111/j.1755-148X.2010.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66*.Maeda Y, Nishikawa H, Sugiyama D, Ha D, Hamaguchi M, Saito T, Nishioka M, Wing JB, Adeeqbe D, Katayama I, Sakaguchi S. Detection of self-reactive CD8+ T cells with an anergic phenotype in healthy individuals. Science. 2014;346(6216):1536–40. doi: 10.1126/science.aaa1292. * Through in vitro experiments, this study found that human Tregs render low-affinity self-reactive CD8+ T cell clones unresponsive, preventing their activation following exposure to antigen. This Treg-mediated control was marked by expression of CTLA4 and CCR7 on the effector cells, a phenotype that was prominent on melanocyte-specific CD8+ T cells in healthy controls, but decreased in vitiligo patients. It suggests that vitiligo may be due in part to failed Treg-mediated suppression of autoreactive T cells in vivo.
- 67.Igyártó BZ, Haley K, Ortner D, Bobr A, Gerami-Nejad M, Edelson BT, Zurawski SM, Malissen B, Zurawski G, Berman J, Kaplan DH. Skin-Resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper responses. Immunity. 2011;35(2):260–72. doi: 10.1016/j.immuni.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kashem SW, Igyártó BZ, Gerami-Nejad M, Kumamoto Y, Mohammed J, Jarrett E, Drummond RA, Zurawski SM, Zurawski G, Berman J, et al. Candida albicans morphology and dendritic cell subsets determine T helper cell differentiation. Immunity. 2015;42(2):356–66. doi: 10.1016/j.immuni.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Henri S, Poulin LF, Tamoutounour S, Ardouin L, Guilliams M, de Bovis B, Devilard E, Viret C, Azukizawa H, Kissenpfennig A, Malissen B. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. The Journal of Experimenal Medicine. 2010;207(1):189–206. doi: 10.1084/jem.20091964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lowes MA, Suárez-Fariñas M, Krueger JG. Immunology of Psoriasis. Annual Review of Immunology. 2014;32(1):227–55. doi: 10.1146/annurev-immunol-032713-120225. [DOI] [PMC free article] [PubMed] [Google Scholar]