Abstract
Nanoarchitectures of titania (TiO2) have been widely investigated for a number of medical applications including implants and drug delivery. Although titania is extensively used in the food, drug and cosmetic industries, biocompatibility of nanoscale titania is still under careful scrutiny due to the conflicting reports on its interaction with cellular matter. For an accurate insight, we performed in vitro studies on the response of human dermal fibroblast cells toward pristine titania nanotubes fabricated by anodic oxidation. The nanotubes at low concentrations were seen to induce toxicity to the cells, whereas at higher concentrations the cell vitality remained on par with controls. Further investigations revealed an increase in the G0 phase cell population depicting that majority of cells were in the resting rather than active phase. Though the mitochondrial set-up did not exhibit any signs of stress, significantly enhanced reactive oxygen species production in the nuclear compartment was noted. The TiO2 nanotubes were believed to have gained access to the nuclear machinery and caused increased stress leading to genotoxicity. This interesting property of the nanotubes could be utilized to kill cancer cells, especially if the nanotubes are functionalized for a specific target, thus eliminating the need for any chemotherapeutic agents.
Recent advances in the synthesis and development of nanomaterials (NMs) of diverse origins, structures and properties have immensely impacted various fields ranging from consumer electronics to medical diagnosis and therapy. At such a rapid pace of NMs’ intrusion into virtually every aspect of life, the concern over their possible adverse or deleterious effects on life forms has become a major topic of discussion1. As the NMs differ widely with respect to their physicochemical parameters such as size, shape and elemental constituents, analysis of their toxicity involves complex challenges2. In addition, cell culture conditions, cell age and cell density can also influence the toxicity of the nanostructures3. Research so far has revealed different modes of NMs induced toxicity including the induction of oxidative stress due to reactive oxygen species (ROS) generation, inflammation, genetic damage and arresting of cell division, all culminating in cell death4,5,6,7. Different routes of exposure have also been shown to cause NM accumulation in major organs and induce toxicity8,9,10,11,12,13,14.
Among the various kinds of NMs currently in focus, the nanotubes (NTs) have been proven most useful for many applications due to their architecture derived unique functionalities15,16. The tubular shape, inner and outer surfaces and the nanoscale wall, all provide them distinct advantages over other nanoarchitectures. A prerequisite for their in vivo use is an in-depth understanding on their toxicological effects. The toxicity of nanoparticles of iron oxide, gold and cadmium chalcogenides, were investigated but the studies were limited to nearly isotropic particles17,18,19 (i.e., with a low aspect ratio compared to fibers). Most studies on the bio-applications and toxicity have so far almost been exclusively focused on carbon based NTs20,21,22,23,24,25,26. Similar studies were also reported on other one dimensional materials including silver nanowires (NWs), silicon NWs and germanium-imogolite nanotubes27,28,29,30. Although there were some attempts (discussed later), the toxicity of oxide nanotubes31 such as titanium dioxide (TiO2) NTs has not been addressed in detail. We note that titanium and its alloys are currently the most widely employed materials for dental and orthopedic implants owing to their non-toxicity and biocompatibility along with commendable mechanical properties and corrosion resistance32,33.
While titania nanotubes can be fabricated by a number of physical, chemical and electrochemical methods, the self-assembled nanotube arrays grown by anodic oxidation has received incredible attention due to the ease in fabrication as well as the exceptional structural, electrical, thermal and optical properties exhibited by them34,35,36. These nanotubes find potential use in in-vivo applications also. In a number of studies on the cell response to TiO2 nanotubes, nanosize effects were demonstrated for a variety of cells37,38. It was reported that the surface topography of nanoscale TiO2 strongly affected the proliferation, migration, and differentiation of mesenchymal stem cells and hematopoietic stem cells, as well as the behavior of osteoblasts and osteoclasts39 which prevailed even after coating the nanotubes with an alloy40. It was established that the geometric factors of nanotubes greatly influenced cell vitality. We note that most of the so called ‘biocompatible materials’ including titanium and its alloys, have shown to instigate inflammatory cell recruitment, granulation tissue formation, foreign body reaction, fibrosis and other related biological events41,42. Such unwarranted events may lead to poor skin–material integration, which is one of the major causes for transcutaneous implantable devices’ rejection and undesired side effects43,44,45. Therefore, it is of extreme importance that nanomaterials, regardless of their physicochemical characteristics, be thoroughly investigated under in vitro and in vivo conditions prior to human and environmental exposure. Herein, we discuss the results of our comprehensive study on the anodically grown TiO2 nanotubes for their compatibility towards human dermal fibroblasts. We used a variety of assays ranging from cell-vitality to mitochondrial, cytoskeletal and nuclear integrity. Our investigations revealed for the first time the titania nanotube induced cyto/genotoxicity.
Results and Discussion
TiO2 Nanotubes
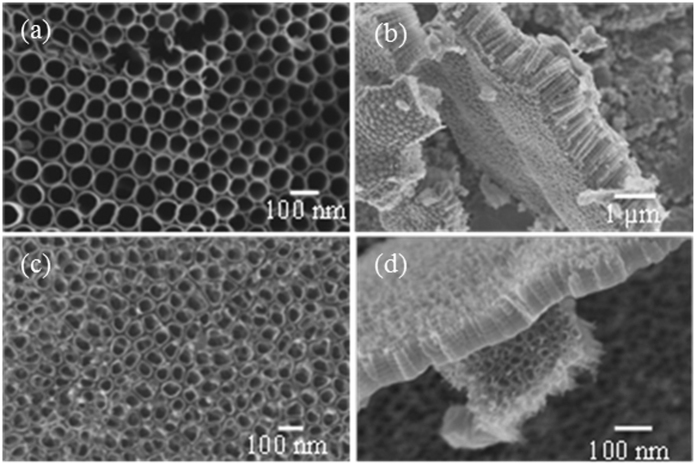
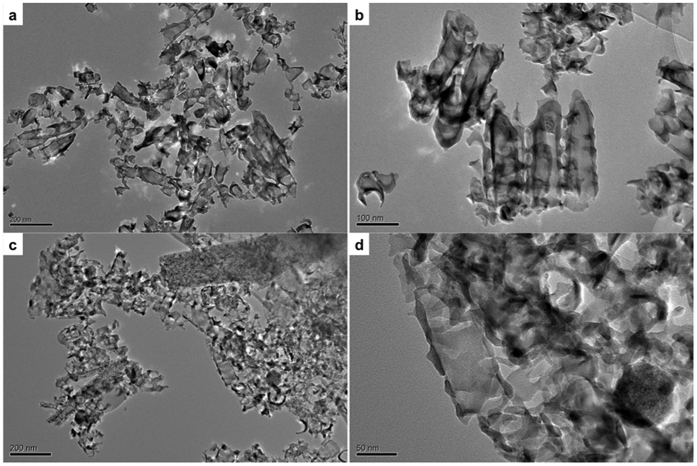
As discussed in detail in Materials and Methods, we used titania nanotube arrays anodically grown in hydrofluoric acid (HF)/water and ethylene glycol (EG)/Ammonium fluoride (NH4F) electrolytes for the study. Fig. 1 shows the field emission scanning electron microscope (SEM; LEO 1525) images of the as-fabricated nanotube arrays. The nanotubes grown in HF/H2O electrolyte had an average length of 146 nm and pore diameter 35 nm and those grown in EG/NH4F electrolyte had these dimensions 970 nm and 70 nm respectively. These nanotube arrays, after heat treatment and detachment from the substrate, were sonicated in water to obtain dispersed individual nanotubes for further experiments. The transmission electron microscope (TEM; JEOL JEM-2100) images of these dispersed nanotubes are shown in Fig. 2. It is evident from the TEM images that nanotubes were broken during detachment and were of similar length (~150 nm) regardless of the fabrication conditions. The NTs were devoid of any metal substrate elements as NT array films were detached electrochemically from the substrates (see Materials and Methods).
Figure 1. SEM images of TiO2 nanotube array films separated from substrates.
(a) Top and (b) lateral views of the nanotubes fabricated using the EG/NH4F electrolyte. (c) and (d) show the images of the corresponding regions in films grown in the water/HF electrolyte.
Figure 2. TEM micrographs of TiO2 nanotubes.
(a) Low magnification and (b) high magnification images of ethylene glycol (organic) electrolyte fabricated TiO2 nanotubes. (c) Low magnification and (d) high magnification images of HF/H2O (aqueous) electrolyte fabricated TiO2 nanotubes.
The anodically grown NTs are generally amorphous upon formation and are known to crystallize at ~280 °C with anatase phase formed in the NT walls46,47. The selected area diffraction pattern and the lattice image showing polycrystalline anatase phase present in the wall of an NT prepared using EG/NH4F electrolyte and heat treated at 530 °C are given in Supporting Information Fig. S1. The HF and EG based NTs used in the present study were crystallized by heat treating at 500 and 450 °C respectively.
Cytotoxicity Analyses
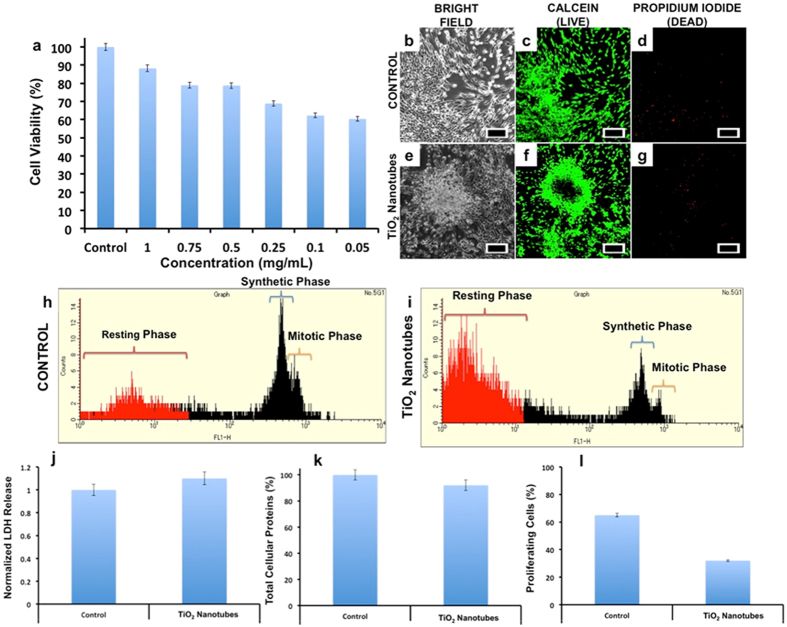
Nanotubes generally share morphological similarities with asbestos fibrils, which are linked to progressive fibrotic ailments48. On this basis we explored the acute cytotoxicity of NTs on human dermal fibroblast cells (HDAF). The effect of NTs on the proliferation and metabolic status of the cells was first investigated by alamar blue assay. The NTs fabricated by both aqueous and organic electrolytes were slightly toxic at lower concentrations (0.05, 0.1 and 0.25 mg/mL) when compared to the higher concentrations (0.5–1.0 mg/mL) after the incubation period of 72 h. In the case of samples fabricated using EG electrolyte, the viability reduced to 60.37, 62.26 and 68.86% for 0.05, 0.1 and 0.25 mg/mL NTs treated set, respectively (Fig. 3a). Higher concentrations i.e., 0.5, 0.75 and 1.0 mg/mL, showed 78.77, 78.94 and 88.22% viabilities, respectively. Although the nanotubes fabricated by HF/H2O electrolyte were significantly shorter (by a factor of ~6) originally, similar results were obtained from these nanotubes also (see Supporting Information Fig. S2). Nonetheless, as mentioned earlier, the dimensions of nanotubes prepared in both the electrolytes became similar after sonication. It is reasonable to believe that the similarity in the toxic nature of both types of nanotubes arises from their comparable dimensions. The study also confirmed that the nanotube pedigree had no apparent role in determining the toxicity. For these reasons, we used the samples prepared using EG/NH4F electrolyte for all subsequent studies.
Figure 3. Cellular toxicity analysis for the effects of TiO2 nanotubes on human dermal fibroblasts.
The alamar blue assisted assay revealed a dose independent viability chart, with viability decreasing with decrease in concentration (a). Though an overall maximum viability remained at approximately 60%. (Columns, means of three independent experiments; bars ± SD). The live dead analysis (b–g) revealed a generally live cell population in the NTs treated group (e–g) which could be correlated to the residual esterase even in the cells undergoing apoptosis (f) (scale bar represents 500 μm). In the cell cycle analysis a predominant synthetic and mitotic phase was observed with the control cells (h), however in the NTs group a prominent resting phase was observed (i). The LDH release was found to be insignificant in treatment group when compared to control (j); The TCP expression were found to be unaffected (k) whereas the number of proliferating cells in the treatment group dropped by half when compared to control (l).
The broken NTs were found to be aggregated and difficult to disperse by sonication. Post sonication, uneven polydisperse size distribution in solutions was evident from Fig. 2a. This observation was similar to earlier studies on carbon nanotubes, where the length and morphology of the carbon nanotubes were distorted after sonication49,50. The extent of aggregation increased when NTs were added to the cell culture media which could likely have prevented cellular cytosol from being exposed to individual NTs at higher concentrations. It is worth mentioning that the NTs were bare and not surface functionalized with biocompatible/surfactant coating, which not only enhances the compatibility of a material but also renders significant and uniform dispersion of the nanomaterial under investigation. Due to the bare nature of NTs, exposure to cell culture medium (containing serum and various proteins) induces aggregation and formation of large clusters that cannot be efficiently endocytosed by cells and therefore may not have shown toxicity. Whereas at lower concentrations, the clusters are expected to be within the endocytosis limit, leading to relatively higher uptake by cells facilitating interaction with intracellular components. Though the dosing metric is considered to be a common way to assess viability, the method is complicated because very little is known about how the aggregation behavior of the NTs affects the dosage/concentration-based toxicity. TiO2 nanoparticles and nanotubes tend to aggregate easily in solution when not surface modified. Such aggregation behavior was reported in a few uptake and localization studies51,52,53,54. Though several studies were reported on toxicity analysis of TiO2 NTs, not much was discussed about the dosage-based aggregation of the NTs. In most of the reported studies, contradictory results were presented with some finding NTs as highly biocompatible, while some others observing toxicity in them55. TiO2 nanomaterials, especially the architectures with high aspect ratio such as NTs, tend to aggregate in solution. This characteristic made preparation of the dosing solutions in this study difficult because the conventional mechanical dispersion methods such as sonication provided only polydispersed solutions, which contained a mixture of individual nanotubes, nanotube aggregates, and particle debris that might have contributed to the interesting results presented here.
We point out that the nanotubes synthesized with aqueous as well as organic electrolytes, showed a similar toxicity profile, i.e., lower viability at lower concentration and higher viabilities at higher concentrations. The TEM images clearly display the fractured forms of nanotubes (Fig. 2). Though both nanotube groups differed in their structural dimensions immediately after synthesis, their morphological features became similar on sonication. We did not see any distinct difference in the cytotoxicity profiles of amorphous and polycrystalline nanotubes for a given nanotube concentration. Furthermore, as the metal substrate elements were completely removed from the nanotubes during detachment, their role in the recorded toxicity could be completely ignored. Therefore, the observed interesting cytotoxicity profile could be correlatively attributed to the size and dimensions of the test materials. The aggregation behavior was visible through microscope as well as with naked eye. The large clusters were seen to be floating in the media or attached to the cell/substratum. Therefore, it was concluded that the larger aggregates remained ineffective while the smaller aggregates or individual broken NTs specifically gained access to the cellular interior and exerted the effects observed thereof.
To assess the possible reasons for the observed toxicity of NTs based on alamar blue analysis, 0.05 mg/mL of the nanomaterial was used for all further experiments. Fluorometric live/dead cell viability and fluorometric cell cycle analysis were assayed. Using the cell viability assay that stains individual cells and measures the number of live versus dead population, it could be observed that most cells exhibited esterase staining, depicting viability (Fig. 3c,f). With alamar blue based cytotoxicity analysis, we found that nearly 40% of cell population was dead when exposed to 0.05 mg/mL of NTs after 72 h. However, with live/dead staining, almost all the cells exhibited bright green fluorescence, which we believe is from the residual esterase in the cells. It could be seen that the cells were aggregated with reduced cellular volume depictive of stress conditions (Fig. 3e), which eventually culminates in cell death. Very few cells in treatment group exhibited propidium iodide (PI) staining (Fig. 3g) which confirms that the major mode of cell-death is via the apoptotic pathway rather than necrotic. To understand whether NTs could disrupt the cell cycle progression, we analyzed the cell cycle phases of the control and treatment groups. Control group cells (Fig. 3h) were predominantly seen in synthetic and mitotic phase (S and M phase), whereas very few cells were seen in resting phase (sub G0 phase). However, with test group (Fig. 3i), we observed a clear decline of cell population in S and M phase and a relative increase in sub G0 phase. The cells in sub G0 phase are indicative of cells which are no longer active part of cell-cycle.
Lactate dehydrogenase (LDH) is an important cytosolic enzyme and its release is considered to be a vital tool to understand the extent of plasma membrane damage when cells are exposed to toxic substances56. Assessing the cell membrane integrity is one of the most common ways to measure cell viability and cytotoxic effects. When tested, the NTs exposed cells exhibited a slight increase in the LDH release as compared to the control groups (Fig. 3j); however, the release was insignificant. If correlated with live/dead analysis, the esterase activity remained active and the cells exhibited bright green fluorescence indicative of their viable status. If the plasma membrane integrity were compromised, the esterases would have leaked out, leading to meager viable fluorescence. Furthermore, in this case the PI would have gained nuclear access exhibiting necrotic signals; the results obtained with live/dead assay clearly supports the retention of plasma membrane integrity that is observed with the LDH assay.
Total cellular proteins (TCP) constitute about 35% of cell volume and their expression could be affected when cells exhibit distress signals57. When the TCP were quantified (Fig. 3k), the amount of proteins expressed by treatment group remained very much comparable to the control group. Though a slight decrease in protein concentrations is accounted, we believe the values are insignificant and the decrease can be attributed to treated cells that are rich in resting phase rather than in synthetic phase as seen in controls.
Cell proliferation analysis kinetics is considered as an important parameter that can critically explain about the cell’s metabolic behavior at a given time58. EDU based cell proliferation assay is considered to be easy and reliable when compared to radioactive thymidine or BrDU based analysis. EDU binds to the newly actively synthesizing DNA by click chemistry. Nearly 65% of the controls exhibited EDU staining whereas only 30% of the treated cells were positive to EDU (Fig. 3l). According to the cell cycle analyses, we believe as most of the NTs treated cells were in the resting phase with less cells in S phase, the cell proliferation ability of this group was diminished.
Cytoskeletal Organization
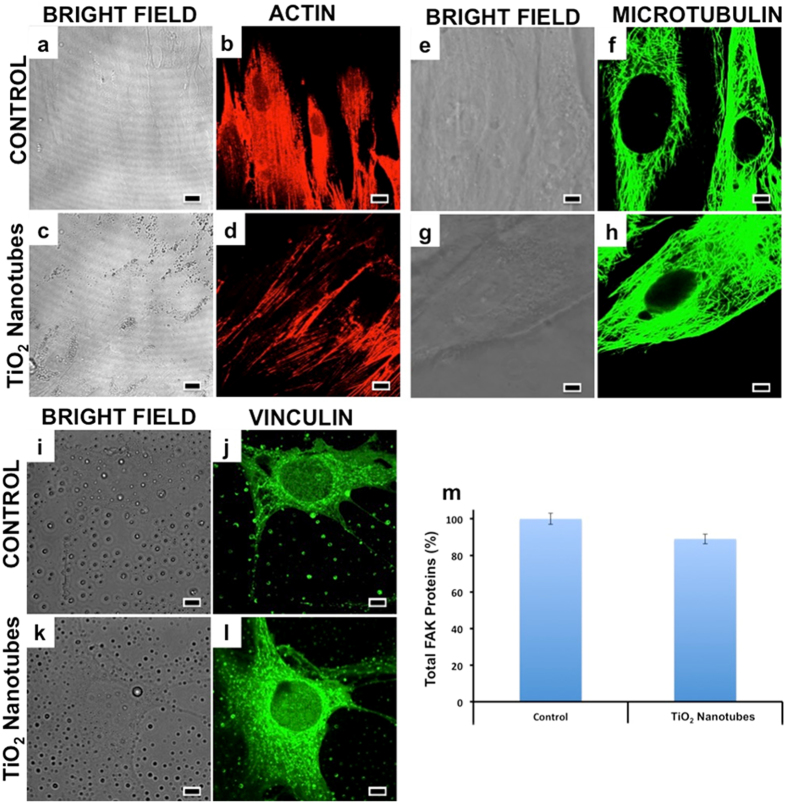
Cytoskeletal disorganization could also lead to serious disorientation in organelle and protein transport within the cell’s cytosol and could fatally affect the cell’s survival59. A major component of cellular skeleton is actin. The actins form rigid but flexible scaffolding, facilitating the mechanical stability and mobility of cells60. Typically, cells are flat adherent to the substratum with strong peripheral F-actin staining along the cell edge, indicative of cell cortical actin fibers. The microtubules form a dense network that is uniformly distributed around the nucleus. Microtubules are mainly responsible for mobility and intracellular transport and their proper functioning is essential for the optimal metabolic output of a cell61. Compared to control cells which displayed an elaborate network of finely arranged actin fibers (Fig. 4b), cells treated with NTs exhibited F-actin thinner and less organized within the main body (Fig. 4d), however, the microtubule structures in the central and peripheral domain remained comparable with control cells (Fig. 4f,h). This result suggested a slight disorganization of the actin cytoskeleton when exposed to NTs, which could be correlated to the observations of cellular aggregation previously mentioned. This disorganization might cause a series of signal pathway disruptions, though concrete conclusions could be made only after a detailed study of downstream processes.
Figure 4. Cytoskeletal integrity analysis of the control and NTs treated group were performed with emphasis on actin, micotubulins and vinculin.
With respect to the actin filaments, a slight reduction in the network of the fibres was observed with the NT group (d) when compared with the control (b). In the case of microtubulins the control (f) and test groups (h) exhibited comparable tubular network. The vinculin expression was found to be undisturbed in the treatment group (l) when compared to control (j). (Scale bars represent 10 μm). Total FAK proteins were quantified with ELISA and their expression in control and treatment groups remained comparable (m). Overall, the cytoskeletal components, which form the basic backbone of a cell and are responsible for their structural integrity and cell-cell/cell-matrix interactions, remained unaffected by the TiO2 NTs, with the exception of actin where a slight rearrangement of the fibers was observed.
Vinculin, a major membrane-cytoskeletal protein, serves as focal adhesion plaque that is involved in linking integrins to the actins and is associated with cell-cell and cell-matrix junctions62. Vinculin disruption can cause a loss in balance within cytoskeletal organization and can lead to contact inhibition between cells and the extra cellular matrix. When investigated, the vinculin immunomapping remained same for the control and treated group (Fig. 4j,l) depicting more or less proper cytoskeletal organization.
We investigated the expression of total focal adhesion kinase (FAK) proteins that work primarily as signal-mediating molecules that direct actin based signaling pathway63. FAK disruption is known to affect cellular homeostasis. When quantified, the FAK levels of the treatment group were slightly decreased as compared to the control group (Fig. 4m). This approximately 8% decrease can be attributed to the actin disorganization that was previously observed. Though a slight modification in actin and FAK levels were noted, the cytoskeletal integrity remained comparable to the control groups. Earlier, single wall carbon nanotubes were shown to cause disorientation and bundling in actins in vitro leading to chronic changes in cell behavior without any observed acute toxicity64. We believe that the observed slight actin re-modeling arose from the shape-effect of the NTs rather than their intrinsic nature bound toxicity
Mitochondrial Health
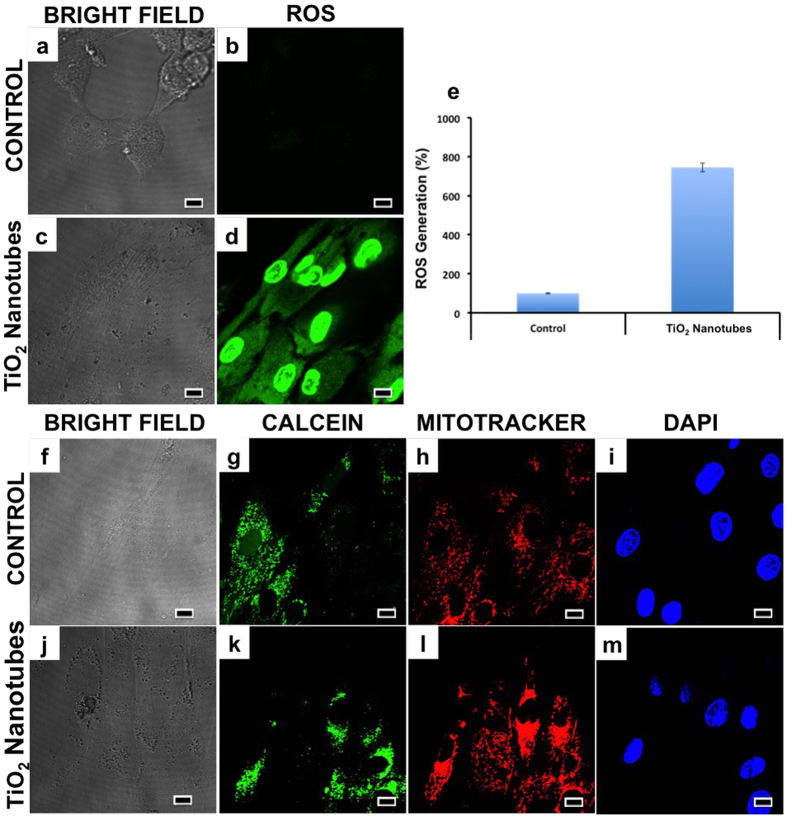
Earlier reports have emphasized the role played by oxidative stress in nanoparticle toxicity that causes serious oxidative damage to proteins and deoxyribonucleic acid (DNA)1. To establish the role of oxidative stress as a decisive factor in NT induced toxicity, ROS tracer based studies were performed. Previous studies proved a direct link between the ROS production in cells and tissues upon exposure to titania based nanostructures65. Untreated cells were used as standards to calculate the extent of ROS production by measuring the percentage of cells with increased fluorescence (directly corresponding to the ROS production) intensity. The analysis showed slight superoxide production in cellular cytosol, treated with 0.05 mg/mL of NTs and highly elevated levels of ROS in the nuclear compartment (Fig. 5d). As one could visualize, a dramatic increase (by 8-fold) of ROS generation could be seen in the cells that were exposed to the NTs (Fig. 5e).
Figure 5. Mitochondrial integrity and condition were assayed with the ROS generation and transition pore formation tests.
It was clearly evident that the NTs induced ROS production, especially nuclear ROS (d). The nuclear ROS in the treatment group was found to be increased by 7 fold than the control (e). The mitochondrial pore induction investigation revealed the absence of any distorted signals from the mitochondrial region (l) with discrete calcein signaling (k), indicative of a properly functioning mitochondrial system. (Scale bars represent 10 μm). It is evident from this assay that though the mitochondrial apparatus seems to be healthy and functioning properly, nuclear ROS is significantly enhanced on TiO2 NTs exposure, which may be the precursor for numerous distortions in nuclear functioning.
Further, we investigated the mitochondrial health, as the main cause of mitochondrial dysfunction arises from ROS production and subsequent oxidative stress. Increased oxidative stress can lead to transitional pore formation in mitochondrial membrane resulting in distorted mitochondrial geometry and function66. The discrete calcein and mitochondrial staining in the treatment group (Fig. 5g,h) revealed a normal and functioning mitochondrial apparatus. The ROS produced due to NT induced oxidative stress was likely counteracted by anti-oxidant systems/or acted upon by other organelles (especially nucleus as observed from the image), thereby nullifying their deleterious effect on mitochondria (Fig. 5k,l).
Genotoxicity
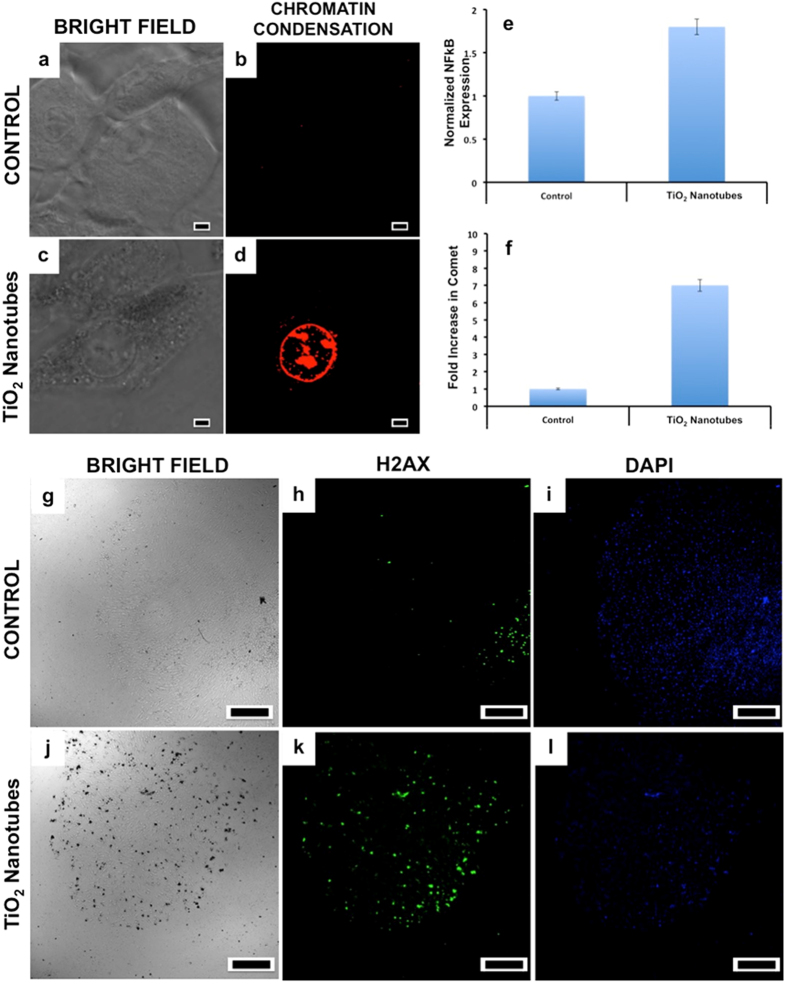
Genotoxicity studies that measure DNA damage such as mutations, chromosomal damage and single/double strand breaks are important part of cancer research and risk assessment of potential carcinogens. The oxidative stress, induction of high levels of ROS in nucleus upon NT exposure can cause spontaneous damage to DNA thus arresting the cell cycle progression. It was reported by Wang et al. that TiO2 NTs could gain access into nuclear region effectively67. To analyze whether NTs could induce genotoxicity, we assessed the chromosomal condensation and double strand DNA breaks. When compared to the normal nuclear content (Fig. 6b), cells treated with NTs for 72 h displayed nuclear chromatin condensation and compromised plasma membrane permeability (Fig. 6d) suggesting cell death via an apoptotic mode. To analyze the secondary effects of ROS, immediate mediator of ROS-induced cell death mediated signaling, NF-κB activation was profiled. As expected, nearly twice the increase in NF-κB levels were noted when compared to controls (Fig. 6e). NF-κB is considered as primary transcription factor of apoptosis that are expressed when cells are in oxidative distress. Their cytosolic expression increases immediately after an oxidative stress, and is transported to nucleus to induce apoptotic transcription leading to programmed cell death68. A clear correlation between the ROS levels and NF-κB expression could be made, which confirmed that the nuclear oxidative stress was the reason for observed genotoxic behavior.
Figure 6. Chromatin condensation and DNA break studies were carried out to assess the condition of the nuclear set up of the control and NT treated cells.
Prominent chromatin condensation and plasma membrane permeability (c,d) was recorded with the NTs treated group. (Scale bars represent 10 μm). The NF-κB expression was elevated in the treatment group when compared to control (e). Comet analysis confirmed significant increase in number of comet tails in treatment group (f). Consistent DNA breaks could also be observed with the H2AX test in the NTs group (k) when compared to the controls (h). (Scale bars represent 500 μm). This nuclear condensation and DNA alteration study is correlative of the ROS induction previously observed.
To analyze whether chromosomal distortion occurred in the treatment groups, the cells were subjected to comet assay. A distorted chromosomal content, leads to tail-comet whereas the healthy chromosomal content appears spherical when subjected to comet electrophoresis. As expected, cells exposed to NTs exhibited nearly 7-fold increase in comet tail formation (Fig. 6f) again affirming the nuclear degeneration behavior of NTs. TiO2 nanoparticles might damage DNA directly or indirectly via oxidative stress and/or inflammatory responses. Recent studies showed a direct chemical interaction between TiO2 nanoparticles and DNA damage69,70. However, other studies showed that TiO2 nanoparticles could cause DNA damage through inflammation71,72 and/or ROS generation73,74. We performed H2AX staining, that is indicative of DNA breaks. The treatment group cells exhibited numerous DNA breaks in the nuclear compartment that are indicative of spontaneous DNA damage due to NT exposure (Fig. 6k). It was evident that oxidative stress could be sensed by the nucleus and that ROS formation was directly generated by the nucleus. Increased ROS in the nuclear region due to NT exposure attacked the nuclear components leading to genotoxicity by inducing nuclear condensation and chromosomal distortion.
Conclusion
The results from our study indicated induction of ROS by NTs that in turn set off DNA damage and chromosomal aberrations, which are believed to be the primary factors resulting in cell cycle arrest. Cells resting at sub G0 interface showed no significant death, indicating the involvement of an active DNA repair pathway. Cells with a robust repair mechanism in place could re-enter the cell cycle, while those with extensive irreparable damage could not repair the DNA effectively, consequently undergoing apoptosis. The NTs displayed the potential to cause toxicity to the cells as analyzed by the cyto- and genotoxicity parameters. Keeping in mind that the NTs were not surface modified with cyto-, biocompatible moieties as polyethylene glycol etc., it is imperative that the biological applications employing NTs should be given special attention and further studies must be conducted in this field to achieve deeper understanding of NT toxicity. As mostly all the nanomaterials currently being examined for various applications including nanomedicine, pose toxicity in vitro/in vivo, and surface functionalization is mandatory, we believe that the TiO2 NTs utilized in the present study could hold better performance once a biocompatible coating is rendered to them. Also, as the cause of cell death is proven to be ROS instigated genotoxicity, these TiO2 nanotubes, after proper surface functionalization and with the assistance of cell-specific markers (antibodies/peptides) could be utilized as specific-cell killing tools, as in the case of anti-cancer therapies. For example, our current interest includes the utilization of such a properly functionalized and targeted TiO2 nanotube to specifically induce ROS and genotoxic effects in cancer cells and kill them as such, negating the need for any chemo agent or external stimuli.
Materials and Methods
Titania nanotube synthesis
The titania nanotube array samples were fabricated by anodic oxidation of titanium (Ti) foils (0.25 mm thick, 99.7% pure, Sigma Aldrich) in a two-electrode cell consisting of a titanium foil anode and a platinum foil cathode immersed in an aqueous or organic electrolyte. The aqueous electrolyte consisted of 0.5 vol% HF in deionized (DI) water. The anodization of Ti foil at 10 V for 1 h in this electrolyte yielded nanotubes having morphology shown in Fig. 1a,b. The organic electrolyte was prepared by mixing EG with 0.7 vol% ammonium fluoride (NH4F) and 50 vol% DI water. The nanotubes (Fig. 1c,d) were grown by anodizing Ti foil at 15 V for 1 h. The titania nanotube array samples prepared in these aqueous and organic electrolytes were then crystallized by heat treating respectively at 500 °C and 450 °C for 30 minutes in oxygen environment. The heat treated samples were anodized again in the respective electrolytes and voltages to detach the crystallized nanotubes from the metal substrate.
Cell culture details
Human Dermal Adult Fibroblasts (HDAF) were obtained from ATCC. The cells were maintained in T25 flasks using CSC complete medium kit (Cell Systems), and incubated in a 5% CO2 incubator at 37 °C. The cells were sub-cultured every three days. Approximately 5000 cells were seeded into each well of 96 well plates. After attaining visual confluency, NTs were added at different concentrations (0.05 mg/mL- 1 mg/mL). Control group was devoid of NTs. After 72 h of incubation with NTs, cells were washed with PBS and 0.2 ml of respective medium was added. Cell viability was assayed with alamar blue in a 96 well plate reader (Multidetection microplate scanner, Dainippon Sumitomo Pharma). For other microplate assays, NTs at a concentration of 0.05 mg/mL were added to the cells and incubated for 72 h. Post 72 h, the cells were quantified for lactate dehydrogenase (LDH) release (Pierce LDH cytotoxicity kit), total cellular protein contents (Pierce BCA protein assay kit), total focal adhesion kinase (FAK) expression (FAK ELISA kit, Thermo Fisher), reactive oxygen species (ROS) generation (DCFH-DA Assay, Sigma) and nuclear factor-κB (NF-κB) expression (NF-κB transcription factor assay kit, Thermo Fisher) according to the respective manufacturer’s instructions.
For flow cytometry (JSAN cell sorter, Bay Bioscience) based cell cycle analysis, approximately 200,000 cells were seeded in 6 well plates and cultured for 24 h. NTs (0.05 mg/mL) were added to the cultures and incubated for 72 h. Post incubation, cells were washed in PBS and flow cytometry performed according to manufacturer’s instructions (Click-iT EDU Alexa fluor 488 flow cytometry assay kit, Life technologies).
For all confocal (CLSM, Olympus IX 81 under DU897 mode; for blue, green and red excitations, wavelength 405, 488 and 561 were used respectively) experiments, approximately 50,000 cells were cultured in 35 mm glass base dishes (Iwaki) for 24 h. The cells were incubated with the NTs (0.05 mg/mL) for 72 h. Live/dead (Live/dead cell double staining kit, Sigma-Aldrich), EDU cell proliferation (Click-iT® Plus EdU, Life Technologies), Actin (Phalloidin rhodamine, Life technologies), Microtubulin (Tubulin tracker, Life technologies), ROS (Cell-ROX, Life technologies), Mitochondrial pore induction (Mitochondrial membrane transition pore assay, Life technologies), Chromatin condensation (Life technologies), DNA break (HCS DNA damage kit, Life technologies) assays were performed as per the respective manufacturer’s instructions.
For comet assay, cells were grown until 80% confluency in T25 flasks. Treatment groups were exposed to 0.05 mg/mL of NTs whereas controls were devoid of any treatment. Post 72 h exposure, cells were collected and processed for comet assay as per manufacturer’s instructions (Alkaline Comet, Trevigen). The comet formations were counted manually and were graphed.
For all cell culture experiments, the cells were washed thoroughly with PBS after incubation with the NTs for the specified time period to remove any unbound or free floating NTs, prior to observations.
Statistical analysis
All quantitative experiments were conducted with at least three independent experiments, each with triplicates. Statistical evaluation was performed using Origin Pro Software.
Additional Information
How to cite this article: Mohamed, M. S. et al. Anodically Grown Titania Nanotube Induced Cytotoxicity has Genotoxic Origins. Sci. Rep. 7, 41844; doi: 10.1038/srep41844 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
A part of this study was supported by a grant for the program of strategic research foundation at private universities S1101017, organized by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan since April 2012.
Footnotes
The authors declare no competing financial interests.
Author Contributions M.S.M., D.S.K. and O.K.V. conceived and designed the work; A.T. and M.P. performed the synthesis of NTs. The characterizations were performed by M.S.M. and M.P. M.S.M. conducted and analyzed in vitro cellular investigations. M.S.M., A.T., M.P., O.K.V. and D.S.K. critically analyzed the results. M.S.M and O.K.V. wrote the manuscript with input from co-authors.
References
- Shvedova A. A., Pietroiusti A., Fadeel B. & Kagan V. E. Mechanisms of carbon nanotube-induced toxicity: focus on oxidative stress. Toxicol. Appl. Pharm. 261, 121–133 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pojlak-Blazi M., Jaganjac M. & Zarkovic N. Cell oxidative stress: risk of metal nanoparticles. Handbook of Nanophysics: Nanomedicine and Nanorobotics, CRC Press, New York, NY, USA, 1–17 (2010). [Google Scholar]
- Papavlassopoulos H. et al. Toxicity of functional nano-micro zinc oxide tetrapods: impact of cell culture conditions, cellular age and material properties. PLoS One. 9, e84983 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju-Nam Y. & Lead J. R. Manufactured nanoparticles: an overview of their chemistry, interactions and potential environmental implications. Sci. Tot. Environ. 400, 396–414 (2008). [DOI] [PubMed] [Google Scholar]
- Li N., Xia T. & Nel A. E. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic. Biol. Med. 44, 1689–1699 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone V., Johnston H. & Cli M. J. D. Air pollution, ultra fine and nanoparticle toxicology: cellular and molecular interactions. IEEE Trans. Nanobioscience. 6, 331–340 (2007). [DOI] [PubMed] [Google Scholar]
- Johnston H. J. et al. A review of the in vivo and in vitro toxicity of silver and gold particulates: particle attributes and biological mechanisms responsible for the observed toxicity. Crit. Rev. Toxicol. 40, 328–346 (2010). [DOI] [PubMed] [Google Scholar]
- Sotiriou G. A. et al. A novel platform for pulmonary and cardiovascular toxicological characterization of inhaled engineered nanomaterials. Nanotoxicology. 6, 680–690 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi L., Yousefi Babadi V. & Espanani H. R. Toxic effects of the Fe2O3 nanoparticles on the liver and lung tissue. Bratisl Lek Listy. 116, 373–378 (2015). [DOI] [PubMed] [Google Scholar]
- Vandebrial R. J. & De Jong W. H. A review of mammalian toxicity of ZnO nanoparticles. Nanotechnol. Sci. Appl. 5, 61–71 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D., Tian Y., Zhang T., Ren G. & Yang Z. Nano-zinc oxide damages spatial cognition capability via over-enhanced long-term potentiation in hippocampus of Wistar rats. Int. J. Nanomedicine. 6, 1453–1461 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.-R. et al. Toxicity of 100 nm zinc oxide nanoparticles: a report of 90-day repeated oral administration in Sprague Dawley rats. Int. J. Nanomedicine. 9, 109–126 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari M., Kumari S. I. & Grover P. Genotoxicity analysis of cerium oxide micro and nanoparticles in Wistar rats after 28 days of repeated oral administration. Mutagenesis, 29, 467–479 (2014). [DOI] [PubMed] [Google Scholar]
- Morimoto Y. et al. Pulmonary toxicity of well-dispersed cerium oxide nanoparticles following intratracheal instillation and inhalation. J. Nanopart. Res. 17, 442 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P. et al. Stable and efficient perovskite solar cells based on titania nanotube arrays. Small. 11, 5533–5539 (2015). [DOI] [PubMed] [Google Scholar]
- Wijeratne A. B. et al. Phosphopeptide separation using radially aligned titania nanotubes on titanium wire. ACS Appl. Mater. Interfaces. 7, 11155–11164 (2015). [DOI] [PubMed] [Google Scholar]
- Stern S. T. & McNeil S. E. Nanotechnology: safety concerns revisited. Toxicol Sci. 101, 4–21 (2008). [DOI] [PubMed] [Google Scholar]
- Lewinski N., Colvin V. L. & Drezek R. Cytotoxicity of nanoparticles. Small. 4, 26–49 (2008). [DOI] [PubMed] [Google Scholar]
- Poulose A. C. et al. Characterizing the biocompatibility and tumor imaging capability of Cu2S nanocrystals in vivo. Nanoscale. 7, 13061–13074 (2015). [DOI] [PubMed] [Google Scholar]
- Helland A., Wick P., Koehler A., Schmid K. & Som C. Reviewing the environmental and human health knowledge base of carbon nanotubes. Environ. Health Perspect. 115, 1125–1131 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam C. W., James J. T., McCluskey R. & Hunter R. L. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 Days after Intractracheal Instillation. Toxicol. Sci. 77, 126–134 (2004). [DOI] [PubMed] [Google Scholar]
- Warheit D. B. et al. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol. Sci. 77, 117–125 (2004). [DOI] [PubMed] [Google Scholar]
- Shvedova A. A. et al. Exposure to carbon nanotube material: assessment of nanotube cytotoxicity using human keratinocyte cells. J. Toxicol. Environ. Health A. 66, 1909–1926 (2003). [DOI] [PubMed] [Google Scholar]
- Maynard A. D. et al. Exposure to carbon nanotube material: aerosol release during the handling of unrefined single-walled carbon nanotube material. J. Toxicol. Environ. Health A. 67, 87–107 (2004). [DOI] [PubMed] [Google Scholar]
- Monteiro-Riviere N. A., Nemanich R. J., Inman A. O., Wang Y. Y. & Riviere J. E. Multi-walled carbon nanotube interactions with human epidermal keratinocyte. Toxicol. Lett. 155, 377–384 (2005). [DOI] [PubMed] [Google Scholar]
- Panessa-Warren B. J., Warren J. B., Wong S. S. & Misewich J. A. Biological cellular response to carbon nanoparticle toxicity. J. Phys. Condens. Mater. 18, S2185–S2201 (2006). [Google Scholar]
- van den Brule S. et al. Nanometer-long Ge-imogolite nanotubes cause sustained lung inflammation and fibrosis in rats. Part. Fibre Toxicol. 11, 67 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva R. M. et al. Short versus long silver nanowires: a comparison of in vivo pulmonary effects post instillation. Part. Fibre Toxicol. 11, 52 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinwald A. & Donaldson K. Use of back-scatter electron signals to visualise cell/nanowires interactions in vitro and in vivo; frustrated phagocytosis of long fibres in macrophages and compartmentalisation in mesothelial cells in vivo. Part. Fibre Toxicol. 9, 34 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. R. et al. Pulmonary toxicity, distribution, and clearance of intratracheally instilled silicon nanowires in rats. Journal of Nanomaterials 2012, 398302 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao B. M., Torabi A. & Varghese O. K. Anodically grown functional oxide nanotubes and applications, MRS Communications, doi: https://doi.org/10.1557/mrc.2016.46.
- Niinomi M. Mechanical biocompatibilities of titanium alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 1, 30–42 (2008). [DOI] [PubMed] [Google Scholar]
- Wang K. The use of titanium for medical applications in the USA. Mater. Sci. Eng. A. 213, 134–137 (1996). [Google Scholar]
- Gong D. et al. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 16, 3331–3334 (2001). [Google Scholar]
- Rani S. et al. Synthesis and applications of electrochemically self-assembled titania nanotube arrays. Phys. Chem. Chem. Phys. 12, 2780–2800 (2010). [DOI] [PubMed] [Google Scholar]
- Brahmi H. et al. Thermal-structural relationship of individual titania nanotubes. Nanoscale. 7, 19004–19011 (2015). [DOI] [PubMed] [Google Scholar]
- Popat K. C., Leoni L., Grimes C. A. & Desai T. A. Influence of engineered titania nanotubular surfaces on bone cells. Biomaterials. 28, 3188–3197 (2007). [DOI] [PubMed] [Google Scholar]
- Bauer S., Park J., von der Mark K. & Schmuki P. Improved attachment of mesenchymal stem cells on super-hydrophobic TiO2 nanotubes. Acta. Biomater. 4, 1576–1582 (2008). [DOI] [PubMed] [Google Scholar]
- Park J., Bauer S., von der Mark K. & Schmuki P. Nanosize and vitality: TiO2 nanotube diameter directs cell fate. Nano Lett. 7, 1686–1691 (2007). [DOI] [PubMed] [Google Scholar]
- Bauer S. et al. Size selective behavior of mesenchymal stem cells on ZrO2 and TiO2 nanotube array. Integr. Biol. 1, 525–532 (2009). [DOI] [PubMed] [Google Scholar]
- Smith C. M. et al. Engineering a titanium and polycaprolactone construct for a biocompatible interface between the body and artificial limb. Tissue Eng. A. 16, 717–724 (2010). [DOI] [PubMed] [Google Scholar]
- Kang N. V., Pendegrass C., Marks L. & Blunn G. Osseocutaneous integration of an intraosseous transcutaneous amputation prosthesis implant used for reconstruction of a transhumeral amputee: case report. J. Hand. Surg. 35, 1130–1134 (2010). [DOI] [PubMed] [Google Scholar]
- Tillander J., Hagberg K., Hagberg L. & Brånemark R. Osseointegrated titanium implants for limb prostheses attachments: infectious complications. Clin. Orthop. Relat. Res. 468, 2781–2788 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney V., Predecki P. K., Renning J. & Gray J. Skeletal extension of limb prosthetic attachments – Problems in tissue reaction. J. Biomed. Mater. Res. 5, 143–159 (1971). [Google Scholar]
- Winter G. D. Transcutaneous implants: reactions of the skin-Implant interface. J. Biomed. Mater. Res. 8, 99–113 (1974). [DOI] [PubMed] [Google Scholar]
- Varghese O. K., Gong D., Paulose M., Grimes C. A.& Dickey E. C. Crystallization and high temperature structural stability of titanium oxide nanotube arrays. J. Maer. Res. 18, 156–165 (2003). [Google Scholar]
- Paulose M., Shankar K., Yoriya S., Prakasam H. E., Varghese O. K., Mor G. K., LaTempa T. A., Fitzgerald A. & Grimes C. A. Anodic growth of highly ordered TiO2 nanotube arrays to 134 μm in length. J. Phys. Chem. B. 110, 16179–16184 (2006). [DOI] [PubMed] [Google Scholar]
- Horváth L. et al. In vitro investigation of the cellular toxicity of boron nitride nanotubes. ACS Nano. 5, 3800–3810 (2011). [DOI] [PubMed] [Google Scholar]
- Huang Y. Y. & Terentjev E. M. Dispersion of carbon nanotubes: mixing, sonication, stabilization, and composite properties. Polymers. 4, 275–295 (2012). [Google Scholar]
- Pagani G., Green M. J., Poulin P. & Pasquali M. Competing mechanisms and scaling laws for carbon nanotube scission by ultrasonication. PNAS. 109, 11599–11604 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love S. A., Maurer-Jones M. A., Thompson J. W., Lin Y. S. & Haynes C. L. Assessing nanoparticle toxicity. Annu. Rev. Anal. Chem. 5, 181–205 (2012). [DOI] [PubMed] [Google Scholar]
- Maurer-Jones M. A., Lin Y. S. & Haynes C. L. Functional assessment of metal oxide nanoparticle toxicity in immune cells. Am. Chem. Soc. Nano. 4, 3363–3373 (2010). [DOI] [PubMed] [Google Scholar]
- Ji Z. et al. Dispersion and stability optimization of TiO2 nanoparticles in cell culture media. Environ. Sci. Technol. 44, 7309–7314 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedja R., Marquis C., Lim M. & Amal R. Biological impacts of TiO2 on human lung cell lines A549 and H1299: particle size distribution effects. J. Nano Res. 13, 3801–3813 (2011). [Google Scholar]
- Tilly T. B., Kerr L. L., Braydich-Stolle L. K., Schalger J. J. & Hussain A. M. Dispersions of geometric TiO2 nanomaterials and their toxicity to RPMI 2650 nasal epithelial cells. J. Nanopart. Res. 16, 2695 (2014). [Google Scholar]
- Chan F. K., Moriwaki K. & De Rosa M. J. Detection of necrosis by release of lactate dehydrogenase activity. Methods Mol. Biol. 979, 65–70 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. C. Total cell protein concentration as an evolutionary constraint on the metabolic control distribution in cells. J. Theor. Biol. 153, 195–203 (1991). [DOI] [PubMed] [Google Scholar]
- Riss T. L., Moravec R. A. & Niles A. L. Cytotoxicity testing: measuring viable cells, dead cells, and detecting mechanism of cell death. Methods Mol. Biol. 740, 103–114 (2011). [DOI] [PubMed] [Google Scholar]
- Nunez R., Sancho-Martinez, Novoa J. M. L. & Lopez-Hernandez F. J. Apoptotic volume decrease as a geometric determinant for cell dismantling into apoptotic bodies. Cell Death. Differ. 17, 1665–1671 (2010). [DOI] [PubMed] [Google Scholar]
- Ono S. Mechanism of depolymerization and severing of actin filaments and its significance in cytoskeletal dynamics. Int. Rev. Cytol. 258, 1–82 (2007). [DOI] [PubMed] [Google Scholar]
- Vale R. D. The molecular motor toolbox for intracellular transport. Cell. 112, 467–480 (2003). [DOI] [PubMed] [Google Scholar]
- Carisey A. & Ballestrem C. Vinculin, an adapter protein in control of cell adhesion signaling. Eur. J. Cell. Biol. 90, 157–163 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grashoff C. et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 466, 263–266 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt B. D. et al. Carbon nanotubes reorganize actin structures in cells and ex vivo. ACS Nano. 4, 4872–4878 (2010). [DOI] [PubMed] [Google Scholar]
- Huang K. T. et al. Titanium nanoparticle inhalation induces renal fibrosis in mice via an oxidative stress upregulated transforming growth factor-β pathway. Chem. Res. Toxicol. 28, 354−364 (2015). [DOI] [PubMed] [Google Scholar]
- Mohamed M. S. et al. Cytological and subcellular response of cells exposed to the type-1 RIP curcin and its hemocompatibility analysis. Sci. Rep. 4, 5747 (2014). [Google Scholar]
- Wang Y. et al. Direct imaging of titania nanotubes located in mouse neural stem cell nuclei. Nano Res. 2, 543–552 (2009). [Google Scholar]
- Wan F. & Leonardo M. J. The nuclear signaling of NF-κB: current knowledge, new insights, and future perspectives. Cell Res. 20, 24–33 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. et al. Impact and mechanism of TiO2 nanoparticles on DNA synthesis in vitro. Sci. Chin. Ser. B Chem. 51, 367–372 (2008). [Google Scholar]
- Zhu R. R., Wang S. L., Zhang R., Sun X. Y. & Yao S. D. A novel toxicological evaluation of TiO2 nanoparticles on DNA structure. Chin. J. Chem. 25, 958–961 (2007). [Google Scholar]
- Chen H. W. et al. Titanium dioxide nanoparticles induce emphysema-like lung injury in mice. FASEB J. 20, 2393–2395 (2006). [DOI] [PubMed] [Google Scholar]
- De Haar C., Hassing I., Bol M., Bleumink R. & Pieters R. Ultrafine but not fine particulate matter causes airway inflammation and allergic airway sensitization to co-administered antigen in mice. Clin. Exp. Allergy. 36, 1469–1479 (2006). [DOI] [PubMed] [Google Scholar]
- Gurr J. R., Wang A. S., Chen C. H. & Jan K. Y. Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicol. 213, 66–73 (2005). [DOI] [PubMed] [Google Scholar]
- Veranth J. M., Kaser E. G., Veranth M. M., Koch M. & Yost G. S. Cytokine responses of human lung cells (BEAS-2B) treated with micron-sized and nanoparticles of metal oxides compared to soil dusts. Part. Fibre. Toxicol. 4, 2 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.