Abstract
Background
Although full costs (including direct and indirect costs) that incurred during the process of chemotherapy administration should be measured, many studies estimate only direct labor and medication costs associated with various chemotherapy delivery systems.
Objectives
To estimate the total costs for dispensing and administration of fluorouracil when administered with leucovorin, by intravenous infusion or bolus, using a microcosting approach from the perspective of a provider or health system.
Methods
A time-and-motion study was used to measure the time spent by (1) pharmacy staff in the handling, admixture, and dispensing of fluorouracil and (2) patients in the clinic. The study was performed at The Cancer Institute of New Jersey for an 8-month period. Costs of dispensing and administering fluorouracil were calculated per patient visit on the basis of resources used in the processing of fluorouracil and time spent by pharmacy staff and patient. All costs were standardized to 2005 dollars.
Results
A total of 275 observations were made, and 74 (26.9%) of these were associated with fluorouracil-based chemotherapy. Pharmacy staff spent an average of 11 minutes for bolus fluorouracil with leucovorin infusion (fluorouracil/LCV-IV) and 8 minutes for bolus fluorouracil with bolus leucovorin (fluorouracil/LCV-B). Patients who received fluorouracil/LCV-IV spent an average of 203 minutes in the clinic, whereas patients who received fluorouracil/LCV-B spent 110 minutes. The average cost of administering fluorouracil/LCV-IV was $933, which comprised drug costs ($279), dispensing costs ($189), and administration costs ($465). The average cost of fluorouracil/LCV-B was $474, which comprised drug costs ($65), dispensing costs ($141), and administration costs ($268).
Conclusions
This is the first study to formally demonstrate the high cost of administering the injectable form of fluorouracil chemotherapy with leucovorin, despite relatively low drug acquisition cost. Therefore, reimbursement rates for fluorouracil should be calculated in such a way that covers all costs, including overhead costs for the department.
Keywords: Keywords: Chemotherapy cost, Chemotherapy administration, Time-and-motion study, Microcosting method, Pharmacy dispensing cost
Background
Cancer is the second leading cause of death and accounts for 23% of all mortality in the United States.1 The total economic costs of cancer were estimated at $206.3 billion ($78.2 billion for direct medical costs, $17.9 billion for indirect morbidity costs, and $110.2 billion for indirect mortality costs) in 2006.2 Chemotherapy is a frequent component of cancer care, and its costs can be substantial to third-party payers. 5-fluorouracil (fluorouracil), administered alone or as a part of a combination therapy, is the basis of cancer treatment regimens for a variety of solid tumors and produces desirable functional outcomes.3,4 As a cytotoxic drug that potentiates the effects of radiation therapy, fluorouracil is also used extensively in cancers of the breast, esophagus, rectum/anus, head and neck, pancreas, cervix, stomach, and bladder.5–9
Providing cost-effective cancer treatment has far-reaching implications for both patients and the institution. As the expenses related to cancer treatment continue to rise, the adequacy of institutional billing and payment methods are also becoming increasingly important.10,11 Presently, reimbursements for providing cancer chemotherapy do not reflect the actual costs and thus remain a challenge for practitioners in private practice and in hospital outpatient departments. In 2006, reimbursement for medications in either setting by the Centers for Medicare and Medicaid Services (CMS) was based on the average sales price (ASP) plus 6%, with no provision for pharmacy handling, admixture, and dispensing costs (referred to as pharmacy dispensing costs from here forward).
Because professionals continue to search for ways to deliver chemotherapy to cancer patients as safely and efficiently as possible, it is equally as important to determine the total (ie, direct and indirect) costs using precise and appropriate methods. Fluorouracil is commonly used for a variety of cancers with a relatively low drug acquisition cost in comparison to other cancer medications, but previous cost analyses conducted for treatment with fluorouracil have addressed only the direct costs associated with the treatment and have reported direct costs ranging from $1057 to 3120 per month (in 2005 US dollar).12–14 To our best knowledge, the full costs of fluorouracil therapy, which consider both direct and indirect costs of this regimen, have not yet been evaluated.
This study was conducted to estimate the full costs of fluorouracil when dispensed and administered with leucovorin intravenous (IV) infusion or bolus from the perspective of a health care provider, using a time-and-motion approach.
Methods
Study patients and data collection
The study included patients who visited the outpatient infusion clinic of The Cancer Institute of New Jersey, which is one of 39 National Cancer Institute-designated Comprehensive Cancer Centers in the United States. Patients treated with fluorouracil during the entire study period were observed to measure time spent from the beginning until the completion of fluorouracil chemotherapy using a time-and-motion study.15 The time spent by the pharmacy was measured from the beginning of preparation to the completion of fluorouracil dispensing for each patient.
A pilot study was conducted to estimate the amount of time spent by 14 patients to receive fluorouracil therapy to determine the number of observations needed for the main study. From the pilot study, it was determined that at least 66 observations would be needed to establish the mean time for handling, admixing, dispensing, and administering fluorouracil chemotherapy, with a 95% confidence level at a significance level of 5%.15 We observed patients who received a fluorouracil-based regimen to measure their time spent undergoing chemotherapy and continued this process until 82 observations were obtained between November 1, 1998, and July 30, 1999. We also observed pharmacy staff to determine time associated with chemotherapy dispensing for these patients.
The sample included all patients who received care at the outpatient infusion clinic from June 14, 1999, to June 18, 1999 (1 week), to measure the total amount of time spent by all of those patients. A total of 275 patients spent a total of 34,617 minutes to receive care. The total time spent for dispensing chemotherapy by pharmacy staff during the same week (pharmacy staff spent a total of 1921 minutes) was also measured. This specific week was selected after consulting a panel of nursing and pharmacy staff, and it was determined that the number of patients in this week was most representative of the mean of weekly totals of patients treated during the past 6 months at the study site. These total minutes spent by patients were used as a denominator to calculate per minute cost associated with chemotherapy administration within the nursing department, and total minutes spent by pharmacy staff were used to calculate per minute cost for chemotherapy dispensing within the pharmacy department, respectively.
A time-and-motion study, which is a recommended approach for use of a microcosting method, identifies in detail and precisely measures all resources used in the production process.16,17 It was used to collect resources in the process of fluorouracil therapy and the time spent by patients and pharmacy staff. The time spent by a patient to receive fluorouracil chemotherapy was used to calculate per minute cost for administration of fluorouracil chemotherapy. The purchase prices of prescription drugs and supplies as well as detailed financial information of The Cancer Institute of New Jersey were obtained from its accounting, pharmacy, and nursing departments. The study was reviewed and approved by the UMDNJ/Robert Wood Johnson University Hospital Institutional Review Board.
Definition of dispensing and administration costs
The full cost of dispensing and administering fluorouracil chemotherapy included both direct costs and indirect costs. Direct costs (ie, drug, supply, and direct labor costs) were defined as those costs incurred at the pharmacy and nursing departments during the process of fluorouracil dispensing and administration. Indirect costs were defined as departmental overhead costs occurred at the pharmacy and nursing departments to support clinical staff’s chemotherapy and institute overhead costs incurred by supporting/service departments (excluding pharmacy and nursing departments) to facilitate the activities of operating departments.18,19 A detailed definition of costs and source of cost information is presented in Table 1.
Table 1.
Definitions of costs and sources of cost information
| Cost type | Definition | Sources of cost information | Basis of cost estimation method |
|---|---|---|---|
| Drug | Cost of fluorouracil and other medications administered concurrently with fluorouracil plus costs associated with wastage due to prepackaged volumes and nonadministration of chemotherapy due to side effects. | Purchasing prices from pharmacy department | Drugs used |
| Supplies | Cost of all disposable supplies used (IV catheter, needle, syringe, central ports, etc) during the preparation and administration of the study medications and costs associated with wastage of supplies. | Purchasing prices from pharmacy and nursing departments | Supplies used |
| Direct labor | Salaries and fringe benefits for clinical staff who administer or dispense chemotherapy (ie, nurses, nurse aides, pharmacists, and pharmacy technicians) directly involved in fluorouracil preparation and administration. | Salaries for clinical staff from pharmacy and nursing departments | Time spent by patients or pharmacy staff |
| Departmental overhead | Departmental overhead costs incurred in the nursing and pharmacy departments for the support of fluorouracil chemotherapy activities. These costs include cost of supervisory and administrative personnel, departmental equipment (computer, copy machines, etc), office supplies and telephone, educational activities, etc. | Pharmacy and nursing departmental annual budget (except budget for drug, suppliers, and clinical staff’s salaries) | Time spent by patients or pharmacy staff |
| Institute overhead | Costs not directly traceable to fluorouracil administration but deemed necessary in supporting the operation of the pharmacy and nursing departments. Institute overhead costs included expenses generated by the supporting departments excluding pharmacy and nursing departments (administration, accounting, computers, patient services including reception desk and waiting room, purchasing etc) and facility-related costs (utilities, building maintenance, janitor, building insurance, etc). | Annual budget of supporting departments from the study site’s accounting department | Time spent by patients or pharmacy staff |
IV, intravenous.
Cost calculation
The costs of dispensing and administering fluorouracil were calculated on a per patient visit basis. The drug and supply costs were calculated using acquisition costs at the study site. The direct labor cost associated with fluorouracil administration (ie, nursing department) was calculated as follows:
Equation (1) = Weekly paid salaries including fringe benefits (ie, 28%) for relevant personnel directly involved in the process of fluorouracil administration (ie, annual salaries divided by 34.2 weeks on the assumption that the clinic operates 252 days a year and provides 12 days of paid vacation per employee per year)
Equation (2) = Total observed time spent by all patients to receive fluorouracil chemotherapy during 1 week (ie, 34,621 minutes)
Equation (3) = Direct labor cost per minute was calculated by dividing (Equation 1) by (Equation 2)
Equation (4) = Direct nursing labor cost for each patient was obtained by multiplying direct nursing labor cost per minute (Equation 3) by the time spent by each patient
Nursing labor costs per minute were estimated by dividing weekly nursing salary by total minutes spent by patients (ie, total minutes served by nursing staff). This method was developed to consider variations in nursing staff services provided to patients and to account for clinical staff providing care to several patients simultaneously under the assumption that the study site served the maximum number of patients at any one time to fully use the capacity of nursing department. This assumption was made because it is common for institutes to use the nursing staff at maximum capacity, as the nurses and nurse aids are paid regardless of the number of patients they served.
Departmental overhead costs included the costs accrued to support chemotherapy administration, and these costs were calculated as follows: first, we calculated per minute departmental overhead cost by dividing the weekly nursing departmental budget (except salaries for clinical staff who administered chemotherapy) by the total amount of time spent by all patients for 1 week. Then, departmental overhead cost per patient was calculated by multiplying per minute departmental overhead cost by the time spent by each patient.
Equipment cost per minute, which was calculated by dividing the annual cost of equipment by the total amount of time spent by all patients while receiving fluorouracil therapy. Annual costs of equipment were determined using the length of useful life of the equipment, purchase price, and maintenance costs. The purchasing prices were obtained from the study site, and the costs were amortized over the useful lifetime to estimate the annual cost, based on the assumption of no salvage value of the equipment by the time of replacement. This method incorporates the opportunity cost and the depreciation aspect of the capital cost.18 Then, equipment cost per patient was calculated by multiplying per minute equipment cost by the time spent by each patient.
Less technology-related equipment (patient IV chairs and tables, furniture, refrigerator, etc) was assumed to have a useful lifespan of 10 years, and electronic equipment was assumed to have a lifespan of 5 years (for computers and copy/fax machines) and 3 years (for blood pressure and thermometer machines). To calculate the capacity of the equipment, the following 2 assumptions were made: the equipment was used 40 hours per week for 50 weeks per year, and the equipment was operated at 80% of its capacity at the study site.19
The institute overhead costs were estimated based on the following procedures: first, 18% of the weekly budget for supporting departments at the institute (based on the amount suggested by the institute’s accounting department) was allocated to the nursing department. Then, the institute overhead cost per minute was calculated by dividing the weekly indirect expenses by total minutes spent receiving therapy by all patients in 1 week. The institute overhead cost for each patient was calculated by multiplying per minute overhead cost by time spent by each patient.
Likewise, the costs of labor, direct departmental overhead, and institute overhead (14% of the institute budget, as suggested by the accounting department) for the pharmacy department were estimated on the basis of time spent by the pharmacy staff on dispensing fluorouracil (total minutes spent by pharmacies and technicians for 1 week: 1921 minutes), which included the handling and admixing of fluorouracil and leucovorin for study patients.
Statistical analysis
Means, SDs, and medians were calculated for the time spent by patients in the ambulatory infusion clinic as well as for the time required by the pharmacy staff in dispensing fluorouracil chemotherapy. Because time spent per patient and costs per fluorouracil chemotherapy were highly skewed due to the variable nature of the duration of infusion for individual patients, a 95% confidence interval for average time and costs associated with dispensing and administering fluorouracil was calculated using the bootstrap method with a resampling of 1000 times.20 The dollar values were converted to 2005 dollars using the consumer price indexes for medical care, prescription drugs, and medical supplies accordingly.21 All data analyses were performed using SAS (SAS Institute Inc., Cary, NC) statistical software.22
Results
Characteristics of patients
The characteristics of the patients who received fluorouracil chemotherapy with leucovorin during the study period are shown in Table 2. A total of 82 observations of dispensing and administration activities associated with fluorouracil chemotherapy were collected during the study period. For the calculation of the full cost associated with fluorouracil chemotherapy, 3 patients received other chemotherapy (eg, methotrexate, carboplatin, or etoposide) orally or subcutaneously before fluorouracil chemotherapy and 8 patients who did not receive chemotherapy as scheduled because of side effects of the treatment (eg, low white blood cell counts, low platelet counts) were excluded.
Table 2.
Characteristics of study patients who received fluorouracil chemotherapy
| Category | Leucovorin IV | Leucovorin bolus | Total |
|---|---|---|---|
| No. of observations | 53 | 18 | 71 |
| Age (mean ± SD), yr | 68 ± 10 | 53 ± 5 | 63 ± 11 |
| Sex, n (%) | |||
| Male | 29 (55) | 1 (6) | 30 (42) |
| Female | 24 (45) | 17 (94) | 41 (58) |
| Insurance, n (%) | |||
| Private insurance or HMO | 9 (17) | 18 (100) | 27 (38) |
| Medicare | 43 (81) | 0 (0) | 43 (61) |
| No insurance | 1 (2) | 0 (0) | 1 (1) |
| Type of cancer, n (%) | |||
| Colon and rectum | 44 (83) | 17 (94) | 61 (86) |
| Gastric | 7 (13) | 0 (0) | 7 (10) |
| Others | 2 (4) | 1 (6) | 3 (4) |
| Race, n (%) | |||
| Caucasian | 26 (49) | 16 (89) | 42 (59) |
| African American | 8 (15) | 0 (0) | 8 (11) |
| Latino American | 8 (15) | 2 (11) | 10 (14) |
| Others | 11 (21) | 0 (0) | 11 (15) |
HMO, health maintenance organization.
The overall average age of patients being treated with fluorouracil was 63 years; patients received leucovorin as an infusion averaged 68 years in age, whereas patients received leucovorin as a bolus averaged 53 years in age. Forty-two percent of the observations involved male patients, whereas 61% of the observations involved patients who were covered by Medicare. Because 86% of patients had colorectal cancer, it is likely that the higher average age of patients receiving fluorouracil is because of the higher incidence of colorectal cancer in the elderly population. Most observations involved Caucasian patients (59%) followed by Latinos (14%).
Time spent on dispensing and administration of chemotherapy
Table 3 summarizes the time spent by patients who were treated with fluorouracil depending on administration route of leucovorin and the time spent by the pharmacy staff for handling, admixing, and dispensing, classified according to chemotherapy type.
Table 3.
Time spent per patient who received fluorouracil chemotherapy (minutes)a
| Variable measured | Fluorouracil with leucovorin-IV | Fluorouracil with leucovorin-B | Average |
|---|---|---|---|
| No. of observations (n) | 53 | 18 | 71 |
| Pharmacy dispensing time (min) | |||
| Mean (bootstrap 95% CI) | 11 (10–12) | 8 (7–10) | 11 (10–11) |
| Median | 10 | 7 | 10 |
| Interquartile range | 10–13 | 7–8 | 8–12 |
| Patient time for chemotherapy (min) | |||
| Mean (bootstrap 95% CI) | 203 (193–213) | 110 (97–124) | 180 (168–193) |
| Median | 210 | 113 | 185 |
| Interquartile range | 178–227 | 91–134 | 145–220 |
IV, intravenous; B, bolus; CI, confidence interval.
The table does not present 3 patients who received fluorouracil concurrently with other chemotherapies (eg, methotrexate, carboplatin, or etoposide).
The average of time spent by the pharmacy for the handling, admixing, and dispensing of fluorouracil was 11 minutes. The dispensing time of fluorouracil with leucovorin as an infusion was 11 minutes, whereas the time for fluorouracil with leucovorin as a bolus was 8 minutes.
Because fluorouracil was always given as a bolus injection at the study site at the time of this study, the differences in time spent receiving treatment were primarily because of the method of administering leucovorin. Patients spent an average of 110 minutes receiving treatment when their fluorouracil was given with leucovorin as a bolus injection. However, they spent almost twice as much time for therapy (203 minutes) when leucovorin was given by IV infusion, even though fluorouracil was administered as a bolus injection over approximately 4 minutes in both cases.
Dispensing and administration costs of chemotherapy with 5-fluorouracil
The various types of mean costs associated with fluorouracil chemotherapy are presented in Table 4. The different types of costs were calculated by multiplying the time spent by the cost per minute accordingly. The full costs associated with fluorouracil therapy were calculated by adding up the individual cost factors, which have already been discussed in the Methods section.
Table 4.
Costs of fluorouracil acquisition, dispensing, and administration
| Administration of chemotherapy | Fluorouracil with leucovorin IV | Fluorouracil with leucovorin bolus | ||
|---|---|---|---|---|
|
|
|
|||
| Mean | Bootstrap 95% CI | Mean | Bootstrap 95% CI | |
| Drug costs ($) | ||||
| Leucovorin | 272.26 | 263.32–283.35 | 58.07 | 58.07–58.07 |
| Fluorouracil | 6.67 | 6.30–6.86 | 6.61 | 6.61–6.61 |
| Subtotal | 278.92 | 269.98–289.96 | 64.68 | 64.68–64.68 |
| Dispensing costs ($) | ||||
| Supply | 6.27 | 6.07–6.52 | 5.20 | 4.89–5.62 |
| Direct labor | 36.04 | 33.79–39.75 | 26.84 | 23.44–33.64 |
| Departmental overhead | 26.10 | 24.47–28.78 | 19.44 | 16.98–24.23 |
| Institute overhead | 120.53 | 113.01–132.52 | 89.77 | 78.40–111.31 |
| Subtotal | 188.93 | 177.52–207.80 | 141.25 | 124.23–174.77 |
| Administration costs ($) | ||||
| Supply | 53.98 | 52.04–57.94 | 44.35 | 42.53–46.64 |
| Direct labor | 148.68 | 141.98–155.23 | 80.89 | 72.10–89.72 |
| Departmental overhead | 106.40 | 101.61–111.09 | 57.89 | 51.60–64.21 |
| Institute overhead | 156.00 | 148.88–162.88 | 84.88 | 75.70–94.31 |
| Subtotal | 465.07 | 445.89–483.01 | 268.00 | 242.89–293.10 |
| Total costs ($) | 932.93 | 907.88–955.97 | 473.93 | 436.90–518.80 |
IV, intravenous; CI, confidence interval.
This study found that the total cost of therapy varied depending on the modes of leucovorin administration. The full cost of providing fluorouracil with leucovorin chemotherapy was approximately $933 when leucovorin was administered as an infusion compared with $474 when leucovorin was given by bolus injection. The cost of providing fluorouracil therapy was predominantly related to drug administration, which accounted for 50% of the costs for IV leucovorin and 57% for bolus leucovorin, respectively.
As presented in Table 4, the acquisition cost of fluorouracil was relatively low but a substantial difference in the costs of drugs was found between IV ($279) and bolus ($65) leucovorin. With regard to costs associated with the dispensing of the chemotherapy by the pharmacy staff, they were found to be generally consistent among the patient groups. The total cost of dispensing fluorouracil chemotherapy treatment was $189 for cancer patients given IV leucovorin and $141 for patients given bolus leucovorin. The direct pharmacy labor costs associated with fluorouracil and leucovorin infusion totaled $36, and similarly, the labor cost associated with fluorouracil and leucovorin bolus was $27. However, the institute overhead was $120.53 for leucovorin infusion and $89.77 for leucovorin bolus when the institute overhead costs assigned to pharmacy department were allocated based on the time spent for fluorouracil dispensing by pharmacists and technicians.
Furthermore, the direct labor cost of nursing administration was considerably higher when the leucovorin was administered by infusion ($149) rather than by bolus injection ($81). The differences in overhead costs were also observed between the administration modes, with departmental overhead costs of nursing administration amounting to $106 for leucovorin infusion and $58 for leucovorin bolus injection. Similar to dispensing costs, the institute overhead cost was a major component of the total nursing administration costs. Institute overhead costs were $156 for leucovorin given by infusion and $85 for leucovorin given as a bolus injection.
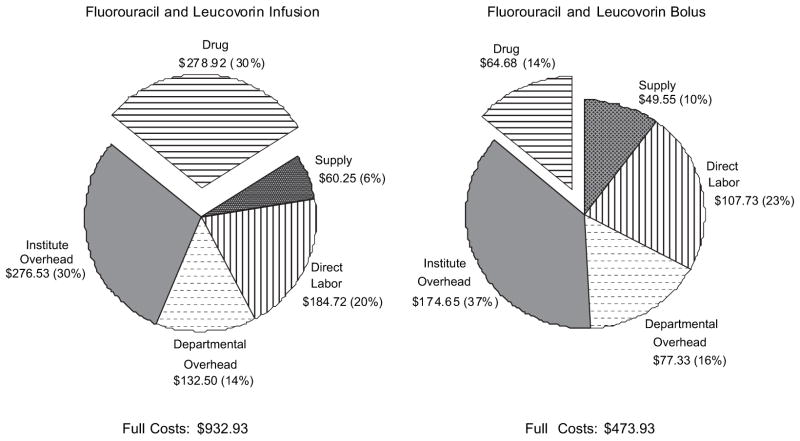
Fig. 1 illustrates the relative proportions for each type of cost associated with fluorouracil chemotherapy. Drug costs accounted for 30% of total costs when leucovorin is given as an infusion and 14% of total costs when leucovorin is given as a bolus injection. The total overhead costs, including the departmental overhead costs at the nursing and pharmacy departments and the institute overhead costs, accounted for 44% of total costs for infusion leucovorin and 53% for bolus leucovorin.
Fig. 1.
Proportion of costs associated with fluorouracil chemotherapy.
Discussion
The present study measured the time spent by patients and pharmacy staff on fluorouracil chemotherapy and calculated the cost of resources used in the process of dispensing and administering fluorouracil using a time-and-motion study. Cancer patients who received bolus fluorouracil spent about 2–3 hours in the clinic depending on the type of leucovorin administration. The average time spent on dispensing chemotherapy by pharmacy staff was about 8–11 minutes, depending on the route of leucovorin administration. The full cost of dispensing and administering fluorouracil was $933 per visit when IV leucovorin was administered and $474 per visit when bolus leucovorin was administered. The acquisition cost of fluorouracil with leucovorin for the treatment of colorectal cancer is insignificant compared with the costs of other injectable drugs such as irinotecan, oxaliplatin, bevacizumab, or cetuximab.23 Therefore, basing the pharmacy fees for handling, admixing, and dispensing a drug on the acquisition cost of the product rather than the actual cost of this process is flawed24 because the costs for pharmacy preparation substantially exceeded the acquisition costs in this study.
CMS currently reimburses medications based on the acquisition cost (ie, estimated by ASP plus 6%) without considering pharmacy handling costs, which are classified as pharmacy labor and departmental overhead costs in our study.25,26 However, our study reveals that these costs accounted for approximately 44–53% of total chemotherapy costs depending on whether leucovorin was administered as infusion or bolus. CMS reimbursement presents a unique financial challenge in the hospital outpatient department because most cancer therapies require reconstitution and preparation by the pharmacy before infusion, which can require a lengthy amount of time, and use specialized safety equipment in accordance with mandates such as the US Pharmacopeia document 797 (USP 797). Such mandates have resulted in significant economic burdens to pharmacies attempting to be in compliance with these requirements.27 Another organization, the National Institute of Occupational Safety and Health (NIOSH), is tasked to enforce safety in pharmaceutical workplaces and released updated guidelines for preventing occupational exposure to antineoplastic and other hazardous drugs in health care settings in September 2004. Because these guidelines require the use of a closed system device to minimize exposure, compliance with both USP 797 and NIOSH results in the pharmacy incurring increased handling and preparation costs. Both of these mandates were not in place when our study was performed. Therefore, this study’s estimated costs for the handling, admixing, and dispensing by the pharmacy may be artificially low.
The direct costs calculated in this study were compared with those of previous studies that estimated direct costs in most instances. Based on the assumption that patients receive fluorouracil chemotherapy for 5 days per month, we extrapolated the full cost of fluorouracil chemotherapy to $4665 per patient per month (including direct costs of $2619) with infusion leucovorin and $2370 per patient per month (including direct costs of $1110) with bolus leucovorin.
A study comparing the costs of infusion versus bolus chemotherapies found the direct cost of bolus fluorouracil and leucovorin to be $1250 per month (equivalent to $1770 per month and $354 per visit in 2005 US dollars) when they were given on a daily schedule for 5 days at 5-week intervals and $2081 per month (equivalent to $2947 per month and $736 per visit in 2005 US dollars) when they were administered weekly.12 The previous study included direct costs only; thus, it should be expected that our estimations were slightly higher because the present study attempted to measure the full costs of chemotherapy treatment by including departmental and institute overhead costs.
Costs of IV fluorouracil and leucovorin in colorectal cancer patients were also calculated in other countries.13,14,28 In Israel, the cost of IV fluorouracil and leucovorin given for 5 days per month was estimated to be $2203 (equivalent to $3120 per month and $625 per visit in 2005 US dollars) for drug costs and clinical services.13 In Canada, the average cost of fluorouracil per patient per cycle was Can$1507-$2274 (equivalent to $1571-$2370 in 2005 US dollars) from the hospital perspective.28 However, the results of these studies cannot be directly compared with those of our study, because in other studies, fluorouracil was administered as an infusion as opposed to bolus injection and not all direct and indirect costs were included. Another study found fluorouracil (425 mg/m2) plus low-dose leucovorin (20 mg/m2) given by bolus injection daily for 5 days repeated every 28 days to have a mean direct cost of £462 per month (equivalent to $1057 per month and $211 per visit in 2005 US dollars).14 Because their study considered patient-specific variable costs and fixed costs of inpatient stays and operations from the hospital viewpoint, our results are found to be very comparable, after selecting equivalent cost components of direct costs from their study. A recent study conducted in England estimated the direct medical costs of fluorouracil with bolus leucovorin to be £959 per cycle (equivalent to $1799 per cycle and $360 per visit in 2005 US dollars) to the England National Health Service.29 All these studies presented the direct costs associated with fluorouracil chemotherapy administration with a wide range of variation.
The present study calculated full costs of fluorouracil chemotherapy per visit using a time-and-motion method as a microcosting approach. Because microcosting is an approach that most precisely estimates and measures all resources used in the production process, this approach is particularly well suited when the purpose of a study is to calculate marginal use of resources among alternatives.16,17,30,31 The actual time spent on chemotherapy by the clinical staff underestimates the total time spent caring for patients because it may not take into account the time spent on cognitive services alongside drug administration such as evaluation of appropriate dose, drug interactions, and laboratory values for appropriateness of care, or patient education and counseling that are not directly related to dispensing of fluorouracil chemotherapy. To allocate the time spent on these types of activities performed by both the pharmacy and the nursing staff to the actual time spent, our study measured time spent on dispensing by the pharmacy as well as the patients’ time spent receiving chemotherapy provided by the nursing staff.15,32
To the authors’ best knowledge, this was the first study calculating the full costs (including direct and indirect costs) of fluorouracil dispensed and administered with leucovorin, thus demonstrating the importance of considering indirect costs for estimation of reimbursement from third-party payers. We estimated per minute nursing costs using patient time spent on receiving chemotherapy rather than nurse time spent on patient care because total patient time reflects real productivity of the nursing department and reimbursement is determined based on how long a patient receives services. In addition, per minute pharmacy cost was estimated by total pharmacy staff time spent on dispensing to measure real productivity at the pharmacy department. Because of the common use of fluorouracil, pharmacy directors or financial administrators in different facilities may be interested in learning more about the importance of overhead costs for chemotherapy. The results of this study will assist them in negotiating reimbursement rates, managing personnel time, and processing budgets.
The method of reimbursement for oncology therapies remains controversial. The Prospective Payment Assessment Commission reported that Medicare and Medicaid underestimated true costs by covering only 89% and 93%, respectively, of what hospitals spent for purchasing oncology treatments.33 However, this was estimated before the current reimbursement rates of the CMS. Between 1991 and 1996, Medicare reimbursement for administration of chemotherapy decreased approximately 57%, which was partially because of the elimination of payment for chemotherapy administration supplies as well as reimbursement barriers secondary to clinical investigation and “off-label” usage.11,34 It has also been shown that considerable geographical variability exists in the reimbursement for chemotherapy treatment.35
It has been suggested that considerable cost savings can be realized by using oral dosage formulations rather than parenteral chemotherapy. 28,36,37 However, the advent of new oral chemotherapy agents with a much higher drug acquisition cost creates the need for estimation of total costs, including administration and overhead costs, to compare the net financial impact of oral chemotherapy with IV chemotherapy.38
The present study is subject to a number of limitations. The results of the study are based on a single institution, and overhead costs, salaries, and drug procurement costs will differ across institutions. In addition, because a few patients required particularly expensive materials at every treatment (eg, central ports), a larger sample of patients may have provided a better estimate of the direct costs of materials, although the cost of materials is often small compared with the total costs of treating a patient. Despite using the microcosting method, we did not estimate marginal costs of fluorouracil chemotherapy by a different dosing regimen of leucovorin (low vs high dose), by different infusion rates, or by use of different chemotherapies besides fluorouracil. However, drug costs in our study can be replaced with those of other regimens to obtain analogous estimates of total costs for other regimens. In addition, this study presented nontrivial fixed cost per patient encounter because our estimation was based on the assumption that costs were linear in time spent for chemotherapy. The constant costs per unit of time may bias the cost estimates for treatments of longer duration upward and those for shorter treatments downward. Finally, one significant limitation of the time study is that there is a potential for observer-induced bias in which participants change their behavior while being directly observed.39
Conclusions
This study measured the costs associated with dispensing and administration of fluorouracil chemotherapy in outpatients using the microcosting approach. The average full cost of administering one dose of fluorouracil and leucovorin chemotherapy was approximately $932 when leucovorin was infused and nearly half as expensive ($474) when leucovorin was given by bolus injection. This study demonstrates the importance of accurately identifying and measuring chemotherapy costs by the method of leucovorin administration rather than estimating average costs of IV chemotherapy. This study also demonstrates that the injectable chemotherapeutic treatment regimen of fluorouracil with leucovorin is costly to provide, even though the acquisition cost of this formulation is low.
Acknowledgments
This study was supported in part by an unrestricted grant from Bristol-Myers Squibb, Co, and The Cancer Institute of New Jersey’s National Cancer Institute Comprehensive Cancer Center Grant P30 CA72720-01-03. The authors thank Dr Joice Huang, Dr Gerry Oster, and Mr Thomas Delea for their valuable comments and recommendations in the designing of this study.
References
- 1.Centers for Disease Control and Prevention. Health, United States, 2006 With Chartbook on Trends in the Health of Americans. Hyattsville, MD: 2006. [Google Scholar]
- 2.American Cancer Society. Cancer Facts and Figures. Atlanta, GA: American Cancer Society; 2007. [Google Scholar]
- 3.Rich TA, Shepard RC, Mosley ST. Four decades of continuing innovation with fluorouracil: current and future approaches to fluorouracil chemoradiation therapy. J Clin Oncol. 2004;22:2214–2232. doi: 10.1200/JCO.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Moertel CG. Chemotherapy for colorectal cancer. N Engl J Med. 1994;330(16):1136–1142. doi: 10.1056/NEJM199404213301608. [DOI] [PubMed] [Google Scholar]
- 5.Thomas CT, Ammar A, Farrell JJ, Elsaleh H. Radiation modifiers: treatment overview and future investigations. Hematol Oncol Clin North Am. 2006;20:119–139. doi: 10.1016/j.hoc.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Sato Y, Takayama T, Sagawa T, Okamoto T, Miyanishi K, Sato T. Aphase I/II study of nedaplatin and 5-fluorouracil with concurrent radiotherapy in patients with esophageal cancer. Cancer Chemother Pharmacol. 2006;58:570–576. doi: 10.1007/s00280-006-0193-x. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt J, Patrut EM, Ma J, Jager D, Knaebel H-P, Buchler MW. Immunomodulatory impact of interferon-alpha in combination with chemoradiation of pancreatic adenocarcinoma. Cancer Immunol Immunother. 2006;55:1396–1405. doi: 10.1007/s00262-006-0140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Q-T, Taira A, Budenz S, et al. Mature results from a randomized phase II trial of cisplatin plus 5-fluorouracil and radiotherapy with or without tirapazamine in patients with resectable state IV head and neck squamous cell carcinomas. Cancer. 2006;106:1940–1949. doi: 10.1002/cncr.21785. [DOI] [PubMed] [Google Scholar]
- 9.Fiore FD, Lecleire S, Rigal O, et al. Predictive factors of survival in patients treated with definitive chemoradiotherapy for squamous cell esophageal carcinoma. World J Gastroenterol. 2006;12(26):4185–4190. doi: 10.3748/wjg.v12.i26.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerr D, O’Connor K. The costs of managing advanced colorectal cancer: a broad perspective. Anticancer Drugs. 1997;8:S23–S26. doi: 10.1097/00001813-199708002-00005. [DOI] [PubMed] [Google Scholar]
- 11.DeMario MD, Ratain MJ. Oral chemotherapy: rationale and future directions. J Clin Oncol. 1998;16(7):2557–2567. doi: 10.1200/JCO.1998.16.7.2557. [DOI] [PubMed] [Google Scholar]
- 12.Lokich JJ, Moore CL, Anderson NR. Comparison of costs for infusion versus bolus chemotherapy administration: analysis of five standard chemotherapy regimens in three common tumors—Part one. Model projections for cost based on charges. Cancer. 1996;78:294–299. doi: 10.1002/(SICI)1097-0142(19960715)78:2<294::AID-CNCR16>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 13.Ron IG, Lotan A, Inbar MJ, Chaitchik S. Advanced colorectal carcinoma: redefining the role of oral ftorafur. Anticancer Drugs. 1996;7:649–654. doi: 10.1097/00001813-199608000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Ross P, Heron J, Cunningham D. Cost of treating advanced colorectal cancer: a retrospective comparison of treatment regimens. Eur J Cancer. 1996;32A(Suppl 5):S13–S17. doi: 10.1016/s0959-8049(96)00334-6. [DOI] [PubMed] [Google Scholar]
- 15.Niebel BW, Freivalds A. Methods, Standards, and Work Design. 11. Burr Ridge, IL: McGraw-Hill; 2002. [Google Scholar]
- 16.Drummond MF, O’Brien B, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. 2. New York, NY: Oxford University Press; 1997. [Google Scholar]
- 17.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 18.United States Department of Health & Human Services. Determining the Unit Cost of Services. Washington, DC: Public Health Service; 1990. [Google Scholar]
- 19.Deakin EB, Maher MW. Cost Accounting. 3. New York, NY: McGraw-Hill; 1991. Professional Publishing. [Google Scholar]
- 20.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Boca Raton, FL: Chapman & Hall/CRC; 1993. [Google Scholar]
- 21. [Accessed 03.10.06];Consumer price indexes. 2006 Available at: http://www.bls.gov/cpi/
- 22.SAS Institute Inc. SAS/STAT User’s Guide, Version 8. Cary, NC: SAS Institute; 1999. [Google Scholar]
- 23.Schrag D. The price tag on progress—chemotherapy for colorectal cancer. N Engl J Med. 2004;351(4):317–319. doi: 10.1056/NEJMp048143. [DOI] [PubMed] [Google Scholar]
- 24.Medicare Payment Advisory Commission. Payment for Pharmacy Handling Costs in Hospital Outpatient Departments. Issues in a Modernized Medicare Program. Washington, DC: Medicare Payment Advisory Commission; 2005. pp. 137–155. [Google Scholar]
- 25.American Society of Clinical Oncology. [Accessed 07.09.06];CMS Releases Proposed Medicare Payment Regulations for 2007. 2006 Available at: http://www.asco.org/portal/site/ASCO.
- 26.Center for Medicare and Medicaid Services. [Accessed 06.09.06];Hospital Outpatient PPS. 2006 Available at: http://www.cms.hhs.gov/HospitalOutpatientPPS/01_overview.asp.
- 27.U.S. Pharmacopeia. [Accessed 03.10.06];USP<797>Guidebook to Proposed Revisions Pharmaceutical Compounding— Sterile Preparations Product Information. 2006 Available at: http://www.usp.org/USPNF/pf/generalChapter797.html.
- 28.Maroun JA, Asche C, Romeyer F, et al. A cost comparison of oral tegafur plus uracil/folic acid and parenteral fluorouracil for colorectal cancer in Canada. Pharmacoeconomics. 2003;21(14):1039–1051. doi: 10.2165/00019053-200321140-00004. [DOI] [PubMed] [Google Scholar]
- 29.Cassidy J, Douillard JY, Twelves C, et al. Pharmacoeconomics analysis of adjuvant oral capecitabine vs intravenous 5-FU/LV in Dukes’ C colon cancer: the X-ACT trial. Br J Cancer. 2006;94:1122–1129. doi: 10.1038/sj.bjc.6603059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castel LD, Bajwa K, Markle JP, Timbie JW, Zacker C, Schulman KA. A microcosting analysis of zoledronic acid and pamidronate therapy in patients with metastatic bone disease. Support Care Cancer. 2001;9:545–551. doi: 10.1007/s005200100249. [DOI] [PubMed] [Google Scholar]
- 31.Dranove D. Measuring costs. In: Sloan FA, editor. Valuing Health Care. New York, NY: Cambridge University Press; 1995. [Google Scholar]
- 32.Barnes RM. Motion and Time Study: Design and Measurement of Work. 7. New York, NY: John Wiley & Sons; 1980. [Google Scholar]
- 33.Ruchlin HS, Elkin EB, MacKenzie CR, Williams-Russo P, Allegrante JP. Determining the cost of a clinical intervention through the use of shadow pricing. Arthritis Care Res. 1997;10(5):343–351. doi: 10.1002/art.1790100509. [DOI] [PubMed] [Google Scholar]
- 34.Bailes JS. Payment and coverage issues affecting medical oncology. Breast Cancer Res Treat. 1993;25:119–126. doi: 10.1007/BF00662137. [DOI] [PubMed] [Google Scholar]
- 35.Cowan DH. The effect of Medicare reimbursement policies on the cost and setting of cancer chemotherapy. Cancer Invest. 1996;14(2):184–186. doi: 10.3109/07357909609018896. [DOI] [PubMed] [Google Scholar]
- 36.Scuplher M, Palmer MK, Heyes A. Costs incurred by patients undergoing advanced colorectal cancer therapy. Pharmacoeconomics. 2000;17(4):361–370. doi: 10.2165/00019053-200017040-00006. [DOI] [PubMed] [Google Scholar]
- 37.Jansman FG, Postman MJ, van Hartskamp D, Willemse PH, Brouwers JR. Cost benefit analysis of capecitabine versus 5-fluorouracil/leucovorin in the treatment of colorectal cancer in the Netherlands. Clin Ther. 2004;26(4):579–589. doi: 10.1016/s0149-2918(04)90060-4. [DOI] [PubMed] [Google Scholar]
- 38.Curtiss FR. Pharmacy benefit spending on oral chemotherapy drugs. J Manag Care Pharm. 2006;12(7):570–577. doi: 10.18553/jmcp.2006.12.7.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burke TA, McKee JR, Wilson HC, Donahue RMJ, Batenhorst AS, Pathak DS. A comparison of time and motion and self-reporting methods of work measurement. J Nurs Adm. 2000;30(3):118–125. doi: 10.1097/00005110-200003000-00003. [DOI] [PubMed] [Google Scholar]