Abstract
A recurring finding in autism spectrum disorder research is that head and brain growth is disproportionate to body growth in early childhood. Nordahl et al. (2011) demonstrated that this occurs in approximately 15% of boys with autism. While the literature suggests that brain growth normalizes at older ages, this has never been evaluated in a longitudinal study. The current study evaluated head circumference and total cerebral volume in 129 male children with autism and 49 age-matched, typically developing controls. We determined whether 3-year-old boys with brain size disproportionate to height (which we call disproportionate megalencephaly) demonstrated an abnormal trajectory of head growth from birth and whether they maintained an enlarged brain at 5 years of age. Findings were based on longitudinal, structural MRI data collected around 3, 4, and 5 years of age and head circumference data from medical records. At 3 years of age, 19 boys with autism had enlarged brains while 110 had brain sizes in the normal range. Boys with disproportionate megalencephaly had greater total cerebral, gray matter, and white matter volumes from 3–5 years compared to boys with autism and normal sized brains and typically developing boys, but no differences in body size. While head circumference did not differ between groups at birth, it was significantly greater in the disproportionate megalencephaly group by around 2 years. These data suggest that there is a subgroup of boys with autism who have brains disproportionate to body size and that this continues until at least 5 years of age.
Keywords: autism spectrum disorder, MRI, longitudinal, brain development, disproportionate megalencephaly
Introduction
Autism spectrum disorder (ASD) is a complex and heterogeneous neurodevelopmental disorder characterized by deficits in social communication and social interaction, as well as restricted and repetitive behaviors. While there has been substantial effort to determine alterations of neural structure and function in ASD [Anagnostou & Taylor, 2011], much is still not understood, particularly of the early stages of abnormal brain development. Previous head circumference and magnetic resonance imaging studies have suggested that there is a period of precocious brain growth in children with ASD [Courchesne, 2004; Courchesne & Pierce, 2005; Courchesne et al., 2007; Redcay & Courchesne, 2005a]. However, it is far less common to observe abnormal brain size in adolescents and adults with ASD [Aylward, Minshew, Field, Sparks, & Singh, 2002a; Aylward et al., 1999; Hardan, Muddasani, Vemulapalli, Keshavan, & Minshew, 2006; Herbert et al., 2004; Schumann et al., 2004]. The question arises, therefore, whether the abnormal trajectory of brain growth in ASD has both a progressive phase early on followed by a regressive phase that ultimately leads to normal brain size in adults with ASD. Alternatively, the proposed trajectory of brain growth from the ASD literature [Redcay & Courchesne, 2005a] may be an artifact due to analyses of cohorts with very different characteristics at different ages. Longitudinal studies spanning early childhood through adulthood are needed to clarify trajectories of neurodevelopment in ASD.
Increased total cerebral volume (TCV) in very young children with ASD has been replicated in a number of studies [Courchesne, Campbell, & Solso, 2011a; Courchesne et al., 2001; Hazlett et al., 2005; Piven, Arndt, Bailey, & Andreasen, 1996; Piven, Arndt, Bailey, Havercamp, Andreasen, & Palmer, 1995; Schumann et al., 2010; Sparks et al., 2002] and is supported by a comprehensive meta-analysis that found a significant age by total brain volume interaction with larger brain size associated with early childhood in ASD [Sacco, Gabriele, & Persico, 2015]. However, most studies with adolescents or adults with ASD report no significant differences in TCV [Aylward et al., 1999; Aylward, Minshew, Field, Sparks, & Singh, 2002b; Greimel et al., 2013; Hallahan et al., 2009; Hardan et al., 2006, 2008; Herbert et al., 2004; Jou, Minshew, Keshavan, & Hardan, 2010; McAlonan et al., 2002, 2005; Schumann et al., 2004; Tamura, Kitamura, Endo, Hasegawa, & Someya, 2010; Tepest et al., 2010]. One study, using a cohort sequential (accelerated longitudinal) design, found increased whole brain volumes in children with ASD and reduced whole brain volumes in adolescents and adults [Lange et al., 2015]. Normal or reduced TCV in adolescents and adults, however, is not consistently observed. Four previous studies reported larger TCV in adolescents and adults with ASD [Freitag et al., 2009; Hazlett, Poe, Gerig, Smith, & Piven, 2006; Palmen et al., 2005; Piven et al., 1995]. In addition, three previous postmortem studies reported conflicting brain weight findings with one study reporting that four of six brains were megalencephalic [Bailey et al., 1998], another study reporting significantly increased brain weights in children with ASD but a nonsignificant reduction in brain weight in adults [Kemper & Bauman, 1998], and one study reporting all children and adults had brain weights that were normal for their age [Williams, Hauser, Purpura, DeLong, & Swisher, 1980]. These studies do not allow one to conclude whether abnormalities in brain size persist beyond early childhood in ASD.
We have been following the trajectory of brain growth in a large cohort of young children with ASD and age-matched typically developing controls as part of the UC Davis MIND Institute Autism Phenome Project (APP). We had previously shown that approximately 15% of the boys in this study demonstrated megalencephaly at the time of their first MRI scan at 3 years of age [Nordahl et al., 2011]. The remainder of the boys and virtually all of the girls had brain sizes within 1.5 standard deviations of the mean of the typically developing children. Megalencephaly generally refers to abnormal size of the brain. However, brain size can be confounded by body size, and there is evidence indicating that children with ASD are taller than typically developing children [Chawarska et al., 2011; Davidovitch, Patterson, & Gartside, 1996; Dissanayake, Bui, Huggins, & Loesch, 2006; Lainhart et al., 1997; Miles, Hadden, Takahashi, & Hillman, 2000]. Therefore, we have since revised our definition of this subgroup to include only children with brain size that is disproportionate to height or disproportionate megalencephaly (ASD-DM). Many of the APP children have now had two additional MRI scans at approximately 4 and 5 years of age. The goal of the current study was to determine whether those boys with ASD-DM at 3 years of age retained the enlarged brain size until 5 years of age or whether there was any indication that brain growth in the ASD-DM boys was decelerating relative to the typically developing (TD) boys.
Materials and Methods
Participants
All aspects of the study protocol were approved by the University of California Davis Institutional Review Board, and informed consent was obtained from the guardian of each participant. At entry to the study, when the participant was between 2 and 3⅓ years of age, height, weight, and head circumference were measured. At Time 1, MRIs were carried out on 129 ASD and 49 TD boys at a mean age of 3.1 years. Longitudinal MRIs were collected 1 year after baseline at Time 2 for 84 ASD and 39 TD returning boys (mean age 4.1 years). A third MRI was collected 1 year later at Time 3 (mean 5.3 years) for 65 ASD and 31 TD participants. Height, weight, and head circumference measurements were also collected at the Time 3 visit. Data from subsets of these participants have been reported previously [Nordahl et al., 2011, 2012, 2013].
A variety of diagnostic and neuropsychological assessments are carried out as part of the APP. Diagnostic measures included the Autism Diagnostic Observation Schedule-Generic (ADOS-G) [DiLavore, Lord, & Rutter, 1995; Lord et al., 2000] and the Autism Diagnostic Interview-Revised [Lord, Rutter, & Le Couteur, 1994]. Diagnostic criteria for ASD were based on DSM-IV and criteria established by the Collaborative Programs of Excellence in Autism network. Developmental quotient (DQ) was measured at Time 1 using the Mullen Scales of Early Learning (MSEL) [Mullen, 1995]. At Time 3 the MSEL and the Differential Ability Scale (DAS) [Elliot, 1990] (depending on the child’s language abilities) were used to measure DQ. In order to perform longitudinal analysis on cognitive data collected from the MSEL and DAS, a method of standardizing overall scores across the two measures was used. T-scores from both the MSEL and DAS subtests were converted to standardized scores with a mean of 100 and standard deviation of 15. These subtest scores were then averaged to obtain an overall standardized cognitive score. Additional behavioral measures collected at Time 3 include the Social Responsiveness Scale (SRS) [Constantino & Gruber, 2002], and the Vineland Adaptive Behavior Scales-II (VABS-II) [Sparrow, Cicchetti, & Balla, 1989]. The ADOS-G was administered at both Time 1 and Time 3. ADOS-G severity scores were calculated to allow for comparison of autism severity across all participants [Gotham, Pickles, & Lord, 2009]. Socioeconomic status information (maternal and paternal education, as well as total annual family income in dollars) was collected from each family.
TD boys were screened and excluded for ASD using the Social Communication Questionnaire (scores below 11) [Rutter, Bailey, & Lord, 2003]. TD boys were excluded if they had first-degree relatives with ASD. Medical interviews were conducted by a licensed pediatrician to rule out other neuropsychiatric disorders in the TD controls. TD boys included in the current study had developmental scores within two standard deviations on all scales of the MSEL. All boys included in the study (both ASD and TD) were native English speakers, ambulatory, and had no vision or hearing problems, or known genetic disorders. Children were not included in the study if they had physical contraindications to MRI (e.g., teeth braces).
Neuroimaging
MRI data were collected at the UC Davis Imaging Research Center using a 3T Siemens TIM Trio MRI scanner (Siemens Medical Solutions, Erlangen, Germany) with an 8-channel head coil. MRI scans were conducted during natural nocturnal sleep (see Nordahl et al. [2008] for a description of the protocol). T1-weighted three-dimensional sagittal magnetization prepared rapid acquisition gradient echo (MPRAGE) scans (TR = 2170 ms, TE = 4.86 ms, matrix size = 256 × 256, slice thickness = 1.0 mm, isotropic voxel size = 1 mm) were acquired for each child. T2-weighted images were also collected and reviewed by a neurologist for clinical abnormalities.
The longitudinal brain imaging began in October 2007. In August 2009, the Siemens 3T Trio MRI system was upgraded to a Trio TIM MRI system (see Nordahl et al. [2015] for more details). Throughout all scanning, a calibration phantom (ADNIMAGPHAM, Phantom Laboratory, Inc., Salem, New York) was scanned at the end of each MRI session using an MPRAGE pulse sequence matched to the study sequence and using the same landmark and shim as the corresponding participant to ensure accurate measurement of spatial characteristics of the MRI volume. A 3D image distortion map was derived to correct for hardware-induced geometric distortion and subject brain images were corrected using this map (Image Owl, Inc., Salem, New York). This mitigated the effect of the MRI upgrade on these volumetric analyses. All TCV measurements were made on distortion corrected images, as in Nordahl et al. [2012].
TCV and Subgroup Classification
Images were preprocessed by removing nonbrain tissue and correcting inhomogeneity. An automated template-based method was used to measure TCV (see Nordahl et al. [2011, 2012] for previous description). Gray matter and white matter volumes were estimated using FSL FAST (FMRIB’s automated segmentation tool) with partial volume estimation for more accurate quantification of subcortical tissue [Zhang, Brady, & Smith, 2001].
Any cutoff of a continuous variable is somewhat arbitrary. To operationalize our analyses, we adopted the definition of disproportionate megalencephaly (ASD-DM) as having a standardized ratio of TCV to height that was 1.5 standard deviations above the mean for the TD group. Based on this classification, at Time 1 the ASD participants were divided into two subgroups: ASD-DM and ASD-N.
Head Circumference
Head circumference (HC) was measured during the medical examinations conducted at Time 1 and Time 3. Retrospective HC measurements were also obtained from pediatric medical records (well-baby visits) for every subject. Birth HC measurements were obtained from labor and delivery records. A total of 1162 measurements (mean = 6.5 per subject, standard deviation = 4.12, range = 18) were collected from birth through 36 months of age. The HC measurements for the TD group fall within the interquartile ranges of the World Health Organization Child Growth Standards at each time point, thus ASD group comparisons were conducted relative to the current TD group sample. Retrospective reports of gestational age were collected from pediatric medical records.
Statistical Analyses
Groups were compared on age, height (age as a covariate) and weight (age as a covariate) using repeated measures analysis of variance (RM ANOVA). To determine whether subject attrition could systematically bias the longitudinal growth models for TCV, Little’s Missing Completely at Random (MCAR) test was conducted for all participants in the ASD and TD groups, as well as the ASD subgroups (ASD-N and ASD-DM) [Little, 1988]. The three subgroups were compared on DQ at Time 1 and Time 3 using ANCOVA, including age as a covariate. The two ASD subgroups (ASD-DM and ASD-N) were compared at Time 1 and Time 3 on behavioral and clinical measures (DQ, ADOS-G severity scores, SRS total t-score, VABS-II adaptive behavior composite score) using ANCOVA, including age as a covariate. Subscale scores from the ADOS-G, SRS, and VABS-II, and verbal and nonverbal DQ were also compared between groups (results did not differ from the total scores, so only the results from analyses including the total standard scores are reported here). Chi-square tests were conducted to compare the three subgroups on maternal education, paternal education, and annual family income. ANCOVAs were conducted to directly compare TCV, gray matter, and white matter content at each time point (age as a covariate) between all ASD and TD participants and between the three subgroups (ASD-N, ASD-DM, and TD). The distributions of standardized ratios of TCV to height were compared between ASD and TD groups using a two-sample Kolmogorov-Smirnov test. To compare body size and brain growth between groups, longitudinal height, HC, total gray matter volume, total white matter volume, and TCV measures were analyzed using linear and nonlinear mixed-effects models [Davidian & Giltinan, 1995; Littell, Stroup, Milliken, Wolfinger, & Schabenberger, 2006; Raudenbush & Bryk, 2002]. In the linear and nonlinear mixed effects models employed, there were some subjects who did not have MRI data collected at all three MRI time points. Preliminary analyses were conducted removing those subjects with any missing data. The main findings of these analyses related to the continuity of brain grown in the ASD-DM group were consistent with the models including all subjects regardless of missing data, thus the results of the models including all subjects are reported here. For the nonlinear mixed effects models of MRI measures and height, it is important for the intercept to fall within the range of the actual data collected in order to provide a meaningful comparison of brain size and body size between groups during early childhood. Thus, age was centered at the mean age of all boys at Time 1 (37.1 months). For analyses of HC, age was not centered because the starting point of data collection began with measurements collected at birth. The fit of the models was assessed with log likelihood and Bayesian information criterion (BIC) [Schwarz, 1978] (see Supporting Information Table 1). In all cases, smaller values indicate a better fit. For height, as there are only two data points collected, the linear model of growth is reported. For gray matter, white matter, and TCV, quadratic models achieved good fit and are reported here. The data for HC follow a clearly nonlinear pattern. To characterize HC, we fitted linear, quadratic, and exponential models [Kail & Ferrer, 2007; McArdle, Ferrer-Caja, Hamagami, & Woodcock, 2002]; the exponential model had the best statistical fit and is reported in the results. Pearson correlation coefficients were also calculated to determine the relationship between HC measurements and TCV at Times 1 and 3.
Results
Participant Characteristics
At Time 1, 19 ASD participants (14.7% of the total ASD sample) were classified as ASD-DM, while 110 ASD participants were considered to have TCV in the normal range (ASD-N). See Table 1 for total number of MRI scans acquired for each group at each time point, and group data for age, height, weight, and clinical measure scores. Based on a standard normal distribution, we would expect 6.7% of the total sample to fall above the 1.5 standard deviation cutoff. However, 14.7% of the ASD sample falls above 1.5 standard deviations for the ratio of TCV to height. Results of a two-sample Kolmogorov-Smirnov test revealed a trend level difference between the ASD and TD groups in the distribution of standardized ratios of TCV to height (D = 1.33, P = 0.058). The ASD distribution is characterized by a wider range of standard TCV/height ratios (ASD range = 6.18, TD range = 4.8) and more boys falling 1 standard deviation beyond the mean, compared to the TD distribution which has most boys falling within ±1 standard deviation of the mean (Fig. 1).
Table 1.
Participant Characteristics (Reported As Means (Standard Deviations)) and Number of MRIs Collected for Each Subgroup: Typically Developing (TD), ASD With Normal-Sized Brains (ASD-N), and ASD With Disproportionate Megalencephaly (ASD-DM)
| Time 1
|
Time 2
|
Time 3
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| TD | ASD-N | ASD-DM | TD | ASD-N | ASD-DM | TD | ASD-N | ASD-DM | |
| N | 49 | 110 | 19 | 39 | 69 | 15 | 31 | 54 | 11 |
| Age, mo. | 35.9 (4.6) | 37.5 (6.1) | 36.8 (5.4) | 49.0 (4.6) | 49.9 (5.5) | 49.7 (6.7) | 62.4 (4.9) | 63.6 (5.5) | 64.1 (6.5) |
| Age range | 27.2–44.0 | 25.7–53.6 | 26.0–46.2 | 40.8–56.7 | 39.8–59.5 | 37.4–58.3 | 53.4–69.8 | 52.8–75.8 | 52.0–74.0 |
| Height, in. | 36.8 (2.4) | 37.5 (2.5) | 37.1 (2.3) | – | – | – | 44.9 (3.0) | 44.6 (3.6) | 44.7 (3.1) |
| Weight, lbs. | 33.1 (3.8) | 34.4 (8.0) | 33.8 (4.7) | – | – | – | 44.3 (5.6) | 45.1 (9.4) | 46.9 (7.0) |
| DQ | 105.5 (11.9) | 63.7 (21.3)* | 56.4 (22.4)* | – | – | – | 108.3 (5.8) | 84.4 (23.2)* | 72.7 (26.8)* |
| ADOS-G | – | 7.9 (1.7) | 7.7 (2.0) | – | – | – | – | 7.2 (2.0) | 6.6 (3.3) |
| SRS | – | 76.0 (13.2) | 81.4 (8.7) | – | – | – | – | 74.6 (10.9) | 76.7 (19.4) |
| VABS-II | – | 77.4 (11.2) | 69.3 (21.1)** | – | – | – | – | 77.2 (16.7) | 75.2 (22.1) |
| Maternal Education Bachelor’s degree or higher |
50.0% | 48.3% | 38.8% | – | – | – | – | – | – |
| Paternal Education Bachelor’s degree or higher |
43.4% | 42.0% | 61.1% | – | – | – | – | – | – |
| Annual Family Income $75,000 or greater |
61.2% | 45.4% | 40.0% | – | – | – | – | – | – |
Note. DQ: developmental quotient, ADOS-G: Autism Diagnostic Observation Schedule-Generic severity score, SRS: Social Responsiveness Scale total t-score, VABS-II: Vineland Adaptive Behavior Scale total composite score. Significant contrast between TD boys and ASD-N and ASD-DM boys
P < 0.001; significant contrast between ASD-N and ASD-DM
P < 0.05.
Figure 1.
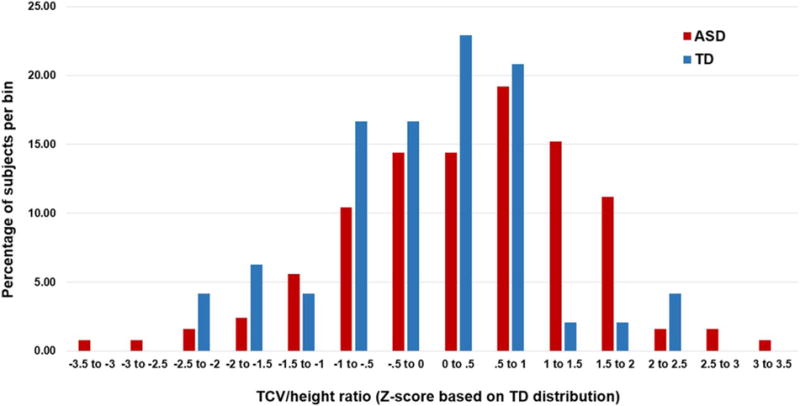
Distribution of subjects. The percentage of subjects in each bin for each group (ASD and TD) based on their standardized total cerebral volume (TCV) to height ratio. Z-scores are based on the TD distribution.
In our assessment of subject attrition, we found that the Little’s MCAR test indicated all missing TCV data are missing completely at random (TD X2 = 4.4, P = 0.49; all ASD X2 = 7.8, P = 0.16; ASD-N X2 = 6.9, P = 0.22; ASD-DM X2 = 6.1, P = 0.29). In other words, boys who did not return at Times 2 or 3 do not systematically bias group comparisons on brain size and growth.
There were no significant differences in age across the three MRI time points between any of the three groups. No significant differences were found between the three groups on height or weight across the Time 1 and Time 3 scans. There were also no significant differences in gestational age between the three groups (Table 1).
As expected, TD controls had significantly higher DQ than the two ASD subgroups at Time 1 (ASD-DM 56.4, sd 22.4; ASD-N 63.7, sd 21.3; TD 105.5, sd 11.9; F[2,174] = 86.24, P < 0.001) and Time 3 (ASD-DM 72.7, sd 26.8; ASD-N 84.4, sd 23.2; TD 108.3, sd 5.8; F[2, 84] = 17.1, P < 0.001). At Time 1, there were no significant differences between the two ASD subgroups for DQ (F[1,126] = 2.00, P = 0.15), ADOS-G severity score (F[1,126] = 0.15, P = 0.70), SRS total t-score (F[1,113] = 2.86, P = 0.093), but a significant difference in VABS-II adaptive behavior composite score (F[1,119] = 6.12, P = 0.015) such that the ASD-DM boys had poorer adaptive behavior skills. At Time 3 there were also no significant differences between the two ASD subgroups for DQ (F[1,56] = 1.78, P = 0.18), ADOS-G severity score (F[1,56] = 0.53, P = 0.46), SRS total t-score (F[1,45] = 0.18, P = 0.67), or VABS-II adaptive behavior composite score (F[1,49] = 0.086, P = 0.77). No significant differences were found in socioeconomic status between the three subgroups (maternal education, X2 = 0.67, P = 0.71; paternal education, X2 = 2.23, P = 0.32; annual family income, X2 = 2.43, P = 0.29).
TCV Cross-Sectional Findings
All ASD boys compared to TD boys
All ASD boys combined had significantly greater TCV than the TD boys at Time 1 (F[1,172] = 8.06, P = 0.005), Time 2 (F[1,116] = 7.74, P = 0.006), and Time 3 (F[1,91] = 5.22, P = 0.025). All ASD boys combined also had significantly greater total gray matter volume than TD boys at Time 1 (F[1,171] = 6.31, P = 0.013), Time 2 (F[1,116] = 6.52, P = 0.012), and Time 3 (F[1,89] = 7.05, P = 0.009), and significantly enlarged white matter volume at Time 1 (F[1,171] = 8.61, P = 0.004) and Time 2 (F[1,116] = 6.70, P = 0.011), but not at Time 3 (F[1,89] = 0.55, P = 0.45) (Table 2).
Table 2.
Group Means and Standard Deviations for Total Cerebral Volume, Total Gray Matter Volume, and Total White Matter Volume for Typically Developing (TD) Boys and All Boys With ASD
| Time 1
|
Time 2
|
Time 3
|
||||
|---|---|---|---|---|---|---|
| TD | ASD | TD | ASD | TD | ASD | |
| Total cerebral volume | 986.26 (76.89) | 1032.57 (91.07)* | 1028.56 (80.88) | 1082.36 (97.25)* | 1065.03 (81.20) | 1115.42 (96.84)** |
| Gray matter | 622.71 (45.11) | 647.66 (54.35)** | 633.16 (48.57) | 664.05 (61.05)** | 636.60 (49.88) | 671.82 (57.64)*** |
| White matter | 363.51 (34.13) | 384.90 (40.26)*** | 395.40 (40.51) | 418.30 (42.60)** | 433.71 (47.18) | 443.59 (45.69) |
Note. Significant contrast between TD and ASD
P < 0.01,
P < 0.05,
P < 0.005.
Subgroup comparisons
The ASD-DM group had significantly greater TCV than the ASD-N and TD groups at Time 1 (F[2,171] = 39.25, P < 0.001), Time 2 (F[2,115] = 29.59, P < 0.001), and Time 3 (F[2,90] = 26.01, P < 0.001). The ASD-DM group also had significant enlargement at all three time points in total gray matter volume (Time 1: F[2,170] = 41.08, P < 0.001; Time 2: F[2,115] = 26.39, P < 0.001; Time 3: F[2,88] = 23.45, P < 0.001), and total white matter volume (Time 1: F[2,170] = 28.39, P < 0.001; Time 2: F[2,115] = 20.52, P < 0.001; Time 3: F[2,88] = 14.89, P < 0.001).
The ASD-N group did not significantly differ from the TD group for TCV at any of the three time points (Time 1: F[1,153] =2 .28, P = 0.13; Time 2: F[1,102] = 2.24, P = 0.13; Time 3: F[1,80] = 1.10, P = 0.29). The ASD-N and TD groups also did not significantly differ at any of the time points on gray matter volume (Time 1: F[1,152] = 1.21, P = 0.27; Time 2: F[1,102] = 1.56, P = 0.21; Time 3: F[1,78] = 2.35, P = 0.12) or white matter volume (Time 1: F[1,152] = 3.26, P = 0.07; Time 2: F[1,102] = 2.21, P = 0.14; Time 3: F[1,78] = 0.20, P = 0.65). See Table 3 for subgroup means for TCV, total gray matter volume, and total white matter volume.
Table 3.
Group Means and Standard Deviations for Total Cerebral Volume, Total Gray Matter Volume, and Total White Matter Volume for Each Subgroup: Typically Developing (TD), ASD With Normal-Sized Brains (ASD-N), and ASD With Disproportionate Megalencephaly (ASD-DM)
| Time 1
|
Time 2
|
Time 3
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| TD | ASD-N | ASD-DM | TD | ASD-N | ASD-DM | TD | ASD-N | ASD-DM | |
| Total cerebral volume | 986.26 (76.89) |
1011.42 (75.20) |
1151.67 (81.59)*,** |
1028.56 (80.88) |
1056.10 (76.68) |
1208.02 (88.27)*,** |
1065.03 (81.20) |
1086.62 (72.36) |
1251.56 (82.06)*,** |
| Gray matter | 622.71 (45.11) |
634.46 (44.45) |
721.97 (44.85)*,** |
633.16 (48.57) |
648.08 (50.78) |
740.46 (47.35)*,** |
636.60 (49.88) |
655.52 (46.48) |
748.92 (40.74)*,** |
| White matter | 363.51 (34.13) |
376.95 (35.01) |
429.70 (39.74)*,** |
395.40 (40.51) |
408.02 (33.09) |
467.55 (49.52)*,** |
433.71 (47.18) |
431.10 (34.16) |
502.63 (48.44)*,** |
Note. Significant contrast between TD and ASD-DM
P < 0.001; significant contrast between ASD-N and ASD-DM
P < 0.001.
Rates of Brain Growth
All ASD boys compared to TD boys
In the mixed effects model analyses, across all subjects, TCV increased over time (t[209] = 21.00, P < 0.0001). The mixed effects model confirmed that all ASD boys combined had significantly greater TCV at Time 1 compared to TD boys (t[173] = 3.07, P = 0.002). The model indicated that the whole ASD group also had a slight but not significant increase in the rate of TCV growth compared to TD boys across Times 1 through 3 (t[207] = 1.70, P = 0.09) (Table 4 and Fig. 2). The model also confirmed that all ASD boys had significantly greater total gray matter volume compared to TD boys at Time 1 (t[176] = 3.24, P = 0.001), but not a different rate of growth compared to TD boys (t[213] = 0.91, P = 0.36). For white matter, the model indicated that the whole ASD group had both significantly greater white matter volume compared to TD boys at Time 1 (t[176] = 3.04, P = 0.002) and significantly greater white matter expansion across Times 1 through 3 (t[213] = 3.23, P = 0.001).
Table 4.
Parameter Estimates From Nonlinear Mixed Effects (Quadratic) Models Fitted to Total Cerebral Volume (TCV), Gray Matter, and White Matter Data for the Typically Developing (TD) Boys, and All Boys With Autism Spectrum Disorder (ASD). The MRI Data Were Collected at Time 1 (Mean Age 3.1 Years), 1 Year After Baseline at Time 2 (Mean Age 4.1 Years), and Approximately 1 Year Later at Time 3 (Mean 5.3 Years). For Each Quadratic Model, Age Was Centered at the Mean Age of Time 1 MRI Collection (37.1 Months)
| Measure | Parameter (SE) | Group
|
|
|---|---|---|---|
| TD | ASD | ||
| TCV | Intercept (cm3) | 991.08 (13.92) | 1033.81 (7.37)** |
| Linear slope | 3.86 (0.32) | 4.09 (0.18) | |
| Quadratic slope | −0.039 (0.010) | −0.057 (0.005) | |
| Gray matter | Intercept (cm3) | 625.67 (8.11) | 651.94 (4.27)** |
| Linear slope | 1.52 (0.31) | 1.45 (0.17) | |
| Quadratic slope | −0.042 (0.011) | −0.032 (0.006) | |
| White matter | Intercept (cm3) | 364.87 (5.82) | 382.60 (3.06)* |
| Linear slope | 2.23 (0.19) | 2.34 (0.11) | |
| Quadratic slope | −0.005 (0.007) | −0.028 (0.003)* | |
Note. Significant contrast between TD and ASD
P < 0.01,
P < 0.001.
Figure 2.
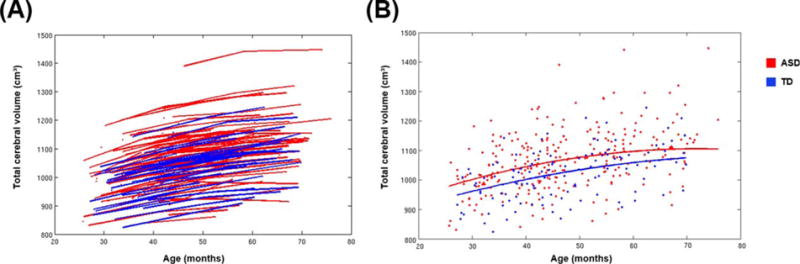
Results from longitudinal analyses of total cerebral volume. (A) Total cerebral volume data for ASD and TD subjects. Each line connects the data points collected from an individual subject. (B) Individual subject data are represented as individual data points. The lines represent the predicted growth trajectories of total cerebral volume for TD boys and all boys with ASD. All ASD boys combined had significantly greater TCV at the earliest age, and a slight but not significant increase in the rate of TCV growth over time, compared to TD boys.
Subgroup comparisons
The mixed effects model indicated that at Time 1 ASD-N boys did not significantly differ from TD controls in TCV (t[172] = 1.75, P = 0.08). Nor was there a significant difference in the overall rate of growth for TCV between the ASD-N and TD boys (t[205] = 1.17, P = 0.24). The mixed effects model did confirm that the ASD-DM boys had significantly greater TCV at Time 1 compared to both ASD-N and TD boys (t[172] = 8.65, P < 0.0001). Importantly, the longitudinal analysis indicated that the ASD-DM boys also had a significantly greater rate of growth between Times 1 and 3 compared to the TD boys (t[205] = 2.52, P = 0.01) and a marginally increased rate of growth compared to the ASD-N boys (t[138] = 1.88, P = 0.062) (see Table 5 and Fig. 3).
Table 5.
Parameter Estimates From Nonlinear Mixed Effects Models Fitted to Total Cerebral Volume (TCV), Gray Matter, and White Matter Data for the Typically Developing (TD), ASD With Normal-Sized Brains (ASD-N), and ASD With Disproportionate Megalencephaly (ASD-DM) Subgroups. The MRI Data Were Collected at Time 1 (Mean Age 3.1 Years), 1 Year After Baseline at Time 2 (Mean Age 4.1 Years), and 1 Year Later at Time 3 (Mean 5.3 Years). For Each Nonlinear Mixed Effects Model, Age Was Centered at the Mean Age of Time 1 MRI Collection, Thus the Reported Intercepts Correspond to 37.1 Months of Age
| Measure | Parameter (SE) | Group
|
||
|---|---|---|---|---|
| TD | ASD-N | ASD-DM | ||
| TCV | Intercept (cm3) | 991.02 (10.00) | 1012.20 (12.08) | 1154.90 (18.93)†,‡ |
| Linear slope | 3.88 (0.26) | 3.98 (0.33) | 4.70 (0. 51) | |
| Quadratic slope | −0.040 (0.008) | −0.052 (0.01) | −0.081 (0.01)*** | |
| Gray matter | Intercept (cm3) | 625.59 (5.96) | 640.07 (7.18)* | 719.69 (11.30)†,‡ |
| Linear slope | 1.54 (0.25) | 1.36 (0.31) | 1.96 (0.49) | |
| Quadratic slope | −0.043 (0.009) | −0.028 (0.01) | −0.055 (0.01) | |
| White matter | Intercept (cm3) | 364.85 (4.36) | 374.45 (5.25) | 429.46 (8.27)†,‡ |
| Linear slope | 2.23 (0.16) | 2.34 (0.20) | 2.38 (0.31) | |
| Quadratic slope | −0.0055 (0.006) | −0.028 (0.007)** | −0.023 (0.01) | |
Note. Significant contrast between TD and ASD-N
P < 0.05,
P < 0.01; significant contrast between TD and ASD-DM
P < 0.05,
P < 0.001; significant contrast between ASD-N and ASD-DM
P < 0.001.
Figure 3.
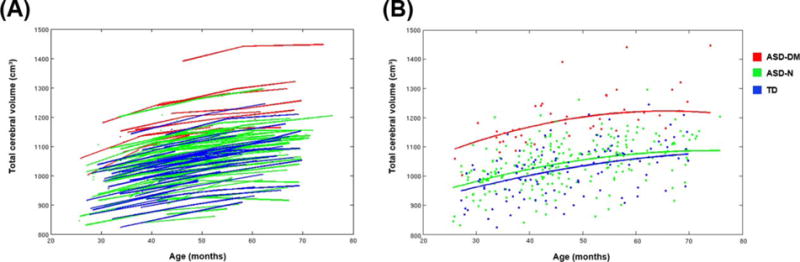
Results from longitudinal analyses of total cerebral volume for subgroups. (A) Total cerebral volume data for the three subgroups: ASD-DM, ASD-N, and TD boys. Each line connects the data points from each individual subject. The ASD-DM group (red) falls in the upper end of the distribution, while there is almost complete overlap between the ASD-N (green) and TD (blue) participants. (B) Individual subject data are represented as individual data points. The lines represent the predicted growth trajectories of total cerebral volume for each of the three subgroups, ASD-DM, ASD-N, and TD. ASD-DM boys had significantly greater TCV at the earliest age and also a significantly greater rate of growth from ages 3–5 years, compared to ASD-N and TD boys.
While the cross-sectional analyses yielded no significant difference in gray matter volume for the ASD-N group at Time 1, the mixed effects model indicated a greater total gray matter volume compared to TD controls at Time 1 (t[175] = 2.02, P = 0.045), but no significant difference in the rate of growth (t[211] = 1.27, P = 0.20). The mixed effects model indicated that the ASD-DM boys had significantly greater total gray matter volume compared to TD and ASD-N boys (t[175] = 8.33, P < 0.001) at Time 1 but the rate of growth was not predicted to be greater (t[211] = 0.70, P = 0.48).
The mixed effects model indicated that white matter volume in the ASD-N children did not differ from TD children at Time 1 (t[175] = 1.83, P = 0.07), but rate of growth was greater across Times 1 through 3 (t[211] = 3.26, P = 0.001). The model indicated that the ASD-DM boys had significantly greater total white matter volume compared to TD and ASD-N boys at Time 1 (t[175] = 7.81, P < 0.0001) and a marginally increased growth rate (t[211] = 1.67, P = 0.09).
Rate of HC Growth
All ASD boys compared to TD boys
The mixed effects model indicated that there were no differences between all ASD boys and TD boys for HC at birth (t[175] = 0.24, P = 0.80). But, the ASD boys had a significantly greater HC at 36 months (t[175] = 3.23, P = 0.001) and a significantly greater rate of growth (t[175] = 3.44, P = 0.007) (Table 6 and Fig. 4).
Table 6.
Parameter Estimates From the Nonlinear Mixed Effects (Exponential) Model Fitted to Head Circumference Data for the Typically Developing (TD) Boys, and All Boys With Autism Spectrum Disorder (ASD). Retrospective Head Circumference (HC) Measurements Were Obtained From Pediatric Medical Records (Well-Baby Visits) for Every Subject. The Exponential Model Was Based on a Total of 1162 Measurements (Mean = 6.5 Per Subject, Standard Deviation = 4.12, Range = 18) That Spanned Birth Through 36 Months of Age
| Model | Parameter(SE) | Group
|
|
|---|---|---|---|
| TD | ASD | ||
| Exponential | Asymptote (cm) | 49.67 (0.19) | 50.32 (0.13)* |
| Intercept (cm) | 34.93 (0.23) | 35.12 (0.18) | |
| Rate | 0.146 (0.004) | 0.160 (0.003)* | |
| Asymptote variance | 1.23 (0.31) | 1.45 (0.24) | |
| Intercept variance | 1.60 (0.49) | 2.27 (0.47) | |
| Rate variance | 0.0001 (0.0001) | 0.0002 (0.0001) | |
| Residual variance | 0.49 (0.04) | 0.71 (0.04) | |
Note. Significant contrast between TD and ASD
P < 0.01.
Figure 4.
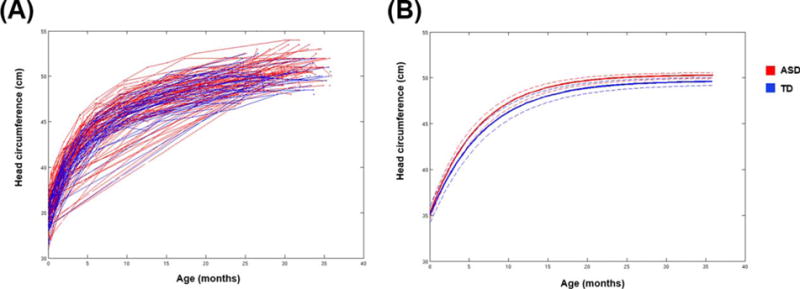
Results from longitudinal analyses of head circumference. (A) Head circumference data for ASD and TD subjects collected retrospectively from medical records. Each line connects the data points collected from an individual subject. A total of 1162 measurements (6.5 measurements per subject on average) were collected from medical records, spanning from birth to 36 months of age. (B) Predicted growth trajectories for head circumference for TD boys and all boys with ASD. Dashed lines indicate the lower and upper bounds of the 95% confidence intervals. There were no differences between ASD and TD boys in head circumference at birth, but the ASD boys had a significantly greater final overall head circumference and a significantly greater rate of growth
Subgroup comparisons
None of the subgroups significantly differed in HC at birth. However, the ASD-DM group reached a significantly larger head size at 3 years of age (Time 1) compared to both the ASD-N (t[126] = 4.40, P < 0.0001) and TD (t[65] = 6.01, P < 0.0001) groups. The ASD-N group also reached a significantly larger final head size compared to the TD group (t[165] = 2.16, P = 0.03) (Table 7 and Fig. 5).
Table 7.
Parameter Estimates From the Nonlinear Mixed Effects (Exponential) Model Fitted to Head Circumference Data for the Typically Developing (TD), ASD With Normal-Sized Brains (ASD-N), and ASD With Disproportionate Megalencephaly (ASD-DM) Subgroups. Retrospective Head Circumference (HC) Measurements Were Obtained From Pediatric Medical Records (Well-Baby Visits) for Every Subject. The exponential Model Was Based on a Total of 1162 Measurements (Mean = 6.5 Per Subject, Standard Deviation = 4.12, Range = 18) That Spanned Birth Through 36 Months of Age
| Model | Parameter (SE) | Group
|
||
|---|---|---|---|---|
| TD | ASD-N | ASD-DM | ||
| Exponential | Asymptote (cm) | 49.67 (0.19) | 50.03 (0.14)* | 51.69 (0.25)**,*** |
| Intercept (cm) | 34.93 (0.23) | 34.92 (0.21) | 36.16 (0.31) | |
| Rate | 0.146 (0.004) | 0.164 (0.004)* | 0.144 (0.006) | |
| Asymptote variance | 1.23 (0.31) | 1.34 (0.25) | 0.46 (0.27) | |
| Intercept variance | 1.60 (0.49) | 2.64 (0.57) | 0.73 (0.58) | |
| Rate variance | 0.0001 (0.0001) | 0.0004 (0.0002) | 0.00002 (0.0001) | |
| Residual variance | 0.49 (0.04) | 0.64 (0.04) | 0.95 (0.13) | |
Note. Significant contrast between TD and ASD-N
P < 0.05; significant contrast between TD and ASD-DM
P < 0.0001; significant contrast between ASD-N and ASD-DM
P < 0.0001.
Figure 5.
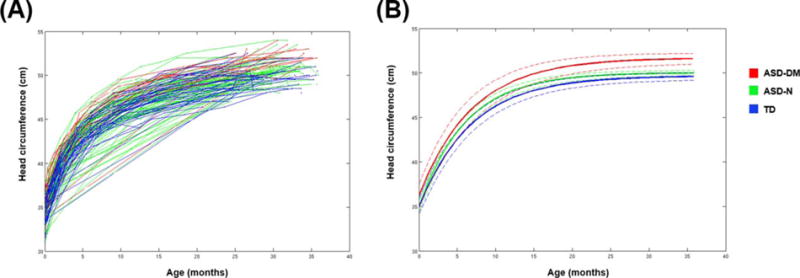
Results from longitudinal analyses of head circumference for subgroups. (A) Data for head circumference for participants from each of the three subgroups: ASD-DM, ASD-N, and TD. Each line connects the data points from each individual subject. The ASD-DM group (red) fall in the upper end of the distribution, while there is a large amount of overlap between the ASD-N (green) and TD (blue) boys. (B) Predicted growth trajectories for head circumference for each of the three subgroups. Dashed lines indicate the lower and upper bounds of the 95% confidence intervals. The ASD-DM group did not differ in head circumference at birth, but reached a significantly larger final overall head size compared to the ASD-N and TD boys.
Body Size
All subjects experienced an increase in height over time (linear mixed-effects model analysis for height, t[113] = 33.25, P < 0.001). There were no significant differences between the groups on height at Time 1 and no significant differences in the overall rate of body growth between the groups (Fig. 6).
Figure 6.
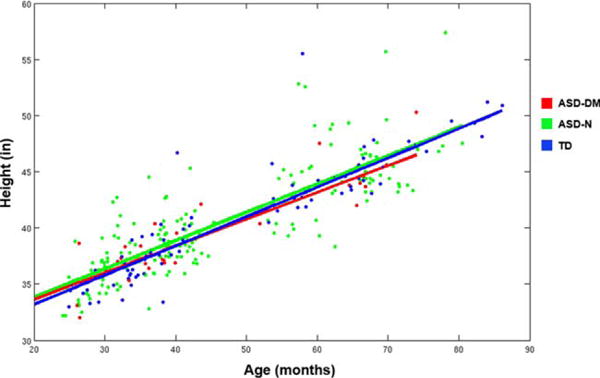
Distribution of height for subjects in this study. Based on these distributions of height we obtained the predicted linear growth trajectories for body height for each subgroup as indicated by the solid lines. No significant differences were found between the three subgroups for height or rate of growth for height.
Classification Change
Of the participants classified as ASD-DM at Time 1, one child was reclassified as ASD-N at Time 3. Of the boys classified as ASD-N at Time 1, three were reclassified as ASD-DM. None of the TD boys were reclassified.
Discussion
Summaries of ASD neurodevelopment highlight a pattern of abnormal acceleration of brain growth from birth until about 4 years of age, which is followed by a normalization of brain size in middle childhood [Redcay and Courchesne, 2005a]. This perspective has been supported by studies reporting increased TCV in very young children with ASD [Courchesne et al., 2011a; Courchesne et al., 2001; Hazlett et al., 2005; Piven et al., 1995, 1996; Schumann et al., 2010; Sparks et al., 2002] but no significant differences in TCV in older individuals with ASD [Aylward et al., 1999, 2002b; Hardan et al., 2006; Herbert et al., 2004; Schumann et al., 2004]. However, the lifespan trajectory of brain development in autism has never been evaluated in a large-scale, longitudinal analysis of children with ASD and age-matched typically developing children. Moreover, it is now very clear that ASD is a heterogeneous disorder with different altered trajectories of brain development in different subsets of affected individuals. We previously reported, for example, that only about 15% of boys with ASD have abnormal early enlargement of their brains [Nordahl et al., 2011]. When taking body size into account, we have labeled this subgroup disproportionate megalencephaly (ASD-DM). Interestingly, girls with ASD do not generally have this abnormal form of brain development.
Children with ASD-DM are defined as having a ratio of brain size to height at the first neuroimaging time point (approximately 3 years of age) that is at least 1.5 standard deviations above the mean of the typically developing children. An overarching goal of the current study was to determine whether the ASD-DM boys persisted in having enlarged brains across the early childhood years, or whether their brain growth plateaued during this time period. The data show quite clearly that the ASD-DM subgroup of boys continue to have disproportionately large brains for the second and third imaging time points which brings the average age of the cohort to just over 5 years. While there has been an ongoing debate about whether brain growth differences in ASD are due to enlargement in gray matter [Hazlett et al., 2006; Palmen et al., 2005] or white matter [Herbert et al., 2004], our findings indicate that there were similar levels of enlargement of both gray matter and white matter compartments for the ASD-DM boys. It is worth noting that when all ASD subjects were combined, the entire group has increased brain volume and growth. However, by separating the ASD-DM children from the rest of the ASD cohort, we found that this pattern is largely driven by the ASD-DM group which displays significantly increased TCV and rate of TCV growth, while the ASD-N group alone does not differ from TD boys in TCV.
We also wanted to examine when brain development in this subgroup diverged from other boys. While we did not have MRI data prior to age three, the Autism Phenome Project collects all available medical records that includes head circumference data from birth onward. We also measured head circumference at the first and third MRI time points. Head circumference has previously been shown to be an accurate proxy of brain size in young children [Bartholomeusz, Courchesne, & Karns, 2002] but is less correlated with brain size in older children. Our own data are consistent with this finding in that HC is a better predictor of TCV at Time 1 (r = 0.65, P < 0.001) than at Time 3 (r = 0.50, P < 0.001). The head circumference data show that while the ASD-DM children do not differ in head size at birth, their trajectory of head growth results in head circumference that is significantly greater than boys from the other groups by around 24 months of age. Thus, it appears that the increased brain size may be due, in large part, to abnormal expansion that starts very early in postnatal life. Some have argued [Chawarska et al., 2011] that the increased brain size in ASD is simply a reflection of overall greater somatic growth. We have examined this directly and found that the ASD-DM children are not taller than the ASD-N and TD children. Thus, the abnormal brain size appears to be organ specific.
A possible explanation for the discrepancy between the results of this longitudinal investigation (in which only a subgroup of children with ASD have persistent brain overgrowth from ages 3–5 years) and the previous perspective (that development in ASD is characterized by accelerated brain growth until around 4 years of age, followed by arrested growth [Redcay & Courchesne, 2005b]) may be that cross-sectional neuroimaging studies of ASD have historically used cohorts with vastly different characteristics at different ages. Studies of young children have typically evaluated a segment of the autism spectrum that has additional developmental delay as indicated by reported mean subject IQ scores ranging from 54.1 to 66.7 [Hazlett et al., 2005; Nordahl et al., 2011, 2013; Schumann et al., 2010]. In contrast, studies including only adolescents and adults have typically reported IQ scores above 80 (ranging from 91 to 105 for those reporting means) [Aylward et al., 1999, 2002b; Freitag et al., 2009; Hardan et al., 2006; Hazlett et al., 2006; Herbert et al., 2004; Palmen et al., 2005; Piven et al., 1996]. The older subjects tend to have less intellectual disability because they are selected to be compliant with the demands of the MRI protocol. Young subjects are typically imaged when they are asleep or anesthetized, whereas older subjects are imaged while awake. Thus, the proposal that individuals with autism have enlarged brains as youngsters and normal-sized brains as adolescents or adults may be an artifact of the samples that are imaged at the different ages. The only way to reliably chart the trajectories of brain development of ASD is through longitudinal studies that span early childhood into adulthood.
While the current study helps clarify longitudinal brain growth in ASD during early childhood, questions still remain as to how the brain continues to develop into adolescence and beyond. By the age of six, TCV is 90–95% of the maximum (adult) size [Dekaban & Sadowsky, 1978; Giedd, 2004; Lenroot & Giedd, 2006]. While it appears that the ASD-DM boys maintain brain enlargement up to 5 years of age, it will be interesting to determine whether this is sustained at later ages. We are currently carrying out additional imaging of these children as they enter middle childhood (i.e., 9–11 years of age).
Although MRI enables the study of global brain development in vivo, it does not provide a clear understanding of the cellular mechanisms underlying any alterations in trajectory. It is currently unclear what the neurobiological basis is for the enlarged brains in the ASD-DM cohort of children. Both increased [Bailey et al., 1998; Courchesne et al., 2011b] and decreased [Bailey et al., 1998; Bauman, 1991; Schumann & Amaral, 2006] neuron counts have been reported in studies using postmortem tissue of individuals with ASD. Since only around 15% of boys with ASD have enlarged brains, there is clearly a need for a greater number of brain donations for study and a comprehensive quantitative assessment of neuron and glial number and size.
In summary, the current study evaluated a subgroup of boys with ASD who have an unusual trajectory of brain enlargement which cannot be accounted for by body size and which persists until at least 5 years of age. All analyses indicated that there was no indication of a slowdown of brain growth in the ASD-DM group relative to the TD group. Our working hypothesis, therefore, is that this subgroup of individuals will maintain an enlarged brain size at later ages – a hypothesis that we are currently testing. Moreover, head circumference data from this cohort indicates that the onset of the abnormal growth trajectory started early in postnatal life. Given that the ASD-DM group defines a neurophenotype of ASD, one of the challenges will be to determine whether the behavioral profile of the ASD-DM is different in any meaningful way.
Acknowledgments
The authors would like to thank the families and children who are participating in the Autism Phenome Project study who have generously given their time to provide the data reported in this paper. The authors thank Wesley Burge for his assistance with the figures. Funding for this study was provided by the National Institute of Mental Health (1R01MH089626, 1R01MH103371, U24MH081810 and 1K99MG085099) and the University of California Davis Medical Investigation of Neurodevelopmental Disorders (MIND) Institute. This project was also supported by the MIND Institute Intellectual and Developmental Disabilities Research Center (U54HD079125).
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
References
- Anagnostou E, Taylor MJ. Review of neuroimaging in autism spectrum disorders: What have we learned and where we go from here. Molecular Autism. 2011;2:4. doi: 10.1186/2040-2392-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward E, Minshew N, Field K, Sparks B, Singh N. Effects of age on brain volume and head circumference in autism. Neurology. 2002a;59:175–183. doi: 10.1212/wnl.59.2.175. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Minshew NJ, Goldstein G, Honeycutt NA, Augustine AM, Yates KO, Pearlson GD. MRI volumes of amygdala and hippocampus in non–mentally retarded autistic adolescents and adults. Neurology. 1999;53:2145–2145. doi: 10.1212/wnl.53.9.2145. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Minshew N, Field K, Sparks B, Singh N. Effects of age on brain volume and head circumference in autism. Neurology. 2002b;59:175–183. doi: 10.1212/wnl.59.2.175. [DOI] [PubMed] [Google Scholar]
- Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, Lantos P. A clinicopathologi-cal study of autism. Brain. 1998;121:889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- Bartholomeusz H, Courchesne E, Karns C. Relationship between head circumference and brain volume in healthy normal toddlers, children, and adults. Neuropediatrics. 2002;33:239–241. doi: 10.1055/s-2002-36735. [DOI] [PubMed] [Google Scholar]
- Bauman ML. Microscopic neuroanatomic abnormalities in autism. Pediatrics. 1991;87:791–796. [PubMed] [Google Scholar]
- Chawarska K, Campbell D, Chen L, Shic F, Klin A, Chang J. Early generalized overgrowth in boys with autism. Archives of General Psychiatry. 2011;68:1021–1031. doi: 10.1001/archgenpsychiatry.2011.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. The social responsiveness scale. Los Angeles, CA: Western Psychological Services; 2002. [Google Scholar]
- Courchesne E. Brain development in autism: Early overgrowth followed by premature arrest of growth. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10:106–111. doi: 10.1002/mrdd.20020. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Campbell K, Solso S. Brain growth across the life span in autism: Age-specific changes in anatomical pathology. Brain Research. 2011a;1380:138–145. doi: 10.1016/j.brainres.2010.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Karns C, Davis H, Ziccardi R, Carper R, Tigue Z, Courchesne RY. Unusual brain growth patterns in early life in patients with autistic disorder an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Mouton PR, Calhoun ME, Semendeferi K, Ahrens-Barbeau C, Hallet MJ, Pierce K. Neuron number and size in prefrontal cortex of children with autism. Journal of the American Medical Association. 2011b;306:2001–2010. doi: 10.1001/jama.2011.1638. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Brain overgrowth in autism during a critical time in development: Implications for frontal pyramidal neuron and interneuron development and connectivity. International Journal of Developmental Neuroscience. 2005;23:153–170. doi: 10.1016/j.ijdevneu.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, Morgan J. Mapping early brain development in autism. Neuron. 2007;56:399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Davidian M, Giltinan DM. Nonlinear models for repeated measurement data. Vol. 62. New York: CRC Press; 1995. [Google Scholar]
- Davidovitch M, Patterson B, Gartside P. Head circumference measurements in children with autism. Journal of Child Neurology. 1996;11:389–393. doi: 10.1177/088307389601100509. [DOI] [PubMed] [Google Scholar]
- Dekaban AS, Sadowsky D. Changes in brain weights during the span of human life: Relation of brain weights to body heights and body weights. Annals of Neurology. 1978;4:345–356. doi: 10.1002/ana.410040410. [DOI] [PubMed] [Google Scholar]
- DiLavore PC, Lord C, Rutter M. The pre-linguistic autism diagnostic observation schedule. Journal of Autism and Developmental Disorders. 1995;25:355–379. doi: 10.1007/BF02179373. [DOI] [PubMed] [Google Scholar]
- Dissanayake C, Bui QM, Huggins R, Loesch DZ. Growth in stature and head circumference in high-functioning autism and Asperger disorder during the first 3 years of life. Development and Psychopathology. 2006;18:381–393. doi: 10.1017/S0954579406060202. [DOI] [PubMed] [Google Scholar]
- Elliot C. Differential abilities scale. San Antonio, TX: Psychological Corporation; 1990. [Google Scholar]
- Freitag CM, Luders E, Hulst HE, Narr KL, Thompson PM, Toga AW, Konrad C. Total brain volume and corpus callosum size in medication-naive adolescents and young adults with autism spectrum disorder. Biological Psychiatry. 2009;66:316–319. doi: 10.1016/j.biopsych.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39:693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greimel E, Nehrkorn B, Schulte-Ruther M, Fink GR, Nickl-Jockschat T, Herpertz-Dahlmann B, Eickhoff SB. Changes in grey matter development in autism spectrum disorder. Brain Structure and Function. 2013;218:929–942. doi: 10.1007/s00429-012-0439-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallahan B, Daly E, McAlonan G, Loth E, Toal F, O’brien F, Murphy DG. Brain morphometry volume in autistic spectrum disorder: A magnetic resonance imaging study of adults. Psychological Medicine. 2009;39:337–346. doi: 10.1017/S0033291708003383. [DOI] [PubMed] [Google Scholar]
- Hardan A, Muddasani S, Vemulapalli M, Keshavan M, Minshew N. An MRI study of increased cortical thickness in autism. American Journal of Psychiatry. 2006;163:1290–1292. doi: 10.1176/appi.ajp.163.7.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardan AY, Minshew NJ, Melhem NM, Srihari S, Jo B, Bansal R, Stanley JA. An MRI and proton spectroscopy study of the thalamus in children with autism. Psychiatry Research: Neuroimaging. 2008;163:97–105. doi: 10.1016/j.pscychresns.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, Piven J. Magnetic resonance imaging and head circumference study of brain size in autism: Birth through age 2 years. Archives of General Psychiatry. 2005;62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe MD, Gerig G, Smith RG, Piven J. Cortical gray and white brain tissue volume in adolescents and adults with autism. Biological Psychiatry. 2006;59:1–6. doi: 10.1016/j.biopsych.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ, Caviness VS., Jr Localization of white matter volume increase in autism and developmental language disorder. Annals of Neurology. 2004;55:530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- Jou RJ, Minshew NJ, Keshavan MS, Hardan AY. Cortical gyrification in autistic and Asperger disorders: A preliminary magnetic resonance imaging study. Journal of Child Neurology. 2010;25:1462–1467. doi: 10.1177/0883073810368311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kail RV, Ferrer E. Processing speed in childhood and adolescence: Longitudinal models for examining developmental change. Child Development. 2007;78:1760–1770. doi: 10.1111/j.1467-8624.2007.01088.x. [DOI] [PubMed] [Google Scholar]
- Kemper TL, Bauman M. Neuropathology of infantile autism. Journal of Neuropathology & Experimental Neurology. 1998;57:645–652. doi: 10.1097/00005072-199807000-00001. [DOI] [PubMed] [Google Scholar]
- Lainhart JE, Piven J, Wzorek M, Landa R, Santangelo SL, Coon H, Folstein SE. Macrocephaly in children and adults with autism. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:282–290. doi: 10.1097/00004583-199702000-00019. [DOI] [PubMed] [Google Scholar]
- Lange N, Travers BG, Bigler ED, Prigge MB, Froehlich AL, Nielsen JA, Lainhart JE. Longitudinal volumetric brain changes in autism spectrum disorder ages 6–35 years. Autism Research. 2015;8:82–93. doi: 10.1002/aur.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neuroscience & Biobehavioral Reviews. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Littell RC, Stroup WW, Milliken GA, Wolfinger RD, Schabenberger O. SAS for Mixed Models. Cary, NC: SAS Institute; 2006. [Google Scholar]
- Little RJ. A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association. 1988;83:1198–1202. [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Rutter M. The Autism Diagnostic Observation Schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Cheung V, Cheung C, Suckling J, Lam GY, Tai K, Chua SE. Mapping the brain in autism. A voxel-based MRI study of volumetric differences and intercorrelations in autism. Brain. 2005;128:268–276. doi: 10.1093/brain/awh332. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Daly E, Kumari V, Critchley HD, van Amelsvoort T, Suckling J, Murphy DG. Brain anatomy and sensorimotor gating in Asperger’s syndrome. Brain. 2002;125:1594–1606. doi: 10.1093/brain/awf150. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Ferrer-Caja E, Hamagami F, Woodcock RW. Comparative longitudinal structural analyses of the growth and decline of multiple intellectual abilities over the life span. Developmental Psychology. 2002;38:115–142. [PubMed] [Google Scholar]
- Miles J, Hadden L, Takahashi T, Hillman R. Head circumference is an independent clinical finding associated with autism. American Journal of Medical Genetics. 2000;95:339–350. [PubMed] [Google Scholar]
- Mullen EM. Mullen scales of early learning. Circle Pines, MN: AGS; 1995. [Google Scholar]
- Nordahl CW, Braunschweig D, Iosif AM, Lee A, Rogers S, Ashwood P, Van de Water J. Maternal auto-antibodies are associated with abnormal brain enlargement in a subgroup of children with autism spectrum disorder. Brain, Behavior, and Immunity. 2013;30:61–65. doi: 10.1016/j.bbi.2013.01.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl CW, Iosif AM, Young GS, Perry LM, Dougherty R, Lee A, Amaral DG. Sex differences in the corpus callosum in preschool-aged children with autism spectrum disorder. Molecular Autism. 2015;6:26. doi: 10.1186/s13229-015-0005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl CW, Lange N, Li DD, Barnett LA, Lee A, Buonocore MH, Amaral DG. Brain enlargement is associated with regression in preschool-age boys with autism spectrum disorders. Proceedings of the National Academy of Sciences. 2011;108:20195–20200. doi: 10.1073/pnas.1107560108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl CW, Scholz R, Yang X, Buonocore MH, Simon T, Rogers S, Amaral DG. Increased rate of amygdala growth in children aged 2 to 4 years with autism spectrum disorders: A longitudinal study. Archives of General Psychiatry. 2012;69:53–61. doi: 10.1001/archgenpsychiatry.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl CW, Simon TJ, Zierhut C, Solomon M, Rogers SJ, Amaral DG. Brief report: Methods for acquiring structural MRI data in very young children with autism without the use of sedation. Journal of Autism and Developmental Disorders. 2008;38:1581–1590. doi: 10.1007/s10803-007-0514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmen SJ, Hulshoff Pol HE, Kemner C, Schnack HG, Durston S, Lahuis BE, Van Engeland H. Increased gray-matter volume in medication-naive high-functioning children with autism spectrum disorder. Psychological Medicine. 2005;35:561–570. doi: 10.1017/s0033291704003496. [DOI] [PubMed] [Google Scholar]
- Piven J, Arndt S, Bailey J, Andreasen N. Regional brain enlargement in autism: A magnetic resonance imaging study. Journal of the American Academy of Child & Adolescent Psychiatry. 1996;35:530–536. doi: 10.1097/00004583-199604000-00020. [DOI] [PubMed] [Google Scholar]
- Piven J, Arndt S, Bailey J, Havercamp S, Andreasen NC, Palmer P. An MRI study of brain size in autism. American Journal of Psychiatry. 1995;152:1145–1149. doi: 10.1176/ajp.152.8.1145. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. Vol. 1. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- Redcay E, Courchesne E. When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biological Psychiatry. 2005a;58:1–9. doi: 10.1016/j.biopsych.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Redcay E, Courchesne E. When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biological Psychiatry. 2005b;58:1–9. doi: 10.1016/j.biopsych.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. The social communication questionnaire: Manual. Torrance, CA: Western Psychological Services; 2003. [Google Scholar]
- Sacco R, Gabriele S, Persico AM. Head circumference and brain size in autism spectrum disorder: A systematic review and meta-analysis. Psychiatry Research. 2015;234:239–251. doi: 10.1016/j.pscychresns.2015.08.016. [DOI] [PubMed] [Google Scholar]
- Schumann C, Amaral DG. Stereological analysis of amygdala neuron number in autism. Journal of Neuroscience. 2006;26:7674–7679. doi: 10.1523/JNEUROSCI.1285-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann C, Bloss CS, Barnes CC, Wideman GM, Carper RA, Akshoomoff N, Courchesne E. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. Journal of Neuroscience. 2010;30:4419–4427. doi: 10.1523/JNEUROSCI.5714-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann C, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, Amaral DG. The amygdala is enlarged in children but not adolescents with autism: The hippocampus is enlarged at all ages. Journal of Neuroscience. 2004;24:6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz G. Estimating the dimension of a model. Annals of Statistics. 1978;6:461–464. [Google Scholar]
- Sparks B, Friedman S, Shaw D, Aylward E, Echelard D, Artru A, Dager SR. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV, Balla DA. The vine-land adaptive behavior scales. Major Psychological Assessment Instruments. 1989;2:199–231. [Google Scholar]
- Tamura R, Kitamura H, Endo T, Hasegawa N, Someya T. Reduced thalamic volume observed across different subgroups of autism spectrum disorders. Psychiatry Research: Neuroimaging. 2010;184:186–188. doi: 10.1016/j.pscychresns.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Tepest R, Jacobi E, Gawronski A, Krug B, Moller-Hartmann W, Lehnhardt FG, Vogeley K. Corpus callosum size in adults with high-functioning autism and the relevance of gender. Psychiatry Research: Neuroimaging. 2010;183:38–43. doi: 10.1016/j.pscychresns.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Williams RS, Hauser SL, Purpura DP, DeLong GR, Swisher CN. Autism and mental retardation: Neuropathologic studies performed in four retarded persons with autistic behavior. Archives of Neurology. 1980;37:749–753. doi: 10.1001/archneur.1980.00500610029003. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]


