Abstract
Cardiac tumors are exceedingly rare (0.001–0.03% in most autopsy series). They can be present anywhere within the heart and can be attached to any surface or be embedded in the myocardium or pericardial space. Signs and symptoms are nonspecific and highly variable related to the localization, size and composition of the cardiac mass. Echocardiography, typically performed for another indication, may be the first imaging modality alerting the clinician to the presence of a cardiac mass. Although echocardiography cannot give the histopathology, certain imaging features and adjunctive tools such as contrast imaging may aid in the differential diagnosis as do the adjunctive clinical data and the following principles: (1) thrombus or vegetations are the most likely etiology, (2) cardiac tumors are mostly secondary and (3) primary cardiac tumors are mostly benign. Although the finding of a cardiac mass on echocardiography may generate confusion, a stepwise approach may serve well practically. Herein, we will review such an approach and the role of echocardiography in the assessment of cardiac masses.
Keywords: ventricular mass, atrial mass, cancer, echocardiography
Introduction
Cardiac masses have captured the attention since the beginning of echocardiography. A key reason lies in the fact that the overall prevalence of primary cardiac tumors is exceedingly rare, less than 0.1% in large autopsy series (1, 2). Accordingly, most cardiac tumors are found incidentally during routine cardiac imaging. Most commonly, as widely available and applicable, echocardiography is the imaging technique of choice. It can delineate multiple cardiac structures and characteristics of a mass such as its mobility, attachment and potential hemodynamic consequences. It also allows for serial imaging over time without the need for radiation, iodine or gadolinium contrast agents.
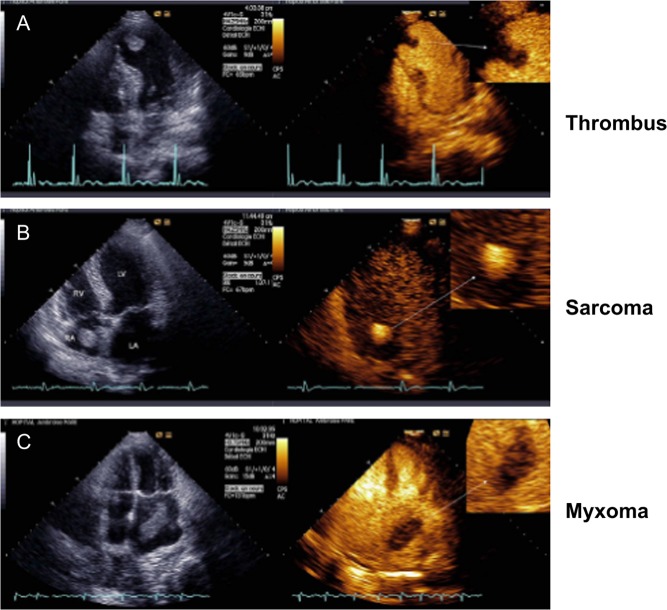
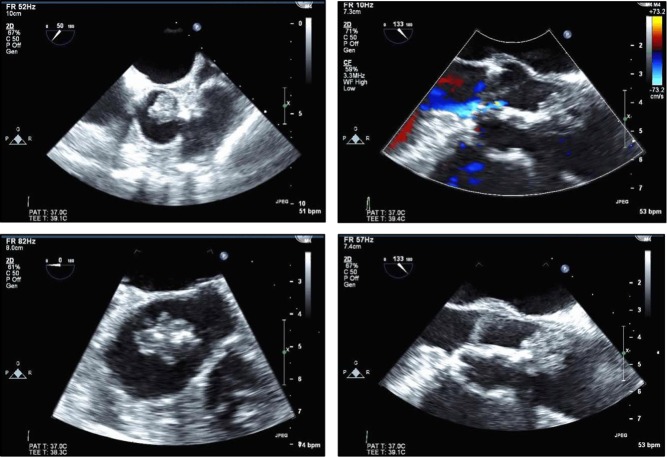
However, echo contrast agents are useful to confirm the presence of an intracardiac mass and to characterize it further by virtue of the extent of contrast enhancement, which is a marker of vascularity (Fig. 1). Accordingly, malignant and highly vascular tumors demonstrate hyper-enhancement with contrast, whereas thrombi do not appear to enhance at all; myxomas tend to be partially enhanced (3, 4). Attachment of a mass to an area of abnormal wall motion, typically akinesis or dyskinesis, makes the diagnosis of thrombus more likely than that of a tumor. Similarly, a valvular mass should raise greater concerns in a thrombotic (sterile) or infectious vegetation than a benign or malignant mass.
Figure 1.
Illustration of the differences in presentation by echo contrast imaging of cardiac thrombus (A), sarcoma (B) and myxoma (C). Reproduced, with permission, from Mansencal N, Revault-d’Allonnes L, Pelage J-P, Farcot J-C, Lacombe P, Dubourg O. Usefulness of contrast echocardiography for assessment of intracardiac masses. Archives of Cardiovascular Diseases 2009; volume 102 (issue 3): 177–183. Copyright 2009 Elsevier Masson SAS. All rights reserved. (4).
Transesophageal echocardiography (TEE), commonly used when a valvular lesion is suspected, may also become necessary to better characterize a cardiac tumor in terms of size, morphology, attachment site, extension and hemodynamic affects. However, not infrequently, this role is taken by cardiac magnetic resonance imaging (MRI) or cardiac computed tomography (CT). Echocardiography has a narrower field of view than MRI or CT in general and can be limited further by poor acoustic windows. Artifacts can be mistaken for true pathology, and echocardiography is unable to give a definite tissue histology, which is a general limitation.
The use of 3-dimensional (3-D) echocardiography is the newest approach in the assessment of intracardiac masses. Three-dimensional echocardiography allows for a volumetric assessment of a mass over a linear measurement as it is obtained with 2-dimensional imaging. With the cropping techniques available with 3D, various aspects of the mass can be better visualized including point of attachment, homogenity, vascularity, and calcification (5).
The symptoms a cardiac tumor causes are usually related to its cardiac location, although some may produce systemic complaints. Embolic phenomena are an example of the latter and may be the clinical first presentation of the tumor. Most commonly, these are noted in the systemic circulation as tumors on the left side of the heart are associated with a greater risk of embolization (e.g. myxoma), but tumor embolism into the pulmonary circulation has been described as well (e.g. lymphoma) (6, 7). Hemodynamically, tumors reduce cardiac output (stroke volume) y causing intra-cavity obstruction or valvular dysfunction. Malignant tumors may invade the myocardium and pericardium resulting in arrhythmias, heart failure and pericardial effusions. Sudden death may also occur, and although infrequent, it can unfortunately be the initial and final manifestation of the tumor (8). In general, early identification of a cardiac tumor is ideal for treatment considerations and prognosis.
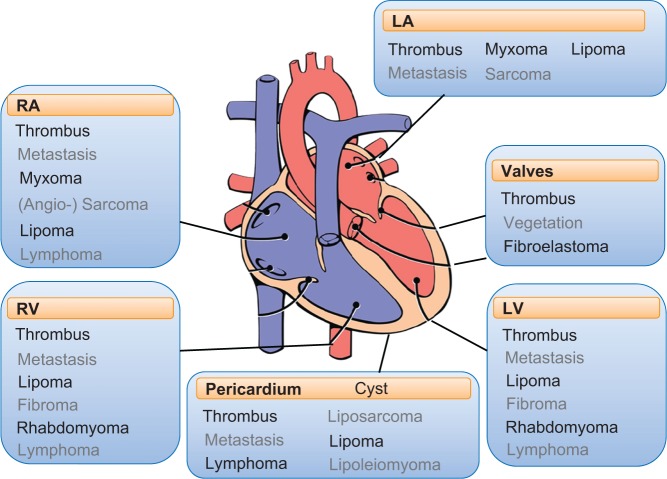
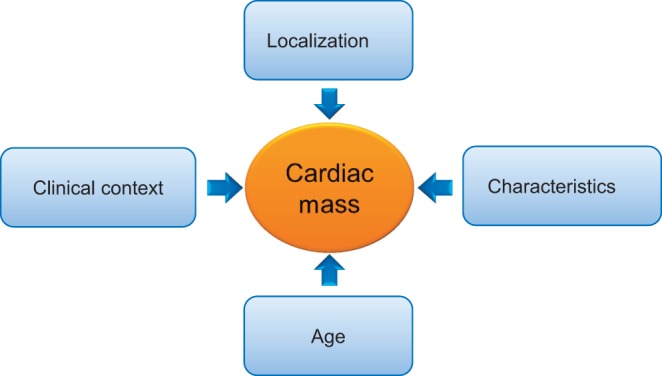
In clinical practice, whenever a cardiac mass is encountered on echocardiography, a pragmatic approach is to focus on the location first, and then on the imaging characteristics and subsequently the clinical context and presentation to reach the correct conclusions (Fig. 2). For instance, a mass on the aortic valve, pedunculated with sea anemone appearance in an asymptomatic adult patient most likely represents a fibroelastoma. A ‘site differential’ is provided in Fig. 3, and details of the specific entities by type of cardiac tumor will be provided in the following sections with particular attention given to the echocardiographic imaging characteristics (1, 9, 10).
Figure 2.
Conceptual initial approach to evaluate a cardiac mass.
Figure 3.
Outline of the distribution of subtypes of cardiac masses by anatomic location. Reprinted from Journal of the American Society of Echocardiography, Vol 13 issue 12, Dujardin KS, Click RL & Oh TK,The role of intraoperative transesophageal echocardiography in patients undergoing cardiac mass removal, Pages 1080–1083, Copyright (2000), with permission from Elsevier. (61).
Benign primary cardiac tumors
The majority of primary cardiac tumors are benign in nature (11, 12, 13). Of these benign cardiac tumors, cardiac myxomas by far are the most common entities in adults and rhabdomyomas in the pediatric population.
Myxoma
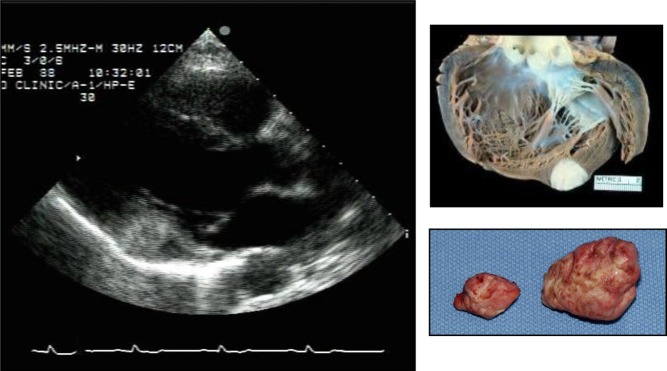
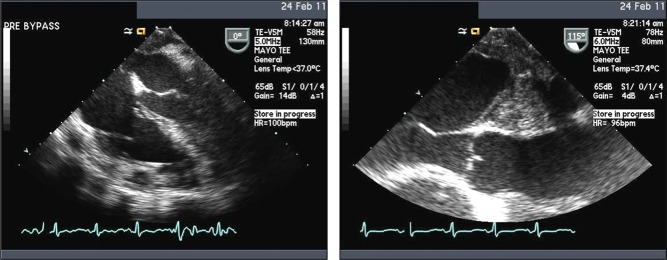
Cardiac myxomas are the most common primary cardiac tumor in adults, typically presenting between the ages of 30 and 60 years and more commonly in women (14, 15, 16). The majority (75–80%) are located within the left atrium, characteristically originating from the mid-portion of the atrial septum by a narrow stalk; 15–20% originate within the right atrium (Fig. 4) (17). It is exceedingly rare to find a myxoma on the cardiac valves or within the left or right ventricle (Fig. 5) (18).
Figure 4.
Atrial myxoma diagnosed in a 69-year-old female presenting with recurrent stroke, history of paroxysmal atrial fibrillation, on therapeutic doses of warfarin. Typical echocardiography images and their surgical pathology correlate on the left- and right-sided panels.
Figure 5.
Right ventricular myxoma diagnosed in a 79-year-old woman who presented with nonexertional chest pain that was worse when supine. Remote history of breast cancer treated with radiation to the left chest.
On echocardiogram, a myxoma presents as a heterogeneous mobile mass with one of the two basic appearances (Figs 4 and 5). Polypoid myxomas are larger with a smooth surface and a rough core including lucencies and cystic areas due to hemorrhage and necrosis. Papillary myxomas tend to be smaller and have a stretched appearance with multiple villi. The latter subtype is associated with embolic phenomena, whereas the former subtype tends to obstruct the blood flow with possible heart failure symptoms (19). These cardiovascular symptoms are seen more frequently than the embolic phenomena (16). Patients with myxomas may report constitutional symptoms, such as fever and weight loss, and these patients tend to exhibit laboratory abnormalities such as anemia and elevations in inflammatory markers (16, 17).
There are a few myxoma syndromes in which a genetic disorder is present, resulting in multiple tumors. The Carney complex is one such syndrome, inherited in an autosomal dominant pattern, in which atrial and noncardiac myxomas, schwannomas and various endocrine tumors are present, along with various skin pigmentation abnormalities (20). The myxomas in this syndrome occur at an earlier age and tend to recur more frequently (21). Screening first-degree relatives with echocardiography is appropriate when a familial myxoma syndrome is identified.
The diagnosis of an atrial myxoma warrants resection due to the risk of embolization, cardiovascular complications and sudden death. Surgical resection is associated with a low operative mortality and good long-term outcome (22, 23, 24). A small percentage of patients (up to 5%), typically those with a family history, smaller tumors or ventricular locations, are at risk of recurrent or new myxomas, which underscores the importance of serial follow-up (16, 24, 25).
Papillary fibroelastoma
Papillary fibroelastomas (PFE) rank third in the prevalence of benign cardiac tumors in adults based on autopsy series (26) but may be more common based on echocardiographic studies and referral patterns (27). PFE can arise from any endocardial surface with a highly mobile stalk and are singular more often than multiple. They are most commonly on the valves, primarily the aortic valve followed by the mitral valve, mainly in the downstream side, and most commonly 2 to 40 mm in size. Larger PFEs are seen when they occur on the right-sided valves.
Echocardiographic features of a PFE are small size, with independent motion and attachment to an endocardial surface (Fig. 6). Especially on TEE, the borders appear stippled or shimmering, which is due to the vibration at the tumor–blood interface due to the finger-like projections (28). The latter has been likened to a ‘sea anemone’ due to the frond-like arms attached to a central pedicle, best seen when the tumor is viewed under water. Distinguishing a PFE from a Lambl’s excrescence may be difficult, but the latter is typically smaller and more linear (Fig. 7). TEE detects more PFEs than transthoracic echocardiography due to the typical small size of these tumors, thus reinforcing the role of TEE in the evaluation of an embolic event (27).
Figure 6.
Multiple frames of a fibroelastoma diagnosed in an 84-year-old woman who underwent echocardiography because of a heart murmur and a history of dizziness and imbalance but no strokes or TIAs.
Figure 7.
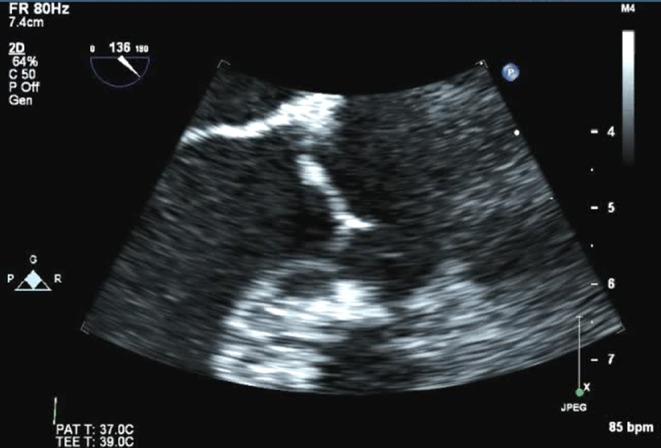
TEE image of a Lambl’s in a 70-year-old male who presented with dysarthria and mild left-sided weakness, which resolved within an hour.
A significant association is seen between a clinically diagnosed PFE and a thromboembolic event (27, 29, 30). Either the tumor itself or thrombi that have formed on the tumor embolize, most commonly causing a transient ischemic attack or stroke (29, 30). These tumors are more common in women and older patients (27, 29, 30). Although these tumors arise from the valvular endocardium, valvular dysfunction is rare (28).
Surgical excision is recommended for larger (≥1 cm), left-sided PFEs in patients who are deemed appropriate surgical candidates (young age, low surgical risk) or at the time of cardiac surgery for another cardiovascular condition (31). Right-sided PFEs should be removed only if large or mobile and associated with hemodynamically significant obstruction or risk of embolism such as can be seen with a patent foramen ovale with right-to-left shunting. Excision dramatically decreases the risk of stroke from a PFE (27). This approach may be prudent even for the incidental finding of a PFE in an asymptomatic patient unless they are small, without a stalk and thus non-mobile. In this case, and if surgery is not undertaken due to patient preference or high surgical risk, treatment with antiplatelet agents is recommendable despite limited supporting data (27).
Rhabdomyoma
Rhabdomyomas are the most common primary cardiac tumor in children, typically appearing before the first year. The majority are associated with tuberous sclerosis (32) and tend to be multiple. Rarely are these tumors seen in adults. The rhabdomyomas are usually seen on the ventricular walls (left ventricle or ventricular septum) or on the atrioventricular valves. On the echocardiogram, they may appear as small, well-circumscribed (multiple) nodules or a pedunculated mass in a cardiac cavity. Myocardial embedding is possible, emerging as a lobulated, homogeneous and hyperechogenic mass (often brighter than the surrounding myocardium). Extensive growth and shrinkage are seen, and most of these tumors regress spontaneously (33). Thus, serial echocardiographic follow-up is key and often sufficient. Resection may be required if the cardiac mass causes obstructive symptoms or arrhythmias (33, 34).
Fibroma
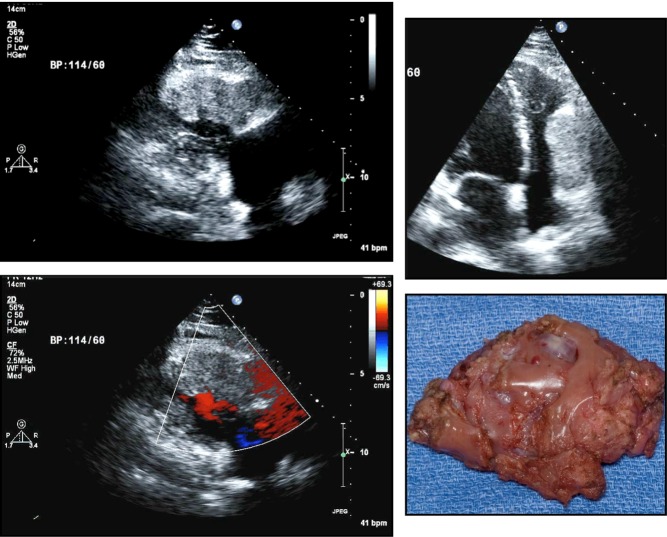
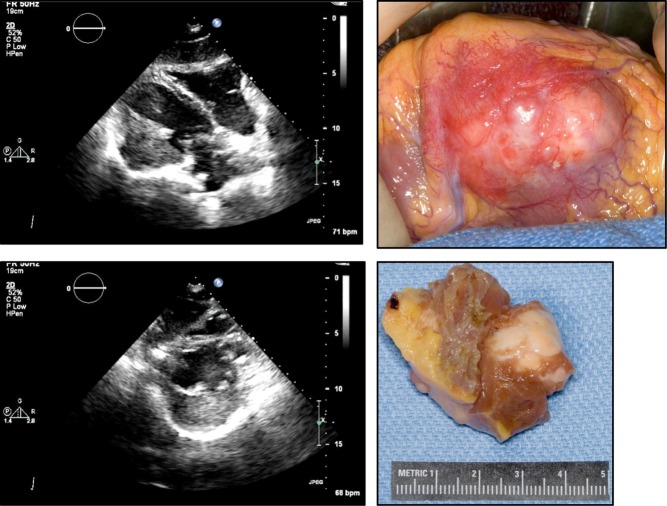
Cardiac fibromas are rare, though still the second most common benign cardiac tumor in the pediatric population (35). On the echocardiogram, they usually appear as a distinct, well-demarcated, non-contractile and solid, highly echogenic mass within the myocardium; calcification is possible (Fig. 8). The most common locations are the left ventricular free wall, anterior free wall or ventricular septum. Extension into the cavity is not infrequent, which can lead to obstruction and heart failure symptoms. Otherwise, the myocardial location can lead to arrhythmias. These tumors do not spontaneously regress and frequently require resection. Patients with multiple basal cell carcinomas should be screened for cardiac fibromas (Gorlin syndrome).
Figure 8.
Echocardiographic images of a fibroma of a 47-year-old female with recent episode of chest pain and a history of possible tuberous sclerosis.
Lipoma
Lipomas are composed of benign adipose tissue. Within the heart, the majority of these arise in the subendocardium, but they can also occur in the pericardium and on the cardiac valves. Tumor size can range from a few to several centimeters. On echocardiography, lipomas tend to be broad based, immobile, without a pedicle and well circumscribed (Fig. 9). They are homogenous without evidence of calcification, hyperechoic in the cavity but hypoechoic in the pericardium. Typically lipomas are asymptomatic, but may cause arrhythmias or valvular dysfunction. Subepicardial lipomas may cause compression of the coronary arteries, leading to ischemic chest pain. Due to the progressive growth of lipomas along with the potential symptom profile, excision may be required.
Figure 9.
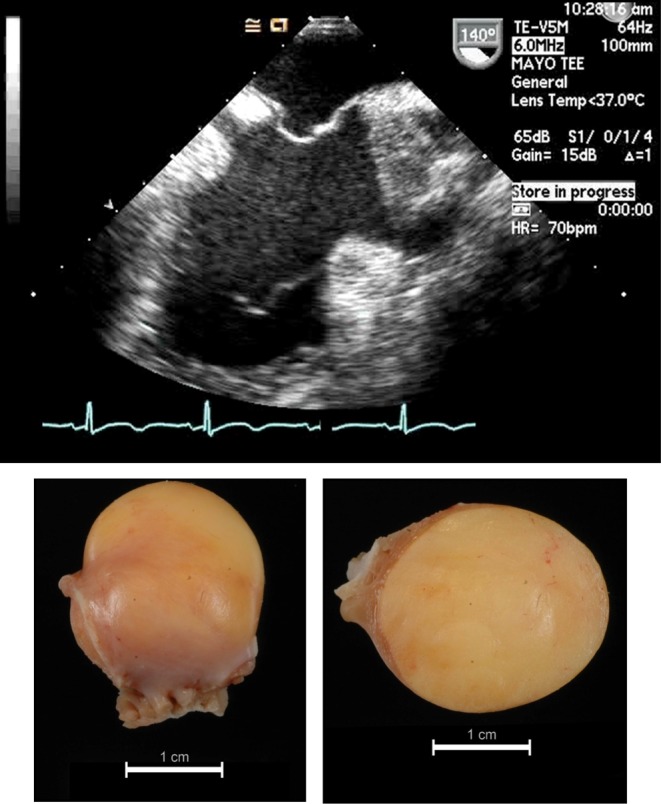
Lipoma diagnosed in a 47-year-old female presenting with chest pain and history of possible tuberous sclerosis. The echocardiographic image and its surgical specimen and cut section are presented in the top and the bottom left and right panel, respectively.
Lipomatous hypertrophy of the interatrial septum is due to fatty infiltration of the proximal and distal portions of the atrial septum, generally with sparing of the fossa ovalis and most commonly seen in the elderly and obese (36). It is not a true tumor, although it can be mistaken for one, namely a myxoma or lipoma. The characteristic appearance and location aids in the differentiation. Resection is not required unless extremely rare symptoms related to it, such as atrial arrhythmias, heart block or caval obstruction, need to be addressed (37, 38).
Other benign primary cardiac tumors
Hemangiomas, atrioventricular node tumors and teratomas are even rarer benign tumors that may occur within the heart. Hemangiomas are made up of dilated vascular channels; thus, their echocardiographic appearance is one of an echogenic mass with echolucencies (Fig. 10). They can be located within the endocardium, myocardium, epicardium or pericardium, and are commonly seen in the right ventricular free wall or the left ventricular lateral wall. Teratomas, which are extremely rare in adults, arise in the pericardium and can cause tamponade or extrinsic compression of the heart.
Figure 10.
Hemangioma found accidentally in a 46-year-old male presenting with palpitations. The two upper panel images illustrate the echocardiographic presentation, the left lower panel illustrates the color Doppler mode indicating vascularity; the corresponding surgical specimen is presented in the lower right panel.
Malignant primary cardiac tumors
Malignant cardiac tumors constitute 15% of primary cardiac tumors (12). However, a tumor present only on the right side of the heart has an almost 50% chance of being a malignant primary cardiac tumor (39). It is very rare for a malignant cardiac tumor to occur exclusively in places other than those within the cardiac chambers. Evidence of rapid growth, local invasion and hemorrhagic pericardial effusion are all additional signs of malignancies. A pericardial tumor is more commonly malignant as well (39). As with benign cardiac tumors, the clinical presentation can vary but typically is related to the location of the tumor. Precordial pain usually indicates a malignant rather than a benign process.
Sarcomas
Sarcomas are the most common of the malignant cardiac tumors typically diagnosed in individuals in their mid-40s (40). Undifferentiated sarcomas appear as a broad-based mass on an echocardiogram, typically in the left atrium (differential diagnosis is a myxoma) with heterogeneous echogenicity; hypoechogenic areas may indicate tumor necrosis (Fig. 11).
Figure 11.
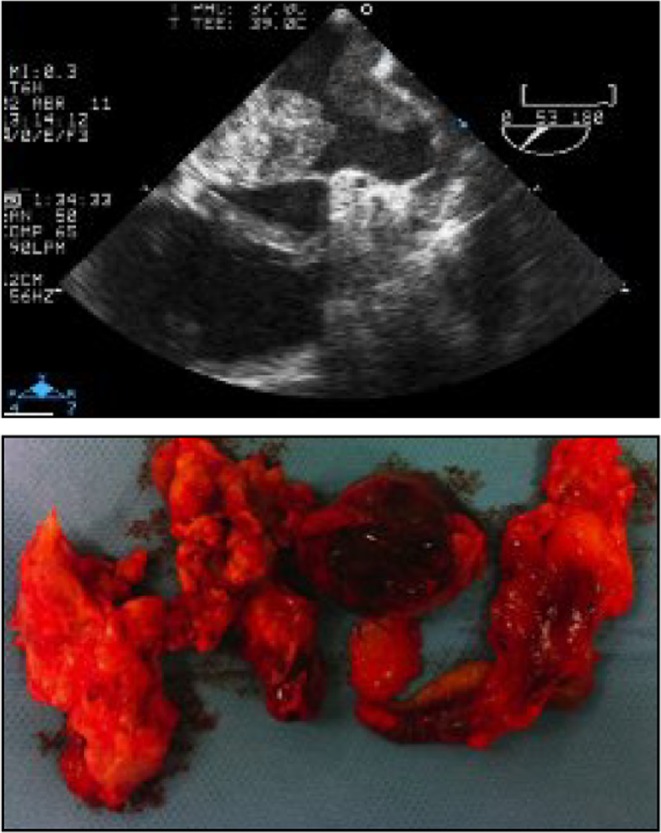
Typical echocardiographic presentation and surgical correlate (upper and lower panels) of a cardiac sarcoma. Reproduced with permission of the Publisher. Original source: Solla-Ruiz I, Villanueva-Benito I, Begoña Iglesias-Rodriguez M, Asorey-Veiga V, Yas SR, Pereira-Loureiro MA. Hemoptysis as the presenting symptom in cardiac sarcoma. Revista Portuguesa de Cardiologia 2012; 31: 463–464. Copyright 2012 Sociedade Portuguesa de Cardiologia. Published by Elsevier España, S.L. All rights reserved. (62)..
Any type of sarcoma may develop in the heart (41), but the most common is the angiosarcoma. The predilection site is the right side of the heart, particularly the right atrium. Echocardiographically they emerge as lobulated masses, distinctly heterogeneous with an area of necrosis or hemorrhage (Fig. 12). They have no stalk, differentiating them from myxomas or PFEs. Even though vascular, contrast echocardiography may not always reveal significant enhancement, which may be due to the slit-like vascular channels and the solid spindle cell areas (42). These tumors tend to have direct involvement with the pericardium, resulting in pericardial effusions, with or without tamponade. Interestingly, however, cytology on the pericardial fluid is frequently unrevealing (42). Angiosarcomas also tend to replace the right atrial wall and fill the cardiac chamber. In addition, as they develop near the right atrioventricular groove, the function of the tricuspid valve can be affected as well (42, 43). The signs and symptoms related to a cardiac angiosarcoma therefore often include pericardial chest pain, obstruction, congestion, dyspnea and fatigue (44). Unfortunately, metastatic disease (often to the lungs) is common at the time of diagnosis in the majority of patients (45, 46). The prognosis is poor, and without resection, 90% of patients die within one year of diagnosis (47). Improved survival is seen with left-sided tumors that have not metastasized at the time of diagnosis (48). Even with metastatic disease, surgical debulking does have a survival advantage (42).
Figure 12.
Example of an angiosarcoma diagnosed in a 63-year-old woman presenting with syncope and atypical chest pain. Reproduced, with permission, from Yang HS, Lee KS, Chaliki HP, Tazelaar HD, Lusk JL, Chandrasekaran K & Tajik AJ, Anomalous insertion of the papillary muscle causing left ventricular outflow obstruction: visualization by real-time three-dimensional echocardiography, European Heart Journal: Cardiovascular Imaging, 2008, volume 9, pages 733–738, by permission of Oxford University Press. (63).
Rhabdomyosarcomas in some series are the second most common primary cardiac sarcoma and can arise from any cardiac structure; they do not seem to have a preference for any particular location (26). These tumors tend to occur in multiple sites within the heart and can cause obstruction at multiple levels (49). They grow rapidly and invade the pericardium early in their course resulting in a poor prognosis (48).
Leimyosarcomas, osteosarcomas, fibrosarcomas and undifferentiated sarcomas are other rare primary cardiac sarcomas. Fibrosarcomas and undifferentiated sarcomas tend to be white fleshy tumors that have prominent areas of necrosis and hemorrhage (50). Leimyosarcomas and osteosarcomas tend to occur more commonly in the left atrium.
Sarcomas in general grow rapidly, extensively and metastasize early. Complete resection is the most ideal option; however, metastatic disease and recurrence limit long-term survival even with complete excision (51, 52). Adjuvant chemotherapy and radiation have been attempted to improve the poor overall survival; however, no randomized trials have been undertaken (40). Radiation typically is used for metastatic disease (53).
Lymphoma
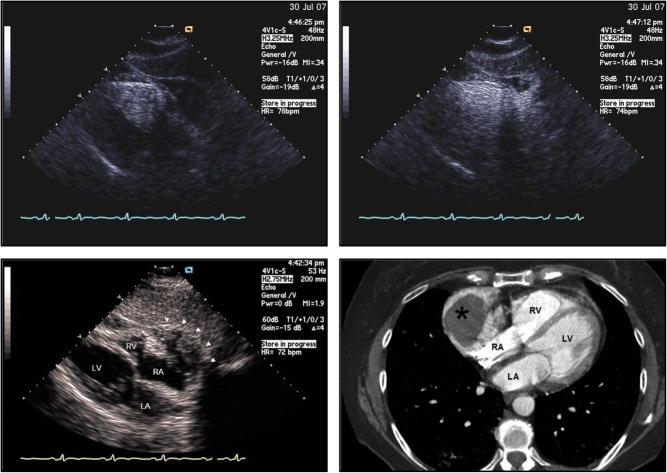
Cardiac lymphomas are less common than sarcomas. On autopsy, 16% of patients with Hodgkin disease and 18% of patients with non-Hodgkin lymphoma had notable cardiac involvement at a median of 20 months after initial diagnosis (54). Primary cardiac lymphomas on the other hand have been seen with increasing frequency in the immunocompromised host (55, 56), such as post-transplant or HIV patients. These tumors are most commonly diffuse large B-cell lymphomas. On echocardiography, they can appear as homogeneous, infiltrating masses leading to ‘wall thickening’ and restrictive hemodynamics or as nodular masses intruding into the heart chambers, preferentially the right heart chambers and especially the right atrium (Fig. 13). The AV groove can be affected as well, encasing the right coronary artery, as well as the pericardium with effusion or enfacement. TEE is the superior imaging technique and should be pursued when there is a need to identify these tumors (56). Depending on the location of the mass, signs and symptoms of inflow obstruction can be noted ranging from cava syndromes to constriction/restriction, heart failure and arrhythmias such as AV nodal block, and phenomena of embolism. Diagnosis can be made with cytology testing of pericardial fluid or by transvenous biopsy under echocardiographic guidance (56). This is a key step as the prognosis of lymphomas is generally much better than that of other primary cardiac masses if patients are eligible for suitable chemotherapy. Radiation therapy is less favorable, and surgical resection of the entire tumor is extremely difficult (56).
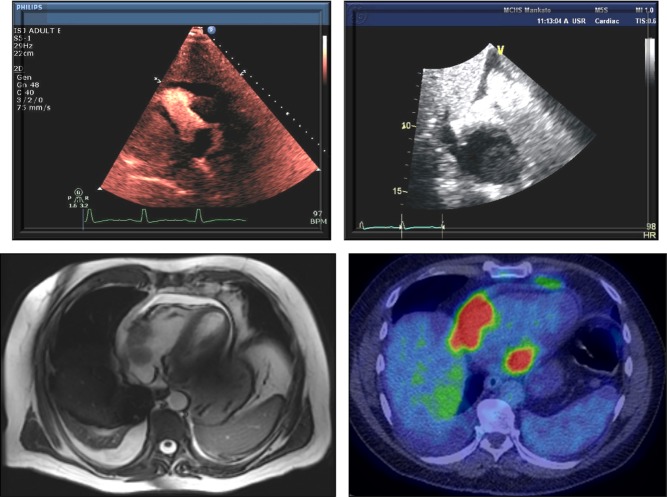
Figure 13.
Cardiac lymphoma in a 55-year-old male presenting with RUQ abdominal pain. Echocardiogram, especially with echo contrast, shows a mass in the right ventricle (upper left panel) and right atrium (upper right panel), close to the lateral tricuspid valve, confirmed by cardiac MRI (lower left panel) and with high metabolic uptake on FTG-PET (lower right panel).
Mesothelioma
Malignant mesotheliomas account for half of the primary pericardial tumors. The other half of primary pericardial tumors is benign (teratomas, fibromas and lipomas). Symptoms from a pericardial mesothelioma include chest pain, cough and palpitations (57). These tumors form bulky nodules within the pericardial cavity, encircling the heart, mimicking pericarditis, tamponade or pericardial constriction (57). Echocardiography typically reveals a pericardial effusion and a tumor encasing the heart; a discrete mass may not be seen (Fig. 14). Prognosis is poor, although surgery plus radiation can provide some palliative benefit (57).
Figure 14.
Pericardial mesothelioma in a 41-year-old female referred to cardiology due to concerns about pericardial constriction and a six-month history of fevers, night sweats, weight loss, profound dyspnea, edema and cough.
Metastatic cardiac tumors
Metastatic involvement of the heart is not an uncommon occurrence. In autopsy series, metastasis to the heart occurs at a frequency of 0.7–3.5% in the general population, but increases to an average of 7.1% in persons with known malignancies (58). Metastases may occur through direct invasion of a nearby tumor, hematogenous spread, transvenous extension through the inferior vena cava or lymphatic spread. The most common tumors with metastatic potential to the heart are lung carcinoma, breast carcinoma, esophageal carcinoma, malignant lymphoma, leukemia and malignant melanoma. Malignant melanoma, due to its hematogenous spread, has the highest propensity to have cardiac involvement (59). The pericardium tends to be most commonly involved with metastases (60) and typically presents with a pericardial effusion, which may be asymptomatic. Renal cell carcinoma reaches the heart by extension through the inferior vena cava, but hematogenous metastases can also be seen (Fig. 15). Cardiac involvement should be suspected or sought in any patient with a known malignancy, who develops new cardiovascular signs or symptoms. Echocardiography should then be undertaken as the initial diagnostic test to evaluate for the presence of cardiac metastatic disease. However, other imaging modalities are frequently used to provide additional information and characterization of potential metastatic disease. Unfortunately, the presence of cardiac metastases occurs in patients with disseminated disease burden. Therefore, prognosis is typically poor when cardiac metastases are identified.
Figure 15.
Metastasis of a renal cell carcinoma (RCC) in the left ventricular lateral wall in a 69-year-old man with a history of RCC, s/p nephrectomy, who presented with atypical chest pain.
Conclusion
Cardiac tumors, both primary and metastatic, are frequently first found and/or evaluated by transthoracic echocardiography. Thrombus (Fig. 16) and vegetation are the two most common entities. Most true cardiac tumors are secondary, i.e. metastatic, whereby malignant melanoma has the highest propensity to metastasize to the heart. Primary cardiac tumors are mostly benign and have a good prognosis, whereas malignant primary cardiac tumors are most commonly sarcomas and have a poor prognosis. Echocardiography is a readily available, portable, low-cost imaging modality, which gives the first clue as to the etiology of a cardiac mass; characteristics of a mass such as location, mobility, attachment and appearance can help determine whether a mass is benign or malignant. New techniques such as contrast echocardiography and three-dimensional echocardiography may further enhance the ability to determine with confidence the etiology of a cardiac mass. At the end, entertaining all possibilities, a stepwise approach may serve well in clinical practice Figs. 16, 17, 18, 19).
Figure 16.
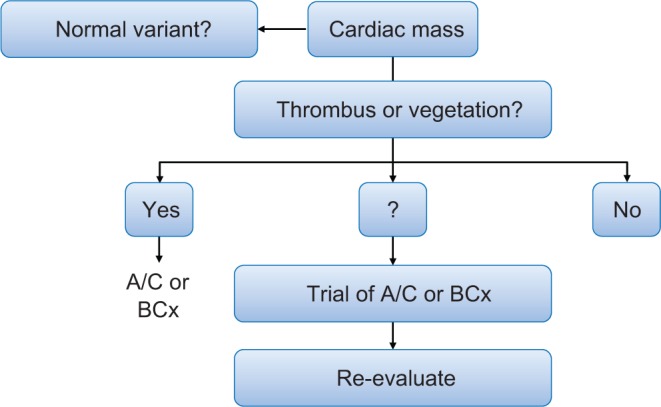
Illustration of the differential diagnostic algorithm for the diagnosis of a cardiac mass and principle #1 that thrombus or vegetation is often most likely. Reproduced from Heart, Bruce CJ, volume 97, pages 151–160, copyright 2011; with permission from BMJ Publishing Group Ltd. (31).
Figure 17.
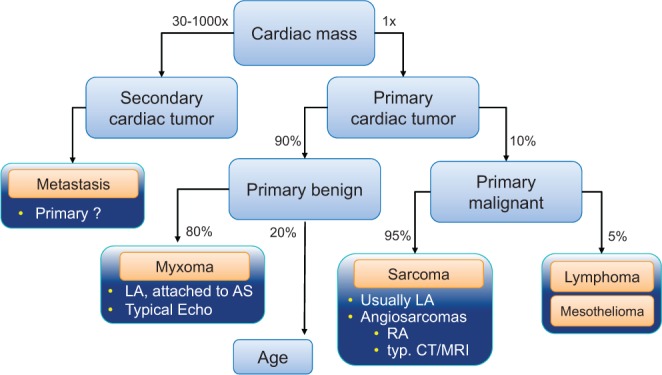
Illustration of the differential diagnostic algorithm for the diagnosis of a cardiac mass and principle #2 that cardiac tumors are most secondary and principle #3 that primary cardiac tumors are mostly benign. Reproduced from Heart, Bruce CJ, volume 97, pages 151–160, copyright 2011; with permission from BMJ Publishing Group Ltd. (31).
Figure 18.
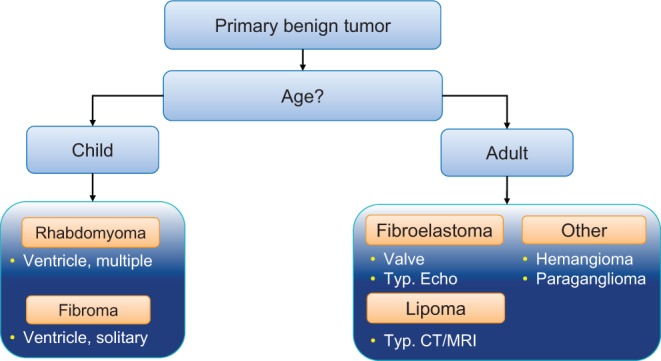
Illustration of the differential diagnoses for primary cardiac tumors by age. Reproduced from Heart, Bruce CJ, volume 97, pages 151–160, copyright 2011; with permission from BMJ Publishing Group Ltd. (31).
Figure 19.
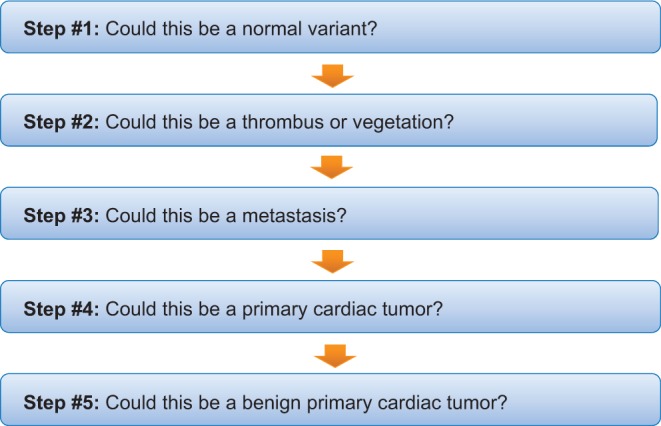
Pragmatic steps to be taken with the finding of a cardiac mass on echocardiogram.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- 1.Lam KY, Dickens P, Chan AC. 1993. Tumors of the heart. A 20-year experience with a review of 12,485 consecutive autopsies. Archives of Pathology and Laboratory Medicine 117 1027–1031. [PubMed] [Google Scholar]
- 2.Reynen K. 1996. Frequency of primary tumors of the heart. American Journal of Cardiology 77 107 ( 10.1016/S0002-9149(97)89149-7) [DOI] [PubMed] [Google Scholar]
- 3.Kirkpatrick JN, Wong T, Bednarz JE, Spencer KT, Sugeng L, Ward RP, DeCara JM, Weinert L, Krausz T, Lang RM. 2004. Differential diagnosis of cardiac masses using contrast echocardiographic perfusion imaging. Journal of the American College of Cardiology 43 1412–1419. ( 10.1016/j.jacc.2003.09.065) [DOI] [PubMed] [Google Scholar]
- 4.Mansencal N, Revault-d’Allonnes L, Pelage JP, Farcot JC, Lacombe P & Dubourg O. 2009. Usefulness of contrast echocardiography for assessment of intracardiac masses. Archives of Cardiovascular Diseases 102 177–183. ( 10.1016/j.acvd.2008.12.007) [DOI] [PubMed] [Google Scholar]
- 5.Zaragoza-Macias E, Chen MA, Gill EA. 2012. Real time three-dimensional echocardiography evaluation of intracardiac masses. Echocardiography 29 207–219. ( 10.1111/j.1540-8175.2011.01627.x) [DOI] [PubMed] [Google Scholar]
- 6.Elbardissi AW, Dearani JA, Daly RC, Mullany CJ, Orszulak TA, Puga FJ, Schaff HV. 2009. Embolic potential of cardiac tumors and outcome after resection: a case-control study. Stroke 40 156–162. ( 10.1161/STROKEAHA.108.525709) [DOI] [PubMed] [Google Scholar]
- 7.Perez Baztarrica G, Nieva N, Gariglio L, Salvaggio F, Porcile R. 2010. Images in cardiovascular medicine. Primary cardiac lymphoma: a rare case of pulmonary tumor embolism. Circulation 121 2249–2250. ( 10.1161/CIRCULATIONAHA.109.863126) [DOI] [PubMed] [Google Scholar]
- 8.Cina SJ, Smialek JE, Burke AP, Virmani R, Hutchins GM. 1996. Primary cardiac tumors causing sudden death: a review of the literature. American Journal of Forensic Medicine and Pathology 17 271–281. ( 10.1097/00000433-199612000-00001) [DOI] [PubMed] [Google Scholar]
- 9.Salcedo EE, Cohen GI, White RD, Davison MB. 1992. Cardiac tumors: diagnosis and management. Current Problems in Cardiology 17 73–137. ( 10.1016/0146-2806(92)90025-J) [DOI] [PubMed] [Google Scholar]
- 10.Silvestri F, Bussani R, Pavletic N, Mannone T. 1997. Metastases of the heart and pericardium. Giornale Italiano di Cardiologia 27 1252–1255. [PubMed] [Google Scholar]
- 11.Larrieu AJ, Jamieson WR, Tyers GF, Burr LH, Munro AI, Miyagishima RT, Gerein AN, Allen P. 1982. Primary cardiac tumors: experience with 25 cases. Journal of Thoracic and Cardiovascular Surgery 83 339–348. [PubMed] [Google Scholar]
- 12.Molina JE, Edwards JE, Ward HB. 1990. Primary cardiac tumors: experience at the University of Minnesota. Thoracic and Cardiovascular Surgeon 38 (Supplement 2) 183–191. ( 10.1055/s-2007-1014064) [DOI] [PubMed] [Google Scholar]
- 13.Tazelaar HD, Locke TJ, McGregor CG. 1992. Pathology of surgically excised primary cardiac tumors. Mayo Clinic Proceedings 67 957–965. ( 10.1016/S0025-6196(12)60926-4) [DOI] [PubMed] [Google Scholar]
- 14.Sarjeant JM, Butany J, Cusimano RJ. 2003. Cancer of the heart: epidemiology and management of primary tumors and metastases. American Journal of Cardiovascular Drugs 3 407–421. ( 10.2165/00129784-200303060-00004) [DOI] [PubMed] [Google Scholar]
- 15.St John Sutton MG, Mercier LA, Giuliani ER, Lie JT. 1980. Atrial myxomas: a review of clinical experience in 40 patients. Mayo Clinic Proceedings 55 371–376. [PubMed] [Google Scholar]
- 16.Pinede L, Duhaut P, Loire R. 2001. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine 80 159–172. ( 10.1097/00005792-200105000-00002) [DOI] [PubMed] [Google Scholar]
- 17.Reynen K. 1995. Cardiac myxomas. New England Journal of Medicine 333 1610–1617. [DOI] [PubMed] [Google Scholar]
- 18.Javed A, Zalawadiya S, Kovach J, Afonso L. 2014. Aortic valve myxoma at the extreme age: a review of literature. BMJ Case Reports 2014. bcr2013202689 ( 10.1136/bcr-2013-202689) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee VH, Connolly HM, Brown RD., Jr 2007. Central nervous system manifestations of cardiac myxoma. Archives of Neurology 64 1115–1120. ( 10.1001/archneur.64.8.1115) [DOI] [PubMed] [Google Scholar]
- 20.Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL. 1985. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine 64 270–283. ( 10.1097/00005792-198507000-00007) [DOI] [PubMed] [Google Scholar]
- 21.Vidaillet HJ, Jr, Seward JB, Fyke FE, 3rd, Su WP, Tajik AJ. 1987. ‘Syndrome myxoma’: a subset of patients with cardiac myxoma associated with pigmented skin lesions and peripheral and endocrine neoplasms. British Heart Journal 57 247–255. ( 10.1136/hrt.57.3.247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keeling IM, Oberwalder P, Anelli-Monti M, Schuchlenz H, Demel U, Tilz GP, Rehak P, Rigler B. 2002. Cardiac myxomas: 24 years of experience in 49 patients. European Journal of Cardio-Thoracic Surgery 22 971–977. ( 10.1016/S1010-7940(02)00592-4) [DOI] [PubMed] [Google Scholar]
- 23.D’Alfonso A, Catania S, Pierri MD, Matteucci SL, Rescigno G, Münch C, Staine J, Iacobone G, Piccoli GP. 2008. Atrial myxoma: a 25-year single-institutional follow-up study. Journal of Cardiovascular Medicine 9 178–181. ( 10.2459/jcm.0b013e3281ac22cb) [DOI] [PubMed] [Google Scholar]
- 24.Shah IK, Dearani JA, Daly RC, Suri RM, Park SJ, Joyce LD, Li Z, Schaff HV. 2015. Cardiac myxomas: a 50-year experience with resection and analysis of risk factors for recurrence. Annals of Thoracic Surgery 100 495–499. ( 10.1016/j.athoracsur.2015.03.007) [DOI] [PubMed] [Google Scholar]
- 25.Bhan A, Mehrotra R, Choudhary SK, Sharma R, Prabhakar D, Airan B, Kumar AS, Venugopal P. 1998. Surgical experience with intracardiac myxomas: long-term follow-up. Annals of Thoracic Surgery 66 810–813. ( 10.1016/S0003-4975(98)00591-8) [DOI] [PubMed] [Google Scholar]
- 26.McAllister HA, Hall RJ, Cooley DA. 1999. Tumors of the heart and pericardium. Current Problems in Cardiology 24 57–116. ( 10.1016/S0146-2806(99)80001-2) [DOI] [PubMed] [Google Scholar]
- 27.Tamin SS, Maleszewski JJ, Scott CG, Khan SK, Edwards WD, Bruce CJ, Oh JK, Pellikka PA, Klarich KW. 2015. Prognostic and bioepidemiologic implications of papillary fibroelastomas. Journal of the American College of Cardiology 65 2420–2429. ( 10.1016/j.jacc.2015.03.569) [DOI] [PubMed] [Google Scholar]
- 28.Klarich KW, Enriquez-Sarano M, Gura GM, Edwards WD, Tajik AJ, Seward JB. 1997. Papillary fibroelastoma: echocardiographic characteristics for diagnosis and pathologic correlation. Journal of the American College of Cardiology 30 784–790. ( 10.1016/S0735-1097(97)00211-8) [DOI] [PubMed] [Google Scholar]
- 29.Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC, Sacchi TJ. 2003. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. American Heart Journal 146 404–410. ( 10.1016/S0002-8703(03)00249-7) [DOI] [PubMed] [Google Scholar]
- 30.Sun JP, Asher CR, Yang XS, Cheng GG, Scalia GM, Massed AG, Griffin BP, Ratliff NB, Stewart WJ, Thomas JD. 2001. Clinical and echocardiographic characteristics of papillary fibroelastomas: a retrospective and prospective study in 162 patients. Circulation 103 2687–2693. ( 10.1161/01.CIR.103.22.2687) [DOI] [PubMed] [Google Scholar]
- 31.Bruce CJ. 2011. Cardiac tumours: diagnosis and management. Heart 97 151–160. ( 10.1136/hrt.2009.186320) [DOI] [PubMed] [Google Scholar]
- 32.Beghetti M, Gow RM, Haney I, Mawson J, Williams WG, Freedom RM. 1997. Pediatric primary benign cardiac tumors: a 15-year review. American Heart Journal 134 1107–1114. ( 10.1016/S0002-8703(97)70032-2) [DOI] [PubMed] [Google Scholar]
- 33.Smythe JF, Dyck JD, Smallhorn JF, Freedom RM. 1990. Natural history of cardiac rhabdomyoma in infancy and childhood. Amerian Journal of Cardiology 66 1247–1249. ( 10.1016/0002-9149(90)91109-J) [DOI] [PubMed] [Google Scholar]
- 34.Jacobs JP, Konstantakos AK, Holland FW, 2nd, Herskowitz K, Ferrer PL, Perryman RA. 1994. Surgical treatment for cardiac rhabdomyomas in children. Annals of Thoracic Surgery 58 1552–1555. ( 10.1016/0003-4975(94)91963-1) [DOI] [PubMed] [Google Scholar]
- 35.Elbardissi AW, Dearani JA, Daly RC, Mullany CJ, Orszulak TA, Puga FJ, Schaff HV. 2008. Survival after resection of primary cardiac tumors: a 48-year experience. Circulation 118 (Supplement 14) S7–S15. ( 10.1161/CIRCULATIONAHA.107.783126) [DOI] [PubMed] [Google Scholar]
- 36.Basu S, Morshuis W, Heijmen R, Jaarsma W. 1994. Lipomatous hypertrophy of the interatrial septum. Cardiovascular Surgery 2 229–231. [PubMed] [Google Scholar]
- 37.Zeebregts CJ, Hensens AG, Timmermans J, Pruszczynski MS, Lacquet LK. 1997. Lipomatous hypertrophy of the interatrial septum: indication for surgery? European Journal of Cardio-Thoracic Surgery 11 785–787. ( 10.1016/S1010-7940(96)01078-0) [DOI] [PubMed] [Google Scholar]
- 38.Cale R, Andrade MJ, Canada M, Hernandez-Estefania R, Lima S, Abecasis M, Vitorino E, Gouveia R, Gouveia R, Silva JA. 2009. Lipomatous hypertrophy of the interatrial septum: report of two cases where histological examination and surgical intervention were unavoidable. European Journal of Echocardiography 10 876–879. ( 10.1093/ejechocard/jep080) [DOI] [PubMed] [Google Scholar]
- 39.Meng Q, Qian Y, Lai H, Lai S, Lima J, Tong W. 2002. Echocardiographic and pathologic characteristics of primary cardiac tumors: a study of 149 cases. International Journal of Cardiology 84 69–75. ( 10.1016/S0167-5273(02)00136-5) [DOI] [PubMed] [Google Scholar]
- 40.Simpson L, Kumar SK, Okuno SH, Schaff HV, Porrata LF, Buckner JC, Moynihan TJ. 2008. Malignant primary cardiac tumors. Cancer 112 2440–2446. ( 10.1002/cncr.23459) [DOI] [PubMed] [Google Scholar]
- 41.Vander Salm TJ. 2000. Unusual primary tumors of the heart. Seminars in Thoracic and Cardiovascular Surgery 12 89–100. ( 10.1053/ct.2000.5080) [DOI] [PubMed] [Google Scholar]
- 42.Kupsky DF, Newman DB, Kumar G, Maleszewski JJ, Edwards WD, Klarich KW. 2016. Echocardiographic features of cardiac angiosarcomas: the mayo clinic experience (1976–2013). Echocardiography 33 186–192. ( 10.1111/echo.13060) [DOI] [PubMed] [Google Scholar]
- 43.Neragi-Miandoab S, Kim J, Vlahakes GJ. 2007. Malignant tumours of the heart: a review of tumour type, diagnosis and therapy. Clinical Oncology 19 748–756. ( 10.1016/j.clon.2007.06.009) [DOI] [PubMed] [Google Scholar]
- 44.Burnside N, MacGowan SW. 2012. Malignant primary cardiac tumours. Interactive Cardiovascular and Thoracic Surgery 15 1004–1006. ( 10.1093/icvts/ivs350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bear PA, Moodie DS. 1987. Malignant primary cardiac tumors. The Cleveland Clinic experience, 1956 to 1986. Chest 92 860–862. ( 10.1378/chest.92.5.860) [DOI] [PubMed] [Google Scholar]
- 46.Butany J, Yu W. 2000. Cardiac angiosarcoma: two cases and a review of the literature. Canadian Journal of Cardiology 16 197–205. [PubMed] [Google Scholar]
- 47.Truong PT, Jones SO, Martens B, Alexander C, Paquette M, Joe H, Hart J, Allan SJ. 2009. Treatment and outcomes in adult patients with primary cardiac sarcoma: the British Columbia Cancer Agency experience. Annals of Surgical Oncology 16 3358–3365. ( 10.1245/s10434-009-0734-8) [DOI] [PubMed] [Google Scholar]
- 48.Burke AP, Cowan D, Virmani R. 1992. Primary sarcomas of the heart. Cancer 69 387–395. () [DOI] [PubMed] [Google Scholar]
- 49.Roberts WC. 1997. Primary and secondary neoplasms of the heart. American Journal of Cardiology 80 671–682. ( 10.1016/S0002-9149(97)00587-0) [DOI] [PubMed] [Google Scholar]
- 50.Okamoto K, Kato S, Katsuki S, Wada Y, Toyozumi Y, Morimatsu M, Aoyagi S, Imaizumi T. 2001. Malignant fibrous histiocytoma of the heart: case report and review of 46 cases in the literature. Internal Medicine 40 1222–1226. ( 10.2169/internalmedicine.40.1222) [DOI] [PubMed] [Google Scholar]
- 51.Centofanti P, Di Rosa E, Deorsola L, Dato GM, Patanè F, La Torre M, Barbato L, Verzini A, Fortunato G, di Summa M. 1999. Primary cardiac tumors: early and late results of surgical treatment in 91 patients. Annals of Thoracic Surgery 68 1236–1241. ( 10.1016/S0003-4975(99)00700-6) [DOI] [PubMed] [Google Scholar]
- 52.Bakaeen FG, Michael JR, Joseph SC, Charles CM, Jimmy FH, Gerald ML, Rafael E, Mahesh KR, George PN, Donald GW, et al. 2003. Surgical outcome in 85 patients with primary cardiac tumors. American Journal of Surgery 186 641–647; discussion 647. ( 10.1016/j.amjsurg.2003.08.004) [DOI] [PubMed] [Google Scholar]
- 53.Bakaeen FG, Jaroszewski DE, Rice DC, Walsh GL, Vaporciyan AA, Swisher SS, Benjamin R, Blackmon S, Reardon MJ. 2009. Outcomes after surgical resection of cardiac sarcoma in the multimodality treatment era. Journal of Thoracic and Cardiovascular Surgery 137 1454–1460. ( 10.1016/j.jtcvs.2008.11.026) [DOI] [PubMed] [Google Scholar]
- 54.Petersen CD, Robinson WA, Kurnick JE. 1976. Involvement of the heart and pericardium in the malignant lymphomas. American Journal of the Medical Sciences 272 161–165. ( 10.1097/00000441-197609000-00005) [DOI] [PubMed] [Google Scholar]
- 55.Ragland MM, Tak T. 2006. The role of echocardiography in diagnosing space-occupying lesions of the heart. Clinical Medicine Research 4 22–32. ( 10.3121/cmr.4.1.22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miguel CE, Bestetti RB. 2011. Primary cardiac lymphoma. International Journal of Cardiology 149 358–363. ( 10.1016/j.ijcard.2010.02.016) [DOI] [PubMed] [Google Scholar]
- 57.Grebenc ML, Rosado de Christenson ML, Burke AP, Green CE, Galvin JR. 2000. Primary cardiac and pericardial neoplasms: radiologic-pathologic correlation. Radiographics 20 1073–1103; quiz 1110–1111, 1112. ( 10.1148/radiographics.20.4.g00jl081073) [DOI] [PubMed] [Google Scholar]
- 58.Al-Mamgani A, Baartman L, Baaijens M, de Pree I, Incrocci L, Levendag PC. 2008. Cardiac metastases. International Journal of Clinical Oncology 13 369–372. ( 10.1007/s10147-007-0749-8) [DOI] [PubMed] [Google Scholar]
- 59.Reynen K, Kockeritz U, Strasser RH. 2004. Metastases to the heart. Annals of Oncology 15 375–381. ( 10.1093/annonc/mdh086) [DOI] [PubMed] [Google Scholar]
- 60.Goldberg AD, Blankstein R, Padera RF. 2013. Tumors metastatic to the heart. Circulation 128 1790–1794. ( 10.1161/circulationaha.112.000790) [DOI] [PubMed] [Google Scholar]
- 61.Dujardin KS, Click RL, Oh TK. 2000. The role of intraoperative transesophageal echocardiography in patients undergoing cardiac mass removal. Journal of the American Society of Echocardiography 13 1080–1083. [DOI] [PubMed] [Google Scholar]
- 62.Solla-Ruiz I, Villanueva-Benito I, Begoña Iglesias-Rodriguez M, Asorey-Veiga V, Pereira-Loureiro MA. 2012. Hemoptysis as the presenting symptom in cardiac sarcoma. Revista Portuguesa de Cardiologia 31 463–464. [DOI] [PubMed] [Google Scholar]
- 63.Yang HS Lee KS Chaliki HP Tazelaar HD Lusk JL Chandrasekaran K, & Tajik AJ. 2008. Anomalous insertion of the papillary muscle causing left ventricular outflow obstruction: visualization by real-time three-dimensional echocardiography. European Heart Journal: Cardiovascular Imaging 9 733–738. [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a