Abstract
Background
Recently, there is an increasing interest in developing specific treatments while managing lung cancer cases. We tested the expressions of six molecular markers in the primary tumor and the metastatic lymph nodes of lung cancer patients at a single institution in China.
Material/Methods
A total of 48 patients with lung cancer who were admitted to the Department of Cardiothoracic Surgery, the First Affiliated Hospital of General Hospital of the Chinese People’s Liberation Army, from September 2010 to February 2011 were retrospectively reviewed.
Results
One of the six biomarkers’ expressions, excision repair cross-complementation group 1 (ERCC-1), was found to be significantly different in primary tumors and metastatic sites in different cancer subtypes.
Conclusions
The onset and pathogenesis of small-cell lung carcinoma (SCLC) and non-small-cell lung carcinoma (NSCLC) are not completely understood, and the predictions of prognosis are not very reliable. The use of molecular markers to guide treatment of these cancers is currently in its initial stages.
MeSH Keywords: Lung Neoplasms, Lymph Nodes, Molecular Medicine
Background
Lung cancer has the highest incidence and mortality rate among all the cancers worldwide [1]. Lung cancer mortality has declined in the recent years. However, because of the increase of the cigarette smoking, the incidence of lung cancer is increasing in China [2]. In 2005, nearly 429,000 people died from lung cancer in China, and this number is expected to increase dramatically in the coming years[ 2].
Most lung cancers are carcinomas that are histologically classified as small-cell lung carcinoma (SCLC) and non-small-cell lung carcinoma (NSCLC) [3]. NSCLC accounts for about 80% of all lung cancers and can be further subdivided into squamous cell carcinoma, adenocarcinoma, and large-cell lung carcinoma. Squamous NSCLC accounts for about 30% of all NSCLC cases [4]. This subtype of NSCLC, which tends to be slow growing, is mainly related to tobacco smoking and is more common in men [5]. NSCLC adenocarcinomas and NSCLC large-cell lung carcinomas tend to metastasize soon after the onset the disease, and patients with these subtypes typically have poor prognosis and high mortality [6].
Surgery is one of the options in treatment of lung cancer, but it is unsuitable for patients with advanced disease. Chemotherapy or radiotherapy, which may be combined with surgery, is another important treatment option, and there is great interest in the development of patient-specific targeted drugs that consider the molecular characteristics of the disease [7]. Recent studies in Asian and Western patients have indicated that lipoprotein receptor-related protein 1 (LRP-1), ribonucleotide reductase M 1 (RRM-1), epidermal growth factor receptor (EGFR), and excision repair cross-complementing gene 1 (ERCC-1) may be useful molecular markers to guide drug selection in patients with lung cancer [8–13]. Determining the K-RAS and EGFR mutation status in both primary and metastatic tumors may be critical for making meaningful decisions regarding the appropriate use of targeted therapies. There were tight relationships between K-RAS and EGFR in NSCLC. Anaplastic lymphoma kinase (ALK) is present in a subgroup of NSCLCs, representing 2% to 7% of such tumors. At present, targeted chemotherapy of cancer is based on the sensitivity of the primary tumor site to specific drugs. For example, Iressa and Tarceva target the tyrosine kinase domain of EGFR, so these drugs are often given to patients whose tumors have high expression of this marker. However, expression of biomarkers may be different in the primary tumors and residual metastatic lymph nodes, so these tissues may have different drug sensitivities. The different expression of these drug selection-associated genes between the primary tumor site and the metastatic lymph nodes might account for the variable chemotherapeutic effects of the drugs, which are mainly based on gene expression level at the primary tumor site.
Several molecular markers can be the diagnosis molecular marker [14–16]. In most cases, one or two markers were selected to be researched. In the present retrospective study, we compared the expression of six molecular markers (MDR-1, LRP, RRM-1, EGFR, ERCC-1, and BRCA-1) in the primary tumor tissues and metastatic lymph nodes of patients with NSCLC. The more molecular markers used, the more facts reflected. This study focused on the characteristics of molecules’ expressions and aimed at identifying the differences within NSCLC patients between primary tumor and metastatic lymph nodes, so we only choose the markers (MDR-1, LRP, RRM-1, EGFR, ERCC-1, and BRCA-1) as representative.
Material and Methods
Specimen
This retrospective study included all the pathologically confirmed lymph node metastasis patients who opted for surgical removal of primary or primary and metastatic lesions, and were admitted to our hospital, Department of Cardiothoracic Surgery, the First Affiliated Hospital of General Hospital of the Chinese People’s Liberation Army, from September 2010 to October 2011. Based on these criteria, a total of 48 patients were considered eligible. In all cases, lymph node metastasis was limited to the ipsilateral side lobe bronchus N1, N2 and the ipsilateral mediastinum that could be completely removed by surgery. Chemotherapy consisted of paclitaxel with carboplatin, docetaxel with cisplatin, or gemcitabine with cisplatin. Some patients received targeted therapy with gefitinib. NSCLC staging was based on the TNM Classification of Malignant Tumors, 7th Edition [17]. This study was conducted in accordance with the Declaration of Helsinki and with approval from the Ethics Committee of the General Hospital of People’s Liberation Army. Written informed consent was obtained from all participants.
Specimen processing
The primary lesions and lymph nodes were surgically removed,and specimens were stored in 10% neutral formalin for 24 h. Samples were trimmed to 2 mm, dehydrated, embedded in paraffin, cut into 5 μm sections, and dried overnight at 37°C.
For hematoxylin-eosin stain, samples were immersed in a jar containing 15% fresh H2O2 for 10 min and washed thoroughly in tap water. Slides were then stained in Harris’s hematoxylin for 15 min followed by tap water washing, and counterstained by using 1% aqueous eosin Y with 2 drops of concentrated acetic acid, incubating for 5 min. Slides were then washed thoroughly and allowed to air dry. Subsequently, the samples were washed again, dehydrated, and mounted with coverslips using Permount before microscopic examination.
Samples stained for multidrug resistant 1 (MDR-1), LRP, RRM-1, EGFR, ERCC-1, and breast cancer 1 (BRCA-1) were prepared with the same basic procedures, as described by Selvaggi et al. [6]. All reagents and antibodies were purchased from Beijing Golden Bridge Biotechnology Co, Ltd. The proper dilutions and applications of primary antibodies followed the manufacturer’s instructions. The secondary antibody was horseradish peroxidase, and 3,3′-diaminobenzidine was the chromogenic substrate.
Immunohistochemical analysis
Samples were viewed at high magnification (×400), and 8 fields were selected for analysis. The total score was based on a system previously described by Fromowitz et al. [18]. (i) Each positive cell scored 0 points (no color), 1 point (yellow), 2 points (brown), or 3 points (dark brown); (ii) the percentage of positive cells scored 0 points (<5%), 1 point (5–25%), 2 points (25–50%), 3 points (50–75%), or 4 points (>75%); (iii) these two scores were added, and the final score was classified as − and +/− (<2 points), + (2–3 points), ++ (4–5 points), or +++ (6–7 points). We defined negative expression as the final score no higher than 2 points and positive expression as final score higher than 2 points.
Statistical analysis
Because of the small size of the sample, the 4 subtypes of lung cancer were compared by the Kruskal-Wallis test for tumor markers and Fisher’s exact test for categorical variables. A multiple comparisons post hoc test was performed by using Bonferroni correction for type I error adjustment when there were apparently significant differences between groups. Results are given as median (interquartile range, IQR) for tumor markers and as number (percentage) for categorical data. The Wilcoxon signed rank test was used to compare differences in the expression of molecular markers in primary lesions and metastatic lymph nodes. All statistical assessments were two-sided and evaluated at the 0.05 level of significance. Statistical analyses were performed using SPSS1 5.0 statistics software (SPSS Inc., Chicago, IL, USA).
Results
We retrospectively analyzed the records of all lung cancer patients who underwent thoracic surgery in the hospital from September 2010 to October 2011, as shown in Table 1. A total of 48 patients with a history of primary lung cancer lesions and more than one metastatic lymph node were ultimately included. All patients underwent surgery for removal of the primary and metastatic lesions. The patients included 39 men and 9 women ranging in age from 38 to 78 years, and the average age was 50.06±10.03 years. Five patients (10%) had SCLC and the other 43 patients (90%) had NSCLC. Among the 43 NSCLC patients, 4 patients had large-cell lung carcinoma, 24 had squamous cell lung carcinoma, and 15 had adenocarcinoma. A total of 37 patients (77.1%) were tobacco smokers.
Table 1.
Demographic and clinical characteristics of enrolled lung cancer patients (n=48).
| Variable | Values |
|---|---|
| Age, years ± standard deviation | 50.06±10.03 |
| Gender, n (%) | |
| Male | 39 (81.3%) |
| Female | 9 (18.8%) |
| Subtype of lung cancer, n (%) | |
| Small-cell lung cancer | 5 (10.4%) |
| Large-cell lung cancer | 4 (8.3%) |
| Squamous-cell carcinoma lung cancer | 24 (50.0%) |
| Adenocarcinoma lung cancer | 15 (31.3%) |
| Smoker, n (%) | 37 (77.1%) |
We examined the expression of 6 molecular biomarkers (MDR-1, LRP, RRM-1, EGFR, ERCC-1, and BRCA-1) in the primary lung carcinoma and the metastatic lymph nodes of all patients. The differences among the expression of molecular markers in primary lesions and metastatic lymph nodes were defined as the biomarker expression levels. They were categorized by different scores between primary lesions and metastatic lymph nodes in patients with different subtypes of lung cancer. The results indicated no significant differences in the expression of these 6 biomarkers in the primary lesions and metastatic lymph nodes among the groups, as shown in Table 2. P values are from Fisher’s exact test
Table 2.
Bio-marker expression between primary lesions and metastatic lymph nodes in patients with different subtypes of lung cancer.
| Small-cell (n=5) | Large-cell (n=4) | Squamous-cell (n=24) | Adenocarcinoma (n=15) | P value | |
|---|---|---|---|---|---|
| MDR-1 | 1 (25%) | 1 (25%) | 6 (25%) | 3 (20%) | 1.000 |
| LRP | 1 (20%) | 3 (75%) | 9 (37%) | 5 (33%) | 0.449 |
| RRM-1 | 3 (60%) | 1 (25%) | 7 (29%) | 9 (60%) | 0.190 |
| EGFR | 2 (40%) | 1 (25%) | 10 (42%) | 7 (47%) | 0.965 |
| ERCC-1 | 0 (0.0%) | 2 (50%) | 8 (33%) | 6 (40%) | 0.345 |
| BRCA-1 | 2 (40%) | 2 (50%) | 3 (13%) | 7 (47%) | 0.051 |
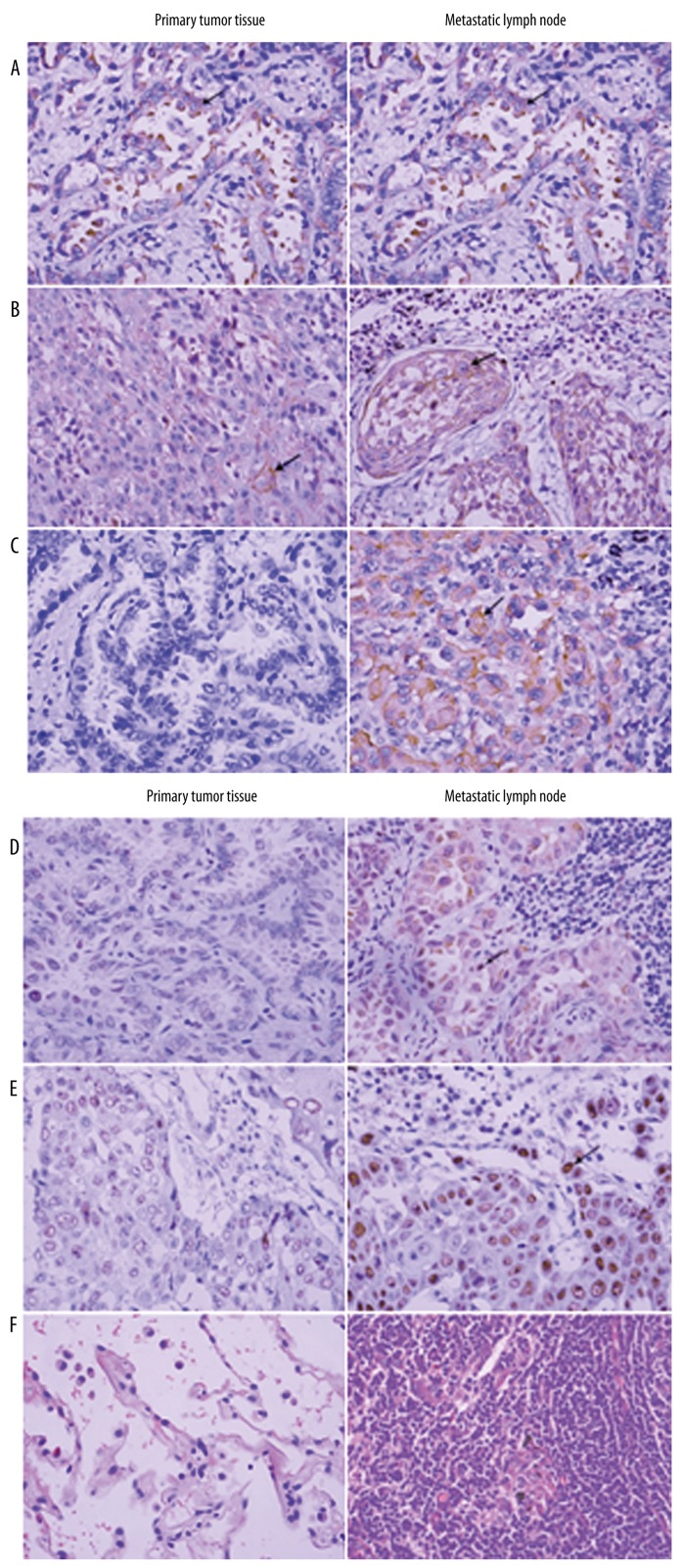
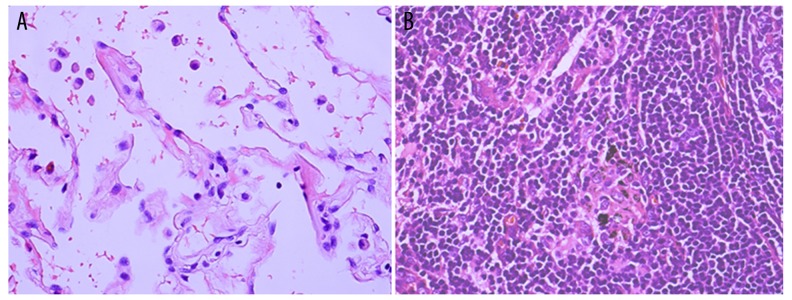
Next, we compared the level of expression of the molecular markers between primary lesions and metastatic lymph nodes of all 48 patients. The results of representative immunohistochemical stainings for MDR-1, LRP, RRM-1, EGFR, ERCC-1, and BRCA-1 in primary lesions and the stainings in metastatic lymph nodes were compared (Figure 1). Stronger and more intensified positive staining was seen in metastatic lymph nodes compared with primary lesions for all 6 different markers. The hematoxylin-eosin–stained normal lung tissue and lymph node (Figure 2) adjacent to primary lesions served as controls. We summarized these results and indicated that ERCC-1 expression was significantly different in primary lesions and metastatic lymph nodes, but that there were no significant differences in the other 5 biomarkers (Table 3). P values are from Wilcoxon signed rank test.
Figure 1.
Representative immunohistochemical staining results for (A) LRP, (B) RRM-1, (C) EGFR, (D) ERCC-1 (E), BRCA-1, and (F) MDR-1 in a primary tumor (adenocarcinoma) and a metastatic lymph node (squamous cell carcinoma) (hematoxylin-eosin [HE], ×400). Arrows indicate strong positive staining in the cytoplasm and the nucleus.
Figure 2.
(A, B) Hematoxylin-eosin (HE) staining of normal lung tissue and lymph node (HE, ×400).
Table 3.
Expression of molecular markers in the primary lesions and metastatic lymph nodes of patients with lung cancer (n=48).
| Primary lesion | Metastatic lymph nodes | P value | |
|---|---|---|---|
| MDR-1 | 1.000 | ||
| − | 41 (85%) | 41 (85%) | |
| −/+ | 3 (6.3%) | 3 (6.3%) | |
| + | 3 (6.3%) | 3 (6.3%) | |
| ++ | 1 (2.1%) | 2 (2.1%) | |
| +++ | 0 (0.0%) | 0 (0.0%) | |
| LRP | 0.080 | ||
| − | 15 (31%) | 18 (38%) | |
| −/+ | 0 (0.0%) | 2 (4.2%) | |
| + | 16 (33%) | 15 (31%) | |
| ++ | 9 (19%) | 5 (10%) | |
| +++ | 8 (17%) | 8 (17%) | |
| RRM-1 | 0.071 | ||
| − | 10 (21%) | 10 (21%) | |
| −/+ | 3 (6.3%) | 0 (0.0%) | |
| + | 13 (27%) | 13 (27%) | |
| ++ | 14 (29%) | 13 (27%) | |
| +++ | 8 (17%) | 12 (25%) | |
| EGFR | 0.392 | ||
| − | 11 (23%) | 8 (17%) | |
| −/+ | 2 (4.2%) | 2 (4.2%) | |
| + | 7 (15%) | 9 (19%) | |
| ++ | 21 (44%) | 20 (43%) | |
| +++ | 7 (15%) | 9 (19%) | |
| ERCC-1 | 0.007* | ||
| − | 37 (77%) | 31 (65%) | |
| −/+ | 3 (6.3%) | 3 (6.3%) | |
| + | 6 (13%) | 6 (13%) | |
| ++ | 2 (4.2%) | 6 (13%) | |
| +++ | 0 (0.0%) | 2 (4.2%) | |
| BRCA-1 | 0.057 | ||
| − | 29 (60%) | 34 (71%) | |
| −/+ | 2 (4.2%) | 5 (10%) | |
| + | 16 (33%) | 8 (17%) | |
| ++ | 61 (2.1%) | 0 (0.0%) | |
| +++ | 0 (0.0%) | 1 (2.1%) |
P<0.05.
Discussion
In the present study of lung cancer patients, we measured the expression of 6 molecular markers (MDR-1, LRP, RRM-1, EGFR, ERCC-1 and BRCA-1) in primary tumors and metastatic lymph nodes in the same patients. Our results indicate that ERCC-1 is the only molecular marker with significantly different expression in the primary tumor compared with metastatic lymph nodes. There was no significant difference in the expression of the six markers in patients with different histological subtypes of lung cancer. This research represents an initial step toward the development of individualized lung cancer therapy based on the expression of biomarkers in primary tumor tissue and metastatic lymph nodes.
Individualized treatment of cancer is believed to have great promise, and many clinical and experimental studies have used tumor-specific molecular markers to identify differences in patients in order to better estimate prognosis and select treatments [19]. This motivated our comparison of markers in patients with different subtypes of lung cancer. In particular, LRP is the major vault protein and its elevated expression is associated with poor response to chemotherapy [20,21]. Rybárová et al. [22] reported that LRP expression was significantly greater in patients with NSCLC than in those with SCLC, which is in line with the general clinical finding that untreated SCLC is more chemosensitive than untreated NSCLC.
Ribonucleotide reductase is a rate-limiting enzyme for the synthesis of DNA and has two subunits, RRM-1 and RRM-2. The RRM-1 gene is a target of numerous chemotherapeutic agents [18]. Previous clinical studies have shown that lung cancer patients with low levels of RRM-1 mRNA are more sensitive to gemcitabine and have a longer duration of survival [23–25]. The recent U.S. National Comprehensive Cancer Network (NCCN) NSCLC treatment guidelines recommend measurement of RRM-1 expression before implementation of gemcitabine therapy in NSCLC patients [26]. Our results indicated no significant differences in RRM-1 expression either between primary and metastatic lesions or in their subtypes.
EGFR is a receptor tyrosine kinase involved in activation of transcription factors that regulate gene transcription, cell migration, adhesion, differentiation, and apoptosis [27]. EGFR-positive lung cancer patients are more responsive to gefitinib (Iressa) and erlotinib (Tarceva) [28], which are classified as EGFR tyrosine kinase inhibitors (TKIs). The presence of an EGFR gene mutation is activating, causing a constant signal to be generated, which leads to cell proliferation and other cancer processes. We found no significant differences in EGFR expression either between primary and metastatic lesions or in their subtypes.
ERCC-1 is a key enzyme in nucleotide excision repair (NER), and mutations in this gene appear to play a role in cancer pathogenesis [29]. Previous clinical studies have demonstrated that down-regulation of ERCC-1 in NSCLC patients is associated with increased sensitivity to platinum-based chemotherapy [30]. The recent NCCN NSCLC treatment guidelines recommend measurement of ERCC-1 before implementation of platinum-based chemotherapy in NSCLC patients [26]. Interestingly, we found significantly elevated expression of ERCC-1 in metastatic lesions compared with that in primary lesions. It is possible that the cells of the primary lung tumor that become metastatic are genetically unique from the bulk of the primary tumor cells. It is reported that down-regulation of ERCC-1 in NSCLC patients is associated with increased sensitivity to platinum-based chemotherapy. Low expression of ERCC1 on circulating tumor cells (CTCs) correlated with progression-free survival. These findings provide general support for our finding of the importance of measuring markers outside the primary tumor [31].
Previous studies indicate that genetic testing should be performed on lung cancer patients and that the treatment should be customized according to the results. Our results indicate that MDR-1, LRP, RRM-1, EGFR, and BRCA-1 levels were similar in the primary and metastatic lesions of all patients. It is not surprising that these lesions ultimately have the same sources. However, ERCC-1 levels were significantly different in the primary and metastatic lesions. At present, the cause and clinical significance of this difference are uncertain. We suggest that future studies measure ERCC-1 in primary and metastatic lesions and determine the association of altered ERCC-1 levels with patient prognosis and responsiveness to chemotherapy.
Our study had several limitations that should be noted. First, our sample size was relatively small, and patients had diverse histological subtypes. In particular, only 5 patients had SCLC and only 4 patients had NSCLC of the large cell subtype. This clearly limited the statistical power of our results. Second, this study was performed at a single institution, so the results should not be generalized to other institutions. Third, this was a retrospective study, so there may have been significant selection bias.
Conclusions
At present, the onset and pathogenesis of SCLC and NSCLC are not completely understood and the predictions of prognosis are not very reliable. The use of molecular markers to guide treatment of these cancers is currently in its initial stages. Our results suggest that expression of ERCC-1 was significantly different in primary tumors and metastatic sites with different lung cancer subtypes. Large-scale prospective studies are needed to validate these findings before we can make definitive suggestions about the use of individualized treatment based on measurement of this biomarker.
Footnotes
Conflicts of interest
All of the authors declare that they have no conflicts of interest regarding this paper.
Source of support: Departmental sources
References
- 1.Polo V, Zago G, Frega S, et al. Non-small cell lung cancer in a very young woman: A case report and critical review of the literature. Am J Case Rep. 2015;16:367–70. doi: 10.12659/AJCR.894426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He F, Xiao R, Yu T, et al. Explore the influence factors on primary lung cancer in Fujian province Han population under the use of generalized linear model. Zhonghua Liu Xing Bing Xue Za Zhi. 2015;36:896–99. [PubMed] [Google Scholar]
- 3.Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10:1243–60. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 4.Travis WD. Pathology of lung cancer. Clin Chest Med. 2002;23:65–81. doi: 10.1016/s0272-5231(03)00061-3. [DOI] [PubMed] [Google Scholar]
- 5.Sun L, Chen Y, Su Q, et al. Increased plasma miRNA-30a as a biomarker for non-small cell lung cancer. Med Sci Monit. 2016;22:647–55. doi: 10.12659/MSM.897330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selvaggi G, Scagliotti GV. Histologic subtype in NSCLC: Does it matter? Oncology (Williston Park) 2009;23:1133–40. [PubMed] [Google Scholar]
- 7.Vadakara J, Borghaei H. Personalized medicine and treatment approaches in non-small-cell lung carcinoma. Pharmgenomics Pers Med. 2012;5:113–23. doi: 10.2147/PGPM.S24258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shames DS, Wistuba II. The evolving genomic classification of lung cancer. J Pathol. 2014;232:121–33. doi: 10.1002/path.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurzrock R, Gabrail N, Chandhasin C, et al. Safety, pharmacokinetics, and activity of GRN1005, a novel conjugate of angiopep-2, a peptide facilitating brain penetration, and paclitaxel, in patients with advanced solid tumors. Mol Cancer Ther. 2012;12:308–16. doi: 10.1158/1535-7163.MCT-11-0566. [DOI] [PubMed] [Google Scholar]
- 10.Nie X, Cheng G, Ai B, Zhang S. The tailored chemotherapy based on RRM1 immunohistochemical expression in patients with advanced non-small cell lung cancer. Cancer Biomark. 2013;13:433–40. doi: 10.3233/CBM-130381. [DOI] [PubMed] [Google Scholar]
- 11.Janku F, Garrido-Laguna I, Petruzelka LB, et al. Novel therapeutic targets in non-small cell lung cancer. J Thorac Oncol. 2011;6:1601–12. doi: 10.1097/JTO.0b013e31822944b3. [DOI] [PubMed] [Google Scholar]
- 12.Qiu ZQ, Zhao K. Expression of ERCC1, RRM1 and LRP in non-small cell lung cancers and their influence on chemotherapeutic efficacy of gemcitabine concomitant with nedaplatin. Asian Pac J Cancer Prev. 2014;15:7303–7. doi: 10.7314/apjcp.2014.15.17.7303. [DOI] [PubMed] [Google Scholar]
- 13.Duncan SJ, Kadaria D. A woman with a lung mass and multiple pulmonary nodules. Am J Case Rep. 2014;16:367–70. doi: 10.12659/AJCR.893749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taira N, Kawabata T, Ichi T, et al. Utility of the serum ProGRP level for follow-up of pulmonary carcinoid tumors. Am J Case Rep. 2014;15:337–39. doi: 10.12659/AJCR.890692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Chen YY, Li SQ, et al. Expression of miR-148/152 family as potential biomarkers in non-small-cell lung cancer. Med Sci Monit. 2014;21:1155–61. doi: 10.12659/MSM.892940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brattström D, Bergqvist M, Wester K, et al. Endothelial markers and circulating angiogenic factors and p53 may be potential markers for recurrence in surgically resected non-small cell lung cancer patients. Med Sci Monit. 2004;10:331–38. [PubMed] [Google Scholar]
- 17.Novák J, Fabian P. Comments on the TNM classification of malignant tumours – 7th edition. Klin Onkol. 2011;24:149–50. [PubMed] [Google Scholar]
- 18.Fromowitz FB, Viola MV, Chao S, et al. ras p21 expression in the progression of breast cancer. Hum Pathol. 1987;18:1268–75. doi: 10.1016/s0046-8177(87)80412-4. [DOI] [PubMed] [Google Scholar]
- 19.Yu Y, He J. Molecular classification of non-small-cell lung cancer: Diagnosis, individualized treatment, and prognosis. Front Med. 2013;7:157–71. doi: 10.1007/s11684-013-0272-4. [DOI] [PubMed] [Google Scholar]
- 20.Huang W, Mao Y, Zhan Y, et al. Prognostic implications of survivin and lung resistance protein in advanced non-small cell lung cancer treated with platinum-based chemotherapy. Oncol Lett. 2016;11:723–30. doi: 10.3892/ol.2015.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang C, Lin X, Song Y, et al. Overexpression of glucosylceramide synthase and its significance in the clinical outcome of non-small cell lung cancer. Chin Med J (Engl) 2014;127:3071–76. [PubMed] [Google Scholar]
- 22.Rybárová S, Hajduková M, Hodorová I, et al. Expression of the multidrug resistance-associated protein 1 (MRP1) and the lung resistance-related protein (LRP) in human lung cancer. Neoplasma. 2004;51:169–74. [PubMed] [Google Scholar]
- 23.Bepler G, Kusmartseva I, Sharma S, et al. RRM1 modulated in vitro and in vivo efficacy of gemcitabine and platinum in non-small-cell lung cancer. J Clin Oncol. 2006;24:4731–37. doi: 10.1200/JCO.2006.06.1101. [DOI] [PubMed] [Google Scholar]
- 24.Rosell R, Danenberg KD, Alberola V, et al. Ribonucleotide reductase messenger RNA expression and survival in gemcitabine/cisplatin-treated advanced non-small cell lung cancer patients. Clin Cancer Res. 2004;10:1318–25. doi: 10.1158/1078-0432.ccr-03-0156. [DOI] [PubMed] [Google Scholar]
- 25.Davidson JD, Ma L, Flagella M, et al. An increase in the expression of ribonucleotide reductase large subunit 1 is associated with gemcitabine resistance in non-small cell lung cancer cell lines. Cancer Res. 2004;64:3761–76. doi: 10.1158/0008-5472.CAN-03-3363. [DOI] [PubMed] [Google Scholar]
- 26.NCCN Clinical Practice Guidelines in Oncology for NSCLC.V2.2009. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 27.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 28.Gupta R, Dastane AM, McKenna R, Marchevsky AM. The predictive value of epidermal growth factor receptor tests in patients with pulmonary adenocarcinoma: review of current “best evidence” with meta-analysis. Hum Pathol. 2009;40:356–65. doi: 10.1016/j.humpath.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Tufman A, Huber RM. Biological markers in lung cancer: A clinician’s perspective. Cancer Biomark. 2010;6:123–35. doi: 10.3233/CBM-2009-0124. [DOI] [PubMed] [Google Scholar]
- 30.Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983–91. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 31.Das M, Riess JW, Frankel P, et al. ERCC1 expression in circulating tumor cells (CTCs) using a novel detection platform correlates with progression-free survival (PFS) in patients with metastatic non-small-cell lung cancer (NSCLC) receiving platinum chemotherapy. Lung Cancer. 2012;77:421–26. doi: 10.1016/j.lungcan.2012.04.005. [DOI] [PubMed] [Google Scholar]