Abstract
Most drugs are intended to act on molecular targets residing within a specific tissue or cell type. Therefore, the drug concentration within the target tissue or cells is most relevant to its pharmacological effect. Increasing evidences suggest that drug transporters not only play a significant role in governing systemic drug levels, but are also an important gate keeper for intra-tissue and intracellular drug concentrations. This review focuses on polyspecific organic cation transporters, which include the organic cation transporters 1-3 (OCT1-3), the multidrug and toxin extrusion proteins 1-2 (MATE1-2) and the plasma membrane monoamine transporter (PMAT). Following an overview of the tissue distribution, transport mechanisms, and functional characteristics of these transporters, we highlight the studies demonstrating the ability of locally expressed OCTs to impact intracellular drug concentrations and directly influence their pharmacological and toxicological activities. Specifically, OCT1-mediated metformin access to its site of action in the liver is impacted by genetic polymorphisms and chemical inhibition of OCT1. The impact of renal OCT2 and MATE1/2-K in cisplatin intrarenal accumulation and nephrotoxicity is reviewed. New data demonstrating the role of OCT3 in salivary drug accumulation and secretion is discussed. Whenever possible, the pharmacodynamic response and toxicological effects is presented and discussed in light of intra-tissue and intracellular drug exposure. Current challenges, knowledge gaps, and future research directions are discussed. Understanding the impact of transporters on intra-tissue and intracellular drug concentrations has important implications for rationale-based optimization of drug efficacy and safety.
Keywords: Organic Cation Transporter, Intracellular Drug Concentration, Intra-Tissue Drug Concentration, Pharmacodynamics, OCT1, OCT2, OCT3, MATE1, MATE2-K, PMAT, Drug Transporter
1. Introduction
The ability of a drug molecule to move through cell membranes is a vital property affecting its pharmacokinetic and pharmacodynamics properties. Lipophilic drugs generally have high membrane permeability and their movement across cell membranes occurs primarily through passive diffusion, a non-mediated process discussed in great details elsewhere in this issue. Hydrophilic drugs, on the other hand, have low membrane permeability, and their efficient uptake into cells and tissues often involve facilitated mechanisms mediated by membrane transporters (also known as carriers). Different from passive diffusion where a drug molecule moves across membranes down its concentration gradient without energy input, carrier-mediated transport can be coupled to a cellular energy source to power uphill transport against the drug concentration gradient. Further, carrier-mediated drug transport is saturable, inhibitable, and highly dependent on the functional characteristics of the membrane transporters expressed in the specific tissues or cell types. In mammalian cells, there are two major types of membrane proteins involved in drug and solute transport: the solute carrier (SLC) and the ATP-binding cassette (ABC) transporters. The past two decades have witnessed an explosion of knowledge in our understandings of the basic biology and pharmacology of various SLC and ABC drug transporters. The in vivo roles of these transporters in drug disposition, efficacy, and toxicity are increasingly being appreciated. The clinical significance of transporters as a site of drug-drug interaction and a source for interindividual variability in drug response is also begining to be acknowledged [1–3].
Most drugs are intended to act on targets residing within a specific tissue or cells. While some drugs bind to external cell surface targets (e.g. G protein-coupled receptors), others act on intracellular enzymes and receptors residing inside the cell. Thus, it is the unbound drug concentration within the target tissue or cells that is directly responsible for eliciting its pharmacological effect. However, in the clinical setting, direct measurement of drug concentrations in target tissues and cells is difficult to achieve. Measurement of blood or plasma drug concentrations is thus commonly used to establish pharmacokinetic–pharmacodynamic relationships. For drugs that rapidly cross membranes by passive diffusion, plasma concentration is often a good surrogate for tissue concentration because the unbound drug concentration in tissue/cells is at equilibrium with its unbound concentration in plasma at steady state [4,5]. However, if a drug is transported by active uptake and/or efflux drug transporters, such a relationship may no longer exist. For drugs that are substrates of uptake transporters, tissue and/or intracellular drug concentrations can be much higher than drug concentrations in plasma. Conversely, for drugs that are substrates of efflux transporters, concentrations in tissues and cells may be substantially lower than predicted from plasma levels. Increasing evidences suggest that transporters expressed in specific tissues and cells can exert a great impact on local and intracellular drug concentrations, directly influencing their pharmacological and toxicological activities [4,5].
This review focuses on a special group of SLC drug transporters—the polyspecific organic cation transporters, which mediate cellular uptake and efflux of a broad spectrum of drugs, toxins, and endogenous compounds. We first briefly review the molecular and functional characteristics of major organic cation transporters with a special emphasis on their tissue distribution, cellular localization and transport mechanisms. We then highlight the impact of these transporters in controlling tissue and intracellular drug concentrations using literature examples where the roles of locally expressed organic cation transporters have been clearly demonstrated in several tissues (liver, kidney, salivary glands) in in vivo or clinical studies. The resulting consequence on pharmacodynamic response and toxicological effects of clinically used organic cation drugs is presented and discussed alongside. Lastly, the current challenges, knowledge gaps and future research directions in this field are briefly summarized and discussed.
2. Molecular and Functional Characteristics of Polyspecific Organic Cation Transporters
Organic cations are structurally diverse endogenous compounds (e.g. biogenic amines) and xenobiotics (e.g. drugs, environmental toxins) that carry a net positive charge at physiological pH. About 40% of the commonly prescribed drugs exist as organic cations at physiological pH [6]. Many organic cations are hydrophilic and rely on transporters to move across cell membranes. In humans and other mammals, there are a number of SLC transporters that appear to be evolved specifically to handle these structurally diverse organic cations. These polyspecific (or multispecific) organic cation transporters including the classic organic cation transporters 1-3 (OCT1-3) from the SLC22 family, the multidrug and toxin extrusion proteins 1-2 (MATE1-2) from the SLC47 family, and the plasma membrane monoamine transporter (PMAT) from the SLC29 family [7–12]. The molecular and functional characteristics of the major human polyspecific organic cation transporters are summarized below and in Table 1. A variety of clinically used drugs have been identified as the substrates of these transporters, and some selected drug substrate are listed in Table 1.
Table 1.
Molecular and Functional Characteristics of Major Human Polyspecific Organic Cation Transporters.
| Transporters | Gene | Transport Mode | Tissue Distribution | Selected Drug Substrates | Reference |
|---|---|---|---|---|---|
| OCT1 | SLC22A1 | Electrogenic | Liver, small intestine, kidney, lung, brain, heart, skeletal muscle, placenta, mammary gland, adrenal gland, immune cells, adipose tissue | Acyclovir, atenolol, debrisoquine, furamidine, ganciclovir, lamivudine, lamotrigine, metformin, oxaliplatin, pentamidine, picoplatin, tropisetron, zalcitabine | [51,57,136–138] |
| OCT2 | SLC22A2 | Electrogenic | Kidney, brain, lung, small intestine, thymus, placenta | Amantadine, amiloride, atenolol, cimetidine, cisplatin, famotidine, ifosfamide, lamivudine, memantine, metformin, oxaliplatin, picoplatin | [51,57,136,139] |
| OCT3 | SLC22A3 | Electrogenic | Liver, skeletal muscle, heart, placenta, brain, kidney, small intestine, urinary bladder, cornea, mammary gland, lung | Cisplatin, etilefrine, lamivudine, lidocaine, metformin, oxaliplatin, pramipexole, quinidine | [57,97,136] |
| MATE1 | SLC47A1 | Electroneutral (H+/OC+ exchange) | Liver, kidney, skeletal muscle, adrenal gland | Acyclovir, atenolol, cimetidine, cisplatin, fexofenadine, guanidine, metformin, oxaliplatin, procainamide, topotecan | [22,51,109,140,141] |
| MATE2/2-K | SLC47A2 | Electroneutral (H+/OC+ exchange) | Kidney | Atenolol, cimetidine, cisplatin, guanidine, metformin, oxaliplatin, procainamide, topotecan | [16,22,51,140] |
| PMAT | SLC29A4 | Electrogenic pH sensitive | Brain, heart, small intestine, kidney, liver | Metformin, ritonavir | [11,12,25,46,47] |
2.1. Molecular Features of OCTs, MATEs, and PMAT
The human OCTs are encoded by the SLC22 gene family and consist of three closely-related members: OCT1 (SLC22A1), OCT2 (SLC22A2) and OCT3 (SLC22A3). hOCT1 and hOCT2 are 70% identical in protein sequence, whereas hOCT3 shares 50% sequence identity with hOCT1 and hOCT2 [13]. The OCT proteins contain 542–556 amino acids with 12 predicted α-helical transmembrane domains (TMDs) [3]. The COOH- and NH2-terminal ends of the OCTs are intracellular. One large hydrophilic loop is localized to the extracellular side between TMD1 and TMD2 and contains several N-glycosylation sites. A large intracellular loop is localized between TMD6 and TMD7 with potential protein kinase C-dependent phosphorylation sites [14].
In excretory organs, OCTs frequently team up with the multidrug and toxin extrusion (MATE) proteins to mediate transepithelial transport of organic cations [15]. Encoded by the SLC47A gene family in humans, MATEs include two members: MATE1 (SLC47A1) and MATE2 (SLC47A2) [15]. Human MATE1 has only one isoform with 570 amino acids in length, while human MATE2 has three isoforms: the full length isoform hMATE2 (602 amino acids), hMATE2-K (566 amino acids) and hMATE2-B (220 amino acids) [9,16]. Both hMATE2 and hMATE2-K are functional, whereas hMATE2-B possesses no transport activity [16]. Human MATEs are predicted to have 13 TMDs with an extracellular carboxyl terminus and an intracellular amino terminus [15,17].
Beside OCTs and MATEs, a newer polyspecific organic cation transporter, the plasma membrane monoamine transporter (PMAT), was recently cloned and characterized by our laboratory [11,12]. By gene ontology, PMAT (SLC29A4) belongs to the equilibrative nucleoside transporter (SLC29) family. However, detailed functional characterization work demonstrated that PMAT functions as a polyspecific organic cation transporter that shares similar substrate specificity and functional characteristics to the OCTs [11,12,18,19]. PMAT is predicted to have 11 TMDs with an intracellular N- and an extracellular C-terminus [11].
2.2. Driving Forces of OCTs, MATEs, and PMAT
OCTs-mediated organic cations transport is independent of the sodium and chloride ions [20,21]. OCTs functions as electrogenic, facilitative transporters, and the transport direction is dependent on the electrochemical gradient of the transported organic cations [7,22]. In animal cells, the universally existing inside-negative membrane potential is used by the OCTs to drive cellular uptake of the organic cation substrate [23]. This allows the OCTs to accumulate a substrate with intracellular concentrations much higher (up to 10 fold) than its extracellular concentration [24]. Similar to the OCTs, PMAT also functions as an electrogenic transporter that utilizes the physiological inside-negative membrane potential as a driving force [25]. The transport activity of PMAT can be further stimulated by an acidic pH [25,26]. MATEs, however, are proton/organic cation exchangers [16]. They couple a transmembrane proton gradient to drive the transport of organic cations in the opposite direction [17], a process involving an electroneutral exchange of proton for a monovalent organic cation [27].
2.3. Tissue distribution and expression of OCTs, MATEs, and PMAT
Despite the similar structure and transport function, tissue distribution of OCT1-3 varies greatly. Oct1, the first OCT isoform identified from rat kidney, is highly expressed in the kidney, liver and small intestine in rodents [28,29]. In humans, however, OCT1 is mainly found in the liver and localized to the basolateral membrane of hepatocytes [30,31]. Besides, low expression of human OCT1 is also detected in other tissues including small intestine, colon, kidney, lung, brain, heart, skeletal muscle, peripheral leukocytes, adrenal gland, mammary gland, immune cells and adipose tissue [29,32,33]. Oct2 was isolated by homology screening from rat kidney and human OCT2 was also cloned later [32,34]. In humans, OCT2 is predominantly expressed on the basolateral membrane of renal proximal tubule cells in the kidney, and low expression has also been reported in brain, lung, small intestine, thymus, placenta and the inner ear [29,32]. Oct3 was independently cloned from rat brain and placenta [35,36], while human OCT3 was cloned from Caki-1 cells and originally identified as an extraneuronal monoamine transporter [37]. Different from hOCT1 and hOCT2, hOCT3 has a broader tissue distribution with relatively high expression in skeletal muscle, placenta, salivary glands, heart, brain, adrenal gland, trachea, small intestine, and uterus [29,35,38–40]. The cellular localization of OCT3 is also tissue-specific. For instance, it is expressed on the basolateral membrane of hepatocytes and placental epithelium [29,41], but in the lung, it is localized to the luminal membrane of bronchial epithelial cells [42]. In salivary glands, the OCT3 protein is localized at both basolateral and apical membranes of the secretory epithelial cells [43].
Human MATE1 was first cloned and characterized as an efflux transporter of organic cations [9]. hMATE1 is highly expressed in the liver, kidney, adrenal gland and skeletal muscle [9], and it is localized to the apical membrane of renal proximal tubule cells and hepatocytes [44]. In humans, MATE2 and MATE2-K are mainly expressed in the kidney, even though they are also detectable in various tissues [16,45]. In the kidney, MATE2 and MATE2-K are also restricted to the apical membrane of renal proximal tubule cells [45].
PMAT mRNA is most strongly expressed in the brain; but lower levels of expression are also found in heart, small intestine, kidney, and liver [11,46,47]. In the brain, PMAT transcripts are widely distributed in many brain regions and are particularly abundant in brain cortex, hippocampus, cerebellum and epithelial cells of the choroid plexus [11,48]. The PMAT protein is localized to the apical membranes of the enterocytes in the intestine and epithelial cells of the choroid plexus [19,49].
2.4. Models of Organic Cation Transport across Excretory Epithelia
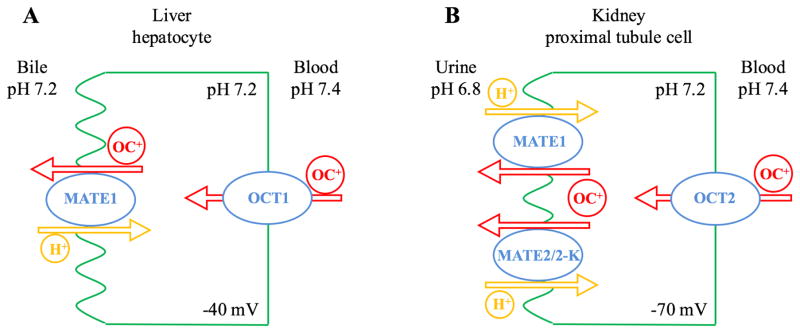
In secretory organs such as the kidney and liver, the OCTs and MATEs form a functional alliance to mediate organic cation secretion from the body (Figure 1). For instance, renal secretion of organic cations consists of two steps. Circulating organic cations in blood are first transported into the renal proximal tubule cells by the basolateral OCT2 driven by negative membrane potential [50]. Once inside the tubular cells, organic cations are effluxed into urine by the MATE1 and MATE2-K on the apical membrane (Figure 1A) [51]. The physiological pH of urine is slightly acidic ( pH 6.0–6.8), which provides an inwardly-directed proton gradient that can efficiently drive MATE-mediated organic cation efflux [52,53]. A similar model has been proposed for organic cation transport in hepatocytes. Located on the basolateral (sinusoidal) membrane of hepatocytes, OCT1, the most abundant OCT isoform in human liver, plays a pivotal role in the uptake of organic cations from blood into the liver [54]. Once inside the cells, organic cations may be further secreted into the bile by MATE1 on the apical (canalicular) membrane (Figure 1B) [9]. However, the canalicular pH is 7.2 or higher [55]. Without an inwardly directed proton gradient, it is unclear if MATE1-mediated efflux of organic cations across canalicular membrane is likely to occur efficiently or rely on some other transporters at the canaliculi.
Figure 1.
Models of organic cation transport in liver hepatocytes (A) and kidney proximal tubule cells (B).
3. Impact of OCTs and MATEs on Intracellular Levels, Pharmacodynamics, and Toxicity
3.1. Impact of OCT1/Oct1 on Hepatic Drug Levels and Action
Located in the sinusoidal membrane of hepatocytes, OCT1 has been identified as a main organic cation transporter in the liver and is responsible for the uptake of basic compounds in hepatocytes [3,29,56,57]. Although OCT3 and MATE1 are also expressed in hepatocytes, their roles in hepatic drug disposition and elimination have not been well established as compared to OCT1 [29,56,58,59]. MATE1 appears to mediate some biliary excretion but its activity with in vivo probes is generally low and appears to be significantly less than in the kidney [59–62]. The apparent low activity of MATE1 was thought to be due to the lack of a significant pH gradient between hepatocytes and bile [63], which is necessary to drive MATE1-mediated organic cation efflux as a proton/organic cation antiporter [59]. The importance of OCT1 in influencing liver intracellular concentrations and pharmacodynamics can be best appreciated with studies on the antidiabetic drug, metformin, and antiviral drugs such as lamivudine [64–70].
Metformin is a first line oral antihyperglycemic agent used for the treatment of type II diabetes [71]. Carrier-mediated transport across cell membranes is particularly important for metformin because it is a hydrophilic (LogP=-1.43) drug with very low passive permeability [60]. In vitro and preclinical in vivo studies have demonstrated metformin is a substrate of hepatic OCT1 as well as OCT2, OCT3, MATEs and PMAT [19,43,62,70,72]. Metformin ameliorates hyperglycemia by decreasing hepatic glucose production, reducing gastrointestinal glucose absorption, and improving peripheral sensitivity to insulin [63]. In the liver, metformin acts on intracellular AMP-activated protein kinase (AMPK) to suppress glucose production [73,74]. As the liver is a major site of metformin action, OCT1, the major gate keeper for organic cation uptake into the liver, acts as an important determinant of intracellular levels and the pharmacodynamics of metformin. Indeed, the impact of Oct1/OCT1 on hepatic metformin concentrations and therapeutic response has been well demonstrated in a series of investigations including Oct1 knockout mouse studies as well as pharmacogenetics and drug-drug interaction studies in humans [64,65,72,75,76].
Shu et al. first demonstrated OCT1 regulates hepatic metformin levels and response in vivo [64]. They showed that metformin hepatic concentration was 4.2-fold greater in wildtype Oct1 (Oct1+/+) mice than in Oct1 knockout (Oct1−/−) mice while metformin concentrations in the plasma and other organs were similar between Oct1+/+ and Oct1−/− mice. When the pharmacodynamic action of metformin was measured, hepatic AMPK and ACC phosphorylation was reduced in Oct−/− mice. Additionally, metformin was unable to reduce the fasting glucose level in Oct1−/− mice in a hyperglycemic mouse model but did reduce fasting glucose levels more than 30% in Oct1+/+ mice [64]. The role of OCT1 in metformin pharmacokinetics and pharmacodynamics in humans was then investigated in a pharmacogenetics study in healthy volunteers carrying either the normal reference OCT1 allele or variant alleles (OCT1-R61C, -G401S, -G465R, and 420del) that showed reduced metformin transport activity in vitro. Metformin’s pharmacokinetics were determined and its glucose-lowering effects were measured with an oral glucose tolerance test (OGTT) in the two groups [64,77]. Before treatment with metformin, subjects with reference and variant alleles had similar baseline plasma glucose levels and area under the glucose concentration-time curve (AUCs) after OGTT. However, after metformin treatment, volunteers carrying the OCT1 polymorphisms had significantly higher plasma glucose levels than those carrying the reference allele in OGTT. The reduced pharmacological effect of metformin in individuals with reduced OCT1 function was unlikely to be due to a change in metformin systemic exposure because metformin plasma concentration-time curve (AUC) and maximal plasma concentration (Cmax) was even increased in volunteers with the reduced OCT1 function polymorphisms [77]. These results are consistent with the data from the mice, further supporting that OCT1 is a critical determinant of liver concentrations of metformin, which then directly affects the therapeutic response. More recently, Cho et al. examined the impact of OCT1 on metformin hepatic concentrations and glucose-lowering action by increasing OCT1 expression using the pregnane X receptor (PXR) agonist rifampin in humans [75]. Their data showed OCT1 mRNA levels were increased 4.1-fold in peripheral blood cells as a surrogate for hepatic induction in vivo. Metformin’s OGTT glucose AUCs were reduced by more than 50% with treatment of rifampin. Rifampin also affected renal clearance and absorption with a net effect of slightly increased systemic exposure (13%). In spite of confounding factors, this study suggests metformin intracellular hepatic concentrations and activity can be increased with induction of OCT1.
Besides metformin, OCT1 may play a similar role for organic cation drugs with a hepatic site of action. A similar concept can be further extended to non-hepatic tissues where transporter-mediated drug uptake is needed for the drug molecule to reach its intracellular target. One of these areas is the treatment of viral hepatitis where drug access to the liver is imperative for interacting with replicating virus [78–80]. A number of anti-retrovirals have been identified as interacting with OCTs [81–83]. Lamivudine (3TC), a treatment for chronic hepatitis B virus (HBV) as well as HIV infection, and its OCT mediated cellular accumulation is one of the better studied examples [67,78,84,85]. In vitro studies showed that lamivudine was a substrate of OCT1-3 [67,68], and several genetic variants of OCT1 were shown to have reduced the uptake activity for lamivudine in vitro [69].
To date, few studies have examined the role of OCT1 in mediating lamivudine uptake into the liver and its impact on HBV treatment outcome. However, ex vivo experiments showed that lamivudine uptake into peripheral blood mononuclear cells (PBMC) was mediated by OCT1 and OCT2 [67,85]. Uptake of lamivudine into PBMCs is important for the treatment of HIV, and a similar effect may be anticipated for OCT1 in hepatic disposition of lamivudine [78]. In one study, the ex vivo uptake of lamivudine into CD4 cells correlated well (r>0.80) with the expression of OCT1 and OCT2 mRNA [67]. This study further demonstrated that OCT inhibitors could reduce the uptake into cells ex vivo. Others have shown that OCT1 polymorphisms can have a large impact on lamivudine uptake in vitro [69]. The role of these polymorphisms on the clinical efficacy of lamivudine has not yet been determined.
The impact of hepatic OCT1 on liver drug disposition and action highlighted the fact that pharmacodynamic responses do not always correlate with plasma drug concentration data (i.e. pharmacokinetics). Rather, drug concentrations in target tissues are more relevant to therapeutic activity. As discussed, a decreased pharmacodynamic response of metformin was observed in Oct1−/− mice without a corresponding change in plasma exposure [64]. In contrast, this decreased pharmacodynamic response in Oct−/− mice corroborated with a substantial reduction in metformin concentrations in the liver. A decreased response to metformin was observed in volunteers carrying OCT1 polymorphisms in spite of an increased metformin systemic exposure [64,77]. These studies clearly demonstrated the ability of locally expressed uptake transporters to impact intracellular drug concentrations at the site of action, and thus directly influencing the pharmacological activity of a medication. However, unlike plasma, in vivo drug concentrations in tissues are often difficult to obtain. Development of biomarkers or use of whole body imaging approaches may offer unique opportunities to probe the impact of uptake transporters on intracellular drug levels at a specific tissue of drug action or toxicity. In addition, genetically modified animals are particularly useful in proof-of-concept studies to dissect the tissue-specific role for uptake transporters in vivo.
3.2. Impact of OCT2/Oct2 on Renal Drug Accumulation and Nephrotoxicity
In human kidneys, OCT2 is the primary blood-facing organic cation uptake transporter [29,57,86,8]. The luminal-facing MATE1 and MATE2-K work in concert with OCT2 to mediate active renal secretion of basic drugs [51,56,87,88] (Figure 1). The roles of OCT2 and the MATE transporters in renal elimination of organic cations are well established [72,89–91]. Many cationic drugs, such as metformin and atenolol, are eliminated by active renal secretion by the OCT2/MATE pathway [66,90,92]. Changes in the activity of OCT2, MATE1, or MATE2-K can impact systemic levels of renally cleared drugs. Furthermore, an imbalance between OCT2-mediated uptake and MATE-mediated efflux may result in drug accumulation in proximal tubule cells, leading to drug-induced nephrotoxicity and kidney injury. This scenario can occur clinically and is thought to underlie the differential nephrotoxicity of platinum-based anticancer agents [93].
Cisplatin is a chemotherapeutic agent used in the treatment of lung, bladder, colon, testis, and brain cancer [94]. Nephrotoxicity, primarily in proximal tubules, is a major dose limiting toxicity of cisplatin [95]. In dogs, cisplatin accumulated between 4 to 8-fold in the kidney as compared with plasma concentrations [96]. Cisplatin is an excellent OCT2 substrate; however, it is a poor substrate of either MATE1 or MATE2-K [97–100]. The in vivo role of OCT2 in cisplatin-induced nephrotoxicity has been investigated in an elegant study by Filipski et al [101]. Unlike human kidneys which primarily express OCT2, both Oct1 and Oct2 are expressed in the rodent kidney [29]. Therefore, a mouse model, in which genes encoding both Oct1 and Oct2 were deleted, was used. It was shown that deletion of Oct1 and Oct2 resulted in significantly reduced cisplatin renal accumulation and impaired urinary excretion of cisplatin without an apparent influence on plasma levels. Further, the Oct1/Oct2-deficient mice were protected from severe cisplatin-induced renal tubular damage [101]. In cancer patients receiving cisplatin treatment, a nonsynonymous single-nucleotide polymorphism (rs316019) in the SLC22A2 gene was associated with reduced cisplatin-induced nephrotoxicity [101]. These studies established a critical role of OCT2 in the renal accumulation and nephrotoxicity of cisplatin. In addition, OCT2 is expressed in hair cells of the cochlea and OCT2-mediated cisplatin accumulation may, by the same token, underline cisplatin-induced ototoxicity [102,103].
The discovery of the critical role of OCT2 in cisplatin toxicity provided a rationale for using OCT2-selective inhibitors to mitigate the debilitating side effect of cisplatin. Indeed, rodent studies suggested a protective effect of Oct inhibitors against cisplatin-induced nephrotoxicity [102,104,105]. For example, co-administration of the OCT2 inhibitor imatinib reduced nephrotoxicity as demonstrated by kidney histology and renal biomarkers [105]. However, the complex interplay between OCT2 and MATE activity plays a crucial role in the nephrotoxicity of cisplatin [93]. Many OCT inhibitors also inhibits MATEs, which may increase intracellular cisplatin accumulation and toxicity. Selective inhibition of MATE transporters with pyrimethamine or ondansetron as well as genetic ablation of Mate1 was shown to increase the nephrotoxicity of cisplatin in mice [106,107]. Cimetidine is a selective inhibitor of MATE transporters at therapeutic doses due to its differential potencies for OCT2 (IC50 ~100–150 μM) and MATE transporters (IC50s~1–7 μM). Cimetidine can inhibit both OCT2 and MATEs in vivo at supratherapeutic doses [91]. A human study using high doses of cimetidine to inhibit OCT2 reduced cisplatin induced nephrotoxicity as measured by effective renal plasma flow and glomerular filtration rate [108]. A more recent high dose cimetidine study also demonstrated minimal changes in human pharmacokinetics or antitumor activity of cisplatin with cimetidine co-administration [104]. Nevertheless, the risk of using chemical inhibitors as a cisplatin nephroprotectant should be carefully addressed given the opposing effect of OCT2 and MATEs in cisplatin intrarenal accumulation and toxicity.
Newer platinum-based chemotherapeutic agent such as carboplatin, oxaliplatin, and nedaplatin have reduced risks of nephrotoxicity [93,97]. Differences in transport activity by OCT2 and MATEs account, at least in part, for their reduced toxicity [93,97,105]. Carboplatin and nedaplatin are not transported by OCT2; and therefore do not accumulate as highly in the kidney [97,109]. Interestingly, oxaliplatin is transported by OCT2 and does not have the same nephrotoxic effects as cisplatin [97,109,110]. Oxaliplatin is also a good substrate of the apical transporters MATE1 and MATE2-K, which efflux oxaliplatin out of tubular cells, thus minimizing the level of tissue accumulation and toxicity [97]. The dependence of oxaliplatin to be efficiently effluxed from proximal tubule cells by the MATE transporters, however, raises a potential concern that drugs selectively inhibiting MATE1 and MATE2-K may promote a nephrotoxic effect of oxaliplatin.
3.3. Impact of OCT3/Oct3 on Drug Accumulation and Secretion in Salivary Glands
The salivary glands play an important role in oral health, nutrient digestion, and immunity to microbial infection [111]. Dysfunction of the salivary glands can lead to xerostomia and dysgeusia [112]. Saliva drug concentrations have been used for therapeutic drug monitoring and well as illicit drug testing due to the ease of access to the diagnostic fluid and other potential benefits [113–116]. The impact of efflux transporters on salivary drug accumulation has been reported, and we recently demonstrated an impact of uptake transporters, particularly OCT3, in salivary gland drug accumulation, secretion and drug-induced taste disturbance [43,117–121].
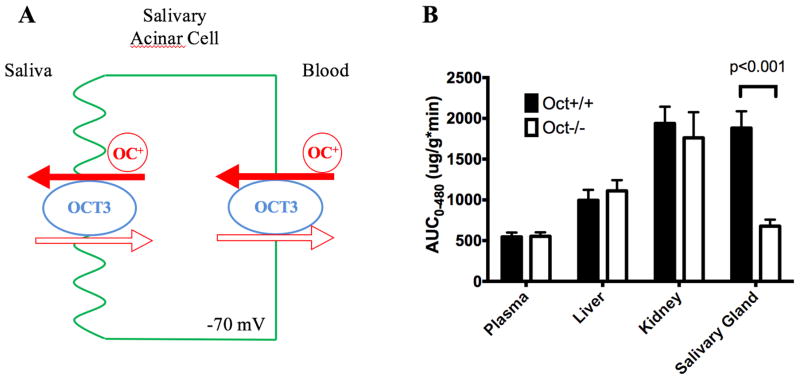
Most drugs are assumed to be secreted into saliva by passive diffusion. This process is characterized by downhill, non-mediated diffusion of drug molecules across the membranes of the salivary gland secretory epithelial cells [122]. However, passive diffusion can neither explain salivary secretion of hydrophilic drugs nor account for high drug accumulation in salivary glands. We found that OCT3 is highly expressed in human parotid, submandibular, and sublingual salivary glands [43]. Other polyspecific organic cation transporters, including OCT1, 2, MATEs, and PMAT, are minimally expressed in human or rodent salivary glands. OCT3 protein is localized at both basolateral (blood-facing) and apical (saliva-facing) membranes of salivary gland acinar cells. OCT3 appears to mediate both epithelial uptake and efflux of organic cations in the secretory cells of salivary glands, where the OCT3-mediated drug uptake is likely to be facilitated by the inside negative membrane potential and the efflux is dependent on the electrochemical potential of the substrate (Figure 2A).
Figure 2.
(A) A proposed model of OCT3-mediated metformin transport in salivary gland epithelial cells. OCT3 on the basolateral membrane of epithelial cells mediates metformin uptake from the blood into the cells. Once metformin is highly concentrated inside the cells, OCT3 on the apical membrane can facilitate efflux of metformin into the saliva. (B) Impact of Oct3 on metformin exposure, defined as area under the concentration-time curve (AUC), in plasma, salivary glands and kidney. Data were compiled from [43].
Metformin, a widely used anti-diabetic drug, is known to induce taste disturbance. Patients on metformin therapy frequently complain about a lingering metallic taste in the mouth [123,124]. When dosed to humans (oral or IV), metformin is readily detectable in the saliva [125]. We found metformin is efficiently transported by human and mouse OCT3/Oct3. When dosed to wild-type mice, metformin was actively transported into the salivary glands and achieved very high level accumulation. The overall exposure in salivary gland tissue, defined as area-under-the-concentration (AUC), is as high as in the kidney, and much higher than plasma and the liver. In contrast, active uptake and accumulation of metformin in salivary glands were substantially attenuated in Oct3−/− mice, and its salivary exposure was reduced by more than 50% in Oct3−/− mice as compared with wildtype mice (Figure 2B). Our studies demonstrated a critical role of OCT3 in the salivary glands drug accumulation and secretion. The high levels of drug accumulation achieved in salivary tissue are alarming with respect to potential tissue-specific adverse effects.
The primary function of salivary glands is to secrete saliva, and about 0.75–1.5 liters of salivary fluid are secreted each day in healthy adults. Dysfunction of the salivary glands can lead to xerostomia (or dry mouth). Although xerostomia has many origins [126], excessive accumulation of foreign chemicals in salivary glands may lead to tissue toxicity and dysfunction of the salivary glands. More than 500 drugs list xerostomia as a side effect and 25% of elderly patients on polytherapy report dry mouth [127–129]. Dry mouth can have a major adverse impact on patients’ quality of life [127,130–132]. The more severe case of oral mucositis by cancer therapeutic agents can also be dose-limiting [129,131,133]. Drug-induced xerostomia and oral mucositis are severe side effects that may result from salivary gland drug accumulation and toxicity. Our in vivo studies revealed that Oct3-mediated active uptake can lead to very high drug accumulation in salivary glands. Oct3-mediated drug uptake and accumulation may thus intensify drug toxicity in salivary gland epithelial cells. As OCT3/Oct3 is a polyspecific transporter, it can also transport other circulating drugs or toxins into salivary glands. Therefore, it is possible that Oct3-mediated or other carrier-mediated drug accumulation may interfere with the normal secretory function of salivary glands, contributing to hyposalivation and xerostomia. In this regard, it is interesting to note that oxaliplatin, a known substrate of OCT3, can cause severe oral mucositis [97,134]. Several other cytotoxic cancer chemotherapeutics including irinotecan, vincristine, and melphalan have been reported to have increased sensitivity in cells expressing OCT3 [135]. Whether OCT3 plays a role in salivary gland accumulation and toxicity of these anticancer drugs has yet to be investigated.
4. Conclusions
In the past two decades, great progress has been made in molecular and functional characterization of drug transporters and understanding their roles in drug disposition and response. It is now becoming increasingly recognized that locally expressed transporters can exert a large impact on tissue and intracellular drug levels, directly influencing their pharmacological and toxicological activities. Drug concentrations in target tissues do not always correlate with plasma drug concentrations when transporters are involved. As exemplified with the OCTs in this review, a change in transporter activity either through drug-drug interactions or genetic polymorphisms, can substantially alter tissue and intracellular drug concentrations in target organs without significantly changing systemic drug exposure. This review highlights the importance of locally expressed organic cation transporters in controlling tissue drug concentrations and pharmacodynamics, similar results have been reported or anticipated for other drug uptake or efflux transporters [4,5]. As in vivo drug levels in tissues are often difficult to obtain, this presents a challenge in predicting in vivo drug effects that depend on the local drug concentration at the site of action. Thus it is critically important to understand the impact of drug transporters on the modulation of tissue and intracellular drug concentrations in vivo.
Understanding the impact of transporters on the modulation of tissue intracellular drug concentrations in vivo is still a challenging area due to significant difficulties and knowledge gaps in the field. First, drug distribution into tissues is a complex process that can be affected by many factors including tissue blood flow, passive permeability, tissue binding and sequestration, as well as expression of compensatory transport mechanisms. To definitively establish the impact of a specific transporter in vivo, these confounding factors should be considered. In this regard, genetically engineered animal models have been proven to be particularly useful, although species differences in transporter function and expression are still of concerns. As shown with the OCTs, pharmacogenetics and clinical drug-drug interactions studies are currently the major approaches to infer the in vivo roles of transporters in humans. However, the lack of specific drug probes and the difficulty to directly measure drug concentrations in target tissues and cells make it challenging to correlate pharmacodynamics effect with the tissue-specific roles of the transporter. This can be further complicated if the transporter is expressed at multiple tissues especially in a drug elimination organ (e.g. kidney, liver). For example, a reduction in OCT-mediated liver uptake can be compensated by an elevated drug concentration in the plasma due to a simultaneously impaired renal clearance, which may result in no change in drug exposure in the target tissue [72]. Accordingly, the roles of transporters in both systemic exposure and local tissue distribution need to be considered. Second, the ability for a drug transporter to accumulate a drug against its concentration gradient is ultimately determined by its energy coupling mechanism or its transport mode. While this has been defined for some drug transporters, it is still unclear for many others including the hepatic OATP transporters. Clarifying the cellular energy source and the coupling modes for these transporters will help to understand and predict the rate and extent of tissue drug uptake mediated by these transporters. Third, there are few well-established methods that can be commonly used to directly measure tissue and intracellular drug concentrations in vivo [4]. New experimental approaches and technologies are clearly needed in this regard to allow direct correlation of tissue and intracellular drug levels with pharmacological response. Finally, as stated earlier, it is the unbound drug concentration at the target site that is responsible for eliciting its pharmacological effect. After transporting into cells, drugs can be further sequestered or bind to intracellular organelles. Little is currently know regarding these processes. Understanding intracellular drug transport processes represents yet another frontier in our understanding of the intra-tissue and intracellular pharmacology of therapeutics.
Acknowledgments
This study was supported by the National Institutes of Health National Institute on Drug Abuse Grant P01DA032507 (JW) and National Institutes of Health General Medical Sciences Grants R01GM066233 (JW) and T32GM07750 (DW). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- ABC
ATP-Binding Cassette
- ACC
Acetyl-CoA Carboxylase
- AMP
Adenosine Monophosphate
- AMPK
AMP-Activated Protein Kinase
- AUC
Area Under the Concentration Time Curve
- Cmax
Maximum Plasma Concentration
- DDI
Drug-Drug Interaction
- MATE
Multidrug and Toxin Extrusion
- OAT
Organic Anion Transporter
- OATP
Organic Anion-transporting Polypeptide
- OCT
Organic Cation Transporter
- OGGT
Oral Glucose Tolerance Test
- PBMC
Peripheral Blood Mononuclear Cell
- PMAT
Plasma Membrane Monoamine Transporter
- PXR
Pregnane X Receptor
- SLC
Solute Carrier
- TMD
Transmembrane Domain
References
- 1.DeGorter MK, Xia CQ, Yang JJ, Kim RB. Drug transporters in drug efficacy and toxicity. Annu Rev Pharmacol Toxicol. 2012;52:249–273. doi: 10.1146/annurev-pharmtox-010611-134529. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human ATP-binding cassette and solute carrier transporter superfamilies. Drug Metab Pharmacokinet. 2005;20:452–477. doi: 10.2133/dmpk.20.452. [DOI] [PubMed] [Google Scholar]
- 3.Giacomini KM, Tweedie DJ, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu X, Korzekwa K, Elsby R, Fenner K, Galetin A, Lai Y, et al. Intracellular drug concentrations and transporters: measurement, modeling, and implications for the liver. Clin Pharmacol Ther. 2013;94:126–41. doi: 10.1038/clpt.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith Da, Di L, Kerns EH. The effect of plasma protein binding on in vivo efficacy: misconceptions in drug discovery. Nat Rev Drug Discov. 2010;9:929–939. doi: 10.1038/nrd3287. [DOI] [PubMed] [Google Scholar]
- 6.Neuhoff S, Ungell AL, Zamora I, Artursson P. pH-dependent bidirectional transport of weakly basic drugs across Caco-2 monolayers: implications for drug-drug interactions. Pharm Res. 2003;20:1141–1148. doi: 10.1023/a:1025032511040. [DOI] [PubMed] [Google Scholar]
- 7.Koepsell H, Endou H. The SLC22 drug transporter family. Pflugers Arch. 2004;447:666–676. doi: 10.1007/s00424-003-1089-9. [DOI] [PubMed] [Google Scholar]
- 8.Wright SH, Dantzler WH. Molecular and cellular physiology of renal organic cation and anion transport. Physiol Rev. 2004;84:987–1049. doi: 10.1152/physrev.00040.2003. [DOI] [PubMed] [Google Scholar]
- 9.Otsuka M, Matsumoto T, Morimoto R, Arioka S, Omote H, Moriyama Y. A human transporter protein that mediates the final excretion step for toxic organic cations. Proc Natl Acad Sci U S A. 2005;102:17923–17928. doi: 10.1073/pnas.0506483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maeda K, Tian Y, Fujita T, Ikeda Y, Kumagai Y, Kondo T, et al. Inhibitory effects of p-aminohippurate and probenecid on the renal clearance of adefovir and benzylpenicillin as probe drugs for organic anion transporter (OAT) 1 and OAT3 in humans. Eur J Pharm Sci. 2014;59:94–103. doi: 10.1016/j.ejps.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Engel K, Zhou M, Wang J. Identification and characterization of a novel monoamine transporter in the human brain. J Biol Chem. 2004;279:50042–50049. doi: 10.1074/jbc.M407913200. M407913200 [pii] [DOI] [PubMed] [Google Scholar]
- 12.Engel K, Wang J. Interaction of organic cations with a newly identified plasma membrane monoamine transporter. Mol Pharmacol. 2005;68:1397–1407. doi: 10.1124/mol.105.016832. mol.105.016832 [pii] [DOI] [PubMed] [Google Scholar]
- 13.Sala-Rabanal M, Li DC, Dake GR, Kurata HT, Inyushin M, Skatchkov SN, et al. Polyamine transport by the polyspecific organic cation transporters OCT1, OCT2, and OCT3. Mol Pharm. 2013;10:1450–1458. doi: 10.1021/mp400024d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koepsell H, Busch A, Gorboulev V, Arndt P. Structure and Function of Renal Organic Cation Transporters. News Physiol Sci. 1998;13:11–16. doi: 10.1152/physiologyonline.1998.13.1.11. [DOI] [PubMed] [Google Scholar]
- 15.Nies AT, Damme K, Schaeffeler E, Schwab M. Multidrug and toxin extrusion proteins as transporters of antimicrobial drugs. Expert Opin Drug Metab Toxicol. 2012;8:1565–1577. doi: 10.1517/17425255.2012.722996. [DOI] [PubMed] [Google Scholar]
- 16.Masuda S, Terada T, Yonezawa A, Tanihara Y, Kishimoto K, Katsura T, et al. Identification and functional characterization of a new human kidney-specific H+/organic cation antiporter, kidney-specific multidrug and toxin extrusion 2. J Am Soc Nephrol. 2006;17:2127–2135. doi: 10.1681/ASN.2006030205. [DOI] [PubMed] [Google Scholar]
- 17.Motohashi H, Inui K. Organic cation transporter OCTs (SLC22) and MATEs (SLC47) in the human kidney. AAPS J. 2013;15:581–588. doi: 10.1208/s12248-013-9465-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho HT, Pan Y, Cui Z, Duan H, Swaan PW, Wang J. Molecular analysis and structure-activity relationship modeling of the substrate/inhibitor interaction site of plasma membrane monoamine transporter. J Pharmacol Exp Ther. 2011;339:376–385. doi: 10.1124/jpet.111.184036. jpet.111.184036 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou M, Xia L, Wang J. Metformin transport by a newly cloned proton-stimulated organic cation transporter (plasma membrane monoamine transporter) expressed in human intestine. Drug Metab Dispos. 2007;35:1956–1962. doi: 10.1124/dmd.107.015495. dmd.107.015495 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koepsell H. Substrate recognition and translocation by polyspecific organic cation transporters. Biol Chem. 2011;392:95–101. doi: 10.1515/BC.2011.009. [DOI] [PubMed] [Google Scholar]
- 21.Koepsell H, Schmitt BM, Gorboulev V. Organic cation transporters. Rev Physiol Biochem Pharmacol. 2003;150:36–90. doi: 10.1007/s10254-003-0017-x. [DOI] [PubMed] [Google Scholar]
- 22.Ciarimboli G. Role of organic cation transporters in drug-induced toxicity. Expert Opin Drug Metab Toxicol. 2011;7:159–174. doi: 10.1517/17425255.2011.547474. [DOI] [PubMed] [Google Scholar]
- 23.Koepsell H. Polyspecific organic cation transporters: their functions and interactions with drugs. Trends Pharmacol Sci. 2004;25:375–381. doi: 10.1016/j.tips.2004.05.005S0165614704001476. [pii] [DOI] [PubMed] [Google Scholar]
- 24.Chien H-C, Zur AA, Maurer TS, Yee S-W, Tolsma J, Jasper P, et al. Rapid Method to Determine Intracellular Drug Concentrations in Cellular Uptake Assays: Application to Metformin in OCT1-transfected HEK Cells. Drug Metab Dispos. 2015 doi: 10.1124/dmd.115.066647. dmd.115.066647–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itagaki S, Ganapathy V, Ho HT, Zhou M, Babu E, Wang J. Electrophysiological characterization of the polyspecific organic cation transporter plasma membrane monoamine transporter. Drug Metab Dispos. 2012;40:1138–1143. doi: 10.1124/dmd.111.042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou M, Duan H, Engel K, Xia L, Wang J. Adenosine transport by plasma membrane monoamine transporter: reinvestigation and comparison with organic cations. Drug Metab Dispos. 2010;38:1798–1805. doi: 10.1124/dmd.110.032987. dmd.110.032987 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright SH, Wunz TM. Transport of tetraethylammonium by rabbit renal brush-border and basolateral membrane vesicles. Am J Physiol. 1987;253:F1040–50. doi: 10.1152/ajprenal.1987.253.5.F1040. [DOI] [PubMed] [Google Scholar]
- 28.Grundemann D, Gorboulev V, Gambaryan S, Veyhl M, Koepsell H. Drug excretion mediated by a new prototype of polyspecific transporter. Nature. 1994;372:549–552. doi: 10.1038/372549a0. [DOI] [PubMed] [Google Scholar]
- 29.Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: Structure, function, physiological roles, and biopharmaceutical implications. Pharm Res. 2007;24:1227–1251. doi: 10.1007/s11095-007-9254-z. [DOI] [PubMed] [Google Scholar]
- 30.Motohashi H, Sakurai Y, Saito H, Masuda S, Urakami Y, Goto M, et al. Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J Am Soc Nephrol. 2002;13:866–874. doi: 10.1681/ASN.V134866. [DOI] [PubMed] [Google Scholar]
- 31.Nies AT, Koepsell H, Winter S, Burk O, Klein K, Kerb R, et al. Expression of organic cation transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) is affected by genetic factors and cholestasis in human liver. Hepatology. 2009;50:1227–1240. doi: 10.1002/hep.23103. [DOI] [PubMed] [Google Scholar]
- 32.Gorboulev V, Ulzheimer JC, Akhoundova A, Ulzheimer-Teuber I, Karbach U, Quester S, et al. Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol. 1997;16:871–881. doi: 10.1089/dna.1997.16.871. [DOI] [PubMed] [Google Scholar]
- 33.Zhang L, Dresser MJ, Gray AT, Yost SC, Terashita S, Giacomini KM. Cloning and functional expression of a human liver organic cation transporter. Mol Pharmacol. 1997;51:913–921. doi: 10.1124/mol.51.6.913. [DOI] [PubMed] [Google Scholar]
- 34.Okuda M, Saito H, Urakami Y, Takano M, Inui K. cDNA cloning and functional expression of a novel rat kidney organic cation transporter, OCT2. Biochem Biophys Res Commun. 1996;224:500–507. doi: 10.1006/bbrc.1996.1056. [DOI] [PubMed] [Google Scholar]
- 35.Kekuda R, Prasad PD, Wu X, Wang H, Fei YJ, Leibach FH, et al. Cloning and functional characterization of a potential-sensitive, polyspecific organic cation transporter (OCT3) most abundantly expressed in placenta. J Biol Chem. 1998;273:15971–15979. doi: 10.1074/jbc.273.26.15971. [DOI] [PubMed] [Google Scholar]
- 36.Wu X, Kekuda R, Huang W, Fei YJ, Leibach FH, Chen J, et al. Identity of the Organic Cation Transporter OCT3 as the Extraneuronal Monoamine Transporter (uptake2) and Evidence for the Expression of the Transporter in the Brain. J Biol Chem. 1998;273:32776–32786. doi: 10.1074/jbc.273.49.32776. [DOI] [PubMed] [Google Scholar]
- 37.Grundemann D, Schechinger B, Rappold GA, Schomig E. Molecular identification of the corticosterone-sensitive extraneuronal catecholamine transporter. Nat Neurosci. 1998;1:349–351. doi: 10.1038/1557. [DOI] [PubMed] [Google Scholar]
- 38.Shang T, Uihlein AV, Van Asten J, Kalyanaraman B, Hillard CJ. 1-Methyl-4-phenylpyridinium accumulates in cerebellar granule neurons via organic cation transporter 3. J Neurochem. 2003;85:358–367. doi: 10.1046/j.1471-4159.2003.01686.x. 1686 [pii] [DOI] [PubMed] [Google Scholar]
- 39.Duan H, Wang J. Selective transport of monoamine neurotransmitters by human plasma membrane monoamine transporter and organic cation transporter 3. J Pharmacol Exp Ther. 2010;335:743–753. doi: 10.1124/jpet.110.170142. jpet.110.170142 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee N, Hebert MF, Prasad B, Easterling TR, Kelly EJ, Unadkat JD, et al. Effect of gestational age on mRNA and protein expression of polyspecific organic cation transporters during pregnancy. Drug Metab Dispos. 2013;41:2225–2232. doi: 10.1124/dmd.113.054072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sata R, Ohtani H, Tsujimoto M, Murakami H, Koyabu N, Nakamura T, et al. Functional analysis of organic cation transporter 3 expressed in human placenta. J Pharmacol Exp Ther. 2005;315:888–895. doi: 10.1124/jpet.105.086827. [DOI] [PubMed] [Google Scholar]
- 42.Lips KS, Volk C, Schmitt BM, Pfeil U, Arndt P, Miska D, et al. Polyspecific cation transporters mediate luminal release of acetylcholine from bronchial epithelium. Am J Respir Cell Mol Biol. 2005;33:79–88. doi: 10.1165/rcmb.2004-0363OC. [DOI] [PubMed] [Google Scholar]
- 43.Lee N, Duan H, Hebert MF, Liang CJ, Rice KM, Wang J. Taste of a Pill: ORGANIC CATION TRANSPORTER-3 (OCT3) MEDIATES METFORMIN ACCUMULATION AND SECRETION IN SALIVARY GLANDS. J Biol Chem. 2014;289:27055–27064. doi: 10.1074/jbc.M114.570564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Q, Shu Y. Role of solute carriers in response to anticancer drugs. Mol Cell Ther. 2014;2:15. doi: 10.1186/2052-8426-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Komatsu T, Hiasa M, Miyaji T, Kanamoto T, Matsumoto T, Otsuka M, et al. Characterization of the human MATE2 proton-coupled polyspecific organic cation exporter. Int J Biochem Cell Biol. 2011;43:913–918. doi: 10.1016/j.biocel.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 46.Xia L, Engel K, Zhou M, Wang J. Membrane localization and pH-dependent transport of a newly cloned organic cation transporter (PMAT) in kidney cells. Am J Physiol Ren Physiol. 2007;292:F682–90. doi: 10.1152/ajprenal.00302.2006. 00302.2006 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou M, Engel K, Wang J. Evidence for significant contribution of a newly identified monoamine transporter (PMAT) to serotonin uptake in the human brain. Biochem Pharmacol. 2007;73:147–154. doi: 10.1016/j.bcp.2006.09.008. S0006-2952(06)00580-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dahlin a, Xia L, Kong W, Hevner R, Wang J. Expression and immunolocalization of the plasma membrane monoamine transporter in the brain. Neuroscience. 2007;146:1193–1211. doi: 10.1016/j.neuroscience.2007.01.072. S0306-4522(07)00153-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duan H, Wang J. Impaired monoamine and organic cation uptake in choroid plexus in mice with targeted disruption of the plasma membrane monoamine transporter (Slc29a4) gene. J Biol Chem. 2013;288:3535–3544. doi: 10.1074/jbc.M112.436972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Budiman T, Bamberg E, Koepsell H, Nagel G. Mechanism of electrogenic cation transport by the cloned organic cation transporter 2 from rat. J Biol Chem. 2000;275:29413–29420. doi: 10.1074/jbc.M004645200. [DOI] [PubMed] [Google Scholar]
- 51.Yin J, Duan H, Shirasaka Y, Prasad B, Wang J. Atenolol Renal Secretion Is Mediated by Human Organic Cation Transporter 2 and Multidrug and Toxin Extrusion Proteins. Drug Metab Dispos. 2015;43:1872–1881. doi: 10.1124/dmd.115.066175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hagenbuch B. Drug uptake systems in liver and kidney: a historic perspective. Clin Pharmacol Ther. 2010;87:39–47. doi: 10.1038/clpt.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meijer DK, Mol WE, Muller M, Kurz G. Carrier-mediated transport in the hepatic distribution and elimination of drugs, with special reference to the category of organic cations. J Pharmacokinet Biopharm. 1990;18:35–70. doi: 10.1007/BF01063621. [DOI] [PubMed] [Google Scholar]
- 54.Moule SK, McGivan JD. Regulation of the plasma membrane potential in hepatocytes--mechanism and physiological significance. Biochim Biophys Acta. 1990;1031:383–397. doi: 10.1016/0304-4157(90)90016-6. [DOI] [PubMed] [Google Scholar]
- 55.Strazzabosco M, Sakisaka S, Hayakawa T, Boyer JL. Effect of UDCA on intracellular and biliary pH in isolated rat hepatocyte couplets and perfused livers. Am J Physiol. 1991;260:G58–69. doi: 10.1152/ajpgi.1991.260.1.G58. [DOI] [PubMed] [Google Scholar]
- 56.Hillgren KM, Keppler D, Zur AA, Giacomini KM, Stieger B, Cass CE, et al. Emerging transporters of clinical importance: an update from the International Transporter Consortium. Clin Pharmacol Ther. 2013;94:52–63. doi: 10.1038/clpt.2013.74. [DOI] [PubMed] [Google Scholar]
- 57.Koepsell H. The SLC22 family with transporters of organic cations, anions and zwitterions. Mol Asp Med. 2013;34:413–435. doi: 10.1016/j.mam.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 58.Staud F, Cerveny L, Ahmadimoghaddam D, Ceckova M. Multidrug and toxin extrusion proteins (MATE/SLC47); role in pharmacokinetics. Int J Biochem Cell Biol. 2013;45:2007–2011. doi: 10.1016/j.biocel.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 59.Zamek-Gliszczynski MJ, Bao JQ, Day JS, Higgins JW. Metformin sinusoidal efflux from the liver is consistent with negligible biliary excretion and absence of enterohepatic cycling. Drug Metab Dispos. 2013;41:1967–1971. doi: 10.1124/dmd.113.053025. [DOI] [PubMed] [Google Scholar]
- 60.Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50:81–98. doi: 10.2165/11534750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 61.Scheen AJ. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 1996;30:359–371. doi: 10.2165/00003088-199630050-00003. [DOI] [PubMed] [Google Scholar]
- 62.Ito S, Kusuhara H, Kuroiwa Y, Wu C, Moriyama Y, Inoue K, et al. Potent and specific inhibition of mMate1-mediated efflux of type I organic cations in the liver and kidney by pyrimethamine. J Pharmacol Exp Ther. 2010;333:341–350. doi: 10.1124/jpet.109.163642. [DOI] [PubMed] [Google Scholar]
- 63.Kirpichnikov D, Mcfarlane SI, Sowers JR. Metformin: An Update. Ann Intern Med. 2002 doi: 10.7326/0003-4819-137-1-200207020-00009. [DOI] [PubMed] [Google Scholar]
- 64.Shu Y, Sheardown SAS, Brown C, Owen RP, Zhang S, Castro RA, et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J Clin …. 2007;117:1422–31. doi: 10.1172/JCI30558DS1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takane H, Shikata E, Otsubo K, Higuchi S, Ieiri I. Polymorphism in human organic cation transporters and metformin action. Pharmacogenomics. 2008;9:415–422. doi: 10.2217/14622416.9.4.415. [DOI] [PubMed] [Google Scholar]
- 66.Gong L, Goswami S, Giacomini KM, Altman RB, Klein TE. Metformin pathways. Pharmacogenet Genomics. 2012;22:820–827. doi: 10.1097/FPC.0b013e3283559b22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jung N, Lehmann C, Rubbert a, Schömig E, Fätkenheuer G, Hartmann P, et al. Organic cation transporters OCT1 and OCT2 determine the accumulation of lamivudine in CD4 cells of HIV-infected patients. Infection. 2013;41:379–385. doi: 10.1007/s15010-012-0308-8. [DOI] [PubMed] [Google Scholar]
- 68.Minuesa G, Volk C, Molina-arcas M, Gorboulev V, Erkizia I, Arndt P, et al. Transport of Lamivudine [(_)-_-L-2_,3_-Dideoxy-3_-thiacytidine] and High-Affinity Interaction of Nucleoside Reverse Transcriptase Inhibitors with Human Organic Cation Transporters 1, 2, and 3. J Pharmacol Exp Ther. 2009;329:252–61. doi: 10.1124/jpet.108.146225. [DOI] [PubMed] [Google Scholar]
- 69.Choi CI, Bae JW, Keum SK, Lee YJ, Lee HI, Jang CG, et al. Effects of OCT2 c.602C > T genetic variant on the pharmacokinetics of lamivudine. Xenobiotica. 2013;43:636–640. doi: 10.3109/00498254.2012.747710. [DOI] [PubMed] [Google Scholar]
- 70.Wang DSS, Jonker JW, Kato Y, Kusuhara H, Schinkel AH, Sugiyama Y. Involvement of organic cation transporter 1 in hepatic and intestinal distribution of metformin. J Pharmacol Exp Ther. 2002;302:510–515. doi: 10.1124/jpet.102.034140. [DOI] [PubMed] [Google Scholar]
- 71.Rojas LBA, Gomes MB. Metformin: an old but still the best treatment for type 2 diabetes. Diabetol Metab Syndr. 2013;5:1. doi: 10.1186/1758-5996-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Higgins JW, Bedwell DW, Zamek-Gliszczynski MJ. Ablation of both organic cation transporter (oct)1 and oct2 alters metformin pharmacokinetics but has no effect on tissue drug exposure and pharmacodynamics. Drug Metab Dispos. 2012;40:1170–1177. doi: 10.1124/dmd.112.044875. [DOI] [PubMed] [Google Scholar]
- 73.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI200113505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abbud W, Habinowski S, Zhang JZ, Kendrew J, Elkairi FS, Kemp BE, et al. Stimulation of AMP-activated protein kinase (AMPK) is associated with enhancement of Glut1-mediated glucose transport. Arch Biochem Biophys. 2000;380:347–352. doi: 10.1006/abbi.2000.1935. [DOI] [PubMed] [Google Scholar]
- 75.Cho SK, Yoon JS, Lee MG, Lee DH, Lim LA, Park K, et al. Rifampin enhances the glucose-lowering effect of metformin and increases OCT1 mRNA levels in healthy participants. Clin Pharmacol Ther. 2011;89:416–421. doi: 10.1038/clpt.2010.266. [DOI] [PubMed] [Google Scholar]
- 76.Shikata E, Yamamoto R, Takane H, Shigemasa C, Ikeda T, Otsubo K, et al. Human organic cation transporter (OCT1 and OCT2) gene polymorphisms and therapeutic effects of metformin. J Hum Genet. 2007;52:117–122. doi: 10.1007/s10038-006-0087-0. [DOI] [PubMed] [Google Scholar]
- 77.Shu Y, Brown C, Castro RA, Shi RJ, Lin ET, Owen RP, et al. Effect of genetic variation in the organic cation transporter 1, OCT1, on metformin pharmacokinetics. Clin Pharmacol Ther. 2008;83:273–280. doi: 10.1038/sj.clpt.6100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jarvis B, Faulds D. Lamivudine. A review of its therapeutic potential in chronic hepatitis B. Drugs. 1999;58:101–141. doi: 10.2165/00003495-199958010-00015. http://dx.doi.org/10.2165/00003495-199958010-00015. [DOI] [PubMed] [Google Scholar]
- 79.Kunze A, Huwyler J, Camenisch G, Gutmann H. Interaction of the antiviral drug telaprevir with renal and hepatic drug transporters. Biochem Pharmacol. 2012;84:1096–1102. doi: 10.1016/j.bcp.2012.07.032. [DOI] [PubMed] [Google Scholar]
- 80.Konig SK, Herzog M, Theile D, Zembruski N, Haefeli WE, Weiss J. Impact of drug transporters on cellular resistance towards saquinavir and darunavir. J Antimicrob Chemother. 2010;65:2319–2328. doi: 10.1093/jac/dkq324. dkq324 [pii] [DOI] [PubMed] [Google Scholar]
- 81.Minuesa G, Huber-Ruano I, Pastor-Anglada M, Koepsell H, Clotet B, Martinez-Picado J. Drug uptake transporters in antiretroviral therapy. Pharmacol Ther. 2011;132:268–279. doi: 10.1016/j.pharmthera.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 82.Moss DM, Liptrott NJ, Siccardi M, Owen A. Interactions of antiretroviral drugs with the SLC22A1 (OCT1) drug transporter. Front Pharmacol. 2015;6 doi: 10.3389/fphar.2015.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jung N, Lehmann C, Rubbert A. Relevance of the organic cation transporters 1 and 2 for antiretroviral drug therapy in human immunodeficiency virus infection. Drug Metab …. 2008;36:1616–1623. doi: 10.1124/dmd.108.020826.Understanding. [DOI] [PubMed] [Google Scholar]
- 84.Jung N, Lehmann C, Rubbert A, Knispel M, Hartmann P, van Lunzen J, et al. Relevance of the organic cation transporters 1 and 2 for antiretroviral drug therapy in human immunodeficiency virus infection. Drug Metab …. 2008;36:1616–1623. doi: 10.1124/dmd.108.020826.Understanding. [DOI] [PubMed] [Google Scholar]
- 85.Zhou Z, Rodman JH, Flynn PM, Robbins BL, Wilcox CK, D’Argenio DZ. Model for intracellular lamivudine metabolism in peripheral blood mononuclear cells ex vivo and in human immunodeficiency virus type 1-infected adolescents. Antimicrob Agents Chemother. 2006;50:2686–2694. doi: 10.1128/AAC.01637-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morrissey KM, Stocker SL, Wittwer MB, Xu L, Giacomini KM. Renal transporters in drug development. Annu Rev Pharmacol Toxicol. 2013;53:503–29. doi: 10.1146/annurev-pharmtox-011112-140317. [DOI] [PubMed] [Google Scholar]
- 87.Lai Y, Sampson KE, Balogh LM, Brayman TG, Cox SR, Adams WJ, et al. Preclinical and clinical evidence for the collaborative transport and renal secretion of an oxazolidinone antibiotic by organic anion transporter 3 (OAT3/SLC22A8) and multidrug and toxin extrusion protein 1 (MATE1/SLC47A1) J Pharmacol Exp Ther. 2010;334:936–944. doi: 10.1124/jpet.110.170753. [DOI] [PubMed] [Google Scholar]
- 88.Müller F, König J, Hoier E, Mandery K, Fromm MF. Role of organic cation transporter OCT2 and multidrug and toxin extrusion proteins MATE1 and MATE2-K for transport and drug interactions of the antiviral lamivudine. Biochem Pharmacol. 2013;86:808–815. doi: 10.1016/j.bcp.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 89.Yoon H, Cho HY, Yoo HD, Kim SM, Lee YB. Influences of organic cation transporter polymorphisms on the population pharmacokinetics of metformin in healthy subjects. AAPS J. 2013;15:571–80. doi: 10.1208/s12248-013-9460-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang ZJ, Yin OQ, Tomlinson B, Chow MS. OCT2 polymorphisms and in-vivo renal functional consequence: studies with metformin and cimetidine. Pharmacogenet Genomics. 2008;18:637–645. doi: 10.1097/FPC.0b013e328302cd4101213011-200807000-00010. [pii] [DOI] [PubMed] [Google Scholar]
- 91.Ito S, Kusuhara H, Yokochi M, Toyoshima J, Inoue K, Yuasa H, et al. Competitive inhibition of the luminal efflux by multidrug and toxin extrusions, but not basolateral uptake by organic cation transporter 2, is the likely mechanism underlying the pharmacokinetic drug-drug interactions caused by cimetidine in the kidney. J Pharmacol Exp Ther. 2012;340:393–403. doi: 10.1124/jpet.111.184986. [DOI] [PubMed] [Google Scholar]
- 92.Song IS, Shin HJ, Shim EJ, Jung IS, Kim WY, Shon JH, et al. Genetic variants of the organic cation transporter 2 influence the disposition of metformin. Clin Pharmacol Ther. 2008;84:559–62. doi: 10.1038/clpt.2008.61. [DOI] [PubMed] [Google Scholar]
- 93.Yonezawa A, Inui KI. Organic cation transporter OCT/SLC22A and H+/organic cation antiporter MATE/SLC47A are key molecules for nephrotoxicity of platinum agents. Biochem Pharmacol. 2011;81:563–568. doi: 10.1016/j.bcp.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 94.de Jongh FE, van Veen RN, Veltman SJ, de Wit R, van der Burg MEL, van den Bent MJ, et al. Weekly high-dose cisplatin is a feasible treatment option: analysis on prognostic factors for toxicity in 400 patients. Br J Cancer. 2003;88:1199–206. doi: 10.1038/sj.bjc.6600884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dobyan DC, Levi J, Jacobs C, Kosek J, Weiner MW. Mechanism of cis-platinum nephrotoxicity: II. Morphologic observations. J Pharmacol Exp Ther. 1980;213:551–6. [PubMed] [Google Scholar]
- 96.Litterst CL, Gram TE, Dedrick RL, Leroy AF, Guarino AM. Distribution and disposition of platinum following intravenous administration of cis diamminedichloroplatinum(II) (NSC 119875) to dogs. Cancer Res. 1976;36:2340–2344. [PubMed] [Google Scholar]
- 97.Yonezawa A, Masuda S, Yokoo S, Katsura T, Inui KI. Cisplatin and oxaliplatin, but not carboplatin and nedaplatin, are substrates for human organic cation transporters (SLC22A1-3 and multidrug and toxin extrusion family) J Pharmacol Exp Ther. 2006;319:879–886. doi: 10.1124/jpet.106.110346.lato. [DOI] [PubMed] [Google Scholar]
- 98.Siddik ZH, Newell DR, Boxall FE, Harrap KR. The comparative pharmacokinetics of carboplatin and cisplatin in mice and rats. Biochem Pharmacol. 1987;36:1925–1932. doi: 10.1016/0006-2952(87)90490-4. [DOI] [PubMed] [Google Scholar]
- 99.Kern W, Braess J, Böttger B, Kaufmann CC, Hiddemann W, Schleyer E. Oxaliplatin pharmacokinetics during a four-hour infusion. Clin Cancer Res. 1999;5:761–765. [PubMed] [Google Scholar]
- 100.Takimoto CH, Graham MA, Lockwood G, Ng CM, Goetz A, Greenslade D, et al. Oxaliplatin pharmacokinetics and pharmacodynamics in adult cancer patients with impaired renal function. Clin Cancer Res. 2007;13:4832–4839. doi: 10.1158/1078-0432.CCR-07-0475. [DOI] [PubMed] [Google Scholar]
- 101.Filipski KK, Mathijssen RH, Mikkelsen TS, Schinkel AH, Sparreboom A. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin Pharmacol Ther. 2009;86:396–402. doi: 10.1038/clpt.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ciarimboli G, Deuster D, Knief A, Sperling M, Holtkamp M, Edemir B, et al. Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions. Am J Pathol. 2010;176:1169–1180. doi: 10.2353/ajpath.2010.090610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lanvers-Kaminsky C, Sprowl JA, Malath I, Deuster D, Eveslage M, Schlatter E, et al. Human OCT2 variant c.808G>T confers protection effect against cisplatin-induced ototoxicity. Pharmacogenomics. 2015;16:323–332. doi: 10.2217/pgs.14.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sprowl Ja, van Doorn L, Hu S, van Gerven L, de Bruijn P, Li L, et al. Conjunctive therapy of cisplatin with the OCT2 inhibitor cimetidine: influence on antitumor efficacy and systemic clearance. Clin Pharmacol Ther. 2013;94:585–92. doi: 10.1038/clpt.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tanihara Y, Masuda S, Katsura T, Inui KI. Protective effect of concomitant administration of imatinib on cisplatin-induced nephrotoxicity focusing on renal organic cation transporter OCT2. Biochem Pharmacol. 2009;78:1263–1271. doi: 10.1016/j.bcp.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 106.Nakamura T, Yonezawa A, Hashimoto S, Katsura T, Inui K. Disruption of multidrug and toxin extrusion MATE1 potentiates cisplatin-induced nephrotoxicity. Biochem Pharmacol. 2010;80:1762–1767. doi: 10.1016/j.bcp.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 107.Li Q, Guo D, Dong Z, Zhang W, Zhang L, Huang SM, et al. Ondansetron can enhance cisplatin-induced nephrotoxicity via inhibition of multiple toxin and extrusion proteins (MATEs) Toxicol Appl Pharmacol. 2013;273:100–109. doi: 10.1016/j.taap.2013.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sleijfer DT, Offerman JJ, Mulder NH, Verweij M, van der Hem GK, Schraffordt Koops HS, et al. The protective potential of the combination of verapamil and cimetidine on cisplatin-induced nephrotoxicity in man. Cancer. 1987;60:2823–2828. doi: 10.1002/1097-0142(19871201)60:11<2823::AID-CNCR2820601138>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 109.Yokoo S, Yonezawa A, Masuda S, Fukatsu A, Katsura T, Inui KI. Differential contribution of organic cation transporters, OCT2 and MATE1, in platinum agent-induced nephrotoxicity. Biochem Pharmacol. 2007;74:477–487. doi: 10.1016/j.bcp.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 110.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Deliv. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 111.Holsinger F, Bui D. Salivary Gland Disord. Pittsburgh: Springer; 2007. Anatomy, function, and evaluation of the salivary glands. [Google Scholar]
- 112.Ackerman BH, Kasbekar N. Disturbances of taste and smell induced by drugs. Pharmacotherapy. 1996;17:482–496. [PubMed] [Google Scholar]
- 113.SIEGEL IA. The Role of Saliva in Drug Monitoring. Ann N Y Acad Sci. 1993;694:86–90. doi: 10.1111/j.1749-6632.1993.tb18345.x. [DOI] [PubMed] [Google Scholar]
- 114.Takahashi T, Fujiwara Y, Sumiyoshi H, Isobe T, Yamaoka N, Yamakido M. Salivary drug monitoring of irinotecan and its active metabolite in cancer patients. Cancer Chemother Pharmacol. 1997;40:449–452. doi: 10.1007/s002800050685. [DOI] [PubMed] [Google Scholar]
- 115.Liu H, Delgado MR. Therapeutic Drug Concentration Monitoring Using Saliva Samples. Clin Pharmacokinet. 1999;36:453–470. doi: 10.2165/00003088-199936060-00006. [DOI] [PubMed] [Google Scholar]
- 116.Navarro M, Pichini S, Farré M, Ortuño J, Roset PN, Segura J, et al. Usefulness of saliva for measurement of 3,4-methylenedioxymethamphetamine and its metabolites: Correlation with plasma drug concentrations and effect of salivary pH. Clin Chem. 2001;47:1788–1795. [PubMed] [Google Scholar]
- 117.Uematsu T, Yamaoka M, Doto R, Tanaka H, Matsuura T, Furusawa K. Expression of ATP-binding cassette transporter in human salivary ducts. Arch Oral Biol. 2003;48:87–90. doi: 10.1016/S0003-9969(02)00159-0. [DOI] [PubMed] [Google Scholar]
- 118.Uematsu T, Yamaoka M, Matsuura T, Doto R, Hotomi H, Yamada A, et al. P-glycoprotein expression in human major and minor salivary glands. Arch Oral Biol. 2001;46:521–527. doi: 10.1016/s0003-9969(01)00012-7. [DOI] [PubMed] [Google Scholar]
- 119.Wijnholds J, Scheffer GL, van der Valk M, van der Valk P, Beijnen JH, Scheper RJ, et al. Multidrug resistance protein 1 protects the oropharyngeal mucosal layer and the testicular tubules against drug-induced damage. J Exp Med. 1998;188:797–808. doi: 10.1084/jem.188.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sun QF, Sun QH, Du J, Wang S. Differential gene expression profiles of normal human parotid and submandibular glands. Oral Dis. 2008;14:500–509. doi: 10.1111/j.1601-0825.2007.01408.x. [DOI] [PubMed] [Google Scholar]
- 121.Ikarashi R, Shibasaki K, Yamaguchi A. Immunohistochemical studies of organic anion transporters and urate transporter 1 expression in human salivary gland. Acta Odontol Scand. 2012:1–5. doi: 10.3109/00016357.2012.680904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Aps JKM, Martens LC. Review: The physiology of saliva and transfer of drugs into saliva. Forensic Sci Int. 2005;150:119–131. doi: 10.1016/j.forsciint.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 123.Bristol-Myers Squibb. Glucophage (metformin hydrochloride) package insert. 2009. [Google Scholar]
- 124.Lee AJ. Metformin in noninsulin-dependent diabetes mellitus. Pharmacotherapy. 1996;16:327–51. [PubMed] [Google Scholar]
- 125.Pentikäinen PJ, Neuvonen PJ, Penttilä A. Pharmacokinetics of metformin after intravenous and oral administration to man. Eur J Clin Pharmacol. 1979;16:195–202. doi: 10.1007/BF00562061. [DOI] [PubMed] [Google Scholar]
- 126.Cassolato SF, Turnbull RS. Xerostomia: clinical aspects and treatment. Gerodontology. 2003;20:64–77. doi: 10.1111/j.1741-2358.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 127.Jensen SB, Vissink A. Salivary gland dysfunction and xerostomia in Sjögren’s syndrome. Oral Maxillofac Surg Clin North Am. 2014;26:35–53. doi: 10.1016/j.coms.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 128.Sreebny LM, Valdini a. Xerostomia. Part I: Relationship to other oral symptoms and salivary gland hypofunction. Oral Surg Oral Med Oral Pathol. 1988;66:451–458. doi: 10.1016/0030-4220(88)90268-X. [DOI] [PubMed] [Google Scholar]
- 129.Epstein JB, Tsang AHF, Warkentin D, Ship Ja. The role of salivary function in modulating chemotherapy-induced oropharyngeal mucositis: A review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:39–44. doi: 10.1067/moe.2002.126018. [DOI] [PubMed] [Google Scholar]
- 130.Scully C, Epstein J, Sonis S. Oral mucositis: A challenging complication of radiotherapy, chemotherapy, and radiochemotherapy: Part 1, pathogenesis and prophylaxis of mucositis. Head Neck. 2003;25:1057–1070. doi: 10.1002/hed.10318. [DOI] [PubMed] [Google Scholar]
- 131.Scully C, Epstein J, Sonis S. Oral mucositis: A challenging complication of radiotherapy, chemotherapy, and radiochemotherapy. Part 2: Diagnosis and management of mucositis. Head Neck. 2004;26:77–84. doi: 10.1002/hed.10326. [DOI] [PubMed] [Google Scholar]
- 132.Skopouli FN, Dafni U, Ioannidis JP, Moutsopoulos HM. Clinical evolution, and morbidity and mortality of primary Sjögren’s syndrome. Semin Arthritis Rheum. 2000;29:296–304. doi: 10.1016/S0049-0172(00)80016-5. [DOI] [PubMed] [Google Scholar]
- 133.Cawley MM, Benson LM. Current trends in managing oral mucositis. Clin J Oncol Nursing. 2005;9:584–592. doi: 10.1188/05.CJON.584-592. [DOI] [PubMed] [Google Scholar]
- 134.Saadeh CE. Chemotherapy- and radiotherapy-induced oral mucositis: review of preventive strategies and treatment. Pharmacotherapy. 2005;25:540–554. doi: 10.1592/phco.25.4.540.61035. http://dx.doi.org/10.1592/phco.25.4.540.61035. [DOI] [PubMed] [Google Scholar]
- 135.Shnitsar V, Eckardt R, Gupta S, Grottker J, Müller GA, Koepsell H, et al. Expression of human organic cation transporter 3 in kidney carcinoma cell lines increases chemosensitivity to melphalan, irinotecan, and vincristine. Cancer Res. 2009;69:1494–1501. doi: 10.1158/0008-5472.CAN-08-2483. [DOI] [PubMed] [Google Scholar]
- 136.Nies AT, Koepsell H, Damme K, Schwab M. Organic cation transporters (OCTs, MATEs), in vitro and in vivo evidence for the importance in drug therapy. Handb Exp Pharmacol. 2011:105–167. doi: 10.1007/978-3-642-14541-4_3. [DOI] [PubMed] [Google Scholar]
- 137.Iwai M, Minematsu T, Li Q, Iwatsubo T, Usui T. Utility of P-glycoprotein and organic cation transporter 1 double-transfected LLC-PK1 cells for studying the interaction of YM155 monobromide, novel small-molecule survivin suppressant, with P-glycoprotein. Drug Metab Dispos. 2011;39:2314–2320. doi: 10.1124/dmd.111.040733. [DOI] [PubMed] [Google Scholar]
- 138.Saadatmand AR, Tadjerpisheh S, Brockmoller J, Tzvetkov MV. The prototypic pharmacogenetic drug debrisoquine is a substrate of the genetically polymorphic organic cation transporter OCT1. Biochem Pharmacol. 2012;83:1427–1434. doi: 10.1016/j.bcp.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 139.Ishiguro N, Shimizu H, Kishimoto W, Ebner T, Schaefer O. Evaluation and prediction of potential drug-drug interactions of linagliptin using in vitro cell culture methods. Drug Metab Dispos. 2013;41:149–158. doi: 10.1124/dmd.112.048470. [DOI] [PubMed] [Google Scholar]
- 140.Tanihara Y, Masuda S, Sato T, Katsura T, Ogawa O, Inui KI. Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H(+)-organic cation antiporters. Biochem Pharmacol. 2007;74:359–371. doi: 10.1016/j.bcp.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 141.Ohta KY, Inoue K, Yasujima T, Ishimaru M, Yuasa H. Functional characteristics of two human MATE transporters: kinetics of cimetidine transport and profiles of inhibition by various compounds. J Pharm Pharm Sci. 2009;12:388–396. doi: 10.18433/j3r59x. [DOI] [PubMed] [Google Scholar]