Abstract
Reprogramming of human somatic cells into induced pluripotent stem (iPS) cells has greatly expanded the set of research tools available to investigate the molecular and cellular mechanisms underlying central nervous system (CNS) disorders. Reali zing the promise of iPS cell technology for the identification of novel therapeutic targets and for high-throughput drug screening requires implementation of methods for the large-scale production of defined CNS cell types. Here we describe a protocol for generating stable, highly expandable, iPS cell-derived CNS neural progenitor cells (NPC) using multidimensional fluorescence activated cell sorting (FACS) to purify NPC defined by cell surface markers. In addition, we describe a rapid, efficient, and reproducible method for generating excitatory cortical-like neurons from these NPC through inducible expression of the pro-neural transcription factor Neurogenin 2 (iNgn2-NPC). Finally, we describe methodology for the use of iNgn2-NPC for probing human neuroplasticity and mechanisms underlying CNS disorders using high-content, single-cell level automated microscopy assays.
Keywords: Induced pluripotent stem cell, neuroplasticity, neurodegeneration, neuropsychiatric, high-throughput screening, high-content imaging, drug discovery
INTRODUCTION
The initial discoveries of Yamanaka and colleagues (Takahashi and Yamanaka, 2006; Takahashi et al., 2007) that enable generation of human induced pluripotent stem (iPS) cells from patients with virtually any disease have greatly expanded the set of research tools available to study human disease at the cellular level. This is particularly true for disorders of the brain, since living human brain tissue has previously been largely inaccessible to researchers. Based on advances in our understanding of human central nervous system (CNS) development (e.g. Staccia et al. 2015; Figure 1A) a variety of protocols that neuralize iPS cells to produce neuroectodermal neural progenitor cells (NPC) that can be patterned to specify neuronal cell types of forebrain, midbrain, hindbrain and spinal cord origins have recently been reported (Muratore et al., 2014; Doi et al., 2014; Mattis and Svendsen, 2015; Watson et al., 2015; Sareen et al., 2014). Patient iPS cell-derived neuronal lines now allow for laboratory grown genetically accurate human neuronal cell models for disease modeling and drug discovery (Haggarty et al., 2016).
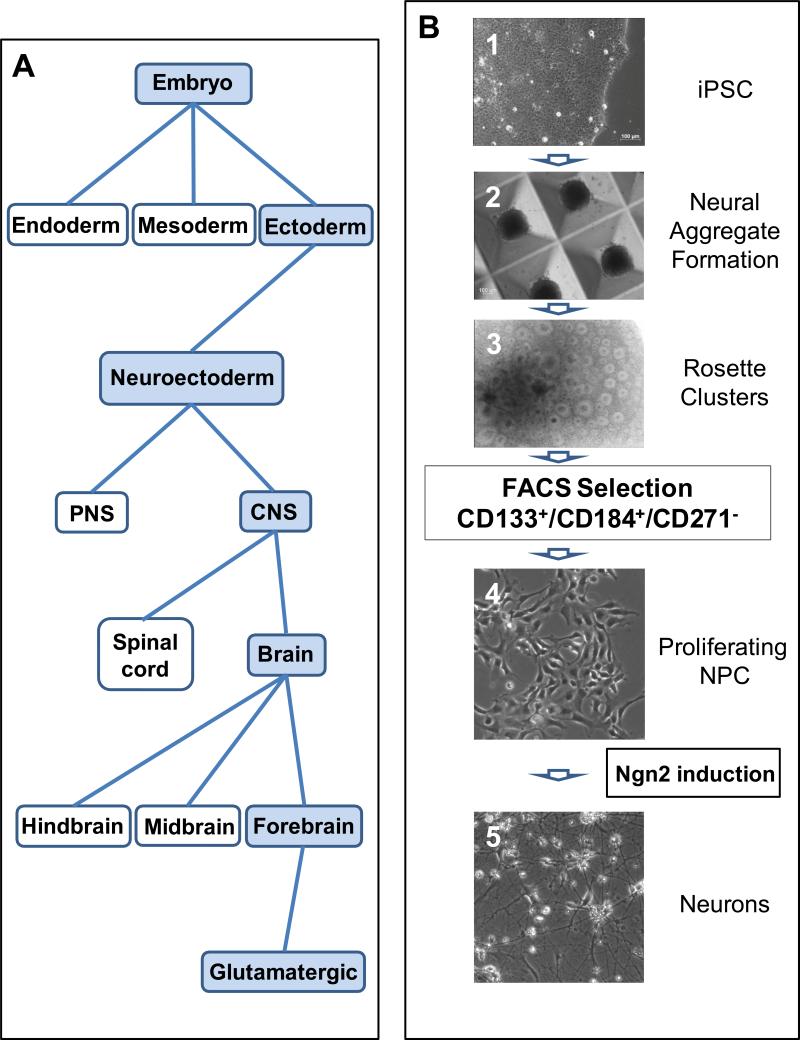
Figure 1. Generation of iPS cell-derived neurons along a human CNS developmental path.
A. Schematic of human CNS development, with the specific path from embryo to excitatory glutamatergic neurons shaded in blue. B. Generation of expandable iPS cell-derived NPC and neurons. Phase contrast images of the cells during the steps in generating the NPC: 1) iPS cells in colonies; 2) formation of aggregates in microwells (time duration 5 days) allows development of three dermal layers but favors neuralization; 3) picking neural rosette structures from clusters in the aggregates (time duration 10-14 days) yields neuroectoderm; 4) sorting NPC from rosette clusters with the selection of viable CD133+/CD184+/CD271− cells yields a CNS-specific population (the expansion and stabilization of nascent NPC is 5 weeks with the first passage of the NPC after FACS occurring within 2 weeks). The resulting stable proliferating NPC can be expanded into billions of cells over many passages while retaining the ability to differentiate into excitatory glutamatergic neurons following Ngn2 induction (5).
While there are many reports of studying CNS disorders using neuronal cell models derived by direct differentiation of iPS cells (e.g. Pasca et al., 2011, Shi et al., 2012; HD iPSC Consortium, 2012; Wen et al., 2014), this typically requires repeated highly labour-intensive and time-consuming procedures that lack the necessary scalability and reproducibility required for use in drug discovery assays. Having a stable, expandable lineage appropriate NPC intermediate between the pluripotent iPS cells and the terminally differentiated neurons is advantageous in reducing the time, effort and variability in generating the eventual cell model desired. While there are reports of generating human NPC for longer-term use, the majority of these are from human embryonic stem (hES) cells (e.g. Hook et al., 2011, Koch et al. 2009, Kim et al., 2012). In the following, we describe a protocol for generating stable, expandable, human iPS cell-derived NPC that can be cryopreserved, thawed out when needed, and grown for at least 50 passages. This methodology allows for precisely timed and scalable initiation of terminal neuronal differentiation that is highly amenable to neuronal phenotyping and drug discovery.
Our protocol has two steps: 1) neural induction using the embryoid aggregate method, which was originally based on methods developed for hES cells (e.g. Li et al., 2009); combined with 2) fluorescence-activated cell sorting (FACS) using neural cell surface markers, similar to Yuan et al. (2011), to enrich for the expandable NPC, and then growing the nascent NPC in cell culture conditions that promote retention of the CNS NPC (over that of other cell types, notably the peripheral nervous system neural crest cells). Lastly, we also describe a rapid, efficient method to generate excitatory forebrain neurons for applications such as screening that demand high reproducibility from culture to culture over time, through generation of an inducible Ngn2 NPC (iNgn2-NPC) stable cell line. Using the methods described in this unit we have generated multiple neuronal cell models to study the mechanisms underlying the pathophysiology of a form of frontotemporal dementia (Silva et al., 2016), and we have also developed a high-content cell-based screening assay to probe for novel therapeutics (Cheng et al., 2016).
BASIC PROTOCOL 1
Maintenance and neural induction of iPS cells
In this protocol, neural induction of iPS cells occurs via formation of embryoid body-like aggregates of uniform size in the presence of a neural induction medium (NIM) from Stemcell Technologies; both aggregate size and neural induction media usage are helpful in maximizing the number of neural rosette structures containing NPC that occur in the aggregates. To obtain maximal neural induction, we recommend growing the iPS cells in mTeSR1 medium, instead of other more simplified commercially available iPS cells media, for a few passages prior to the neural induction. It is also possible to generate NPC of different passages as a monolayer culture after dissociation of the neural rosette structures. However, we have found that further purification of the nascent NPC provides a key step in producing (almost infinitely) expandable NPC (basic protocol 2, below).
Materials
Human iPS cells (e.g. Silva et al., 2016)
Matrigel (Corning cat# 354277)
mTeSR1 medium (Stemcell Technologies cat# 05850)
Phosphate-buffered saline (PBS, MediaTech cat# 21-040-CV)
AggreWell 800 plate (Stemcell Technologies cat# 27865)
DMEM/F12 (Gibco cat# 11330)
Neural induction medium (NIM, Stemcell Technologies cat# 05831)
Y-27632 (EMD Millipore cat# 688001)
Humidified incubator at 37°C with 5% CO2
Accutase (Sigma cat# A6964-100ML)
Countess automated cell counter (Thermo Fisher Scientific cat# C1022; can use other hemacytometer-based, trypan-blue exclusion cell counting)
Cell strainer, 40um nylon mesh (BD Falcon ref # 352340)
Poly-ornithine/laminin(POL)-coated 6-well tissue culture plates (POL-coating see recipe)
Neural Rosette Selection Reagent (NRSR, Stemcell Technologies cat# 05832)
DMEM (Gibco cat# 11995)
Neural proliferation media (NPM, see recipe)
Day 0 – Start neural induction of iPS cells
Prior to neural induction, iPS cells should be maintained in Matrigel-coated 6-well plates, fed daily with mTeSR1 media (containing 1% penicillin/streptomycin), and passaged once per week. The protocol for neural induction of iPS cells typically begins the day before the day the iPS cells are slated to be passaged; at this point the iPS cells are growing in medium- to large-sized colonies, with >90% of cells having little or no differentiation. The few differentiated cells should be picked off manually using an angled fire-polished pasteur pipette before starting neural induction.
- In preparation for plating the iPS cells into well(s) of the AggreWell 800 plate, rinse each well to be used once with 1mL DMEM/F12 media, and after removing the DMEM/F12, add 0.5mL NIM containing 10μM Y-27632. Spin the plate at 2000×g for 5min and then place the plate in the incubator until cell plating.
- The plate is spun to get rid of any potential air bubbles within the well. If after the initial spin, air bubbles are still observed in the wells, repeat the spin until there are no air bubbles in the wells.
- Remove media from wells containing iPS cells and wash once with 2mL DMEM/F12/well.
- On average, will need 2-3 wells of 90% confluent iPS cells for one well of an AggreWell plate.
- Remove DMEM/F12 and add 750μL Accutase/well and incubate in 37°C incubator for approximately 7min (at 5min, take out and rock plate to dislodge cells, then return to incubator).
- The frozen aliquot of Accutase is thawed from −20°C to room temperature (RT) by leaving the Accutase at RT >1hr. In handling Accutase prior to applying to the cells, Accutase is kept at ≤20°C (RT) since it loses its enzymatic activity when at 37°C for a prolonged period of time (manufacturer's instructions).
- After incubation with Accutase, use a 5mL pipet to pipette the cell mixture up and down 2-3 times to dislodge and break up cells.
- Not all cells will be dislodged, as the clumps of cells remain attached to the bottom of the well while the single cells have detached.
Transfer the cell mixture to a 15mL conical tube. Add another 3mL DMEM/F12 to the Accutase-treated well to dislodge remaining cells, and transfer contents of well to conical tube. Repeat once more for a final volume of 9mL of cell mixture in 15 mL conical tube.
Spin cells at 300×g for 5min; remove supernatant and resuspend pellet in 1.5mL NIM containing 10μM Y-27632.
- Count cells using the Countess cell counter and plate 3×106 viable cells/well of the AggreWell 800 plate (in wells prepared beforehand, in step 2). Add additional NIM containing 10μM Y-27632 in each well, if needed, to reach a total well volume of 2mL. Gently pipette the contents of well up and down a few times to distribute the cells evenly throughout the microwells.
- There is some flexibility in the cell density plated. Successful neural induction is achieved with cell density between 2.5-3×106 cells/well, but should not exceed 3×106 cells/well. A suggestion for the initial neural induction experiment would be to plate two wells, one at each end of the cell density range. Plating at a cell density of 3×106cells/well will result in 1×104 cells aggregating within each microwell.
- Centrifuge the AggreWell plate (containing the iPS cells) at 100×g for 3min before placing the plate of cells in the incubator.
- After spinning the cells, check with a microscope that the cells are distributed evenly in the 300 microwells within each well. When placing the plate of cells in the incubator, move the plate gently (do not rock or shake the plate) so as to keep the cells evenly distributed across the microwells in each well. After placing the plate of cells in the incubator, leave undisturbed for 24hr to allow the cells within each microwell to form an aggregate.
Days 1-4—Daily feeding of aggregates
-
10.Prior to the first feeding (24hr after plating cells in the well), should see one aggregate/microwell.
- Not all cells may have coalesced into the aggregate in each microwell, but the majority of cells should be in the aggregate (see Figure 1B).
-
11.Very gently, remove 1.5mL media from each well, and add 1.5mL fresh NIM very carefully, trying not to disturb the aggregates from the microwells. This is especially critical on Day 1, the first feeding after plating.
- In our hands, the most effective method of feeding the cells is to use a 1mL pipetman, keeping the pipette tip as horizontal as possible, and add media slowly and steadily with the tip at the interface of the air and media. Keeping the aggregates within the microwell is important because if the aggregates from different microwells contact each other at this point, they tend to form larger aggregates and subsequently do not effectively form neural rosette structures.
Day 5—Transferring aggregates from AggreWell plate to a POL-coated plate
-
12.
Prepare the POL-coated 6-well plate by removing the solution from each well and wash once with 4mL PBS/well before replacing the PBS with 1mL NIM/well and placing plate in the incubator until use.
-
13.Dislodge the aggregates from microwells by pipetting media firmly into the middle of the well using a P1000 pipetman, and then quickly transfer the aggregates to top of a cell strainer over a 50mL conical tube. Repeat until all the aggregates are out of the microwells.
- To minimize damaging the aggregates when transferring them out of the microwells, it may be useful to use a big bore tip on the pipetman (or cut off tip of a regular-sized P1000 pipette tip).
-
14.Turn the strainer over the top of a well of a POL-coated 6-well plate containing 1mL NIM (prepared beforehand, step 12), and flow 1mL NIM through the strainer. During this process, the aggregates will transfer into the well.
- In this way, the aggregates from each well of an AggreWell 800 plate are transferred to one well of POL-coated 6-well plate.
-
15.Place the plate containing the aggregates in the incubator.
- For an even distribution of the aggregates within the well, once the plate of aggregates is on a shelf in the incubator, shake the plate gently but firmly left/right twice and back/forth twice before leaving plate undisturbed overnight to allow aggregates to attach.
Days 6-11—Daily feeding of attached aggregates
-
16.Feed attached aggregates with fresh 2mL NIM/well daily.
- By the second day of feeding the attached aggregates, you should be able to see rosette clusters with the aid of a microscope under low magnification. It is optimal to continue the protocol only if the majority of the aggregates contain rosette clusters (>80%).
Day 12—Isolation and transfer of rosette clusters
-
17.The isolation and transfer of the rosette clusters is performed one well at a time. Remove media from well containing rosette clusters within aggregates, wash once with 1mL DMEM/F12/well, then replace the DMEM/F12 with 1mL NRSR/well and incubate at 37°C for 30min.
- The incubation time in NRSR may need to be determined empirically for each end-user. It may be possible to isolate and transfer the rosette clusters with the use of NRSR only. This will require a NRSR incubation closer to 1hr, but with longer NRSR incubations, it may be difficult to also prevent the transfer of the surrounding flat cells. Following the 30min NRSR incubation, the rosette clusters should still be lightly attached to the well and the flat cells remain attached during steps 18 and 19.
-
18.
Remove NRSR and wash gently once with 2mL DMEM/F12/well and replace DMEM/F12 with 1mL NIM/well.
-
19.Using an angled fire-polished pasteur pipette, score around the rosette cluster and detach the cluster from the surrounding flat cells. Batch-pick all the rosette clusters to a well in a new POL-coated 6-well plate (prepared as described in step 12).
- Picking the rosette clusters can be a lengthy process (we typically have greater than 200 rosette clusters per well), so attempt to limit the amount of time the cells are out of the incubator to under an hour. Ideally the cluster-picking step should be <30min per well.
-
20.Place the plate containing the rosette clusters in the incubator.
- For an even distribution of the rosette clusters within the well, once the plate of rosette clusters is on a shelf in the incubator, shake plate gently but firmly left/right twice and back/forth twice before leaving plate undisturbed overnight to allow the rosette clusters to attach.
Day 14—Change media from NIM to NPM
-
21.
Remove media from each well and replace with 3mL NPM/well.
Days 16-20—Feed with fresh NPM
-
22.As needed, feed the rosette clusters with 3mL NPM/well.
- There should be a large number of cells within each well of the 6-well plate, thus feed the cells with fresh media when the media on the cells turns an orange-tinged colour, typically either daily or every other day.
BASIC PROTOCOL 2
Fluorescence-activated cell sorting (FACS) and maintenance of nascent NPC
In this protocol, after the neural induction of iPS cells into neural rosettes (clusters), FACS is used to enrich for the nascent NPC. Like Muratore et al. (2014), we have found that when applied to different patient derived cells, other purification methods such as magnetic affinity cell sort (MACS) yielded variable results. While FACS selection can be time-consuming and/or expensive, we too have found that it yields NPC of the highest purity in terms of morphological homogeneity (see Figure 1B). The cell surface markers that we use in the cell sorting are a combination of: 1) the cell adhesion molecule prominin-1 (CD133), 2) the G protein-coupled receptor CXCR4 (CD184) and 3) the low-affinity nerve growth factor receptor LNGFR (CD271). We arrived at this combination by modification of methods reported by others: 1) CD133 is considered a neural stem cell marker and FACS on CD133 has been used to isolate NPC from human brain tissue as early as over a decade and a half ago (Uchida et al. 2000); and 2) CD184 and CD271 were used as part of cell surface marker signatures in an elegant study by Yuan et al. (2011) to isolate neural stem cells and neurons by FACS. After the cell sorting, the nascent NPC are maintained as high density cultures for a period of time in order to optimize the retention of the nascent NPC. Nementi et al. (2011) also noted that high cell density in the initial passages is important for promoting the survival and stabilization of NPC lines. Once the nascent NPC have stabilized, they can be maintained as an attached monolayer cell line with fairly standard cell culture techniques, including cryopreservation, for many passages (>50).
Materials
FACS buffer (see recipe)
TrypLE select (Gibco cat# 12563-029)
Reference NPC line (e.g. we use 8330-8 NPC; Sheridan et al., 2011)
35μm mesh strainer cap with FACS tube (BD Falcon Tube with Cell Strainer Cap product# 352235)
DNase I (Sigma cat# D4527-40KU)
BSA (Sigma cat# A7906-100G)
Countess automated cell counter (Thermo Fisher Scientific cat# C1022; can use other hemacytometer-based, trypan-blue exclusion cell counting)
PE-CD133 (Miltenyi Biotec cat# 130-080-801)
APC-CD184 (BD Biosciences cat# 555976)
PerCP-Cy5.5-CD271 (BD Biosciences cat# 560834)
200μg/mL DAPI (Molecular Probes cat# D1306)
Neural proliferation media (NPM, see recipe)
Humidified incubator at 37°C with 5% CO2
FACS machine (e.g. BD FACSAria IIu in biosafety cabinet)
FACS collection tube (Falcon #352063)
Cell preparation including immunostaining with FACS markers
- Warm up an aliquot of FACS buffer to RT, keeping most of the FACS buffer on ice.
- Remember to add 20ng/mL bFGF to the FACS buffer on day of use (as noted in recipe section).
- Detach Day 20-21 rosette cluster cells with TrypLE by adding 1mL TrypLE/well, rock plate back and forth a few times, then incubate at RT for 4-5min.
- The rosette clusters may be a little resistant to dissociation (clumpy), so use the P1000 pipetman to break up the clumps by pipetting the cells up and down a few times. Start this step approximately an hour before beginning of FACS. Similarly, detach and immunostain the cells one cell line at a time—the FACS step can take up to two hours—and it is not optimal to keep these cells in single cell suspension for a prolonged period then followed by FACS. The plate of reference NPC should be dissociated and immunostained with the first cell line to be FACS’ed each day.
- We tested the effect of the following cell dissociation methods on the resulting viable CD133+/CD184+/CD271− FACS cells (of our reference 8330-8 NPC): 1) manual cell scraping, 2) TrypLE proteolysis and 3) Accutase proteolysis. Manual cell scraping resulted in the lowest proportion of viable single cells (40%) and TrypLE had the highest proportion (58%). Both TrypLE and Accutase treatment yielded comparable levels of CD133+/CD184+/CD271− sorted cells.
After incubation of the cells with TrypLE, add 4-5mL RT FACS buffer, separate the single cells from the cellular clumps by passing the cells through a cell strainer.
- Count cells using the Countess cell counter to estimate the amount of cells collected.
- Typically, there should ~7-12×106 viable cells in the plate of reference NPC, and at least 1×107 viable cells from one well of rosette clusters.
Spin cells at 233×g for 5min.
Remove the supernatant and add 200μL FACS buffer containing 100U/mL DNaseI to the cell pellet, resuspend the cell pellet and incubate at RT for 10min.
After the DNaseI incubation, add additional 200μL FACS buffer and 40μL 5% BSA to each cell suspension.
- For each cell line, add the following together and incubate at 4°C for 20min, flicking tubes every 5min to mix.
Tube label Amount of cell suspension Volume of Fluorochrome-Labelled Antibody Unstained 25μL — PE-CD133 25μL 10μL PE-CD133 APC-CD184 25μL 10μL APC-CD184 PerCP-Cy5.5-CD271 25μL 2μL PerCP-Cy5.5-CD271 3-colour combo Remainder of cells (approx 300μL) 20μL PE-CD133
20μL APC-CD184
5μL PerCP-Cy5.5-CD271 After the antibody incubation, add 500μL cold FACS buffer to 25μL cell suspension and 5mL to remaining (approximately 300μL) cell mixture and spin cells at 700×g and 233×g, respectively.
Remove the supernatant and gently resuspend the cell pellet in cold FACS buffer, 500μL for the unstained and single antibody-stained cells and 1mL for the 3-colour antibody-stained cells and store at 4°C until cell sorting (soon after staining).
FACS of single cells for CD133+/CD184+/CD271−
State-of-the-art FACS provides an integral technique for the successful analysis and recovery of viable NPC based upon a defined cell surface code. For this purpose, we have utilized a 5-laser BD FACSAria IIu high-speed cell sorter inside a biosafety cabinet driven by the BD FACSDiva software (HSCI-CRM Flow Cytometry Core Facility). If using another FACS instrument, the essential components of the equipment are lasers at excitation of 405nm, 488nm and 640nm wavelengths and the ability to sort using more “gentle” instrument settings than those typically used for non-neural cells and rodent cells as detailed below.
-
11.Prior to cell sorting, adjust the FACS equipment to sort for the more sensitive human neural cells with a 100μm nozzle and a steady 20psi sheath pressure, with 0.9% saline used as a sheath fluid.
- Even though most cell sorters are not located in a sterile environment, it is essential to maintain proper tissue culture cell handling practices such as wearing gloves and spraying all surfaces with 70% ethanol. It is also highly recommended to have an equipment cleaning protocol such as flushing the instrument's fluid lines with 10% bleach between users, and soaking the fluid lines in 70% ethanol when not in use (e.g. over the weekend).
-
12.Set the sorting parameters (outlined in Figure 2) initially with the reference NPC line (e.g. 8330-8 NPC) after passing the cells through a 35μm cell strainer and gently vortexing the cells before placing the cells in the FACS machine. 1μL 200μg/mL DAPI was added to fully-stained samples to gate for viable cells.
- Gate the cells for size and complexity (FSC (forward scatter) versus SSC (side scatter)) and to exclude aggregates (FSC-A (Area) vs. FSC-W (Width) and SSC-A vs. SSC-W).
- Excite the following fluorochromes with the instrument's lasers
Fluorochrome Excitation Laser Line (nm) Emission Filter DAPI 405 450/50 PerCP-Cy5.5 488 695/40 PE 532 575/26 APC 640 675/25
-
13.Finalize the sorting gates with the fully-stained sample cells with conservative gating of CD271− cells (approx 95-99% of unstained cells) and gating of double-positive CD133+/CD184+ cells (<0.1% of the CD133+/CD184+ single colour controls). The CD133+/CD184+/CD271− cells are sorted into a FACS collection tube containing 1mL NPM.
- The event rate under these conditions is approximately 2000 events/sec. It may be necessary to add more FACS buffer to the fully-stained sample and gently vortex the sample occasionally during the cell sorting to achieve sort efficiency >80%. It takes between 1 and 1.5hr to complete sorting of a starting population of 1×107 cells, with replacement of the FACS collection tube when the initial tube is full. Once the tube of sorted cells is removed from the FACS machine, place cells at 4°C until plating.
-
14.
During the cell sorting, once the number of cells that will be sorted can be estimated, prepare the appropriate POL-coated plate. Ideally, with optimal neural induction of the iPS cells (at least 90% of aggregates contain rosette clusters) and collecting all the rosette clusters, approximately 1.5×106 viable CD133+/CD184+/CD271− cells can be collected. Since this number of sorted cells is plated into one well of a 6-well plate, the subsequent directions will be for 6-well plates. To prepare the POL-coated 6-well plate, remove the solution from well and wash once with 4mL PBS/well before replacing the PBS with 1mL NPM/well. Place the prepared POL-coated plate in the incubator until the sorted cells is ready to be plated.
-
15.After sorting, spin cells at 233×g for 5min, resuspend the cell pellet gently and plate cells into the prepared POL-coated plate (prepared above in step 14). Note that the cells are at passage 0.
- If there are less than 1×106 sorted cells, plate the cells into the following: 200K cells into a well of 24-well plate and 500K cells into a well of 12-well plate.
-
16.Place the plate of sorted cells in the incubator.
- For even distribution of the cells within the well, once the plate is on a shelf in the incubator, shake plate gently but firmly left/right twice and back/forth twice before leaving plate undisturbed overnight to allow the cells to attach. There will be sorted cells that are not viable and thus not all cells will attach.
-
17.
Feed the cells with fresh NPM every 3-4 days until the cells are confluent, typically up to two weeks after FACS.
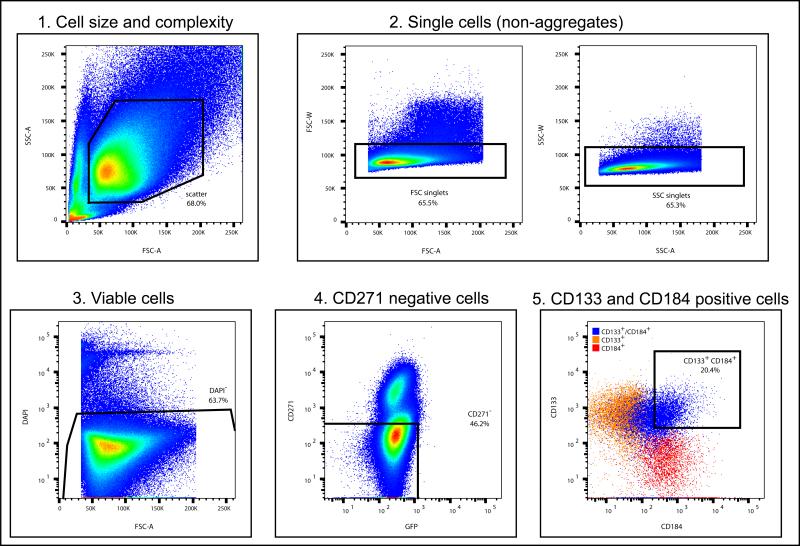
Figure 2. Steps in NPC FACS gating methodology.
All cells are initially gated for size and complexity (1), then singlets (2). Singlets of the correct size and complexity are then gated for viability (3). Viable singlet cells are then gated for CD271 negative cells (4). Finally, the viable singlet CD271- cells are gated for double CD133 and CD184 positive cells (5). The pseudocolour plots in 1-4 is represented as a gradient with blue the least and red the most number of cells. The noted percentage (of total cells) in each graph is an example of representative amount of cells with the appropriate parameters.
Cell culture maintenance of emerging NPC
In our experience, maintaining the nascent NPC at high density (as described below) is important for preservation and stabilization of expandable NPC.
-
18.The sorted cells are ready for the first passage when the cells are tightly packed (at least 3×105 cells/cm2 or 3×106 cells in a well of 6-well plate). Prepare a POL-coated 6-well plate (as described in step 14).
- If the cells were plated in a smaller welled plate, prepare a 6-well plate for cells in a 12-well plate and prepare a 12-well plate for cells in a 24-well plate.
-
19.Remove the media from the well and add 0.5mL TrypLE/well and incubate at RT for 5min.
- During the TrypLE incubation, periodically tap and rock the plate to aid in detaching the cells.
-
20.
Add 2.5mL NPM/well and pipette the cell mixture up and down a few times to mix.
-
21.Divide the cell mixture (from one well) into 3 wells of the POL-coated 6-well plate for a 1:3 split. The cells are at passage 1.
- Optional to count the cells. Cells should be plated at least 2×105 cells/cm2.
- For cells in smaller welled plate, plate cells from a well of 12-well plate into a well of 6-well plate and cells from a well of 24-well plate into a well of 12-well plate.
-
22.Place the plate of emergent NPC in the incubator.
- For an even distribution of the cells within the well, once the plate is on a shelf in the incubator, shake plate gently but firmly left/right twice and back/forth twice before leaving plate undisturbed at least overnight to allow the cells to attach.
-
23.For approximately the first ten passages, continue to passage the cells as described in steps 18-22, typically every 3-4 days.
- To cryopreserve the NPC, detach the cells with TrypLE as described above (step 19), spin at 233×g for 5min, resuspend cell pellet in cold freezing media (10% DMSO in NPM), transfer the cell mixture to a cryovial and place the cryovial in a cell freezing container (e.g. a container that will gradually decrease the temperature when placed in −80°C freezer from RT) and leave in −80°C freezer at least 20hr before transferring the cryovial to a liquid nitrogen cryotank.
-
24.After passage 10, the NPC can be passaged with TrypLE at a confluent density between 1.2-2.1×105/cm2 and plating density of 4.2×104 cells/cm2 maintained in NPM.
- We have NPC lines that maintain the ability to differentiate into neurons up to passage 50+. These neurons are immunopositive for neuronal markers such as tau, SMI312, MAP2 and βIII-tubulin.
BASIC PROTOCOL 3
Generation of iNgn2-NPC stable line for precisely timed and scalable initiation of differentiation into excitatory forebrain neurons
In modelling CNS diseases, it is of great interest to generate specific cell types that may contribute to the underlying pathophysiology. To use disease modeling to discover novel targets for therapeutic development and lead structures, there is the additional need for an appropriate neuronal cell model that can satisfy criteria for high-throughput drug screening purposes. Here, we describe a protocol that rapidly and efficiently generates excitatory glutamatergic forebrain neurons using a doxycycline-inducible Neurogenin 2 (iNgn2) expression cassette that is stably incorporated into NPC (Figure 3). The iNgn2-NPC method of neural induction has the advantages of 1) phenotypically similar neurons are produced from distinct iPS cells-derived NPC lines, as assessed by gene expression analysis; and 2) the cells acquire neuronal gene expression patterns and morphology within two weeks, and can become electrically active to glutamate receptor antagonists within three weeks (Zhang et al., 2013); and 3) cultures grown to large scale can be easily differentiated by doxycycline treatment; this would be much more difficult if it were necessary to deliver the iNgn2 construct to a large number of naive NPC, e.g. by lentiviral infection. The creation of iNgn2-NPC cell lines allows, therefore, for ease of use and decreased variability in large scale production of neuronal cultures, rendering these cultures suitable for cell-based screening assays. An example of using the iNgn2 neurons in a high-content imaging assay is shown in Figure 4.
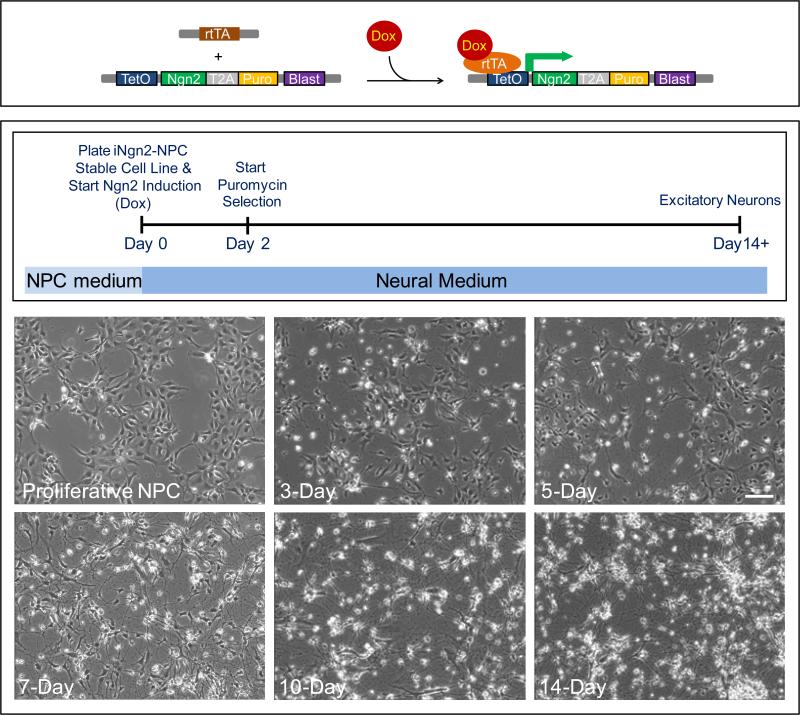
Figure 3. Development of neurons from iNgn2-NPC stable cell lines.
Top panel illustrates the action of the transgenes in inducing expression of Ngn2 in generating the iNgn2 neurons. The addition of doxycycline (Dox) results in the binding of Dox to the transactivator (rtTA, driven by the constitutively active human ubiquitin C promoter), changing the conformation of rtTA to promote binding of the rtTA-Dox complex to the inducible TetO promoter, thereby activating the expression of Ngn2 and the puromycin (Puro) resistance gene. The bottom panel contains the timeline of iNgn2 neuron generation, as well as phase contrast images of live iNgn2-NPC stable cells at various time points before and after the initiation of Ngn2 induction. The iNgn2-NPC stable cells are plated and neuronal induction started at Day 0; Puro selection can be performed at Days 2 and 4 (optional as the cells have already been selected with blasticidin (Blast) at the proliferative NPC stage); excitatory glutamatergic neurons are generated by Day 14. Scale bar = 100μm.
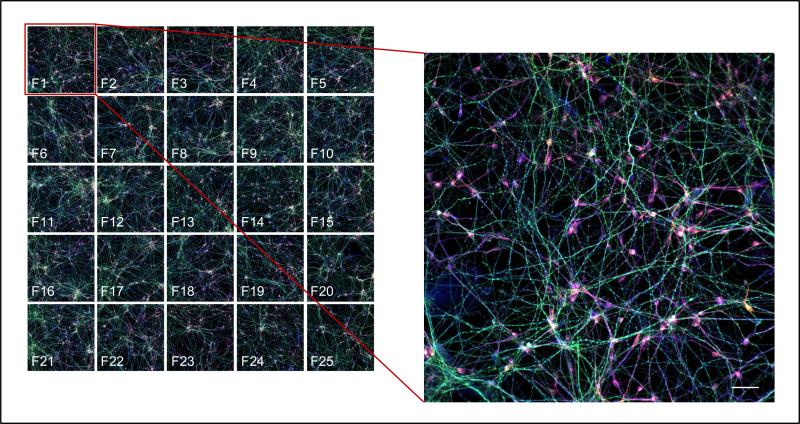
Figure 4. An example of using the iNgn2 neurons in a high-content imaging assay.
Left image illustrates 4 channel (phosphorylated Tau (PHF-1)/ total Tau (K9JA)/neuronal (βIII-Tubulin) and Hoechst 33342 (DNA) immunostaining) acquisition of 25 fields (F1-F25) per each well in a 96-well plate. Right image is zoomed in composite image of a field (F1) of cells immunostained for phosphorylated Tau (PHF-1, green), total Tau (K9JA, red) and neurons (βIII-Tubulin, blue). Scale bar = 50μm.
Materials
pTetO-mNgn2-TA-puro (Zhang et al., 2013; available at Addgene plasmid ID# 52047)
pLX-304 (Addgene plasmid ID# 25890)
Phusion Hot Start II DNA polymerase (ThermoFisher Scientific cat# F549L)
PacI restriction endonuclease (NEB cat# R0547S)
Calf intestinal alkaline phosphatase (NEB cat# M0290S)
Quick Ligation kit (NEB cat# M2200S)
Stbl3 E. coli strain (ThermoFisher Scientific cat# C737303)
Qiagen Plasmid Maxi Kit (cat# 12162)
Transactivator plasmid pFUW-M2rtTA (Hockemeyer et al., 2008, Addgene plasmid ID# 20342
Lentiviral packaging plasmid pCMV-dR8.2 dvpr (Stewart et al., 2003, Addgene plasmid ID# 8455
VSV-G envelope expressing plasmid pMD2.G (Addgene plasmid ID# 12259)
Humidified incubator at 37°C with 5% CO2
HEK293T cells (ATCC 293T/17)
Poly-L-Lysine (Sigma cat# P5899)
D10 medium (see recipe)
Lipofectamine 2000 (Thermo Fisher Scientific cat# 11668-019)
Opti-MEM (Gibco cat# 31985)
Neural proliferation media (NPM, see recipe)
POL-coated plates (see recipe)
Blasticidin (Gibco cat# A11139-03)
POLS-coated plates
iNgn2 neural media (N3aM, see recipe)
Puromycin (Sigma cat# P8833-25MG)
Cytosine arabinoside (AraC; Sigma cat# C6645)
Generation of iNgn2-BSDR construct
There are numerous selection markers and many methods to insert the selection marker of choice into the inducible Ngn2 construct (pTetO-mNgn2-TA-puro). Below is a protocol that we used to insert the blasticidin S deaminase resistance gene (BSDR) into the inducible Ngn2 construct (pTetO-mNgn2-TA-puro), in preparation for generating iNgn2-NPC stable cells.
- Generate a PCR fragment of the blasticidin S deaminase resistance gene (BSDR) driven by the human phosphoglyercerate kinase 1 (PGK) promoter from plasmid pLX-304 with PacI restriction sites on both ends with Phusion Hot Start II DNA polymerase. Gel purify the fragment.
- The primer sequences are:
- forward primer = atttaattaatctctggaacagatttg
- reverse primer = ctttaattaactgccatttgtctcaag.
Linearize the pTetO-mNgn2-TA-puro plasmid with PacI restriction endonuclease, and dephosphorylate the ends of the vector with calf intestinal alkaline phosphatase. Clean up the reaction with using a Qiagen PCR purification kit.
Ligate the PCR fragment into the PacI-linearized pTetO-mNgn2-puro plasmid using, for example, the Quick Ligation kit, and transform the reaction into Stbl3 competent cells according to manufacturer's instructions.
Confirm the expected sequence of the resulting plasmid pTetO-mNgn2-puro-PGK-BSDR with Sanger sequencing.
Expand the construct in the Stbl3 E. coli strain grown at 30°C and isolate the DNA using a plasmid maxiprep kit (e.g. Qiagen Plasmid Maxi Kit).
Generation of iNgn2-BSDR/rtTA lentiviruses
Production of the lentiviral stocks of the iNgn2-BSDR (pTetO-mNgn2-puro-PGK-BSDR plasmid) and rtTA (pFUW-M2rtTA plasmid) constructs are based on a method described previously (Wang et al. 2014).
-
6.Grow HEK293T cells in poly-L-lysine-coated flasks to 95% confluency in D10 media.
- To coat flasks with poly-L-lysine, add 4μg poly-L-lysine/cm2 to each flask, mix to distribute the solution evenly in the flask, and then incubate at RT for 5min. Wash once with H2O, then remove H2O and let the flask dry for 2hr before use.
-
7.
Mix together the following plasmids in Opti-MEM: pTetO-mNgn2-puro-PGK-BSDR or pFUW-M2rtTA or with the helper plasmids pCMV-dR8.2 and pMD2.G at 0.145, 0.109 and 0.073μg of each DNA per cm2 tissue culture flask/plate, respectively. Mix gently.
-
8.
Dilute the stock Lipofectamine 2000 reagent in Opti-MEM at 1μL/cm2 and mix. Incubate at RT for 5min.
-
9.
Mix together the DNA complex and the diluted Lipofectamine 2000 reagent, mix gently and incubate at RT for 20min.
-
10.
Change the media on the HEK293T cells to Opti-MEM, and then add the DNA complex/Lipofectamine 2000 mixture to the cells. Mix gently by rocking the flask back and forth a few times, then place the cells mixed with the DNA complex/Lipofectamine 2000 mixture in the incubator for 4hr.
-
11.
After the 4hr incubation with the DNA complex/Lipofectamine 2000 mixture in Opti-MEM, return the cells back to D10 media.
-
12.
Collect the media from the cells 48hr later. Clear the supernatant by spinning the lentiviral-containing media at 500×g for 5min, and then pass the supernatant through a low protein binding 0.45μm filter (e.g. PES membrane).
-
13.Titer the lentiviral supernatant (e.g. using a p24 ELISA kit, Lenti-X P24 Rapid Titer Kit, Clontech, cat# 632200). Store aliquots of the lentiviral supernatants at −80°C until use.
- Determine an appropriate aliquot volume so that the number of freeze-thaw cycles of the lentiviral supernatants is limited to 3 or less.
Generation and maintenance of the iNgn2-NPC stable cells
-
14.
When NPC reach approx 90% confluency, dissociate with TrypLE and plate into a POL-coated 24-well plate at a density of 8×104 cells/well.
-
15.Approximately an hour after cell plating, start transduction with the addition of iNgn2-BSDR and rtTA lentiviral supernatants to the cells at a multiplicity of infection (MOI) of 10.
- Optimal MOI for lentiviral transduction can be determined using a blasticidin cell toxicity assay (e.g. using AlamarBlue cell viability reagent, ThermoFisher Scientific cat# DAL1100) with varying amounts of the lentivirus on naive NPC.
-
16.
Spin the newly transduced NPC plates at 930×g for 30min before returning to the incubator.
-
17.
Begin selection of transduced cells at 24hr after start of transduction with the addition of 20μg/mL blasticidin in fresh NPM.
-
18.
Feed the cells with fresh NPM containing 20μg/mL blasticidin every 3-4 days until the cells are confluent, and then passage the cells with TrypLE into wells of POL-coated 6-well plates at 4×105 cells/well.
-
19.Maintain the iNgn2-NPC stable cell line by passaging every 3-4 days with TrypLE at a confluent density between 1.2-2.1×105/cm2 and plating density of 4.2×104 cells/cm2 maintained in NPM containing 20μg/mL blasticidin.
- It is optional to include the blasticidin in the media after several passages (we did not observe obvious differences in growth of the iNgn2-NPC stable cell line with or without blasticidin up to 18-20 passages). If maintained in NPM only, it is advisable to grow cells from subsequent passages in blasticidin-containing media periodically, especially for stock cultures. At this point, the iNgn2-NPC stable cell line can be cryopreserved and thawed out as needed to generate neurons.
Generation of excitatory glutamatergic neurons from iNgn2-NPC stable cell line
Day 0—Plating and start of Ngn2 induction
-
20.Prepare POLS-coated plate by removing the solution from each well, wash once with 2X volume of PBS (e.g. for 6-well plates containing 2mL of POLS solution, wash with 4mL PBS), then replace the PBS with N3aM (contains doxycycline to induce Ngn2 expression and differentiation). Place plate in incubator until plating cells.
- For plates smaller than 6-well plates, it is advisable to add a 1X volume DMEM/F12 wash between PBS and N3aM.
-
21.Dissociate confluent iNgn2-NPC stable cells with TrypLE and plate into the prepared POLS-coated plate at a cell density of 8.5×104 cells/cm2.
- This is a suggested starting cell density to plate the iNgn2-NPC stable cells, and may need to be adjusted to optimize for the end point assay that is used.
Days 2 and 4—Select with puromycin (optional)
-
22.Feed the cells with fresh N3aM every two days. On day 2 and 4, the iNgn2 cells can be fed with N3aM containing 1μg/mL puromycin to select for cells expressing Ngn2.
- For cells plated into 96-well plates, do half-media changes starting at day 2 to minimize detachment of cells, which has a tendency to occur with complete media changes. For cells plated into larger-sized wells, do complete media changes up to day 6 after which point half-media changes are recommended. If the intended use of these neuronal cultures involves measuring electrophysiological or synaptic activity, the addition of astrocyte-conditoned media or co-culture with astrocytes may be advisable, starting at day 2.
Day 6—Treat with AraC
-
23.
Feed the cells with fresh N3aM containing 5μM AraC.
Day 8-end
-
24.
From day 8 onward, feed iNgn2 neurons with fresh N3aM every two days until cell treatment/collection for end point assay.
Reagents and Solutions
POL-coated plates
Initially add 20μg/mL poly-ornithine (Sigma cat# P3655-50MG) in H2O to each well of plate and incubate at 37°C for 2hr. Remove poly-ornithine solution and wash once with 2X volume of H2O (optional for 6-well plates, recommended for smaller-sized plates). Replace H2O with 5μg/mL laminin (Sigma cat# L-2020-1MG) in PBS and incubate at 37°C for at least two 2hr (preferably overnight) before use or storing plates (in laminin solution) wrapped in aluminum foil at 4°C up to 2-3 weeks.
POLS-coated plates
Add together in PBS, 20μg/mL poly-ornithine and 5μg/mL laminin, mix, then dispense to each well of plate. Rock plate to distribute the solution evenly over the bottom of well, then incubate at 37°C overnight before use or storing plates wrapped in aluminum foil at 4°C for approximately a week.
NPM (neural proliferation media)
70% DMEM (Gibco cat# 11995)
30% Ham's F12 (Mediatech cat# 10-080-CV)
2% B27 (50X stock, Gibco cat# 17504-044)
1% penicillin/streptomycin (100X stock or 10,000 U/mL, Gibco cat# 15140-122)
20ng/mL EGF (Sigma cat# E9644-.2MG)
20ng/mL bFGF (Stemgent cat# 03-0002)
5μg/mL heparin (Sigma cat# H3149-100KU)
Sterile filter with 0.22μm bottle filter after making up the media.
Note: EGF, bFGF and heparin are made as 1000X stock (20μg/mL, 20μg/mL and 5mg/mL, respectively; frozen stocks are stored −20°C; working stocks are kept at 0-4°C and are added to media just before feeding cells
FACS buffer
Make in HBSS (Gibco cat# 14175095)
2% B27 (50X stock, Gibco cat# 17504-044)
20mM glucose (Sigma cat# G8270)
1% penicillin/streptomycin (100X stock or 10,000 U/mL, Gibco cat# 15140-122)
1mM EDTA
20ng/mL bFGF (Stemgent cat# 03-0002)
Sterile filter with 0.22μm bottle filter after making up the buffer and keep cold (e.g. make day before use and leave in fridge overnight).
Note: bFGF is made as 1000X stock (20μg/mL; frozen stocks are stored −20°C; working stocks are kept at 0-4°C), and are added to buffer just before use.
D10 Media
89% DMEM (Gibco cat#11995)
10% fetal bovine serum (FBS, Gemini Bio-Products cat# 100-106)
1% penicillin/streptomycin (100X stock or 10,000 U/mL, Gibco cat# 15140-122)
Sterile filter with 0.22μm bottle filter after making up the media.
N3aM (iNgn2 neural media)
48% Neurobasal Medium (Gibco cat# 21103-049)
48% DMEM/F12 (Gibco cat# 11330)
1% B27 (Gibco cat# 17504-044)
0.5% N2 (Gibco cat# 17502-048)
0.75% GlutaMax (Gibco cat# 35050-061)
1% penicillin/streptomycin (Gibco cat# 15140-122)
0.5% MEM NEAA (Gibco cat# 11140)
50μM β-mercaptoethanol (BioRad cat# 161-0710)
0.2% bovine serum albumin (Sigma cat# A7906-100G)
2μg/mL doxycycline (Clontech cat# 631311)
10ng/mL BDNF (Peprotech cat# 450-02)
10ng/mL NT3 (Peptrotech cat# 450-03)
Note: doxycycline, BDNF and NT3 are made as 1000X stock (2mg/mL, 10μg/mL and 10μg/mL, respectively; frozen stocks are stored −20°C; working stocks are kept at 0-4°C); BSA is made as 10X stock in N3aM (minus doxycycline, BDNF and NT3) and stored at 4°C; and are added to the basic media just before media is applied to cells.
COMMENTARY
Background Information
Many techniques have been developed to differentiate human ES or iPS cells into forebrain neuronal types through a NPC intermediate (e.g. Hook et al., 2011, Koch et al. 2009, Kim et al., 2012; Muratore et al. 2014). These range from the formation of embryoid bodies to cells grown in a monolayer that are neurally induced with or without inhibitors to SMAD or WNT signaling pathways, with or without selective purification of NPC. A comparison of some of these methods to differentiate iPS cells into forebrain cortical neurons is described in Muratore et al. (2014). We have tested a number of these different techniques, and we have found that the protocol described in this unit represents the most consistent and reproducible method of generating (almost infinitely) expandable NPC from a number of different patient iPS cells that retain the ability to differentiate into neurons upon command, even after many passages, and can be used in cell-based screening assays.
Critical Parameters & Troubleshooting
There are a few aspects of the protocol in this unit that require careful implementation in order to successfully obtain expandable NPC capable of differentiating into neurons over many passages. One key factor is generating sufficient emerging NPC to sort and then plate at high density to facilitate survival. This requires efficient neural induction of the aggregates into rosette clusters. During neural induction, it is critical to use high quality iPS cells as a starting material, and to plate iPS cells in AggreWell plates at a density between 2.5-3×106 cells/well and not to exceed 3×106 cells/well. After plating, attached rosette clusters optimally produce satisfactorily expandable NPC when dissociated and sorted at Day 20-21. On the day of cell sorting, it is important to proceed quickly through the steps of immunostaining, FACS, and plating the sorted cells because cell viability will be greatly reduced by excessive periods of time in suspension. It is also important to adjust the FACS instrument to gentle cell handling settings, e.g. decrease sheath pressure. Lastly, FACS parameters should be set with conservative gating of CD271− cells (it is preferable to exclude cells that score as borderline between CD271− and CD271+), and to collect only the double positively stained CD184+/CD133+ cells, since single positives of either CD184+ or CD133+ do not yield optimal NPC. After sorting, plate the sorted cells into an appropriately-sized well (e.g. 1.5×106 cells into a well of 6-well plate). There will be some cell death in the days following cell sorting as the FACS procedure is harsh on these nascent NPC, but it is critical to keep these cells at high density for the first 10 passages to allow for preservation and stabilization of expandable CNS NPC.
Anticipated Results
The protocol described in this unit comprises a highly reproducible way to generate NPC lines, from a number of different patient iPS cells, that retain the ability to differentiate into neurons even after many passages (>50). With these NPC, we have also found that the generation of stable NPC lines with iNgn2 expression provides a facile way to obtain cortical, glutamatergic neurons en masse without the need for constant reintroduction of virus.
Time Considerations
The neural induction of iPS cells (Basic Protocol 1) will take three weeks. It will take approximately 1.5 to 2hrs to FACS each cell line. The maintenance of the nascent NPC (up to passage 10) can take up to five weeks with the first passage after FACS typically occurring within two weeks. Once the CNS NPC have stabilized, they can be cryogenically preserved or expanded into upwards of billions of cells. The generation of the iNgn2-NPC stable line can take 1-2 weeks. Upon neuronal induction of the iNgn2-NPC, neurite formation can be detected within 3-4 days (Figure 3).
Acknowledgements
We additionally thank Laura Prickett-Rice of the HSCI-CRM Flow Cytometry Core Facility at the Massachusetts General Hospital for her superb technical assistance with FACS. We thank the Tau Consortium, Association for Frontotemporal Dementia (AFTD), and National Institute of Health/National Institute of Neurological Disorders and Stroke (R21NS085487) for funding and continued support; members of the Haggarty laboratory for helpful discussions; Dr. Marius Wernig (Stanford University) for sharing of reagents; and Dr. Peter Davies (Albert Einstein College of Medicine) for the generous contribution of the PHF-1 antibody.
Literature Cited
- Cheng C, et al. Development of an image-based high-content screening assay for tau clearing drugs in a human iPSC-derived neuronal cell model of frontotemporal dementia. Manuscript in preparation. 2016 [Google Scholar]
- Doi D, Samata B, Katsukawa M, Kikuchi T, Morizane A, Ono Y, Sekiguchi K, Nakagawa M, Parmar M, Takahashi J. Isolation of human induced pluripotent stem cell-derived dopaminergic progenitors by cell sorting for successful transplantation. Stem Cell Reports. 2014;2(3):337–350. doi: 10.1016/j.stemcr.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggarty SJ, Silva MC, Cross A, Brandon NJ, Perlis RH. Advancing drug discovery for neuropsychiatric disorders using patient-specific stem cell models. Mol Cell Neurosci. 2016;73:104–115. doi: 10.1016/j.mcn.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HD iPSC Consortium Induced pluripotent stem cells from patients with Huntington's disease show CAG-repeat-expansion-associated phenotypes. Cell Stem Cell. 2012;11(2):264–278. doi: 10.1016/j.stem.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockemeyer D, Soldner F, Cook EG, Gao Q, Mitalipova M, Jaenisch R. A drug-inducible system for direct reprogramming of human somatic cells to pluripotency. Cell Stem Cell. 2008;3(3):346–353. doi: 10.1016/j.stem.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook L, Vives J, Fulton N, Leveridge M, Lingard S, Bootman MD, Falk A, Pollard SM, Allsopp TE, Dalma-Weiszhausz D, Tsukamoto A, Uchida N, Gorba T. Non-immortalized human neural stem (NS) cells as a scalable platform for cellular assays. Neurochem. Int. 2011;59(3):432–444. doi: 10.1016/j.neuint.2011.06.024. [DOI] [PubMed] [Google Scholar]
- Kim DS, Lee DR, Kim HS, Yoo JE, Jung SJ, Lim BY, Jang J, Kang HC, You S, Hwang DY, Leem JW, Nam TS, Cho SR, Kim DW. Highly pure and expandable PSA-NCAM-positive neural precursors from human ESC and iPSC-derived neural rosettes. PLoS One. 2012;7(7):e39715. doi: 10.1371/journal.pone.0039715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch P, Opitz T, Steinbeck JA, Ladewig J, Brüstle O. A rosette-type, self-renewing human ES cell-derived neural stem cell with potential for in vitro instruction and synaptic integration. Proc. Natl. Acad. Sci. U.S.A. 2009;106(9):3225–3230. doi: 10.1073/pnas.0808387106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XJ, Zhang X, Johnson MA, Wang ZB, Lavaute T, Zhang SC. Coordination of sonic hedgehog and Wnt signaling determines ventral and dorsal telencephalic neuron types from human embryonic stem cells. Development. 2009;136(23):4055–4063. doi: 10.1242/dev.036624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattis VB, Svendsen CN. Modeling Huntington's disease with patient-derived neurons. Brain Res. 2015:S0006–8993(15)00738-6. doi: 10.1016/j.brainres.2015.10.001. 2015, [Epub ahead of print http://dx.doi.org/10.1016/j.brainres.2015.10.001] [DOI] [PubMed]
- Muratore CR, Srikanth P, Callahan DG, Young-Pearse TL. Comparison and optimization of hiPSC forebrain cortical differentiation protocols. PLoS One. 2014;9(8):e105807. doi: 10.1371/journal.pone.0105807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemati S, Hatami M, Kiani S, Hemmesi K, Gourabi H, Masoudi N, Alaei S, Baharvand H. Long-term self-renewable feeder-free human induced pluripotent stem cell-derived neural progenitors. Stem Cells Dev. 2011;20(3):503–514. doi: 10.1089/scd.2010.0143. [DOI] [PubMed] [Google Scholar]
- Paşca SP, Portmann T, Voineagu I, Yazawa M, Shcheglovitov A, Paşca AM, Cord B, Palmer TD, Chikahisa S, Nishino S, Bernstein JA, Hallmayer J, Geschwind DH, Dolmetsch RE. Using iPSC-derived neurons to uncover cellular phenotypes associated with Timothy syndrome. Nat Med. 2011;17(12):1657–1662. doi: 10.1038/nm.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sareen D, Gowing G, Sahabian A, Staggenborg K, Paradis R, Avalos P, Latter J, Ornelas L, Garcia L, Svendsen CN. Human induced pluripotent stem cells are a novel source of neural progenitor cells (iNPC) that migrate and integrate in the rodent spinal cord. J Comp Neurol. 2014;522(12):2707–2728. doi: 10.1002/cne.23578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan SD, Theriault KM, Reis SA, Zhou F, Madison JM, Daheron L, Loring JF, Haggarty SJ. Epigenetic characterization of the FMR1 gene and aberrant neurodevelopment in human induced pluripotent stem cell models of fragile X syndrome. PLoS One. 2011;6(10):e26203. doi: 10.1371/journal.pone.0026203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Kirwan P, Smith J, Robinson HP, Livesey FJ. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat. Neurosci. 2012;15(3):477–486. doi: 10.1038/nn.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MC, Cheng C, Mair W, Almeida S, Fong H, Biswas HUM, Zhang Z, Huang Y, Temple S, Coppola G, Geschwind DH, Karydas A, Miller BL, Kosik KS, Gao FB, Steen JA, Haggarty SJ. Human iPSC-Derived Neuronal Model of Tau-A152T Frontotemporal Dementia Reveals Tau-Mediated Mechanisms of Neuronal Vulnerability. Stem Cell Reports. 2016;7(3):325–340. doi: 10.1016/j.stemcr.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart SA, Dykxhoorn DM, Palliser D, Mizuno H, Yu EY, An DS, Sabatini DM, Chen IS, Hahn WC, Sharp PA, Weinberg RA, Novina CD. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA. 2003;9(4):493–501. doi: 10.1261/rna.2192803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straccia M, Garcia-Diaz Barriga G, Sanders P, Bombau G, Carrere J, Mairal PB, Vinh NN, Yung S, Kelly CM, Svendsen CN, Kemp PJ, Arjomand J, Schoenfeld RC, Alberch J, Allen ND, Rosser AE, Canals JM. Quantitative high-throughput gene expression profiling of human striatal development to screen stem cell-derived medium spiny neurons. Mol. Ther. Methods Clin. Dev. 2015;2:15030. doi: 10.1038/mtm.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc. Natl. Acad. Sci. U.S.A. 2000;97(26):14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JL, Shamah SM, Sun AX, Waldman ID, Haggarty SJ, Perlis RH. Label-free, live optical imaging of reprogrammed bipolar disorder patient-derived cells reveals a functional correlate of lithium responsiveness. Transl. Psychiatry. 2014;4:e428. doi: 10.1038/tp.2014.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson LM, Wong MM, Becker EB. Induced pluripotent stem cell technology for modelling and therapy of cerebellar ataxia. Open Biol. 2015;5(7):150056. doi: 10.1098/rsob.150056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Nguyen HN, Guo Z, Lalli MA, Wang X, Su Y, Kim NS, Yoon KJ, Shin J, Zhang C, Makri G, Nauen D, Yu H, Guzman E, Chiang CH, Yoritomo N, Kaibuchi K, Zou J, Christian KM, Cheng L, Ross CA, Margolis RL, Chen G, Kosik KS, Song H, Ming GL. Synaptic dysregulation in a human iPS cell model of mental disorders. Nature. 2014;515(7527):414–418. doi: 10.1038/nature13716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan SH, Martin J, Elia J, Flippin J, Paramban RI, Hefferan MP, Vidal JG, Mu Y, Killian RL, Israel MA, Emre N, Marsala S, Marsala M, Gage FH, Goldstein LS, Carson CT. Cell-surface marker signatures for the isolation of neural stem cells, glia and neurons derived from human pluripotent stem cells. PLoS One. 2011;6(3):e17540. doi: 10.1371/journal.pone.0017540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Pak C, Han Y, Ahlenius H, Zhang Z, Chanda S, Marro S, Patzke C, Acuna C, Covy J, Xu W, Yang N, Danko T, Chen L, Wernig M, Südhof TC. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron. 2013;78(5):785–798. doi: 10.1016/j.neuron.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]