Significance
The kinase WNK4 (with-no-lysine kinase 4) is an important regulator of the Na-Cl cotransporter (NCC) in the renal distal convoluted tubule (DCT). Volume depletion induces angiotensin II, activating PKC, which prevents WNK4 degradation by phosphorylating the KLHL3/CUL3 ubiquitin ligase. We now show that PKC also directly phosphorylates WNK4 at multiple sites in cell culture. Phosphorylation of two of these sites, S64 and S1196, promotes increased WNK4 kinase activity by increasing autophosphorylation of the WNK4 T-loop at S332. Volume depletion also induces phosphorylation of WNK4-S64 in the DCT in vivo, promoting NCC activity. These findings provide insights into the mechanisms regulating activity of NCC and the promotion of renal Na-Cl reabsorption without concomitant K+ secretion in volume depletion.
Keywords: renin-angiotensin-aldosterone system, NCC, hypertension, renal electrolyte transport, distal convoluted tubule
Abstract
With-no-lysine kinase 4 (WNK4) regulates electrolyte homeostasis and blood pressure. WNK4 phosphorylates the kinases SPAK (Ste20-related proline alanine-rich kinase) and OSR1 (oxidative stress responsive kinase), which then phosphorylate and activate the renal Na-Cl cotransporter (NCC). WNK4 levels are regulated by binding to Kelch-like 3, targeting WNK4 for ubiquitylation and degradation. Phosphorylation of Kelch-like 3 by PKC or PKA downstream of AngII or vasopressin signaling, respectively, abrogates binding. We tested whether these pathways also affect WNK4 phosphorylation and activity. By tandem mass spectrometry and use of phosphosite-specific antibodies, we identified five WNK4 sites (S47, S64, S1169, S1180, S1196) that are phosphorylated downstream of AngII signaling in cultured cells and in vitro by PKC and PKA. Phosphorylation at S64 and S1196 promoted phosphorylation of the WNK4 kinase T-loop at S332, which is required for kinase activation, and increased phosphorylation of SPAK. Volume depletion induced phosphorylation of these sites in vivo, predominantly in the distal convoluted tubule. Thus, AngII, in addition to increasing WNK4 levels, also modulates WNK4 kinase activity via phosphorylation of sites outside the kinase domain.
The distal portion of the mammalian nephron plays a key role in water, electrolyte, and blood pressure homeostasis. Mutations that alter normal renal Na-Cl homeostasis in this nephron segment modulate blood pressure and result in diverse electrolyte abnormalities (1). One such rare Mendelian trait is Pseudohypoaldosteronism type II (PHAII, OMIM 145260), which is characterized by hypertension, hyperkalemia, and metabolic acidosis; these features can be corrected by low doses of thiazide diuretics, inhibitors of the Na-Cl cotransporter of the distal convoluted tubule (NCC).
Genetic analysis of PHAII has revealed a previously unrecognized pathway that regulates blood pressure and electrolyte homeostasis in the distal nephron. Causative mutations have been found in four genes; two encode the serine–threonine kinases with-no-lysine 1 and 4 (WNK1 and WNK4) (2), and two encode Cullin 3 (CUL3) and Kelch-like 3 (KLHL3), components of an E3-RING ubiquitin ligase complex (3). At the time of the discovery of their causal relationship to this Mendelian disease, none of these proteins were known to play a role in electrolyte or blood pressure homeostasis. The biochemical mechanisms that link mutations to clinical phenoytpes are becoming understood. WNK4 is a Cl−-regulated kinase (4); when active, the kinase phosphorylates the kinases SPAK (Ste20-related proline alanine-rich kinase) and OSR1 (oxidative stress responsive kinase), which in turn phosphorylate and activate the thiazide-sensitive Na-Cl cotransporter of the renal distal convoluted tubule (DCT). The phenotype of WNK4 knockout (WNK4-KO) mice recapitulates Gitelman syndrome (OMIM 263800), the mirror image of PHAII, suggesting that in vivo WNK4 is mainly present in the active state and that it plays a central role in NCC regulation (5). In addition to this kinase-dependent regulation of the Na-Cl contransporter, WNK4 also regulates the activity of other mediators of distal renal electrolyte transport, for example, inhibiting the K+ channel ROMK (6). It thus appears that increased WNK4 activity can explain the increased Na-Cl reabsorption and hypertension seen in PHAII; the inhibition of ROMK can explain the inability to excrete K+, accounting for hyperkalemia (7). Inhibition of NCC activity can reverse both the hypertension and hyperkalemia seen with PHAII.
Nonetheless, the mechanisms that regulate the activity of WNK4 were obscure until the discovery that mutations in CUL3 and KLHL3 can also cause PHAII (3, 8). Subsequent work has shown that the kelch-like domain of KLHL3 specifically binds to WNK1 and WNK4, leading to their ubiquitylation and degradation (9, 10). Disease-causing dominant mutations in KLHL3 and CUL3 result in impaired binding and degradation of WNKs (9, 11, 12). Conversely, disease-causing mutations in WNK4 are predominantly missense mutations in a short acidic domain (2), which also prevent binding and ubiquitylation by the KLHL3–CUL3–RING complex; this acidic domain of WNK4 and WNK1 physically binds to KLHL3 (13).
Recent work has shown that the binding of WNK4 by KLHL3 is regulated by Angiotensin II (AngII) via protein kinase C (PKC). Activation of PKC by AngII results in phosphorylation of S433 in the kelch domain, preventing WNK4 binding (14). S433 is also targeted by PKA, presumably downstream of vasopressin (AVP) (15). The importance of this site is strongly supported by the observation that S433 is recurrently mutated in PHAII (3, 8). Thus, the level of WNK4 clearly plays a critical role in regulation of electrolyte homeostasis, with single amino acid substitutions that result in increased WNK4 levels, leading to hypertension and hyperkalemia in humans. This pathogenic mechanism is supported by studies of BAC-transgenic PHAII mice, which show that mutant WNK4 levels are elevated in vivo (9, 11).
These findings nonetheless leave open the question of whether elevated levels are all that is necessary for increased WNK4 activity. Specifically, we considered whether PKC and PKA, in addition to phosphorylating KLHL3, might also impart effects by phosphorylating WNK4. Consistent with this possibility, we noted that WNK4 contains multiple consensus sites for PKC and PKA. We report herein systematic identification of phosphorylation sites in WNK4 and demonstration of their effects on WNK4’s biochemical function.
Results
WNK4 Phosphorylation Sites in HEK293 Cells.
To examine steady-state Ser/Thr phosphorylation of WNK4, we expressed WNK4 tagged at the C terminus with the HA epitope in HEK293 cells and performed immunoprecipitation with anti-HA followed by mass spectrometry (MS) (16). Three mapping experiments were performed, capturing 56% of the WNK4 protein sequence. Eighteen phosphorylation sites were reproducibly identified (Fig. S1A and Table S1). Four of these sites were at RRXS motifs, which are commonly phosphorylated by PKC and PKA (S64, S1169, S1180, S1196); the peptide containing an additional RRXS motif, at Ser-47, was not observed in MS. The three most C-terminal RRXS sites are clustered and conserved across all vertebrates and lie close to functionally important domains (Fig. 1), whereas the two N-terminal sites are clustered and conserved in vertebrates, other than fish (Fig. 1A). Non-RRXS phosphorylation sites S1172 and S1176, which lie close to the C-terminal RRXS sites, and S332, which is the T-loop phosphorylation site required for activation of the kinase, are also highly conserved, whereas others are not at highly conserved positions in the protein (Table S1).
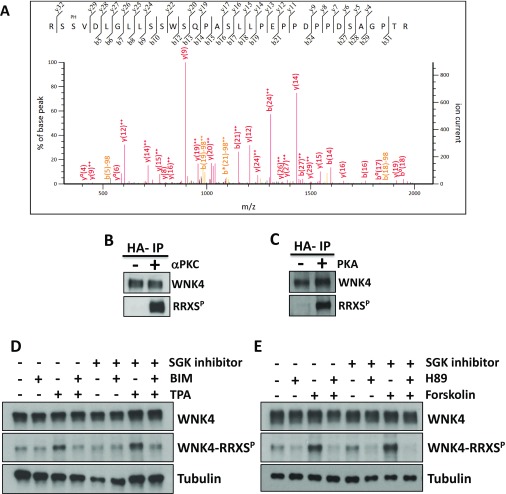
Fig. S1.
WNK4 phosphorylation at RRXS sites. (A) Representative MS/MS spectrum of one of the WNK4 phosphopeptides identified. Specific y and b fragment ions allowed unambiguous identification of the precursor peptide and phosphorylation at specific residues. Fragment ions with neutral loss of phosphate (H3PO4; −98 Da) are indicated (b5–98, etc.). The phosphorylated residue (Ser-64) is indicated with the PH label above the sequence. (B and C) WNK4 is a substrate for in vitro phosphorylation by PKCα and PKA. Kinase inactive WNK4 was purified by immunoprecipitation from extracts of COS-7 cells that were serum-depleted for 12 h before lysis. This decreased the basal WNK4 phosphorylation observed. Immunopurified WNK4 was used as substrate for in vitro phosphorylation by PKCα (B) or PKA (C). WNK4 phosphorylation was assessed by Western blot using the RRXSP antibody. (D and E) The increase in WNK4–RRXS phosphorylation induced by PKC or PKA activation is independent of SGK1 activity. COS-7 cells were transfected with wild-type WNK4 and at 48 h posttransfection, cells were stimulated with the indicated drugs. The SGK1 inhibitor GSK65039, which has been shown to be effective in cell culture experiments (23), was added 30 min before the addition of other drugs. Cells were lysed and extracts were blotted with the indicated antibodies. Similar observations were made in two independent experiments.
Table S1.
LC-MS/MS results for WNK4 expressed in HEK293 cells
| Mod. | Start | End | Sequence | Score | M+ | m/z | Conserv. in n species |
| 6 | 35 | NTETGVPMSQTEADLALRPSPALTSTGPTR | 15.68 | 3 | 1040.2 | ||
| Phos (S64) | 62 | 94 | RSSVDLGLLSSWSQPASLLPEPPDPPDSAGPTR | 58.72 | 3 | 1170.6 | 19 |
| Phos (S63/S100) | 62 | 101 | RSSVDLGLLSSWSQPASLLPEPPDPPDSAGPTRSPPSSSK | 24.84 | 4 | 1072.3 | 19 (S63), 16 (S100) |
| 63 | 94 | SSVDLGLLSSWSQPASLLPEPPDPPDSAGPTR | 56.57 | 3 | 1091.9 | ||
| Phos (S99) | 63 | 101 | SSVDLGLLSSWSQPASLLPEPPDPPDSAGPTRSPPSSSK | 101.2 | 3 | 1342.0 | 14 |
| Phos (S95) | 95 | 115 | SPPSSSKEPPEGTWMGAAPVK | 92.7 | 3 | 744.7 | 13 |
| 102 | 115 | EPPEGTWMGAAPVK | 94.46 | 2 | 738.4 | ||
| ✓ | 116 | 134 | AVDSACPELTGSSGGPGSR | 4.8 | 3 | 719.9 | |
| 135 | 145 | EPPRVPDAAAR | 42.58 | 2 | 589.8 | ||
| 148 | 169 | RREQEEKEDTETQAVATSPDGR | 64.05 | 3 | 844.7 | ||
| 149 | 169 | REQEEKEDTETQAVATSPDGR | 78.91 | 3 | 792.7 | ||
| 150 | 169 | EQEEKEDTETQAVATSPDGR | 96.41 | 2 | 1110.5 | ||
| 155 | 169 | EDTETQAVATSPDGR | 118.55 | 2 | 788.9 | ||
| 173 | 179 | FDIEIGR | 58.07 | 2 | 425.2 | ||
| ✓ | 180 | 187 | GSFKTVYR | 4.15 | 2 | 607.2 | |
| 215 | 223 | FSEEVEMLK | 77.09 | 2 | 567.3 | ||
| 224 | 232 | GLQHPNIVR | 49.46 | 2 | 517.3 | ||
| ✓ | 243 | 260 | GQVCIVLVTELMTSGTLK | 4.79 | 4 | 519.3 | |
| 284 | 291 | GLHFLHSR | 54.9 | 2 | 483.8 | ||
| 292 | 298 | VPPILHR | 58.29 | 2 | 416.3 | ||
| 302 | 315 | CDNVFITGPSGSVK | 51.68 | 2 | 712.3 | ||
| 316 | 326 | IGDLGLATLKR | 84.62 | 2 | 578.9 | ||
| 316 | 325 | IGDLGLATLK | 97.46 | 2 | 500.8 | ||
| Phos (S332) | 332 | 348 | SVIGTPEFMAPEMYEEK | 74.26 | 2 | 1022.4 | 28 |
| 349 | 383 | YDEAVDVYAFGMCMLEMATSEYPYSECQNAAQIYR | 18.41 | 3 | 1386.9 | ||
| 385 | 396 | VTSGTKPNSFYK | 68.07 | 2 | 664.8 | ||
| 418 | 430 | FTIQDLLAHAFFR | 100.25 | 2 | 794.9 | ||
| 434 | 451 | GVHVELAEEDDGEKPGLK | 108.49 | 2 | 961.5 | ||
| 467 | 479 | DNQAIEFLFQLGR | 115.16 | 2 | 775.9 | ||
| ✓ | 480 | 505 | DAAEEVAQEMVALGLVCEADYQPVAR | 5.91 | 3 | 956.4 | |
| ✓ | 627 | 657 | SGPGSDFSPGDSYASDAASGLSDMGEGGQMR | 86.58 | 3 | 1030.4 | |
| 672 | 683 | LRVTSVSDQSDR | 36.3 | 2 | 691.9 | ||
| Phos (S676) | 674 | 683 | VTSVSDQSDR | 71.39 | 2 | 592.2 | 15 |
| 684 | 695 | VVECQLQTHNSK | 85.85 | 2 | 696.4 | ||
| 701 | 726 | FDLDGDSPEEIAAAMVYNEFILPSER | 47.76 | 3 | 982.1 | ||
| 727 | 732 | DGFLSR | 22.3 | 1 | 704.4 | ||
| 733 | 739 | IREIIQR | 36.38 | 2 | 464.3 | ||
| 740 | 746 | VETLLKR | 39.42 | 2 | 429.8 | ||
| Phos (S759) | 747 | 780 | DAGPPEAAEDALSPQEEPAALPALPGPPNAEPQR | 85.86 | 3 | 1161.5 | 14 |
| Phos (S783) | 781 | 787 | SISPEQR | 43.32 | 2 | 453.7 | 26 |
| 971 | 981 | NPAQPLLGDAR | 78.66 | 2 | 581.3 | ||
| 982 | 996 | LAPISEEGKPQLVGR | 96.89 | 2 | 797.4 | ||
| 997 | 1003 | FQVTSSK | 52.25 | 2 | 401.7 | ||
| Phos (S1014) | 997 | 1019 | FQVTSSKEPAEPPLQPASPTLSR | 79.88 | 2 | 1274.1 | 11 |
| Phos (S1014) | 1004 | 1019 | EPAEPPLQPASPTLSR | 58.14 | 2 | 885.4 | 11 |
| Phos (S1025) | 1020 | 1046 | SLKLPSPPLTSESSDTEDSAAGGPETR | 54.65 | 3 | 942.4 | 16 |
| Phos (S1033) | 1023 | 1046 | LPSPPLTSESSDTEDSAAGGPETR | 83.42 | 2 | 1241.0 | 16 |
| Phos (S1052) | 1047 | 1054 | EALAESDR | 37.99 | 2 | 485.7 | 14 |
| 1055 | 1071 | AAEGLGVAVDDEKDEGK | 116.81 | 2 | 851.9 | ||
| 1055 | 1067 | AAEGLGVAVDDEK | 109.36 | 2 | 637.3 | ||
| 1122 | 1136 | QKHLSEVEALQTLQK | 87.6 | 2 | 876.5 | ||
| 1124 | 1136 | HLSEVEALQTLQK | 118.72 | 2 | 748.4 | ||
| 1138 | 1145 | EIEDLYSR | 51.83 | 2 | 512.7 | ||
| 1146 | 1166 | LGKQPPPGIVAPAAMLSCRQR | 2.95 | 2 | 1116.6 | ||
| Phos (S1169) | 1167 | 1177 | RLSKGSFPTSR | 28.51 | 2 | 698.3 | 27 |
| Phos (S1172) | 1168 | 1177 | LSKGSFPTSR | 35.83 | 2 | 580.3 | 26 |
| Phos (S1176) | 1171 | 1177 | GSFPTSR | 24.25 | 2 | 416.2 | 23 |
| ✓ | 1178 | 1194 | RNSLQRSDLPGPGIMRR | 2.07 | 3 | 710.3 | |
| Phos (S1180) | 1178 | 1183 | RNSLQR | 30.84 | 2 | 427.2 | 27 |
| 1184 | 1193 | SDLPGPGIMR | 80.16 | 2 | 521.8 | ||
| Phos (S1196) | 1194 | 1208 | RNSLSGSSTGSQEQR | 112.17 | 2 | 837.4 | 24 |
| ✓ | 1194 | 1211 | RNSLSGSSTGSQEQRASK | 7.52 | 3 | 733.9 | |
| Phos (S1196) | 1195 | 1208 | NSLSGSSTGSQEQR | 82.09 | 2 | 759.3 | 24 |
| 1212 | 1221 | GVTFAGDIGR | 70.07 | 2 | 496.8 |
Observed peptides are presented. Mod, variable modification; Phos, phosphorylation; Score, MASCOT score. Peptide sequences are numbered according to position in mouse WNK4. Phosphorylated residues are underlined. Check mark means that the peptide was phosphorylated but the phosphorylated residue could not be assigned. Conservation of the identified phosphorylation sites was analyzed in 28 vertebrate species (including fish, birds, reptiles, amphibians, and mammals). The last column indicates the number of species in which the residue was found to be conserved. N-terminal RRXS sites lie within a conserved region of the protein across vertebrate classes, except fish. C-terminal phosphorylation sites located after S1169 lie within a region that is conserved among all vertebrates analyzed. S783 is conserved in most vertebrates; however, the surrounding sequence is only conserved among mammals. S332 is the T-loop phosphorylation site, which lies within the kinase domain and is conserved in all orthologs. All other sites are generally only conserved among mammals.
Fig. 1.
Phosphorylation sites in WNK4 present within a RRXS sequence. (A) Alignment of amino acid sequences of WNK4 from different vertebrate species. The five RRXS motifs present in WNK4 and the phosphorylatable serine in each of them are indicated. Numbering corresponds to mouse WNK4. (B) Schematic representation of WNK4. Known domains and motifs are indicated. Location of the RRXS sites is shown with asterisks. CC, coiled-coil domain (2); CC*, coiled-coil domain that is implicated in WNK homo- and heterodimerization (39); PF2-like, domain similar to the PF2 domain present in SPAK/OSR1 (49); PP1-BS, protein phosphatase 1 binding site (50); SPAK-BS: SPAK binding site (49). PHAII-causing mutations found in WNK4 lie within the acidic box. Only one has been reported outside this region (R1185C; R1164C in mouse WNK4) (2) and its position is indicated. See also Tables S1 and S2.
Activation of PKC and PKA Promotes Phosphorylation of WNK4–RRXS Sites.
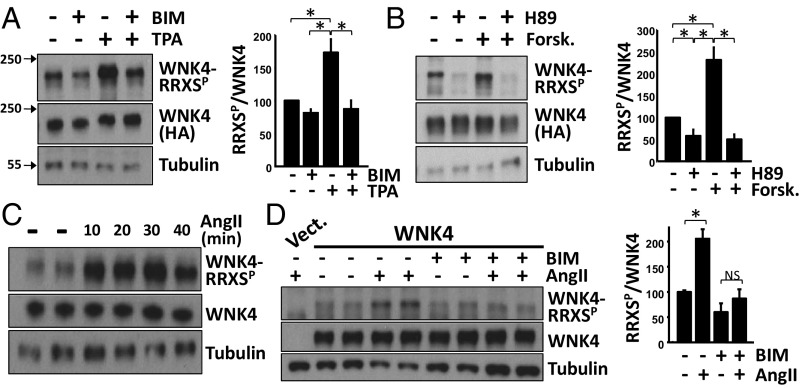
WNK4-HA–transfected COS-7 cells were treated acutely with the PKC inhibitor bisindolylmaleimide I (BIM), the PKC activator 12-O-tetradecanoylphorbol-13-acetate (TPA), or both. Changes in phosphorylation of WNK4–RRXS sites was examined by Western blotting using a well-established antibody with high specificity for RRXSP (Materials and Methods). A clear WNK4–RRXSP signal was detected in total cell extracts (Fig. 2A). TPA stimulation produced a significant increase in WNK4–RRXS phosphorylation (Fig. 2A) that was prevented in the presence of BIM. This finding strongly supports TPA-stimulated WNK4 phosphorylation because of activation of PKC rather than a different TPA-responsive kinase (17, 18). Because PKA also phosphorylates the RRXS motif (19), we probed the effect of pharmacologic inhibition and activation of PKA on WNK4–RRXS phosphorylation. Forskolin-induced PKA activation promoted WNK4–RRXS phosphorylation, which was prevented by coincubation with forskolin and PKA inhibitor, H89 (Fig. 2B).
Fig. 2.
Phosphorylation of WNK4–RRXS sites is triggered by AngII-PKC and PKA signaling. (A) WNK4-HA–transfected COS-7 cells were treated with the PKC inhibitor or activator, BIM (4 μM), or TPA (200 nM), respectively. Lysates were blotted with the RRXSP antibody. (B) Same as in A, but cells were treated with the PKA inhibitor or activator, H89 (20 μM) or forskolin (30 μM), respectively. Bar graphs show the results of quantitation for A and B (n = 5). Data are means ± SEM; *P < 0.05. (C) COS-7 cells cotransfected with WNK4 and ATI were serum-depleted overnight and then stimulated with AngII (100 nM). AngII induced an increase in RRXSP at all time points tested. (D) The indicated groups were preincubated with BIM for 15 min before stimulation with AngII (30 min). Blockade of PKC with BIM prevented the AngII-induced increase in WNK4–RRXSP. Bar graphs summarize results of three experiments. Data are means ± SEM; *P < 0.05; NS, not significant. See also Fig. S1.
To further demonstrate the direct effect of PKC and PKA on WNK4–RRXS phosphorylation, in vitro kinase reactions were performed. Purified PKCα (one of the most highly expressed diacylglycerol-dependent PKC isoforms in kidney epithelium) or PKA efficiently phosphorylated WNK4–RRXS sites, demonstrating that WNK4 is a substrate for in vitro phosphorylation by both kinases (Fig. S1 B and C).
Some of the RRXS sites in WNK4 have also been described as targets for serum glucocorticoid kinase 1 (SGK1)-mediated phosphorylation (20–22). However, TPA or forskolin-induced increases in WNK4–RRXS phosphorylation were not prevented by SGK1 inhibition with GSK65039 (23), suggesting that these effects were independent of SGK1 (Fig. S1 D and E).
PKC can be activated by AngII via downstream signaling via Gq and phospholipase C (24). COS-7 cells that express the AngII receptor show Gq-dependent activation of PKC in response to AngII (25). In these cells, we found that AngII promoted an increase in WNK4–RRXS phosphorylation within 10 min (Fig. 2C). This increase was prevented with BIM, consistent with AngII inducing PKC-dependent phosphorylation (Fig. 2D).
Phosphorylation of Individual Sites Probed with Phospho-Specific Antibodies.
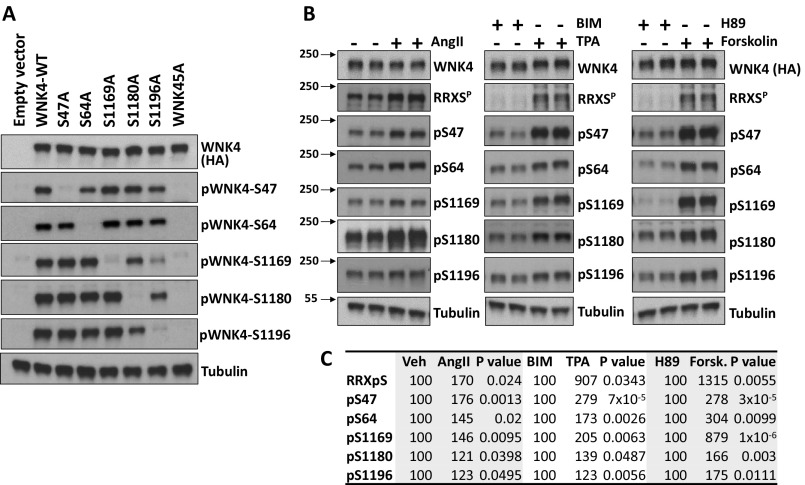
We developed phosphosite-specific antibodies for each of the five RRXS sites in WNK4 (Materials and Methods). Each of these was highly specific for phosphorylation at the specified site, as shown in each case by the loss of signal when the targeted serine residue was mutated to alanine (Fig. S2A).
Fig. S2.
Analysis of WNK4 phosphorylation dynamics in COS-7 cells using phosphosite-specific antibodies. (A) Demonstration of specificity of WNK4 phosphosite-specific antibodies. COS-7 cells were transfected with empty vector, wild-type WNK4, or the indicated mutant forms of WNK4. At 48 h posttransfection, cells were stimulated with TPA for 30 min before lysis and extracts were blotted with the indicated antibodies. The results demonstrate that the antibodies mainly detect the expected phosphorylated epitope. (B) Characterization of the dynamics of WNK4 phosphorylation in HEK293T cells using phosphospecific antibodies. HEK293T cells were transfected with WNK4-HA and 48 h posttransfection they were stimulated with the indicated drugs for 30 min and then lysed. For the AngII experiment, cells were serum-depleted overnight before stimulation. Extracts were immunoblotted with the indicated antibodies. pS47, pS64, pS1169, pS1180, and pS1196 are WNK4 phosphosite-specific antibodies. (C) Results of quantitation of blots in B (n = 3 or higher). Band intensity values obtained were normalized, establishing the vehicle, BIM, or H89 groups as 100%. Data are expressed as means.
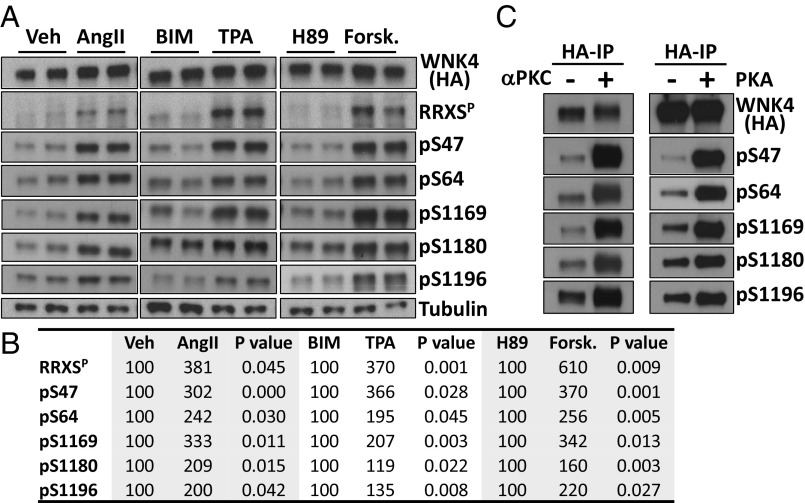
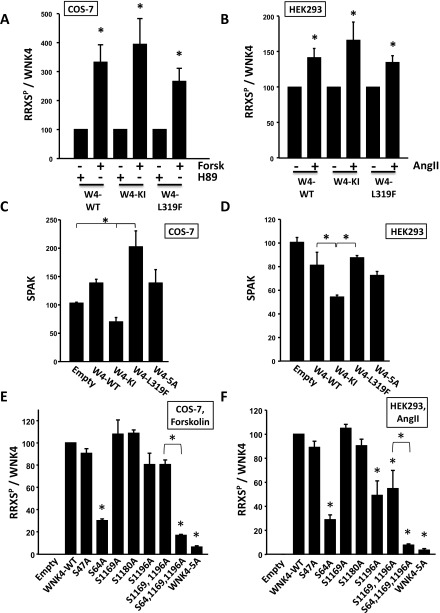
Phosphorylation of each site was assessed in COS-7 and HEK293T cells following transfection with WNK4-HA and AT1 receptor and incubation with AngII, TPA (PKC activator), BIM (PKC inhibitor), forskolin (PKA activator), or H89 (PKA inhibitor). In both cell lines, treatment with AngII, TPA, and forskolin induced increased phosphorylation of all five sites (Fig. 3 A and B and Fig. S2 B and C). In addition, in vitro kinase assays performed with the purified PKCα and PKA produced phosphorylation of each of the five RRXS sites (Fig. 3C).
Fig. 3.
Characterization of WNK4 phosphorylation dynamics using phosphosite-specific antibodies. (A) COS-7 cells transfected with WNK4-HA and AT1 were stimulated with the indicated drugs for 30 min. Lysates were immunoblotted with the indicated antibodies. pS47, pS64, pS1169, pS1180, and pS1196 are WNK4 phosphosite-specific antibodies. (B) Results of quantitation of blots in A (n ≥ 4, in at least three independent experiments). Band intensity values obtained with ImageJ were normalized, establishing vehicle, BIM, or H89 groups as 100%. (C) Immunopurified WNK4-HA was incubated with recombinant αPKC and PKA in the presence of all of the components necessary for kinase activity (30 min). Proteins were blotted with the indicated antibodies. See also Fig. S2.
Functional Relevance of WNK4–RRXS Phosphorylation.
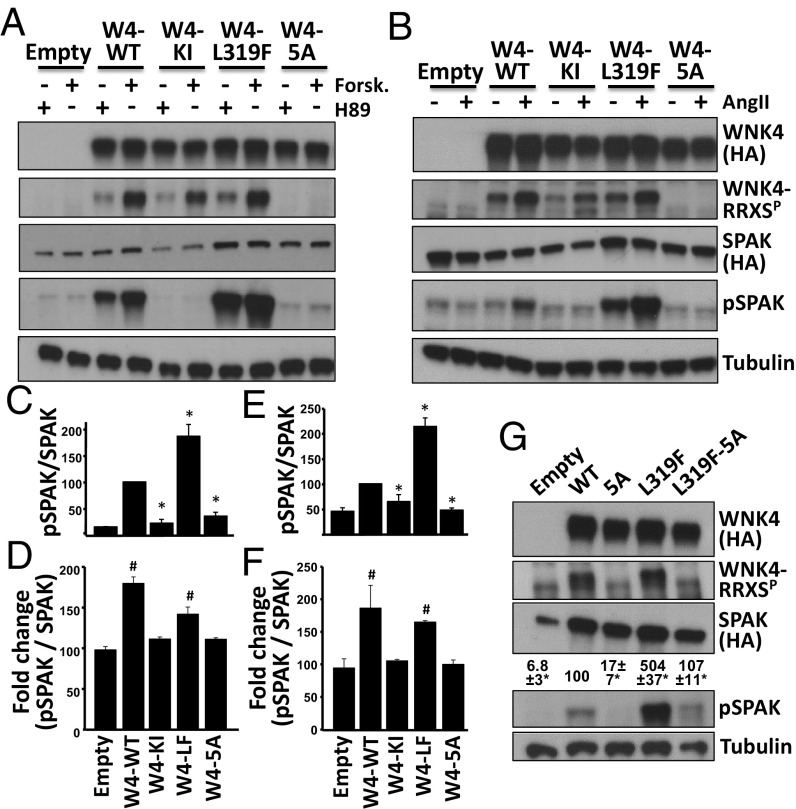
We next evaluated the effect of RRXS phosphorylation on WNK4’s kinase activity using a well-known target, SPAK, as a substrate. COS-7 cells were transfected with SPAK and WNK4 phosphorylation mutants. Cells were incubated with H89 or forskolin to alter WNK4 phosphorylation and SPAK phosphorylation (S373) was assessed (Fig. 4 A, C, and D, and Fig. S3). In the absence of WNK4, SPAK phosphorylation (pSPAK) levels were low and similar between H89- or forskolin-treated cells. However, in WNK4-transfected cells, pSPAK levels significantly increased despite the presence of H89, the PKA inhibitor, but were further augmented by forskolin (Fig. 4 A and D). This forskolin-induced increase in pSPAK was not observed in cells transfected with kinase inactive WNK4 (WNK4-KI; D318A) (26). Cotransfection of SPAK with a WNK4 (L319F mutant), which is not inhibitable by chloride (4, 27), promoted an even stronger increase in pSPAK that was again augmented by forskolin treatment (Fig. 4 A, C, and D). Mutation of all five serines in RRXS motifs to alanine (5A mutant) had no effect on WNK4 level, but drastically reduced the ability of WNK4 to phosphorylate SPAK. The 5A mutant showed pSPAK levels that were not significantly different from levels seen in the absence of WNK4, indicating a critical role of phosphorylation at these sites in modulation of WNK4’s phosphorylation of SPAK. Furthermore, in the presence of this mutant, forskolin stimulation did not increase pSPAK, suggesting that phosphorylation of RRXS sites is necessary for this effect (Fig. 4 A, C, and D).
Fig. 4.
Functional relevance of WNK4 phosphorylation in RRXS sites. (A) COS-7 cells were transfected with SPAK-HA and WNK4-HA or the indicated WNK4 mutants and stimulated with H89 (20 μM) or forskolin (30 μM). Lysates were blotted with the indicated antibodies. (B) HEK293T cells were transfected with SPAK-HA, ATI receptor and WNK4-HA, or the indicated mutants. After overnight serum depletion, cells were stimulated with vehicle or AngII (100 nM). Lysates were blotted with the indicated antibodies. KI, kinase inactive; 5A, mutant in which the Ser residues of the five RRXS motifs were substituted for Ala. (C) Quantitation of pSPAK/SPAK for H89-treated groups represented in A. (D) Fold-change in pSPAK/SPAK between H89 and forskolin groups. (E) Quantitation of pSPAK/SPAK for AngII-treated groups represented in B. (F) Fold-change in pSPAK/SPAK between vehicle and AngII groups. All data are means ± SEM; n ≥ 3. *P < 0.05 vs. WT; #P < 0.05 vs. Empty. Results of quantitation for RRXSP and SPAK blots are shown in Fig. S3. (G) Cells were transfected with SPAK and different WNK4 mutants. Note that L319F-5A makes reference to a single clone that presents six mutations. SPAK phosphorylation was assessed. Results of quantitation are shown above pSPAK blot. All data are means ± SEM; n = 3. See also Figs. S3 and S4.
Fig. S3.
Results of the quantitation of band intensities of the blots presented in Figs. 4 and 5. (A and B) Bar graphs show the results of quantitation of the RRXSP blots of Fig. 4 A and B, respectively. Results were normalized, establishing the H89 or vehicle groups as 100%. (C and D) Results of quantitation of the SPAK blots of Fig. 4 A and B, respectively. Because no difference was observed in SPAK band intensities between the H89 and forskolin groups or the vehicle and AngII groups, these values were averaged for comparison with values obtained for other WNK4 mutants. Note that WNK4 activity levels in the cells had an impact on the level of total SPAK expression. (E and F) Results of quantitation of the RRXSP blots of Fig. 5 A and B, respectively. Results were normalized, establishing the wild-type groups as 100%. *P < 0.01.
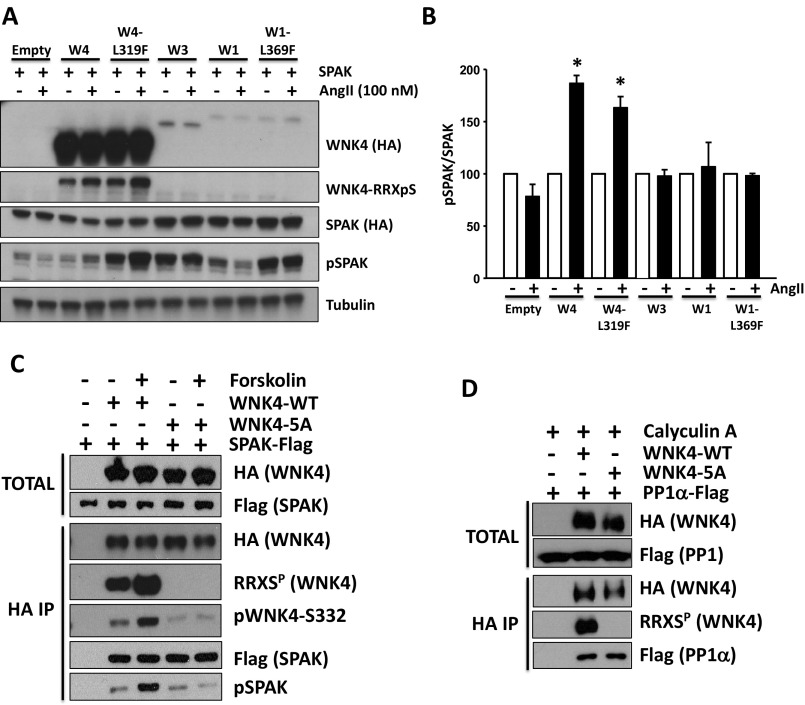
Similarly, AngII stimulation of HEK293T cells expressing WNK4 and SPAK also showed augmented SPAK phosphorylation that was abolished by the WNK4-5A mutations (Fig. 4 B, E, and F). Interestingly, AngII did not induce increased pSPAK in cells cotransfected with WNK1 or WNK3 instead of WNK4 (Fig. S4 A and B), indicating that WNK4 is required for AngII-induced phosphorylation of SPAK (5).
Fig. S4.
AngII-induced phosphorylation of SPAK is exclusively mediated by WNK4. (A) HEK293T cells were transfected with SPAK-HA, ATI receptor and the indicated WNK kinase. At 48 h posttransfection, cells were stimulated with 100 nM AngII for 30 min and then lysed. Protein extracts were immunoblotted with the indicated antibodies. The increase in pSPAK signal promoted by AngII stimulation was only observed in the presence of WNK4 or WNK4-L319F. (B) Band intensities from the pSPAK blots from three different experiments were quantified and the results are represented in the graph as means ± SEM. Values were normalized, establishing the vehicle groups as 100%. *P < 0.05 vs. Veh. (C) SPAK binding to WNK4 is not affected by WNK4–RRXS phosphorylation. HEK293T cells were transfected with SPAK-FLAG and wild-type WNK4-HA or WNK4-5A-HA. After overnight serum depletion, the indicated groups of cells were treated with forskolin to promote WNK4-RRXS phosphorylation. WNK4 was immunoprecipitated using Sepharose beads bound to anti-HA antibody and levels of coimmunoprecipitated SPAK were analyzed by Western blot. Three independent experiments were performed with similar results. (D) PP1α binding to WNK4 is not affected by WNK4–RRXS phosphorylation. HEK293T cells were transfected with PP1α-FLAG and wild-type WNK4-HA or WNK4-5A-HA. All groups were treated with calyculin A for 20 min before lysis to prevent RRXS dephosphorylation by PP1α. Three independent experiments were performed with similar results.
To explore the hierarchy of regulatory mechanisms, we tested the activity of the chloride-insensitive mutant (W4-L319F) in the 5A mutant. This sextuple mutant showed markedly lower phosphorylation of SPAK than the W4-L319 mutant (Fig. 4G).
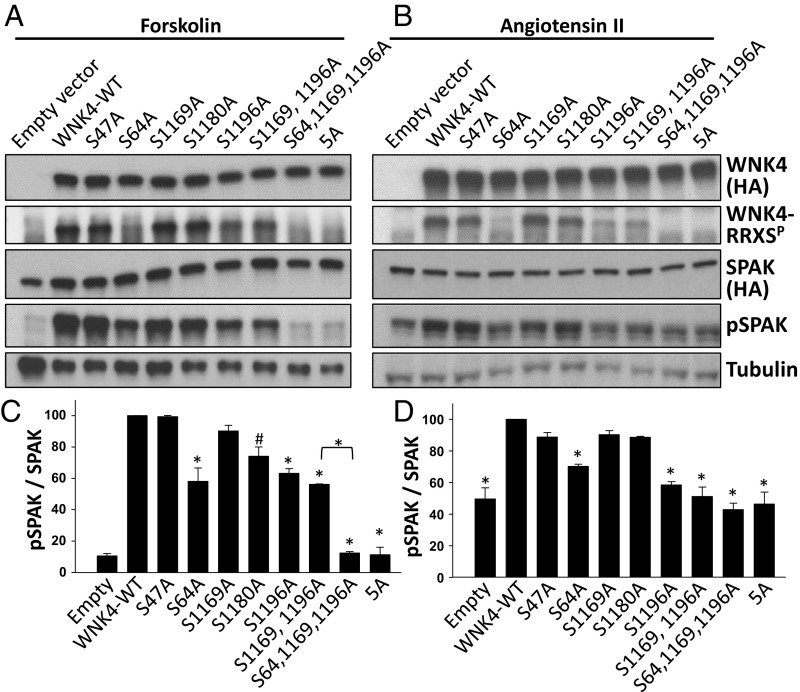
We next tested the importance of individual WNK4–RRXS phosphorylation sites on pSPAK and WNK4–RRXSP levels in forskolin-stimulated COS-7 cells. We found that the S64A and S1196A mutations were the only ones that significantly reduced both total RRXS phosphorylation and SPAK phosphorylation (Fig. 5 A and C and Fig. S3E). When both mutations were present, the signal was virtually completely abolished. This observation, along with evidence that individual site mutations do not alter phosphorylation at other RRXS sites (Fig. S2A), suggests that S64 and S1196 are the main sites of WNK4 phosphorylation detected by the RRXSP antibody and are the main sites required for phosphorylation of SPAK (Fig. 5 A and C). Similar results demonstrating the primacy of phosphorylation at S64 and S1196 in WNK4-mediated phosphorylation of SPAK were observed in HEK293T cells stimulated with AngII (Fig. 5 B and D and Fig. S3F).
Fig. 5.
Role of individual WNK4-RRXSP sites in the regulation of WNK4-mediated SPAK phosphorylation. (A) COS-7 cells were transfected with SPAK-HA and WNK4-HA or the indicated WNK4 mutants. All cells were stimulated with forskolin (30 μM). Total cell extracts were blotted. (B) Same as in A, but instead HEK29T cells were used and cells were stimulated with AngII (100 nM). (C and D) Results of quantitation of pSPAK blots of A and B, respectively (n = 3). Band intensities were normalized establishing the WNK4-WT group as 100%. Data are means ± SEM. 5A, mutant in which the Ser residues of the five RRXS sites were substituted for Ala. *P < 0.01; #P < 0.05. Quantitation for RRXSP and SPAK blots is shown in Fig. S3.
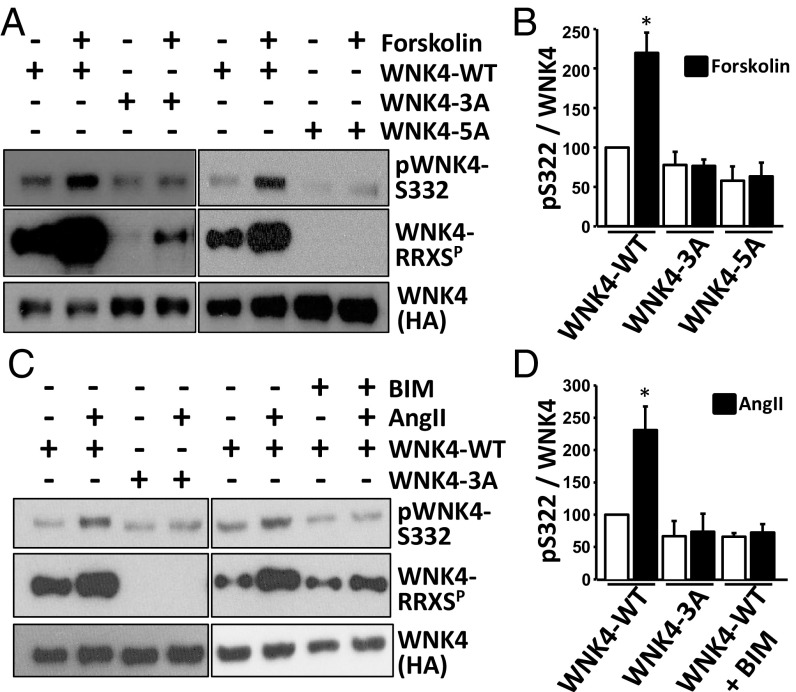
Phosphorylation of S64 and S1196 Regulates Phosphorylation of the WNK4 T-Loop.
Activation of WNK4 kinase is known to require autophosphorylation of S332 of the T-loop in the kinase catalytic domain (4, 28). We found that forskolin and AngII markedly increased T-loop phosphorylation, consistent with this being a primary mechanism by which forskolin and AngII increased downstream SPAK phosphorylation (Fig. 6). Moreover, we found that this increased phosphorylation at S332 was abolished following mutation of all RRXS sites; this effect was mediated by alanine substitution at S64 and S1196. In addition, we observed that AngII-induced S332 phosphorylation was dependent on PKC activation. In contrast, the alanine mutants showed no impairment of WNK4 binding to SPAK or protein phosphatase 1 (PP1) (Fig. S4 C and D). Thus, phosphorylation of these sites is implicated in the activation of WNK4 kinase activity rather than in WNK4’s ability to bind to downstream targets or associated phosphatases.
Fig. 6.
WNK4-RRXS phosphorylation promotes WNK4 T-loop autophosphorylation. (A) WNK4 Ser-322 phosphorylation was assessed in immunoprecipitated WNK4 from HEK293 cells transfected with WNK4-WT, WNK4-3A (S64A, S1169A, S1196A) or WNK4-5A (S47A, S64A, S1169A, S1180A, S1196A) and stimulated with vehicle or forskolin for 30 min before lysis. (B) Results of quantitation. White bars, nonstimulated cells; Black bars, forskolin-stimulated cells. (C) Same as in A, but WNK4 Ser-322 phosphorylation was assessed in immunoprecipitated WNK4 from lysates of cells stimulated with vehicle or AngII for 30 min before lysis. The PKC inhibitor BIM was also added in the indicated groups. (D) Results of quantitation. White bars, nonstimulated cells; Black bars, AngII-stimulated cells. Data are means ± SEM; *P < 0.01 vs. WT-nonstimulated (n = 3–6 in each group). See also Fig. S4 C and D.
Volume Depletion in Mice Promotes Phosphorylation of S64, S1169, S1180, and S1196.
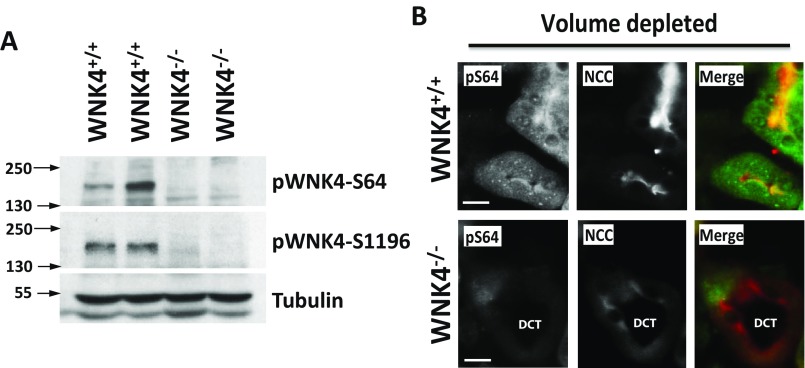
We used phosphosite-specific antibodies to test phosphorylation at specific sites in WNK4 in mouse kidney. Proteins from kidneys of WNK4-KO mice (5) were used to confirm the specificity of identified signals. We detected signal indicating phosphorylation at S64 and S1196 in Western blots of whole kidney protein extracts (Fig. S5A), and also at S1169 and S1180 in blots of renal extracts immunoprecipitated with anti-WNK4 (Fig. S6). We have not detected phosphorylation at S47 in either analysis.
Fig. S5.
Demonstration of specificity of pWNK4 antibodies for in vivo studies. (A) Kidney lysates from WNK+/+ and WNK4−/− mice were blotted with the different WNK4 phosphoantibodies. Only the pS64 antibody and the previously generated pS1196 antibody (50) gave a specific signal. This is, a band of the expected size was observed that was absent in the samples from the WNK4−/− mice. Nonspecific binding with the remaining four antibodies, masked the bands corresponding to phospho-WNK4. (B) Kidney sections from volume depleted WNK4+/+ and WNK4−/− were stained in parallel with pWNK4 antibodies. Only the pS64 antibody gave a specific signal that was not observed in the WNK4−/− mice. This signal was observed in NCC-positive tubules (DCTs). Note that a lower NCC signal was observed in the WNK4−/− mice. This was expected as it has been previously reported that NCC expression is greatly decreased in WNK4−/− mice (5). (Scale bars, 10 μm.)
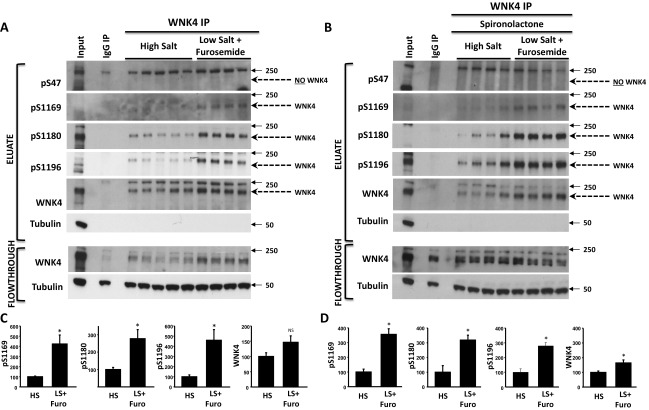
Fig. S6.
Volume depletion in mice promotes phosphorylation of WNK4-S1169 and WNK4-S1180 in addition to WNK4-S64 and WNK4-S1196. (A and B) The same treatment groups presented in Fig. 6 were studied. WNK4 was immunoprecipitated from kidney lysates and the obtained purified proteins (eluate) were subjected to Western blotting with the indicated antibodies. All antibodies, except pS47-WNK4 antibody detected a band of the expected size for WNK4 (indicated by pointed arrow). (C and D) Results of quantitation for experiments represented in A and B, respectively. Data are expressed as means ± SEM (n = 6). *P < 0.05; NS, not significant.
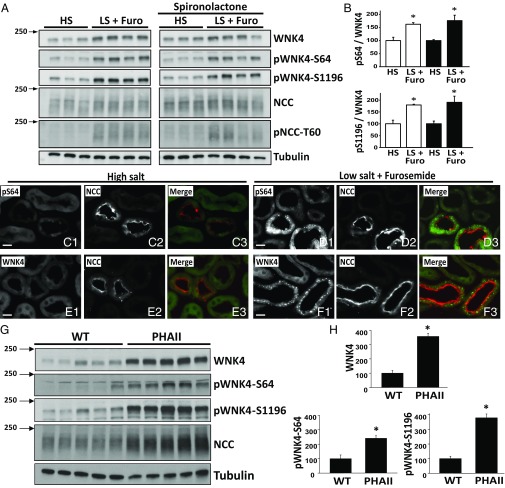
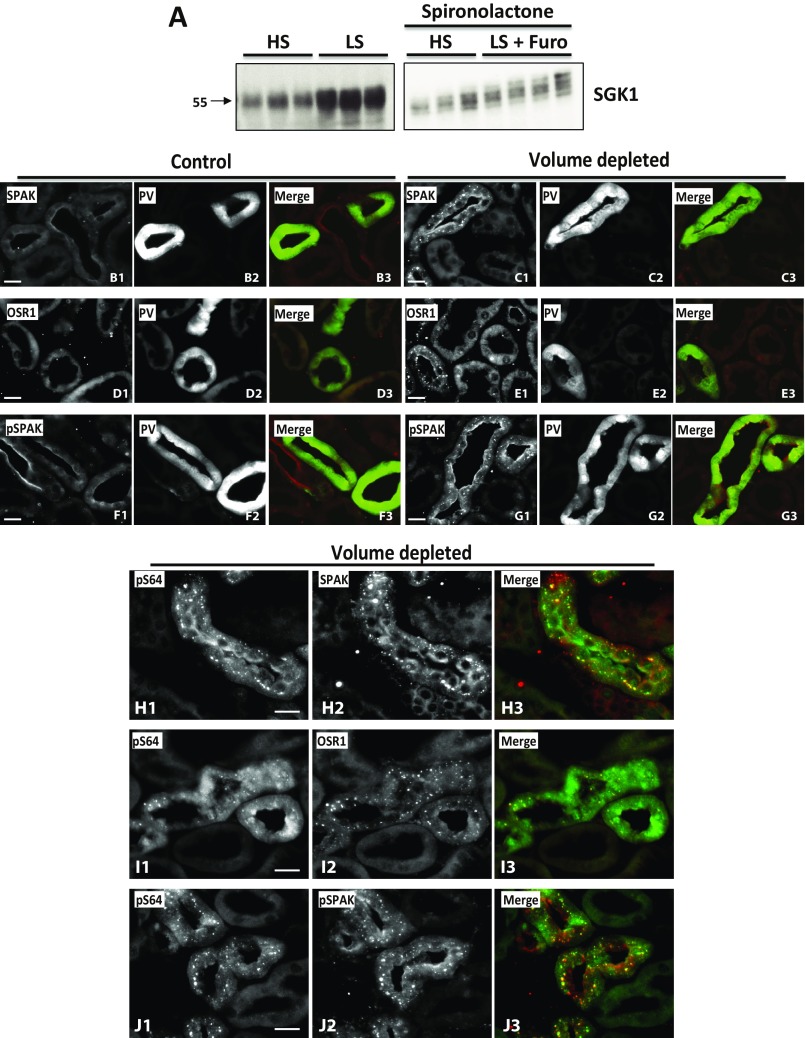
We tested whether phosphorylation at these sites increases in response to AngII in the setting of volume depletion. As positive controls, we observed increased phosphorylation of T60 in NCC and increased levels of WNK4 (5, 14). We observed significantly increased phosphorylation of S64, S1169, S1180, and S1196 in the volume-depleted group (Fig. 7A and Fig. S6A). The ratio of pWNK4 to WNK4 was also significantly increased (Fig. 7B and Fig. S6C), indicating that WNK4 phosphorylation was stimulated and the increase was not simply the consequence of higher WNK4 levels. The increases in S64, S1169, S1180, and S1196 phosphorylation were not prevented by spironolactone (Fig. 7 A and B and Fig. S6 B and D), despite effective blockade of aldosterone signaling, as shown by blunting of the induction of SGK1 expression (Fig. S7A).
Fig. 7.
In vivo phosphorylation of WNK4-S64 and S1196. (A) Mice were administered a low salt (LS) diet and given intraperitoneal furosemide for 3 d, or maintained on high salt (HS) diet and injected with vehicle. Treatments were replicated in two groups of mice, in which spironolactone was also administered. Kidney lysates were subjected to Western blot. Volume depletion promoted S64 and S1196 phosphorylation in mice. (B) Band intensities were quantified and the phosphorylated/total ratios were calculated. HS groups were established as 100%. White and black bars are groups without and with spironolactone treatment, respectively. Data are means ± SEM (n = 6 in each group). *P < 0.05. (C–F) Immunofluorescent staining of kidney sections from volume-replete and volume-depleted mice. Volume depletion-induced changes in signal intensity and staining pattern are observed in the DCTs of mice with the pS64-WNK4 and WNK4 antibodies. In the HS group, pS64 and WNK4 signals are virtually undetectable (C and E). (Scale bars, 10 μm.) (G) Kidney extracts from wild-type mice or PHAII mice carrying the WNK4–Q562E transgene (32) were blotted with the indicated antibodies. (H) Band intensities were quantified and results are presented relative to wild-type (100%). Data are expressed as means ± SEM; *P < 0.05. See also Figs. S5–S7.
Fig. S7.
Volume depletion-induced changes in SPAK and OSR1. (A) SGK1 expression levels in volume-depleted mice. SGK1 blots were performed with kidney extracts of mice treated with high salt (HS) diet and low salt (LS) diet. As expected, a significantly higher expression was observed in mice on LS diet. In contrast, in spironolactone-treated mice, volume depletion induced by LS + furosemide did not promote a significant increase in SGK1 expression, suggesting that aldosterone signaling was effectively blocked. (B–G) Effect of volume depletion on SPAK, OSR1, and pSPAK levels and intracellular distribution in parvalbumin (PV)-positive tubules. Volume depletion-induced changes in signal intensity and staining pattern are observed in DCT1 (PV+) of mice with the SPAK, OSR1, and pSPAK antibodies. (B) SPAK staining in control mice can be observed in thick ascending limb (TAL) and DCT apical membranes. Signal is more intense in TAL. Volume depletion promotes an increase in the SPAK signal observed in the PV+ tubules and under this condition cytoplasmic puncta become apparent (C). (D) OSR1 is almost undetectable in DCT of control mice; however, in volume-depleted mice a punctuate staining pattern is observed in DCT1 (E). (F) Phosphorylated SPAK/OSR1 is detected in the apical membrane of TALs and DCTs in control mice. The signal observed in DCT is weak. In contrast, in volume-depleted mice, DCT apical signal is intensified and cytoplasmic puncta appear (G). (H–J) Phosphorylated WNK4 at S64 colocalizes with SPAK, OSR1, and pSPAK/OSR1. Kidney sections of volume-depleted mice were stained with the indicated antibodies. Cytoplasmic puncta observed with the pS64-WNK4 antibody in DCT cells colocalize with the puncta observed with the SPAK (H), OSR1 (I), and pSPAK/OSR1 (J) antibodies. Similar observations were made in sections from at least three different mice. (Scale bars, 10 μm.)
Volume Depletion Induces WNK4-S64 Phosphorylation Mainly in the DCT.
To determine the renal cell types in which AngII increases phosphorylation of WNK4, we performed immunofluorescence microscopy using the anti-pS64 antibody, which gave a clear signal in volume-depleted wild-type mice that was eliminated in WNK4-KO mice (Fig. S5B). Staining was identified exclusively in nephron segments that also expressed NCC, localizing this phosphorylation to the DCT. As previously reported, NCC membrane abundance increased in the high AngII state (29). The staining patterns observed with the pS64-WNK4 and WNK4 antibodies were strikingly different between volume-depleted and volume-replete wild-type mice. In mice on a high salt diet, no pS64-WNK4 signal was detected in DCT cells (identified by NCC expression), whereas in volume-depleted mice, diffuse cytoplasmic staining with cytoplasmic puncta were clearly observed with anti-pS64 in all NCC-positive tubules (Fig. 7 C–F). Interestingly, this pattern of cytoplasmic puncta has been observed by others when staining with antibodies to SPAK, OSR1, and WNK4 (30, 31). We reproduced this pattern with antibodies to SPAK, OSR1, and pSPAK-S383 (Fig. S7 B–G). Costaining with WNK4-pS64 and SPAK, OSR1, or pSPAK antibodies revealed colocalization of these signals (Fig. S7 H–J).
S64 and S1196 Phosphorylation in PHAII Mice.
We tested phosphorylation at S64 and S1196 in kidney samples from mice carrying a transgene with a WNK4–PHAII mutation (32). As expected, these mice had higher levels of WNK4 expression (Fig. 7 G and H). This result was accompanied by a higher level of S64 and S1196 phosphorylation. However, the fold-change in S64 phosphorylation and S1196 phosphorylation were, respectively, lower or similar to the fold-change observed for WNK4 total expression.
Discussion
The ability of increased renal salt reabsorption to cause hypertension is well established (1). AngII has been shown to promote renal Na+ reabsorption in an aldosterone-independent manner (33–35), in part by increasing NCC activity (33) in a WNK4-dependent mechanism (5). We have previously shown that part of this mechanism is via increased levels of WNK4, because of AngII-PKC–mediated phosphorylation of KLHL3, which disrupts WNK4 binding and degradation (14). Here we describe an additional mechanism contributing to AngII’s effect on WNK4. Upon AngII stimulation, PKC phosphorylates WNK4 sites, which in turn increase phosphorylation of the T-loop of WNK4, increasing kinase activity and phosphorylation of downstream targets. Prior work has shown that WNK4 regulates diverse electrolyte flux mediators by kinase-dependent and independent mechanisms. The ability to regulate kinase activity independently of WNK4 level permits differential regulation of downstream targets (7).
Of the two most critical PKC phosphorylation sites for kinase activation, serine 64 has not previously been reported, whereas S1196 has also been identified as a target of the aldosterone-induced kinase SGK1 (20–22). It seems clear that phosphorylation at this site is substantially attributable to direct phosphorylation by PKC/PKA because this site is directly phosphorylated by these enzymes in vitro, is regulated in cell culture by activators and inhibitors of PKC/PKA, and this site is phosphorylated in vivo by volume depletion despite inhibition of aldosterone signaling by spironolactone. We cannot exclude the possibility that PKC and PKA may phosphorylate additional sites in WNK4. For example, four phosphorylation sites identified by MS had variations of the PKC phosphorylation consensus sequence defined by basic amino acids at positions −3, −2, and +2, and in some cases, a hydrophobic amino acid at position +1 (36, 37) (Table S2). Nevertheless, data show that elimination of S64 and S1196 is sufficient to prevent AngII-induced WNK4 activation.
Table S2.
Observed peptides which contain features of the PKC consensus motif
| Mod. | Start | End | Sequence | Score | M+ | m/z | Nearby sequence |
| Phos (S63/S100) | 62 | 101 | RSSVDLGLLSSWSQPASLLPEPPDPPDSAGPTRSPPSSSK | 24.84 | 4 | 1072.3 | SRRS*SV |
| Phos (S64) | 62 | 94 | RSSVDLGLLSSWSQPASLLPEPPDPPDSAGPTR | 58.72 | 3 | 1170.6 | RRSS*VD |
| Phos (S676) | 674 | 683 | VTSVSDQSDR | 71.39 | 2 | 592.2 | RVTS*VS |
| Phos (S1025) | 1020 | 1046 | SLKLPSPPLTSESSDTEDSAAGGPETR | 54.65 | 3 | 942.4 | KLPS*PP |
| Phos (S1169) | 1167 | 1177 | RLSKGSFPTSR | 28.51 | 2 | 698.3 | RRLS*KG |
| Phos (S1172) | 1168 | 1177 | LSKGSFPTSR | 35.83 | 2 | 580.3 | SKGS*FP |
| Phos (S1180) | 1178 | 1183 | RNSLQR | 30.84 | 2 | 427.2 | RRNS*LQ |
| Phos (S1196) | 1194 | 1208 | RNSLSGSSTGSQEQR | 112.17 | 2 | 837.4 | RRNS*LS |
| Phos (S1196) | 1195 | 1208 | NSLSGSSTGSQEQR | 82.09 | 2 | 759.3 | RRNS*LS |
MS/MS results for WNK4 expressed in HEK293 cells. Mod, variable modification; PHOS, phosphorylation; Score, MASCOT score. Peptide sequences are numbered according to the position in mouse WNK4. Phosphorylated residues are underlined in the fourth column or marked in bold in the last column.
It is interesting that the critical phosphorylation sites mediating the PKC/PKA-induced increase in WNK4 activity lie at the extreme amino and carboxyl-terminal ends of the protein. The N-terminal domain of WNK4 has previously been shown to have an inhibitory effect on WNK4 activity: when the N-terminal domain of WNK4 is substituted with the N-terminal domain of WNK3, the kinase becomes active in Xenopus laevis oocytes (38). Similarly, phosphorylation of S1196 has been shown to diminish WNK4’s inhibitory effect on NCC in X. laevis oocytes, perhaps because of the attenuation of a dominant-negative effect (21). Our data suggest that phosphorylation of these sites via PKC/PKA relieves this inhibition via a mechanism that promotes phosphorylation of the T-loop of the kinase domain. This may occur via induction of a conformational change in WNK4, allowing access for intermolecular T-loop phosphorylation (39), by reducing catalytic domain binding and inhibition by Cl−, or by modulating the binding of an as yet unidentified protein that alters WNK4 activation.
In addition to PKC, we show that PKA also regulates WNK4. This finding is interesting because phosphorylation and activity of SPAK/OSR1-NCC is also increased by AVP (30, 40, 41). Binding of AVP to V2 receptors in renal epithelia leads to PKA activation; phosphorylation of WNK4 by PKA may be the next step mediating SPAK/OSR1-NCC activation.
PHAII is caused by increased activity of WNK1 and WNK4 (9, 11, 12). We propose that WNK4–RRXS phosphorylation is important for its activity; thus, we predict that WNK4–RRXS sites are phosphorylated in PHAII. Consistently, we observed that mice carrying a WNK4–PHAII mutation (32) have higher WNK4 expression and phosphorylation at S64 and S1196 (Fig. 7). The fold-change in pS1196, pS64, and WNK4 were similar; therefore, the higher phosphorylation observed may have been because of higher availability of substrate. Note that these sites are phosphorylated despite the low activity of the renin–angiotensin system. One possibility is that AVP-induced PKA activation may sustain RRXS phosphorylation. AVP levels have not been reported in PHAII patients or animals. However, AVP levels may be increased in states of increased salt reabsorption to maintain plasma isosmolarity, similar to the increased levels seen with high salt intake (42–44). Additionally, high plasma [K+] may also promote AVP secretion (45). It is also intriguing that in PHAII, WNK4 remains activated despite hyperkalemia, because high plasma K+ would be expected to inhibit WNK activity by modulation of intracellular chloride (31).
Finally, these findings provide insight into the biochemical mechanism by which the kidney responds rapidly to reduce salt excretion in the setting of volume depletion (46). NCC activation by AngII occurs within minutes of the stimulus onset (29). AngII action, via WNK4 and SPAK, to activate NCC provides a response to volume depletion that is more rapid than the response via aldosterone, which requires time for adrenal induction of CYP11B2 mRNA and protein synthesis followed by aldosterone biosynthesis and its own induction of target genes in the kidney.
Materials and Methods
Cell Culture and Transient Transfection.
COS-7 and HEK293T cells were used for transient expression of WNK4-HA, WNK4-FLAG, SPAK-HA, AT1R, WNK1-HA, and WNK3-HA. Cells were grown to 70–80% confluency and transfected with lipofectamine 2000 (Life Technologies). Forty-eight hours posttransfection, cells were lysed with a lysis buffer containing protease (Complete, Roche) and phosphatase inhibitors (Mixture 3, Sigma, P0044). Protein concentration was quantified by the BCA protein assay. Western blot assays and immunoprecipitation experiments were performed as described in SI Materials and Methods. Antibodies are also described. For treatment with BIM (Cell Signaling Technology #9841), TPA (Cell Signaling Technology #4174), H-89, Dihydrochloride (Cell Signaling Technology #9844), and forskolin (Cell Signaling Technology #3828), drugs were added to the culture media 30 min before lysis. The final concentration in the culture wells was 4 μM for BIM, 200 nM for TPA, 20 μM for H89, and 30 μM for forskolin. For acute treatment with AngII, cells were serum-depleted overnight and then incubated with 100 nM AngII for 30 min.
Mutations were introduced into the WNK4 clone by site directed mutagenesis using Pfu turbo DNA polymerase (Agilent) and confirmed by Sanger sequencing.
Phosphopeptide Enrichment and MS.
The procedures followed were similar to those described previously (16). Briefly, HA-tagged WNK4 was immunoprecipitated from lysates of transiently transfected HEK293 cells and then resolved on SDS/PAGE. Protein bands were excised from the gel and digested in a trypsin solution (20 μg/mL, sequence grade; Promega). Extracted peptides were applied to pre-equilibrated TiO2 TopTip microspin columns (Glygen). Eluate fractions were injected onto the HPLC column (Atlantis, 100 μm × 150 mm; Waters) directly interfaced to an electrospray ionization-quadrupole time-of-flight (ESI-QTOF) mass spectrometer (Waters/Micromass Q-Tof Ultima). Data were analyzed with Mascot 2.1 with improved phosphopeptide scoring. Identified phosphopeptides were confirmed by manual inspection of the spectra (for details SI Materials and Methods).
Mouse Studies.
Animal studies were approved by the Yale Institutional Animal Care and Use Committee (protocol no. 10018). Most studies were performed in wild-type C57BL/6 mice. WNK4-knockout and PHAII-transgenic mouse strains were also used (5, 32).
Volume-depletion model.
Both the control and volume-depleted groups were kept on high salt diet [8% (wt/wt) NaCl] for ∼7 d before the beginning of treatment with furosemide. Half of the mice were then switched to low sodium diet (0.01–0.02% Na+) and given intraperitoneal injections of furosemide (15 mg·kg−1, every 12 h for 3 d) (47). The control group was injected with saline and kept on high salt diet. Before being killed, mice were anesthetized with 100 mg·kg−1 ketamine plus 10 mg·kg−1 xylazine, the right renal artery was ligated, and the right kidney was collected and flash-frozen in liquid nitrogen. Mice were then perfused as described in SI Materials and Methods and the left kidney was harvested and treated as indicated for immunofluorescence (SI Materials and Methods).
Spironolactone treatment.
The volume-depletion protocol was carried out as described in the previous section, but spironolactone was administered in both groups, control and furosemide-treated. Spironolactone treatment began 2 d before the first injection of furosemide and continued throughout experiment. Mice were injected every 12 h (intraperitoneally) with spironolactone (40 mg·kg−1·d−1) (48).
Statistical Analysis.
For comparison between two groups, unpaired Student’s t test (two tailed) was used. For comparison between multiple groups, ANOVA tests were performed, followed by Tukey post hoc tests. A difference between groups was considered significant when P < 0.05.
SI Materials and Methods
Phosphopeptide Enrichment and MS.
HA-tagged WNK4 was immunoprecipitated and then resolved on SDS/PAGE. Protein bands, visualized with Coomassie blue staining, were excised from the gel and washed in 50% (vol/vol) CH3CN, 50 mM NH4HCO3. The gel fragments were then crushed and resuspended in a trypsin solution (20 μg·mL−1, sequence grade; Promega) with 10 mM NH4HCO3, and incubated overnight at 37 °C. Peptides were extracted with 0.5% trifluoroacetic acid (TFA)/50% (vol/vol) acetonitrile (ACN), dried, resuspended, and applied to pre-equilibrated TiO2 TopTip microspin columns (Glygen). Unbound nonphosphopeptides were washed off with 0.5% TFA/50% (vol/vol) ACN, and bound phosphopeptides were eluted with a 1:33 solution of saturated ammonia. Eluate fractions were dried and dissolved in 70% (vol/vol) formic acid and then diluted with 0.1% TFA. Sample was injected onto the HPLC column (Atlantis; 100 μm × 150 mm, Waters) directly interfaced to an ESI-QTOF mass spectrometer (Waters/Micromass Q-Tof Ultima). Data were analyzed with Mascot 2.1 with improved phosphopeptide scoring. A WNK4 residue was considered phosphorylated if it was observed in at least two experiments or was identified in a single experiment with a Mascot score greater than 30. Identified phosphopeptides were confirmed by manual inspection for precise match of the m/z ratio of the observed peptide precursor ions and their fragment ions to those predicted to result from trypsin cleavage of WNK4. For further details, see refs. 16 and 51.
Immunoprecipitation and Western Blotting.
For immunoprecipitation of HA-tagged WNK4, clarified lysates prepared from transfected, cultured cells (0.5–2 mg of protein) were incubated with monoclonal anti-HA agarose (Sigma, A2095) overnight, at 4 °C. Beads were washed five times with ice-cold PBS and bound protein was eluted either with glycine buffer (pH 2) or loading buffer containing SDS.
Western blots were performed as described previously (5). For sample preparation we used the following lysis buffer: 250 mM sucrose, 10 mM triethanolamine, 1× protease inhibitors (Roche), 1× phosphatase inhibitors (Sigma). The following antibodies were used: polyclonal antibodies raised in sheep against NCC (S965B), WNK4 (S121B), phosphorylated NCC at threonine 58 (in mouse) (S995B), and phosphorylated SPAK at serine 383 (in mouse) (S670B). This pSPAK antibody is able to recognize both pSPAK-S383 and pOSR1-S325. The concentrations used were 1–3 μg·mL−1. These antibodies were produced at the Medical Research Council phosphorylation unit at Dundee University, and their specificity has been demonstrated previously. Other antibodies used were: anti-HA (Sigma, H6908), anti–α-tubulin (Sigma, T5168), peroxidase conjugated anti-mouse IgG, anti-sheep IgG, and anti-rabbit IgG (Jackson ImmunoResearch).
For characterization of WNK4 phosphorylation, we used an antibody that recognizes protein motifs containing phospho-Ser/Thr residue with arginine residues at the −3 and −2 positions (RRXSP motifs; Cell signaling technology #9624) (14). Phosphosite-specific antibodies were produced by Covance Immunology Services. Rabbits were immunized with the following mouse WNK4 phospho-peptides: CRRF(pS)GKAEP (for phospho-Ser-47), CRRS(pS)VDLGL (for phospho-Ser-64), CRRL(pS)KGSFP (for phospho-Ser-1169), CRRN(pS)LQRSD (for phospho-Ser-1180), and CRRN(pS)LSGSS (for phospho-Ser-1196). Sera from three different production bleeds per rabbit were tested and the samples with the highest titer and specificity were selected and pooled for affinity purification. Antibodies were purified following a two-step procedure. First, nonphospho-antibodies were removed with an affinity column bound to the nonphophorylated peptide. Second, columns with bound phospho-peptides were used to isolate antibodies that recognize the phosphorylated epitopes.
In Vitro Kinase Assays.
Assays were performed as described previously (14). Briefly, kinase inactive WNK4-HA immunoprecipitated from COS-7 lysates was incubated with PKCα (100 ng; ProSpec, #pKa-218) or PKA catalytic subunit (100 ng; Promega #V5161) in a total assay volume of 50 mL of buffer containing 20 mM Mops, pH 7.2, 25 mM b-glycerolphosphate, 1 mM sodium orthovanadate, 1 mM EGTA, 1 mM DTT, 1 mM CaCl2, 15 mM MgCl2, and 100 μM ATP. For PKC assays, 0.5 mg·mL−1 phosphatidylserine and 0.05 mg·mL−1 diacylglycerol were also added and, for PKA assays, 2 μM cAMP was included in the reaction. After incubation for 30 min at 30 °C, reactions were stopped by addition of 5% (wt/vol) SDS loading buffer and proteins were fractionated by SDS/PAGE. Phosphate incorporation into WNK4–RRXS sites was determined by blotting with an antibody specific for RRXSP motifs (see above).
Immunoprecipitation of WNK4 from Mouse Tissue.
Clarified kidney lysates were incubated with 10 μg of sheep anti-WNK4 antibody (39) and protein A/G magnetic beads (Pierce) overnight at 4 °C. Beads were washed five times with a buffer containing 0.025 M Tris pH 7.4, 0.15 M NaCl, 0.001 M EDTA, 1% Nonidet P-40, 5% (vol/vol) glycerol. Bound proteins were eluted by incubation in glycine buffer, pH 2 for 10 min, and then prepared for Western blot analysis.
Immunofluorescence.
Mice were anesthetized with 100 mg·kg−1 ketamine plus 10 mg·kg−1 xylazine and perfused with 40 mL PBS followed by 50 mL 4% paraformaldehyde in PBS. Kidneys were harvested, incubated in 4% (wt/vol) formaldehyde in PBS for at least 3 h, and then incubated in 30% (wt/vol) sucrose in PBS overnight at 4 °C. The kidneys were mounted in OCT (Tissue-Tek) and 5-μm sections were prepared. For immunostaining, tissues were hydrated TBS-tween 0.1%. Antigen retrieval was performed in 100 mM citrate buffer, pH 6. Sections were incubated in blocking buffer for 1 h [10% (wt/vol) BSA in TBS-tween 0.1%], following by incubation with primary and secondary antibodies dissolved in 1% BSA in TBS-tween. The following antibodies were used: antiparvalbumin (Swant PV235; 1:2,000); anticalbindin (Swant CB-300; 1:2,000); anti-NCC (52) (1:100); anti–WNK4-pS64 (1:50); anti-WNK4 (kindly provided by David H. Ellison, Oregon Health & Science University, Portland, OR) (31); Alexa Fluor 594, donkey anti-sheep (1:400); Alexa Fluor 488 donkey anti-rabbit (1:400); Alexa Fluor 594, donkey anti-mouse (1:400) from Life Technologies.
Acknowledgments
We thank Carol Nelson-Williams for advice and helpful discussions; Dr. Jeremy Nichols (Parkinson's Institute and Clinical Center, Sunnyvale, CA) for kindly providing the PPIα clone; and Dr. David H. Ellison (Oregon Health & Science University, Portland, OR) for kindly providing the WNK4 antibody used for immunofluorescence studies. This work was supported by National Institutes of Health Grants P01DK17433 (to J.R. and R.P.L.), K01DK089006 (to J.R.), and R01DK51496 (to G.G.); Yale O’Brien Center Grant P30DK079310 (to P. Aronson and R.P.L.); and Conacyt Grants 165815 (to G.G.) and 257726 (to M.C.-B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1620315114/-/DCSupplemental.
References
- 1.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104(4):545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 2.Wilson FH, et al. Human hypertension caused by mutations in WNK kinases. Science. 2001;293(5532):1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 3.Boyden LM, et al. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature. 2012;482(7383):98–102. doi: 10.1038/nature10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bazúa-Valenti S, et al. The Effect of WNK4 on the Na+-Cl- cotransporter is modulated by intracellular chloride. J Am Soc Nephrol. 2015;26(8):1781–1786. doi: 10.1681/ASN.2014050470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castañeda-Bueno M, et al. Activation of the renal Na+:Cl- cotransporter by angiotensin II is a WNK4-dependent process. Proc Natl Acad Sci USA. 2012;109(20):7929–7934. doi: 10.1073/pnas.1200947109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahle KT, et al. WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat Genet. 2003;35(4):372–376. doi: 10.1038/ng1271. [DOI] [PubMed] [Google Scholar]
- 7.Hadchouel J, Ellison DH, Gamba G. Regulation of renal electrolyte transport by WNK and SPAK-OSR1 kinases. Annu Rev Physiol. 2016;78(1):367–389. doi: 10.1146/annurev-physiol-021115-105431. [DOI] [PubMed] [Google Scholar]
- 8.Louis-Dit-Picard H, et al. International Consortium for Blood Pressure (ICBP) KLHL3 mutations cause familial hyperkalemic hypertension by impairing ion transport in the distal nephron. Nat Genet. 2012;44(4):456–460, S1–S3. doi: 10.1038/ng.2218. [DOI] [PubMed] [Google Scholar]
- 9.Shibata S, Zhang J, Puthumana J, Stone KL, Lifton RP. Kelch-like 3 and Cullin 3 regulate electrolyte homeostasis via ubiquitination and degradation of WNK4. Proc Natl Acad Sci USA. 2013;110(19):7838–7843. doi: 10.1073/pnas.1304592110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wakabayashi M, et al. Impaired KLHL3-mediated ubiquitination of WNK4 causes human hypertension. Cell Reports. 2013;3(3):858–868. doi: 10.1016/j.celrep.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 11.Susa K, et al. Impaired degradation of WNK1 and WNK4 kinases causes PHAII in mutant KLHL3 knock-in mice. Hum Mol Genet. 2014;23(19):5052–5060. doi: 10.1093/hmg/ddu217. [DOI] [PubMed] [Google Scholar]
- 12.McCormick JA, et al. Hyperkalemic hypertension-associated cullin 3 promotes WNK signaling by degrading KLHL3. J Clin Invest. 2014;124(11):4723–4736. doi: 10.1172/JCI76126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schumacher FR, Sorrell FJ, Alessi DR, Bullock AN, Kurz T. Structural and biochemical characterization of the KLHL3-WNK kinase interaction important in blood pressure regulation. Biochem J. 2014;460(2):237–246. doi: 10.1042/BJ20140153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shibata S, et al. Angiotensin II signaling via protein kinase C phosphorylates Kelch-like 3, preventing WNK4 degradation. Proc Natl Acad Sci USA. 2014;111(43):15556–15561. doi: 10.1073/pnas.1418342111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshizaki Y, et al. Impaired degradation of WNK by Akt and PKA phosphorylation of KLHL3. Biochem Biophys Res Commun. 2015;467(2):229–234. doi: 10.1016/j.bbrc.2015.09.184. [DOI] [PubMed] [Google Scholar]
- 16.Rinehart J, et al. Sites of regulated phosphorylation that control K-Cl cotransporter activity. Cell. 2009;138(3):525–536. doi: 10.1016/j.cell.2009.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Springett GM, Kawasaki H, Spriggs DR. Non-kinase second-messenger signaling: New pathways with new promise. BioEssays. 2004;26(7):730–738. doi: 10.1002/bies.20057. [DOI] [PubMed] [Google Scholar]
- 18.Wilkinson SE, Parker PJ, Nixon JS. Isoenzyme specificity of bisindolylmaleimides, selective inhibitors of protein kinase C. Biochem J. 1993;294(Pt 2):335–337. doi: 10.1042/bj2940335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Songyang Z, Cantley LC. The use of peptide library for the determination of kinase peptide substrates. Methods Mol Biol. 1998;87:87–98. doi: 10.1385/0-89603-392-9:87. [DOI] [PubMed] [Google Scholar]
- 20.Ring AM, et al. An SGK1 site in WNK4 regulates Na+ channel and K+ channel activity and has implications for aldosterone signaling and K+ homeostasis. Proc Natl Acad Sci USA. 2007;104(10):4025–4029. doi: 10.1073/pnas.0611728104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rozansky DJ, et al. Aldosterone mediates activation of the thiazide-sensitive Na-Cl cotransporter through an SGK1 and WNK4 signaling pathway. J Clin Invest. 2009;119(9):2601–2612. doi: 10.1172/JCI38323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Na T, Wu G, Zhang W, Dong WJ, Peng JB. Disease-causing R1185C mutation of WNK4 disrupts a regulatory mechanism involving calmodulin binding and SGK1 phosphorylation sites. Am J Physiol Renal Physiol. 2013;304(1):F8–F18. doi: 10.1152/ajprenal.00284.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sherk AB, et al. Development of a small-molecule serum- and glucocorticoid-regulated kinase-1 antagonist and its evaluation as a prostate cancer therapeutic. Cancer Res. 2008;68(18):7475–7483. doi: 10.1158/0008-5472.CAN-08-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52(3):415–472. [PubMed] [Google Scholar]
- 25.Hansen JL, et al. Lack of evidence for AT1R/B2R heterodimerization in COS-7, HEK293, and NIH3T3 cells: how common is the AT1R/B2R heterodimer? J Biol Chem. 2009;284(3):1831–1839. doi: 10.1074/jbc.M804607200. [DOI] [PubMed] [Google Scholar]
- 26.Wilson FH, et al. Molecular pathogenesis of inherited hypertension with hyperkalemia: The Na-Cl cotransporter is inhibited by wild-type but not mutant WNK4. Proc Natl Acad Sci USA. 2003;100(2):680–684. doi: 10.1073/pnas.242735399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piala AT, et al. Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci Signal. 2014;7(324):ra41. doi: 10.1126/scisignal.2005050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zagórska A, et al. Regulation of activity and localization of the WNK1 protein kinase by hyperosmotic stress. J Cell Biol. 2007;176(1):89–100. doi: 10.1083/jcb.200605093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandberg MB, Riquier AD, Pihakaski-Maunsbach K, McDonough AA, Maunsbach AB. ANG II provokes acute trafficking of distal tubule Na+-Cl(-) cotransporter to apical membrane. Am J Physiol Renal Physiol. 2007;293(3):F662–F669. doi: 10.1152/ajprenal.00064.2007. [DOI] [PubMed] [Google Scholar]
- 30.Saritas T, et al. SPAK differentially mediates vasopressin effects on sodium cotransporters. J Am Soc Nephrol. 2013;24(3):407–418. doi: 10.1681/ASN.2012040404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terker AS, et al. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab. 2015;21(1):39–50. doi: 10.1016/j.cmet.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lalioti MD, et al. Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet. 2006;38(10):1124–1132. doi: 10.1038/ng1877. [DOI] [PubMed] [Google Scholar]
- 33.van der Lubbe N, et al. Angiotensin II induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter independent of aldosterone. Kidney Int. 2011;79(1):66–76. doi: 10.1038/ki.2010.290. [DOI] [PubMed] [Google Scholar]
- 34.Mamenko M, et al. Chronic angiotensin II infusion drives extensive aldosterone-independent epithelial Na+ channel activation. Hypertension. 2013;62(6):1111–1122. doi: 10.1161/HYPERTENSIONAHA.113.01797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crowley SD, et al. Role of AT1 receptor-mediated salt retention in angiotensin II-dependent hypertension. Am J Physiol Renal Physiol. 2011;301(5):F1124–F1130. doi: 10.1152/ajprenal.00305.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pearson RB, Kemp BE. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- 37.Nishikawa K, Toker A, Johannes FJ, Songyang Z, Cantley LC. Determination of the specific substrate sequence motifs of protein kinase C isozymes. J Biol Chem. 1997;272(2):952–960. doi: 10.1074/jbc.272.2.952. [DOI] [PubMed] [Google Scholar]
- 38.San-Cristobal P, Ponce-Coria J, Vázquez N, Bobadilla NA, Gamba G. WNK3 and WNK4 amino-terminal domain defines their effect on the renal Na+-Cl- cotransporter. Am J Physiol Renal Physiol. 2008;295(4):F1199–F1206. doi: 10.1152/ajprenal.90396.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thastrup JO, et al. SPAK/OSR1 regulate NKCC1 and WNK activity: Analysis of WNK isoform interactions and activation by T-loop trans-autophosphorylation. Biochem J. 2012;441(1):325–337. doi: 10.1042/BJ20111879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pedersen NB, Hofmeister MV, Rosenbaek LL, Nielsen J, Fenton RA. Vasopressin induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter in the distal convoluted tubule. Kidney Int. 2010;78(2):160–169. doi: 10.1038/ki.2010.130. [DOI] [PubMed] [Google Scholar]
- 41.Mutig K, et al. Short-term stimulation of the thiazide-sensitive Na+-Cl- cotransporter by vasopressin involves phosphorylation and membrane translocation. Am J Physiol Renal Physiol. 2010;298(3):F502–F509. doi: 10.1152/ajprenal.00476.2009. [DOI] [PubMed] [Google Scholar]
- 42.Cowley AW, Jr, Skelton MM, Merrill DC, Quillen EW, Jr, Switzer SJ. Influence of daily sodium intake on vasopressin secretion and drinking in dogs. Am J Physiol. 1983;245(6):R860–R872. doi: 10.1152/ajpregu.1983.245.6.R860. [DOI] [PubMed] [Google Scholar]
- 43.Matsuguchi H, Schmid PG, Van Orden D, Mark AL. Does vasopressin contribute to salt-induced hypertension in the Dahl strain? Hypertension. 1981;3(2):174–181. doi: 10.1161/01.hyp.3.2.174. [DOI] [PubMed] [Google Scholar]
- 44.Kjeldsen SE, et al. Dietary sodium intake increases vasopressin secretion in man. J Clin Hypertens. 1985;1(2):123–131. [PubMed] [Google Scholar]
- 45.Riphagen IJ, et al. Effects of potassium supplementation on markers of osmoregulation and volume regulation: Results of a fully controlled dietary intervention study. J Hypertens. 2016;34(2):215–220. doi: 10.1097/HJH.0000000000000786. [DOI] [PubMed] [Google Scholar]
- 46.Rafiqi FH, et al. Role of the WNK-activated SPAK kinase in regulating blood pressure. EMBO Mol Med. 2010;2(2):63–75. doi: 10.1002/emmm.200900058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee CT, Chen HC, Lai LW, Yong KC, Lien YH. Effects of furosemide on renal calcium handling. Am J Physiol Renal Physiol. 2007;293(4):F1231–F1237. doi: 10.1152/ajprenal.00038.2007. [DOI] [PubMed] [Google Scholar]
- 48.Castañeda-Bueno M, et al. Modulation of NCC activity by low and high K(+) intake: Insights into the signaling pathways involved. Am J Physiol Renal Physiol. 2014;306(12):F1507–F1519. doi: 10.1152/ajprenal.00255.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gagnon KB, Delpire E. Molecular physiology of SPAK and OSR1: Two Ste20-related protein kinases regulating ion transport. Physiol Rev. 2012;92(4):1577–1617. doi: 10.1152/physrev.00009.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin DH, et al. Protein phosphatase 1 modulates the inhibitory effect of With-no-Lysine kinase 4 on ROMK channels. Am J Physiol Renal Physiol. 2012;303(1):F110–F119. doi: 10.1152/ajprenal.00676.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rinehart J, et al. WNK2 kinase is a novel regulator of essential neuronal cation-chloride cotransporters. J Biol Chem. 2011;286(34):30171–30180. doi: 10.1074/jbc.M111.222893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richardson C, et al. Activation of the thiazide-sensitive Na+-Cl- cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci. 2008;121(Pt 5):675–684. doi: 10.1242/jcs.025312. [DOI] [PubMed] [Google Scholar]