Significance
Rift Valley fever (RVF) is an emerging, mosquito-borne viral infection of ruminants, transmissible to people, and linked to rainfall. By investigating a wider range of possible drivers this study confirms the assumption that RVF occurrence can also be dependent on nonenvironmental drivers. In Madagascar, human and ruminant infections were geographically distinct, with introduction and long-distance disease spread linked to livestock trading. Human infections were highest in those involved with slaughtering and handling fresh meat. The identification of possible introduction routes, major cattle trade hubs, and areas of high risk to ruminants has shown how multidisciplinary analyses are needed to properly understand disease dynamics and spread, thereby improving early detection and prevention of RVF in humans.
Keywords: vector-borne infection, zoonosis, El Niño, cattle trade, One Health
Abstract
Rift Valley fever (RVF) is a vector-borne viral disease widespread in Africa. The primary cycle involves mosquitoes and wild and domestic ruminant hosts. Humans are usually contaminated after contact with infected ruminants. As many environmental, agricultural, epidemiological, and anthropogenic factors are implicated in RVF spread, the multidisciplinary One Health approach was needed to identify the drivers of RVF epidemics in Madagascar. We examined the environmental patterns associated with these epidemics, comparing human and ruminant serological data with environmental and cattle-trade data. In contrast to East Africa, environmental drivers did not trigger the epidemics: They only modulated local Rift Valley fever virus (RVFV) transmission in ruminants. Instead, RVFV was introduced through ruminant trade and subsequent movement of cattle between trade hubs caused its long-distance spread within the country. Contact with cattle brought in from infected districts was associated with higher infection risk in slaughterhouse workers. The finding that anthropogenic rather than environmental factors are the main drivers of RVF infection in humans can be used to design better prevention and early detection in the case of RVF resurgence in the region.
Rift Valley fever (RVF) is a vector-borne infection caused by the RVF virus (RVFV). Mosquito species from the Aedes, Culex, and Mansonia genera are the main RVFV vectors (1). The virus persists in the environment either by vertical transmission occurring in some Aedes species or by an enzootic cycle involving ruminants and mosquitoes. When environmental conditions are favorable to mosquito proliferation, this cycle is amplified by Culex populations, leading to RVF epidemics (2). In the Horn of Africa, these circumstances are met during the warm phases of El Niño southern oscillation (ENSO) and the Indian Ocean dipole zonal mode: Warm sea-surface temperatures in the equatorial eastern-central Pacific Ocean and the western equatorial Indian Ocean result in heavy autumn rainfall and subsequent greening of the vegetation (3–5). The latter can be monitored by the remotely sensed normalized difference vegetation index (NDVI). Besides these environmental conditions triggering epidemics, long-distance RVFV dissemination is often related to ruminant trade (6), as reported in Madagascar and Saudi Arabia (7–9).
In domestic ruminants, the RVFV causes mass abortions and high neonatal mortality (10). Humans may get infected after the bites of infected mosquitoes or the consumption of raw milk. However, most clinical cases occur after contact with blood, aborted fetuses, and placenta of viremic animals. Farmers, veterinarians, slaughterhouse workers, and butchers are thus the most exposed to the risk of RVF, as well as any people attending the slaughtering of viremic animals (11): Blood aerosol produced on this occasion is highly infectious (12). The virus is not transmitted from person to person.
In Madagascar, the first known RVF epidemic occurred in 1990–1991 on the East Coast and in the central highlands (7). A second epidemic occurred in 2008–2009 with official reports suggesting at least 700 suspected cases and 26 RVFV laboratory-confirmed fatalities. However, due to underreporting, an excess of 10,000 human cases was estimated (13–15).
Madagascar is one of the poorest countries in the World, with 90% of the population living with less than USD 1.5 inhab.− 1d− 1 (16). Because public health resources are limited in such a setting, it is crucial to identify the most exposed populations, based on scientific evidence. To this end, we aimed at understanding the circumstances in which RVF epidemics occurred. We first assessed the risk of RVFV introduction to ruminants via livestock trade. Then, we described the environmental conditions associated with the start of the 1990 and 2008 epidemics and with local RVFV transmission. We then aimed to build risk indexes of RVFV human infection related to the local environment (local risk) and cattle trade (remote risk), assess their relative importance, identify and map high-risk areas, and assess the consequences for human health.
Results
Risk of Virus Introduction by Livestock Trade.
Queries in the United Nations (UN) ComTrade database did not reveal direct importations of live ruminants from mainland Africa to Madagascar. However, they highlighted several official importations from the Union of Comoros in 2005–2007 (Table 1). Although the numbers are small, they confirmed the risk of RVFV introduction in Madagascar from an infected country, via livestock trade.
Table 1.
Importation of live ruminants in Madagascar from the Union of Comoros between 2005 and 2007 (source: UN ComTrade)
Estimated number from the reported financial value. No imports of live ruminants were reported from the Union of Comoros in 2003 and 2004.
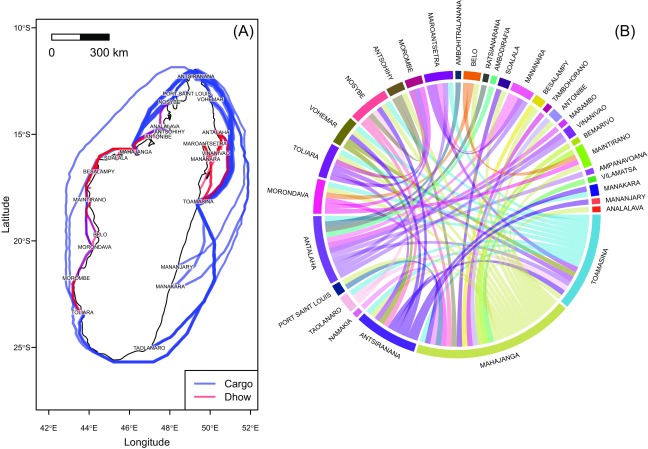
In addition to this official trade, illegal cattle movements between the Comoros Archipelago and Madagascar were probably much more frequent. Informal surveys conducted in 2009 and 2010 in the main Comoros harbors and in the northwest of Madagascar (Mahajanga and Antsiranana) revealed the frequent presence of cattle and small ruminants on board freighters and botry (dhows) traveling from the Comoros Islands to Madagascar and from port to port. This coastal navigation is widespread in Madagascar, given the weakness of the terrestrial-road network (Fig. S1). Therefore, RVFV could have been introduced to Madagascar through ruminant trade from Comoros Islands—which were previously infected (17, 18)—and further disseminated through coastal navigation between Malagasy sea ports.
Fig. S1.
Sea trade traffic in Madagascar: coastal navigation by cargoes and dhows. (A) Pseudoroutes were computed using a least-cost distance algorithm applied on a friction surface defined by bathymetric data (The Satellite Geodesy research group at Scripps Institution of Oceanography, University of California, San Diego). Route width is proportional to the traffic intensity (tonsy− 1 in 2004). (B) Chord diagram showing the trade flows (number of flows). Data for A and B: Scetauroute International (57).
Environmental Conditions and RVFV Epidemics.
Triggering RVF epidemics.
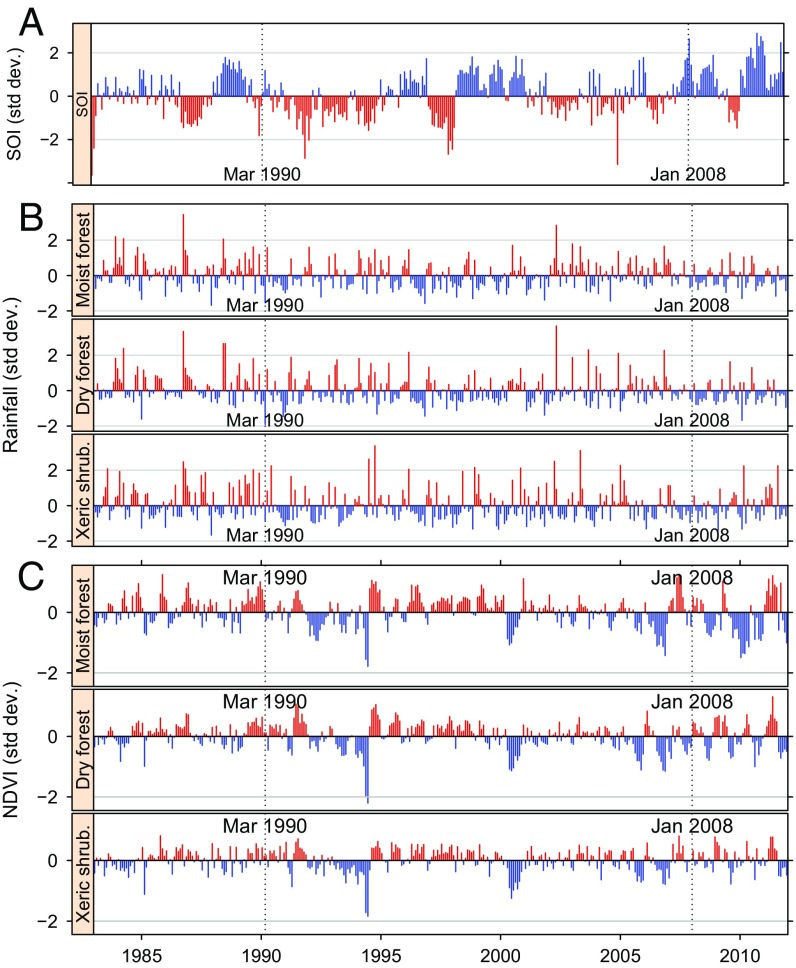
Conditions at the start of the two RVF epidemics in humans (March 1990 and January 2008) are shown on Fig. 1. For the three indicators [southern oscillation index (SOI), rainfall, and NDVI] and three biomes (Fig. S2A), the two epidemics did not occur in typical RVF conditions according to the eastern African standards (14). In March 1990, a marked negative anomaly was observed for SOI, one of the main indicators of ENSO (Fig. 1A). Rainfall was lower than normal in all three biomes (Fig. 1B). March 1990 was also at the end of a period of positive NDVI anomalies and before a short period of negative anomalies for the dry forest and xeric shrubland. The NDVI pattern was not clear for the moist forest where the epidemics started (Fig. 1C). Conditions during the 2008 epidemic were almost the reverse as it occurred during a cold (positive) anomaly of SOI, when rainfall was close to normal in the moist forest and somewhat higher than normal in the two other biomes.
Fig. 1.
Environmental conditions (monthly average) in the main Malagasy biomes with respect to the 1990–1991 and 2008–2009 RVF epidemics. (A) Standardized southern oscillation index (19). (B) Standardized rainfall anomalies. Source: National Oceanic and Atmospheric Administration (NOAA)’s precipitation reconstruction over Land (PREC/L) provided by the NOAA/Office of Oceanic and Atmospheric Research/Earth Sciences Research Laboratory - Physical Sciences Divisions, Boulder, CO. (C) Standardized NDVI anomalies. Source: Global Inventory Modeling and Mapping Studies (GIMMS), Advanced Very High Resolution Radiometer (AVHRR) NDVI version 3 (20). On each plot, favorable conditions for RVF are shown in red.
Fig. S2.
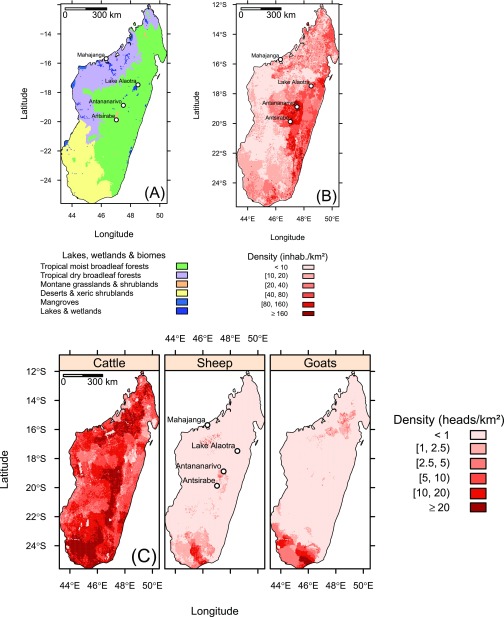
Biomes (A) and human and ruminant population densities (B and C), Madagascar. Sources: Olson et al. (44) for the biomes, Tatem et al. (51) for the human population, and Robinson et al. (53) for ruminant populations.
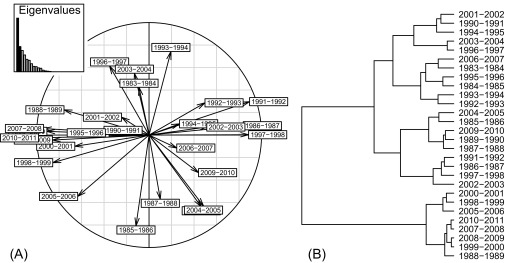
These general impressions were corroborated by the results of partial triadic analysis and hierarchical clustering (Fig. S3): The environmental conditions of RVF epidemics fell into quite different clusters in 1990–1991 and in 2008–2009: With a three-class partition, the 1990 epidemics occurred in a category of rainy seasons with close-to-normal mean values for SOI (0.01), rainfall (0.16), and NDVI (−0.11). In 2008, the epidemic fell into a category with a high mean SOI (1.38) and close-to-normal mean values for NDVI (0.07) and rainfall (−0.16).
Fig. S3.
Typology of 28 rainy seasons (1983–2011) in the dry-forest biome of Madagascar, according to anomalies of the SOI, of rainfall and of NDVI. (A) Correlation circle of the 28 rainy seasons. (B) Dendrogram of a hierarchical cluster analysis on rainy seasons.
RVFV spread at the end of 2008 epidemic.
We modeled the sero-prevalence rate of immunoglobulins of type G (IgG) in ruminant sera collected at the end of the 2008 epidemic to assess the role of environmental conditions in RVF spread in livestock. The subset of plausible models according to the available data is shown in Table S1. The importance of environmental predictors with respect to the selected subset of plausible models is displayed in Table S2. Multimodel averaged coefficients are shown in Table S3. Higher rainfall, municipalities within 50 km from a sea port, and lower altitude were associated with higher sero-prevalence rate in ruminants. See SI Results and Figs. S4 and S5 for details on exploratory data analysis.
Table S1.
Binomial logistic regression models of anti-RVFV IgG sero-prevalence rate in cattle, Madagascar ( = 4,427 sera) selected according to AICc
| Variables | df* | log(likelihood) | AICc | Weight | |
| 1,3,4,6 | 7 | -315.9 | 646.7 | 0.00 | 0.252 |
| 1,2,3,4,6 | 8 | -315.0 | 647.2 | 0.49 | 0.198 |
| 1,2,3,4,5,6 | 10 | -313.1 | 648.1 | 1.41 | 0.125 |
| 1,3,4 | 6 | -318.0 | 648.7 | 1.96 | 0.095 |
| 1,2,3,4,5 | 9 | -314.7 | 649.0 | 2.26 | 0.081 |
| 1,2,3,4 | 7 | -317.0 | 649.0 | 2.28 | 0.081 |
| 3,4,6 | 5 | -319.7 | 649.9 | 3.16 | 0.052 |
| 2,3,4,6 | 6 | -319.0 | 650.6 | 3.93 | 0.035 |
| 1,3,4,5,6 | 9 | -315.6 | 650.7 | 4.02 | 0.034 |
| 1,3,6 | 6 | -319.4 | 651.4 | 4.72 | 0.024 |
| 1,3,4,5 | 8 | -317.6 | 652.4 | 5.64 | 0.015 |
| 1,2,3,6 | 7 | -319.3 | 653.6 | 6.91 | 0.008 |
Labels of variables: 1, average altitude; 2, 10-d average NDVI; 3, 10-d average rainfall; 4, municipality within 50 km of the nearest sea port; 5, 10 d average DLST; 6, municipality-level water/surface ratio.
Degrees of freedom.
Table S2.
Importance of predictors in the averaged beta-binomial logistic regression model of anti-RVFV IgG sero-prevalence rate in ruminants
| Predictor | Importance | No. of models |
| Rainfall | 1.000 | 12 |
| Within 50 km of a seaport | 0.968 | 10 |
| Altitude | 0.913 | 10 |
| Water surface ratio | 0.728 | 8 |
| NDVI | 0.529 | 6 |
| DLST | 0.255 | 4 |
Table S3.
Coefficient of the averaged beta-binomial logistic regression model, selected according to AICc, to explain the RVFV IgG sero-prevalence rate in cattle, Madagascar
| Term | Estimate | SE | value | |
| Intercept | -1.474 | 1.095 | -1.347 | 0.1780 |
| Alti2 | -0.719 | 0.258 | -2.782 | 0.0054 |
| Alti3 | -0.885 | 0.359 | -2.466 | 0.0137 |
| Rainfall | 0.058 | 0.012 | 5.040 | 0.0000 |
| Buffer 50 km | -0.858 | 0.306 | -2.802 | 0.0051 |
| Water surface ratio | 1.219 | 0.601 | 2.028 | 0.0425 |
| NDVI | -1.963 | 1.386 | -1.416 | 0.1566 |
| Daytime temp2 | -0.306 | 0.249 | -1.230 | 0.2186 |
| Daytime temp3 | -0.557 | 0.352 | -1.581 | 0.1138 |
Altitude (Alti) and DLST were split into three equal-size categories defined by their 1/3 quantiles numbered 1 (reference category), 2, and 3. The municipality-level water/surface ratio was transformed into its square root. Municipalities located within 50 km from a seaport were coded as 0 (reference category); others were coded as 1.
Fig. S4.
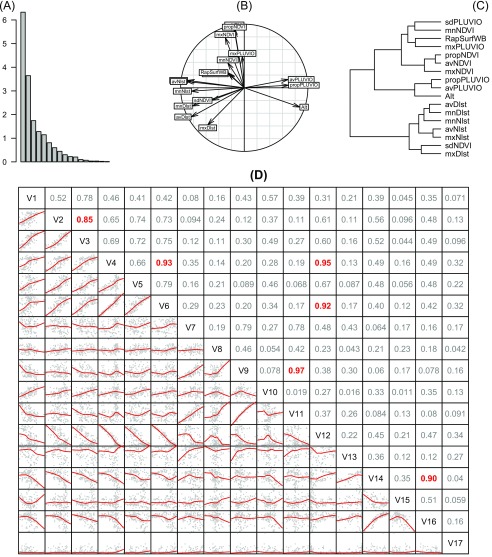
Exploratory data analysis for the 18 predictors of sero-prevalence rate in cattle. (A–C) PCA (A) eigenvalues, (B) correlation circle for the variables, and (C) hierarchical clustering on the PCA scores. (D) Matrix of scatter plots: variable names on the diagonal: V1, mxDlst; V2, mnDlst; V3, avDlst; V4, mxNlst; V5, mnNlst; V6, avNlst; V7, mxNDVI; V8, mnNDVI; V9, avNDVI; V10, sdNDVI; V11, propNDVI; V12, Alt; V13, mxPLUVIO; V14, avPLUVIO; V15, sdPLUVIO; V16, propPLUVIO; V17, RapSurfWB. Below the diagonal, red curves are loess smoothing lines. Above the diagonal, numbers are the linear correlation coefficients, in red for .
Fig. S5.
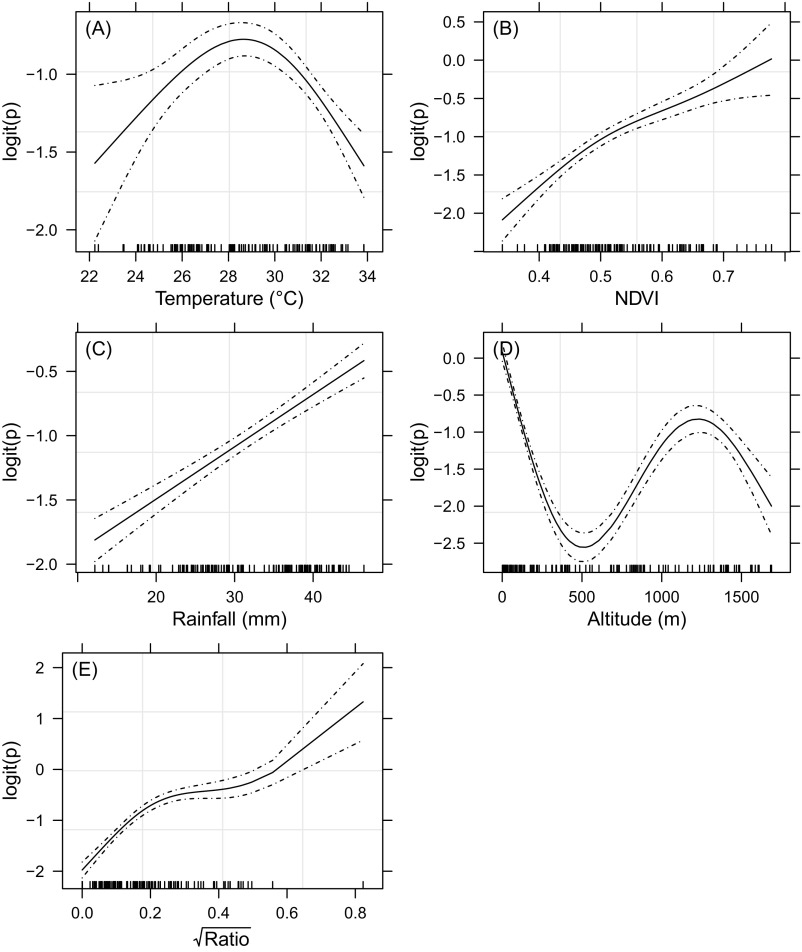
Trend in anti-RVFV IgG sero-prevalence rate in cattle (logit scale) as a smoothing-spline function of the predictor. (A) Average DLST. (B) Average NDVI. (C) Average monthly rainfall. (D) Altitude. (E) Square root of the surface water ratio. Observed data are indicated by small tick marks on the axis; the solid and dashed lines show the trend and its 95% CI.
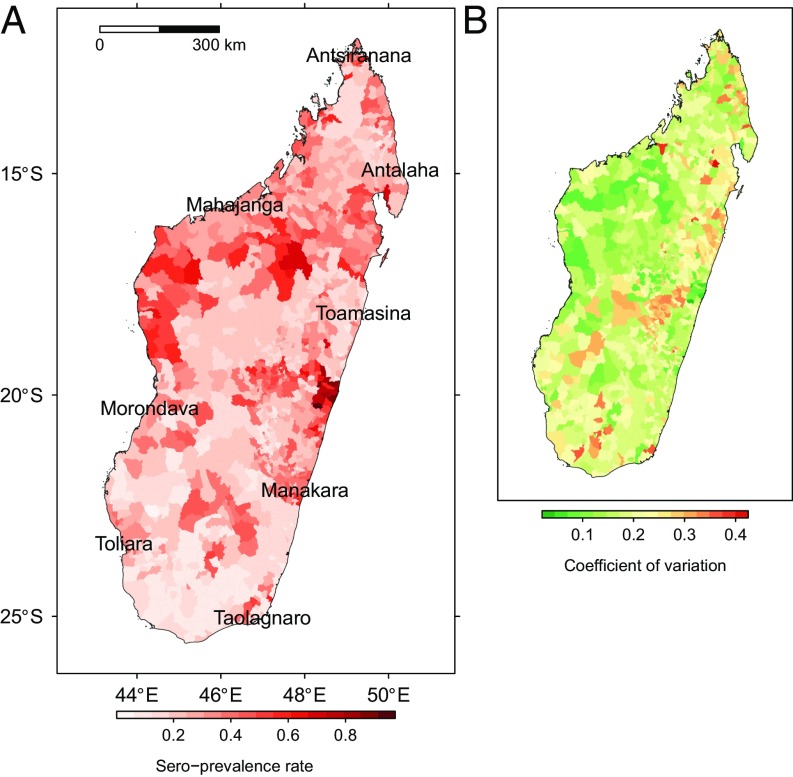
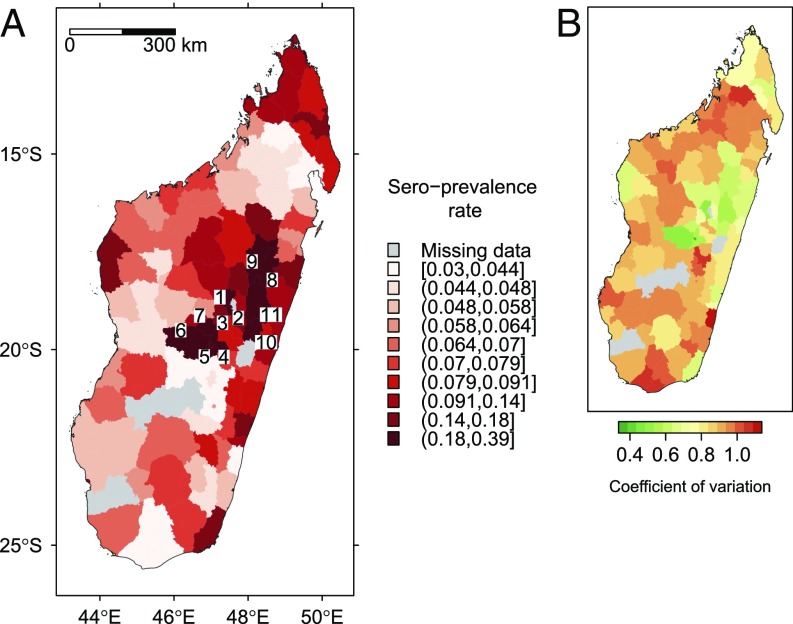
The receiver operating characteristic (ROC) curve for the averaged model had an area under the curve of 74%. The map of predicted sero-prevalence rate showed high-risk areas on the northwestern and northeastern coasts (lowlands). Southern regions and highlands were less affected with the exception of sea-port municipalities (Fig. 2). See SI Results for details.
Fig. 2.
Sero-prevalence rate of anti-RVFV IgG in ruminants (Madagascar) predicted by the averaged beta-binomial logistic regression model: (A) predicted rate and (B) coefficient of variation.
The Cattle Trade Network and RVFV Dissemination.
Cattle trade data were collected monthly from 2007 to 2011, with large variations in numbers across the years. The overall network activity is presented in Fig. S6A: Each segment corresponds to a link between two nodes; a segment is drawn if at least one movement has been recorded along that link on a given month; its color is related to its recorded frequency during the 5-y survey. To assess the influence of the cattle trade network on the risk of RVFV in humans, we quantified the trade flows using the most comprehensive dataset, collected in 2010. This network had the largest number of nodes (257 farms, markets, and slaughterhouses) and links (346). We could distinguish two sets of links: those active throughout the year (56% of the links) constituting the backbone of the network and those occasional links active for 1 mo or 2 mo. Despite their large number, only a small volume (3%) of animals was traded on these occasional links. Most of the backbone nodes and links active in 2010 were already present in the previous year.
Fig. S6.
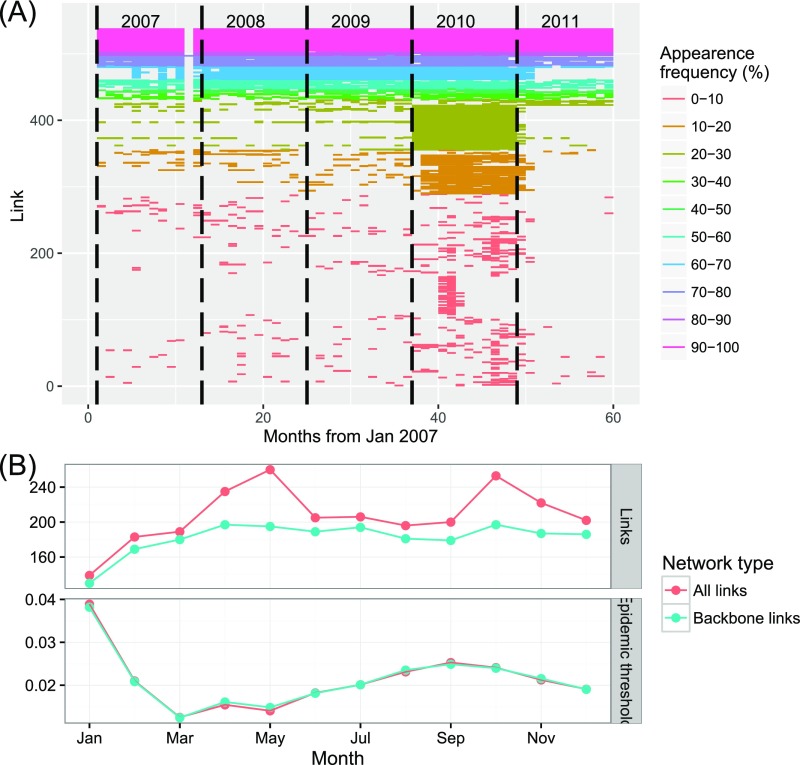
(A) Monthly activity (number of links) in the cattle trade network, Madagascar. Each colored line on the graph corresponds to a link. The color of the line corresponds to the frequency of movements on that particular link during the 5 y of the survey. (B, Top) Number of links for the monthly networks. (B, Bottom) The corresponding epidemic threshold. The color corresponds to the type of network considered: “Backbone link” is for the backbone structure alone; “All links” also includes the occasional links.
To assess the role of the occasional links on RVFV spread, we considered separate monthly network snapshots and we evaluated the corresponding epidemic threshold (21), which provides an estimate of the critical probability for the virus to spread through the network: The lower its value is, the higher the risk of spread. The backbone network was the smallest in January (rainy season) and then increased until reaching its plateau size in April (early dry season) (Fig. S6B). The number of occasional links peaked in May and October. Despite these large variations, was slightly affected by the presence of these occasional links. Conversely, it was strongly influenced by the variations of trade volume in the backbone network. The whole network was prone to the diffusion of RVFV: The average epidemic threshold was , the period of maximum risk from March to May, with , and the minimum risk () in January.
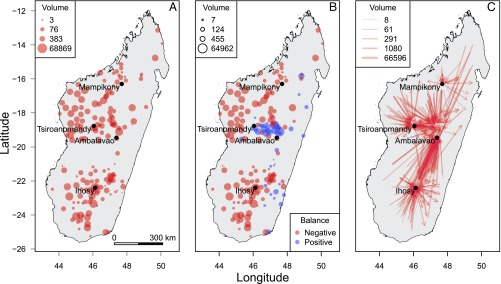
The distribution of in/out degrees (number of incoming/outgoing links for a given node) matched a power-law distribution. Only a few nodes (Ihosy, Tsiroanomandy, Ambalavao, and Mampikony) were highly connected hubs and also had the highest betweenness. The four network hubs belonged to three different giant strong components (GSC)—Ambalavao and Ihosy were in the same one. Nodes belonging to these GSC were strongly connected between themselves, thus increasing the risk of infection for other locations in their neighborhood (22). These hubs were also cut points in the network: Their removal would disrupt the network connectedness and thus limit the diffusion of an infectious agent like RVFV.
The pastoral areas of southwest and northwest of Madagascar were the major sources of the traded cattle (Fig. 3A) and were characterized by high cattle density and low human density. The great majority of markets had a negative balance in cattle flows: They were sources in the network, with low local consumption (Fig. 3B). On the other hand, two hubs (Ambalavao and Tsiroanomandy) had a large, positive balance. Further information is provided on Fig. 3C: Cattle were collected in the markets of Tsiroanomandy and Ihosy and then sent to Ambalavao for slaughtering, Tsiroanomandy also being a consumption center. In addition, a close examination of these four hubs showed that many small, outgoing flows offered numerous opportunities for long-distance RVFV spread.
Fig. 3.
Cattle flows in the Malagasy cattle trade network, 2010. (A) Municipality of origin (number of flows). (B) Balance in cattle flows at the municipality level (number of heads). (C) Directed flows (number of heads).
Cattle Trade and the Risk of RVFV Infection in Humans.
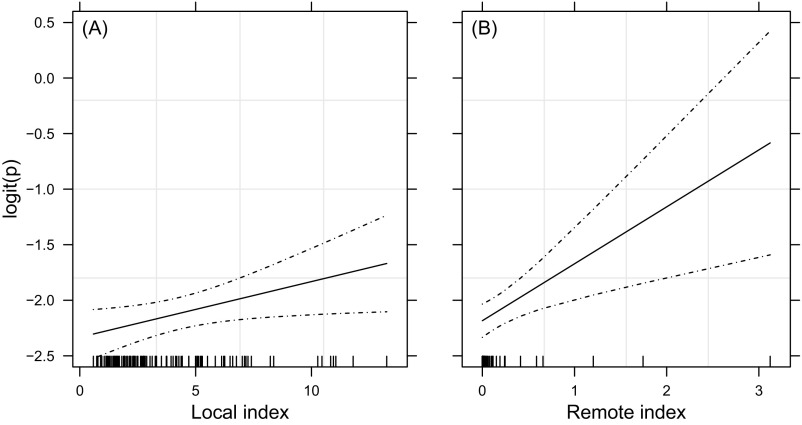
The coefficient for the local, environment-related index of RVFV transmission to humans was not significant, in contrast to the coefficient for the remote, cattle trade-related index (Table S4 and Fig. S7). The odds ratio (OR) for the latter was ORC = 1.7 (95% confidence interval (CI): [1.1; 2.7]). The risk of humans getting infected with RVFV increased with the intensity of cattle trade from areas with infected livestock.
Table S4.
Fixed-effect coefficients for the beta-binomial logistic regression model of sero-prevalence rate of anti-RVFV IgM in humans ( = 1,975 sera)
| Term | Estimate | SE | value | P () |
| Intercept | -2.351 | 0.186 | -12.608 | |
| Local risk index | 0.017 | 0.039 | 0.425 | 0.671 |
| Cattle trade-related index | 0.540 | 0.229 | 2.354 | 0.019 |
Fig. S7.
Trend in anti-RVFV IgM sero-prevalence rate in humans (logit scale) as a smoothing-spline function of the RVF risk index. (A) Local, environment-related index. (B) Remote, cattle trade-related index. The scale is common to A and B. Observed data are indicated by small tick marks on the axis; the solid and dashed lines show the trend and its 95% CI.
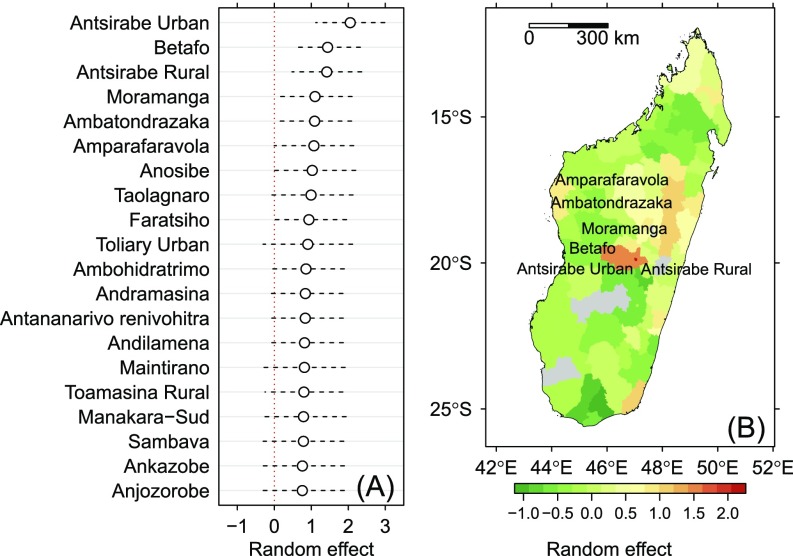
The map of predicted sero-prevalence rate in humans (Fig. 4) and the plot of random variations (Fig. S8) both highlighted large spatial variations in the infection rate, with a clear spatial pattern. The highest sero-prevalence rates were encountered in the densely populated areas of central highlands. This pattern is emphasized in Fig. S8B: A strong additional risk (with respect to cattle trade-related risk) was found in the regions of Antsirabe and Lake Alaotra. See SI Results for details.
Fig. 4.
Sero-prevalence rate in humans predicted by a mixed-effect binomial logistic regression model: (A) predicted rate and (B) coefficient of variation. In A, numbers were placed at the centroid of districts with the highest predicted rates (10th decile): 1, Ambohidratrimo; 2, Antananarivo Renivohitra; 3, Antananarivo-Sud; 4, Antsirabe Rural; 5, Antsirabe Urban; 6, Betafo; 7, Faratsiho; 8, Ambatondrazaka; 9, Amparafaravola; 10, Anosibe; and 11, Moramanga.
Fig. S8.
Random variations around the population means predicted by the mixed-effect logistic regression model of sero-prevalence rate in humans. (A) Estimates and 95% CI for the 20 highest random variations. (B) Geographical variations. Named districts are those with a random variation significantly >0 ().
SI Results
Ruminant Sero-Prevalence Rate.
The estimated sero-prevalence rate in cattle was 25.9% (), 95% CI [24.6; 27.2]. PCA and correlation plots on the set of explanatory variables showed high correlations between many of these variables (Fig. S4). Out of 18 possible predictors, 5 were kept for modeling: altitude, average DLST, average NDVI, average monthly rainfall, and proportion of municipality area covered by water bodies. Further analyses on the selected predictors revealed nonlinear relationships between the predictors and the response on the logit scale (Fig. S5). Therefore, we cut altitude and DLST into three categories defined by their 1/3 empirical quantiles. Also, we transformed the proportion of municipality area covered by water bodies into its square root. To account for the risk of RVFV introduction through sea trade, we added a predictor taking the value 0 if a given municipality was within 50 km of a sea port and 1 elsewhere.
Pearson’s test for the full BBLR model with the six predictors did not allow rejecting the null hypothesis of a good fit: , df = 118, . We selected the subset of models with AICc difference <7 with respect to the AICc-best model. This subset included 12 models that were used for model averaging (Table S1).
Human Sero-Prevalence Rate.
The observed sero-prevalence rate of IgM was 10.6% (, 95% CI [8.4%; 12.9%]). Results of exploratory data analysis are shown in Fig. S7. These indexes were the predictors in a BBLR model of human sero-prevalence rate. Pearson’s test for the overall goodness of fit did not allow rejecting the null hypothesis that this model correctly fitted the data (, df = 101, ). The external validation test was successful (, ). Model coefficients are reported in Table S4. The coefficient for the local index was not significant (), conversely to the coefficient for the remote, cattle trade-related index ().
A MBLR model was then used to study the variance components of sero-prevalence rate. It had the same fixed effects as the BBLR (local and cattle trade-related indexes) and two nested random effects associated with the intercept: (district: ) and (region: ). The standard deviations of and were large and significantly >0 (): , 95% CI [0.35; 0.90], , 95% CI [0.21; 0.91].
Discussion
Cattle Trade and the Risk of RVFV Introduction.
Imports of live ruminants from Comoros were the main driver for RVFV introduction to Madagascar livestock and subsequent trade-related movements of cattle led to its spread to humans, at least in 2008. Phylogenetic studies showing that Malagasy RVFV were closely related to viruses previously circulating in mainland Africa (15) suggest that RVFV was probably introduced into the Comoros Islands through cattle trade with East Africa (23). The existence of illegal livestock importation from the Comoros Islands provided opportunities for the introduction of RVFV in Madagascar. Preventing such introductions is therefore essential to avoid further RVF epidemics in Madagascar. In practice, strengthening communication between African, Comoros, and Malagasy public health and veterinary services would be important to share early detection in the event of new RVFV circulation. Also, quarantine measures should be reinforced for ruminants exported from continental Africa to the Comoros Islands.
Environmental Conditions and RVF Epidemics.
Anyamba et al. (14) has already pointed out the contrasting behavior of the disease in Madagascar and East and South Africa. Prolonged heavy rainfall and positive NDVI anomalies occurred after the first known epidemics of 1990, e.g., in 1994 with the occurrence of the Geralda cyclone (24). More recently, torrential rainfall occurred in February–March 2015 in Madagascar, related to the strong El Niño conditions (25). No RVF outbreak could be detected despite specific surveillance measures in 1994 (shortly after the 1990–1991 RVF epidemic) and strengthened national and regional surveillance in 2015 (26, 27).
RVF epidemics usually start in the arid environments of dambos (East Africa) and/or pans (southern Africa) (1), which are similar to ecosystems in the Toliara region of southwest Madagascar that are covered by xeric shrublands (Fig. S2A). Although this sea-port municipality was hit by RVF, the sero-prevalence rate in ruminants was low in the neighboring municipalities (Fig. 2), probably because the virus was introduced in suboptimal conditions for the vectors (no heavy rainfall, no flooding). Conversely, high sero-prevalence rates were observed in cattle in the northwest of the island, covered by the dry forest. Moreover, in East Africa, Aedes mosquitoes are the primary RVFV vectors (1): Their biology and ecology are well adapted to arid environments. Regarding Madagascar, 23 mosquito species might be considered as potential RVFV vectors, including no floodwater Aedes mosquito species (28–30). A single species, Culex antennatus, meets all criteria for formal classification as an RVFV vector (31). This mosquito is widespread in Madagascar (except in the North), including in rice paddies that cover large areas of the island. The introduction of infected animals in conjunction with Culex hatching, during a standard rainy season profile, might have amplified the outbreak locally, in many places.
Two major differences are thus highlighted between Madagascar and East Africa: (i) the lack of connection between the start of 1990–1991 and 2008–2009 RVF epidemics and El Niño events and, more generally, with anomalous heavy rainfall and (ii) no obvious role of Aedes mosquitoes in the primary RVFV transmission cycle, as well as the wide distribution of Culex (and other mosquito species).
Apparently, the climatic conditions observed during the two epidemics are common in Madagascar: The drivers triggering RVFV epidemics must therefore be sought elsewhere. Nevertheless, these climatic conditions remain important for the amplification of the primary epidemiological cycle between mosquitoes and ruminants (Table S2).
Cattle Trade as a Driver of RVF Epidemics.
The Malagasy population is growing fast, from 16 million to 24 million between 2000 and 2015 (16), and is concentrating in Antananarivo and other large cities (Fig. S2B). Cattle are omnipresent in Malagasy agriculture, economy, and culture. This leads to an ever-growing demand for cattle meat and draught power for crops. Consequently, the increasing cattle trade provides more opportunities for RVF epidemics to spread (7, 8). Our description of the national cattle trade network strongly supports this assumption and further extends a previous analysis in the North of Madagascar (8). The connectedness of the cattle trade network and its low percolation threshold make the risk of seeding epidemics high.
Lake Alaotra and Antsirabe (Fig. S2A) are two major crop and livestock farming regions. The former is the largest rice-production basin in Madagascar, with many paddies and swamps favorable to mosquito proliferation. The two regions are densely populated, with cities harboring big livestock markets and slaughterhouses to match local red meat needs. Therefore, there are many opportunities for human exposure to the RVFV when viremic animals are slaughtered.
Additionally, using the network of markets, butcheries, and hotely (cheap restaurants for travelers), farmers try to sell their sick animals at the first clinical sign of any disease, to mitigate economic losses. When they do not succeed, they slaughter them and eat their meat (32). This practice was probably at the origin of many unreported human infections during the 2008–2009 RVF epidemics in Madagascar.
More collaboration with sociologists and anthropologists is needed to decipher farmers’ perceptions of animal diseases and to assess the social acceptability of prevention, surveillance, and control measures, such as cattle vaccination to protect people (should enough vaccine be available) or cattle movement restrictions to avoid RVFV spread through hubs in the cattle trade network (33). If RVFV introduction into Malagasy livestock continues, targeted (risk-based) vaccination campaigns of ruminants might be organized to protect human populations. Individual protection measures, such as vaccination when the human vaccine becomes available or wearing personal protective equipment such as gowns, gloves, safety glasses, and masks when slaughtering ruminants would also be important to implement in the most exposed categories of people (34, 35), together with dissemination and training programs. This implementation of coordinated actions between Public Health and Veterinary Services would represent an important advance in the so-called “One Health” joint approach of human and animal health (27, 36).
Materials and Methods
To assess the risk of RVFV introduction, we scanned national and international databases on sea trade from 2003 to 2008. We also implemented informal surveys in harbors of the Comoros Islands and northwestern Madagascar during and after this epidemic (up to 2010).
To describe the environmental conditions of the past RVF epidemics, we used global datasets available as long-term time series: (i) the SOI as a main indicator of ENSO of major importance for the climatic conditions in East Africa and the southwest tropical Indian Ocean (3); (ii) rainfall data, of crucial importance for the epidemiology of vector-borne diseases (37); and (iii) the NDVI as an indicator of rainfall impact on the vegetation (38): forage resources for ruminants and resting sites for mosquitoes. These indicators were averaged over the main biomes (SI Materials and Methods). First, we plotted the time series for each biome. Second, we selected data from the dry-forest biome where the highest sero-prevalence rate was observed in cattle in 2008 (39). We then used partial triadic analysis (PTA), a multitable version of principal component analysis (PCA) (40), to identify common or contrasted patterns in rainy seasons (November to March) from 1983 to 2011 (28 y).
To assess the risks of RVFV spread within Madagascar, we used two types of data: (i) environmental data selected among the factors of interest for mosquito-borne infections, i.e., related to the availability of resting or breeding sites or the seasonal changes in conditions favoring the development of their immature stage, etc. (37) (see the list in SI Materials and Methods), which were obtained from international databases and processed using standard methodology; and (ii) cattle trade data collected during repeated nationwide surveys in livestock markets and slaughterhouses, implemented from 2007 to 2011 for the purpose of this study.
Environmental data were used in beta-binomial logistic regression (BBLR) models to predict the RVFV sero-prevalence rate for cattle at the municipality level. The serological data were collected after the 2008 epidemics and published (39). Sera were tested for the presence of anti-RVFV IgG. We adopted a multimodel inference approach to select the best predictors. A set of plausible models was kept for model averaging and prediction of sero-prevalence rate in cattle at the municipality level.
Cattle trade data were used (i) to assess the risk of RVFV spread through the network and (ii) to assess the risk of human infection with RVFV. Farms, markets, and slaughterhouses were the nodes of a directed cattle trade network. A link between two nodes corresponded to a trade movement of animals. The direction indicated the origin and destination places, and the volume was the number of traded animals.
We assessed the risk of RVFV infection in humans, using sera collected during a nationwide survey of slaughterhouse workers performed in 2008 and 2009. The study was approved by the Malagasy National Ethical Committee. Participants were included if they gave written informed consent. Sera were tested for the presence of anti-RVFV IgM, indicating a recent infection, and the results were published (13). We defined two indexes for the risk of RVFV infection in humans: (i) We created an index of local RVFV transmission risk, defined by the product of local predicted sero-prevalence rate in cattle and cattle density. This index was built to capture the risk associated with the primary RVFV epidemiological cycle involving mosquitoes and domestic ruminants, in the absence of known wild hosts for RVFV in Madagascar (41, 42). Humans are not involved in this cycle. Therefore, their density was not considered in the index. (ii) We created an index of RVFV transmission risk related to cattle trade. It was defined as the product of cattle incoming flow (number of head), predicted sero-prevalence rate in cattle at the origin, and local human density. This latter variable was included because meat consumption is proportionally higher in urban than in rural areas (43), with higher densities of slaughterhouses, butchers, and meat markets. Consequently, the risk of RVFV infection related to cattle trade should be positively correlated with human density.
The effect of these two indexes on human sero-prevalence rate was assessed with a BBLR model. Finally, a mixed-effect binomial logistic regression model was used to study the local variations of sero-prevalence rate in humans, with the administrative district and region as the nested random effects associated with the intercept. See SI Materials and Methods for detailed information on data sources and statistical methods, as well as links to download the datasets used in the analyses.
SI Materials and Methods
Data.
Environmental conditions of RVF epidemics.
Three variables were used to characterize the environmental conditions of RVF epidemics in Madagascar: (i) the SOI, (ii) rainfall, and (iii) NDVI. SOI is a standardized index based on the observed sea-level pressure differences between Tahiti and Darwin, Australia. Its negative (warm) anomalies are often associated with RVF epidemics in eastern Africa (3). Other indicators related to the sea-surface temperature (SST) in the western tropical Indian Ocean, such as the dipole mode index (4), account for a lower proportion of SST variability; they were not considered here. We used time series of standardized values (data expressed in standard deviations) for each of these indicators: Southern oscillation index provided by National Centers for Environmental Information (NOAA) (19); for rainfall, NOAA’s PREC/L provided by the NOAA/OAR/ESRL PSD, Boulder, CO; and NDVI: GIMMS AVHRR NDVI version 3 (20).
Madagascar is a large island (1,580 km long, 590,000 km2) with contrasted climatic and other environmental conditions. We computed standardized rainfall and NDVI for each of the three main biomes in terms of land cover: (i) tropical and subtropical moist broad-leaf forests (referred to as “moist forest”), located on the eastern coast and highlands of Madagascar; (ii) tropical and subtropical dry broad-leaf forests (referred to as “dry forest”), located in the northwestern part of the island; and (iii) deserts and xeric shrublands (referred to as “xeric shrublands”), covering the southwestern region of Madagascar (Fig. S2A). The definition and geographical distribution of these biomes were taken from Olson et al. (44) and associated datasets.
Sero-prevalence data in ruminants.
Data were kindly provided by Malagasy Veterinary Services, Pasteur Institute of Madagascar, and Food and Agriculture Organization of the United Nations. A partial analysis of these data has been published (39). They were collected during the last RVF epidemic in 2008 in Madagascar. Animals were sampled in local livestock market places or before slaughtering. Their birth places were recorded at the municipality level. In total, 316 municipalities were involved in this survey. Blood was taken from 4,427 ruminants, i.e., 3,438 cattle, 227 sheep, and 762 goats. Sera were tested for the presence of anti-RVFV antibodies—immunoglobulins of type G (IgG) and M (IgM), with commercial enzyme-linked immunosorbent assays (ELISA). IgG appear soon after the rise of IgM and persist much longer: Animals surviving a RVF epidemic can develop a life-long immunity to RVF infections (45). In our sample, the median age of animals was 4 y, ranging from 3 mo to 19 y; 357 animals were born during the 2008–2009 RVF epidemic, and only 8 animals were born during or before the 1990–1991 RVF epidemic. Therefore, the risk of sampling animals infected during this earlier epidemic was limited. In addition, results of phylogenetic studies of viruses sampled during epidemics, of event-based surveillance, and of cross-sectional and prospective serological surveys in cattle did not provide strong evidence of long-term and widespread persistence of RVFV circulation between successive epidemics in Madagascar (15, 34). Therefore, we used IgG for data analysis, considering that their sero-prevalence was a good indicator of RVFV infection during the 2008–2009 epidemic.
Sero-prevalence data in humans.
We used the dataset described and analyzed by Andriamandimby et al. (13). A nationwide cross-sectional serological survey was performed in 2008 and 2009, which was approved by the Malagasy National Ethical Committee. It covered people at risk for RVFV exposure in all 111 districts of Madagascar. Participants were included if (i) they had been working in slaughterhouses located in the district since 2007 or had been exposed to fresh meat or blood of ruminants and if (ii) they had been residents of the district when they started this work. Five milliliters of blood was then collected from each participant and samples were tested for anti-RVFV IgG and IgM, using ELISA (46). Because IgG may persist more than 10 y in humans after a natural infection with RVFV, we used IgM serological prevalence rate as the response in this analysis.
Environmental data.
We used remotely sensed data as detailed below and computed their average value over the area of each municipality.
We used elevation data with a 90-m resolution, as a general indicator of climatic conditions and agro-ecosystem. This dataset was available from the Consortium for Spatial Information of the Consultative Group for International Agricultural Research (CGIAR-CSI) (47).
Water bodies and vegetation are key variables for the development of mosquitoes and the abundance of their ruminant hosts. Surface water is necessary for the larval development of mosquitoes and for watering cattle and small ruminants in pastoral areas. We calculated the maximum proportion of water bodies in each municipality during the study period.
Live green vegetation, characterized by NDVI, is important for adult mosquito mating and resting. Also, NDVI is an indicator of relative humidity, which is a major factor for mosquito survival. And finally, NDVI is related to the amount of vegetal biomass that is used by ruminants to cover their nutritional needs. We computed minimum, average, maximum, and SD of NDVI, together with the proportion of decades with an average value greater than the national average.
Water bodies and NDVI datasets were available from the GEONETCast project and its data repository (48).
Temperature is a major factor for mosquito activity, development, and survival, as well as for the kinetics of the extrinsic virus cycle in RVF-infected mosquitoes (37). We used two moderate resolution imaging spectroradiometer (MODIS) 8-d composite datasets provided by the National Aeronautics and Space Administration (NASA) (49): nighttime land surface temperature (NLST) and daytime land surface temperature (DLST). We used minimum, maximum, and average NLST and DLST values.
Rainfall is important for the larval development of mosquitoes and for the development of herbaceous vegetation (37). We used 10-d composite data provided by the Malaria Early Warning System (MEWS) (50). We extracted minimum, average, maximum, and SD of rainfall precipitation, together with proportion of decades with an average value greater than the national average.
Population data.
We used a raster dataset (≃100 m spatial resolution) of the 2010 Malagasy human population (51) kindly provided by the program WorldPop (52) (Fig. S2B). We computed the total human population in each municipality.
For ruminant population, we used the Gridded Livestock of the World (GLW) database, which provides modeled livestock densities of the world, adjusted to match official national estimates for the reference year 2010, at 1-km spatial resolution (53) (Fig. S2C).
Ruminant trade.
We assumed RVFV might have been introduced in Madagascar via international livestock trade. Looking for data to support this assumption, we queried the United Nations’ ComTrade database (54), a repository of official trade statistics. It contains annual trade statistics starting from 1962 (and monthly trade statistics since 2010). Because (i) a retrospective serological survey using a cattle serum bank showed that RVFV has been circulating in Comoros Archipelago at least since 2004 (18) and (ii) a large RVFV epidemic started in Somalia in 2006 and reached northern Tanzania 1 y later (14, 55, 56), we started the queries in 2005, looking for cattle and small ruminant importations.
We also investigated sea trade data, particularly coastal trade with small freighters and botry (dhows). Because many coastal areas are poorly served by terrestrial roads, a lot of goods—including live animals—are shipped by sea. First, in 2010, we conducted interviews with dhow captains in the main harbors of Comoros Archipelagos and northwestern Madagascar (Mahajanga, Nosy Be, Antsiranana) to assess whether live ruminants are shipped from the Comoros Islands to Madagascar and between Malagasy harbors. Second, we used published data on sea trade traffic between Malagasy harbors to identify a possible RVFV entry point (57).
Nationwide cattle movements were collected by field veterinary officers from 2007 to 2011. Data were taken from trade registries held for tax purposes in primary and secondary cattle markets. Also, a questionnaire survey was implemented by field veterinary officers during the same period with farmers and livestock traders bringing their animals into slaughterhouses. All of the data were cleaned, checked, geo-referenced, and stored in a PostgreSQL database (The PostgreSQL Global Development Group). Elementary data (description of a cattle movement) encompassed the date, origin, destination, source type (farmer, livestock market, or slaughterhouse), and volume (number of animals involved in the movement).
Data availability.
All of the environmental and population data are freely available, following indications given in SI Materials and Methods. The borders of Madagascar administrative units needed for the analyses (municipalities, districts, and regions) are freely available from the GADM database of Global Administrative Areas. Other datasets are available following these links: Coastal navigation data are available at https://figshare.com/s/73ffd8ff3d3011c8e741. Cattle trade data are available at https://figshare.com/s/7a1c14c8f0a5ccb7e7b8. Animal sero-prevalence data are available at https://figshare.com/s/f09650b3309a1a944f15. Correspondence between IDs used in animal sero-prevalence data and in vector layers of administrative units is available at https://figshare.com/s/84924c2fbc37f03f4ab1. Human sero-prevalence data are available at https://figshare.com/s/a0a8ec4be4d78dbd53ac.
Data Analysis.
Partial triadic analysis of climatic variables.
We jointly analyzed a series of 28 tables, each representing a single rainy season. Each table had three columns (SOI, rainfall, and NDVI) and five rows (months from November to March). From these inputs, data were reorganized into new table with the 5 mo three variables (five groups of three) as the rows and 28 rainy seasons as the columns. A PCA was then run on this new table to get the interstructure of the 28 rainy seasons. The correlation circle was drawn to visualize the correlations between the rainy seasons. Finally, their row numbers were used in a hierarchical cluster analysis, and the resulting dendrogram was used to visualize the clusters of rainy seasons.
Modeling ruminant serological prevalence rate.
The goals were (i) to assess whether municipalities located close to a harbor were more at risk for RVFV transmission in cattle than others, (ii) to identify the most important environmental predictors of RVFV transmission in cattle, and (iii) to estimate the population-level sero-prevalence rate in cattle and to map this rate at the municipality level to characterize the risk of RVFV spread through the cattle trade network. For this purpose, we built a statistical model for the sero-prevalence rate of antibodies against RVFV in municipality , computed as the proportion of cattle sera testing positive with ELISA. Predictors were the environmental variables described above. To assess the risk of RVFV introduction and spread related to sea trade, we defined circular buffers of various radii (10 km, 25 km, and 50 km) around sea ports and built a municipality-level indicator, taking the value of 0 if the buffer intersected with the municipality (reference category in regression analyses) and 1 elsewhere. We kept the buffer size minimizing the Akaike information criterion corrected for small-sample size (AICc: see below) in statistical models.
To assess the shape of the relationship between each predictor and the serological prevalence rate, we estimated and plotted the trend in the logit of this rate and a smoothing spline function of the data (58). Those predictors showing a nonlinear trend with the sero-prevalence rate on the logit scale were categorized into three classes according to the 1/3 quantiles. We also checked between-predictor correlations, using PCA on the table of predictors, and hierarchical clustering on the PCA scores. We discarded highly correlated variables (), keeping those with the most straightforward bio-ecological meaning. Sero-prevalence rate was then modeled using BBLR to account for spatial correlation of these rates and their possible overdispersion with respect to the binomial distribution.
For model selection and inference, we adopted an approach based on model comparison with the AICc (59),
with the maximized likelihood, the number of model coefficients, and the sample size. A set of BBLR models was defined using all possible combinations of the selected predictors. Goodness of fit of the most complex model was assessed using Pearson’s test. Models were then ranked according to the difference in AICc with respect to the AICc-best model () and weighted according to their Akaike weight:
Averaged coefficients and standard deviations were computed, as well as confidence intervals for model coefficients and predictions. Averaged BBLR model’s predictive capacity was assessed using the ROC curve, and the corresponding area under this curve (AUC). The averaged model was then used to predict sero-prevalence rates and coefficients of variations for the 1,434 Malagasy municipalities.
Ruminant trade network.
We used the framework of social network analysis (SNA) to describe the links between a set of interconnected so-called nodes, i.e., farmers, livestock markets, or slaughterhouses (60). They were sources or destinations in the cattle movement network. Links were directed: movements from source to destination nodes.
We focused on the 2010 cattle trade network, for which the most comprehensive data were available. These data are represented in Fig. S6A, where each line corresponds to a trade link. A segment was drawn if the link was active in a specific month. The color corresponds to the frequency of activity of the link over 5 y. In 2010, besides the links that were present before, a new set of links appeared and persisted over all the year. Thus, almost 60% of the links were present through all of the years. The remaining links were active only for 2 mo in 2010, at the most.
We described the seasonal and annual distribution of the number of traded cattle, and then we used SNA tools to find flow patterns in the network. Network-centrality measures were computed, such as degrees (in- and out-degrees), betweenness, or closeness, to identify the major nodes with respect to livestock movements and the consecutive risk of disseminating RVFV (61). The average path length provided information about the number of hops to reach a given node. Consequently, it is a measure of the average time before a node can be infected. Correlation between connected nodes and the in-degree () and out-degree() of the nodes were also estimated. Following Volkova et al. (21), we computed the epidemic threshold , which is an estimate of the minimum transmission probability for a newly introduced virus to infect all of the network:
Building indexes of human risk of RVFV infection.
The goals were (i) to build risk indexes of RVFV human infection related to the local environment (local risk) and cattle trade (remote risk), (ii) to assess their relative importance, and (iii) to identify and map high-risk areas.
The local risk was assumed to be proportional to the number of locally infected cattle. Therefore, the local index was defined as the product of the cattle density (heads per square kilometer) and the sero-prevalence rate in cattle predicted by the BBLR model.
The remote risk index was the product of the following three variables:
-
∙
The number of incoming cattle (estimated from the trade data in 2010), aggregated at the district level.
-
∙The average sero-prevalence rate in cattle at the origin. For a given destination (district), we enumerated for the different origins (i) the observed numbers of exported cattle and (ii) the predicted sero-prevalence rates (). Then was computed as
-
∙
The average human density (number of inhabitants per square kilometer) at the district level. This variable was included in the index to account for its anthropogenic nature. As a matter of fact, the socio-economic pattern is quite different in urban and rural populations. Meat consumption is proportionally higher in the former: According to a national survey implemented by the National Institute of Statistics in 2015 (43), meat, fish, and animal products were consumed on average 3.1 d− 1wk− 1 (2.5 d− 1wk− 1) in urban (rural) households (, Wilcoxon test, ). The higher human density combined with higher meat consumption results in a higher density of slaughterhouse workers, butchers, and people exposed to infected blood, blood aerosols, or fresh meat. Therefore, the risk of RVFV infection related to cattle trade should be positively correlated with human density.
These two indexes were included in a BBLR model to assess their adjusted, relative importance. To validate the model, we used the confirmed cases of RVF in humans reported by the national surveillance system (13). The confirmed clinical cases of RVF occurred in 20 districts, mostly located on the highlands. We summed the predicted sero-prevalence rate in humans for these districts, thus forming the value of our statistic of interest. Then, we randomly sampled 20 districts from the list of 105 districts with a predicted sero-prevalence rate and computed the value of the statistic. We iterated the process times, combined the observed and simulated values, and computed , with large values of (e.g., ). The model was validated if .
To study the local variations of sero-prevalence rate in humans around the population mean predicted by the two risk indexes, we used a mixed-effect binomial logistic regression (MBLR) model with two random effects and : the administrative district and the administrative region (grouping level for districts).
The R language and environment for statistical computing was used for data analysis, including plots and maps (62). Specific tasks were accomplished with the following add-on packages: ade4 for PCA and PTA; sna for social network analysis; aods3 and MuMIn for BBLR and multimodel inference; lme4 for MBLR; and sp, rgeos, gdistance, and raster for spatial analyses and mapping. All these packages and their documentation are available on the Comprehensive R Archive Network (https://cran.r-project.org).
Acknowledgments
This study was partially funded by the World Health Organization and the Food and Agriculture Organization of the United Nations through the Central Emergency Response Fund of the United Nations. It was also partially funded by European Union Grant FP7-613996 Emerging Viral Vector-Borne Diseases (VMERGE) and is cataloged by the VMERGE Steering Committee as VMERGE019 (http:/www.vmerge.eu). The contents of this publication are the sole responsibility of the authors and do not necessarily reflect the views of the European Commission.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1607948114/-/DCSupplemental.
References
- 1.Linthicum KJ, Britch SC, Anyamba A. Rift Valley fever: An emerging mosquito-borne disease. Annu Rev Entomol. 2016;61:395–415. doi: 10.1146/annurev-ento-010715-023819. [DOI] [PubMed] [Google Scholar]
- 2.de LaRocque S, Formenty P. Rift Valley fever: Disease ecology and early warning. In: Odongo N, Garcia M, Viljoen G, editors. Sustainable Management of Animal Production and Health. Food and Agriculture Organization of the United Nations; Rome: 2010. pp. 327–333. [Google Scholar]
- 3.Linthicum K, et al. Climate and satellite indicators to forecast Rift Valley fever epidemics in Kenya. Science. 1999;285(5426):397–400. doi: 10.1126/science.285.5426.397. [DOI] [PubMed] [Google Scholar]
- 4.Saji NH, Goswami BN, Vinayachandran PN, Yamagata T. A dipole mode in the tropical Indian Ocean. Nature. 1999;401(6751):360–363. doi: 10.1038/43854. [DOI] [PubMed] [Google Scholar]
- 5.Anyamba A, et al. Prediction of a Rift Valley fever outbreak. Proc Natl Acad Sci USA. 2009;106(3):955–959. doi: 10.1073/pnas.0806490106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chevalier V, Pépin M, Plée L, Lancelot R. Rift Valley fever–A threat for Europe. Euro Surveill. 2010;15(10):19506. [PubMed] [Google Scholar]
- 7.Morvan J, Saluzzo JF, Fontenille D, Rollin PE, Coulanges P. Rift Valley fever on the east coast of Madagascar. Res Virol. 1991;142(6):475–482. doi: 10.1016/0923-2516(91)90070-j. [DOI] [PubMed] [Google Scholar]
- 8.Nicolas G, Durand B, Duboz R, Rakotondravao R, Chevalier V. Description and analysis of the cattle trade network in the Madagascar highlands: Potential role in the diffusion of Rift Valley fever virus. Acta Trop. 2013;126(1):19–27. doi: 10.1016/j.actatropica.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Shoemaker T, et al. Genetic analysis of viruses associated with emergence of Rift Valley fever in Saudi Arabia and Yemen, 2000-01. Emerg Infect Dis. 2002;8(12):1415–1420. doi: 10.3201/eid0812.020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerdes G. Rift Valley fever. Rev Sci Tech. 2004;23(2):613–623. doi: 10.20506/rst.23.2.1500. [DOI] [PubMed] [Google Scholar]
- 11.Anyangu AS, et al. Risk factors for severe Rift Valley fever infection in Kenya, 2007. Am J Trop Med Hyg. 2010;83(2 Suppl):14–21. doi: 10.4269/ajtmh.2010.09-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reed C, et al. Aerosol exposure to Rift Valley fever virus causes earlier and more severe neuropathology in the murine model, which has important implications for therapeutic development. PLoS Negl Trop Dis. 2013;7(4):e2156. doi: 10.1371/journal.pntd.0002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andriamandimby SF, et al. Rift Valley fever during rainy seasons, Madagascar, 2008 and 2009. Emerg Infect Dis. 2010;16(6):963–970. doi: 10.3201/eid1606.091266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anyamba A, et al. Prediction, assessment of the Rift Valley fever activity in East and Southern Africa 2006-2008 and possible vector control strategies. Am J Trop Med Hyg. 2010;83(2 Suppl):43–51. doi: 10.4269/ajtmh.2010.09-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carroll SA, et al. Genetic evidence for Rift Valley fever outbreaks in Madagascar resulting from virus introductions from the East African mainland rather than enzootic maintenance. J Virol. 2011;85(13):6162–6167. doi: 10.1128/JVI.00335-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.TheWorld Bank Group 2015 Countries: Madagascar (The World Bank Group,Washington, DC). Available at www.worldbank.org/en/country/madagascar. AccessedDecember 28, 2015.
- 17.Sissoko D, et al. Rift Valley fever, Mayotte, 2007-2008. Emerg Infect Dis. 2009;15(4):568–570. doi: 10.3201/eid1504.081045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cêtre-Sossah C, et al. Prevalence of Rift Valley fever among ruminants, Mayotte. Emerg Infect Dis. 2012;18(6):972–975. doi: 10.3201/eid1806.111165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.NOAA . 2015. Southern Oscillation Index. Available at https://www.ncdc.noaa.gov/teleconnections/enso/indicators/soi/. Accessed December 30, 2015. [Google Scholar]
- 20.Pinzon JE, Tucker CJ. A non-stationary 1981–2012 AVHRR NDVI3g time series. Remote Sens. 2014;6(8):6929–6960. [Google Scholar]
- 21.Volkova VV, Howey R, Savill NJ, Woolhouse MEJ. Sheep movement networks and the transmission of infectious diseases. PLoS One. 2010;5(6):e11185. doi: 10.1371/journal.pone.0011185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Danon L, et al. Networks and the epidemiology of infectious disease. Interdiscip Perspect Infect Dis. 2011;2011:1–28. doi: 10.1155/2011/284909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maquart M, et al. Phylogeographic reconstructions of a Rift Valley fever virus strain reveals transboundary animal movements from eastern continental Africa to the Union of the Comoros. Transbound Emerg Dis. 2016;63(2):e281–e285. doi: 10.1111/tbed.12267. [DOI] [PubMed] [Google Scholar]
- 24.Fitchett JM, Grab SW. A 66-year tropical cyclone record for south-east Africa: Temporal trends in a global context. Int J Climatol. 2014;34(13):3604–3615. [Google Scholar]
- 25.Monastersky R. Monster El Niño probed by meteorologists. Nature. 2016;529(7586):267–268. doi: 10.1038/529267a. [DOI] [PubMed] [Google Scholar]
- 26.Zeller H, Andriamanana R, Quirin R. 1998. Rift Valley Fever Surveillance in Madagascar, Tech Rep Project IPM/PSE/BM, January 1996–March 1998 (Institut Pasteur de Madagascar, Antananarivo, Madagascar) [Google Scholar]
- 27.Cardinale E, Rasamoelina-Andriamanivo H, Razafimandimby A, Lepec R, Flachet L. A concrete regional “One Health” surveillance system and management of epidemics: A success story in the South West Indian Ocean. In: De Anda JH, editor. ISVEE 14, Yucatan 2015-Veterinary Epidemiology & Economics: Planning Our Future, Session 2: One Health. ISVEE; Merida, Mexico: 2015. [Google Scholar]
- 28.Ratovonjato J, et al. Detection, isolation, and genetic characterization of Rift Valley fever virus from Anopheles (Anopheles) coustani, Anopheles (Anopheles) squamosus, and Culex (Culex) antennatus of the Haute Matsiatra region, Madagascar. Vector Borne Zoonotic Dis. 2011;11(6):753–759. doi: 10.1089/vbz.2010.0031. [DOI] [PubMed] [Google Scholar]
- 29.Nepomichene TJJN, Elissa N, Cardinale E, Boyer S. Species diversity, abundance, and host preferences of mosquitoes (Diptera: Culicidae) in two different ecotypes of Madagascar with recent RVFV transmission. J Med Entomol. 2015;52(5):962–969. doi: 10.1093/jme/tjv120. [DOI] [PubMed] [Google Scholar]
- 30.Tantely LM, Boyer S, Fontenille D. A review of mosquitoes associated with Rift Valley fever virus in Madagascar. Am J Trop Med Hyg. 2015;92(4):722–729. doi: 10.4269/ajtmh.14-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reeves WC. Arthropods as vectors and reservoirs of animal pathogenic viruses. In: Hallauer C, Meyer KF, editors. Handbuch der Virusforschung [Handbook of Virus Research] Springer; New York: 1958. pp. 177–202. [Google Scholar]
- 32.Ribot JJ, Coulanges P. Les zoonoses malgaches [Malagasy zoonoses] Rev Elev Med Vet Pays Trop. 1988;41(1):9–22. [PubMed] [Google Scholar]
- 33.Goutard FL, et al. How to reach the poor? Surveillance in low-income countries, lessons from experiences in Cambodia and Madagascar. Prev Vet Med. 2015;120(1):12–26. doi: 10.1016/j.prevetmed.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 34.Zeller H, Rakotoharinadrasana H, Rakoto-Andrianarivelo M. Rift Valley fever in Madagascar: Infection risks for the abattoir staff in Antananarivo. Revue Elev Méd Vét Pays Trop. 1998;51(1):17–20. [Google Scholar]
- 35.Bausch DG, Senga M. International Encyclopedia of Public Health. 2nd Ed. Vol 2. Elsevier; Amsterdam, The Netherlands: 2017. pp. 396–409. [Google Scholar]
- 36.de LaRocque S, Formenty P. Applying the One Health principles: A trans-sectoral coordination framework for preventing and responding to Rift Valley fever outbreaks. Rev Sci Tech. 2014;33(2):555–567. doi: 10.20506/rst.33.2.2288. [DOI] [PubMed] [Google Scholar]
- 37.Kalluri S, Gilruth P, Rogers D, Szczur M. Surveillance of arthropod vector-borne infectious diseases using remote sensing techniques: A review. PLoS Pathog. 2007;3(10):1361–1371. doi: 10.1371/journal.ppat.0030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anyamba A, Small JL, Tucker CJ, Pak EW. Thirty-two years of Sahelian zone growing season non-stationary NDVI3g patterns and trends. Remote Sens. 2014;6(4):3101–3122. [Google Scholar]
- 39.Jeanmaire E, et al. Prevalence of Rift Valley fever infection in ruminants in Madagascar after the 2008 outbreak. Vector Borne Zoonotic Dis. 2011;11(4):395–402. doi: 10.1089/vbz.2009.0249. [DOI] [PubMed] [Google Scholar]
- 40.Thioulouse J, Simier M, Chessel D. Simultaneous analysis of a sequence of paired ecological tables. Ecology. 2004;85(1):272–283. [Google Scholar]
- 41.Olive M-M, Goodman SM, Reynes JM. The role of wild mammals in the maintenance of Rift Valley fever virus. J Wildl Dis. 2012;48(2):241–266. doi: 10.7589/0090-3558-48.2.241. [DOI] [PubMed] [Google Scholar]
- 42.Olive M-M, et al. Absence of Rift Valley fever virus in wild small mammals, Madagascar. Emerg Infect Dis. 2013;19(6):1025–1027. doi: 10.3201/eid1906.121074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Institut Nationalde la Statistique de Madagascar . 2015. Policy Brief - Les Principaux Résultats sur le tThème “Alimentation et Sécurité Alimentaire” [Main Results for Food and Food Security], Technical Report (INSTAT, Antananarivo, Madagascar). Available at instat.mg/. Accessed May 6, 2016. [Google Scholar]
- 44.Olson DM, et al. Terrestrial ecoregions of the world: A new map of life on Earth. BioScience. 2001;51(11):933–938. [Google Scholar]
- 45.Pépin M, Bouloy M, Bird BH, Kemp A, Paweska J. Rift Valley fever virus (Bunyaviridae: Phlebovirus): An update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet Res. 2010;41(6):61. doi: 10.1051/vetres/2010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madani TA, et al. Rift Valley fever epidemic in Saudi Arabia: Epidemiological, clinical, and laboratory characteristics. Clin Infect Dis. 2003;37(8):1084–1092. doi: 10.1086/378747. [DOI] [PubMed] [Google Scholar]
- 47.The CGIARConsortium forSpatialInformation (CGIAR-CSI) 2015. SRTM 90m Digital Elevation Data, Update Version 4 (International Centre for Tropical Agriculture, Palmira, Colombia). Available at srtm.csi.cgiar.org/. Accessed December 28,2015. [Google Scholar]
- 48.Vito . DevCoCast: The GEONETCast for and by Developing Countries. Flemish Institute for Technological Research NV (Vito); Mol, Belgium: 2015. www.vgt4africa.org [Google Scholar]
- 49.NASA . 2015. MODIS - Moderate Resolution Imaging Spectroradiometer (National Aeronautics and Space Administration, Washington, DC). Available at https://modis.gsfc.nasa.gov/. Accessed December 28, 2015. [Google Scholar]
- 50.InternationalResearchInstitute forClimate andSociety, Earth institute, ColumbiaUniversity . 2015. Malaria Early Warning System (MEWS). Dekadal (10-Day) Precipitation (Earth Institute. Columbia University, New York). Available at ridl.ldeo.columbia.edu/maproom/Health/Regional/Africa/Malaria/MEWS/. Accessed December28, 2015. [Google Scholar]
- 51.Tatem A, Linard C. Population mapping of poor countries. Nature. 2011;474(7349):36. doi: 10.1038/474036d. [DOI] [PubMed] [Google Scholar]
- 52.WorldPopProject . 2015. WorldPop: Open Access Demographic Data (GeoData Institute. Univ of Southampton, Southampton, UK). Available at www.worldpop.org.uk. Accessed December 28, 2015. [Google Scholar]
- 53.Robinson TP, et al. Mapping the global distribution of livestock. PLoS One. 2014;9(5):e96084. doi: 10.1371/journal.pone.0096084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.United Nations Statistics Division . 2016. UN Comtrade Database (United Nations. New York) Available at comtrade.un.org/data/. Accessed January 1, 2016. [Google Scholar]
- 55.Munyua P, et al. Rift Valley fever outbreak in livestock in Kenya, 2006-2007. Am J Trop Med Hyg. 2010;83(2 Suppl):58–64. doi: 10.4269/ajtmh.2010.09-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nderitu L, et al. Sequential Rift Valley fever outbreaks in eastern Africa caused by multiple lineages of the virus. J Infect Dis. 2011;203:655–665. doi: 10.1093/infdis/jiq004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Scetauroute International (2004) Plan national de transport 2004–2020. Evaluation des principaux flux de transport maritime. Rapport final provisoire [National plan for transportation 2004–2020. Assessment of the main sea-traffic flows. Draft final report], (République de Madagascar, Viceprimature chargée des programmes économiques, Ministère des Travaux Publics, des Transports et de l’aménagement du Territoire, Programme sectoriel des transports, Secrétariat exécutif, Antananarivo, Madagascar), Technical Report.
- 58.Wood SN. Thin plate regression splines. J R Stat Soc Series B Stat Methodol. 2003;65(1):95–114. [Google Scholar]
- 59.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. 2nd Ed Springer; New York: 2002. [Google Scholar]
- 60.Wasserman S, Faust K. Social Network Analysis: Methods and Applications. Vol 8 Cambridge Univ Press; Cambridge, UK: 1994. [Google Scholar]
- 61.Ortiz-Pelaez A, Pfeiffer DU, Soares-Magalhães RJ, Guitian FJ. Use of social network analysis to characterize the pattern of animal movements in the initial phases of the 2001 foot and mouth disease (FMD) epidemic in the UK. Prev Vet Med. 2006;76(1-2):40–55. doi: 10.1016/j.prevetmed.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 62.RCore Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, The R Foundation; Vienna: 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All of the environmental and population data are freely available, following indications given in SI Materials and Methods. The borders of Madagascar administrative units needed for the analyses (municipalities, districts, and regions) are freely available from the GADM database of Global Administrative Areas. Other datasets are available following these links: Coastal navigation data are available at https://figshare.com/s/73ffd8ff3d3011c8e741. Cattle trade data are available at https://figshare.com/s/7a1c14c8f0a5ccb7e7b8. Animal sero-prevalence data are available at https://figshare.com/s/f09650b3309a1a944f15. Correspondence between IDs used in animal sero-prevalence data and in vector layers of administrative units is available at https://figshare.com/s/84924c2fbc37f03f4ab1. Human sero-prevalence data are available at https://figshare.com/s/a0a8ec4be4d78dbd53ac.