Significance
Recent studies have revealed that basophils, the rarest granulocytes, have crucial roles in various immune responses. Among their properties, the MHC class II (MHC-II) expression and their function as antigen-presenting cells are matters of considerable controversy. Here we show that basophils indeed express MHC-II on the cell surface, but with little transcription of corresponding genes. This could be achieved by the acquisition of peptide–MHC-II complexes from dendritic cells via cell contact-dependent trogocytosis in vitro and in vivo. The acquired complexes enabled basophils to stimulate and differentiate T cells toward Th2 cells. Thus, the present study clarified the mechanism by which basophils display MHC-II on the cell surface and appears to reconcile some discrepancies observed in previous studies.
Keywords: basophil, dendritic cell, MHC class II, trogocytosis
Abstract
Th2 immunity plays important roles in both protective and allergic responses. Nevertheless, the nature of antigen-presenting cells responsible for Th2 cell differentiation remains ill-defined compared with the nature of the cells responsible for Th1 and Th17 cell differentiation. Basophils have attracted attention as a producer of Th2-inducing cytokine IL-4, whereas their MHC class II (MHC-II) expression and function as antigen-presenting cells are matters of considerable controversy. Here we revisited the MHC-II expression on basophils and explored its functional relevance in Th2 cell differentiation. Basophils generated in vitro from bone marrow cells in culture with IL-3 plus GM-CSF displayed MHC-II on the cell surface, whereas those generated in culture with IL-3 alone did not. Of note, these MHC-II–expressing basophils showed little or no transcription of the corresponding MHC-II gene. The GM-CSF addition to culture expanded dendritic cells (DCs) other than basophils. Coculture of basophils and DCs revealed that basophils acquired peptide–MHC-II complexes from DCs via cell contact-dependent trogocytosis. The acquired complexes, together with CD86, enabled basophils to stimulate peptide-specific T cells, leading to their proliferation and IL-4 production, indicating that basophils can function as antigen-presenting cells for Th2 cell differentiation. Transfer of MHC-II from DCs to basophils was also detected in draining lymph nodes of mice with atopic dermatitis-like skin inflammation. Thus, the present study defined the mechanism by which basophils display MHC-II on the cell surface and appears to reconcile some discrepancies observed in previous studies.
Basophils, the rarest granulocytes, have long been considered erroneously as minor relatives or blood-circulating precursors of tissue-resident mast cells because of phenotypic similarity between them, including basophilic granules in the cytoplasm and the expression of high-affinity IgE receptor FcεRI on the cell surface (1). However, recent studies have revealed that basophils and mast cells play distinct roles in immune responses such as allergic inflammation and protective immunity to parasitic infections (2–4). Basophils also contribute to immune regulation through their interaction with other types of cells, including T cells (5–8), monocytes (9, 10), innate lymphoid cells (11), fibroblasts (12, 13), and endothelial cells (14).
Basophils rapidly secrete larger quantities of IL-4 than Th2 cells on a per cell basis in response to a variety of stimuli (15). IL-4 has an important role in promoting the differentiation of naive T cells to Th2 cells (16). Sokol et al. demonstrated that basophils transiently migrate to draining lymph nodes just before Th2 differentiation occurs there, in response to subcutaneous injection of protease allergens such as papain (17). They are localized in the T-cell zone and express IL-4, and their depletion abolished Th2 differentiation in lymph nodes (17). These results suggested that basophils are a crucial provider of IL-4 necessary for Th2 differentiation. In this setting, it was originally assumed that dendritic cells (DCs) function as antigen-presenting cells (APCs) and induce Th2 differentiation in cooperation with basophil-derived IL-4.
Reports from three independent groups further expanded the role for basophils in Th2 differentiation and demonstrated in three distinct experimental settings that basophils, rather than DCs, are the critical APCs for driving Th2 differentiation (5–7). In all settings, basophils expressed both MHC class II (MHC-II) and costimulatory molecules (CD80, CD86, or CD40) necessary for APC function, and could process and present antigens. Depletion of basophils but not DCs abolished Th2 differentiation in vivo. This paradigm shift was greeted with great enthusiasm, but also with criticism (16, 18, 19). One concern is the method used for the depletion of DCs and basophils, pointing out the possibility that radioresistant DCs may have remained intact in the DC-depleted chimeric mice (20) and that the basophil-depleting anti-FcεRI antibody may have ablated FcεRI-expressing inflammatory DCs (21). Indeed, the crucial role of DCs in Th2 differentiation was demonstrated by later studies (20–23). Of note, basophils reportedly express H-2M and invariant chain, which are key regulators of peptide loading on MHC-II, at very low levels compared with DCs, raising concerns about the basophil’s antigen-processing and presentation ability (21). Moreover, the level of MHC-II expression on basophils varies, depending on experimental conditions, but it is generally much lower than that on professional APCs such as DCs and B cells (5–8). Thus, the significance of MHC-II expression on basophils and the antigen-presentation capacity of basophils remain controversial (24).
In the present study, we revisited MHC-II expression on basophils and examined it in various cytokine milieus. Unexpectedly, MHC-II expression was detected on cultured basophils at protein, but not at transcription level. We here demonstrate that basophils acquired peptide–MHC-II complexes from DCs through cell contact-dependent trogocytosis in vitro and in vivo. Peptide–MHC-II–dressed basophils could function as APCs and induced the proliferation and IL-4 production of T cells. Thus, the present study clarified the mechanism by which basophils display MHC-II on the cell surface and appears to reconcile some discrepancies observed in previous studies.
Results
Basophils Express MHC-II at Protein but Not at Transcription Level.
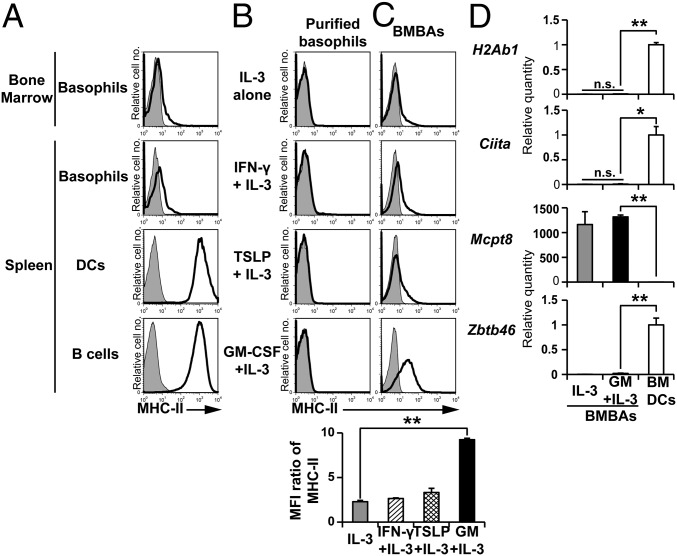
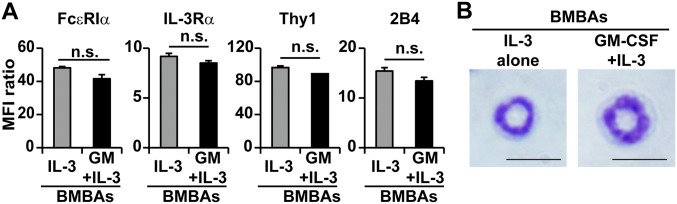
Basophils isolated from the bone marrow and spleen displayed MHC-II on their surface at very low levels, in contrast to high expression on professional APCs such as DCs and B cells (Fig. 1A). Culture of basophils ex vivo with their survival and growth factor IL-3 did not up-regulate the MHC-II expression (Fig. 1B). The addition of other cytokines, including IFN-γ, thymic stromal lymphopoietin (TSLP), and GM-CSF, showed no significant effect on it (Fig. 1B), even though IFN-γ is known to induce MHC-II expression on many types of cells (25). Considering that basophils differentiate from their progenitor cells in the bone marrow, we thought their MHC-II expression might be influenced by cytokine milieu during their differentiation, but not once they mature. Indeed, when bone marrow cells were cultured in the presence of IL-3 for 5 d to generate bone marrow–derived basophils (BMBAs), the addition of GM-CSF, but not IFN- γ or TSLP, significantly up-regulated the MHC-II expression on BMBAs (Fig. 1C), whereas the expression of FcεRIα, IL-3Rα, Thy1, and 2B4 was unaffected (Fig. S1A). Intriguingly, the GM-CSF addition induced little or no transcriptional up-regulation of the H2Ab1 gene encoding MHC-II proteins in BMBAs (Fig. 1D), despite the increased level of MHC-II proteins on their surface (Fig. 1C). This was also the case for the transcription of the Ciita gene, the master regulator of MHC-II transcription (Fig. 1D). GM-CSF/IL-3-elicited BMBAs expressed the basophil-specific Mcpt8 gene, but not the DC-specific Zbtb46 gene (Fig. 1D), and possessed ring-like nuclei (Fig. S1B), as did authentic IL-3-elicited BMBAs. These results suggested that GM-CSF did not directly act on basophils to induce the transcription and translation of MHC-II and might influence the MHC-II expression on basophils indirectly, via the effect on another type of cells.
Fig. 1.
Basophils express MHC-II at protein but not at transcription level. (A) MHC-II expression (open histograms) on bone marrow basophils and splenic basophils, DCs and B cells of C57BL/6 mice. Shaded histograms indicate staining with isotype-matched control antibody. (B) Sort-purified basophils from the bone marrow were cultured for 5 d in the presence of IL-3 alone, IFN-γ plus IL-3, TSLP plus IL-3, or GM-CSF plus IL-3, and were subjected to flow cytometric analysis of surface MHC-II expression. (C) Total bone marrow cells instead of purified basophils were cultured as in B, and the resulting BMBAs (CD200R3+CD49b+c-kit−) were examined for MHC-II expression. (Lower) Relative MHC-II expression on BMBAs generated under different culture conditions (mean ± SEM; n = 3 each), in that the ratio of mean fluorescence intensity (MFI; MFI of MHC-II staining divided by MFI of control staining) in each group was calculated. (D) Relative amounts of indicated mRNAs in IL-3- and GM-CSF/IL-3-elicited BMBAs in comparison with those in BMDCs (mean ± SEM; n = 3 each). The amount of each mRNA in BMDCs is set as 1. Data shown in A–D are representative of at least three independent experiments. n.s., no significant differences. *P < 0.05; **P < 0.01.
Fig. S1.
Surface marker expression and morphology is comparable between GM-CSF/IL-3 and IL-3-elicited basophils. (A) Cell surface expression of FcεRIα, IL-3Rα, Thy1, and 2B4 on IL-3- and GM-CSF/IL-3-elicited BMBAs is shown. Relative expression of each molecule is shown (mean ± SEM; n = 3 each). (B) May-Grünwald-Giemsa staining of IL-3- and GM-CSF/IL-3-elicited BMBAs. (Scale bar, 10 μm.) Data are representative of at least three independent experiments. n.s., no significant differences.
Basophils Acquire MHC-II Proteins from DCs.
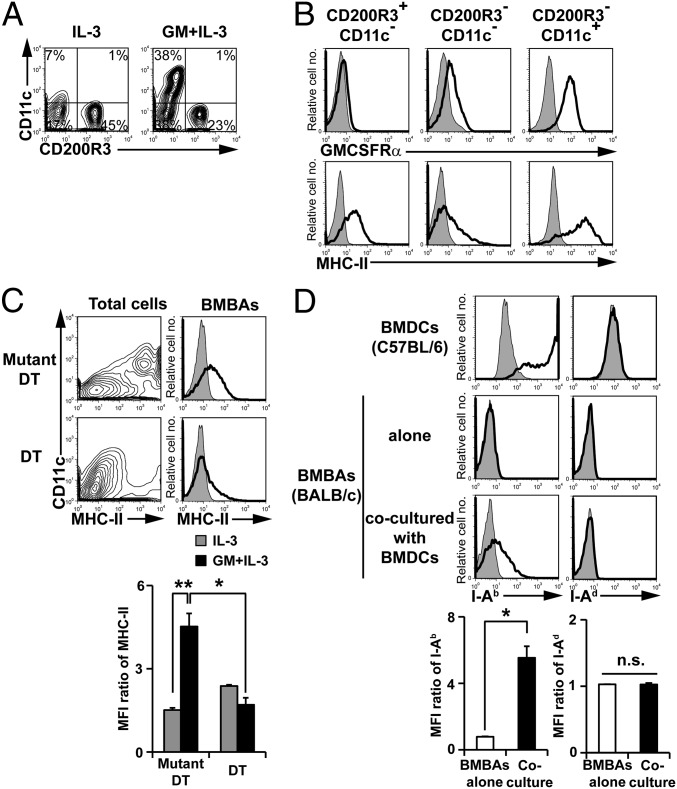
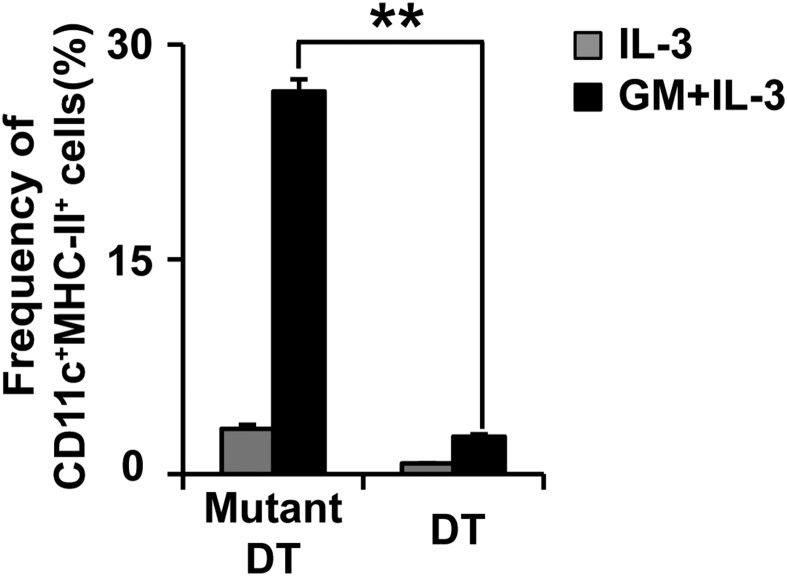
Flow cytometric analysis revealed that the addition of GM-CSF to the IL-3 culture of bone marrow cells expanded the CD200R3−CD11c+ population (Fig. 2A). This population expressed high levels of GM-CSF receptor and MHC-II (Fig. 2B), suggesting BMBAs might have acquired MHC-II proteins from DCs in culture. To explore this possibility, we generated BMBAs from bone marrow cells of CD11cDTR mice, in that the diphtheria toxin receptor (DTR) is selectively expressed in CD11c+ cells. The treatment of their bone marrow cells in vitro with diphtheria toxin depleted most of CD11c+MHC-II+ cells (Fig. 2C; Fig. S2), and the culture of treated cells with IL-3 plus GM-CSF resulted in significant reduction of MHC-II expression on BMBAs in comparison with the expression in the presence of CD11c+ cells (Fig. 2C), suggesting CD11c+ DCs were the major provider of MHC-II for BMBAs. In accordance with this, coculture of IL-3-elicited BMBAs (from H-2d BALB/c mice) with GM-CSF-elicited bone marrow-derived DCs (BMDCs) (from H-2b C57BL/6 mice) in the presence of IL-3 resulted in the appearance of I-Ab molecules on the surface of BMBAs (Fig. 2D), clearly demonstrating the transfer of MHC-II proteins from DCs to basophils.
Fig. 2.
Basophils acquire MHC-II proteins from DCs. (A) The expression of CD11c and CD200R3 on bone marrow cells cultured in the presence of IL-3 alone or GM-CSF plus IL-3. (B) The expression of GMCSFRα and MHC-II (open histograms) on the CD200R3+CD11c− (basophil), CD200R3−CD11c−, CD200R3−CD11c+ fractions of GM-CSF/IL-3-cultured cells. Shaded histograms indicate staining with isotype-matched control antibody. (C) Bone marrow cells isolated from CD11cDTR mice were cultured for 5 d with IL-3 plus GM-CSF in the presence of diphtheria toxin (DT) or its inactive mutant (Mutant DT). CD11c and MHC-II expression on total cells in the culture (Left) and MHC-II expression on BMBAs (Right). (Lower) Relative MHC-II expression on BMBAs in each group (mean ± SEM; n = 3 each). (D) BMBAs and BMDCs were generated from BALB/c and C57BL/6J mice, respectively. BMBAs were cultured in the presence of IL-3 and GM-CSF for 12 h together with or without BMDCs. The expression of MHC-II (I-Ab and I-Ad) on cultured BMBAs is shown in comparison with that on BMDCs (Upper). (Lower) Relative expression of I-Ab and I-Ad on BMBAs is displayed (mean ± SEM; n = 3 each). Data in A–D are representative of at least three independent experiments. n.s., no significant differences. *P < 0.05; **P < 0.01.
Fig. S2.
Depletion of DCs in bone marrow culture. Bone marrow cells isolated from CD11cDTR mice were cultured with IL-3 alone or IL-3 plus GM-CSF in the presence of DT or Mutant DT, as in Fig. 2C. The frequency (percentage) of CD11c+ MHC-II+ cells in the culture is shown (mean ± SEM; n = 3 each). Data are representative of at least three independent experiments. **P < 0.01.
Basophils Capture MHC-II from DCs Through Cell Contact-Dependent Trogocytosis.
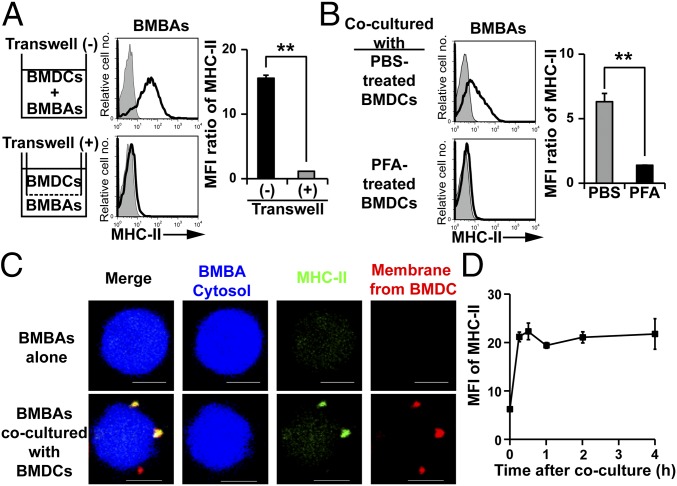
To clarify the molecular mechanism underlying the translocation of MHC-II from DCs to basophils, we first examined whether the cell contact between basophils and DCs during coculture is necessary for the translocation by using the transwell culture apparatus. The separation of IL-3-elicited BMBAs and GM-CSF-elicited BMDCs with 0.4-µm pore semipermeable membrane completely abolished the transfer of MHC-II from DCs to basophils (Fig. 3A), ruling out the exosome-mediated one as the major mechanism of MHC-II transfer. Moreover, once BMDCs were fixed with paraformaldehyde, they could not transfer MHC-II to BMBAs (Fig. 3B), suggesting that the cell–cell contact of living cells is necessary for the MHC-II transfer.
Fig. 3.
Basophils acquire MHC-II from DCs through cell contact-dependent trogocytosis. (A) BMBAs and BMDCs were cultured for 12 h, together in the same chamber or separately in the lower and upper chambers, respectively, in the transwell apparatus. MHC-II expression on BMBAs after the culture (open histograms) is shown (Left), and all data are summarized (Right), showing relative MHC-II expression (mean ± SEM; n = 3 each). (B) BMBAs were cocultured with BMDCs that had been pretreated with paraformaldehyde (PFA) or control PBS for 15 min. MHC-II expression on BMBAs after the 12-h coculture was analyzed (mean ± SEM; n = 3 each). (C) Before the culture, the cytosol of BMBAs was labeled in blue with CellTrace Violet, whereas the plasma membrane of BMDCs was labeled in red with PKH26. BMBAs were cultured with or without BMDCs for 12 h, and then MHC-II molecules on their surface were stained in green with FITC-conjugated anti-I-A/I-E antibody. Representative photographs of BMBAs taken under confocal fluorescence microscope are shown. (Scale bars, 5 μm.) (D) BMBAs were cocultured with BMDCs. Time course of MHC-II expression on BMBAs is shown (mean ± SEM; n = 3 each). Data in A–D are representative of at least three independent experiments. n.s., no significant differences. *P < 0.05; **P < 0.01.
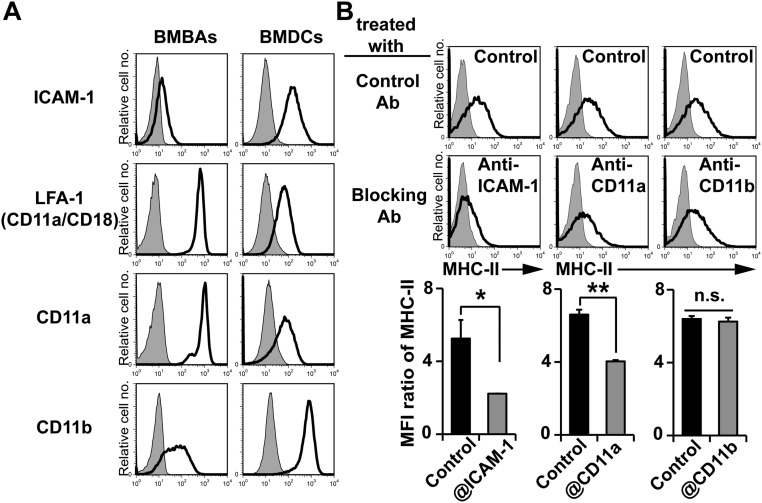
Confocal microscopic examination revealed that fragments of DC plasma membrane were translocated together with MHC-II proteins to the surface of basophils (Fig. 3C). Moreover, the MHC-II transfer from DCs to basophils could be detected as early as 15 min after the start of coculture (Fig. 3D). These observations appeared to be consistent with the phenomenon of trogocytosis that has been demonstrated in lymphocytes when interacted with APCs (26, 27). In trogocytosis, receptor–ligand interactions are thought to play an important role in the cell–cell interaction to trigger the transfer of proteins (28). Both BMBAs and BMDCs expressed LFA-1 (CD11a/CD18), Mac-1 (CD11b/CD18), and their ligand ICAM-1 (Fig. S3A). The addition of anti-ICAM1 blocking mAb to the BMBAs–BMDCs coculture significantly suppressed the MHC-II transfer from BMDCs to BMBAs (Fig. S3B). Moreover, anti-CD11a, but not anti-CD11b, mAb suppressed it (Fig. S3B). Thus, the cell interaction through ICAM-1 and LFA-1 appeared to contribute to the trogocytosis from DCs to basophils.
Fig. S3.
Trogocytosis between BMBAs and BMDCs is dependent on ICAM-1 and LFA-1. (A) Cell surface expression ICAM-1, LFA-1, CD11a, and CD11b on BMBAs and BMDCs is shown (open histogram). Shaded histogram indicates control staining with isotype-matched control. (B) BMBAs were cocultured with BMDCs for 12 h in the presence of indicated blocking antibodies or their control. MHC-II expression on BMBAs after the culture was examined. (Upper and Middle) Representative staining profiles. (Lower) All the data are summarized (mean ± SEM; n = 3 each). Data are representative of at least three independent experiments. n.s., no significant differences. *P < 0.05; **P < 0.01.
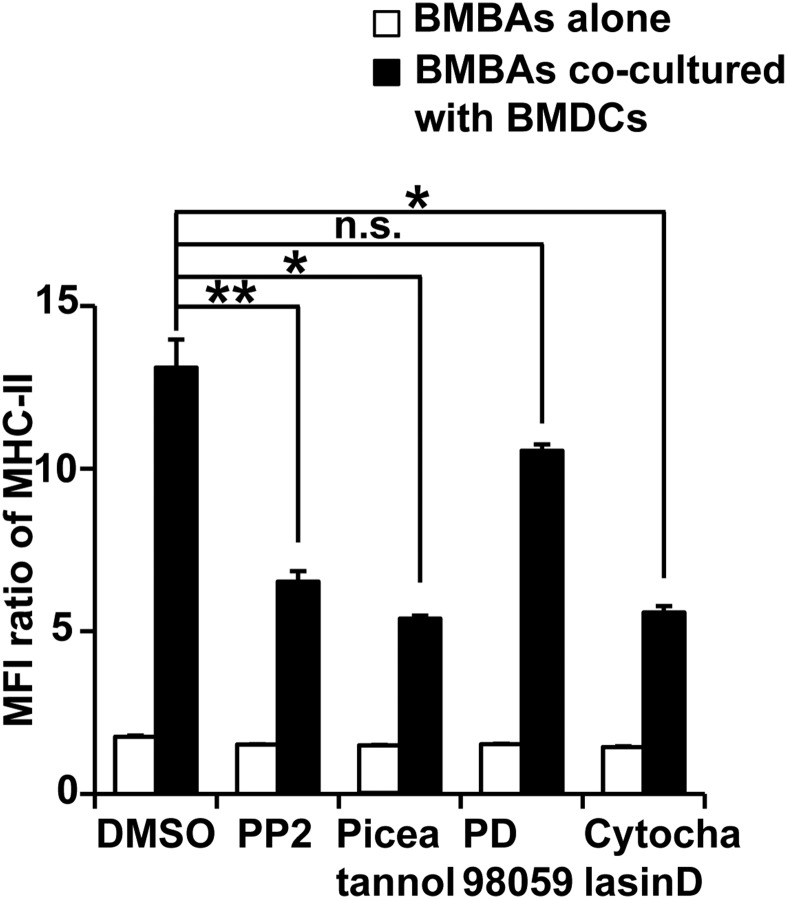
Trogocytosis of MHC-II from DCs to basophils was significantly suppressed by PP2 (Src inhibitor) or piceatannol (Syk inhibitor), but not by PD98059 (MEK inhibitor) (Fig. S4), suggesting that both Src and Syk kinases are involved in the trogocytosis, perhaps through regulation of integrin outside-in signaling (29). Moreover, actin mobilization appears to be important for the trogocytosis because cytochalasin D, an actin cytoskeleton inhibitor, suppressed the MHC-II transfer from DCs to basophils (Fig. S4).
Fig. S4.
Signals from Src/Syk and actin mobilization play important roles in BMBA-BMDC trogocytosis. BMBAs were pretreated for 1 h with indicated reagents or control vehicle (DMSO), and then cultured for 12 h with or without BMDCs in the presence of the same reagents. Relative MHC-II expression on BMBAs is shown (mean ± SEM; n = 3 each). Data are representative of at least three independent experiments. n.s., no significant differences. *P < 0.05; **P < 0.01.
Trogocytosis of Peptide–MHC-II Complexes from DCs Confers Antigen-Presenting Ability on Basophils.
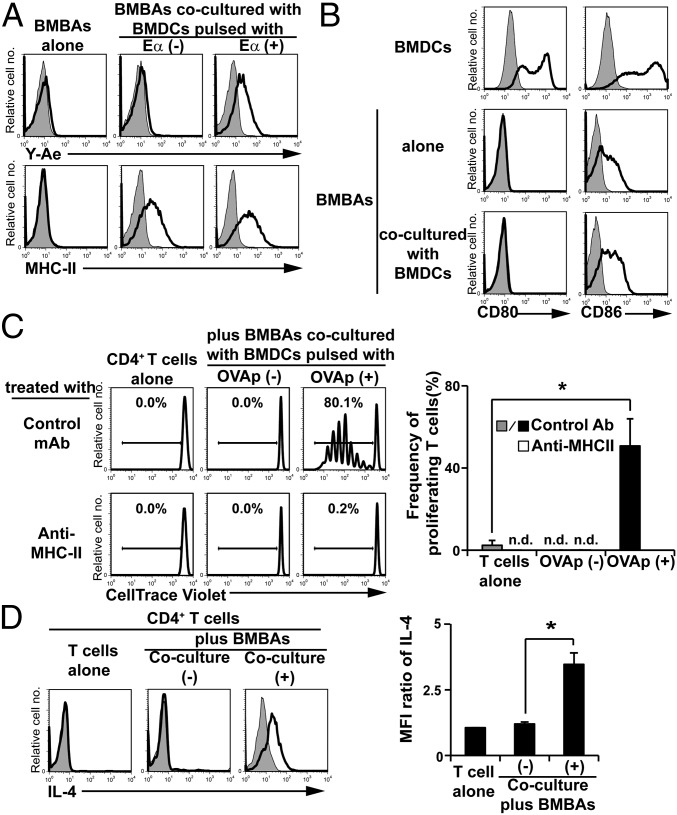
To examine the functional relevance of the trogocytosis of MHC-II from DCs to basophils, we first examined whether peptide–MHC-II complexes generated by DCs can be transferred to basophils through trogocytosis. To this end, we took advantage of the Y-Ae antibody that recognizes the complex of processed H2-Eα peptide and MHC-II (30). Proteolytic processing is necessary for Eα peptide to form a complex with MHC-II. BMDCs were preincubated with or without Eα peptide before coculture with BMBAs. Regardless of preincubation with or without Eα peptide, the coculture resulted in the acquisition of MHC-II on BMBAs (Fig. 4A). Only when BMBAs were cocultured with Eα peptide-pulsed BMDCs did BMBAs become positive for Y-Ae staining (Fig. 4A), indicating that the complex of processed Eα peptide and MHC-II was transferred from BMDCs to BMBAs.
Fig. 4.
Trogocytosis of peptide–MHC-II complexes from DCs confers antigen-presenting ability on basophils. (A) BMBAs were cultured for 12 h with or without BMDCs that had been pulsed with Eα peptide or vehicle (PBS) alone. The expression of MHC-II molecules and processed peptide/MHC-II complexes on BMBAs was detected by using anti-MHC-II and Y-Ae antibodies, respectively. Representative staining profiles are shown. (B) BMBAs were cultured for 12 h with or without BMDCs, and CD80 and CD86 expression on BMBAs was examined in comparison with that on BMDCs. (C) BMBAs were cocultured with BMDCs that had been pulsed with or without OVA peptides. BMBAs were purified from the culture and then cocultured for 5 d with CellTrace Violet-labeled naive CD4+ T cells isolated from the spleen of OT-II Tg mice, in the presence of anti-MHC-II blocking or isotype-matched control Ab. As a control, T cells were cultured without BMDCs. The extent of T-cell proliferation was assessed by dilution of CellTrace Violet. (Left) Representative CellTrace Violet staining profiles. (Right) Frequency (percentage) of divided T cells (showing diluted CellTrace Violet) in each group (mean ± SEM; n = 3 each). (D) BMBAs were cultured with or without BMDCs for 12 h. BMBAs were purified from the culture and then cocultured with naive CD4+ T cells isolated from OT-II Tg mice for 5 d in the presence of OVA peptides as antigens. As a control, T cells were cultured without BMDCs. (Left) IL-4 production of T cells was examined by cytoplasmic staining with anti-IL-4 mAb. (Right) Relative IL-4 staining (mean ± SEM; n = 3 each). Data in A–D are representative of at least three independent experiments. n.d., not detected. *P < 0.05.
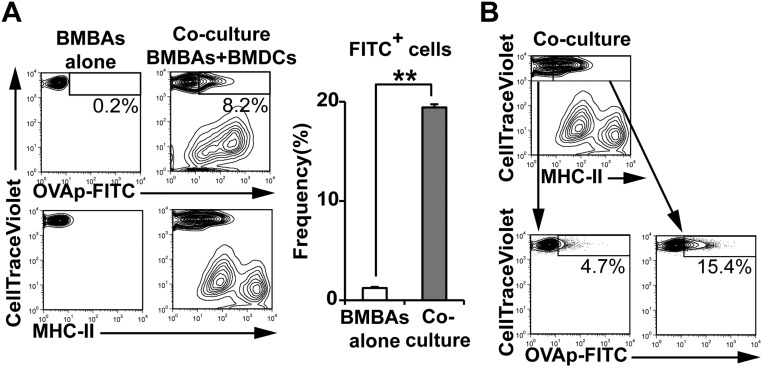
BMBAs constitutively displayed CD86 on their surface, albeit to a lesser extent compared with BMDCs, whereas little or no CD80 expression was detected on BMBAs, unlike on BMDCs (Fig. 4B). Coculture of BMBAs with BMDCs slightly increased the expression of CD86, but not CD80, on BMBAs (Fig. 4B). Therefore, one may assume that peptide–MHC-II–dressed CD86+ BMBAs could function as APCs and stimulate T cells. To address this possibility, we examined the response of ovalbumin (OVA)-specific T-cell receptor (TCR) transgenic T cells to OVA peptide–MHC-II complexes acquired by and displayed on BMBAs. Flow cytometric analysis using FITC-labeled OVA peptides demonstrated that OVA peptides together with MHC-II were transferred from OVA peptide-pulsed BMDCs to BMBAs during their coculture (Fig. S5 A and B), as observed in the Eα peptide–MHC-II complex (Fig. 4A). When OVA-specific TCR transgenic T cells were incubated with BMBAs that had been cocultured with OVA peptide-pulsed BMDCs, prominent T-cell proliferation was observed, and anti-MHC-II blocking mAb almost completely abolished this proliferation (Fig. 4C). Cytoplasmic staining of T cells revealed that proliferating T cells produced IL-4, indicating their differentiation to Th2 cells (Fig. 4D). Such T-cell proliferation and Th2 differentiation were not induced when T cells were incubated with BMBAs that had cocultured with unpulsed BMDCs (Fig. 4 C and D). Thus, peptide–MHC-II–dressed basophils could function as APCs and promote Th2 differentiation.
Fig. S5.
FITC-labeled OVA peptides are transferred from DCs to basophils. BMBAs were cultured for 12 h with or without BMDCs that had been pulsed with FITC-conjugated OVA peptides (OVAp-FITC) for 8 h. (A) Staining profiles of CellTrace Violet, FITC and MHC-II are shown (Left). (Right) MFI of FITC on cultured BMBAs is summarized (mean± SEM; n = 3 each). (B) (Upper) Staining profile of CellTrace Violet and MHC-II. (Left) Staining profiles CellTrace Violet and FITC in MHC-IIlo CellTrace Violethi cells and MHC-IIhi CellTrace Violethi cells. Data in A and B are representative of at least three independent experiments.
Trogocytosis of MHC-II from DCs to Basophils Is Operative in Vivo.
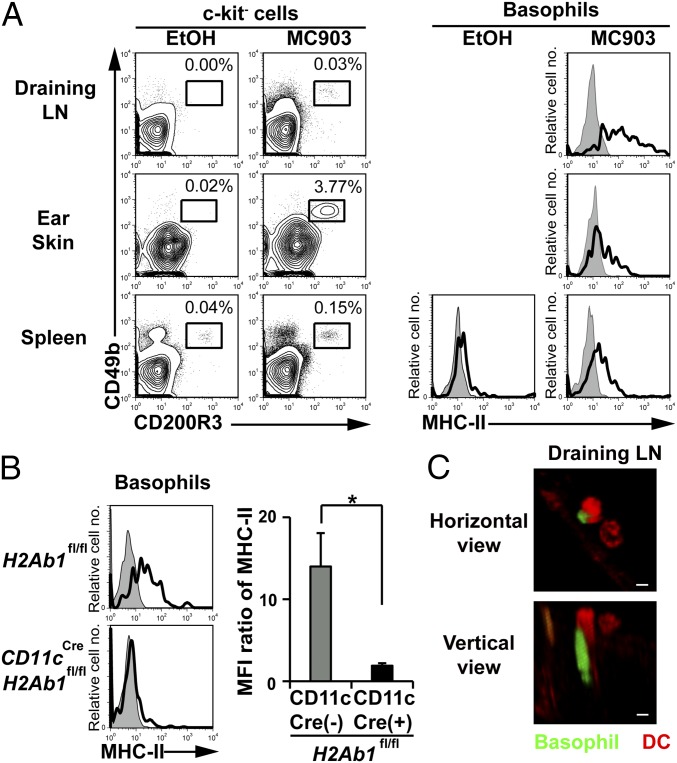
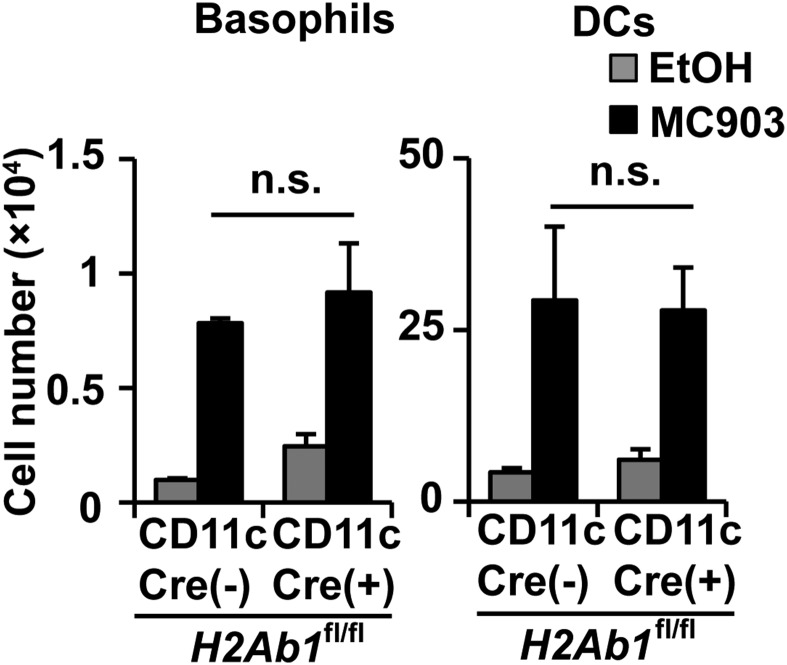
Repeated topical application of a vitamin D3 analog, MC903, induces atopic dermatitis-like allergic inflammation in the skin and promotes basophil recruitment and accumulation in the skin lesion and draining lymph nodes (31, 32). In this model, basophil depletion has been shown to impair Th2 cell differentiation in draining lymph nodes (32). We found that basophils isolated from lymph nodes expressed substantial amounts of MHC-II on their surface (Fig. 5A). The MHC-II expression on basophils isolated from the skin lesion and spleen also increased moderately in MC903-treated mice compared with in control mice (Fig. 5A). To examine whether MHC-II molecules detected on lymph node basophils were derived from DCs, we took advantage of CD11cCreH2Ab1fl/fl C57BL/6 mice, in that the MHC-II expression is defective in CD11c-expressing cells. The number of basophils and CD11c+ cells in draining lymph nodes was comparable in CD11cCreH2Ab1fl/fl and control H2Ab1fl/fl mice when treated with MC903 (Fig. S6). Nevertheless, the MHC-II expression on lymph node basophils was almost completely abolished in the former (Fig. 5B), suggesting the transfer of MHC-II from DCs to basophils could occur in vivo, as observed in the coculture system in vitro. Confocal microscopic examination detected the intimate contact of basophils and DCs in draining lymph nodes of MC903-treated mice (Fig. 5C).
Fig. 5.
Trogocytosis of MHC-II from DCs to basophils occurs in vivo. (A) Ear skin of wild-type C57BL/6 mice was topically treated with MC903 or vehicle EtOH alone for 6 consecutive days. Cells isolated from draining lymph nodes (LNs), ear skin, and spleen were analyzed for the expression of CD49b and CD200R3 (Left). The frequency (percentage) of CD200R3+CD49b+ basophils among isolated cells is shown. (Right) MHC-II expression on basophils (open histograms). Shaded histograms indicate staining with isotype-matched control antibody. (B) Ear skin of CD11cCreH2Ab1fl mice and control H2Ab1fl mice were treated with MC903 or EtOH, as in A. MHC-II expression of draining LN basophils is shown (mean ± SEM; n = 3 each). (C) After the ear skin of Mcpt8GFP mice was treated with MC903 for 6 d, as in A, PE-conjugated anti-CD11c antibody was i.v. administered to visualize CD11c+ cells. Draining LNs were dissected from mice and subjected to confocal fluorescence microscopic analysis. Representative photographs are shown in that basophils and CD11c+ cells are labeled in green and red, respectively. (Scale bars, 10 μm.) Data in A–C are representative of at least three independent experiments. n.s., no significant differences. *P < 0.05.
Fig. S6.
Infiltration of basophils and DCs into draining LNs is independent of MHC-II expression in DCs in MC903-induced skin inflammation. Ear skin of CD11cCreH2Ab1fl mice and control H2Ab1fl mice were treated with MC903 or EtOH, as in Fig. 5A. The numbers of basophils and DCs in draining LNs of treated mice are shown (mean ± SEM; n = 3 each). Data are representative of at least three independent experiments. n.s., no significant differences.
Discussion
The activation of naive CD4 T cells requires interaction with professional APCs that present peptide–MHC-II complexes and provide costimulatory signals and instructive cytokines to T cells (33). Basophils fulfill two of the criteria for APCs (namely, the expression of costimulatory molecules and the production of IL-4), whereas their expression of MHC-II has been a matter of debate. The mechanism by which basophils display MHC-II, if any, remained to be determined. In the present study, we demonstrated that basophils produce little or no MHC-II by themselves, and instead acquire MHC-II from DCs through cell contact-dependent trogocytosis in vitro and in vivo. This appears to explain well why the level of MHC-II expression on basophils varied in different experimental settings (5–8, 21), likely depending on the basophil–DC interaction and the extent of trogocytosis. Of note, once basophils acquired peptide–MHC-II complexes from DCs, they could function as APCs and stimulated T cells to proliferate and produce IL-4. This finding appears to reconcile the discrepancy observed in previous studies demonstrating basophils as important APCs for driving Th2 differentiation under some, but not other, experimental conditions (5–8, 21, 22).
Tang et al. reported that the cooperation of basophils and DCs is important for papain-induced Th2 differentiation in lymph nodes (20). Leyva-Castillo et al. also demonstrated that both types of cells are essential for MC903-elicited Th2 differentiation in lymph nodes (32). In both cases, basophil depletion showed no significant effect on CD4+ T-cell expansion, and it abolished Th2 differentiation. Depletion of DCs also abolished Th2 differentiation. Therefore, these studies suggested that DCs function as APCs, whereas basophils function as IL-4 provider, as originally proposed by Sokol et al. (17). Alternatively, considering our findings in the present study, one may assume that basophils function as Th2-oriented APCs through IL-4 production and trogocytosis-mediated MHC-II acquisition from DCs. In this situation, DC depletion should deprive basophils of APC function, and therefore results in impaired Th2 differentiation, consistent with the reported observations (20, 32). Otsuka et al. reported that basophils cannot take up or process ovalbumin proteins in significant quantities, whereas they can promote Th2 skewing on ovalbumin protein exposure in the presence of DCs (8). This apparently puzzling observation can be readily explained by the trogocytosis of peptide–MHC-II complexes from DCs to basophils.
It remains to be determined how IL-4 production by basophils is triggered to promote Th2 differentiation, but one might assume the following scenario: Formation of the immunologic synapse between peptide–MHC-II–dressed basophils and T cells stimulates T cells to produce IL-3 or other molecules, which in turn activate basophils to secrete IL-4, as suggested by previous report (34). IL-4 produced by basophils conjugated to T cells can efficiently differentiate T cells toward Th2 cells. Alternative or additional stimuli for IL-4 production may include allergen–IgE complexes, cytokines such as IL-33 and TSLP, and ligands for Toll-like receptors (TLR). In addition to IL-4 production, basophils appear to have characteristics suitable for Th2 induction. Given the fact that weak TCR and costimulatory signals preferentially induce Th2 differentiation, lower levels of MHC-II and costimulatory molecules on basophils, compared to those on DCs, favor Th2 skewing (33). Moreover, basophils have been shown to suppress Th1 differentiation in an IL-4-independent and cell contact-dependent manner (35).
BMBAs are commonly used as a source of basophils for both in vitro and in vivo transfer experiments as a result of the limited availability of primary basophils, because of their rarity. We routinely generated BMBAs by culturing bone marrow cells with a relatively low concentration (0.3 ng/mL) of IL-3, as we noticed that a higher IL-3 concentration preferentially expands mast cells, rather than basophils. In contrast, previous studies by others used a much higher concentration (10∼30 ng/mL) of IL-3 to generate BMBAs (5, 7, 8, 17, 21), in that MHC-II+CD11c+ DCs were also generated, even without the addition of GM-CSF (21). Careful and precise purification of BMBAs from such a culture may be able to avoid the contamination of DCs (36), whereas BMBAs likely express MHC-II acquired from DCs during the culture. Thus, our findings in the present study warn that data obtained from experiments using such BMBAs should be carefully interpreted.
Intercellular transfer of cell surface proteins can be achieved by multiple mechanisms, including trogocytosis, secretion of exosome, and tunneling nanotubes (37–39). Trogocytosis is a dynamic transfer of membrane patches that occurs within minutes of conjugate formation of two live cells (26, 27). It is well documented that T cells and natural killer (NK) cells can acquire MHC-I molecules via trogocytosis. CD8 T cells that acquired their peptide–MHC-I ligands become susceptible to antigen-specific cytolysis, leading to effector clearance (40). Acquisition of MHC-I by NK cells from tumor cells leads to a reduced NK cytotoxic function (41). Acquisition of MHC-II from DCs via trogocytosis has been shown in CD4 T cells, NK cells, DCs, macrophages, and lymph node stromal cells (28, 42–45). MHC-II–dressed recipient cells either stimulate or suppress T cells, perhaps depending on the expression and acquisition of costimulatory molecules (28). Innate lymphoid cell (ILC)2s can also acquire MHC-II from DCs, although ILC2s express endogenous MHC-II, and process and present protein antigens to T cells (46). MHC-II+ ILC2s induce T-cell proliferation and differentiation to IL-13-producing Th2 cells in an IL-4-independent manner (46). In the present study, we demonstrated that basophils acquire peptide–MHC-II complexes from DCs via trogocytosis and can function as APCs that preferentially drive T cells to differentiate into IL-4-producing Th2 cells. The relative contribution of basophils and DCs in vivo as APCs in Th2 differentiation may vary, depending on experimental settings, in that the trogocytosis-mediated transfer of peptide–MHC-II complexes from DCs to basophils may occur to various extents. Indeed, we found in the MC903-elicited model of atopic dermatitis that basophils were recruited to both the skin lesion and draining lymph nodes, whereas their MHC-II expression was much higher in lymph nodes than in the skin, despite the fact that both tissues contained DCs. It remains to be determined what makes this difference, including cell density, cytokine and chemokine milieu, expression of adhesion molecules, and the nature of antigens.
In conclusion, we have defined the mechanism by which basophils display MHC-II on the cell surface at the protein, but not transcriptional, level. Trogocytosis-mediated acquisition of peptide–MHC-II complexes from DCs together with CD86 expression confers the Th2-oriented APC activity on basophils in conjunction with their production of IL-4, illustrating the functional significance of physical interaction of basophils with DCs in the immune system.
Materials and Methods
Further details are available in SI Materials and Methods.
Mice.
BALB/c and C57BL/6J mice were purchased from Sankyo Labo Service Corporation, Inc. H2Ab1fl/fl C57BL/6 mice (47) were purchased from the Jackson Laboratory. CD11cDTR (48), OT-II (provided by Francis R. Carbone, University of Melbourne) (49) and CD11cCre (provided by T. Ohteki, Tokyo Medical and Dental University) (50) mice on the C57BL/6 background were described previously. Mcpt8GFP-transgenic mice were established in our laboratory, as written in SI Materials and Methods. Mice were maintained under specific pathogen-free conditions in our animal facilities. All animal studies were approved by the Institutional Animal Care and Use Committee of the Tokyo Medical and Dental University.
Generation and Purification of BMBAs and BMDCs.
BMBAs were generated as described previously (51), with some modifications. Briefly, bone marrow cells were cultured in the presence of murine IL-3 (300 pg/mL; BioLegend) for 7 d. In some experiments, GM-CSF (3 ng/mL), IFN-γ (10 ng/mL; Miltenyi), or TSLP (1 μg/mL; Affymetrix) was included in culture. To purify basophils, CD49b+c-kit−CD11c−FSClowSSClow cells were isolated by using FACSAria II.
BMDCs were generated by culturing bone marrow cells in the presence of murine GM-CSF (20 ng/mL; Miltenyi) for 7 d. On day 6 of culture, cells were stimulated with LPS (1 µg/mL; Sigma-Aldrich). To purify DCs, CD49b−c-kitint-hiCD11c+FSChiSSChi cells were isolated by using FACSAria II.
Coculture of Basophils and DCs.
Sort-purified BMBAs were cocultured with sort-purified BMDCs (5 × 105 cells/well, each) in 96-well round bottom plates at 37 °C for indicated periods. For distinction of BMBAs from BMDCs, BMBAs were stained with CellTrace Violet (LifeTechnologies) before coculture. In the transwell assay shown in Fig. 3A, BMBAs and BMDCs were placed, respectively, into the lower and upper chambers that were separated by 0.4 μm-pore semipermeable membrane (Corning). As controls, BMBAs and BMDCs were cultured together in the lower chamber of a transwell plate.
Flow Cytometric Analyses and Cell Sorting.
For flow cytometric analysis, single-cell suspensions prepared from the bone marrow or spleen were incubated with indicated antibodies after treatment with an anti-CD16/32 mAb (clone name: 2.4G2) and normal rat serum (Merck Millipore) to reduce the nonspecific staining. Dead cells stained with propidium iodide (Sigma-Aldrich) were excluded in the analyses. Stained cells were analyzed with FACS Canto II (BD Biosciences). Data were analyzed using FlowJo software (Treestar). Cell lineages were identified as follows: basophils (CD200R3+CD49b+c-kit−CD11c−), DCs (CD200R3−CD11c+CD49b−c-kitint-hi), B cells (CD19+), and CD4+ T cells (CD3ε+CD4+CD49b−).
For analysis of Th2 differentiation, cells were first stimulated for 6 h with phorbol 12-myristate 13-acetate (PMA 0.1 μg/mL; Sigma-Aldrich) plus ionomycin (1 μM; Sigma-Aldrich) in the presence of monensin (BD GolgiStop; BD Biosciences) for the last 2 h. Stimulated cells were stained with Ghost Dyes (Tonbo Biosciences) to exclude dead cells, followed by staining for cell surface markers (CD3ε, CD4, and CD49b). Subsequently, cells were treated with BD Cytofix/Cytoperm Fixation and Permeabilization Solution (BD Biosciences) and then stained with anti-IL-4 mAb (BVD-24G2).
MC903-Induced Allergic Inflammation in the Skin.
MC903 (calcipotriol; Tocris Biosciences) dissolved in ethanol (EtOH) or control EtOH alone was topically applied on mouse ears (2 nmol in 20 µL per ear) for 6 consecutive days. Ears were excised and treated with collagenase (125 U/mL; Wako Chemicals) in RPMI complete medium at 37 °C for 2 h, followed by depletion of red blood cells to prepare single cell suspension. Cells isolated from ear skin and draining lymph nodes were subjected to flow cytometric analysis.
Statistical Analysis.
Statistical analysis was performed using unpaired Student t test. P value <0.05 was considered statistically significant.
SI Materials and Methods
Establishment of Mcpt8GFP-Transgenic Mice.
Mcpt8GFP-transgenic mice were established using BAC transgenes, in which Mcpt8 exon1 is replaced to eGFP-pA-Neo/Kan sequence from pEGFP-N3 (Clontech). BAC clone (RP23-16G10) was obtained from Children's Hospital Oakland Research Institute and modified by bacterial homologous recombination, using Red/ET Recombination kit (Gene bridges). Modified BAC-transgene was microinjected to fertilized eggs derived from C57BL/6J mice.
Antibodies.
APC-conjugated anti-CD200R3 (Ba13); APC-Cy7-conjugated anti-CD45 (30-F11) streptavidin; biotinylated anti-CD8α (53-6.7), anti-CD11b (M1/70), anti-CD19 (6D5), anti-CD49b (DX5), anti-CD123 (5B11), and anti-I-A/I-E (M5/114.15.2); FITC-conjugated anti-CD11b (M1/70), anti-CD49b (HMα2), anti-CD90.2 (30-H12), anti-CD244.2 (m2B4 (B6)458.1), anti-I-Ad (39-10-8), and anti-I-A/I-E(M5/114.15.2); Pacific Blue-conjugated anti-c-kit (2B8); PE-conjugated anti-CD3ε (145-2C11), anti-CD11a/CD18 (H155-78), anti-CD11a (M17/4), anti-CD86 (GL-1), anti-CD200R3 (Ba13), anti-FcεRIα (MAR-1), anti-I-Ab (AF6-120.1), anti-I-A/I-E (M5/114.15.2), and streptavidin; PE-Cy7-conjugated anti-CD4 (RM4-5) and LEAF-purified anti-I-A/I-E (M5/114.15.2), anti-ICAM-1 (YN1/1.7.4), anti-CD11a (M17/4), anti-CD11b (M1/70), rat IgG2a isotype control antibody (RTK2758), and rat IgG2b isotype control antibody (RTK4530) were from BioLegend. APC-conjugated anti-CD44 (IM7), APC-Cy7-conjugated anti-CD11c (N418), PE-conjugated anti-CD62L (MEL-14), and anti-CD80 (16-10A1), PE-Cy7-conjugated, and anti-CD25 (PC61) were from Tonbo Biosciences. Biotinylated anti-CD11c (N418), anti-ICAM-1 (YN1/1.7.4), and anti-mouse Eα52–68 peptide bound to I-Ab (Y-Ae), FITC-conjugated anti-IL-4 (BVD-24G2), and PE-Cy7-conjugated anti-c-kit (2B8) were from eBiosciences. Biotinylated anti-I-Ab (AF6-120.1) was from BD Biosciences. APC-conjugated GMCSFRα (698423) was from R&D Systems.
Purification of Basophils from Spleen.
For experiments in Fig. 1B, single-cell suspensions from spleen were incubated with biotinylated anti-CD49b antibody, followed by streptavidin-conjugated magnetic particles (BD Pharmingen). After magnetic sorting of CD49b+ cells, basophils were purified by sorting FSClowSSClowCD49b+c-kit−CD11c−CD45int cells with FACSAria II (BD Biosciences).
Depletion of CD11c+ Cells in Cell Culture.
To deplete CD11c+ cells from culture, bone marrow cells isolated from CD11c-DTR mice were cultured in the presence of DT (40 ng/mL; Sigma-Aldrich) or its inactive mutant ([Glu52]-DT, 40 ng/mL; Sigma-Aldrich).
Treatment of BMBAs and BMDCs.
In experiments shown in Fig. 3B, BMDCs were fixed with 4% (wt/wt) paraformaldehyde-PBS (Nacalai tesque) or PBS at 37 °C for 20 min, and extensively washed by PBS before coculture. In experiments shown in Fig. S3B, anti-ICAM-1 (20 μg/mL), anti-CD11a (20 μg/mL), or isotype-matched control antibodies were added to the coculture. In experiments shown in Fig. S4, BMBAs were pretreated with PP2 (10 μM; Sigma-Aldrich), piceatannol (50 μM; Sigma-Aldrich), PD98059 (50 μM; Sigma-Aldrich), or cytochalasin D (50 μM; Sigma-Aldrich) for 1 h, and these inhibitors were also added to the coculture. In the transwell assay shown in Fig. 3A, BMBAs and BMDCs were placed, respectively, into the lower and upper chambers that were separated by 0.4 μm-pore semipermeable membrane (Corning). As controls, BMBAs and BMDCs were cultured together in the lower chamber of a transwell plate.
Assays for Transfer of Peptide–MHC-II Complexes.
Detection of peptide–MHC-II complexes on the cell surface by using Y-Ae antibody was performed as previously described (52). Briefly, the 33-aa-long peptides (RLEEFAKFASFEAQGALANIAVDKANLDVMKKR) derived from H2-Eα were purchased from Eurofins genomics. Sort-purified BMDCs were pulsed with Eα peptides (100 μg/mL) for 8 h, followed by extensive wash with PBS, and then cocultured with sort-purified BMBAs for 12 h. Processed peptide Eα52–68 (ASFEAQGALANIAVDKA; underlined amino acid sequence) in complex with I-Ab expressed on the cell surface was detected using Y-Ae antibody.
Assays using FITC-conjugated OVA peptides were performed as previously described (42). Briefly, BMDCs were pulsed with OVA peptide (10 μg/mL; Sigma-Aldrich) or FITC-conjugated OVA peptide (10 μg/mL; Anaspec) for 8 h, followed by being extensively washed by PBS, and then cocultured with sort-purified BMBAs for 12 h. FITC fluorescence was detected by flow cytometric analysis.
Giemsa Staining.
Cytospin samples were prepared by centrifuging sorted basophils using SHANDON Cytospin2 (Thermo Fisher Scientific), followed by May-Grünwald staining (Wako) for 5 min and then Giemsa staining (Wako) for 5 min. Stained cells were treated with 10% (vol/vol) acetate, dehydrated by acetone, penetrated by xylene, and examined under IX71 microscope (Olympus).
Confocal Microscopic Analysis.
For the experiment in Fig. 3C, BMBAs and BMDCs were purified as follows: BMBAs were isolated by magnetic sorting of CD49b+ cells, followed by sorting of FSClowSSClow cells among them, using FACSAria II. BMDCs were isolated by magnetic sorting of CD11c+ cells, followed by sorting FSChiSSChiI-A/I-Ehi cells using FACSAria II. Sort-purified BMBAs and BMDCs were labeled with CellTrace Violet (Life Technologies Corporation) and PKH26 (Sigma-Aldrich), respectively, and cocultured for 12 h. After coculture, cells were treated with an anti-CD16/32 mAb (2.4G2) and normal rat serum (Merck Millipore), followed by staining with FITC-conjugated anti-I-A/I-E mAb. Cocultured cells were then placed onto the poly-d-lysine-coated glass bottom of culture dishes (MatTek Corporation). Single-plane images were acquired with an A1R confocal laser scanning microscope (Nikon Instech), using a 40× water objective lens and processed with NIS-Elements C software (Nikon Instech).
For experiment in Fig. 5C, LNs were dissected from mice and placed on micro cover glass (Matsunami Glass) with 0.5 mL PBS. Z-stacked images (16 sections, 64 μm in total thickness) were obtained with an A1R confocal laser scanning microscope (Nikon Instech), using 20× water objective lens and processed with NIS-Elements C software (Nikon Instech).
Quantitative RT-PCR.
Total mRNAs were prepared by using Reliaprep RNA cell miniprep system (Promega). The first-strand cDNAs were generated with reverse transcription, using oligo-dT, random primers (Life Technologies Corporation), and ReverTra Ace (Toyobo). Quantitative PCR of the cDNA was performed on Applied Biosystems StepOnePlus Real-Time PCR system, using a Fast SYBR Green Master Mix (Life Technologies Corporation) and the following primer sets: for Mcpt8, forward 5′-CACTGGTCAATGACATCATGCTCC-3′ and reverse 5′-GTCAGACAAAGTGCAATTGGCCAAC-3′; for Prss34, forward 5′-CTGGCTCCTGTTCCTCAGTCT-3′ and reverse 5′-GCTGTGCTCCATGTCGTAGAG-3′; for Zbtb46, forward 5′-AGAGAGCACATGAAGCGACA-3′ and reverse 5′-CTGGCTGCAGACATGAACAC-3′; for H2Ab1, forward 5′-AGCCCCATCACTGTGGAGT-3′ and reverse 5′-GATGCCGCTCAACATCTTGC-3′; for Ciita, forward 5′-CCTATGCCAACATTGCG-3′ and reverse 5′-GGCTTCTGTCCTGCTTCTA-3′; and for ActB, forward 5′-GGCTGTATTCCCCTCCATCG-3′ and reverse 5′-CCAGTTGGTAACAAATGCCATGT-3′. Gene expression levels were standardized using ActB as an endogenous control in each sample.
Assessment of Antigen Presentation and Th2 Differentiation Ability.
The antigen presentation capacity of cells was analyzed as described previously (7), with some modifications. On the one hand, naive CD4+ T cells were purified from the spleen of OT-II transgenic mice by using CD4T Lymphocyte Enrichment Kit (BD Biosciences), followed by sorting of Lin−CD62L+CD44−CD25− cells (Lin is defined as CD8α, CD11b, CD11c, CD19, CD49b, and I-Ab), using FACS Aria II. They were labeled with CellTrace Violet. On the other hand, sort-purified BMDCs were cultured in the presence of GM-CSF (20 ng/mL), with or without OVA323–339 peptides (10 µg/mL; Sigma-Aldrich) for 8 h, followed by extensive wash with PBS, and then cocultured with sort-purified BMBAs for 12 h. BMBAs (5 × 104 per well) isolated from the coculture by sorting were cocultured with CellTrace Violet-labeled naive CD4+ T cells (2 × 105 per well) in a 96-well U-bottom plate in the presence of recombinant IL-2 (2 ng/mL) and IL-3 (10 ng/mL) for 5 d. For experiment in Fig. 4D, recombinant IL-33 (10 ng/mL; BioLegend) is also added to the coculture. The extent of T-cell proliferation was assessed by fluorescence intensity of CellTrace Violet.
Acknowledgments
We thank Dr. Francis R. Carbone from the University of Melbourne and Dr. T. Ohteki from the Tokyo Medical and Dental University for providing mice; M. Kawawa, D. Yamanaka, A. Tomisawa, R. Matsunaga, and H. Ohtsuka for technical support; the members of the H.K. laboratory for helpful discussions; and M. Miki for secretary assistance. This work was supported by research grants from Japan Science and Technology Agency (JST), and the Japanese Ministry of Education, Culture, Sports, Science and Technology (to H.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1615973114/-/DCSupplemental.
References
- 1.Galli SJ. Mast cells and basophils. Curr Opin Hematol. 2000;7(1):32–39. doi: 10.1097/00062752-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Sokol CL, Medzhitov R. Emerging functions of basophils in protective and allergic immune responses. Mucosal Immunol. 2010;3(2):129–137. doi: 10.1038/mi.2009.137. [DOI] [PubMed] [Google Scholar]
- 3.Karasuyama H, Mukai K, Obata K, Tsujimura Y, Wada T. Nonredundant roles of basophils in immunity. Annu Rev Immunol. 2011;29:45–69. doi: 10.1146/annurev-immunol-031210-101257. [DOI] [PubMed] [Google Scholar]
- 4.Siracusa MC, Comeau MR, Artis D. New insights into basophil biology: initiators, regulators, and effectors of type 2 inflammation. Ann N Y Acad Sci. 2011;1217(1):166–177. doi: 10.1111/j.1749-6632.2010.05918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sokol CL, et al. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10(7):713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perrigoue JG, et al. MHC class II–dependent basophil–CD4+ T cell interactions promote TH2 cytokine–dependent immunity. Nat Immunol. 2009;10(7):697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshimoto T, et al. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009;10(7):706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 8.Otsuka A, et al. Basophils are required for the induction of Th2 immunity to haptens and peptide antigens. Nat Commun. 2013;4:1739. doi: 10.1038/ncomms2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egawa M, et al. Inflammatory monocytes recruited to allergic skin acquire an anti-inflammatory M2 phenotype via basophil-derived interleukin-4. Immunity. 2013;38(3):570–580. doi: 10.1016/j.immuni.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Obata-Ninomiya K, et al. The skin is an important bulwark of acquired immunity against intestinal helminths. J Exp Med. 2013;210(12):2583–2595. doi: 10.1084/jem.20130761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motomura Y, et al. Basophil-derived interleukin-4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation. Immunity. 2014;40(5):758–771. doi: 10.1016/j.immuni.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Nakashima C, et al. Basophils regulate the recruitment of eosinophils in a murine model of irritant contact dermatitis. J Allergy Clin Immunol. 2014;134(1):100–107. doi: 10.1016/j.jaci.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 13.Schiechl G, et al. Basophils trigger fibroblast activation in cardiac allograft fibrosis development. Am J Transplant. 2016;16(9):2574–2588. doi: 10.1111/ajt.13764. [DOI] [PubMed] [Google Scholar]
- 14.Cheng LE, et al. IgE-activated basophils regulate eosinophil tissue entry by modulating endothelial function. J Exp Med. 2015;212(4):513–524. doi: 10.1084/jem.20141671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamanishi Y, Karasuyama H. Basophil-derived IL-4 plays versatile roles in immunity. Semin Immunopathol. 2016;38(5):615–622. doi: 10.1007/s00281-016-0568-y. [DOI] [PubMed] [Google Scholar]
- 16.Paul WE, Zhu J. How are TH2-type immune responses initiated and amplified? Nat Rev Immunol. 2010;10(4):225–235. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9(3):310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finkelman FD. Basophils as TH2-inducing APCs: The dog can sing but is it a diva? Immunol Cell Biol. 2009;87(8):568–570. [Google Scholar]
- 19.Maddur MS, Kaveri SV, Bayry J. Basophils as antigen presenting cells. Trends Immunol. 2010;31(2):45–48. doi: 10.1016/j.it.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Tang H, et al. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat Immunol. 2010;11(7):608–617. doi: 10.1038/ni.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammad H, et al. Inflammatory dendritic cells–not basophils–are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010;207(10):2097–2111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phythian-Adams AT, et al. CD11c depletion severely disrupts Th2 induction and development in vivo. J Exp Med. 2010;207(10):2089–2096. doi: 10.1084/jem.20100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumamoto Y, et al. CD301b(+) dermal dendritic cells drive T helper 2 cell-mediated immunity. Immunity. 2013;39(4):733–743. doi: 10.1016/j.immuni.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kambayashi T, Laufer TM. Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell? Nat Rev Immunol. 2014;14(11):719–730. doi: 10.1038/nri3754. [DOI] [PubMed] [Google Scholar]
- 25.Reith W, LeibundGut-Landmann S, Waldburger JM. Regulation of MHC class II gene expression by the class II transactivator. Nat Rev Immunol. 2005;5(10):793–806. doi: 10.1038/nri1708. [DOI] [PubMed] [Google Scholar]
- 26.Joly E, Hudrisier D. What is trogocytosis and what is its purpose? Nat Immunol. 2003;4(9):815–815. doi: 10.1038/ni0903-815. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed KA, Munegowda MA, Xie Y, Xiang J. Intercellular trogocytosis plays an important role in modulation of immune responses. Cell Mol Immunol. 2008;5(4):261–269. doi: 10.1038/cmi.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campana S, De Pasquale C, Carrega P, Ferlazzo G, Bonaccorsi I. Cross-dressing: an alternative mechanism for antigen presentation. Immunol Lett. 2015;168(2):349–354. doi: 10.1016/j.imlet.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Mócsai A, Zhou M, Meng F, Tybulewicz VL, Lowell CA. Syk is required for integrin signaling in neutrophils. Immunity. 2002;16(4):547–558. doi: 10.1016/s1074-7613(02)00303-5. [DOI] [PubMed] [Google Scholar]
- 30.Rudensky AY, Rath S, Preston-Hurlburt P, Murphy DB, Janeway CA., Jr On the complexity of self. Nature. 1991;353(6345):660–662. doi: 10.1038/353660a0. [DOI] [PubMed] [Google Scholar]
- 31.Siracusa MC, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;477(7363):229–233. doi: 10.1038/nature10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leyva-Castillo JM, et al. Skin thymic stromal lymphopoietin initiates Th2 responses through an orchestrated immune cascade. Nat Commun. 2013;4:2847. doi: 10.1038/ncomms3847. [DOI] [PubMed] [Google Scholar]
- 33.Yamane H, Paul WE. Early signaling events that underlie fate decisions of naive CD4(+) T cells toward distinct T-helper cell subsets. Immunol Rev. 2013;252(1):12–23. doi: 10.1111/imr.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sullivan BM, et al. Genetic analysis of basophil function in vivo. Nat Immunol. 2011;12(6):527–535. doi: 10.1038/ni.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh K, Shen T, Le Gros G, Min B. Induction of Th2 type immunity in a mouse system reveals a novel immunoregulatory role of basophils. Blood. 2007;109(7):2921–2927. doi: 10.1182/blood-2006-07-037739. [DOI] [PubMed] [Google Scholar]
- 36.Duriancik DM, Hoag KA. Mistaken identity: purified basophils likely contaminated with dendritic cells. Cytometry A. 2014;85(7):570–572. doi: 10.1002/cyto.a.22476. [DOI] [PubMed] [Google Scholar]
- 37.Davis DM. Intercellular transfer of cell-surface proteins is common and can affect many stages of an immune response. Nat Rev Immunol. 2007;7(3):238–243. doi: 10.1038/nri2020. [DOI] [PubMed] [Google Scholar]
- 38.Rechavi O, Goldstein I, Kloog Y. Intercellular exchange of proteins: the immune cell habit of sharing. FEBS Lett. 2009;583(11):1792–1799. doi: 10.1016/j.febslet.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 39.Carroll-Portillo A, et al. Mast cells and dendritic cells form synapses that facilitate antigen transfer for T cell activation. J Cell Biol. 2015;210(5):851–864. doi: 10.1083/jcb.201412074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang JF, et al. TCR-mediated internalization of peptide-MHC complexes acquired by T cells. Science. 1999;286(5441):952–954. doi: 10.1126/science.286.5441.952. [DOI] [PubMed] [Google Scholar]
- 41.Sjoström A, et al. Acquisition of external major histocompatibility complex class I molecules by natural killer cells expressing inhibitory Ly49 receptors. J Exp Med. 2001;194(10):1519–1530. doi: 10.1084/jem.194.10.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dubrot J, et al. Lymph node stromal cells acquire peptide-MHCII complexes from dendritic cells and induce antigen-specific CD4(+) T cell tolerance. J Exp Med. 2014;211(6):1153–1166. doi: 10.1084/jem.20132000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakayama M, et al. Natural killer (NK)-dendritic cell interactions generate MHC class II-dressed NK cells that regulate CD4+ T cells. Proc Natl Acad Sci USA. 2011;108(45):18360–18365. doi: 10.1073/pnas.1110584108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martínez-Martín N, et al. T cell receptor internalization from the immunological synapse is mediated by TC21 and RhoG GTPase-dependent phagocytosis. Immunity. 2011;35(2):208–222. doi: 10.1016/j.immuni.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mazzini E, Massimiliano L, Penna G, Rescigno M. Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1(+) macrophages to CD103(+) dendritic cells. Immunity. 2014;40(2):248–261. doi: 10.1016/j.immuni.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 46.Oliphant CJ, et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41(2):283–295. doi: 10.1016/j.immuni.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hashimoto K, Joshi SK, Koni PA. A conditional null allele of the major histocompatibility IA-beta chain gene. Genesis. 2002;32(2):152–153. doi: 10.1002/gene.10056. [DOI] [PubMed] [Google Scholar]
- 48.Jung S, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17(2):211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76(1):34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 50.Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J Exp Med. 2007;204(7):1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nei Y, et al. GATA-1 regulates the generation and function of basophils. Proc Natl Acad Sci USA. 2013;110(46):18620–18625. doi: 10.1073/pnas.1311668110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vander Lugt B, et al. Transcriptional programming of dendritic cells for enhanced MHC class II antigen presentation. Nat Immunol. 2014;15(2):161–167. doi: 10.1038/ni.2795. [DOI] [PubMed] [Google Scholar]