Significance
Juvenile hormone (JH) intricately controls molting and metamorphosis in holometabolous insects. Ecdysone-induced protein 93F (E93) functions as an adult specifier gene in the pupal–adult transition. JH is known to repress E93 expression to prevent immature larvae from bypassing the pupal stage and progressing to precocious adult development; however, the molecular mechanism underlying JH-mediated E93 repression remains unknown. Here, we demonstrated that JH-inducible Krüppel homolog 1 functions as a direct transcriptional repressor of E93. This study markedly advances the present understanding of the molecular basis of JH function in repressing insect metamorphosis.
Keywords: metamorphosis, juvenile hormone, E93, Krüppel homolog 1, holometabolous insects
Abstract
Juvenile hormone (JH) represses precocious metamorphosis of larval to pupal and adult transitions in holometabolous insects. The early JH-inducible gene Krüppel homolog 1 (Kr-h1) plays a key role in the repression of metamorphosis as a mediator of JH action. Previous studies demonstrated that Kr-h1 inhibits precocious larval–pupal transition in immature larva via direct transcriptional repression of the pupal specifier Broad-Complex (BR-C). JH was recently reported to repress the adult specifier gene Ecdysone-induced protein 93F (E93); however, its mechanism of action remains unclear. Here, we found that JH suppressed ecdysone-inducible E93 expression in the epidermis of the silkworm Bombyx mori and in a B. mori cell line. Reporter assays in the cell line revealed that the JH-dependent suppression was mediated by Kr-h1. Genome-wide ChIP-seq analysis identified a consensus Kr-h1 binding site (KBS, 14 bp) located in the E93 promoter region, and EMSA confirmed that Kr-h1 directly binds to the KBS. Moreover, we identified a C-terminal conserved domain in Kr-h1 essential for the transcriptional repression of E93. Based on these results, we propose a mechanism in which JH-inducible Kr-h1 directly binds to the KBS site upstream of the E93 locus to repress its transcription in a cell-autonomous manner, thereby preventing larva from bypassing the pupal stage and progressing to precocious adult development. These findings help to elucidate the molecular mechanisms regulating the metamorphic genetic network, including the functional significance of Kr-h1, BR-C, and E93 in holometabolous insect metamorphosis.
Holometabolous insects undergo a complete metamorphosis, which consists of egg, larval, pupal, and adult stages. Larval–pupal and pupal–adult metamorphoses are coordinated by the actions of ecdysteroids and juvenile hormone (JH) (1). In the presence of JH, 20-hydroxyecdysone (20E; the active metabolite of ecdysteroids) induces larval–larval molting, whereas, in the absence of JH, it induces larval–pupal and pupal–adult molts (1). Thus, the major function of JH is to prevent immature larvae from precociously transitioning to pupae and adults (1).
The molecular action of 20E in target cells during the larval–pupal transition was initially proposed in the 1970s (2), and its molecular mechanisms were well characterized by later studies. In particular, the 20E-liganded ecdysone receptor (EcR)/ultraspiracle (USP) complex directly activates the expression of a few early ecdysone-inducible transcription factors, which then regulate a large number of late ecdysone-inducible genes involved in pupal formation (3–5). The molecular mechanism of JH signaling has been clarified more recently (6–8). The JH receptor methoprene tolerant (Met) was found to bind JH (9–12) and subsequently interact with steroid receptor coactivator (SRC; also known as FISC and Taiman) (13–15). The JH/Met/SRC complex then activates Krüppel homolog 1 (Kr-h1) (15, 16), which plays a key role in the repression of metamorphosis (17–20).
The transcription factor Broad-Complex (BR-C), which consists of a Bric-a-brac/Tramtrack/Broad complex and zinc finger domains, is induced by 20E and functions as a “pupal specifier” during the larval–pupal transition (21–24). An amorphic BR-C mutant of Drosophila melanogaster failed to achieve larval–pupal metamorphosis (22, 23), and overexpression of BR-C in penultimate-instar larvae concomitantly suppressed the expression of larval cuticle genes and activated pupal cuticle genes (24). Kr-h1 is required for BR-C repression in the fat body in D. melanogaster (25). In Lepidoptera, JH was shown to repress 20E-dependent induction of BR-C in the larval epidermis (26, 27). We have found that Kr-h1 directly binds to a site within the BR-C promoter and represses 20E-dependent BR-C activation (28). Through these molecular actions, JH prevents immature larvae from undergoing precocious larval–pupal transition.
Ecdysone-induced protein 93F (E93; also known as Eip93F), originally identified as an ecdysone-induced gene from D. melanogaster, is a member of the helix–turn–helix transcription factor family (29). E93 regulates the expression of genes involved in programmed cell death, including apoptosis and autophagy, to facilitate the remodeling of larval to adult tissues (30–33). A recent study in the hemimetabolous German cockroach, Blattella germanica, identified that E93 is an “adult specifier gene” because cockroaches treated with E93RNAi continuously repeated nymphal molts and finally reached the 10th instar, whereas normal cockroaches progress through only six nymphal instars before adult metamorphosis (34). Similarly, E93RNAi prevented the pupal–adult transition and resulted in a supernumerary pupa in Tribolium castaneum and D. melanogaster, suggesting that the function of E93 as an adult specifier gene is conserved in holometabolous insects (34).
A recent analysis demonstrated that E93 expression is induced by 20E and repressed by JH similarly to BR-C (34). Moreover, it was first reported in B. germanica that JH-inducible Kr-h1 suppressed E93 transcript levels (35), and this effect was also observed in D. melanogaster and T. castaneum (36); however, these findings were obtained mainly by RNAi-based genetic analyses using individual insects, and therefore the molecular mechanism underlying JH-mediated repression of E93 in the cells remains poorly understood. In particular, it is unknown whether Kr-h1 represses E93 transcription directly or indirectly, and how Kr-h1 represses the transcript of E93. Therefore, in this study, we sought to elucidate this molecular mechanism in Bombyx mori, and proposed that JH prevents precocious larval–adult metamorphosis via direct Kr-h1–dependent E93 gene repression.
Results
Genomic Structure and Expression Profile of E93 in Vivo and in a Cell Line.
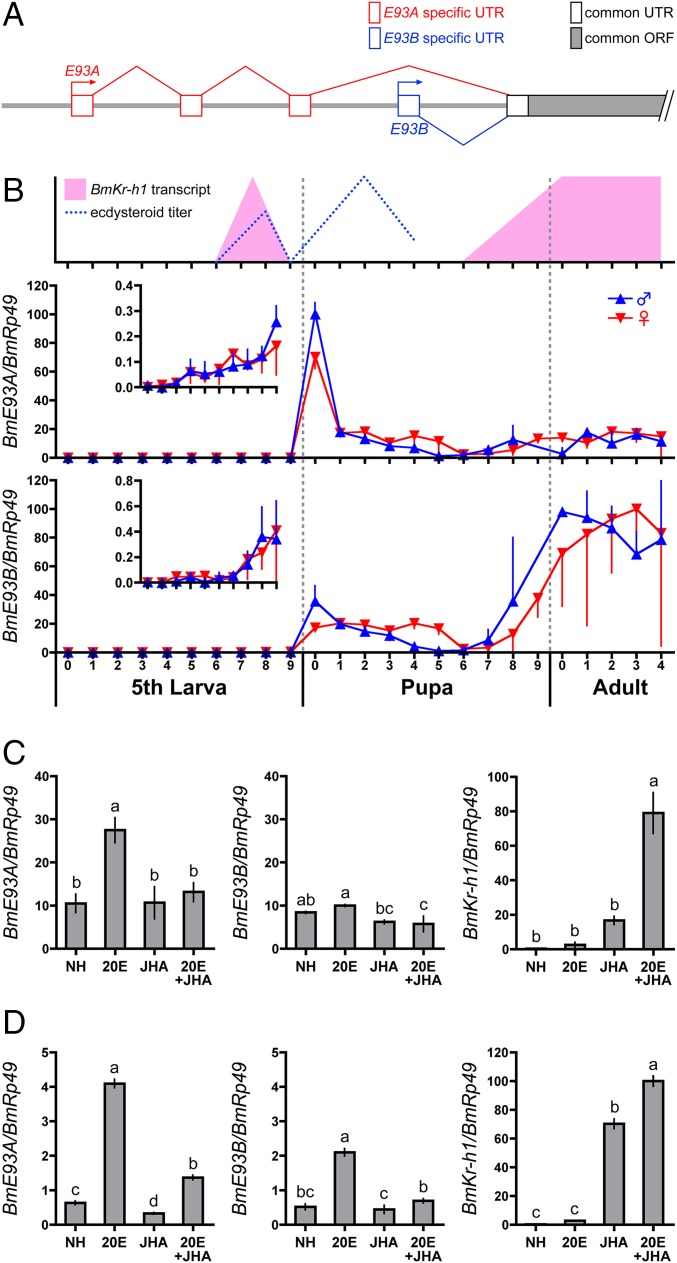
Two BmE93 isoforms with different transcription start sites, BmE93A (LC177616) and BmE93B (LC177617), were identified by rapid amplification of 5′ cDNA ends (5′RACE; Fig. 1A). BmE93A and BmE93B had partially different UTRs but shared a common ORF (Fig. 1A). Comparison of the cDNA sequences to the B. mori genome database (37) revealed that the transcription start site of BmE93B was located downstream of that of BmE93A (Fig. 1A).
Fig. 1.
Gene structure, developmental profile, and hormonal regulation of BmE93. (A) Schematic representation of the BmE93 gene structure. Predicted exons are shown as boxes. The BmE93 gene has two transcriptional start sites [the distal promoter (BmE93A, red) and the proximal promoter (BmE93B, blue)]. (B) Developmental expression profiles of BmE93A and BmE93B in the epidermis of B. mori as determined by qPCR. The numbers under the horizontal axis indicate the days in the respective stage. Blue and red triangles indicate males and females, respectively. (Inset) Vertical axes are scaled to show expression changes at low levels. The changes in the ecdysteroid titer and BmKr-h1 transcripts are depicted based on the data from previous studies. (C) Integuments of day 0 pupae were cultured in the presence of 1 μM 20E and 10 μM JHA for 1 d, and expressions of BmE93A, BmE93B, and BmKr-h1 were monitored. (D) BmE93A, BmE93B, and BmKr-h1 expression were examined in NIAS-Bm-aff3 cells treated with 1 μM 20E and 10 μM JHA for 1 d. The transcript levels of BmE93A in male pupae at day 0, BmE93B in adult males at day 0, and BmKr-h1 in NIAS-Bm-aff3 cells treated with 20E and JHA were set as 100. The data represent means ± SD (n = 3–4). Bars with the same letter (C and D) are not significantly different (Tukey–Kramer test, α = 0.05).
Developmental changes in BmE93A/B expression in the epidermis of B. mori were determined by quantitative PCR (qPCR; Fig. 1B). Only trace expression of the two isoforms was detected in the fifth instar stage (Fig. 1B), but gradually increased toward the pupal stage (Fig. 1B, Inset). BmE93A transcript levels peaked at day 0 of the pupal stage and diminished thereafter, whereas the BmE93B transcript was expressed at low levels during the pupal stage and its levels peaked during the adult stage (Fig. 1B). Little difference was observed in the expression of both isoforms between females and males. Notably, the expression patterns of BmE93A and BmE93B were dominant in the pupal and adult stages, respectively. The expression pattern of BmE93A was highly correlated with the changes in hemolymph ecdysteroid titer (38–41) when BmKr-h1 expression was absent (15, 42) (Fig. 1B), suggesting that BmE93A was induced by 20E and suppressed by BmKr-h1.
Following this result, we examined the effect of 20E and methoprene [a JH analog (JHA)] on the cultured epidermis of pupae at day 0. BmE93A expression of pupa at day 0 was induced by 20E, and JHA repressed this induction; however, these hormones had little effect on BmE93B transcription (Fig. 1C). Our previous studies showed that JHA induced BmKr-h1 expression in the epidermis of fifth-instar larvae at day 5 and adults at day 0 (42). Consistently, JHA also induced BmKr-h1 expression in day 0 pupae, and 20E superinduced it (Fig. 1C).
Next, we examined the hormonal regulation of BmE93 transcripts in a Bombyx cell line (NIAS-Bm-aff3 cells) by qPCR. The BmE93A transcript was weakly detectable at baseline, but it was significantly up-regulated by 20E, and this effect was repressed by JHA (Fig. 1D). The responses to these hormones were weaker for BmE93B transcripts than for BmE93A transcripts (Fig. 1D).
Identification of cis and trans Elements Involved in JH-Dependent BmE93A Repression.
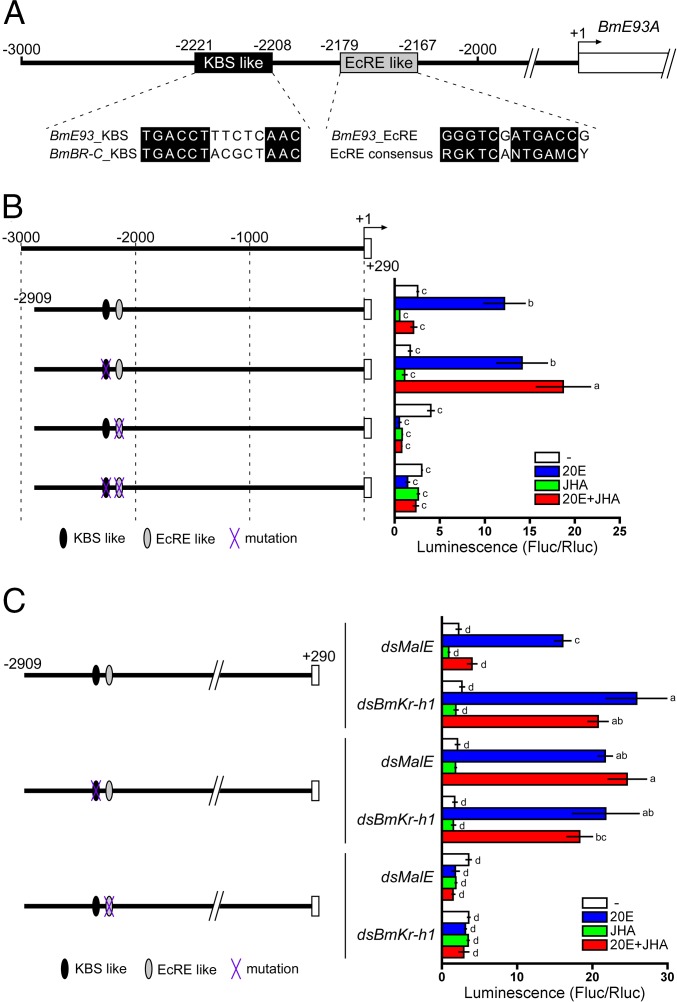
A previous study demonstrated that BmKr-h1 directly binds to a Kr-h1 binding site (KBS) in the BmBR-C promoter and represses 20E-dependent BmBR-C transcription (28). Thus, we hypothesized that BmKr-h1 is also involved in the JH-mediated repression of BmE93A. To test this hypothesis, we searched for sequences with shared similarity to the BmBR-C KBS upstream of BmE93A (<3 kb), and found a candidate KBS (−2221 to −2208) that was highly similar to the BmBR-C KBS (Fig. 2A). Interestingly, a putative ecdysone response element (EcRE; −2179 to −2167) that was homologous to a consensus EcRE sequence (RGKTCANTGAMCY) (43) was located near the putative KBS (Fig. 2A), whereas the upstream region of BmE93B (<3 kb) did not contain this consensus sequence. To characterize the function of the putative KBS and EcRE at the BmE93A locus, we performed reporter assays in NIAS-Bm-aff3 cells using constructs carrying the −2909 to +290 region of BmE93A with or without mutations in these sequences. The WT BmE93A reporter was strongly activated by 20E, and this induction was completely repressed by JHA (Fig. 2B). Mutation in the putative KBS region abolished this JHA-dependent repression (Fig. 2B). Deletion of the putative EcRE from the WT reporter abolished 20E-induced expression (Fig. 2B), indicating that the sequence represents an authentic EcRE. Collectively, these results revealed that the BmE93A transcription was activated by 20E via the EcRE, and that the 20E-induction was repressed by JHA via the putative KBS.
Fig. 2.
Identification of the BmKBS and EcRE in the BmE93A promoter region. (A) The predicted KBS and EcRE in the BmE93A promoter region. The numbers indicate the distance from the BmE93A transcription start site. The putative KBS and EcRE are aligned with the KBS of BmBR-C and EcRE consensus sequences, respectively. (B) Functional characterization of the putative KBS and EcRE in BmE93A. NIAS-Bm-aff3 cells were cotransfected with reporter plasmids carrying the promoter regions indicated in the figure and a reference reporter plasmid. Cells were treated with 1 μM 20E and 10 μM JHA for 2 d, and reporter activity was measured by using a dual-luciferase reporter assay system. Data represent means ± SD (n = 3). Bars with the same letter are not significantly different (Tukey–Kramer test, α = 0.05). Black and gray ellipses indicate the KBS sequence (−2221 to −2208) and EcRE sequence (−2179 to −2167), respectively. The purple “X” indicates a mutated sequence. (C) Functional interaction of KBS and BmKr-h1. NIAS-Bm-aff3 cells were cotransfected with reporter plasmids, a reference reporter plasmid, and dsBmKr-h1 or dsMalE (control) and then treated with 20E or JHA. Other procedures were identical to those in B.
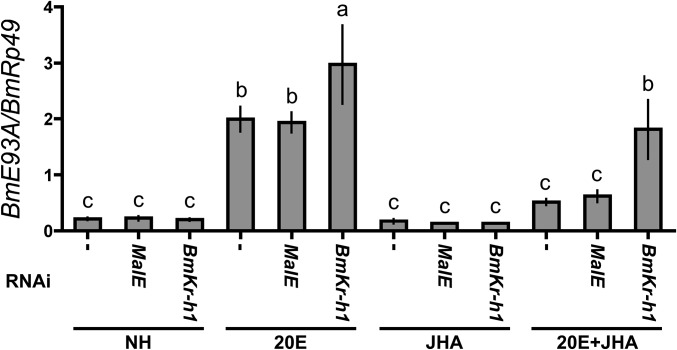
To examine whether BmKr-h1 interacts with the putative KBS in BmE93A, we performed reporter assays in combination with RNAi. BmKr-h1RNAi silencing abolished JHA-dependent repression of BmE93A in the transcript levels (Fig. S1). Notably, BmKr-h1RNAi alleviated the JHA-dependent reporter suppression (Fig. 2C), whereas treatment with dsMalE (RNAi control) had no effect on reporter activity (Fig. 2C), indicating that BmKr-h1 is involved in the repression of 20E-induced BmE93A transcription. Based on these results, we concluded that the KBS (−2221 to −2208; 14 bp) of BmE93A is indeed an authentic KBS that functionally interacts with BmKr-h1.
Fig. S1.
Expression of BmE93A in NIAS-Bm-aff3 cells treated with dsBmKr-h1. NIAS-Bm-aff3 cells were transfected with dsMalE or dsBmKr-h1 by using FuGENE HD (Promega) and then treated with 1 μM 20E and 10 μM JHA for 1 d. The transcript levels of BmE93A were determined by qPCR. The data represent mean ± SD (n = 3). Bars with the same letter are not significantly different (Tukey–Kramer test, α = 0.05).
Conservation of the KBS.
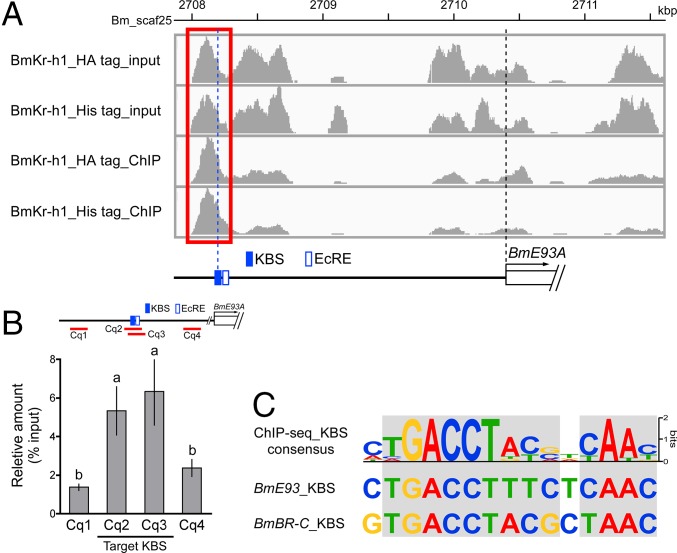
To identify the putative KBSs on a genome-wide basis, we performed ChIP-seq analysis using BmN cells overexpressing BmKr-h1 with a C-terminal HA or V5 epitope tag (BmKr-h1_HA tag and BmKr-h1_V5 tag, respectively). Focusing on the region around the BmE93A locus (2708–2711.5-kbp region in Bm_Scaf25), 10 peaks enriched with read sequences were observed in input samples of BmN cells overexpressing BmKr-h1_HA tag and BmKr-h1_V5 tag (Fig. 3A). An enriched peak between 2708.0 and 2708.5 kbp was observed in both ChIP products (Fig. 3A, red box), and this region contained the KBS identified in this study (Fig. 3A). We designed ChIP-qPCR primers to determine the quantities of enriched read sequences (Fig. 3B, red lines), and confirmed a significant enrichment in the region containing the KBS (Fig. 3B). Moreover, genome-wide motif analysis of the enriched sequences successfully identified a KBS consensus sequence (TGACCTNNNNYAAC; Fig. 3C). The KBSs found upstream of BmE93A and BmBR-C (28) are highly similar to the consensus KBS (Fig. 3C), further confirming that BmKr-h1 indeed interacts with these KBS sequences. This KBS consensus was not found in the upstream region of BmE93B (<3 kb).
Fig. 3.
KBS characterization by ChIP-seq. ChIP-seq was performed by using anti-HA and -V5 tag antibodies in BmN cells overexpressing tagged BmKr-h1. (A) Peak calls in the promoter region of BmE93A (Bm_scaf25: 2708–2711.5 kbp, chromosome 26). The red box indicates a putative ChIP-specific enriched region with the KBS and EcRE locations identified in Fig. 2 shown below. (B) Enrichment of the BmE93A KBS was quantified by ChIP-qPCR. Red lines represent the region amplified by ChIP-qPCR. The Cq2 (−2269 to −2171) and Cq3 (−2265 to −2167) regions contain the KBS, and Cq1 (−3945 to −3842) and Cq4 (−1483 to −1335) regions serve as negative controls. The data represent means ± SD (n = 4). Bars with the same letter are not significantly different (Tukey–Kramer test, α = 0.05). (C) Motif analysis based on BmKr-h1 ChIP-seq. The obtained KBS consensus is aligned with that of BmE93A and BmBR-C. Light gray shading indicates highly conserved nucleotides.
BmKr-h1 Directly Binds to KBS.
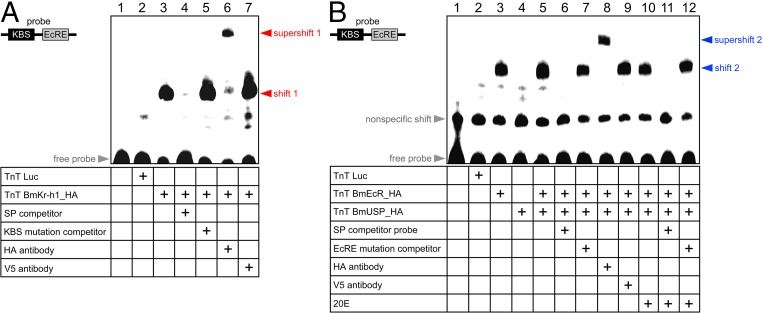
To demonstrate that BmKr-h1 directly binds to the BmE93A KBS, we performed EMSAs using a probe containing the KBS and EcRE sequences. As shown in Fig. 4A, a specific shift band appeared (shift 1) when HA-tagged BmKr-h1 (BmKr-h1_HA) was added to the probe (Fig. 4A, lane 3), whereas no shift band was observed when luciferase was added as a mock binding factor (negative control; Fig. 4A, lane 2). Competition assays showed that the specific band disappeared with the addition of a 100-fold molar excess of unlabeled probe (Fig. 4A, lane 4), but not KBS-mutated probe (Fig. 4A, lane 5, and Table S1). Moreover, the specific band supershifted in the presence of an anti-HA tag antibody (Fig. 4A, lane 6), but not an anti-V5 tag antibody (negative control; Fig. 4A, lane 7). These results clearly indicate that BmKr-h1 directly and specifically bound to the KBS sequence.
Fig. 4.
BmKr-h1 directly binds to the KBS. The BmKr-h1/KBS (A) and BmEcR/EcRE interactions (B) were examined by EMSA. Luciferase (mock), BmKr-h1_HA, BmEcRE_HA, and BmUSP_HA proteins were synthesized with an in vitro transcription and translation system and incubated with biotin-tagged oligonucleotide probes (60 bp) containing the KBS and EcRE sequences. Competition assays were performed by using a 100-fold molar excess of unlabeled specific, (A) KBS-, or (B) EcRE-mutated probes. The specificity of the shifted bands was verified by using polyclonal antibodies against the HA and V5 tags (negative control).
Table S1.
List of oligonucleotides used for PCR and EMSA
| Name | Figure | PCR template | Nucleotide sequence (5′ to 3′) |
| 5′RACE | |||
| BmE93_5′RACE_1st | Fig. 1A | RACE cDNA | CGACTGGAGCACGAGGACACTGA |
| CTGGTTCCTCTAGGACTTCCACTTTCG | |||
| BmE93_5′RACE_2nd | Fig. 1A | 5′RACE 1stPCR product | GGACACTGACATGGACTGAAGGAGTA |
| CGTCTTCTGGCGCGTTGATTTCCTC | |||
| qPCR | |||
| BmE93A_qPCR | Fig. 1 B–D and Fig. S1 | cDNA | GCCCGTACAAAAAAAAGAACTCGAAG |
| TCACGACGTGCTCCATTCCG | |||
| BmE93B_qPCR | Fig. 1 B–D | cDNA | TGCACAATCGGCAGAGAGTCG |
| GCAAAAGAGATGTTTGTAGCGGTTTG | |||
| ChIP-qPCR_1 (Cq1) | Fig. 3B | ChIP DNA & input DNA | CGACTATTATCAAAGCCG |
| CGAAGAGTTTTGTGACTGC | |||
| ChIP-qPCR_2 (Cq2) | Fig. 3B | ChIP DNA & input DNA | TTGAAAGTAAAAGCCCACACGCAC |
| CATCGACCCCCTGTATTCGGC | |||
| ChIP-qPCR_3 (Cq3) | Fig. 3B | ChIP DNA & input DNA | AAGTAAAAGCCCACACGCACACTC |
| CGGTCATCGACCCCCTGTATTC | |||
| ChIP-qPCR_4 (Cq4) | Fig. 3B | ChIP DNA & input DNA | GATTCAGGCATCTGTCAAATATCTTGC |
| GGTTACGTGACGCAAAGGGATCAG | |||
| Reporter plasmid | |||
| pGL4.14_-2909/+290 | Fig. 2 B and C and Fig. 5D | Genomic DNA | AAAAAGCAGGCTNNGCAGCTGCTCTCGAGTTGTTAGGACTC |
| AGAAAGCTGGGTNGTCCACAAAACAAGCCAGCAC | |||
| pGL4.14_-2909/+290_ΔKBS like | Fig. 2 B and C | pGL4.14_-2909/+290 | ATAAGGACTACATACGGCCGAATACAGGG |
| TGTTGTATACAAATTTTGTGAGTGTGCGTGTGG | |||
| pGL4.14_-2909/+290_ΔEcRE like | Fig. 2 B and C | pGL4.14_-2909/+290 | GCGACACCGCCCGAGGCCG |
| CCTGTATTCGGCCGTATGTAGTCCTTATGTT | |||
| pGL4.14_-2909/+290_ΔKBS like &EcRE like | Fig. 2B | pGL4.14_-2909/+290_ΔKBS like | GCGACACCGCCCGAGGCCG |
| CCTGTATTCGGCCGTATGTAGTCCTTATGTT | |||
| EMSA probe and competitor | |||
| Probe | Figs. 4 A and B and 5A and Fig. S2 | — | Biotin-ON-TTGTATACAACACTGACCTTTCTCAACATAAGGACTACATACGGCCGAATACAGGGGGTCGATGACCGGCGACACCGCCC |
| Biotin-ON-GGGCGGTGTCGCCGGTCATCGACCCCCTGTATTCGGCCGTATGTAGTCCTTATGTTGAGAAAGGTCAGTGTTGTATACAA | |||
| KBS Mutation probe | Fig. 5B | — | Biotin-ON-TTGTATACAACAAAAAAAAAAAAAAAAATAAGGACTACATACGGCCGAATACAGGGGGTCGATGACCGGCGACACCGCCC |
| Biotin-ON-GGGCGGTGTCGCCGGTCATCGACCCCCTGTATTCGGCCGTATGTAGTCCTTATTTTTTTTTTTTTTTTTGTTGTATACAA | |||
| Specific competitor | Fig. 4 A and B | — | TTGTATACAACACTGACCTTTCTCAACATAAGGACTACATACGGCCGAATACAGGGGGTCGATGACCGGCGACACCGCCC |
| GGGCGGTGTCGCCGGTCATCGACCCCCTGTATTCGGCCGTATGTAGTCCTTATGTTGAGAAAGGTCAGTGTTGTATACAA | |||
| KBS mutation competitor | Fig. 4A | — | TTGTATACAACAAAAAAAAAAAAAAAAATAAGGACTACATACGGCCGAATACAGGGGGTCGATGACCGGCGACACCGCCC |
| GGGCGGTGTCGCCGGTCATCGACCCCCTGTATTCGGCCGTATGTAGTCCTTATTTTTTTTTTTTTTTTTGTTGTATACAA | |||
| EcRE mutation competitor | Fig. 4B | — | TTGTATACAACACTGACCTTTCTCAACATAAGGACTACATACGGCCGAATACAGGAAAAAAAAAAAAAGCGACACCGCCC |
| GGGCGGTGTCGCTTTTTTTTTTTTTCCTGTATTCGGCCGTATGTAGTCCTTATGTTGAGAAAGGTCAGTGTTGTATACAA | |||
| Expression plasmid | |||
| pIZT_BmKr-h1_HA | Fig. 3 | pIZT_BmKr-h1 | CCGGACTATGCAGGATCCTATCCATATGACGTTCCAGATTACGCTTGAGTTTATCTGACTAAATCTTAGTTTGTATTGTCATG |
| GACGTCATAGGGATAGCCCGCATAGTCAGGAACATCGTATGGGTATTCGAACCGCGGGCCCTCTAGAC | |||
| pIZT_BmKr-h1_V5 | Fig. 3 | pIZT_BmKr-h1 | CATCATCACCATCACCATACCGGTCATCATCACCATCACCATTGAG |
| GCCATGGTGATGGTGATGATGTTCGAACCGCGGGCCCTCTAGAC | |||
| pF25A_EcR | — | RACE cDNA | TTTGCGATCGCATGAGAGTCGAGAACGTGGATA |
| TTTGTTTAAACCTATAGCACCACCGGGTTG | |||
| pF25A_USP | — | RACE cDNA | TTTGCGATCGCATGGAGCCCTCAAGAGAATCA |
| TTTGTTTAAACCTACATGATGTTGGTGTCGATG | |||
| pF25A_EcR_HA | Fig. 4B and 5 A and B and Fig. S2 | pF25A_EcR | CCGGACTATGCAGGATCCTATCCATATGACGTTCCAGATTACGCTTGAGTTTAAACGAATTCGGGCTCGGTACCC |
| GACGTCATAGGGATAGCCCGCATAGTCAGGAACATCGTATGGGTATAGCACCACCGGGTTGGTGGGC | |||
| pF25A_USP_HA | Fig. 4B | pF25A_USP | CCGGACTATGCAGGATCCTATCCATATGACGTTCCAGATTACGCTTGAGTTTAAACGAATTCGGGCTCGGTACCC |
| GACGTCATAGGGATAGCCCGCATAGTCAGGAACATCGTATGGGTACATGATGTTGGTGTCGATGGGCGGC | |||
| pIZT BmKr-h1_ΔCtC | Fig. 5D | pIZT_BmKr-h1 | CCGCCAGCTACAGAATCATAGACCC |
| ATCAGACGGCAACGAAATGTAATGTACGG |
Next, we tested the interaction between BmEcR/USP and EcRE by EMSA (Fig. 4B). A specific shift band (shift 2) appeared when HA-tagged BmEcR (BmEcRE_HA) and BmUSP (BmUSP_HA) were added to the probe (Fig. 4B, lane 5), which supershifted with the addition of the anti-HA antibody (Fig. 4B, lane 8). This shift was not changed by the addition of 20E (Fig. 4B, lanes 10–12). These results reveal that BmEcR specifically binds to the BmE93AEcRE irrespective of the presence of 20E. Curiously, shift 2 was observed on addition of BmEcR alone (Fig. 4B, lane 3), implying that BmEcR alone can bind to the BmE93AEcRE. However, the possibility remains that a remnant USP in the cell extract of the TnT protein expression kit (derived from the Spodoptera frugiperda Sf21 cell line) formed a functional complex with BmEcR and bound the EcRE.
Molecular Mechanism of Transcriptional Repression by BmKr-h1.
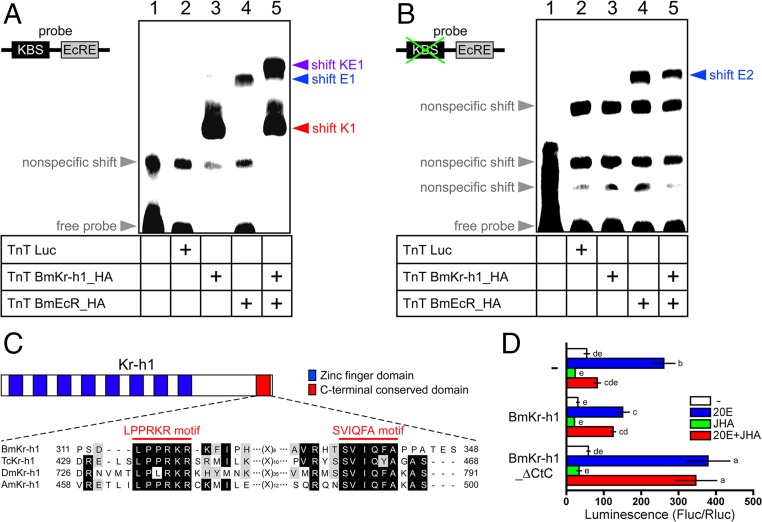
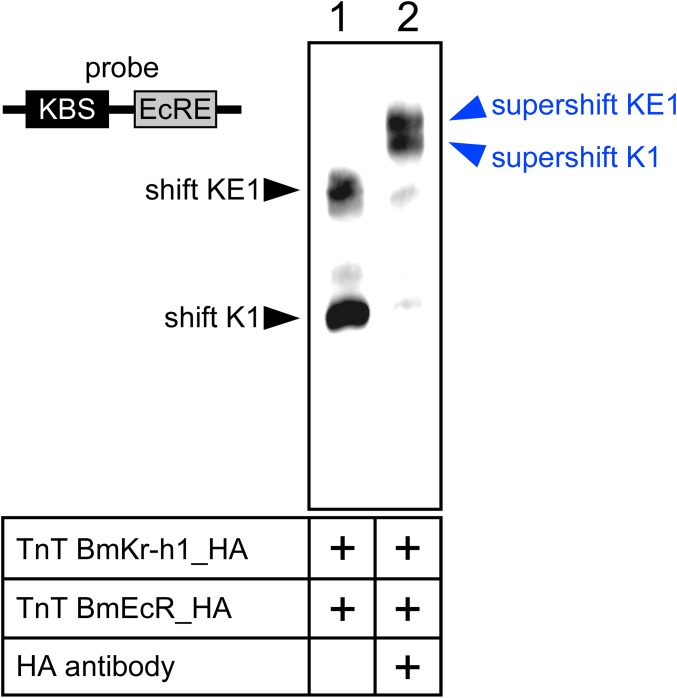
The aforementioned results facilitate us to explore the mechanism how BmKr-h1 represses the 20E-mediated BmE93A induction. Because the KBS and EcRE in the E93 promoter region were separated by only 28 bp (Fig. 2A), we hypothesized that binding of BmKr-h1 to the KBS sterically inhibits the interaction between BmEcR and EcRE. We verified this hypothesis by using EMSA with an oligonucleotide probe that contained the KBS and EcRE sequences in BmE93A (Fig. 5 A and B). Specific shift bands, shift K1 and shift E1, appeared with the addition of BmKr-h1_HA (Fig. 5A, lane 3) and BmEcR_HA (Fig. 5A, lane 4), respectively. The combination of BmKr-h1_HA and BmEcR_HA resulted in a new band (shift KE1) with a lower mobility than shift K1 and E1 (Fig. 5A, lane 5). The shift K1 and KE1 disappeared and were supershifted by the anti-HA tag antibody (Fig. S2), suggesting that shift K1, shift KE1, supershift K1, and supershift KE1 were composed of BmKr-h1, BmKr-h1/BmEcR, BmKr-h1/antibody, and BmKr-h1/BmEcR/antibody, respectively. By using a probe lacking KBS (Fig. 5B), no specific shift band was observed with BmKr-h1_HA (Fig. 5B, lane 3), whereas a specific shift band (shift E2) was observed with BmEcR_HA alone (Fig. 5B, lane 4) and with the combination of BmKr-h1 and BmEcR (Fig. 5B, lane 5). These results clearly indicate that BmKr-h1 and BmEcR independently bind to the KBS and EcRE, respectively, and that these two factors could simultaneously bind the adjacent sequences to form shift KE1. Therefore, our first hypothesis that physical interference occurs between BmKr-h1 and BmEcR on the BmE93A promoter is unlikely.
Fig. 5.
Molecular mechanism of BmKr-h1-mediated transcriptional repression. Concomitant BmKr-h1 and BmEcR binding was examined by EMSA with (A) WT and (B) KBS-mutated probes. (C) Schematic representation of the Kr-h1 structure and sequence alignment. Blue and red boxes indicate the zinc finger and CtC domains, respectively. Alignment of predicted amino acid sequences in the BmKr-h1 C terminus with those of T. castaneum Kr-h1 (TcKr-h1; GenBank accession no. NP_001129235), D. melanogaster Kr-h1 (DmKr-h1; GenBank accession no. NP_477467), and Apis mellifera Kr-h1 (AmKr-h1; GenBank accession no. AB642243). Black and light gray shading indicate identical and similar amino acid residues, respectively. Red bars indicate the putative protein interaction motifs (LPPRKR and SVIQFA) in the CtC domain. (D) Reporter assays using BmKr-h1 constructs lacking the CtC domain were performed to characterize CtC domain function. BmKr-h1_∆CtC refers to BmKr-h1 with the CtC domain deleted. NIAS-Bm-aff3 cells were cotransfected with a reporter plasmid carrying the BmE93A promoter region (−2909 to +290), a reference reporter plasmid, and the mutated BmKr-h1 expression plasmid. The cells were treated with 1 μM 20E and 10 μM JHA for 2 d, and reporter activity was measured by using a dual-luciferase reporter assay system. Data represent means ± SD (n = 3). Bars with the same letter are not significantly different (Tukey–Kramer test, α = 0.05).
Fig. S2.
Specificities of the shifted bands in Fig. 5A were verified by using polyclonal antibodies against the HA tag. EMSA was performed following the same method used for the experiments in Figs. 4 and 5.
More than 60% of BmKr-h1 is composed of zinc finger domains, and the remaining 40% shares no similarity with other insect Kr-h1s (15, 17). However, we discovered two distinctive motifs (LPPRKR and SVIQFA motif) in the Kr-h1 C terminus conserved in four holometabolous insects and termed this region the C-terminal conserved domain (CtC domain; Fig. 5C). To determine whether the CtC domain is involved in the transcriptional inhibitory activity of BmKr-h1, we performed reporter assays in NIAS-Bm-aff3 cells ectopically expressing WT BmKr-h1 or BmKr-h1 with deletion of the CtC domain (BmKr-h1_∆CtC). Notably, ectopic expression of native BmKr-h1 repressed the 20E-dependent activation of a reporter carrying the −2909 to +290 region of BmE93A (Fig. 5D). In contrast, deletion of the CtC domain abolished the repression by BmKr-h1, and ectopic expression of BmKr-h1_∆CtC inhibited the JH-mediated repression through a dominant-negative effect (Fig. 5D). Taken together, these findings support that the CtC domain in Kr-h1 contributes to JH-inducible transcriptional repression of target genes.
Discussion
The pupal stage in holometabolous insects is an important intermediary for the transition from larvae to adults. E93 gene expression during the pupal stage functions as an adult specifier and plays a key role in inducing programmed cell death necessary for tissue remodeling (30–33). Genetic studies have revealed that during the larval stage, JH suppresses E93 expression to prevent larvae from undergoing precocious metamorphosis to adults (34–36). In the present study, we clarified the molecular mechanism underlying the repression of E93 by JH.
RACE and developmental expression profiling revealed that BmE93 has distinct pupal (BmE93A) and adult isoforms (BmE93B) that differ in their transcription start sites. In experiments using cultured epidermis, BmE93A was induced by 20E and repressed by JH, but neither hormone affected BmE93B expression, indicating that differential responses to these hormones enable the varied developmental expression of BmE93 isoforms. Given that both BmE93 isoforms share a common ORF and are involved in programmed cell death, it is likely that BmE93A and BmE93B function to disrupt larval and pupal traits in larval–pupal and pupal–adult metamorphosis, respectively. Additionally, in D. melanogaster, E93 is expressed widely in adult cells during the pupal stage and contributes to many patterning processes at the adult stage (44), suggesting that BmE93B might be required for the patterning process in B. mori.
A transient JH peak during the prepupal stage has been observed in some holometabolous insects and is suggested to prevent precocious adult development (45–47). A recent study using RNAi in T. castaneum showed that a peak of Kr-h1 in the prepupal stage suppressed premature E93 induction (36). However, because all previous studies were performed using whole individuals, it remains unknown whether the suppression of E93 by JH-induced Kr-h1 is a cell-autonomous event. In this study, by using NIAS-Bm-aff3 cells, we demonstrated that the suppression of BmE93A by BmKr-h1 is a cell-autonomous event. BmE93A expression was induced by 20E, and this induction was suppressed by JHA. This hormonal response is reminiscent of that observed in early pupal epidermal cells.
We have identified a putative KBS (−2221 to −2208) upstream of BmE93A that is similar to the KBS previously identified upstream of BmBR-C (28). Through reporter assays using a B. mori cell line, we confirmed that this sequence is indispensable for the JH-mediated suppression of BmE93A. EMSA experiments revealed that BmKr-h1 physically binds to the BmE93A KBS. Thus, the KBS in BmE93A is unequivocally a functional Kr-h1–binding sequence that mediates cell-autonomous JH-dependent suppression of BmE93A. Moreover, we identified a consensus KBS (TGACCTNNNNYAAC) by genome-wide ChIP-seq analysis, and the BmE93A KBS perfectly matches this consensus sequence. Interestingly, we previously observed that two Kr-h1 molecules were bound to the KBS in BmBR-C (28). In fact, subsequent analysis of this site revealed two consensus sequences, suggesting that the two BmKr-h1 molecules bind independently to the two KBSs in this region. Several amino acid residues in the α-helix of the C2H2 zinc finger enable its specific interaction with DNA (48). These residues in Kr-h1 are highly conserved in other insect species (15), suggesting that Kr-h1 homologs in other insects may also recognize the consensus KBS. Previous genetic analyses in some insects demonstrated that Kr-h1 represses BR-C and/or E93 (25, 35, 36). Therefore, the function and mechanism by which Kr-h1 represses the transcription of E93 and/or BR-C are essentially shared by a wide variety of insects.
We have identified an EcRE upstream of BmE93A and demonstrated that physically interacts with BmEcR and is responsible for the induction of BmE93A by 20E. Because the EcRE adjoins the KBS, it is plausible that BmKr-h1 bound to the KBS sterically interferes with the binding of BmEcR to the EcRE, thereby inhibiting induction of BmE93A by 20E; however, the findings of EMSA experiments rejected this simple hypothesis. We therefore alternatively hypothesize that E93 suppression is caused by interaction of a corepressor with Kr-h1. For example, Kr-h1 might interact with general corepressors, such as Groucho (Gro) and C-terminal binding protein (CtBP), which recruit histone deacetylases to inhibit transcription of target genes (49, 50). However, WRPW or PXDLS motifs, which DNA-binding transcription factors use to interact with Gro and CtBP (49, 50), are absent in Kr-h1. Rather, we have identified a CtC domain, which is common in various insects. Reporter assays confirmed that this domain is necessary for BmKr-h1 to function as a transcriptional suppressor. Thus, the CtC motif likely mediates a functional interaction between Kr-h1 and an unknown corepressor.
The pupal specifier BR-C is differentially regulated by Kr-h1 in holometabolous insects; it is induced in response to JH-mediated Kr-h1 expression at the prepupal and pupal stages, whereas Kr-h1 represses BR-C during the larval stage (17, 18, 24, 25, 28, 36). The transcription factor CSL [CBF1/Su(H)/Lag-1] recruits a corepressor complex in the absence of Notch signaling to repress its target genes (51); however, Notch signaling converts the CSL repressor complex to an activator complex, resulting in transcriptional activation of the target genes (51). Thus, the flexibility of Kr-h1 action on BR-C regulation may result from different cofactors such as Notch signaling. Alternatively, because Kr-h1 contains two putative protein interaction motifs (LPPRKR and SVIQFA) in its CtC domain, a coactivator and corepressor might individually interact with either motif. Further exploration of Kr-h1–interacting cofactors would provide a unified understanding of its role in BR-C regulation.
In conclusion, this study yielded important findings on JH-dependent gene regulation during metamorphosis in holometabolous insects. We have identified a consensus KBS sequence, and JH-inducible Kr-h1 directly binds to the KBS in the E93 promoter region and represses its transcription in a cell-autonomous manner. Moreover, we have identified a CtC motif that confers the inhibitory ability of Kr-h1. As this motif is common in holometabolous insects, further analysis of the CtC motif would provide a deep understanding of the fundamental mechanism underlying the “status quo action” of JH, which could provide a substantial contribution to the pest management field and to the effective utilization of insects.
Materials and Methods
A detailed description of the materials and methods used in this study is provided in SI Materials and Methods.
Cell Lines.
The NIAS-Bm-aff3 (52, 53) and BmN cell lines (Katakura) derived from the fat body and ovary of B. mori, respectively, were maintained at 25 °C in IPL-41 medium (Gibco, Invitrogen) containing 10% (vol/vol) FBS (HyClone).
Chemicals.
Methoprene (JH analog [JHA]; SDS Biotech) was a gift from Sho Sakurai, Division of Life Sciences, Graduate School of Natural Science and Technology, Kanazawa University, Japan. The 20E was purchased from Sigma-Aldrich.
SI Materials and Methods
5′RACE.
The BmE93 transcription start sites were identified by 5′RACE with Table S1 primers and the cDNA template using a GeneRacer kit (Invitrogen) as described previously (15). The PCR products were subcloned into the pCR4Blunt-TOPO vector (Invitrogen) and sequenced by using a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems).
Experimental Animals, Dissections, and Tissue Culture.
Bombyx mori (Kinsyu × Showa strain) was reared on an artificial diet (Nosan) at 25 °C under 12-h light/dark cycles. The epidermis was dissected at various stages in PBS solution (137 mM NaCl, 8 mM Na2HPO4, 2.7 mM KCl, and 1.5 mM KH2PO4, pH 7.4). Dorsal abdominal integuments of pupae were cultured in Grace’s insect medium (Gibco, Invitrogen) at 25 °C as previously described (27). The integuments were incubated in medium containing 1 μM 20E or 10 μM JHA, or both, for 24 h.
qPCR.
Total RNA was extracted from the epidermis and NIAS-Bm-aff3 cells with an RNeasy Plus mini kit (Qiagen) and used to synthesize cDNA with the PrimeScript RT reagent kit (Takara Bio). The primers designed to quantify the BmE93A and BmE93B transcripts are shown in Table S1, whereas the BmKr-h1 (common region of two isoforms) and BmRp49 primers were described previously (15). BmRp49 was used as an internal reference. Reactions were performed in a 10-μL volume containing template cDNA derived from 1 ng of total RNA, 5 μL of SYBR Premix Ex Taq (Takara Bio), and each primer at 0.2 μM using the LightCycler 480 real-time thermal cycler (Roche). The PCR conditions were 95 °C for 5 min, followed by 55 cycles of 95 °C for 5 s and 60 °C for 20 s. Relative gene expression was determined by the 2−ΔΔCt method (54).
Construction.
Genomic DNA was extracted from a silkworm of the Daizo p50 strain using conventional methods (55). The 5′-flank (−2909 to +290 region) of BmE93A was amplified from the genomic DNA by PCR using KOD FX DNA polymerase (Toyobo) and gene-specific primers tagged with attB1 or attB2 (Table S1). The amplified DNA was inserted into the pGL4.14 luciferase reporter vector (Promega) modified for the Gateway system (Invitrogen). Mutation vectors were constructed from pGL4.14_−2909/+290 by inverse PCR (iPCR) using the KOD Plus Mutagenesis Kit (Toyobo) with the primers listed in Table S1. The pIZT expression plasmids (Invitrogen) containing C-terminal HA- or V5-tagged BmKr-h1 (BmKr-h1_HA and _V5) were constructed by iPCR using the primers in Table S1 and pIZT_BmKr-h1 (28) as a template. For the in vitro transcription and translation system, BmEcR and BmUSP anchored with Sgf I and Pme I sites were amplified by PCR using primers listed in Table S1 and RACE cDNA. The amplified fragment was digested with Sgf I and Pme I and inserted into the pF25A ICE T7 Flexi plasmid (Promega). The pF25A BmEcR and BmUSP expression plasmids with a C-terminal HA epitope tag (BmEcR_HA and BmUSP_HA) were constructed by iPCR using pF25A_BmEcR or _BmUSP template and primers listed in Table S1.
dsRNA Synthesis.
MalE and BmKr-h1dsRNAs were synthesized as previously described (28).
Transfection and Reporter Assays.
NIAS-Bm-aff3 cells were seeded at a density of 1.5 × 105 cells per well in 200 μL of medium in a 96-well plate (Sumilon) 1 d before transfection. Transfection of NIAS-Bm-aff3 cells was performed by using Fugene HD (Promega). The pIZT_RLuc vector containing the Renilla luciferase gene was used as a reference for NIAS-Bm-aff3 cells (56). The transfected cells were treated with 20E or JHA and then analyzed by using the Dual-Luciferase reporter assay system (Promega) and a luminometer (ARVO; PerkinElmer) according to the manufacturer’s instructions.
ChIP-seq and ChIP-qPCR.
The pIZT_BmKr-h1_HA and pIZT_BmKr-h1_V5 plasmids were transfected into BmN cells with Fugene HD (Promega), and the transfected cells were subjected to Zeocin (InvivoGen) selection to establish stable lines. ChIP was performed by using the ChIP-IT Express Enzymatic Kit (Active Motif) with anti-HA or -V5 tag antibody (Abcam). The input and ChIP DNAs were treated with a TruSeq ChIP Sample Prep Kit (Illumina), and were sequenced by Illumina HiSeq 101-bp paired-end data (Hokkaido System Science). The raw paired reads were filtered by Trimmomatic (57) and then mapped to the silkworm reference genome downloaded from KAIKObase (sgp.dna.affrc.go.jp/KAIKObase/) (37) using Bowtie 2 (58). Peak callings were performed by using uniquely mapped paired reads by MACS2 (59). Motif analysis was performed against sequences within 500 bp of the identified peak summits by using MEME suite (60). To determine the quantities of enriched read sequences in the KBS region of BmE93A, the input and ChIP samples were analyzed by qPCR as described earlier with primers shown in Table S1. Relative enrichment was calculated as input (percentage) = ChIP quantity/input quantity × 100.
EMSA.
EMSA was performed using the LightShift Chemiluminescent EMSA Kit (Pierce Biotechnology). Oligonucleotides labeled with 5′-terminal Biotin-ON were purchased from Eurofins Genomics; the sequences are shown in Table S1. The oligonucleotide pairs were mixed, heated at 95 °C for 5 min, and left to anneal at room temperature for 1.5 h. The resultant dsDNA was used as a probe in EMSA reactions. Specific and mutated competitor probes (Table S1) were prepared by using the same method. The in vitro transcription and translation of BmKr-h1_HA, BmEcR_HA, BmUSP_HA, and luciferase (mock) were performed with the TnT T7 Insect Cell Extract Protein Expression System (Promega). Binding reactions were performed in a 10-μL volume containing 0.02 pmol of probe, 1 μL of TnT reaction, 1 μL of 10× Binding Buffer (Pierce), 0.5 μg of poly(dI-dC), 2.5% (vol/vol) glycerol, 0.05% Nonidet P-40, 10 mM KCl, 1 mM MgCl2, 1 mM ZnSO4, and 2 mM EDTA at 25 °C for 4 h. In competition and antibody assays, the reaction mixture was incubated with 100-fold (2 pmol) unlabeled competitors and 1 μL of tag antibody (Abcam), respectively. The samples were then electrophoresed at room temperature on a 4% (vol/vol) nondenaturing polyacrylamide gel in 0.5× TBE. The probes in electrophoresed gels were transferred to positively charged nylon membranes (Roche) by electrophoresis. The nylon membranes were then processed by using the LightShift Chemiluminescent EMSA Kit according to the manufacturer’s instructions and visualized with an LAS-3000 mini system (Fujifilm).
Acknowledgments
This work was supported by Japan Society for the Promotion of Science KAKENHI Grants 25850230 and 16K15072 (to T.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the DDBJ/EMBL/GenBank database [accession nos. LC177616 (BmE93A) and LC177617 (BmE93B)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1615423114/-/DCSupplemental.
References
- 1.Riddiford LM. Cellular and molecular actions of Juvenile hormone I. General considerations and premetamorphic actions. Adv Insect Physiol. 1994;24:213–274. [Google Scholar]
- 2.Ashburner M, Chihara C, Meltzer P, Richards G. Temporal control of puffing activity in polytene chromosomes. Cold Spring Harb Symp Quant Biol. 1974;38:655–662. doi: 10.1101/sqb.1974.038.01.070. [DOI] [PubMed] [Google Scholar]
- 3.Ashburner M. Puffs, genes, and hormones revisited. Cell. 1990;61(1):1–3. doi: 10.1016/0092-8674(90)90205-s. [DOI] [PubMed] [Google Scholar]
- 4.Dubrovsky EB. Hormonal cross talk in insect development. Trends Endocrinol Metab. 2005;16(1):6–11. doi: 10.1016/j.tem.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Nakagawa Y, Henrich VC. Arthropod nuclear receptors and their role in molting. FEBS J. 2009;276(21):6128–6157. doi: 10.1111/j.1742-4658.2009.07347.x. [DOI] [PubMed] [Google Scholar]
- 6.Riddiford LM. How does juvenile hormone control insect metamorphosis and reproduction? Gen Comp Endocrinol. 2012;179(3):477–484. doi: 10.1016/j.ygcen.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Jindra M, Palli SR, Riddiford LM. The juvenile hormone signaling pathway in insect development. Annu Rev Entomol. 2013;58:181–204. doi: 10.1146/annurev-ento-120811-153700. [DOI] [PubMed] [Google Scholar]
- 8.Jindra M, Belles X, Shinoda T. Molecular basis of juvenile hormone signaling. Curr Opin Insect Sci. 2015;11:39–46. doi: 10.1016/j.cois.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Wilson TG, Fabian J. A Drosophila melanogaster mutant resistant to a chemical analog of juvenile hormone. Dev Biol. 1986;118(1):190–201. doi: 10.1016/0012-1606(86)90087-4. [DOI] [PubMed] [Google Scholar]
- 10.Ashok M, Turner C, Wilson TG. Insect juvenile hormone resistance gene homology with the bHLH-PAS family of transcriptional regulators. Proc Natl Acad Sci USA. 1998;95(6):2761–2766. doi: 10.1073/pnas.95.6.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miura K, Oda M, Makita S, Chinzei Y. Characterization of the Drosophila Methoprene-tolerant gene product. Juvenile hormone binding and ligand-dependent gene regulation. FEBS J. 2005;272(5):1169–1178. doi: 10.1111/j.1742-4658.2005.04552.x. [DOI] [PubMed] [Google Scholar]
- 12.Charles J-P, et al. Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc Natl Acad Sci USA. 2011;108(52):21128–21133. doi: 10.1073/pnas.1116123109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li M, Mead EA, Zhu J. Heterodimer of two bHLH-PAS proteins mediates juvenile hormone-induced gene expression. Proc Natl Acad Sci USA. 2011;108(2):638–643. doi: 10.1073/pnas.1013914108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Z, Xu J, Sheng Z, Sui Y, Palli SR. Steroid receptor co-activator is required for juvenile hormone signal transduction through a bHLH-PAS transcription factor, methoprene tolerant. J Biol Chem. 2011;286(10):8437–8447. doi: 10.1074/jbc.M110.191684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kayukawa T, et al. Transcriptional regulation of juvenile hormone-mediated induction of Krüppel homolog 1, a repressor of insect metamorphosis. Proc Natl Acad Sci USA. 2012;109(29):11729–11734. doi: 10.1073/pnas.1204951109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kayukawa T, Tateishi K, Shinoda T. Establishment of a versatile cell line for juvenile hormone signaling analysis in Tribolium castaneum. Sci Rep. 2013;3:1570. doi: 10.1038/srep01570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minakuchi C, Zhou X, Riddiford LM. Krüppel homolog 1 (Kr-h1) mediates juvenile hormone action during metamorphosis of Drosophila melanogaster. Mech Dev. 2008;125(1-2):91–105. doi: 10.1016/j.mod.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minakuchi C, Namiki T, Shinoda T. Krüppel homolog 1, an early juvenile hormone-response gene downstream of Methoprene-tolerant, mediates its anti-metamorphic action in the red flour beetle Tribolium castaneum. Dev Biol. 2009;325(2):341–350. doi: 10.1016/j.ydbio.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Konopova B, Smykal V, Jindra M. Common and distinct roles of juvenile hormone signaling genes in metamorphosis of holometabolous and hemimetabolous insects. PLoS One. 2011;6(12):e28728. doi: 10.1371/journal.pone.0028728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lozano J, Belles X. Conserved repressive function of Krüppel homolog 1 on insect metamorphosis in hemimetabolous and holometabolous species. Sci Rep. 2011;1:163. doi: 10.1038/srep00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DiBello PR, Withers DA, Bayer CA, Fristrom JW, Guild GM. The Drosophila Broad-Complex encodes a family of related proteins containing zinc fingers. Genetics. 1991;129(2):385–397. doi: 10.1093/genetics/129.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiss I, Bencze G, Fodor G, Szabad J, Fristrom JW. Prepupal larval mosaics in Drosophila melanogaster. Nature. 1976;262(5564):136–138. doi: 10.1038/262136a0. [DOI] [PubMed] [Google Scholar]
- 23.Kiss I, Beaton AH, Tardiff J, Fristrom D, Fristrom JW. Interactions and developmental effects of mutations in the Broad-Complex of Drosophila melanogaster. Genetics. 1988;118(2):247–259. doi: 10.1093/genetics/118.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou X, Riddiford LM. Broad specifies pupal development and mediates the ‘status quo’ action of juvenile hormone on the pupal-adult transformation in Drosophila and Manduca. Development. 2002;129(9):2259–2269. doi: 10.1242/dev.129.9.2259. [DOI] [PubMed] [Google Scholar]
- 25.Huang J, et al. DPP-mediated TGFbeta signaling regulates juvenile hormone biosynthesis by activating the expression of juvenile hormone acid methyltransferase. Development. 2011;138(11):2283–2291. doi: 10.1242/dev.057687. [DOI] [PubMed] [Google Scholar]
- 26.Zhou B, Hiruma K, Shinoda T, Riddiford LM. Juvenile hormone prevents ecdysteroid-induced expression of broad complex RNAs in the epidermis of the tobacco hornworm, Manduca sexta. Dev Biol. 1998;203(2):233–244. doi: 10.1006/dbio.1998.9059. [DOI] [PubMed] [Google Scholar]
- 27.Muramatsu D, Kinjoh T, Shinoda T, Hiruma K. The role of 20-hydroxyecdysone and juvenile hormone in pupal commitment of the epidermis of the silkworm, Bombyx mori. Mech Dev. 2008;125(5-6):411–420. doi: 10.1016/j.mod.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Kayukawa T, et al. Krüppel homolog 1 inhibits insect metamorphosis via direct transcriptional repression of Broad-Complex, a pupal specifier gene. J Biol Chem. 2016;291(4):1751–1762. doi: 10.1074/jbc.M115.686121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baehrecke EH, Thummel CS. The Drosophila E93 gene from the 93F early puff displays stage- and tissue-specific regulation by 20-hydroxyecdysone. Dev Biol. 1995;171(1):85–97. doi: 10.1006/dbio.1995.1262. [DOI] [PubMed] [Google Scholar]
- 30.Lee CY, et al. E93 directs steroid-triggered programmed cell death in Drosophila. Mol Cell. 2000;6(2):433–443. doi: 10.1016/s1097-2765(00)00042-3. [DOI] [PubMed] [Google Scholar]
- 31.Lee CY, Baehrecke EH. Steroid regulation of autophagic programmed cell death during development. Development. 2001;128(8):1443–1455. doi: 10.1242/dev.128.8.1443. [DOI] [PubMed] [Google Scholar]
- 32.Liu H, Wang J, Li S. E93 predominantly transduces 20-hydroxyecdysone signaling to induce autophagy and caspase activity in Drosophila fat body. Insect Biochem Mol Biol. 2014;45:30–39. doi: 10.1016/j.ibmb.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Liu X, et al. 20-Hydroxyecdysone (20E) Primary response gene E93 modulates 20E signaling to promote Bombyx larval-pupal metamorphosis. J Biol Chem. 2015;290(45):27370–27383. doi: 10.1074/jbc.M115.687293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ureña E, Manjón C, Franch-Marro X, Martín D. Transcription factor E93 specifies adult metamorphosis in hemimetabolous and holometabolous insects. Proc Natl Acad Sci USA. 2014;111(19):7024–7029. doi: 10.1073/pnas.1401478111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belles X, Santos CG. The MEKRE93 (Methoprene tolerant-Krüppel homolog 1-E93) pathway in the regulation of insect metamorphosis, and the homology of the pupal stage. Insect Biochem Mol Biol. 2014;52:60–68. doi: 10.1016/j.ibmb.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Ureña E, Chafino S, Manjón C, Franch-Marro X, Martín D. The occurrence of the holometabolous pupal stage requires the interaction between E93, Krüppel homolog 1 and Broad-Complex. PLoS Genet. 2016;12(5):e1006020. doi: 10.1371/journal.pgen.1006020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimomura M, et al. KAIKObase: An integrated silkworm genome database and data mining tool. BMC Genomics. 2009;10:486. doi: 10.1186/1471-2164-10-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Satake S, Kaya M, Sakurai S. Hemolymph ecdysteroid titer and ecdysteroid-dependent developmental events in the last-larval stadium of the silkworm, Bombyx mori: Role of low ecdysteroid titer in larval-pupal metamorphosis and a reappraisal of the head critical period. J Insect Physiol. 1998;44(10):867–881. doi: 10.1016/s0022-1910(98)00075-4. [DOI] [PubMed] [Google Scholar]
- 39.Calvez B, Hirn M, De Reggi M. Ecdysone changes in the haemolymph to two silkworms (Bombyx mori and Philosamia cynthia) during larval and pupal development. FEBS Lett. 1976;72(1):57–61. doi: 10.1016/0014-5793(76)80898-8. [DOI] [PubMed] [Google Scholar]
- 40.Kaneko Y, Takaki K, Iwami M, Sakurai S. Developmental profile of annexin IX and its possible role in programmed cell death of the Bombyx mori anterior silk gland. Zoolog Sci. 2006;23(6):533–542. doi: 10.2108/zsj.23.533. [DOI] [PubMed] [Google Scholar]
- 41.Kamimura M, et al. Fungal ecdysteroid-22-oxidase, a new tool for manipulating ecdysteroid signaling and insect development. J Biol Chem. 2012;287(20):16488–16498. doi: 10.1074/jbc.M112.341180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kayukawa T, et al. Hormonal regulation and developmental role of Krüppel homolog 1, a repressor of metamorphosis, in the silkworm Bombyx mori. Dev Biol. 2014;388(1):48–56. doi: 10.1016/j.ydbio.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 43.Cherbas L, Lee K, Cherbas P. Identification of ecdysone response elements by analysis of the Drosophila Eip28/29 gene. Genes Dev. 1991;5(1):120–131. doi: 10.1101/gad.5.1.120. [DOI] [PubMed] [Google Scholar]
- 44.Mou X, Duncan DM, Baehrecke EH, Duncan I. Control of target gene specificity during metamorphosis by the steroid response gene E93. Proc Natl Acad Sci USA. 2012;109(8):2949–2954. doi: 10.1073/pnas.1117559109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams CM. Juvenile Hormone II. Its role in endocrine control of molting, pupation, and adult development in cecropia silkworm. Biol Bull. 1961;121:572–585. [Google Scholar]
- 46.Kiguchi K, Riddiford LM. Role of juvenile hormone in pupal development of tobacco hornworm, Manduca sexta. J Insect Physiol. 1978;24:673–680. [Google Scholar]
- 47.Nijout HF. The endocrine control of molting and metamorphosis. In: Nijout HF, editor. Insect hormones. Princeton Univ Press; Princeton, NJ: 1994. pp. 89–141. [Google Scholar]
- 48.Iuchi S. Three classes of C2H2 zinc finger proteins. Cell Mol Life Sci. 2001;58(4):625–635. doi: 10.1007/PL00000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turner J, Crossley M. The CtBP family: Enigmatic and enzymatic transcriptional co-repressors. Bio Essays. 2001;23(8):683–690. doi: 10.1002/bies.1097. [DOI] [PubMed] [Google Scholar]
- 50.Jennings BH, Ish-Horowicz D. The Groucho/TLE/Grg family of transcriptional co-repressors. Genome Biol. 2008;9(1):205. doi: 10.1186/gb-2008-9-1-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borggrefe T, Oswald F. Setting the stage for Notch: The DrosophilaSu(H)-Hairless Repressor Complex. PLoS Biol. 2016;14(7):e1002524. doi: 10.1371/journal.pbio.1002524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Imanishi S, Akiduki G, Haga A. 2002. Novel insect primary culture method by using newly developed media and extracellular matrix. In Vitro Cell Dev Biol 38:16-A.
- 53.Takahashi T, et al. Calreticulin is transiently induced after immunogen treatment in the fat body of the silkworm Bombyx mori. J Insect Biotechnol Sericol. 2006;75:79–84. [Google Scholar]
- 54.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔC(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 55.Sambrook J, Fritsch EF, Maniatis T. 1989. Isolation of high-molecular-weight DNA from mammalian cells. Molecular Cloning: A Laboratory Manual, eds Sambrook J, Fritsch EF, Maniatis T (Cold Spring Harbor Lab Press, Cold Spring Harbor, NY), 2nd ed, pp 14–19.
- 56.Kanamori Y, et al. A eukaryotic (insect) tricistronic mRNA encodes three proteins selected by context-dependent scanning. J Biol Chem. 2010;285(47):36933–36944. doi: 10.1074/jbc.M110.180398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bolger AM, Lohse M, Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y, et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9(9):R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bailey TL, et al. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009;37(Web server issue):W202-8. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]