Significance
We developed a platform technology to determine therapeutic mechanisms of human mesenchymal stromal stem cells (hMSCs) in a dorsal root ganglion coculture system and an intraspinal cord implantation model. The unique poly(lactic-co-glycolic) acid scaffolding augments hMSC stemness, engraftment, and function without neural transdifferentiation or mesenchymal lineage development, resulting in robust motosensory improvement, pain and tissue damage mitigation, and myelin preservation in adult rat spinal cord after injury. The scaffolded hMSC-derived neurotrophism, neurogenesis, angiogenesis, antiautoimmunity, and antiinflammation support the propriospinal network, neuromuscular junctions, and serotonergic reticulospinal reinnervation to activate the central pattern generator for restoring hindlimb locomotion. Our findings illuminate “recovery neurobiology”—i.e., the injured spinal cord may deploy polysynaptic neural circuits different from normal adulthood pathways for postinjury improvement.
Keywords: spinal cord injury, recovery neurobiology, mesenchymal stromal stem cell, PLGA, locomotion
Abstract
Mesenchymal stromal stem cells (MSCs) isolated from adult tissues offer tangible potential for regenerative medicine, given their feasibility for autologous transplantation. MSC research shows encouraging results in experimental stroke, amyotrophic lateral sclerosis, and neurotrauma models. However, further translational progress has been hampered by poor MSC graft survival, jeopardizing cellular and molecular bases for neural repair in vivo. We have devised an adult human bone marrow MSC (hMSC) delivery formula by investigating molecular events involving hMSCs incorporated in a uniquely designed poly(lactic-co-glycolic) acid scaffold, a clinically safe polymer, following inflammatory exposures in a dorsal root ganglion organotypic coculture system. Also, in rat T9–T10 hemisection spinal cord injury (SCI), we demonstrated that the tailored scaffolding maintained hMSC stemness, engraftment, and led to robust motosensory improvement, neuropathic pain and tissue damage mitigation, and myelin preservation. The scaffolded nontransdifferentiated hMSCs exerted multimodal effects of neurotrophism, angiogenesis, neurogenesis, antiautoimmunity, and antiinflammation. Hindlimb locomotion was restored by reestablished integrity of submidbrain circuits of serotonergic reticulospinal innervation at lumbar levels, the propriospinal projection network, neuromuscular junction, and central pattern generator, providing a platform for investigating molecular events underlying the repair impact of nondifferentiated hMSCs. Our approach enabled investigation of recovery neurobiology components for injured adult mammalian spinal cord that are different from those involved in normal neural function. The uncovered neural circuits and their molecular and cellular targets offer a biological underpinning for development of clinical rehabilitation therapies to treat disabilities and complications of SCI.
Repair of neurotrauma, stroke, and neurodegenerative diseases remains an unmet medical demand because of their pathophysiological complexity and the limited spontaneous healing capacity of adult mammalian CNS. Human mesenchymal stromal stem cells (hMSCs) offer autologous transplantation feasibility (1–4) and have been studied both experimentally and clinically for traumatic brain injury (TBI) and spinal cord injury (SCI) (5–8). Although MSCs possess homeostatic and proneurogenic activities (7, 9), studies relying on neural transdifferentiation (i.e., putative differentiations of MSCs into neural cells without reentering the pluripotency phase) did not show long-term functional improvement in SCI models. The poor outcomes were attributed mainly to suboptimal survival of MSCs, leaving key therapeutic mechanisms undetermined (10). We previously established a 3D cell delivery technology by seeding neural stem cells (NSCs) in biodegradable polymer scaffolds that significantly improved donor efficacy and enabled investigation of NSC repair mechanisms in the damaged CNS (11, 12). In the present study, to test whether hMSCs might facilitate SCI recovery via multimodal actions of neural protection, plasticity, antiinflammation, and angiogenesis, rather than by transdifferentiation (7, 9, 11–14), we designed a unique microtexture poly(lactic-co-glycolic) acid (PLGA) scaffold that maintains the stemness of hMSCs and in an organotypic dorsal root ganglion (DRG) coculture system, determined that inflammatory agents would induce both antiinflammatory and proneurogenic actions of the scaffolded hMSCs. Moreover, the multifaceted effects of hMSCs in the scaffold-improved survival and stemness status were comprehensively studied in vivo to probe the cellular and circuitry components underlying the “recovery neurobiology” as a defined concept, of injured adult rat spinal cords.
Results
Characterization of Stem Cell Biology of hMSCs in Vitro.
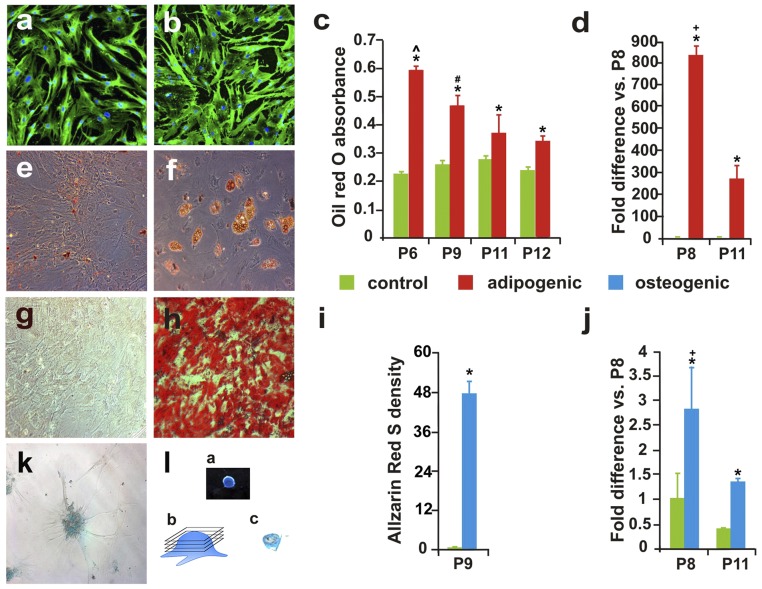
Following established protocols (3), we characterized the stemness status of hMSCs (MSCness) by the expression of representative markers and the capacity for phenotypic differentiation. The passage 6 (P6) hMSCs all expressed CD90 and CD105 markers (Fig. S1 A and B) and were capable of adipogenic, osteogenic, and chondrogenic differentiations as determined by Oil Red O (Fig. S1 C–F), Alizarin Red S (Fig. S1 G–I), and Alcian Blue staining (Fig. S1 K and L), respectively. hMSCs of P9–P12 also demonstrated definitive adipogenic differentiation, although in reduced levels compared with that of P6 cells (Fig. S1C). Additionally, real-time PCR was used to evaluate the adipogenic and osteogenic potencies of hMSCs by quantifying mRNA levels of adiponectin (Fig. S1D) and alkaline phosphatase (Fig. S1J), respectively. P8 cells showed significantly higher folds of mRNA expressions than P11 hMSCs undergoing differentiation. To test our hypothesis that homeostatic restoration of the host environment is a characteristic therapeutic impact of progenitor cells (9, 11), P6–P12 and P5–P7 hMSCs were used for the present in vitro and in vivo experiments, respectively (3, 13, 15).
Fig. S1.
hMSC quality control. (A and B) Immunostaining of MSC markers CD105 (A) and CD90 (B) for passage 12 (P12) hMSCs. (C) Oil Red O staining of adipogenic differentiation of P6–12 hMSCs (n = 6 per group; *, differentiation vs. control, P < 0.05, Student’s t test; ^, P6 vs. P9–12 and #, P9 vs. P11 and 12; P < 0.05, one-way ANOVA with Tukey’s post hoc test). (D) Real-time PCR showed mean expression levels of adiponectin (an adipogenic marker) in P8 and P11 hMSCs cultured in adipogenic or control medium (+, P8 vs. P11; statistical method per C). The real-time PCR finding was corroborated by Oil Red O staining of P12 and P6 hMSC-derived adipogenic cells shown in E and F, respectively. Alizarin Red S staining confirmed osteogenic capability of hMSCs. Whereas P9 hMSCs cultured in control condition showed no reactivity (G), the cells had high osteogenic yield after differentiation medium induction (H and I). Densitometric analysis of Alizarin Red S staining of P9 hMSCs cultured in osteogenic or control medium. Real-time PCR assay detected a significantly higher group mean level of ALP expression, an osteogenic marker, in P8 and P11 hMSCs cultured in osteogenic medium than in control culture and in P8 than in P11 hMSCs (J; statistical methods per C). Alcian Blue staining showed P9 cells with chondrogenic differentiation (K), and one of the intact chondrogenic pellets derived from hMSCs (L, a) that was cut (L, b) into 50-µm sections (L, c).
Antiautoimmunity Actions of Scaffolded hMSCs Determined in Vitro.
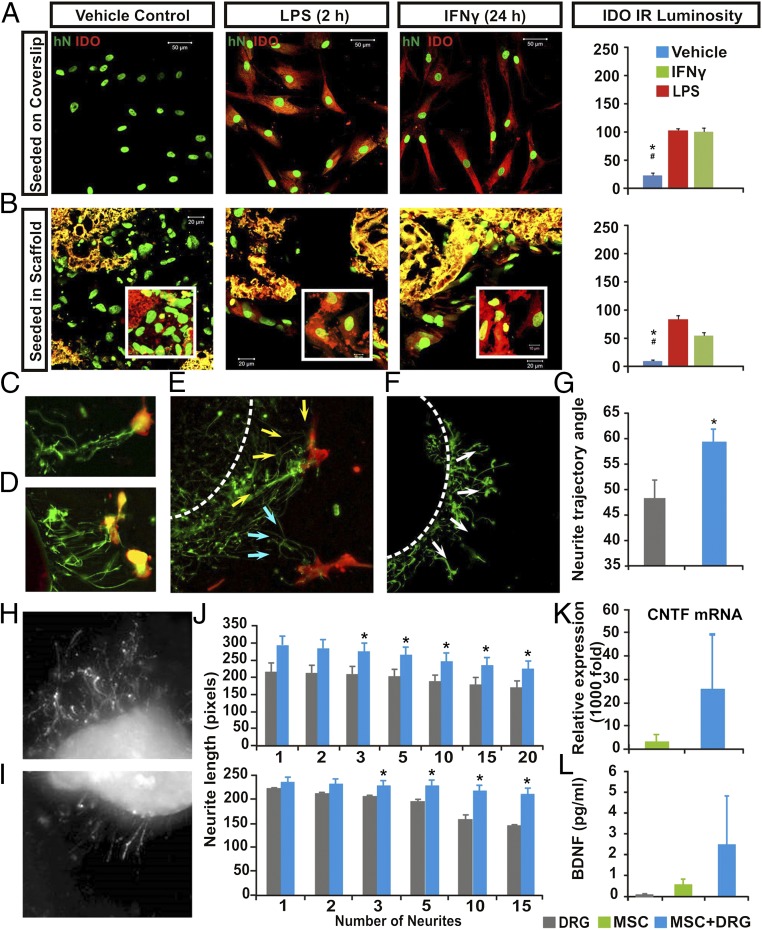
In addition to expressing immunosuppressive factors (e.g., IL-10, TGF-β, prostaglandin E2), hMSCs, after inflammatory insults, increase the expression of indoleamine 2,3-dioxygenase (IDO) that catabolizes tryptophan into kynurenine, picolinic acid, and quinolinic acid, resulting in tryptophan depletion. The combinatorial outcomes drastically eliminate the activated T-cells through apoptosis (15, 16). Although autoimmunity-triggered myelin loss has been reported to contribute to post-SCI disability (17), whether the IDO-mediated immunosuppressive effect is beneficial for repair of injured spinal cord remained to be investigated (18). We herein examined if IDO expression in hMSCs could be induced following direct exposure to the inflammatory mediator LPS, a trigger of autoimmune pathology, or to IFN-γ, a crucial factor for innate and adaptive immunity (19, 20). We found that a period of 2- or 24-h exposure to LPS and INF-γ, respectively, resulted in high levels of IDO expression in the hMSCs (Fig. 1A; P < 0.01), compared with negative IDO immunoreactivity (IR) in the control cells. Similarly, the same regimens of LPS and INF-γ stimulation significantly augmented IDO production in the PLGA-scaffolded hMSCs (Fig. 1B; P < 0.01), validating the study design to examine the construct’s immunomodulatory effect in injured spinal cord (21).
Fig. 1.
Multimodal effects of hMSCs in vitro. Compared with saline control, LPS or IFN exposure significantly augmented IDO expression (red) in both (A) nonscaffolded hMSCs and (B) PLGA-scaffolded hMSCs, as shown by human nuclei (hN) immunostaining (green; P < 0.05: *, control vs. LPS; #, control vs. IFN-γ; n = 5; one-way ANOVA with Tukey’s post hoc test). PLGA scaffolds showed yellow autofluorescence under dual channels. (C–E) DRG in organotypic coculture grew neurites (GAP43+, green) that track toward nearby hMSCs (CD90+, red; arrows), compared with (F) a more radial neurite pattern in DRG cultured with scaffold only (G). There were significantly more angular neurite paths in DRG and scaffolded hMSC cocultures (59.25 ± 2.9°) relative to the DRG and scaffold-alone group (48.15 ± 4.1°; P = 0.026, Student’s t test). Images showed different lengths of neurite outgrowth at the (H) distal and (I) proximal sites of axotomized DRG cocultured with PLGA-scaffolded hMSCs or scaffold alone, respectively. (J, Upper) The scaffolded hMSC and DRG cocultures had significant increases in mean total lengths when 3–20 neurites per DRG were averaged (*P = 0.032, n = 6, Mann–Whitney) and also (J, Lower) significantly increased the maximum absolute length of DRG neurite outgrow when 3–15 neurites per DRG were assessed (*P = 0.026, n = 6, Mann–Whitney). (K) Relative CNTF mRNA expression in scaffolded hMSCs was significantly elevated, as was (L) secretion of human BDNF (P < 0.05, one-way ANOVA) in the scaffolded hMSC + DRG coculture system.
Neurotherapeutic Mechanisms of PLGA-Scaffolded hMSCs Assessed in an Organotypic Coculture System.
To counteract the paucity of well-characterized biomimetic models that permit effective in vitro screening of the complex mechanisms underlying adult stem cell-mediated neural benefits, we established an organotypic DRG and stem cell coculture assay by plating hMSCs around adult rat DRG explants (Materials and Methods). Such coculture settings induced neurite outgrowth from axonomized DRG neurons. The regenerating DRG neurites responded to hMSCs by changing their path to track and home toward hMSCs in closest proximity (Fig. 1 C–E), in contrast to neurite behavior in a DRG-alone preparation, where the less-regrown neurites manifested a nontargeted projection pattern (Fig. 1F). We quantitatively compared the projection trajectory of neurite extension between the emergent segment and the terminal tip of the DRG after 48 h of coculture. The angle between the neurite directions at the two locations was taken as a measure of hMSCs’ neurotropic potency. Whereas the control DRG (cultured alone) showed an average neurite trajectory angle of 48.15° without targeting preference, DRG cocultured with hMSCs showed a significantly increased average neurite path angle of 59.25° (P = 0.026 relative to controls, Student’s t test), further diverging from the original trajectory to home toward hMSCs (Fig. 1G).
We next investigated whether this DRG axonal extension/homing was influenced by hMSC secretion of neurotropic and neurotrophic molecules. A unique type of PLGA scaffold with fine-tuned softness, smoothness, and pore size ranges (see details in Materials and Methods) was synthesized to maximize their protection of hMSC viability and stemness that are crucial for the functional multipotency of stem cells (9–13). Survival of seeded hMSCs with characteristic spreading morphology was supported by the tailored scaffold (Fig. S2A), and these hMSCs expressed BDNF and CD90 (Fig. S2 B and C), indicating neurotrophic/tropic factor production and stemness maintenance (3).
Fig. S2.
Characterization of scaffolded hMSCs. (A) H&E staining of hMSCs seeded in PLGA scaffold. The PLGA polymer scaffold has a porous, soft, and smooth texture (Materials and Methods). (B) Immunostaining revealed extensive BDNF expression in hMSCs seeded in PLGA scaffold (red, BDNF; blue, DAPI staining of cell nuclei; green, PLGA autofluorescence). (C) Immunocytochemical stain for CD90 showed a high level of hMSC engraftment in the tailored PLGA scaffold (red, CD90; blue, DAPI; green, PLGA autofluorescence).
In the organotypic DRG coculture system, hMSCs scaffolded in PLGA or PLGA coated with hMSC medium alone as controls were placed alternately 2 mm away from either the proximal or distal axotomy side of each explanted DRG. After 48-h culture, the length of regenerated neurites in cocultures was measured (e.g., scaffolded hMSC side: Fig. 1H; control side: Fig. 1I). We observed a significant increase in the mean length (22% increase, P = 0.03; Fig. 1J, Upper) and in the maximum absolute distance of growth (45% increase, P = 0.03; Fig. 1J, Lower) of neurites on the DRG side next to the scaffolded hMSCs in comparison with the opposite side exposed to the control scaffold alone. Moreover, the group average number of regrowing neurites was markedly increased on the side of DRG next to the scaffolded hMSCs, relative to the control side exposed to the control scaffold (288% increase; 98 ± 22 vs. 34 ± 6 neurites, n = 6; P < 0.001, paired Student’s t test). We also found that cocultured hMSCs, either scaffolded or not, expressed ciliary neurotrophic factor (CNTF) and BDNF, potent neurogenic and neuroprotective molecules (Fig. 1 K and L). The secretion of CNTF showed a pattern of dependence on target exposure, and BDNF secretion by hMSCs was significantly increased by the presence of dissected/injured DRG explants relative to the scaffold-only controls (P < 0.05; one-way ANOVA; Fig. 1I). Overall, the data suggested that the active context/target-dependent biological responses (i.e., functional multipotency) of the scaffolded hMSCs may hold therapeutic potential for treating SCI (22).
Evaluation of Antiinflammatory Effects of Scaffolded hMSCs in Vitro.
To simulate post-SCI inflammatory pathology (23) and evaluate the antiinflammatory potential of the scaffolded hMSCs, we challenged the organotypic DRG coculture system with LPS at 18 h after the assay initiation (20, 24). Following a dose escalation of LPS (20 pg/mL to 1,000 ng/mL), a graded increase of TNF-α expression was triggered; above the LPS dose of 1,000 ng/mL, TNF-α production in the DRG plateaued (Fig. S3A). DRG treated with 20 ng/mL LPS showed transient spikes of the proinflammatory cytokines TNF-α, IL-1β, and IL-6 expression, whereas coculture with PLGA-scaffolded hMSCs prevented these up-regulations (Fig. S3 B–D) by decreasing the group mean levels of mRNA expressions of TNF-α, IL-6, and IL-1β by 62%, 72%, and 65%, respectively (Fig. S3 E–G). The data demonstrated that we had established an in vitro cellular and molecular screening system for neural protection and repair events mediated by hMSCs (25).
Fig. S3.
Antiinflammatory mechanisms of hMSCs investigated in an organotypic coculture system of axotomized DRG explants. (A–D) Generation of LPS-induced inflammation in an original axotomized DRG explant model. (A) Adult female rat DRG were explanted and embedded in Matrigel and treated for 2 h with LPS at various concentrations. Relative mRNA expression of TNFα, an inflammatory marker, was quantified with real-time PCR. A dose-dependent effect of LPS on DRG expression of TNFα plateaued at LPS doses ≥1,000 ng/mL. (B–D) Rat DRG explants were treated for 0.5, 6, 12, or 24 h with 2 ng/mL LPS. Relative mRNA expression levels of TNFα (B), IL-1 (C), and IL-6 (D) characteristic inflammatory cytokines were quantified by real-time PCR. (E–G) Organotypic DRG and scaffolded hMSCs cocultures were treated with 10 ng/mL LPS for 2 h. Fold difference in rat inflammatory cytokine mRNA levels for (E) TNFα, (F) IL-1, and (G) IL-6 are expressed relative to those of control DRG cultured with PLGA scaffolds coated with hMSC-conditioned medium only. Coculture with scaffolded hMSCs significantly suppressed expression of proinflammatory cytokines by axotomized DRG after LPS exposure (*P < 0.05, n = 6 per group; one-way ANOVA with Tukey’s post hoc test).
Motosensory Recovery After SCI Resulting from Scaffolded hMSC Implantation in Vivo.
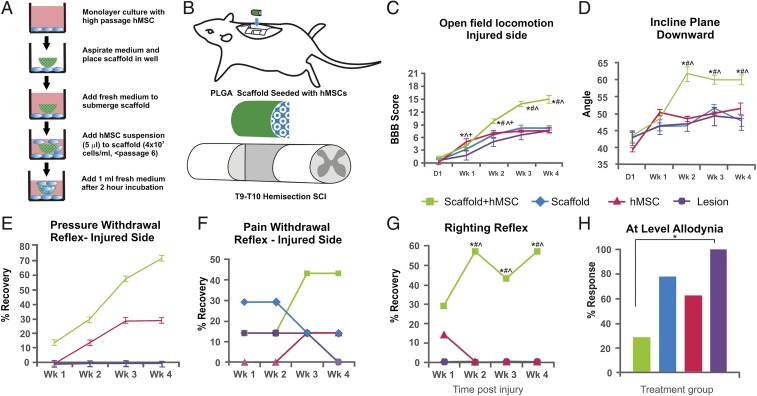
To test our therapeutic device in lesioned spinal cord, the PLGA-scaffolded hMSC construct (Fig. 2A) was surgically implanted into the injury epicenter immediately following T9–T10 midline hemisection in female adult Sprague-Dawley rats (Fig. 2B) (11). We monitored behavioral recovery, focusing on locomotion and evolution of neuropathic pain as clinically relevant outcome measures. The mean Basso, Beattie, and Bresnahan (BBB) score [an established open-field locomotion scale ranging from 0 (paralysis) to 21 (normal)] for the hindlimb ipsilateral to the injury site in the scaffolded hMSC treatment group was significantly higher throughout the 4 wk after SCI relative to control groups that received hMSCs alone, lesion alone, or scaffold alone (Fig. 2C; P < 0.05, repeated-measures ANOVA; n = 7/group). All rats were immunosuppressed with Prograf; Materials and Methods). In addition, an inclined plane assay was performed to test forelimb strength (i.e., general physical condition) in the upward-facing orientation and coordinated hindlimb motor function in the downward-facing orientation (11). The scaffolded hMSC-treated group achieved significantly higher (downward facing) inclined plane mean angle than all control groups (Fig. 2D), whereas no deficits in upward-facing performance were observed in any group.
Fig. 2.
Treatment designs and behavioral outcomes. Schematic presentations of (A) the in vitro hMSC seeding process for a PLGA scaffold and (B) design of the T9–T10 midline hemisection injury followed by implant insertion. Compared with three control groups, treatment with PLGA-scaffolded hMSCs significantly improved overall coordinated motor function as determined by (C) group mean BBB locomotion score of the hindlimb ipsilateral to injury and (D) inclined plane angle. Implantation of scaffolded hMSCs also significantly reduced occurrence of abnormal spinal reflexes in response to (E) pressure and (G) contact-triggered righting. Both (F) brief nociceptive pinch to the toe pads and (H) sensory tests at T9–T10 with standard 2- and 10-g Semmes-Weinstein filaments showed markedly higher hypersensitive responses in the controls relative to the scaffolded hMSC-treated rats (*, treated vs. lesion control, P < 0.05, Fisher’s exact test). Data points (n = 7) represent average ± SEM or percent with normal (E–G) or abnormal (H) responses of each group, analyzed with repeated-measures ANOVA that showed an overall significant effect of treatment (P < 0.05). Symbols indicate that means are significantly different from those of the lesion-only (*), scaffold-only (#), and hMSCs-alone (^) control groups at the specified times after injury (Tukey’s post hoc procedure or Student’s t test).
For changes in spinal reflexes, by 4 wk post-SCI, 71% of the scaffolded hMSC-treated rats displayed a normal reflex response to a brief pressure stimulus to the affected hindlimb, whereas none (0%) of the lesion-only controls showed normal reflexes (Fig. 2E); importantly, 57% of the scaffolded hMSC-treated rats were able to perform a normal contact righting reflex, in contrast to 0% of the lesion-only controls (Fig. 2F). Thus, the scaffolded hMSC treatment was highly beneficial in recovering systemic (e.g., proprioception regarding contact righting) and local (e.g., brief pressure-induced spinal reflex) neural function in the SCI rats.
Sensory perturbations are common debilitating complications of clinical SCI. We evaluated the effects of scaffolded hMSC treatment on postinjury development of hypersensitivity, a type of sensory impairment associated with neuropathic pain conditions such as allodynia (26). The hindlimb response to a quick nociceptive pinch (i.e., pain withdrawal reflex) demonstrated a marked reduction in incidence rate of hyperreflexia in the scaffolded hMSC-treated rats, compared with the controls (Fig. 2G). For more detailed analyses, a barrage of sensory tests with standard 2- and 10-g Semmes-Weinstein filaments was performed weekly for each rat at approximately dermatomes T9–T10, as “at-level neuropathic pain,” which is most frequently observed in clinical SCI (26). At 4 wk post-SCI, rats treated with scaffolded hMSCs demonstrated a significantly lower prevalence of hypersensitivity, relative to non–hMSC-treated controls (Fig. 2H), thus substantiating the therapeutic impact of scaffolded hMSC implantation for sensory disorders after SCI.
Histopathological Evaluation.
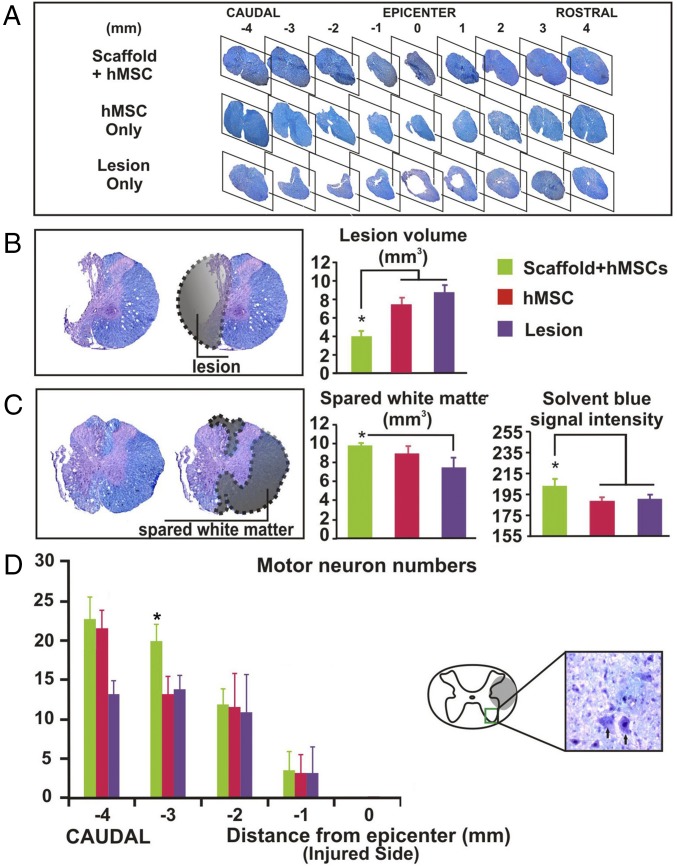
Solvent blue and hematoxylin-stained spinal cord sections showed complete degradation of the PLGA scaffold in vivo ≥6 wk after SCI (Fig. 3A). Based on assessment of tissue sparing in representative spinal cords of each group (i.e., tissue from SCI rats with BBB scores closest to the group mean; n = 4 per group), the scaffolded hMSC treatment showed the most extensive neuroprotection. In addition to an overall difference among groups (one-way ANOVA, P < 0.05), the mean lesion volume was 3.99 mm3 in the scaffolded hMSC group compared with 8.76 mm3 in the lesion-only control group (∼55% reduction in tissue loss; P < 0.05, Tukey’s post hoc test; Fig. 3B). We also quantified the white matter volume (Fig. 3C, Left) and ventral horn motor neuron number (Fig. 3D, Right) of the spinal cord tissue sampled at 1-mm intervals rostral and/or caudal to the injury epicenter of the same set of spinal cords (27, 28). Relative to the controls, treatment with scaffolded hMSCs significantly spared white matter in tissue adjacent to the epicenter and enhanced the morphologic integrity of myelin determined by the solvent blue stain (27) (Fig. 3C, Center and Right, respectively); it also significantly protected motor neurons in spinal cord loci caudal to the lesion epicenter (i.e., −1 to −4 mm; Fig. 3D, Left). We did not observe any ectopic growth/colonization or tumorigenesis of hMSCs in the CNS or in those internal organs collected (lungs, kidneys, liver, and spleen).
Fig. 3.
Histopathological analysis. Solvent blue- and hematoxylin-stained serial transverse spinal cord sections showed (A) that spinal cords with implanted scaffolded hMSC had more spared tissue around the lesion site than hMSC-alone and lesion-only controls and complete degradation of the PLGA scaffolds at ≥6 wk after SCI. Quantitative assessment of representative spinal cords (n = 4 per group) showed that relative to the controls, (B) scaffolded hMSC significantly reduced mean lesion volume and (C) increased white matter sparing in the spinal cord sections ±2 mm from the lesion epicenter. (D) Scaffolded hMSC implantation also preserved ventral horn motor neurons (Left) in spinal cord tissue caudal to the epicenter. Asterisks indicate a significant difference from the control group (P < 0.05, one-way ANOVA or repeated-measures ANOVA with Tukey’s post hoc test).
Fate and Multimodal Effects of Scaffolded hMSCs in Vivo.
hMSC engraftment was evaluated immunocytochemically (ICC) for expression of human CD90 (hCD90) or human heat shock protein 27 (hHSP27) in adjacent sections. We found that in contrast to controls treated with nonscaffolded hMSCs that showed no long-term survival of donor cells, ICC-positive cells for hHSP27 or hCD90 were both detected in the scaffolded hMSC-treated spinal cords, with no statistical difference in the mean number between the two ICC markers (Fig. 4A), suggesting that the scored cells were of human origin with MSCness. The number of such hMSCs was highest at the lesion epicenter and sharply decreased within the surrounding 1–2 mm of host parenchyma in either direction, denoting the critical role of PLGA scaffolding for hMSC survival (11) and a strong tendency of hMSCs to remain close to the lesion epicenter. Surviving hMSCs at the injury site even at ∼6 wk postinjury was, on average, ≤2–5% of the cell number implanted. These hMSCs distributed slightly more in the rostral compared with the caudal side of the epicenter, a pattern similar to that reported previously for motor neuron sparing at T8 contusion epicenter (28).
Fig. 4.
Analysis of fate and multimodal effects of scaffolded hMSCs in vivo. (A) Immunostaining for CD90 and human heat shock protein 27 (hHSP27) showed long-term survival (≥6 wk postinjury) only of scaffolded hMSCs (arrowed cells in Insets for the framed area). The engrafted hMSCs were mostly CD90+ and primarily concentrated in the lesion epicenter, with sharply reduced presence in the adjacent parenchyma. (B) Costaining for hHSP27 demonstrated cytoplasmic BDNF in the engrafted hMSCs (Left); relative to the controls, mean expression level increased nearly fourfold in the scaffolded hMSC-treated group (Right: ^, comparing with hMSC alone or *, lesion only; n = 5 per group; P < 0.05). (C) Costaining for CD90 showed IL-10 expression in the engrafted hMSC cytoplasm (Left); expression level of IL-10 was significantly higher in the scaffolded hMSC-treated spinal cords (^, comparing with hMSC-only group and *, to lesion-only group; +, hMSC only vs. lesion only; n = 5 per group; P < 0.05, one-way ANOVA with Tukey’s post hoc test). (D) Confocal analysis of the scaffolded hMSC-treated spinal cord tissue detected no discernible IR to collagen I, II, and IV or ALP (bone tissue marker), despite persistent presence of CD90+ signals, suggesting that no mesenchymal phenotypic differentiation of hMSCs occurred in the injured spinal cords ≥6 wk after transplantation. (E) Confocal z-stack imaging confirmed that IDO was expressed mainly in the cytoplasm of donor hMSCs (CD90+, green) in the subacutely injured spinal cord (i.e., 7–10 d postinjury). (F) The number of infiltrated CD3+ (red) T-cells was significantly decreased (G) in the white matter areas 3 mm rostral (Left) and caudal (Right) to the epicenter, and in the gray matter 3 mm caudal to the epicenter (G, Center). (H) Scaffolded hMSCs (CD90+, green) discernibly increased the number of macrophages manifesting M2 phenotype polarization (arginase 1 IR: green), whereas the number of activated microglia and macrophage (CD68+) was greatly reduced near the epicenter of the subacutely injured spinal cord, compared with lesion-only or hMSC-alone controls (n = 4 per group).
The functional multipotent status of hMSCs was ICC assessed by their expression of therapeutic mediators (Fig. 1 and Figs. S2 and S3) (9, 11, 15). Relative to the controls, BDNF expression was increased more than threefold (P < 0.05, one-way ANOVA with post hoc Student’s t test) in spinal cords of rats bearing scaffolded hMSCs (Fig. 4B, Right), elucidated in costaining for hHSP27 and DAPI (Fig. 4B). Expression of IL-10 (Fig. 4C, Right) was significantly higher in scaffolded hMSC-treated than in control spinal cords (P < 0.05, one-way ANOVA), largely in the remaining hMSCs, in contrast to the very limited number of host cells that showed IL-10 IR in the hMSC-alone group (Fig. 4C). We next evaluated expression of collagens I, II, and IV by ICC to determine if phenotypic differentiation of hMSCs occurred in the spinal cords ≥6 wk after injury and implantation. The lack of detectable collagen presence, confirmed by confocal microscopy, suggested that hMSCs did not undergo terminal mesenchymal differentiation. Also, there were neither hMSCs-derived osteocytes determined by alkaline phosphatase (ALP) IR (Fig. 4D, Bottom) nor Oil Red O-stained adipocytes. Last, based on our established protocols (14), we found no hMSC-derived neurons, astrocytes, or oligodendrocytes [verified by MAB1273 (Chemicon) staining for human mitochondrial antigen].
To determine whether the neural repair outcomes were derived specifically from multimodal actions of hMSCs (25), we examined the immunoregulatory capability of hMSCs (Fig. 1 A and B) to impede T-cells in the subacutely injured spinal cord (i.e., 7–10 d post-SCI; n = 3 per group). Despite administration of Prograf, CD3+ T cells were still present in the subacutely lesioned control tissue (21); PLGA-scaffolded hMSCs survived well and markedly increased expression of IDO (Fig. 4E) in the subacute SCI tissue, resulting in discernibly diminished presence of CD3+ T-cells in the white and gray matter at the epicenter (15, 25) (Fig. 4 F and G) and significantly mitigated invasion of iNOS-carrying mononuclear leukocytes (21, 25, 29) (Fig. 4H), which likely helped to protect myelin integrity (Fig. 3C). Compared with the lesion control tissue, scaffolded hMSCs with high IDO expression levels significantly polarized macrophage toward an arginase 1-positive M2 phenotype (Fig. 4H, Left and Center) inducible by IDO-generated tryptophan catabolites and considered to be beneficial for neural repair (30), but reduced the number of activated microglia and macrophage (CD68+) in the subacute lesion epicenter (21) (Fig. 4H, Right). Moreover, we noted that most donor hMSCs appeared to have died off by chronic postinjury stages. Thus, the tailored scaffolding approach to implanting hMSCs into adult spinal cord niche may have the combinatorial effect of preventing lineage differentiation, neural transdifferentiation, and tumor formation of MSCs.
Impediment of Chronic Inflammation.
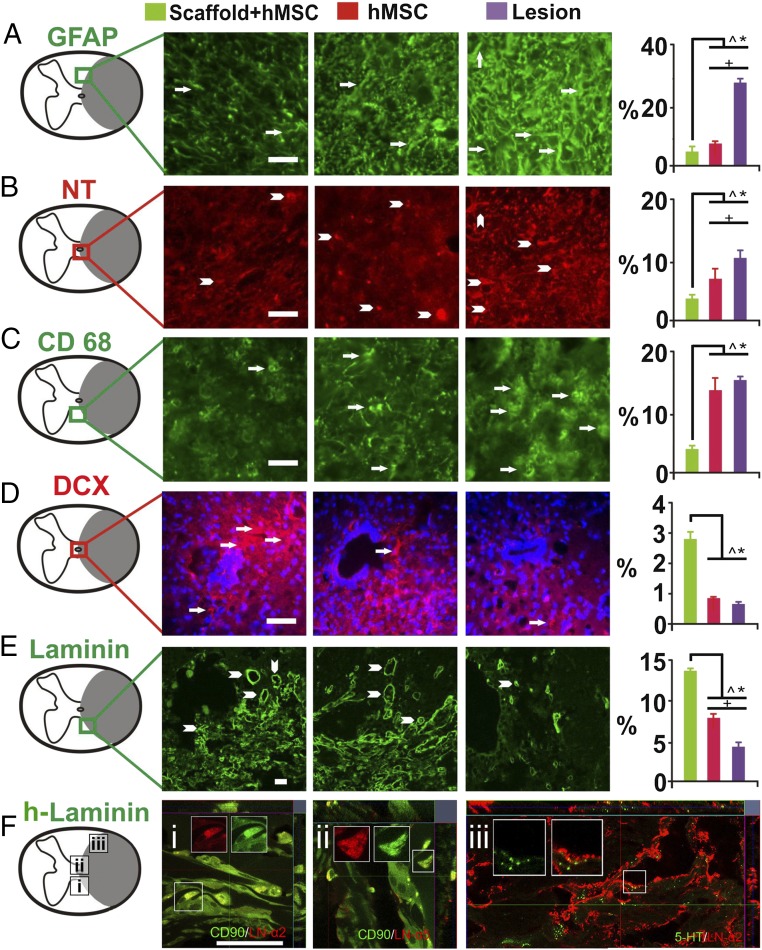
The inflammatory cascade paradoxically contributes to both postinjury neural damage and repair (23, 31), so that subtly tuned antiinflammation capability has become a focus for treating CNS trauma (32–34). Based on the antiinflammatory and antiautoimmunity effects of scaffolded hMSCs in vitro (Fig. 1) and in vivo (Fig. 4), we assessed transverse sections at and adjacent to the lesion epicenter and found that GFAP, a marker of inflammation-triggered reactive astrogliosis (11), was lowest in spinal cords treated with scaffolded hMSCs (Fig. 5A). In addition, IR for nitrotyrosine (Fig. 5B), a “fingerprint” of protein nitration that indicates oxidative damage (29), was also significantly reduced in the treated spinal cords. Furthermore, ICC staining for CD68 (Fig. 5C), a marker of activated microglia and macrophages (35), was significantly decreased in chronic SCI tissue following treatment with scaffolded hMSCs, compared with control groups. Relative to the lesion-alone controls, the PLGA scaffold-only implant did not trigger discernibly worsened inflammatory responses in either subacutely or chronically injured spinal cords.
Fig. 5.
Investigation of neural repair effects of scaffolded hMSC engraftment. Transverse spinal cord sections, 3 mm caudal to the injury epicenters, were immunostained for (A) GFAP for astrogliosis; (B) nitrotyrosine (NT) for oxidative damage; (C) CD68 for activated microglia (and vascular-derived macrophages); (D) DCX for endogenous NSCs; (E) laminin for angiogenesis; and (F) human laminin (h-laminin) to assess if donor hMSCs produced these glycoproteins to promote neurite outgrowth. The scaffolded hMSC treatment significantly reduced (Right) IR of GFAP, NT (protein nitration), and CD68. The treatment significantly augmented endogenous neurogenesis (arrows and arrowheads denote DCX+ NSCs in D) and increased angiogenesis in chronically lesioned spinal cord, indicated by the blood vessel-resembling morphology of pan-laminin IR (arrowheads in E). Moreover, h-laminin secretion from hMSCs (CD90+; green) near the epicenter in the subacutely injured spinal cord was detected (F, i: h-laminin α2 chain; ii: h-laminin α5 chain). Last, h-laminin deposition persisted chronically to ≥6 wk after injury in the same region (F, iii: h-laminin α2 chain) and showed intimate interactions with 5-HT+ axonal fibers, suggesting its promotion of serotonergic reinnervation (F, iii). (Scale bar: A–D, 50 µm: E, 20 µm; F, 25 µm.) P < 0.05, one-way ANOVA with Tukey’s post hoc test: *, scaffolded hMSCs vs. lesion only; #, scaffolded hMSCs vs. hMSC alone, and +, hMSC alone vs. lesion alone.
Neurobiological Mechanisms Underlying Post-SCI Recovery Following Implantation of Scaffolded hMSCs.
We first investigated the impact of donor cells on endogenous NSCs (36) in the host spinal cord on the inference that established roles of mesoderm–ectoderm interaction during development (37, 38) might partly apply in adult injury repair, and the known neural repair effects of activated endogenous NSCs (9, 14). ICC analysis of doublecortin (DCX), a marker of migratory NSCs, revealed the presence of DCX+ NSCs that are normally absent in the intact adult mammalian spinal cord (39). We found (Fig. 5D) the number of DCX+ pixels in NSCs in the scaffolded hMSC-treated adult spinal cords 2 mm caudal to the lesion site to be significantly increased by ∼314%, compared with lesion-only or hMSC-only controls. Although activation by donor hMSCs of endogenous NSC proliferation, migration, and differentiation was previously described in the brain (36), the significant difference between the rats treated with scaffolded and nonscaffolded hMSCs suggests that a synthetic matrix tailored to enhance hMSC survival and stemness potently augmented the location-specific impact of hMSCs on adult spinal cord neurogenesis post-SCI, providing therapeutic benefits (9, 39). We also examined whether intraparenchymal angiogenesis, an important primary tissue repair mechanism that can be induced by MSCs (41), was promoted by the scaffolded hMSC implant around the epicenter; a nearly fourfold increase in group mean IR against laminin, a primary angiogenic marker (41), was detected by L9393, a pan anti-laminin antibody, in the treated spinal cords relative to the controls (P < 0.05; Fig. 5E). The laminin+ structures show morphologic features of blood vessels.
Because laminins, particularly those containing chains α2 and α5, are unique extracellular matrix protein components in the adult CNS neurogenic niche, and in addition to their effects on angiogenesis also promote neurite outgrowth (42), we investigated by ICC whether human laminin containing chains α2 and α5 was deposited by donor hMSCs around the epicenter. Indeed, (Fig. 5F, i and ii) these chains were secreted by scaffolded hMSCs around the implantation site in the subacutely injured spinal cord, and were present in the chronically injured tissue immediately caudal to the epicenter, forming (Fig. 5F, iii) an intertwined pattern of distribution with the sprouting serotonergic (5HT) neurites, indicating likely support for serotonergic reinnervation.
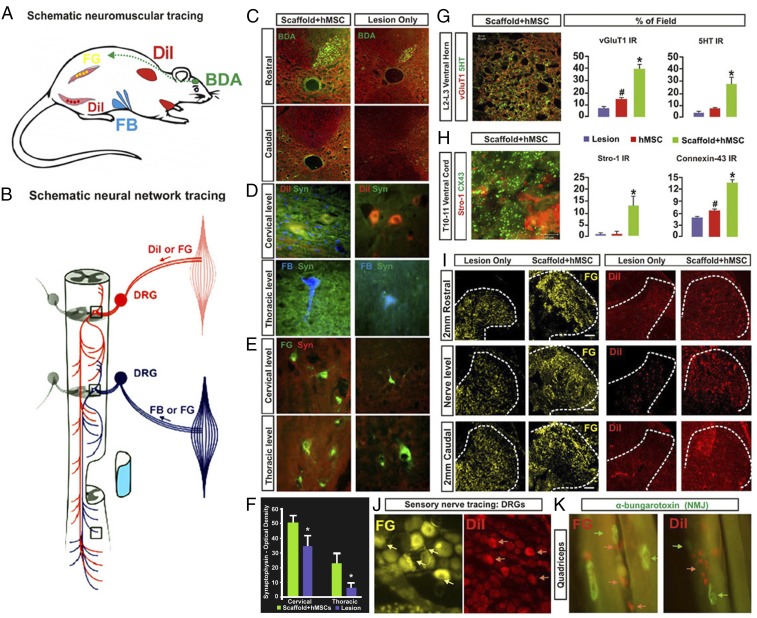
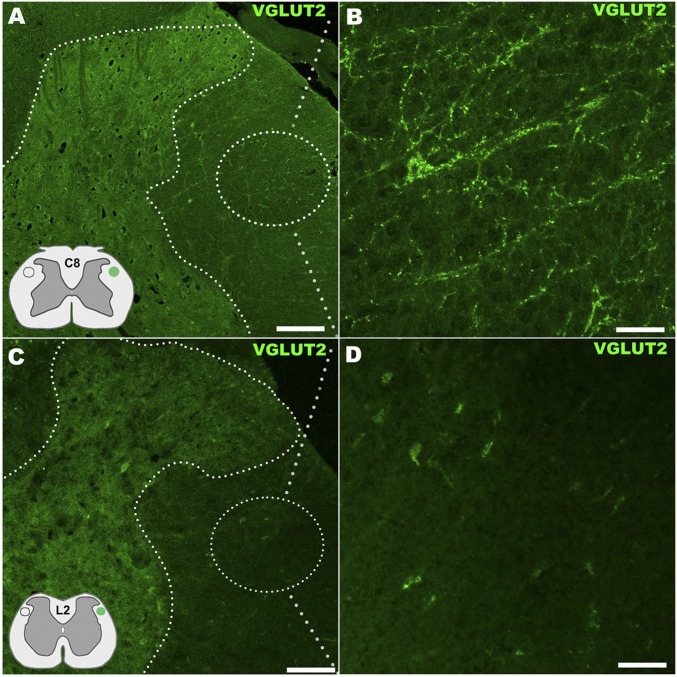
Our biotinylated dextran amine (BDA) anterograde tracing revealed no cross-epicenter regeneration of corticospinal tract (CST) axons in all spinal cord funiculi involved (e.g., Fig. 6 A and C showed the dorsal funiculus data) for all study groups. There was no evidence of rubrospinal tract (RST) regrowth beyond the lesion site, as determined (Fig. S4) by ICC of vGluT2, a primary marker of RST terminals (43). We thereby used a novel “peripheral–central” and “bottom-up” tracing strategy to elucidate the neural pathways responsible for the observed recovery of locomotion in the treated rats. We specifically investigated whether the rodent central pattern generator (CPG) for hindlimb locomotion might be partially activated by submidbrain inputs (40, 44); this was done by injecting different retrograde tracers [i.e., DiI, Fast Blue (FB), and Fluoro-Gold (FG)] into the cervical, thoracic, abdominal, and hindlimb muscles and performing 5HT ICC (Fig. 6 A and B; Materials and Methods) (40, 43, 44). We also immunochemically evaluated the distribution and quantity of synaptophysin, a pansynaptic vesicle protein and vGluT1, the primary proprioceptive terminal glutamate transporter interacting with propriospinal interneurons (PSNs) in Rexed's Laminae (RL) IV–VIII and RL IX motor neurons (45). We found that compared with the lesion-only controls, the spinal cords treated with scaffolded hMSCs had a significantly stronger mean IR for synaptophysin around the ventral horn motor neurons at the cervical and thoracic segments with higher signal levels of DiI and FB (Fig. 6D), respectively, and of FG (Fig. 6E; see statistics in Fig. 6F). The treated spinal cords also contained more 5HT+ fibers and vGluT1-positive buttons in the upper lumbar segments, suggesting improved plasticity of the propriospinal projection system that interacts actively with the 5HT+ reticulospinal fibers in the CPG region (Fig. 6G) (46–49). We also observed that at T10–T11, the engrafted and still undifferentiated hMSCs [i.e., stromal cell antigen 1 (STRO-1)+ cells] significantly augmented surrounding host cells’ expression of connexin 43 (Cx43), the main gap junction-forming protein unit between neural cells, NSCs and inflammatory cells (32, 50), compared with controls (Fig. 6H). Notably, scaffolded hMSC-treated spinal cords, relative to controls, showed nearly normal T6–T8 dorsal horn innervation of primary proprioceptive fibers (Fig. 6I) and DRG neuronal integrity (Fig. 6J) as assessed by intramuscular retrograde tracers, and reduced degeneration of hindlimb neuromuscular junctions (NMJs: labeled by α-bungarotoxin; Fig. 6K). Clearly, the scaffolded hMSC-treated SCI rats showed an overall enhancement of the proprioceptive network and NMJ integrity, which might work together with improved descending serotonergic facilitation to reanimate CPG for locomotion recovery.
Fig. 6.
Neurobiological benefits resulting from the scaffolded hMSC treatment. (A) Tracing regimens: BDA (green; injected into motosensory cortex contralateral to hemisection) for tracing the CST on the lesioned side, whereas DiI, Fast Blue (FB), and Fluoro-Gold (FG) were injected ipsilaterally into cervical region muscles and intercostal and abdominal muscles (Materials and Methods) to trace both primary proprioceptive axons that synapse with the PSN interneurons and with ventral horn motor neurons. DiI and FG were also injected into hindlimb muscles for retrograde tracing of lumbar motor neurons and primary afferent fibers. (B) Schematic of a simplified PSN network consisting of cross-segmental 1° and 2° neurites that project bilaterally and bidirectionally. (C) BDA IR was detected in the ipsilateral CST rostral to the injury epicenter, but not at any levels caudal to the lesion site in both scaffolded hMSC-treated and lesion-only spinal cords, suggesting no CST regeneration. (D and E) Images of DiI-, FB-, or FG-traced cervical and thoracic ventral horn motor neurons, respectively, and the synapses in the surrounding regions that were costained by antibody against synaptophysin (Syn, green), a synaptic vesicle marker. Scaffolded hMSCs-treated spinal cords showed much higher Syn IR density in both cervical and thoracic cord regions, compared with lesion-only (or hMSCs-only) controls (F; P < 0.05, paired Student’s t test, n = 5 per group). (G, Left) Tissue slices selected from upper lumbar segment 5 mm caudal to the epicenter were immunostained for 5-HT (red) and vGluT1 (green). A representative z-stack confocal plane (20 × 0.1 μm optical sections) of the Rexed Lamina VIII area is shown (Inset) at 600× magnification, and presents typical details of the pericellular distribution of axon terminal vGluT1 and beaded varicosities of 5HT. (Scale bar: 5 µm.) (G, Right) Group average IR of vGluT1 (red) that marks primary proprioceptive terminals and 5HT (green) in lumbar Rexed Lamina VIII were significantly stronger in scaffolded hMSC-treated spinal cords compared with controls. (H) Coimmunostaining of STRO-1 (red), an hMSC marker and Cx43 (green), a gap junction protein, revealed significantly higher signal density of Cx43 in the host ventral cord region of sections immediately caudal to the scaffolded hMSC implant site, compared with the control groups (*, scaffolded hMSC vs. hMSC only and lesion only; #, hMSC only vs. lesion only; P < 0.05, n = 5 per group; one-way ANOVA with Tukey’s post hoc test). Cx43 was expressed mainly by host cells (i.e., STRO-1–negative cells). (I) Retrograde tracing with FG or DiI via T6–T8 intercostal nerve showed FG+ or DiI+ primary afferent fibers and (J) DRG neurons. Scaffolded hMSC treatment drastically increased densities and area of both tracers, relative to the lesion controls, and DRG neuron integrity (J). (K) Motor nerve endings are in close contact with α-bungarotoxin+ nicotinic receptors in the muscles, showing near normal morphology of NMJs in the quadriceps of scaffolded hMSC-treated rats.
Fig. S4.
Assessment of RST innervation in the spinal cord by vGluT2 immunostain. (A) RST, located in the dorsal aspect of the lateral funiculus (Insets) showed innervation along the side of the spinal cord ipsilateral to and above T9–T10 hemisection in the scaffolded hMSC implantation group, with immunocytochemistry detecting vGluT2 that is carried by all RST terminals at C8 segment (area details in B). By contrast, no vGlut2 immunoreactive axon terminals or presence of RST neurites were detected in similar areas of spinal cord segments below the SCI site (C: at the L2 level with detail in D), indicating no regeneration of RST axons across the epicenter following scaffolded hMSC treatment. (Scale bars: A and C, 200 µm; B and D, 30 µm.)
Discussion
We have developed an hMSC-delivering platform technology by seeding hMSCs in a uniquely designed PLGA matrix to improve donor cell survival in a nondifferentiated state and in a localized engraftment that fosters implant–host interaction in vivo. Developed concurrently is a neurotrauma-relevant DRG coculture system for in vitro screening of multiple neurotherapeutic effects of stem cells. Our synthetic matrix supports hMSC survival, stemness, and function, avoiding phenotypic differentiation, ectopic donor cell engraftment, and tumorigenesis in lesioned adult rat spinal cord. The scaffolded hMSCs implanted at a T9–T10 hemitransection site robustly restore locomotor function and prevent neuropathic pain by mitigating T-cell–mediated demyelination and augmenting M2 macrophage polarization, by providing BDNF, CNTF, and IL-10 cytokines, serotonergic neurite sprouting, and morphologic integrity of thoracolumbar synapses, and by promoting angiogenesis, endogenous neurogenesis, and gap junction formation. Notably, these biological effects were exerted by hMSCs in the progenitor state, without mesenchymal lineage differentiation or neural transdifferentiation. Thereby, hMSCs may provide their therapeutic impacts mainly through functional multipotency events, conducive to post-SCI coordination of four components essential for hindlimb locomotor recovery: (i) serotonergic reticulospinal reinnervation caudal to the lesion; (ii) propriospinal projection network plasticity; (iii) NMJ integrity; and (iv) CPG reactivation (40, 43–45). In regard to CST axonal regrowth as the frequently pursued central regenerative goal in treating SCI, these outcomes collectively introduce a different theoretical and experimental platform to investigate stem cell-based therapy that sets forth an interactive set of recovery neurobiology mechanisms for lesioned adult mammalian spinal cord.
Although the T9–T10 lesion paradigm we used does not directly model spinal cord contusion, the most common SCI seen clinically, it offers the special opportunity to investigate precisely lesion scale, secondary injury, donor cell fate, neuroprotection, neurite outgrowth, and intraspinal cord pathway plasticity (11, 29, 46). Targeting each of the multiple pathophysiological mechanisms underlying SCI individually has to date yielded a high number of proposals, indicating their inadequacy and occasional mutual contradictions (48). We and others have proposed that the complexity and heterogeneity of SCI pathology necessitates development of multimodal integrated mechanistic and therapeutic regimens (11, 46, 48). We now consider the scaffolded hMSCs to represent an effective strategy based on the multiple therapeutic mechanisms observed in the present study, especially the cooperative effects of tissue sparing, serotonergic axonal sprouting, and propriospinal plasticity. Previously, implantation of a PLGA scaffold by itself that had different texture and composition tailored to enhance spinal cord healing significantly improved function in the same rat SCI model (11). By contrast, the matrix of the present formulation designed to maintain MSCness, when used by itself as an implant, was not sufficiently efficacious for post-SCI hindlimb recovery. Our data suggest that a correctly formulated synthetic matrix is crucial for realization of the intended biological effects without triggering side effects. The porous and flexible attributes of the current PLGA scaffold are essential for maintaining the basic stemness biology of hMSCs, avoiding unwanted mesenchymal differentiation. Compared with other types of polymers, PLGA offers reliable chemical engineering properties that can be adapted to optimize stem cell seeding and fate control (11). It has been reported lately that substrate stiffness could regulate the stemness level of progenitor cells (51). hMSCs under our delivery regimen exercised their homeostatic function while undifferentiated, and later died off, probably by apoptosis, because no necrosis-triggered inflammatory signs were spotted. This process may describe a desirable cell fate for nonneural progenitor cells serving for SCI treatment. The donor cells interacted with host cells to produce molecules that ameliorate local pathology. There was up-regulation of IDO, M2 polarization, endogenous neurogenesis, and gap junction formation, accompanied by down-regulation of proinflammatory cytokines, T-cells, and M1 macrophages (22, 23, 32, 39, 50), all in site-specific actions realized through tailored PLGA scaffolding of hMSCs and in a manner essentially unachievable for repairing open wounds by hMSC-conditioned medium or nonscaffolded cells. Hence, the notion of implanting stem cells pre- or postdifferentiated to a neural lineage may be working at cross-purposes to the developmental imperatives of the multipotent cells in certain cases, particularly for systemic repair of complex organs such as the spinal cord and brain (25, 32, 33). Our results suggest that scaffolded hMSCs may be adaptable for implantation directly into the contused spinal cord of patients in the subacute phase after injury when autoimmune and inflammatory insults peak (4, 33, 41, 48).
Traditional belief has attributed functional recovery observed after SCI in rodents primarily to the regrowth of CST and/or RST axons. Nevertheless, following thoracic lesion of descending motor pathways, neuroplasticity also occurs more caudally in the PSN network and lumbar CPG, a semiautonomous neural circuit for locomotion (45, 49). Studies examining “spinalized” cats or humans with incomplete SCI have demonstrated that motor cues, such as weight-suspended treadmill training or treadmill walk, can reanimate the CPG and its reengagement of arm swing, respectively (52, 53). Previously, a combinatorial application of direct electrical stimulation or administration of 5-HT receptor agonists enabled locomotion in spinalized rodents with dual spinal cord hemisections by igniting the PSN network for CPG activation through relaying CST signals (44). The present data, however, show that scaffolded delivery of hMSCs restored locomotor function in adult rats with T9–10 hemisection without promoting CST and RST regeneration across the injury epicenter or neural transdifferentiation of hMSCs. Our deductive analysis reveals that functional improvement of the affected hindlimb was likely supported by CPG activation via a submidbrain circuitry that is driven by treatment-enhanced reciprocal communication among the serotonergic reticulospinal pathway, PSN, and the neuromuscular system secured by NMJ preservation. We observed significantly increased IR of vGluT1 and synaptophysin expression and augmented presence of serotonergic fibers in RL IV–VIII around the epicenter and in RL IX of lumbar segments in the treated spinal cord relative to the controls. It was shown that after unilateral pyramidal tract section, proprioceptive fibers could sprout into the denervated motoneuron region as an evidence of maladaptive plasticity (54). By contrast, our results demonstrate that the enhanced PSN network, together with improved serotonergic reinnervation and preserved NMJs resulting from scaffolded hMSC implantation, exemplify beneficial plastic adaptations; this, combined with better preserved dorsal horn proprioceptive innervation, may serve as a pivotal mechanism to reactivate CPG and impede neuropathic pain following SCI. We are currently analyzing selective lesion data generated by targeted transgenic models in mice to verify the essential role of each specific neural component in recovering locomotion after SCI.
In conclusion, our findings corroborate the newly emerging observation that adult mammalian spinal cord contains inherent circuits that may be therapeutically recruited and modulated to restore function posttrauma (8, 49, 53, 55). These results collectively suggest it is plausible that direct axonal connections from motor cortex neurons to spinal cord are not essential for reestablishment of basic locomotion after mammalian SCI (55–57). Our results help define recovery neurobiology: the lesioned adult CNS may use neural circuits not identical to those deployed under normal physiological conditions for functional improvement. Therefore, SCI repair studies, in addition to pursuing regeneration of the CST and RST, should investigate recovery requirements that are specific for restoring targeted function. Tailored polymer scaffolding for hMSC delivery offers new opportunities for adult stem cell-based approaches to understanding and treating neurotrauma and perhaps other types of neural degeneration (58).
Materials and Methods
Experimental group size in the in vivo study was set at n = 7 per group based on power analysis of the outcome measure data of a previous study (28). All rats survived the entire study and were tested behaviorally at day 1 and then weekly after injury through ≥4 wk after injury. Remaining information is detailed in SI Materials and Methods.
All surgical procedures were performed in strict compliance with the Laboratory Animal Welfare Act and the Guide for the Care of Use of Laboratory Animals (59) after review and approval by the Institutional Animal Care and Use Committee of the VA Boston Healthcare System or Harvard Medical School.
SI Materials and Methods
After ≥4 wk postinjury, rats were reanesthetized, and neurite tracers, in solution or soaked in a piece of gelfoam, were injected or implanted into the pertinent targets (see Neural Tracing Assays for detailed information). At the experiment’s end, the rats were euthanized and fixed by 4% (wt/vol) paraformaldehyde (PFA) perfusion for histopathological analyses of spinal cord and selected other organs. All values are expressed as mean ± SEM. Statistical significance was defined at the P < 0.05 level. The statistical tests are described in Results and figure legends.
hMSC Characterization and Culture.
hMSCs were obtained from a 26-y-old male donor (7023-R) and a 29-y-old female donor (7038-L) at the Tulane University Center for Gene Therapy, an NIH-funded cell distribution center (3). Standard characterization was performed by the Tulane University Center for Gene Therapy. At passage 2, more than 95% of cells expressed CD90, CD105, CD49c, CD29, CD59, CD73a, and CD44. Ninety to 95% expressed CD147 and HLA-I. Less than 5% expressed CD36, CD34, CD19, CD11b, CD45, CD117, CD3, CD14, CD79a, CD271, or CD106. Five to 10% expressed CD49b or CD184. Passage 3 hMSCs were first evaluated for a sufficient level of stemness based on their capability of >60% osteogenic and adipogenic differentiation under inductions determined by Von Kossa and Oil Red O staining, respectively. Such P3 hMSCs were plated at 5 × 103 cells/cm2 and cultured in DMEM supplemented with 1% penicillin/streptomycin (Invitrogen) and 0.1 mM nonessential amino acids (the combination was hereafter referred to as DMEM), plus 10% (vol/vol) MSC-qualified FBS (Invitrogen) and 1 ng/mL bFGF at 37 °C under a 5% (vol/vol) CO2 atmosphere. hMSCs were passaged 1:2 every 6–7 d when they were at 80–90% confluency. hMSCs used in the in vitro and in vivo experiments were between passages 6 and 12 and 5 and 7, respectively; viability during passages was >98%. We did not observe any discernible difference between cell lines from the two donors and used the cells in approximately equal numbers in our assays.
hMSCs were plated at 5 × 103 cells/cm2 onto glass coverslips in 12-well plates for 1 wk and then fixed for 30 min in 4% PFA in PBS at 37 °C for ICC assays. Cell differentiation into adipogenic/osteogenic/chondrogenic cells is shown Fig. S1. Commercial Oil Red O/Alizarin Red S/Alcian Blue staining kits (StemPro; Invitrogen) were used according to the manufacturer’s instructions. ICC for confirmation of MSCness was performed with antibodies against CD90 (BD Pharmingen, BD 555593; 1:200) and CD105 (Chemicon, MAB2152; 1:200), with the secondary antibody applied alone as a negative control. Stained cell-seeded coverslips were mounted onto slides in an antifade mounting medium containing DAPI (VectaShield; Vector Labs). For PCR analysis, well plates with differentiated cells were frozen at −80 °C after removal of culture medium, and the total RNA was extracted with the RNAqueous kit (Ambion) per manufacturer’s instructions. PCR protocol (Fig. S1) is described below.
DRG Extraction.
Adult female rats (∼250 g) were anesthetized with ketamine and xylazine (75 mg/kg and 10 mg/kg i.p., respectively). Bilateral DR ganglia from L6 to T11 were carefully dissected after laminectomy followed by euthanization. DR ganglia were immediately placed in ice-cold DMEM supplemented with 10% FBS. DRG were maintained in floating culture at 37 °C under a 5% CO2 atmosphere for up to 12 h before plating.
Tailored Fabrication of PLGA Scaffold.
Based on our previous work (11, 29, 46, 60), we used the following refined protocol to fabricate PLGA scaffolds tailored with unique porous, soft, and smooth features to maintain the stemness biology of hMSCs. The scaffolds (pore size diameters: 350–500 µm; dimensions: 1 × 2 × 3 mm for in vitro assays and 1 × 2 × 4 mm for in vivo implantation) were synthesized from a blend of 50:50 PLGA [PolySciTech; 75% (wt/wt), number average molecular weight, Mn ∼40,000] and a block copolymer of poly(lactic-co-glycolic acid)–polylysine [25% (wt/wt), PLGA block Mn ∼30,000, polylysine block Mn ∼2000]. The porous scaffold was made by a salt-leaching process. Briefly, 0.03–0.1% solution of the polymer blend in chloroform (wt/vol, which defines the degree of softness) was cast over salt with a particle diameter range of 350–500 µm inside a glass vial to ∼2:1 volume ratio (salt:PLGA solution); the solvent was allowed to evaporate under an organic solvent-resistant desiccator in a contained environment (61).
After the construct has dried, the salt/PLGA layer was cut/shaved off the glass and the construct was placed in sterilized distilled water in a container to leach the salt sufficiently by either soaking overnight followed by a series of fresh washes with sterile distilled water the following morning, or vice versa. The scaffolds were placed in sterile Petri dishes to further air-dry in a laminar flow hood for a few hours (with germicidal lamp turned off), the scaffold trimmed to the designed sizes and shapes, and individual scaffolds stored in a −20 °C freezer inside parafilm-sealed Eppendorf Safe-Lock microcentrifuge tubes. Importantly, we found that PLGA scaffolds in harder texture, more rigid and uneven fabrication patterns, and smaller pore sizes could induce mesenchymal phenotypic differentiation of hMSCs even under regular maintenance medium incubation, an outcome that should be avoided for therapeutic applications of hMSCs in the CNS.
Scaffold Incorporation of MSCs.
PLGA scaffolds were briefly dipped in sterile distilled water before treatment with 100% FBS overnight to promote cell adhesion and overall fiber smoothness; after washing with DMEM/10% FBS, they were placed in a low-adhesion six-well plate. hMSCs were suspended at 2 × 107 cells/mL DMEM/10% FBS, and then 6 mL of the cell suspension was dropped onto each piece of scaffold. Scaffolds were then incubated for 3 h at 37 °C under a 5% CO2 atmosphere to permit MSC attachment; during this time, scaffolds were constantly observed, and additional 6-mL aliquots of DMEM/10% FBS were added when scaffolds began to dry. After 3 h, 2 mL DMEM/10% FBS was added. The seeding procedure was repeated on each of the following 2 d, and hMSC-incorporated scaffolds (∼5.0 × 105 hMSCs per scaffold) were then incubated overnight until use on the following morning. For confirmation and quantification of seeding, MSC-incorporated scaffolds were fixed for 3 h in 4% PFA, cryosectioned at 50 µm (five sections per slide), and stained with H&E by a standard protocol (Fig. S2). The collective number of hMSCs per millimeter scaffold section was estimated by first obtaining the mean cell number (i.e., N) per 10-µm implant section that was averaged from four slices 250 µm apart from each other. The cell number estimate ∑ 1-mm scaffold = 100 × N, which was used to derive the total cell count per scaffold based on its length (e.g., length of an intraspinal cord implant: 4 mm; see above for implant dimension details).
A Unique Organotypic DRG Coculture System.
For cocultures used in neurite angle experiments and supernatant BDNF measurement, DRG plating was done by making a small pool of growth factor-reduced Matrigel (Corning) in the center of each well of a six-well plate. The growth factor-reduced Matrigel was then aspirated to leave a thin layer. One DRG was placed at the center of the Matrigel. After DR ganglia were seeded in all wells, the plate was incubated at 37 °C under a 5% CO2 atmosphere for 10 min. Wells were then filled with 2 mL DMEM/10% FBS. hMSCs were plated onto DRG-containing wells at 3 × 105 MSCs per well, and cocultures were maintained in 2 mL DMEM/10% FBS at 37 °C under a 5% CO2 atmosphere.
For cocultures used in neurite length and orientation experiments, a pool of 25 µL Matrigel was made in the center of a well in a 12-well plate. The DRG was dropped into the center of the pool. A 1 × 2 × 3-mm piece of hMSC-incorporated scaffold was quickly placed in the pool and positioned with forceps so that it was 2 mm away from either the proximal or distal axotomy site of the DRG before the Matrigel set. A nonseeded scaffold was then placed in the pool facing the opposite axotomy site so that each DRG could be used as its own control, and we could use the natural pseudounipolar morphology of adult DRG neurons in the explants to evaluate the length of regrown neurites. Additionally, the placement of hMSC-incorporated scaffolds was alternated to control for any potential differences in neuritogenic capacity between proximal and distal sides. Next, 75 µL of Matrigel was gently applied to coat the entire assembly, and the plate was incubated at 37 °C under a 5% CO2 atmosphere for 15 min to permit the Matrigel to solidify. Finally, 1 mL DMEM was added to each well, and cocultures were maintained at 37 °C under a 5% CO2 atmosphere; this allowed the generation of cocultures with a fixed distance between scaffolded hMSCs and DRG.
For cocultures in inflammation experiments, DR ganglia were plated in growth factor-reduced Matrigel as described above; however, 24-well plates were used, and a 1 × 2 × 3-mm piece of MSC-seeded scaffold was placed into the Matrigel next to the DRG before the Matrigel was allowed to set. After setting, 500 µL of medium was added to each well. At 24 h after plating, the medium was replaced by serum-free medium containing various concentrations of LPS (Sigma) to induce neuroinflammation.
Immunocytology for Cocultures.
Cocultures were fixed in 4% PFA in 0.1 M phosphate buffer at 37 °C for 3 h. After washing in PBS, cocultures were blocked for 1 h at room temperature in PBS supplemented with 2% (vol/vol) donkey serum and then incubated overnight at room temperature with primary antibodies against β3-tubulin (1:300), human heat shock protein 27 (1:300), or BDNF (1:300). After washing in PBS, incubation with FITC- and Texas Red-labeled secondary antibodies (1:200) was performed at room temperature for 3 h. Cocultures were immediately imaged after the final PBS wash while still immersed in PBS (mounting was not feasible because of the 3D structure of the cocultures; Fig. S2).
Imaging.
All imaging was performed with a Nikon Eclipse TE300 microscope equipped with a Spot RT-Slider CCD camera (Diagnostic Instruments) or a Zeiss Axiovert 200 microscope equipped with an Axiocam CCD camera (Zeiss) or a Zeiss LSM1 confocal microscope equipped with Zeiss Zen 2011 software set (Carl Zeiss Microimaging), with appropriate filters for FITC, Texas Red, and DAPI.
Quantification of Neurite Turning Behavior and Neurite Length.
Neurite orientation behavior was quantified by using the measure angle function of ImageJ software (NIH) to evaluate the angle between the direction of neurite extension at the point of emergence from the DRG and the point of termination at the neurite tip. Thus, neurite extension in a straight line would result in an angle of 0°. Neurite growth pattern was measured with the segmented line function of ImageJ software and defined as the length along the neurite from the point of emergence from the DRG to the point of termination at the tip when both sites were clearly determined. This measurement of neurite length accounted for the tortuosity of the neurites; i.e., if two neurites had the same absolute distance measured by a straight line between their endpoints and the DRG origin sites, the neurite that followed the straighter path without turning or switching back on itself would show a lower total pixel value (Fig. 1). To account for the absolute extension distance aforementioned, we also measured the maximum straight line distance from the point of emergence from the DRG to the end of the neurite, regardless of the path followed by the neurite or its tortuosity (Fig. 1).
ELISAs.
Supernatants were collected from cocultures, snap-frozen in liquid nitrogen, and stored at −80 °C. TNF-α and BDNF were quantified with ELISA kits (R&D Systems, Inc.) according to the manufacturer’s instructions with minor modifications. Optical density was measured in THERMOMax ELISA Reader (Molecular Devices) at a wavelength of 450 nm and a reference wavelength of 570 nm. Density values were correlated linearly with the concentrations of cytokine standards.
Real-Time PCR.
At predetermined times after the application of LPS, the medium was removed from the well plates, and the plates were frozen at −80 °C. While still frozen, DRG and scaffolded hMSCs were removed from the well plates with forceps and placed in the lysis buffer of the RNAqueous kit (Ambion) for total RNA extraction. Per the manufacturer’s instructions, extraction then proceeded and the extracted RNA was concentrated to 15 µL with sodium acetate and linear acrylamide. The RNA was treated with DNase to prevent contamination by genomic DNA. Reverse transcription was then performed with the SuperScript III kit (Invitrogen) per instructions of the manufacturer. Real-time PCR was performed with the 2× SYBR Green Master Mix (Applied Biosystems) per the manufacturer’s instructions on an Applied Biosystems 7900 HT Fast Real-Time PCR system, with a dissociation curve analysis performed to confirm the specificity of the reaction and the lack of primer dimerization. PCR primers were selected from previous reports or designed with Primer Express software. Relative quantification of the amplification data were performed with the ∆∆Ct method.
Spinal Cord Injury and Animal Care.
Surgeries were performed according to a randomized block design where the group size was set at n = 7. Based on power analysis of the outcome measure data of a previous study, there was an 87% probability of detecting an effect ≥50% in ventral horn neuronal sparing, and an 80% probability for detecting an effect ≥47% in intermediate lateral neuronal sparing at a spinal level 3 mm caudal to the injury epicenter (28). Female Sprague–Dawley rats were anesthetized with ketamine (75 mg/kg) and xylazine (10 mg/kg) and placed prone. Following a 3-cm midline incision and surrounding tissue dissection, laminectomy was made at the ninth and 10th thoracic vertebral levels (T9–T10). A midline incision was made with a no. 11 sharp-tip scalpel and transverse hemisections plus myelotomy were carried out to complete a unilateral (left- or right-sided) segmental hemisection, measuring 4 mm rostral–caudal. After hemostasis was achieved and lesion size consistency confirmed by a surgery assistant, PLGA scaffold seeded with hMSCs (∼5.0 × 105 hMSCs per scaffold), hMSCs-alone control (∼5.0 × 105 hMSCs/100 µL medium; i.e., hMSC-alone control), polymer alone, or 100 µL hMSC culture-conditioned medium (i.e., lesion-only control; n = 7 per group) was selected by an independent observer per a block design and administered by the surgeon into the hemisection cavity (Fig. 2) (11). Following implantation, the spinal musculature and fascia were approximated and closed with a 4-0 nonabsorbable suture. The skin was closed with standard wound clips and rats were daily administered Lactated Ringer’s solution (10 mL/day) and ketoprofen (Anafen, Merial; 5 mg/kg) s.c. for 5 d. Bladders were manually expressed twice daily for 5–7 d following SCI, until micturition returned via a reflex bladder. To immunosuppress the animals treated with hMSCs, all groups were given a standard dose of Prograf/Tacrolimus (Astellas; 1 µg/kg, s.c.) daily starting 2 d before surgery and daily afterward until perfusion (14).
Behavior Evaluation.
Behavioral analysis was conducted by two observers, blinded to the treatment identity. Coordinated motor activity was evaluated with the BBB locomotor rating scale (scored on a 21-point scale) (11). Ability to maintain stable body position was assessed with an inclined plane, with the highest degree of inclination defined as that at which the animal could maintain its position for 5 s on two consecutive trials. Reflex tests, including toe spread, withdrawal reflexes to extension, and pain and pressure, as well as placing and righting, were performed and graded as described previously (11). For more detailed sensory measurement, a barrage of sensory tests with standard 2- and 10-g Semmes-Weinstein filaments was performed weekly for each rat in the T9–T10 dermatomes, for detection of “at-level neuropathic pain”-like hypersensitivity response (35). All rats were tested on the first postoperative day and weekly thereafter throughout the entire study.
Neural Tracing Assays.
To evaluate whether the treatments with hMSC-seeded scaffolds enhanced repair of damaged spinal cord, 5–8 wk after the initial SCI surgery, rats were anesthetized as described above and placed on a Kopf stereotaxic frame. Biotinylated dextran amine (BDA; Molecular Probes, 10% wt/vol solution in PBS), an anterograde tracer, was injected into the sensorimotor cortex identified anatomically contralateral to the lesioned T9–T10 side for tracing of the CST as previously described (11). Gelfoam cubes (1 mm3) soaked in the retrograde tracer Fluoro-Gold (Fluorochrome Inc.) in 2% wt/vol solution in PBS or a few crystals of DiI (Molecular Probes, Inc.) were inserted through two small incisions on each side of the hindlimb, respectively, into the quadriceps. Animals were maintained for ≥2–4 wk and then killed. BDA signal was revealed histochemically on floating or mounted 20- to 30-µm spinal cord cross-sections with a Vector Elite ABC kit (Vector Laboratories) and DAB kit (Pierce Biotechnology). Alternatively, Fluorescein Avidin DCS (Vector Laboratories) was used to show BDA labeling, and FG or DiI signals were observed directly with a fluorescent microscope (Carl Zeiss USA).
A subset of rats also received intramuscular administration of FB (Polysciences Inc.) or DiI or FG, as retrograde fluorescent tracers, which, together with synaptophysin ICC (see below) was used to investigate the intraspinal proprioceptive neuronal projection integrity. At 4–8 wk postinjury, the rats were reanesthetized as aforedescribed. The left latissimus dorsi and left pectoralis major muscle innervated by the C7, C8, and T1 nerve roots were exposed. Gelfoams (1 mm3) soaked in 1.5 µL of 2% (wt/vol) DiI in dimethyl sulfoxide or 2% (wt/vol) FG aqueous solution or FB crystals were inserted into four different locations within each muscle at an approximate depth of 2 mm. For tracing T6–T8 motor nerve roots, 1 mm3 gelfoams soaked in 1.5 µL of 2% FB or FG in distilled water or FB crystals were inserted into four different locations within the left external intercostal muscles and anterior abdominus muscle. The soft tissue was closed with suture and standard skin clips. Two weeks later, the spinal cords and muscle samples were excised following euthanization and prepared in standard fashion for immunocytochemical analysis.
Perfusion and Tissue Processing.
Following intracardiac perfusion of 4% PFA (pH 7.4), spinal cords were dissected, postfixed overnight in 4% PFA, dehydrated overnight at 4 °C in 30% sucrose, and frozen in −50 °C isopentane. Two-centimeter blocks of the thoracic region of the cords including injury epicenters were embedded in Tissue-Tek OCT compound (Sakura Finetek) and cryosectioned at a slice thickness of 20 µm.
Histopathological Analysis.
Histopathology was analyzed after staining with H&E and solvent blue. Sections were imaged with a standard upright microscope (Carl Zeiss USA), and digital photographs taken and subsequently analyzed with Adobe Photoshop CS4 11.0.1 (Adobe). Quantifications of lesion volume and white matter sparing, as well as motor neuron quantification, were performed on the three rats in each treatment group whose behavior values most closely approximated the mean for that group. Lesion volume and white matter sparing were approximated by pixel number comparisons for the targeted regions (Fig. 3) (11). Counts of motor neuron number in the ventral horn on each spinal cord side were made per previous methods (28).
Immunocytochemistry.
Immunocytochemistry was performed on 20-µm mounted sections. Primary antibodies for inflammatory markers were against glial fibrillary acidic protein (anti-GFAP rabbit; Millipore; 1:1,000), CD11b (anti-CD11b mouse; AbD Serotec; 1:250), CD68 (anti-CD68 mouse; Chemicon; 1:250), and nitrotyrosine (anti-NT mouse; Santa Cruz; 1:250). ICC for endogenous neural stem cell activity was performed with primary antibodies against nestin (anti-nestin mouse; Santa Cruz; 1:200) and doublecortin (anti-DCX goat; Santa Cruz; 1:250). Angiogenesis was evaluated with primary antibodies to laminin (pan anti-laminin antibody L9393; Sigma; 1:60–100) and CD31 (anti-CD31 goat; Santa Cruz; 1:400). Neurotrophic factors in the transplants were evaluated with ICC primary antibodies against brain-derived neurotrophic factor (anti-BDNF chicken; Promega; 1:250) and IL-10 (anti-IL-10 mouse; Santa Cruz; 1:250). Antibodies against descending serotonin axons (5HT; Immunostar), synaptic vesicles (rabbit anti-synapsin I (1:500; Chemicon), and rabbit anti-synaptophysin (1:100; Zymed) based were used for ICC procedures. Antibodies to evaluate donor cell fate included STRO-1 (sc-47733; Santa Cruz; 1:200), collagen 1 (anti-col1 rabbit), collagen 2 (anti-col2 mouse), collagen 4 (anti-col4 mouse), and anti-ALP (all Santa Cruz; 1:250) as well as for lipids with Oil Red O (Sigma). Costaining for nuclei was performed with DAPI (VectaShield), and for human CD90 (anti-CD90 goat; Santa Cruz; 1:300), human heat shock protein (anti-HSP 27 rabbit; Stressgen Bioreagents; 1:250), and human mitochondria antigen (anti-mitochondria: MAB1273 mouse, Chemicon; 1:100–200). Secondary antibodies included donkey anti-rabbit FITC, donkey anti-mouse Texas Red, donkey anti-mouse FITC, DyLight 594 donkey anti-goat, donkey anti-chicken Texas Red, donkey anti-mouse FITC, and donkey anti-rabbit Texas Red (all Jackson ImmunoResearch and 1:250). For human laminin ICC, CD90 (1:400; Santa Cruz Biotechnology) together with anti-human laminin α5 chain (clone LAM-89; 1:1,000; Sigma) or anti-human laminin α2 chain (clone 5H2; 1:1,000; Millipore) were used with fluorescent chromogen-conjugated secondary antibodies (Jackson ImmunoResearch). To detect vGluT1, we used mouse monoclonal antibody against vGluT1 (1:1,000; EMD Millipore MABS132) plus goat polyclonal secondary antibody to mouse IgG (1:1,000–5000; ab6787; Texas Red; Abcam); for vGlut2, we used guinea pig anti vGluT2 polyclonal antibody (1:6,000; EMD Millipore AB2251) and secondary antibody of FITC-goat anti-guinea pig IgG.
Primary antibodies were generally incubated at 4 °C overnight, followed by secondary antibody incubation at room temperature for 1 h. Blocking was performed for 1 h at room temperature immediately before primary antibody incubation with donkey (or secondary antibody species matching) serum with 5% (wt/vol) BSA. For semiquantification of ICC, signal intensity was measured above a determined threshold level and divided by the total pixel count or units, to yield a percentage above threshold, or relative signal value in arbitrary units reported in Results.
Donor hMSC Survival.
Immunocytochemistry of scaffold + hMSC spinal cords was performed for HSP and DAPI (see above), and the number of surviving cells was reviewed with 5–40× micrographs of 20-µm sections at consecutive millimeters on either side of the section. The number of surviving human cells was estimated by dividing the spinal cord into eight sectors and manually counting the cell number in each sector. These numbers were summed for the total number of survival donor cells per section, and the same method was used with CD90 to confirm the donor cell survival trend in a spatial relationship.
Statistical Analysis.
Unless otherwise specified, statistics were performed with SPSS software version 19 (IBM Corp). Comparisons of behavioral data, motor neuron quantifications, and semiquantification of pixels (for histopathology and ICC) among study groups were performed with repeated-measures ANOVA or one-way ANOVA, followed with Tukey’s post hoc test. Statistical difference between two study groups was determined by the Student t-test. For comparing proportion difference, we used Fisher’s exact test. In all cases, statistical significance was set at P < 0.05.
Acknowledgments
Support for this work was provided by United States Department of Veterans Affairs Rehabilitation Research and Development Grant 1-I01-RX000308-01A1, Center for the Advancement of Science in Space and National Aeronautics and Space Administration Grant GA-2015-222, CIMT-DoD Grant (to Y.D.T.), and a Cele H. and William B. Rubin Family Fund, Inc. Grant for the Gordon Program (to Y.D.T. and R.D.Z.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1616340114/-/DCSupplemental.
References
- 1.Egusa H, et al. Downregulation of extracellular matrix-related gene clusters during osteogenic differentiation of human bone marrow- and adipose tissue-derived stromal cells. Tissue Eng. 2007;13(10):2589–2600. doi: 10.1089/ten.2007.0080. [DOI] [PubMed] [Google Scholar]
- 2.Connick P, et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: An open-label phase 2a proof-of-concept study. Lancet Neurol. 2012;11(2):150–156. doi: 10.1016/S1474-4422(11)70305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lennon DP, Schluchter MD, Caplan AI. The effect of extended first passage culture on the proliferation and differentiation of human marrow-derived mesenchymal stem cells. Stem Cells Transl Med. 2012;1(4):279–288. doi: 10.5966/sctm.2011-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prockop DJ. Concise review: Two negative feedback loops place mesenchymal stem/stromal cells at the center of early regulators of inflammation. Stem Cells. 2013;31(10):2042–2046. doi: 10.1002/stem.1400. [DOI] [PubMed] [Google Scholar]
- 5.Parr AM, Tator CH, Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 2007;40(7):609–619. doi: 10.1038/sj.bmt.1705757. [DOI] [PubMed] [Google Scholar]
- 6.Kumar AA, Kumar SR, Narayanan R, Arul K, Baskaran M. Autologous bone marrow derived mononuclear cell therapy for spinal cord injury: A phase I/II clinical safety and primary efficacy data. Exp Clin Transplant. 2009;7(4):241–248. [PubMed] [Google Scholar]
- 7.Saito F, et al. Administration of cultured autologous bone marrow stromal cells into cerebrospinal fluid in spinal injury patients: A pilot study. Restor Neurol Neurosci. 2012;30(2):127–136. doi: 10.3233/RNN-2011-0629. [DOI] [PubMed] [Google Scholar]
- 8.Jarocha D, et al. Preliminary study of autologous bone marrow nucleated cells transplantation in children with spinal cord injury. Stem Cells Transl Med. 2014;3(3):395–404. doi: 10.5966/sctm.2013-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teng YD, et al. Functional multipotency of stem cells: A conceptual review of neurotrophic factor-based evidence and its role in translational research. Curr Neuropharmacol. 2011;9(4):574–585. doi: 10.2174/157015911798376299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexanian AR, Kwok WM, Pravdic D, Maiman DJ, Fehlings MG. Survival of neurally induced mesenchymal cells may determine degree of motor recovery in injured spinal cord rats. Restor Neurol Neurosci. 2010;28(6):761–767. doi: 10.3233/RNN-2010-0547. [DOI] [PubMed] [Google Scholar]
- 11.Teng YD, et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci USA. 2002;99(5):3024–3029. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park KI, Teng YD, Snyder EY. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat Biotechnol. 2002;20(11):1111–1117. doi: 10.1038/nbt751. [DOI] [PubMed] [Google Scholar]
- 13.Quertainmont R, et al. Mesenchymal stem cell graft improves recovery after spinal cord injury in adult rats through neurotrophic and pro-angiogenic actions. PLoS One. 2012;7(6):e39500. doi: 10.1371/journal.pone.0039500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teng YD, et al. Multimodal actions of neural stem cells in a mouse model of ALS: a meta-analysis. Sci Transl Med. 2012;4(165):165ra164. doi: 10.1126/scitranslmed.3004579. [DOI] [PubMed] [Google Scholar]
- 15.Ren G, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27(8):1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- 16.Meisel R, et al. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103(12):4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 17.Lee KD, et al. FTY720 reduces inflammation and promotes functional recovery after spinal cord injury. J Neurotrauma. 2009;26(12):2335–2344. doi: 10.1089/neu.2008.0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffin MD, et al. Concise review: Adult mesenchymal stromal cell therapy for inflammatory diseases: How well are we joining the dots? Stem Cells. 2013;31(10):2033–2041. doi: 10.1002/stem.1452. [DOI] [PubMed] [Google Scholar]
- 19.Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 20.Chastain EM, Miller SD. Molecular mimicry as an inducing trigger for CNS autoimmune demyelinating disease. Immunol Rev. 2012;245(1):227–238. doi: 10.1111/j.1600-065X.2011.01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trivedi A, Olivas AD, Noble-Haeusslein LJ. Inflammation and spinal cord injury: Infiltrating leukocytes as determinants of injury and repair processes. Clin Neurosci Res. 2006;6(5):283–292. doi: 10.1016/j.cnr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cantinieaux D, et al. Conditioned medium from bone marrow-derived mesenchymal stem cells improves recovery after spinal cord injury in rats: An original strategy to avoid cell transplantation. PLoS One. 2013;8(8):e69515. doi: 10.1371/journal.pone.0069515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.David S, Zarruk JG, Ghasemlou N. Inflammatory pathways in spinal cord injury. Int Rev Neurobiol. 2012;106:127–152. doi: 10.1016/B978-0-12-407178-0.00006-5. [DOI] [PubMed] [Google Scholar]
- 24.Kondo S, Kohsaka S, Okabe S. Long-term changes of spine dynamics and microglia after transient peripheral immune response triggered by LPS in vivo. Mol Brain. 2011;4:27. doi: 10.1186/1756-6606-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ribeiro TB, et al. Neuroprotection and immunomodulation by xenografted human mesenchymal stem cells following spinal cord ventral root avulsion. Sci Rep. 2015;5:16167. doi: 10.1038/srep16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finnerup NB, et al. Phenotypes and predictors of pain following traumatic spinal cord injury: A prospective study. J Pain. 2014;15(1):40–48. doi: 10.1016/j.jpain.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg LJ, Teng YD, Wrathall JR. 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(f)quinoxaline reduces glial loss and acute white matter pathology after experimental spinal cord contusion. J Neurosci. 1999;19(1):464–475. doi: 10.1523/JNEUROSCI.19-01-00464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teng YD, Mocchetti I, Taveira-DaSilva AM, Gillis RA, Wrathall JR. Basic fibroblast growth factor increases long-term survival of spinal motor neurons and improves respiratory function after experimental spinal cord injury. J Neurosci. 1999;19(16):7037–7047. doi: 10.1523/JNEUROSCI.19-16-07037.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu D, et al. Blockade of peroxynitrite-induced neural stem cell death in the acutely injured spinal cord by drug-releasing polymer. Stem Cells. 2009;27(5):1212–1222. doi: 10.1002/stem.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.François M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20(1):187–195. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]
- 31.Schwab JM, Zhang Y, Kopp MA, Brommer B, Popovich PG. The paradox of chronic neuroinflammation, systemic immune suppression, autoimmunity after traumatic chronic spinal cord injury. Exp Neurol. 2014;258:121–129. doi: 10.1016/j.expneurol.2014.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cusimano M, et al. Transplanted neural stem/precursor cells instruct phagocytes and reduce secondary tissue damage in the injured spinal cord. Brain. 2012;135(Pt 2):447–460. doi: 10.1093/brain/awr339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DePaul MA, et al. Intravenous multipotent adult progenitor cell treatment decreases inflammation leading to functional recovery following spinal cord injury. Sci Rep. 2015;5:16795. doi: 10.1038/srep16795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scruggs BA, et al. Multipotent stromal cells alleviate inflammation, neuropathology, and symptoms associated with globoid cell leukodystrophy in the twitcher mouse. Stem Cells. 2013;31(8):1523–1534. doi: 10.1002/stem.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu D, et al. Alleviation of chronic pain following rat spinal cord compression injury with multimodal actions of huperzine A. Proc Natl Acad Sci USA. 2013;110(8):E746–E755. doi: 10.1073/pnas.1300083110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bao X, et al. Transplantation of human bone marrow-derived mesenchymal stem cells promotes behavioral recovery and endogenous neurogenesis after cerebral ischemia in rats. Brain Res. 2011;1367:103–113. doi: 10.1016/j.brainres.2010.10.063. [DOI] [PubMed] [Google Scholar]
- 37.Henrique D, Abranches E, Verrier L, Storey KG. Neuromesodermal progenitors and the making of the spinal cord. Development. 2015;142(17):2864–2875. doi: 10.1242/dev.119768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haragopal H, et al. Stemness enhancement of human neural stem cells following bone marrow MSC coculture. Cell Transplant. 2015;24(4):645–659. doi: 10.3727/096368915X687561. [DOI] [PubMed] [Google Scholar]
- 39.Sabelström H, et al. Resident neural stem cells restrict tissue damage and neuronal loss after spinal cord injury in mice. Science. 2013;342(6158):637–640. doi: 10.1126/science.1242576. [DOI] [PubMed] [Google Scholar]
- 40.Konya D, et al. Functional recovery in T13-L1 hemisected rats resulting from peripheral nerve rerouting: Role of central neuroplasticity. Regen Med. 2008;3(3):309–327. doi: 10.2217/17460751.3.3.309. [DOI] [PubMed] [Google Scholar]
- 41.Assi R, et al. Delivery of mesenchymal stem cells in biomimetic engineered scaffolds promotes healing of diabetic ulcers. Regen Med. 2016;11(3):245–260. doi: 10.2217/rme-2015-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kazanis I, et al. Quiescence and activation of stem and precursor cell populations in the subependymal zone of the mammalian brain are associated with distinct cellular and extracellular matrix signals. J Neurosci. 2010;30(29):9771–9781. doi: 10.1523/JNEUROSCI.0700-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Du Beau A, et al. Neurotransmitter phenotypes of descending systems in the rat lumbar spinal cord. Neuroscience. 2012;227:67–79. doi: 10.1016/j.neuroscience.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 44.Courtine G, et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci. 2009;12(10):1333–1342. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fukuhara K, et al. Specificity of monosynaptic sensory-motor connections imposed by repellent Sema3E-PlexinD1 signaling. Cell Reports. 2013;5(3):748–758. doi: 10.1016/j.celrep.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pritchard CD, et al. Establishing a model spinal cord injury in the African green monkey for the preclinical evaluation of biodegradable polymer scaffolds seeded with human neural stem cells. J Neurosci Methods. 2010;188(2):258–269. doi: 10.1016/j.jneumeth.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flynn JR, Graham BA, Galea MP, Callister RJ. The role of propriospinal interneurons in recovery from spinal cord injury. Neuropharmacology. 2011;60(5):809–822. doi: 10.1016/j.neuropharm.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 48.Kwon BK, et al. A systematic review of directly applied biologic therapies for acute spinal cord injury. J Neurotrauma. 2011;28(8):1589–1610. doi: 10.1089/neu.2009.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain. 2014;137(Pt 5):1394–1409. doi: 10.1093/brain/awu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jaderstad J, et al. Communication via gap junctions underlies early functional and beneficial interactions between grafted neural stem cells and the host. Proc Natl Acad Sci U S A. 2010;107(11):5184–5189. doi: 10.1073/pnas.0915134107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang C, Tibbitt MW, Basta L, Anseth KS. Mechanical memory and dosing influence stem cell fate. Nat Mater. 2014;13(6):645–652. doi: 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lovely RG, Gregor RJ, Roy RR, Edgerton VR. Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Exp Neurol. 1986;92(2):421–435. doi: 10.1016/0014-4886(86)90094-4. [DOI] [PubMed] [Google Scholar]
- 53.Tester NJ, et al. Device use, locomotor training and the presence of arm swing during treadmill walking after spinal cord injury. Spinal Cord. 2011;49(3):451–456. doi: 10.1038/sc.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan AM, Chakrabarty S, Kimura H, Martin JH. Selective corticospinal tract injury in the rat induces primary afferent fiber sprouting in the spinal cord and hyperreflexia. J Neurosci. 2012;32(37):12896–12908. doi: 10.1523/JNEUROSCI.6451-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawai R, et al. Motor cortex is required for learning but not for executing a motor skill. Neuron. 2015;86(3):800–812. doi: 10.1016/j.neuron.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grillner S. Role of motor cortex in movement challenged. Curr Biol. 2016;25:R490–R514. doi: 10.1016/j.cub.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 57.Jarocha D, Milczarek O, Wedrychowicz A, Kwiatkowski S, Majka M. Continuous improvement after multiple mesenchymal stem cell transplantations in a patient with complete spinal cord injury. Cell Transplant. 2015;24(4):661–672. doi: 10.3727/096368915X687796. [DOI] [PubMed] [Google Scholar]
- 58.Snyder EY, Teng YD. Stem cells and spinal cord repair. N Engl J Med. 2012;366(20):1940–1942. doi: 10.1056/NEJMcibr1200138. [DOI] [PubMed] [Google Scholar]
- 59.Committee on Care and Use of Laboratory Animals 1996. Guide for the Care and Use of Laboratory Animals (Natl Inst Health, Bethesda), DHHS Publ No (NIH) 85-23.
- 60.Lavik E, Teng YD, Snyder E, Langer R. Seeding neural stem cells on scaffolds of PGA, PLA, and their copolymers. Methods Mol Biol. 2002;198:89–97. doi: 10.1385/1-59259-186-8:89. [DOI] [PubMed] [Google Scholar]
- 61.Grayson AC, Cima MJ, Langer R. Size and temperature effects on poly(lactic-co-glycolic acid) degradation and microreservoir device performance. Biomaterials. 2005;26(14):2137–2145. doi: 10.1016/j.biomaterials.2004.06.033. [DOI] [PubMed] [Google Scholar]