Significance
Our knowledge of the functions required by extracellular bacterial pathogens to grow in host tissues is still limited. Most available information refers to studies conducted under laboratory growth conditions that mimic host environments but exclude the influence of the host immune system. Tissue dual RNA sequencing allows simultaneous transcript profiling of a pathogen and its infected host. This sensitive approach led to the identification of host immune responses and virulence-relevant bacterial functions that were not previously reported in the context of a Yersinia infection. Application of this tool will allow transcript profiling of other pathogens to unravel concealed gene functions that are crucial for survival in different host niches and will improve identification of potential drug targets.
Keywords: tissue dual RNA-seq, host–pathogen interaction, host-adapted metabolism, noncoding RNAs, Yersinia
Abstract
Pathogenic bacteria need to rapidly adjust their virulence and fitness program to prevent eradication by the host. So far, underlying adaptation processes that drive pathogenesis have mostly been studied in vitro, neglecting the true complexity of host-induced stimuli acting on the invading pathogen. In this study, we developed an unbiased experimental approach that allows simultaneous monitoring of genome-wide infection-linked transcriptional alterations of the host and colonizing extracellular pathogens. Using this tool for Yersinia pseudotuberculosis-infected lymphatic tissues, we revealed numerous alterations of host transcripts associated with inflammatory and acute-phase responses, coagulative activities, and transition metal ion sequestration, highlighting that the immune response is dominated by infiltrating neutrophils and elicits a mixed TH17/TH1 response. In consequence, the pathogen’s response is mainly directed to prevent phagocytic attacks. Yersinia up-regulates the gene and expression dose of the antiphagocytic type III secretion system (T3SS) and induces functions counteracting neutrophil-induced ion deprivation, radical stress, and nutritional restraints. Several conserved bacterial riboregulators were identified that impacted this response. The strongest influence on virulence was found for the loss of the carbon storage regulator (Csr) system, which is shown to be essential for the up-regulation of the T3SS on host cell contact. In summary, our established approach provides a powerful tool for the discovery of infection-specific stimuli, induced host and pathogen responses, and underlying regulatory processes.
All bacterial pathogens encounter rapidly changing environmental conditions and adverse host reactions during the course of infection. Accordingly, a dynamic cascade of events is initiated that triggers a global alteration of gene expression patterns in both interacting organisms to adapt for survival. Monitoring these infection-linked transcriptome alterations represents a powerful approach to identify virulence-related factors and regulatory processes that drive pathogenesis. Over the past years, RNA sequencing (RNA-seq) technologies have revolutionized the analyses of transcriptomes by enabling a highly accurate and sensitive transcriptional profiling on single-nucleotide resolution. Moreover, RNA-seq has permitted the identification of sensory RNA elements and numerous noncoding RNAs (ncRNAs) in pathogenic bacteria. A small subset of the few already characterized riboregulators was found to contribute to pathogenesis (1–3).
Most global gene expression studies of pathogens relied on laboratory growth conditions, which mimic certain host environments (4–6). Only a few RNA-seq studies have used cell culture systems or fluids of infected animals to profile pathogen gene expression in vivo, but they have commonly neglected the host response (7–9). Consequently, our knowledge about the temporal events and gene expression changes in both the pathogen and the host that drive pathogenesis is still very limited.
The recent development of “dual RNA-seq” (using probe-independent parallel cDNA sequencing) now offers the possibility to simultaneously profile RNA expression in the pathogen and infected host cells (10). Taking advantage of this technology, high-resolution transcriptome profiles have lately been obtained from HeLa cells invaded by Salmonella enterica serovar Typhimurium, primary human bronchial epithelial cells infected with Haemophilus influenza, and macrophages with intracellular Mycobacterium tuberculosis (11–13). These studies not only revealed novel insights into pathogen–host cell cross-talk, but they also illustrated the importance of ncRNAs for fine-tuned intracellular bacterial gene expression and how it manipulates host cells.
As a major drawback, this approach does not address pathogens that live and replicate in extracellular spaces either within tissues or on the surface of the epithelia that line body cavities during the natural course of an infection. Moreover, although cell culture models are immensely useful for dissecting out pathways and the molecular details, they exclude the influence of the host immune response and cannot substitute for the whole-animal/tissue context. To overcome this limitation, we established a fast, generic tissue dual RNA-seq approach, which allows comprehensive and simultaneous monitoring of infection-linked gene expression changes of the infected host and the colonizing pathogen. In contrast to traditional microarray-based studies, this approach is species-independent (i.e., it does not require the time-consuming design of pathogen- and host-specific custom chips) and can concurrently capture the full-transcript repertoire of the pathogen and the host. We successfully applied this method to investigate the global gene expression pattern of the enteropathogenic bacterium Yersinia pseudotuberculosis growing extracellularly in the gut-associated lymphoid follicles (Peyer’s patches) of infected mice. The obtained high-resolution transcriptomes confirmed known host–pathogen interactions and revealed insights into infection-specific stimuli that induce host responses and provoke bacterial gene expression driving pathogenesis. Collectively, our findings show that tissue dual RNA-seq is a fast and powerful tool that can be applied to a broad range of bacteria and host tissues/organs to discover virulence-related functions and defense reactions of the host.
Results and Discussion
Tissue Dual RNA-Seq of Y. pseudotuberculosis-Infected Peyer’s Patches.
We established tissue dual RNA-seq using Y. pseudotuberculosis proliferating in the gut-associated lymphoid tissue (Peyer’s patches) of infected mice. The Peyer’s patch is the primary replication site of enteropathogenic yersiniae after transcytosis of the intestinal epithelium. Inside the Peyer’s patches, the bacteria replicate extracellularly to high numbers (107–108 cfu/g tissue) (14). A striking feature of the pathology of an enteric Yersinia infection is inflammation of the intestinal tissue. The murine infection model, which mimics all aspects of the human disease, has been proven extremely valuable for the characterization of host responses (e.g., cytokine signaling pathways) involved in infection control (15–17). The well-defined host response associated with the colonization of the Peyer’s patches (mainly obtained with Yersinia enterocolitica) will allow us to prove the validity of our approach and helps to reveal different and hidden pathogen-triggered host reactions.
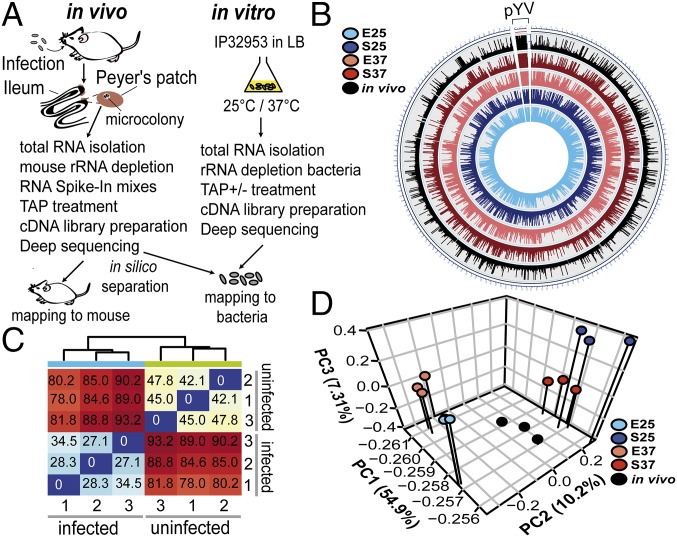
In our tissue dual RNA-seq approach, we systematically cataloged (i) the transcriptome of Y. pseudotuberculosis strain IP32953 grown in murine Peyer’s patches or under different laboratory growth conditions in vitro and (ii) the host transcriptome of uninfected and infected mice (Fig. 1A). To simultaneously capture pathogen and host transcripts, high-quality total RNA was isolated from Peyer’s patches 3 d postinfection (SI Appendix, Fig. S1A). Mouse rRNA, which represents the major RNA species within in vivo RNA pools, was depleted to increase coverage of informative mouse and bacterial transcripts (Fig. 1A). Commercially available RNA spike-in mixes covering a 106-fold concentration range were added to RNA pools from infected and uninfected tissues to determine the dynamic range within a sample and test for accurate fold change responses between different biological treatments (Fig. 1A and SI Appendix, Fig. S1 B and C) (18). Subsequent strand-specific Illumina-based deep sequencing (SI Appendix) generated ∼201 million cDNA reads from biological triplicate cDNA libraries of infected Peyer’s patches (Dataset S1). About 148 million reads mapped to the mm10 genome, whereas more than 8.5 million cDNA reads mapped to the Y. pseudotuberculosis genome. From cDNA libraries of uninfected Peyer’s patches, ∼114 million reads were generated, of which 87 million mapped to the Mus musculus mm10 genome (Dataset S1). Between ∼230,000 and 1.6 million uniquely mapped reads for the pathogen (grown in vivo and in vitro) and between 19 and 68 million mapped reads for the host were obtained from cDNA libraries, providing sufficient coverage for robust RNA profiling (Fig. 1B and Dataset S1) (19, 20). By using stringent filter criteria, we observed linearity between the read density and RNA input over the entire detection range of around 12 log2 units concentration (SI Appendix, Fig. S1B). This span was sufficient to reliably quantify and compare transcript abundance across a wide dynamic range (19, 20). Highly accurate fold change estimates are visualized by the linear fit (SI Appendix, Fig. S1C). Host and pathogen global gene expression profiles were distinct, and profiles of the three biological triplicates clustered together (Fig. 1 C and D). Normalized read counts for all detected mouse (SI Appendix, Fig. S2A) or Yersinia (SI Appendix, Fig. S2B) genes were plotted for the three biological replicates, and the Pearson correlation coefficient (r) is indicated. All samples closely correlated with their respective replicates from the same biological sample group (r > 0.9). For Yersinia, 1,312 transcriptional start sites of mRNAs (mTSSs) were mapped (Dataset S2), and 129 trans-encoded small ncRNAs, 45 antisense RNAs, and 23 3′-UTR–derived RNAs (Dataset S2) were identified. The 5′-UTR size distribution of mRNAs, the transcriptional start site (TSS) nucleotide use, and the core promoter structure are highly similar to Y. pseudotuberculosis YPIII (SI Appendix, Fig. S3) (5) and consistent with reports in Escherichia coli and Salmonella (21, 22).
Fig. 1.
Tissue dual RNA-seq workflow and global reports. (A) For tissue dual RNA-seq, female BALB/c mice were orally infected with 2 × 108 cfu Y. pseudotuberculosis IP32953 (infected) or 1× PBS (uninfected). For in vitro RNA-seq, IP32953 was grown in LB to exponential or stationary phase at 25 °C or 37 °C. Total RNA was isolated from Peyer’s patches of mice or bacterial cultures processed for preparation of strand-specific barcoded cDNA libraries and sequenced (SI Appendix). cDNA reads were separated in silico by mapping to the mm10 and the IP32953 genomes. TAP, tobacco acid pyrophosphatase. (B) Circos plot visualizing reads per kilobase transcript length per million mapped reads-normalized expression values of in vitro and in vivo RNA-seq data for the IP32953 chromosome (NC_006155.1) and pYV virulence plasmid (NC_006153.2). E25, exponential phase 25 °C; E37, exponential phase 37 °C; in vivo, infected Peyer’s patches; S25, stationary phase 25 °C; S37, stationary phase 37 °C. (C) Heat map of Euclidian sample distances of rlog-transformed read counts for triplicate mouse RNA-seq data from RNA pools of uninfected and infected Peyer’s patches. (D) Principal component (PC) analysis of mean centered and scaled rlog-transformed read count values of bacterial in vitro and in vivo RNA-seq data for the Y. pseudotuberculosis IP32953 core genome and the pYV virulence plasmid.
Yersinia-Induced Host Responses.
The host gene expression profile was calculated by the differential expression analysis package DESeq2 (23), and genes showing statistically significant changes in the expression level by at least fourfold were considered. Of 19,993 profiled host transcripts, 1,336 were significantly altered in abundance within infected Peyer’s patches (Fig. 2A and Dataset S3). Although 448 transcripts were more abundant as a response to the bacterial infection, 888 were less abundant (referred to as infection “induced” or “repressed” genes). As a consequence of the Yersinia infection, significant alterations in the host transcriptome were evident, with many of the highly induced genes being associated with (i) inflammatory responses, (ii) acute-phase responses, (iii) coagulative activities, and (iv) transition metal ion sequestration, which are among the top enriched pathways (Fig. 2B and Dataset S3). Detected changes in the abundance of host transcripts reflect expression changes but also, variations in the cell composition of the infected tissues (e.g., because of infiltrating immune cells). DESeq2 estimated fold change responses of selected host transcripts were confirmed by quantitative RT-PCR (qRT-PCR) (SI Appendix, Fig. S4A).
Fig. 2.
The host’s transcriptional response to Y. pseudotuberculosis. (A) Volcano plot obtained from DESeq2 analysis of uninfected and infected Peyer’s patches RNA pools. (B) Heat map of the top 50 enriched and depleted host transcripts. Color-coding is based on rlog-transformed read count values. (C) Infection-mediated activation of extrinsic tissue factor-dependent coagulation cascade genes. On IP32953 colonization, extrinsic coagulation cascade transcripts are highly enriched in infected Peyer’s patches. Fibrin clotting is typically induced by bacterial antigens, such as LPS, to trap the invading bacteria and prevent further dissemination. In addition, stimulation of the extrinsic coagulation pathway is triggered by the neutrophil-specific collagenase MMP8, which activates the tissue factor via cleavage of TFPI.
Inflammatory responses.
Because inflammation is a hallmark of Yersinia infection, it was expected that various transcripts for host immunomodulatory proteins would be strongly enriched in infected Peyer’s patches (Fig. 2B and Dataset S3). The pattern of up-regulated inflammatory cytokines/chemokine receptors and strong enrichment of neutrophil-specific transcripts (e.g., stefin A/cystatin A, prokineticin 2, Schlafen 4, Fpr1, and Clec4e) involved in neutrophil mobilization, neutrophil-mediated phagocytosis, and neutrophil extracellular traps formation (24–26) highlight that the host immune response is dominated by infiltrating neutrophils (Dataset S3). This finding is consistent with previous observations that the number of neutrophils within murine Peyer’s patches increases ∼100-fold (14). Among the highest infection-induced immunomodulatory genes were those of the major proinflammatory cytokines (IL-1α, IL-1β, IL-6, IL-17F, and IFN-γ) and the chemokines (CCL2, CXCL1, CXCL2, CXCL3, CXCL5, and CXCL10). They were previously shown to be strongly up-regulated in Peyer’s patches infected with Y. enterocolitica and/or lymph nodes infected with Yersinia pestis and contribute to the pathology of Yersiniosis and plague (16, 17, 27–29). However, many up-regulated chemokine/lymphokine receptors and immune cell signaling molecules have not yet been described in the context of a Yersinia infection (Dataset S3). Of those listed, IL-22 and IL-23a are known to enhance innate and adaptive immune responses (30, 31). Others (e.g., IL-19 and IL-27) quench the inflammatory effects, similar to IL-10, IL-11, the IL-1 receptor antagonist IL-1Ra, and the decoy receptor IL-1R2, which sequester and inhibit IL-1 (32, 33). Overall, high induction of TH17-type cytokines (IL-17, IL-6, IL-1, and IL-23) and enrichment of the TH1-type cytokine IFN-γ suggest that Y. pseudotuberculosis triggers adaptive immunity toward a mixed TH17/TH1 response.
Acute-phase response.
Another specific cluster of enriched transcripts is part of the acute-phase response—a complex systemic reaction activated by trauma, infection, and inflammation with the goal of clearing potential pathogens, reestablishing homeostasis, and promoting healing (34). This cluster encompasses mainly the serum amyloids A2, A3, A4, and P (Fig. 2B and Dataset S3), which contribute to intestinal immunity. Serum amyloids are up-regulated by proinflammatory signals (e.g., IL-1, TNF-α, and IL-6), which are increased during Yersinia infection. They participate in the systemic modulation of innate and adaptive immune responses (e.g., TH17 responses and migration/activation of monocytes, neutrophils, and T cells) (35–38). Moreover, they have antimicrobial activity and induce expression of matrix metalloproteases, chitinase-like proteins, and collagenases that are pivotal for tissue remodeling after injury (39). Transcripts of the metalloproteases MMP3 and MMP8 and the chitinase-like proteins CHIL1 and CHIL3 were especially significantly enriched in Yersinia-infected Peyer’s patches (Fig. 2 and Dataset S3). A recent study further showed that serum amyloid A binds and transports retinoids, which are induced in response to the infection (Dataset S3). Retinoids promote maturation of immune cells, elicit TH17 responses, and facilitate the regeneration of damaged epithelial barriers (40, 41).
Coagulative activities.
Transcripts related to the coagulation cascade were among the most enriched host messengers, encoding the coagulation factors VII, X, XIIIa1, and FII; the α-, β-, and γ-subunits of the acute-phase reactant fibrinogen (Fga, Fgb, and Fgg); and plasminogen (Plg) (Fig. 2 B and C and Dataset S3). In particular, factor VII, factor X, and fibrinogen transcripts were highly enriched, suggesting that a Yersinia infection triggers activation of the tissue factor-dependent extrinsic coagulation cascade (Fig. 2C). Enhanced fibrin deposition was shown to physically trap invading pathogens and limit their spread (42). Activation of the extrinsic coagulation pathway by Y. pseudotuberculosis is further supported by the vast accumulation of neutrophil-specific Mmp8 transcripts (Fig. 2 and Dataset S3). MMP8 is stored in specific granules and released by neutrophils at sites of acute inflammation (43, 44). It contributes to activation of the extrinsic coagulation pathway because of its capacity to cleave the tissue factor pathway inhibitor (TFPI), which was shown to obtain antibacterial activity on proteolytic processing (45, 46). Similarly, many other coagulation factors, including factor X and prothrombin, contain a catalytic domain at their C terminus with antimicrobial activity after proteolytic processing (47). To date, it is unknown whether coagulation plays a protective role against a Y. pseudotuberculosis infection. However, both plasminogen and fibrinogen have been identified as critical determinants of the inflammatory response and survival after Y. pestis infection. Y. pestis infections normally lead to formation of widespread foci containing large numbers of scattered bacteria with low numbers of infiltrated inflammatory cells and reduced fibrin deposition because of the presence of the plasminogen activator gene pla (48–50). This pathology is contrary to infections caused by Y. pseudotuberculosis, which lead to foci of densely packed bacterial microcolonies with many surrounding inflammatory cells, primarily neutrophils (51, 52). Formation of fibrin-rich matrices at the early stage of microbial invasion seems to support recruitment and attachment of phagocytizing cells as well as T cell-mediated responses that may help to counteract a Yersinia infection (48–50). Impediment of the inflammatory response, neutrophil recruitment, and reduced survival of fibrin(ogen)-deficient mice after a Y. pestis infection undermine the role of fibrin(ogen) as a critical amplifier of the inflammatory response and crucial determinant of the host defense against pathogenic yersiniae (48, 53).
Metal ion sequestration.
Among the strongest induced host factors in the infected lymphatic tissue are metal ion sequestration systems (Fig. 2 A and B and Dataset S3). Vertebrates sequester metal ions from invading pathogens as a first line of defense against bacterial infections, an antimicrobial strategy termed “nutritional immunity” (54). Genes encoding the heme and iron-loaded siderophore sequestration systems haptoglobin and lipocalin 2 (LCN2) are >100-fold induced on infection; transcripts of lactotransferrin and hemopexin were 7- and 14-fold enriched (Fig. 2 A and B and Dataset S3). LCN2 is expressed and secreted in a Toll-like receptor 4-dependent manner in response to TH17 cell-specific proinflammatory cytokines, such as IL-17 and IL-22 (55), which are also up-regulated during the infection (Dataset S3). LCN2 stimulates the secretion of neutrophil-attracting CXC chemokines (55). Moreover, transcripts for the heterodimeric S100A8/S100A9 complex were among the top 10 enriched messengers (83- to 190-fold) (Fig. 2B and Dataset S3). The S100A8/S100A9 complex, also known as calprotectin, sequesters the essential trace elements zinc and manganese (56, 57) and was found to be released from intestinal epithelial cells and infiltrating neutrophils during acute inflammation (55, 58, 59).
In addition, expression of the immunoresponsive gene 1 (Irg1) and the histidine decarboxylase gene (Hdc) was highly induced in response to a Y. pseudotuberculosis infection (Fig. 2B and Dataset S3). HDC is exclusively responsible for production of the biogenic amine histamine. Histamine signaling via the histamine receptor 2 has been previously reported to be important for controlling Y. enterocolitica colonization in the Peyer’s patches, possibly through altering of cytokine production and disruption of the TH1–TH2 balance (60). The Irg1 gene encodes an enzyme synthesizing the metabolite itaconate from the TCA cycle intermediate cis-aconitate. Its production is specifically induced and highly expressed by macrophages on LPS activation, and it is a potent inhibitor of isocitrate lyase, a key enzyme of the glyoxylate shunt, which is required for assimilation of fatty acids (61). Notably, Y. pestis and Y. pseudotuberculosis encode itaconate-degrading enzymes within the ripCBA operon (62), indicating that these bacteria evolved detoxification pathways to prevent itaconate-mediated growth inhibition. However, itaconate degradation and metabolism seem less relevant for Y. pseudotuberculosis during Peyer’s patch colonization, because ripCBA transcripts (YPTB1926-1924) were barely detectable by RNA-seq (Dataset S4).
Other than the identified up-regulated functions, 888 host transcripts were strongly decreased during infection. A large number of down-regulated transcripts encode metabolic enzymes. Among them are several monooxygenases, in particular cytochrome P450 enzymes, solute carriers, and UDP-glucuronosyltransferase systems, which convert lipophilic molecules into excretable compounds (Dataset S3). The infection-induced decrease of these transcripts may simply reflect an overall shutdown of pivotal cellular functions in response to the infection. A down-regulation of monooxygenases was also observed in Y. enterocolitica-infected Peyer’s patches, which led to the assumption that they may reduce oxidative stress and minimize detrimental effects (63). Among the depleted transcripts are several that relate to transport and hydrolytic genes of dietary mono-/disaccharides (e.g., glucose transporters: Slc5a4a, Slc5a11, Slc5a4b, Slc5a1, lactase, trehalase, and sucrose isomaltase) (Fig. 2B and Dataset S3), indicating Yersinia-mediated malnutrition. Based on the fact that unabsorbed carbohydrates increase the flow of water and electrolytes into the lumen (64), it is possible that a decrease of these enzyme activities contributes to the intestinal disorders (diarrhea) associated with a Yersinia infection. Additional analysis of Yersinia-mediated alteration of individual transport functions in the brush border and association with the intestinal disorders is required to confirm this hypothesis.
The Host-Responsive mRNA Repertoire of Yersinia.
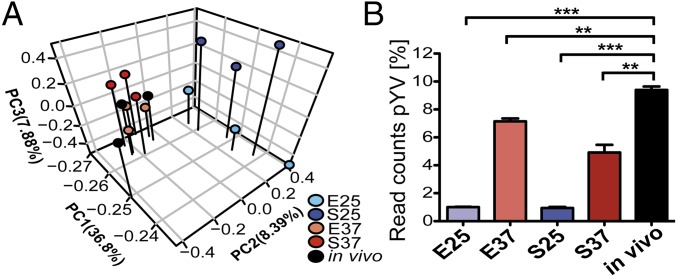
To identify infection-specific gene expression alterations, which consequently lead to the precise adjustment of virulence and fitness programs of the pathogen, we simultaneously profiled the entire transcriptional landscape of Y. pseudotuberculosis during growth under laboratory conditions (in vitro) and Peyer’s patch colonization (in vivo) (Fig. 1A). The bacterial in vivo expression profile of chromosomal genes was very distinct from all in vitro profiles (Fig. 1D), whereas profiles of the virulence plasmid pYV from in vivo- and in vitro-grown bacteria at 37 °C were more alike (Fig. 3 and SI Appendix, Fig. S5 A and B). The Yersinia virulence plasmid pYV encodes the adhesin YadA, the Ysc type III secretion system (T3SS) and the effectors called Yops, which are translocated by the T3SS mainly into neutrophils and macrophages on cell contact. The Yops modulate eukaryotic signaling pathways to counteract phagocytosis and other immune responses, and they induce apoptosis of the infected host cell (65, 66).
Fig. 3.
Temperature as a major stimulus for virulence plasmid expression in vivo. (A) Principal component (PC) analysis of reads per kilobase transcript length per million mapped reads-normalized expression values of in vitro and in vivo RNA-seq data for the IP32953 pYV virulence plasmid (NC_006153.2). (B) Percentage of pYV cDNA read counts obtained from in vitro and in vivo RNA-seq data relative to the read counts obtained for the bacterial chromosome. Unpaired t test (n = 3). E25, exponential phase 25 °C; E37, exponential phase 37 °C; in vivo, infected Peyer’s patches; S25, stationary phase 25 °C; S37, stationary phase 37 °C. **P ≤ 0.01; ***P ≤ 0.001.
Expression of virulence factors.
Similar mRNA profiles of the pYV-encoded virulence genes at 37 °C suggest that the thermal upshift on host entry is the primary signal triggering pYV gene expression. The overall ysc/yop mRNA level is further increased during colonization of Peyer’s patches (Fig. 3B and Dataset S4), showing that additional temperature-independent signals enhance pYV gene expression (e.g., via host cell contact) (67). Notably, the levels of the copB mRNA, encoding a negative regulator of the pYV replicase gene repA (68), were significantly decreased during infection (Dataset S4 and SI Appendix, Fig. S5 B and C). The ratio of repA transcripts to its antisense RNA copA was strongly increased compared with bacteria grown at 37 °C. This transcriptional profile indicated that the replication of pYV is increased during Peyer’s patch colonization and supported a recent observation showing that Yersinia up-regulates the pYV copy number during infection (69).
To explicitly unravel other transcriptional responses that allow the bacteria to resist host defenses and replicate within extracellular lymphatic tissue compartments, we screened for transcripts that differed significantly in their abundance between in vitro and infection conditions (Fig. 4A, Datasets S4 and S5, and SI Appendix, Fig. S6). During Peyer’s patch colonization, 97 mRNAs were consistently enriched, whereas 60 mRNAs were consistently depleted. Similar to host gene expression profiles, DESeq2-estimated fold change responses for selected bacterial messenger and small ncRNAs were confirmed by qRT-PCR (SI Appendix, Fig. S4 B and C).
Fig. 4.
Bacterial global gene expression analysis uncovers infection-specific metabolic adaptations. (A) Four-way Venn diagram illustrating Yersinia transcripts, which are significantly altered (log2fc ≥ 1; log2fc ≤ −1; adjusted P value ≤ 0.05) during Peyer’s patch colonization (in vivo); 283 transcripts were altered in abundance in vivo compared with all four tested in vitro conditions, including those that were enriched under one condition and depleted under another condition. From those 283 transcripts, 116 were consistently enriched, and 62 were consistently depleted in vivo. E25, exponential phase 25 °C; E37, exponential phase 37 °C; in vivo, infected Peyer’s patches; S25, stationary phase 25 °C; S37, stationary phase 37 °C. (B) Heat map of selected bacterial transcripts related to metabolic functions that are strictly enriched (red) or depleted (blue) during infection. Values represent the log2 fold change between indicated conditions. (C) Central carbon metabolism of Y. pseudotuberculosis. Significant changes of the transcriptomic pattern between the in vitro- and in vivo-grown bacteria are indicated. Enriched transcripts during infection are given in red, and decreased transcripts are indicated in blue.
Most of the differentially regulated transcripts encode stress responses and host-adapted metabolic functions but not classical virulence factors (e.g., ail). Evidently, their expression is mainly triggered by temperature and not triggered by infection-specific stimuli (Datasets S4 and S5).
Host-induced bacterial stress responses.
The bacterial in vivo transcriptome revealed that Yersinia is exposed to severe stresses during Peyer’s patch colonization. The overall transcript abundance of important stress resistance genes (e.g., groEL/ES, dnaK, grpE, rpoS, and cspA/B/C/D) was high (Dataset S4). Moreover, several genes involved in detoxification of nitric oxide and other reactive nitrogen species (e.g., nitric oxide dioxygenase: hmp; glutaredoxin: nrdH) were strongly up-regulated. This upregulation counteracts induction of nitric oxide synthase 2 in the infected tissue (Dataset S3). In contrast, classical oxidative stress-related transcripts were not enriched (Datasets S4 and S5). This expression profile suggests that Y. pseudotuberculosis is not exposed to reactive oxygen species produced by neutrophils and other innate immune cells. However, a recent study showed that the production of multiple Yersinia catalases, superoxide dismutases, and thioredoxin (katA, sodB, sodC, and trxA) is controlled on the posttranscriptional level by thermoresponsive RNA structures (RNA thermometers) (70). They act as translational repressor elements at lower temperature but not at 37 °C, and they are thus not detectable by conventional RNA-seq.
The most strongly enriched bacterial transcripts in response to host defense strategies are those implicated in metal ion acquisition (Fig. 4B and Datasets S4 and S5). The great majority encodes uptake systems for iron (e.g., heme uptake system: hmuPRSTUV; ferric/ferrous transporters: yfeABCDE; siderophore systems: fecCD, alcABC, fyuA-ybtUTE-irp1/2-ybtA-ybtPQXS, fcuA, rhbC, sfuB, tonB, and exbBD). Some also or specifically sequester zinc (znuA-znuCB and ybtPQXS) or manganese (mntH and yfeABCDE) (71), most of which are well-known members of the Fur and Zur regulon. The strong increase of multiple redundant systems suggests that especially the availability of iron and zinc is very limited within the infected tissue because of the induction of metal ion sequestration and intracellular retention systems by the host (Fig. 2 A and B and Dataset S3) (72).
Another strategy to overcome metal ion scarcity in the bacteria is the substitution of ion-containing proteins against nonion-containing homologs. The most striking example is the rpmE2J transcript encoding the ribosomal subunits RpmE2 and RpmJ, one of two alternative L31 and L36 proteins, which showed the highest induction (>300-fold) during infection (Datasets S4 and S5). Both proteins lack zinc-binding sites in contrast to their paralogs encoded by YPTB0102 (RpmE) and YPTB3677 (RpmJ). Recent studies in E. coli and Bacillus subtilis suggested that the zinc-containing RpmE protein is replaced by the highly overexpressed nonzinc-containing paralog to mobilize zinc from ribosomes under zinc-limiting conditions (73, 74). A highly conserved Zur box was identified in the Y. pseudotuberculosis rpmE2 regulatory upstream region overlapping the rpmE2 TSS (mRNA mTSS_0385) (Dataset S2 and SI Appendix, Fig. S7A). We could show Zur-dependent expression of the rpmE2J operon (SI Appendix, Fig. S7B). This finding implies that a switch to RpmE2J-containing ribosomes contributes to the survival of the pathogen in low-zinc in vivo environments. However, despite the strong induction during infection, mouse survival and tissue colonization efficiency of the bacteria were not affected by a loss of both alternative ribosomal proteins (SI Appendix, Fig. S7 C and D). This observation illustrates that loss of a single-ion substitution system is insufficient to reduce pathogenesis. Most likely, it is compensated by the up-regulation of other alternative metal ion-independent metabolic functions and scavenging systems (Datasets S4 and S5).
Host-adapted metabolism.
Another prerequisite for the survival of many bacterial pathogens is the adaptation of their metabolism to the nutrients available within the host tissue. We found that yersiniae colonizing the Peyer’s patches use carbohydrates as their primary carbon source. Particularly, transcripts encoding the glucose, mannose (ptsGI/crr and manXYZ), fructose (fruBKA), and glucuronate (uxaABC) uptake systems as well as the upper part of glycolysis (gapA, gmpA, eno, and pykF) were highly abundant or strongly enriched during infection (Fig. 4B, Datasets S4 and S5, and SI Appendix, Fig. S8A). In agreement, an fruBKA deletion mutant was significantly impaired, and a pykF mutant was completely outcompeted in Peyer’s patch colonization (SI Appendix, Fig. S8B) (75). A strong down-regulation of TCA cycle components (acnB, fumA, sdhCDAB, icdA, and mdh), fatty acid oxidation enzymes (fadBA, fadJ, and fadL), and cytochrome o ubiquinol oxidase (cyoABCDE), the terminal complex of the respiratory chain, coupled with a high and/or strongly enhanced in vivo expression of fermentation genes (adhE, focA-pflB, and ldhA) suggest that pyruvate originating from glycolysis is reduced to ethanol, formate, and lactate (Fig. 4 B and C and Datasets S4 and S5). Parallel use of different fermentation pathways and expression of rescuing alternative enzymes (YPTB0382, YPTB1517, and YPTB3387) seem to account for the fact that inactivation of the highest induced adhE gene was insufficient to affect the outcome of the infection (SI Appendix, Fig. S8B).
Overall, the Y. pseudotuberculosis metabolism in Peyer’s patches resembles that of Y. pestis during rat bubo colonization (76–78), indicating that the different lymphatic tissues constitute a similar nutritional environment, which requires comparable metabolic adaptations. Likewise to Y. pseudotuberculosis, Y. pestis was found to strongly up-regulate homologous metal ion acquisition and nitrosative stress systems within bubos (76, 77). Similarly, simple carbohydrates constitute the main carbon source that is metabolized to pyruvate and then, redirected to anaerobic energy production pathways because of the down-regulation of the TCA cycle. However, in contrast to Y. pseudotuberculosis, fructose and mannose are not important carbon sources for Y. pestis (76, 78). Moreover, Y. pestis seems to metabolize its energy sources mainly by anaerobic respiration (e.g., by the dimethyl sulfoxide reductase DmsABC) (76). The dmsABC transcript was not detected at a significant level in our study, indicating that these genes are not required for Y. pseudotuberculosis during Peyer’s patch colonization.
Growth in Lymphatic Tissues Alters the Abundance of Conserved Metabolic ncRNAs.
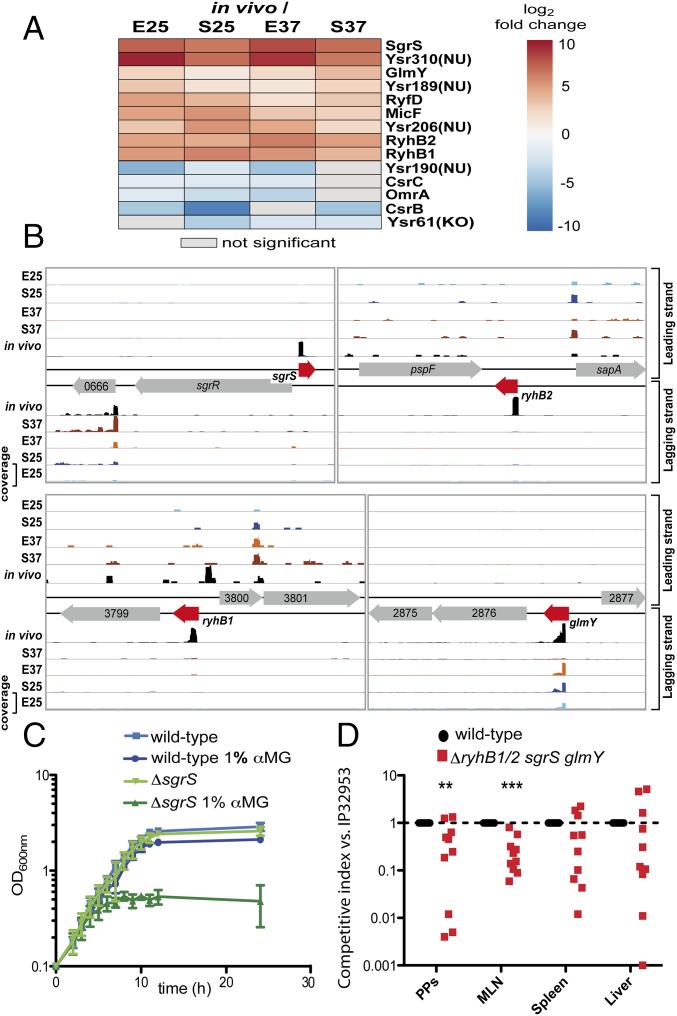
Previous in vitro studies identified numerous ncRNAs in Y. pseudotuberculosis (5, 6). Many of them respond to temperature, but whether they contribute to virulence remained largely unknown. Our in vivo transcriptome analysis revealed that the transcript of the general RNA chaperone Hfq as well as that of the global regulator Crp, which controls large subsets of ncRNAs in Y. pseudotuberculosis (5, 6), changed significantly during infection compared with in vitro growth at 37 °C (Dataset S5). This result indicated that growth in lymphatic tissues alters the abundance of regulatory RNAs. In fact, of 197 identified ncRNAs (Dataset S2), 9 trans-encoded ncRNAs (Fig. 5A) were at least twofold enriched in vivo compared with all in vitro conditions. Some Yersinia-specific in vivo-induced trans-encoded RNAs were identified [Ysr189(NU), Ysr206(NU), and Ysr310(NU)], but their overall expression level was rather low. In contrast, several of the conserved ncRNAs (SgrS, RyhB1, and RyhB2), which were not or were marginally expressed in vitro, were strongly up-regulated (20- to 250-fold) and highly abundant during infection (Fig. 5 A and B).
Fig. 5.
Highly conserved bacterial ncRNAs contribute to pathogenesis. (A) Heat map of in vivo-enriched (red) and -depleted (blue) trans-encoded bacterial ncRNAs. Values represent the log2 fold change of indicated conditions (adjusted P value ≤ 0.05). (B) Read coverage of the RNA-seq analysis of the sgrS, ryhB1, ryhB2, and glmY loci is illustrated in the Integrated Genome Browser (99). The data were normalized according to the number of uniquely mapped reads. E25, exponential phase 25 °C; E37, exponential phase 37 °C; in vivo, infected Peyer’s patches; S25, stationary phase 25 °C; S37, stationary phase 37 °C. (C) Y. pseudotuberculosis WT strain IP32953 and the isogenic ΔsgrS strain were grown in LB at 37 °C in the presence or absence of 1% αMG, a glucose-phosphate stress-inducing agent. (D) Two independent groups of BALB/c mice (2 × n = 5 per group) were orally infected with an equal mixture of 107 cfu IP32953 (WT) and the isogenic mutant YPIP56 (ΔsgrS/ryhB1/ryhB2/glmY). Data are graphed as competitive index values for tissue samples from one mouse in Peyer’s patches (PPs), mesenteric lymph nodes (MLNs), and organs after 3 days. A competitive index score of one denotes no difference in virulence compared with the WT. The statistical significance between the WT and the mutant was determined by a Wilcoxon signed rank test. **P ≤ 0.01; ***P ≤ 0.001.
The sibling RNAs RyhB1/RyhB2 are both activated after iron scarcity by inactivation of the ferric uptake regulator Fur (79). Both ncRNAs are functionally redundant, but RyhB1 seems slightly more sensitive to alterations of degradosome factors (79, 80). They both regulate iron homeostasis by up-regulation of iron-scavenging systems and repression of nonessential iron-containing proteins to liberate iron (79, 81). Notably, in E. coli and Salmonella, the RyhB RNAs were found to repress expression of iron-containing TCA cycle enzymes, such as succinate dehydrogenase and fumarate reductase (sdhCDAB and frdABCD), fumerase (fumA), and aconitase (acnA and acnB), as well as the respiratory-chain components (nuo and fdo) (81, 82). This finding strongly suggests that they contribute to the down-regulation of the TCA cycle and respiration of Y. pseudotuberculosis during infection.
SgrS RNA homologs are found in several γ-proteobacteria, including E. coli and Salmonella (83). SgrS production in these bacteria is induced during accumulation of phosphosugars in the cytoplasm, a harmful growth-restricting condition referred to as glucose-phosphate stress (84, 85). The regulatory function of SgrS involves translational repression and enhanced degradation of mRNAs encoding sugar transporters (ptsG, fruBKA, and manXYZ), which are highly abundant in Yersinia during Peyer’s patch colonization (83, 86, 87). A deletion of sgrS restricted growth of Y. pseudotuberculosis at 37 °C in the presence of the glucose-phosphate stress-inducing agent methyl α-d-glucoside (αMG) (Fig. 5C). This result showed that SgrS fulfills a similar function in Y. pseudotuberculosis, and its up-regulation in vivo supports our data identifying glucose and fructose as dominant carbon sources during Peyer’s patch colonization.
Another abundant in vivo–up-regulated ncRNA, GlmY, is involved in cell wall synthesis. Together with its sibling ncRNA GlmZ, they regulate expression of the glucosamine-6-phosphate synthase GlmS. Transcription of glmY is activated from a σ54-dependent promoter and controlled by the GlrR/GlrK two-component system, which plays a role in virulence (88, 89).
High abundance and strong in vivo induction of the important metabolic ncRNAs SgrS, RyhB1/RyhB2, and GlmY prompted us to test their role for virulence. The lack of GlmY, SgrS, or RyhB1/2 did not have an effect or had only a very mild effect on host colonization (SI Appendix, Fig. S8C). This finding illustrates that the network of cellular functions controlled by the ncRNAs is very robust, because their individual loss can be easily compensated. In contrast, a deletion of all four ncRNA genes significantly impaired colonization of the Peyer’s patches and mesenteric lymph nodes (Fig. 5D), showing that pathogenicity is reduced when their functions are eliminated.
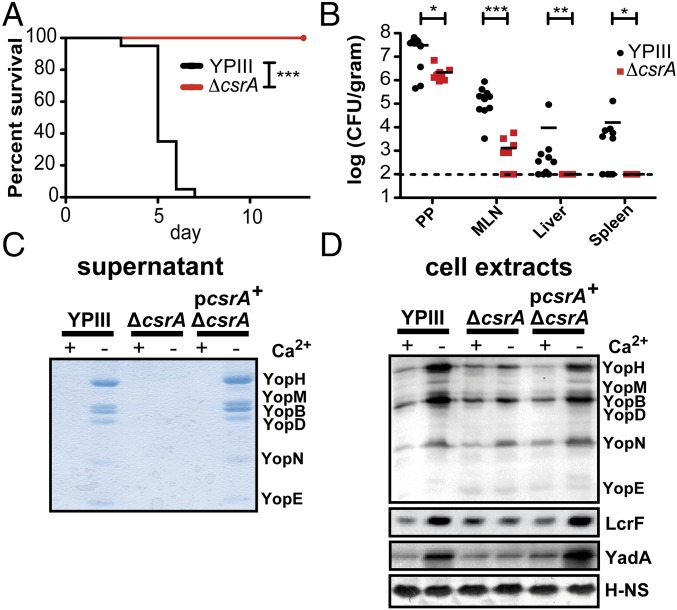
Within the entire ncRNA pool, only a set of five trans-encoded RNAs was depleted in in vivo compared with in vitro growth (Fig. 5A and Dataset S4). The most abundant ncRNAs are carbon storage regulator B (CsrB) and CsrC, of which CsrB showed the strongest decrease under infection conditions (Fig. 5A and Dataset S4). Decrease of CsrB and CsrC levels is likely induced by the down-regulation of Crp and up-regulation of CsrD (Datasets S4 and S5). Crp is a known activator of csrB transcription, and CsrD is involved in the RNase E-mediated decay of CsrB and CsrC (90, 91). CsrB, CsrC, and the RNA-binding protein CsrA form the conserved Csr system, in which both Csr RNAs bind multiple CsrA molecules and prevent CsrA interaction with other target mRNAs. The CsrA regulon of Enterobacteriaceae is very complex and includes colonization factors, infection-linked metabolic processes, and physiological traits (92, 93), indicating that an increase of active CsrA is important under infection conditions. We tested a Y. pseudotuberculosis YPIII csrA mutant in the oral mouse model and found that mice infected with a csrA mutant did not develop any disease symptoms and remained alive 14 d postinfection (Fig. 6A); 10- to 100-fold fewer bacteria were recovered from the Peyer’s patches and mesenteric lymph nodes at day 3 postinfection, and no bacteria could be isolated from the liver and spleen (Fig. 6B), showing that CsrA is crucial for virulence.
Fig. 6.
CsrA is crucial for virulence and mediates expression of T3SS. (A) Mice were orally infected with 2 × 108 cfu Y. pseudotuberculosis WT strain YPIII or the isogenic ΔcsrA strain. Survival of mice was monitored for 12 d in two independent experiments with 2 × 10 mice per group for each genotype. ***P ≤ 0.001; log rank (Mantel–Cox) test. (B) BALB/c mice were orally infected with 107 cfu Y. pseudotuberculosis YPIII WT or the isogenic ΔcsrA strain. Mice were killed 3 d postinfection, and the numbers of bacteria in homogenized Peyer’s patches (PPs), mesenteric lymph nodes (MLNs), liver, and spleen was determined. Data from two independent experiments (2 × 5 mice per group) are presented. Statistical significances between the WT and mutants were determined by a Mann–Whitney test. The detection limit is indicated by the dotted line. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. (C) TCA-precipitated supernatants or (D) Western blots of whole-cell extracts of Y. pseudotuberculosis YPIII WT and the isogenic ΔcsrA strain with and without the complementing csrA+ plasmid pAKH56 grown at 37 °C in the absence of Ca2+ are shown. Yops, LcrF, and YadA identified by (C) Coomassie blue staining or (D) Western blotting are indicated. The nucleoid-associated protein H-NS was used as loading control.
Important Genes for Peyer’s Patch Colonization Are Part of the CsrA Regulon.
A comparison of genes that were differentially expressed during infection with the previously defined CsrA regulon (75) revealed that CsrA governs numerous metabolic genes implicated in the adaptation of Yersinia to the lymphatic environment. We detected a strongly reduced transcript abundance of pyruvate–TCA cycle genes (acs, sdhCDAB, icdA, fumA, and acnB) and fatty acid oxidation genes (fadAB, fadJ, and fadL) in vivo, which are repressed by CsrA, and a high abundance of the adhE mRNA, which is activated by CsrA (Datasets S4 and S5) (75). This finding suggests that adjustment of the CsrABC levels during infection plays a crucial role for the adaptation of the biological fitness during host tissue colonization.
The strong attenuation of a csrA mutant also indicated that essential virulence functions (e.g., T3SS/yop genes) are abolished in the absence of CsrA. To test this hypothesis, we investigated the influence of CsrA on Yop secretion using Ca2+ limitation as a substitute for cell contact. Ca2+ depletion stimulated secretion of Yops (Fig. 6C) by the Y. pseudotuberculosis WT strain YPIII but not by the isogenic csrA mutant. Moreover, levels of the T3SS/Yop regulator LcrF and the adhesin YadA were not increased or only slightly increased in the absence of CsrA on Ca2+ depletion (Fig. 6D). The WT phenotype could be restored by csrA expression in trans, indicating that Yop/YadA expression indeed requires CsrA, which promotes induction of LcrF synthesis on host cell contact. This observation provides evidence that CsrA not only affects the bacterial metabolic fitness but also, is crucial for type III secretion and pathogenesis during colonization of the Peyer’s patches.
Conclusions
One key feature of this work is the ability to simultaneously capture the RNA expression profile of the extracellular pathogen Yersinia and its mammalian host during infection. The established tissue dual RNA-seq approach generated a comprehensive, high-resolution transcriptomic dataset that (i) enabled us to identify immune reactions directed against the pathogen and opposed responses of Yersinia at one time point of the infection that drive pathogenesis with high statistical confidence and (ii) allowed us to discover infection-specific stimuli and bacterial riboregulators shaping host–pathogen interactions. Future studies including earlier and later stages of the infection will provide valuable information about the dynamics of gene expression changes in the pathogen and its host during the course of an infection. The bacterial load within infected tissues is the most limiting factor for tissue dual RNA-seq, and transcripts with very low abundance might have been missed. However, ongoing improvements in sampling, library preparation, and high-throughput sequencing technologies will very likely allow a more sensitive detection and analysis of expression changes in less colonized host tissues in the near future. Moreover, single-cell RNA-seq (94, 95) will enable us to elucidate the transcriptional response of individual host cell types or different bacterial species (e.g., other enteric pathogens and intestinal microbiota) and dissect heterogeneity of bacterial and host cell subpopulations during the infection.
Our data on Y. pseudotuberculosis revealed many similarities but also marked differences in the host response to infection with its close relatives Y. enterocolitica and Y. pestis (29, 63). Specifically, integration of the strong enrichment of neutrophil-specific transcripts for antimicrobial defense and immune cell dynamics during infection illustrates that the immune response directed against Y. pseudotuberculosis is dominated by the massive recruitment and activation of neutrophils and steers adaptive immunity primarily to TH1 and TH17 responses (Fig. 7). This response coincides with an induction of the extrinsic coagulation cascade, shown to form fibrin-rich matrices that encase the bacteria to hamper dissemination and enhance bacterial clearance (42, 51). Consequently, the most important defense mechanism of the bacteria is to adjust to the stressful conditions and counteract neutrophil attack within fibrin clots.
Fig. 7.
Infection-specific changes of the Yersinia–host transcriptomes. Schematic overview of the complex transcript-based adaptations of Y. pseudotuberculosis and the host during colonization of Peyer’s patches. The most strongly affected inflammatory and acute-phase responses, metabolic and virulence adaptations, and identified differentially regulated bacterial riboregulators with impact on pathogenesis are illustrated. DC, dendritic cell; NK, natural killer cell; NO, nitric oxide; PMN, polymorphonuclear leukocyte.
Accordingly, the pathogen’s transcriptional response is characterized by a marked up-regulation of the antiphagocytic T3SS/Yop machinery in response to temperature and an increase of the virulence plasmid copy number, which is likely triggered by host cell contact (69, 96). This defense strategy is supported by the induction of radical detoxifying genes, such as hmp (Fig. 7).
In addition to the immune defense mechanisms, in vivo growth of Yersinia is also accompanied by a strong up-regulation of Fe/Zn/Mn sequestration/uptake systems and a readjustment of metabolic functions that allows the pathogen to overcome host-imposed ion and nutrient constraints (Fig. 7). The plethora of up-regulated redundant ion acquisition systems reflects the high importance of this process to prevent pertinent host measures to restrict bacterial growth. It also illustrates the difficulties that interfere with this process by target-directed antimicrobial therapies.
Our data further suggest that Yersinia assesses and uses a number of diverse nutrients, especially simple sugars, which likely originate from dying cells in the infected tissue (97) and remain available because of the overall reduction of host cell sugar carriers (Fig. 7). They are metabolized to pyruvate and converted by anaerobic fermentation into a variable mixture of short-chain fatty acids. This metabolic diversity relieves Yersinia dependence on a particular energy source and improves survival when individual nutrients are scarce or fluctuating during the infection process. It further explains the remarkable robustness of the pathogen’s metabolism against internal perturbations but pinpoints limited possibilities for antimicrobial targets (e.g., the phosphoenolpyruvate/pyruvate-acetyl-CoA node).
Furthermore, our data provide an overall view of the ncRNA expression profile within lymphatic tissue, which offers a unique resource for the identification of important riboregulators during infection. We show that a successful host infection by Y. pseudotuberculosis relies on the combined action of several highly expressed, conserved ncRNAs, with abundance that is adjusted in response to ion/nutrient availability under infection conditions and/or by the global ncRNA regulators Hfq and Crp. In particular, adjustment of the activity of the global RNA regulator CsrA via the ncRNAs CsrB and CsrC is crucial for virulence. Other than its vital role in the regulation of carbon metabolism (75) and invasin gene expression (98), we show that CsrA is essential for Yop synthesis and secretion to defend against attacks of surrounding phagocytic cells during later stages of the infection.
In summary, these findings argue for the usefulness of the established tissue dual RNA-seq approach to derive meaningful conclusions related to pathogen biology and host response that affect the outcome of an infection. The integration of obtained RNA profile information with data of immune cell migration and bacterial pathogenesis offers insights into the complex host–pathogen interaction network and developing disease. It further creates possibilities to identify critical processes that govern pathogen colonization and increase the potential to identify targets for antimicrobial therapies.
Materials and Methods
Details on methodologies are provided in SI Appendix and include bacterial growth conditions, DNA cloning procedures and mutant constructions, analysis of reporter gene expression, Yop synthesis and secretion assays, animal experiments, RNA isolation, qRT-PCR, cDNA library preparation, read mapping, bioinformatics, and statistics. The custom annotation file including identified ncRNAs is given in Dataset S6. The animal protocols were approved by the Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit: permission no. 33.9.42502-04-12/1010.
Supplementary Material
Acknowledgments
We thank M. Fenner for discussions and W. Weigel for critical reading of the manuscript. We also thank R. Steinmann, M. Kroll, M. Schoof, W. Heine, O. Goldmann, and J. Reinkensmeier for tools and experimental and bioinformatics support and K. Paduch and T. Krause for technical assistance. This work was supported from German Research Foundation Grants DE616/4 (to A.M.N.), DE616/6 (to A.M.N.), and SPP1617–Young Investigator Start Up Funding (to A.M.N.). M.P. and W.O. received fellowships from the Helmholtz Centre for Infection Research Graduate School. P.D. is supported by the German Center of Infection Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The raw high-throughput read data have been deposited as FASTQ files in the European Nucleotide Archive (ENA; accession no. PRJEB14242). Split and processed FASTQ files as well as corresponding BAM files have been deposited in the ENA (accession no. PRJEB17683).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1613405114/-/DCSupplemental.
References
- 1.Papenfort K, Vogel J. Small RNA functions in carbon metabolism and virulence of enteric pathogens. Front Cell Infect Microbiol. 2014;4:91. doi: 10.3389/fcimb.2014.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliva G, Sahr T, Buchrieser C. Small RNAs, 5′ UTR elements and RNA-binding proteins in intracellular bacteria: Impact on metabolism and virulence. FEMS Microbiol Rev. 2015;39(3):331–349. doi: 10.1093/femsre/fuv022. [DOI] [PubMed] [Google Scholar]
- 3.Heroven AK, Nuss AM, Dersch P. RNA-based mechanisms of virulence control in Enterobacteriaceae. RNA Biol. 2016;2016:1–17. doi: 10.1080/15476286.2016.1201617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kröger C, et al. An infection-relevant transcriptomic compendium for Salmonella enterica Serovar Typhimurium. Cell Host Microbe. 2013;14(6):683–695. doi: 10.1016/j.chom.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Nuss AM, et al. Transcriptomic profiling of Yersinia pseudotuberculosis reveals reprogramming of the Crp regulon by temperature and uncovers Crp as a master regulator of small RNAs. PLoS Genet. 2015;11(3):e1005087. doi: 10.1371/journal.pgen.1005087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koo JT, Alleyne TM, Schiano CA, Jafari N, Lathem WW. Global discovery of small RNAs in Yersinia pseudotuberculosis identifies Yersinia-specific small, noncoding RNAs required for virulence. Proc Natl Acad Sci USA. 2011;108(37):E709–E717. doi: 10.1073/pnas.1101655108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srikumar S, et al. RNA-seq brings new insights to the intra-macrophage transcriptome of Salmonella Typhimurium. PLoS Pathog. 2015;11(11):e1005262. doi: 10.1371/journal.ppat.1005262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mandlik A, et al. RNA-Seq-based monitoring of infection-linked changes in Vibrio cholerae gene expression. Cell Host Microbe. 2011;10(2):165–174. doi: 10.1016/j.chom.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Z, et al. The Streptococcus suis transcriptional landscape reveals adaptation mechanisms in pig blood and cerebrospinal fluid. RNA. 2014;20(6):882–898. doi: 10.1261/rna.041822.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westermann AJ, Gorski SA, Vogel J. Dual RNA-seq of pathogen and host. Nat Rev Microbiol. 2012;10(9):618–630. doi: 10.1038/nrmicro2852. [DOI] [PubMed] [Google Scholar]
- 11.Westermann AJ, et al. Dual RNA-seq unveils noncoding RNA functions in host-pathogen interactions. Nature. 2016;529(7587):496–501. doi: 10.1038/nature16547. [DOI] [PubMed] [Google Scholar]
- 12.Baddal B, et al. Dual RNA-seq of nontypeable haemophilus influenzae and host cell transcriptomes reveals novel insights into host-pathogen cross talk. MBio. 2015;6(6):e01765–e15. doi: 10.1128/mBio.01765-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rienksma RA, et al. Comprehensive insights into transcriptional adaptation of intracellular mycobacteria by microbe-enriched dual RNA sequencing. BMC Genomics. 2015;16:34. doi: 10.1186/s12864-014-1197-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pisano F, et al. Influence of PhoP and intra-species variations on virulence of Yersinia pseudotuberculosis during the natural oral infection route. PLoS One. 2014;9(7):e103541. doi: 10.1371/journal.pone.0103541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carter PB. Animal model of human disease. Yersinia enteritis. Animal model: Oral Yersinia enterocolitica infection of mice. Am J Pathol. 1975;81(3):703–706. [PMC free article] [PubMed] [Google Scholar]
- 16.Dube PH, Handley SA, Lewis J, Miller VL. Protective role of interleukin-6 during Yersinia enterocolitica infection is mediated through the modulation of inflammatory cytokines. Infect Immun. 2004;72(6):3561–3570. doi: 10.1128/IAI.72.6.3561-3570.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dube PH, Revell PA, Chaplin DD, Lorenz RG, Miller VL. A role for IL-1 alpha in inducing pathologic inflammation during bacterial infection. Proc Natl Acad Sci USA. 2001;98(19):10880–10885. doi: 10.1073/pnas.191214498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Locati MD, et al. Improving small RNA-seq by using a synthetic spike-in set for size-range quality control together with a set for data normalization. Nucleic Acids Res. 2015;43(14):e89. doi: 10.1093/nar/gkv303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haas BJ, Chin M, Nusbaum C, Birren BW, Livny J. How deep is deep enough for RNA-Seq profiling of bacterial transcriptomes? BMC Genomics. 2012;13:734. doi: 10.1186/1471-2164-13-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ching T, Huang S, Garmire LX. Power analysis and sample size estimation for RNA-Seq differential expression. RNA. 2014;20(11):1684–1696. doi: 10.1261/rna.046011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim D, et al. Comparative analysis of regulatory elements between Escherichia coli and Klebsiella pneumoniae by genome-wide transcription start site profiling. PLoS Genet. 2012;8(8):e1002867. doi: 10.1371/journal.pgen.1002867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kröger C, et al. The transcriptional landscape and small RNAs of Salmonella enterica serovar Typhimurium. Proc Natl Acad Sci USA. 2012;109(20):E1277–E1286. doi: 10.1073/pnas.1201061109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu M, et al. Formylpeptide receptors are critical for rapid neutrophil mobilization in host defense against Listeria monocytogenes. Sci Rep. 2012;2:786. doi: 10.1038/srep00786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma A, Steichen AL, Jondle CN, Mishra BB, Sharma J. Protective role of Mincle in bacterial pneumonia by regulation of neutrophil mediated phagocytosis and extracellular trap formation. J Infect Dis. 2014;209(11):1837–1846. doi: 10.1093/infdis/jit820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang MJ, et al. Role of chitinase 3-like-1 in interleukin-18-induced pulmonary type 1, type 2, and type 17 inflammation; alveolar destruction; and airway fibrosis in the murine lung. Am J Respir Cell Mol Biol. 2015;53(6):863–871. doi: 10.1165/rcmb.2014-0366OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Autenrieth IB, Kempf V, Sprinz T, Preger S, Schnell A. Defense mechanisms in Peyer’s patches and mesenteric lymph nodes against Yersinia enterocolitica involve integrins and cytokines. Infect Immun. 1996;64(4):1357–1368. doi: 10.1128/iai.64.4.1357-1368.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matteoli G, et al. Role of IFN-gamma and IL-6 in a protective immune response to Yersinia enterocolitica in mice. BMC Microbiol. 2008;8:153. doi: 10.1186/1471-2180-8-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Comer JE, et al. Transcriptomic and innate immune responses to Yersinia pestis in the lymph node during bubonic plague. Infect Immun. 2010;78(12):5086–5098. doi: 10.1128/IAI.00256-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nikoopour E, Bellemore SM, Singh B. IL-22, cell regeneration and autoimmunity. Cytokine. 2015;74(1):35–42. doi: 10.1016/j.cyto.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, et al. Jagged-1 signaling suppresses the IL-6 and TGF-β treatment-induced Th17 cell differentiation via the reduction of RORγt/IL-17A/IL-17F/IL-23a/IL-12rb1. Sci Rep. 2015;5:8234. doi: 10.1038/srep08234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mascanfroni ID, et al. IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat Immunol. 2013;14(10):1054–1063. doi: 10.1038/ni.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 34.Cray C, Zaias J, Altman NH. Acute phase response in animals: A review. Comp Med. 2009;59(6):517–526. [PMC free article] [PubMed] [Google Scholar]
- 35.Kanther M, et al. Commensal microbiota stimulate systemic neutrophil migration through induction of serum amyloid A. Cell Microbiol. 2014;16(7):1053–1067. doi: 10.1111/cmi.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Björkman L, et al. Serum amyloid A mediates human neutrophil production of reactive oxygen species through a receptor independent of formyl peptide receptor like-1. J Leukoc Biol. 2008;83(2):245–253. doi: 10.1189/jlb.0607-408. [DOI] [PubMed] [Google Scholar]
- 37.Ather JL, et al. Serum amyloid A activates the NLRP3 inflammasome and promotes Th17 allergic asthma in mice. J Immunol. 2011;187(1):64–73. doi: 10.4049/jimmunol.1100500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ivanov II, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vallon R, et al. Serum amyloid A (apoSAA) expression is up-regulated in rheumatoid arthritis and induces transcription of matrix metalloproteinases. J Immunol. 2001;166(4):2801–2807. doi: 10.4049/jimmunol.166.4.2801. [DOI] [PubMed] [Google Scholar]
- 40.Hall JA, Grainger JR, Spencer SP, Belkaid Y. The role of retinoic acid in tolerance and immunity. Immunity. 2011;35(1):13–22. doi: 10.1016/j.immuni.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Derebe MG, et al. Serum amyloid A is a retinol binding protein that transports retinol during bacterial infection. eLife. 2014;3:e03206. doi: 10.7554/eLife.03206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Poll T, Herwald H. The coagulation system and its function in early immune defense. Thromb Haemost. 2014;112(4):640–648. doi: 10.1160/TH14-01-0053. [DOI] [PubMed] [Google Scholar]
- 43.Weiss SJ, Peppin G, Ortiz X, Ragsdale C, Test ST. Oxidative autoactivation of latent collagenase by human neutrophils. Science. 1985;227(4688):747–749. doi: 10.1126/science.2982211. [DOI] [PubMed] [Google Scholar]
- 44.Hasty KA, Hibbs MS, Kang AH, Mainardi CL. Secreted forms of human neutrophil collagenase. J Biol Chem. 1986;261(12):5645–5650. [PubMed] [Google Scholar]
- 45.Cunningham AC, Hasty KA, Enghild JJ, Mast AE. Structural and functional characterization of tissue factor pathway inhibitor following degradation by matrix metalloproteinase-8. Biochem J. 2002;367(Pt 2):451–458. doi: 10.1042/BJ20020696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Papareddy P, et al. Tissue factor pathway inhibitor 2 is found in skin and its C-terminal region encodes for antibacterial activity. PLoS One. 2012;7(12):e52772. doi: 10.1371/journal.pone.0052772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kasetty G, et al. The C-terminal sequence of several human serine proteases encodes host defense functions. J Innate Immun. 2011;3(5):471–482. doi: 10.1159/000327016. [DOI] [PubMed] [Google Scholar]
- 48.Degen JL, Bugge TH, Goguen JD. Fibrin and fibrinolysis in infection and host defense. J Thromb Haemost. 2007;5(Suppl 1):24–31. doi: 10.1111/j.1538-7836.2007.02519.x. [DOI] [PubMed] [Google Scholar]
- 49.Luo D, et al. Fibrin facilitates both innate and T cell-mediated defense against Yersinia pestis. J Immunol. 2013;190(8):4149–4161. doi: 10.4049/jimmunol.1203253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sodeinde OA, et al. A surface protease and the invasive character of plague. Science. 1992;258(5084):1004–1007. doi: 10.1126/science.1439793. [DOI] [PubMed] [Google Scholar]
- 51.Guinet F, et al. Dissociation of tissue destruction and bacterial expansion during bubonic plague. PLoS Pathog. 2015;11(10):e1005222. doi: 10.1371/journal.ppat.1005222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davis KM, Mohammadi S, Isberg RR. Community behavior and spatial regulation within a bacterial microcolony in deep tissue sites serves to protect against host attack. Cell Host Microbe. 2015;17(1):21–31. doi: 10.1016/j.chom.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Korhonen TK. Fibrinolytic and procoagulant activities of Yersinia pestis and Salmonella enterica. J Thromb Haemost. 2015;13(Suppl 1):S115–S120. doi: 10.1111/jth.12932. [DOI] [PubMed] [Google Scholar]
- 54.Diaz-Ochoa VE, Jellbauer S, Klaus S, Raffatellu M. Transition metal ions at the crossroads of mucosal immunity and microbial pathogenesis. Front Cell Infect Microbiol. 2014;4:2. doi: 10.3389/fcimb.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raffatellu M, et al. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe. 2009;5(5):476–486. doi: 10.1016/j.chom.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kehl-Fie TE, et al. Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus. Cell Host Microbe. 2011;10(2):158–164. doi: 10.1016/j.chom.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Damo SM, et al. Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. Proc Natl Acad Sci USA. 2013;110(10):3841–3846. doi: 10.1073/pnas.1220341110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steinbakk M, et al. Antimicrobial actions of calcium binding leucocyte L1 protein, calprotectin. Lancet. 1990;336(8718):763–765. doi: 10.1016/0140-6736(90)93237-j. [DOI] [PubMed] [Google Scholar]
- 59.Flo TH, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432(7019):917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 60.Handley SA, Dube PH, Miller VL. Histamine signaling through the H(2) receptor in the Peyer’s patch is important for controlling Yersinia enterocolitica infection. Proc Natl Acad Sci USA. 2006;103(24):9268–9273. doi: 10.1073/pnas.0510414103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Michelucci A, et al. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc Natl Acad Sci USA. 2013;110(19):7820–7825. doi: 10.1073/pnas.1218599110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sasikaran J, Ziemski M, Zadora PK, Fleig A, Berg IA. Bacterial itaconate degradation promotes pathogenicity. Nat Chem Biol. 2014;10(5):371–377. doi: 10.1038/nchembio.1482. [DOI] [PubMed] [Google Scholar]
- 63.Handley SA, Miller VL. General and specific host responses to bacterial infection in Peyer’s patches: A role for stromelysin-1 (matrix metalloproteinase-3) during Salmonella enterica infection. Mol Microbiol. 2007;64(1):94–110. doi: 10.1111/j.1365-2958.2007.05635.x. [DOI] [PubMed] [Google Scholar]
- 64.Gericke B, Amiri M, Naim HY. The multiple roles of sucrase-isomaltase in the intestinal physiology. Mol Cell Pediatr. 2016;3(1):2. doi: 10.1186/s40348-016-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cornelis GR, et al. The virulence plasmid of Yersinia, an antihost genome. Microbiol Mol Biol Rev. 1998;62(4):1315–1352. doi: 10.1128/mmbr.62.4.1315-1352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Durand EA, Maldonado-Arocho FJ, Castillo C, Walsh RL, Mecsas J. The presence of professional phagocytes dictates the number of host cells targeted for Yop translocation during infection. Cell Microbiol. 2010;12(8):1064–1082. doi: 10.1111/j.1462-5822.2010.01451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pettersson J, et al. Modulation of virulence factor expression by pathogen target cell contact. Science. 1996;273(5279):1231–1233. doi: 10.1126/science.273.5279.1231. [DOI] [PubMed] [Google Scholar]
- 68.Pinto UM, Pappas KM, Winans SC. The ABCs of plasmid replication and segregation. Nat Rev Microbiol. 2012;10(11):755–765. doi: 10.1038/nrmicro2882. [DOI] [PubMed] [Google Scholar]
- 69.Wang H, et al. Increased plasmid copy-number is essential for Yersinia T3SS function and virulence. Science. 2016;353(6298):492–495. doi: 10.1126/science.aaf7501. [DOI] [PubMed] [Google Scholar]
- 70.Righetti F, et al. Temperature-responsive in vitro RNA structurome of Yersinia pseudotuberculosis. Proc Natl Acad Sci USA. 2016;113(26):7237–7242. doi: 10.1073/pnas.1523004113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Perry RD, Bobrov AG, Fetherston JD. The role of transition metal transporters for iron, zinc, manganese, and copper in the pathogenesis of Yersinia pestis. Metallomics. 2015;7(6):965–978. doi: 10.1039/c4mt00332b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soares MP, Weiss G. The Iron age of host-microbe interactions. EMBO Rep. 2015;16(11):1482–1500. doi: 10.15252/embr.201540558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gabriel SE, Helmann JD. Contributions of Zur-controlled ribosomal proteins to growth under zinc starvation conditions. J Bacteriol. 2009;191(19):6116–6122. doi: 10.1128/JB.00802-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Akanuma G, Nanamiya H, Natori Y, Nomura N, Kawamura F. Liberation of zinc-containing L31 (RpmE) from ribosomes by its paralogous gene product, YtiA, in Bacillus subtilis. J Bacteriol. 2006;188(7):2715–2720. doi: 10.1128/JB.188.7.2715-2720.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bücker R, Heroven AK, Becker J, Dersch P, Wittmann C. The pyruvate-tricarboxylic acid cycle node: A focal point of virulence control in the enteric pathogen Yersinia pseudotuberculosis. J Biol Chem. 2014;289(43):30114–30132. doi: 10.1074/jbc.M114.581348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pradel E, et al. New insights into how Yersinia pestis adapts to its mammalian host during bubonic plague. PLoS Pathog. 2014;10(3):e1004029. doi: 10.1371/journal.ppat.1004029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sebbane F, et al. Adaptive response of Yersinia pestis to extracellular effectors of innate immunity during bubonic plague. Proc Natl Acad Sci USA. 2006;103(31):11766–11771. doi: 10.1073/pnas.0601182103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Palace SG, Proulx MK, Lu S, Baker RE, Goguen JD. Genome-wide mutant fitness profiling identifies nutritional requirements for optimal growth of Yersinia pestis in deep tissue. MBio. 2014;5(4):e01385-14. doi: 10.1128/mBio.01385-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Deng Z, et al. Two sRNA RyhB homologs from Yersinia pestis biovar microtus expressed in vivo have differential Hfq-dependent stability. Res Microbiol. 2012;163(6-7):413–418. doi: 10.1016/j.resmic.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 80.Deng Z, et al. Rapid degradation of Hfq-free RyhB in Yersinia pestis by PNPase independent of putative ribonucleolytic complexes. BioMed Res Int. 2014;2014:798918. doi: 10.1155/2014/798918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Massé E, Gottesman S. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc Natl Acad Sci USA. 2002;99(7):4620–4625. doi: 10.1073/pnas.032066599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim JN, Kwon YM. Identification of target transcripts regulated by small RNA RyhB homologs in Salmonella: RyhB-2 regulates motility phenotype. Microbiol Res. 2013;168(10):621–629. doi: 10.1016/j.micres.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 83.Horler RS, Vanderpool CK. Homologs of the small RNA SgrS are broadly distributed in enteric bacteria but have diverged in size and sequence. Nucleic Acids Res. 2009;37(16):5465–5476. doi: 10.1093/nar/gkp501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vanderpool CK, Gottesman S. Involvement of a novel transcriptional activator and small RNA in post-transcriptional regulation of the glucose phosphoenolpyruvate phosphotransferase system. Mol Microbiol. 2004;54(4):1076–1089. doi: 10.1111/j.1365-2958.2004.04348.x. [DOI] [PubMed] [Google Scholar]
- 85.Wadler CS, Vanderpool CK. A dual function for a bacterial small RNA: SgrS performs base pairing-dependent regulation and encodes a functional polypeptide. Proc Natl Acad Sci USA. 2007;104(51):20454–20459. doi: 10.1073/pnas.0708102104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wadler CS, Vanderpool CK. Characterization of homologs of the small RNA SgrS reveals diversity in function. Nucleic Acids Res. 2009;37(16):5477–5485. doi: 10.1093/nar/gkp591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rice JB, Vanderpool CK. The small RNA SgrS controls sugar-phosphate accumulation by regulating multiple PTS genes. Nucleic Acids Res. 2011;39(9):3806–3819. doi: 10.1093/nar/gkq1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Göpel Y, et al. Common and divergent features in transcriptional control of the homologous small RNAs GlmY and GlmZ in Enterobacteriaceae. Nucleic Acids Res. 2011;39(4):1294–1309. doi: 10.1093/nar/gkq986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Flamez C, Ricard I, Arafah S, Simonet M, Marceau M. Phenotypic analysis of Yersinia pseudotuberculosis 32777 response regulator mutants: New insights into two-component system regulon plasticity in bacteria. Int J Med Microbiol. 2008;298(3-4):193–207. doi: 10.1016/j.ijmm.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 90.Heroven AK, et al. Crp induces switching of the CsrB and CsrC RNAs in Yersinia pseudotuberculosis and links nutritional status to virulence. Front Cell Infect Microbiol. 2012;2:158. doi: 10.3389/fcimb.2012.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suzuki K, Babitzke P, Kushner SR, Romeo T. Identification of a novel regulatory protein (CsrD) that targets the global regulatory RNAs CsrB and CsrC for degradation by RNase E. Genes Dev. 2006;20(18):2605–2617. doi: 10.1101/gad.1461606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Heroven AK, Böhme K, Dersch P. The Csr/Rsm system of Yersinia and related pathogens: A post-transcriptional strategy for managing virulence. RNA Biol. 2012;9(4):379–391. doi: 10.4161/rna.19333. [DOI] [PubMed] [Google Scholar]
- 93.Vakulskas CA, Potts AH, Babitzke P, Ahmer BM, Romeo T. Regulation of bacterial virulence by Csr (Rsm) systems. Microbiol Mol Biol Rev. 2015;79(2):193–224. doi: 10.1128/MMBR.00052-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Avraham R, et al. Pathogen cell-to-cell variability drives heterogeneity in host immune responses. Cell. 2015;162(6):1309–1321. doi: 10.1016/j.cell.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saliba AE, et al. Single-cell RNA-seq ties macrophage polarization to growth rate of intracellular Salmonella. Nat Microbiol. 2016;2:16206. doi: 10.1038/nmicrobiol.2016.206. [DOI] [PubMed] [Google Scholar]
- 96.Böhme K, et al. Concerted actions of a thermo-labile regulator and a unique intergenic RNA thermosensor control Yersinia virulence. PLoS Pathog. 2012;8(2):e1002518. doi: 10.1371/journal.ppat.1002518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Monack DM, Mecsas J, Bouley D, Falkow S. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J Exp Med. 1998;188(11):2127–2137. doi: 10.1084/jem.188.11.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Heroven AK, Böhme K, Rohde M, Dersch P. A Csr-type regulatory system, including small non-coding RNAs, regulates the global virulence regulator RovA of Yersinia pseudotuberculosis through RovM. Mol Microbiol. 2008;68(5):1179–1195. doi: 10.1111/j.1365-2958.2008.06218.x. [DOI] [PubMed] [Google Scholar]
- 99.Nicol JW, Helt GA, Blanchard SG, Jr, Raja A, Loraine AE. The Integrated Genome Browser: Free software for distribution and exploration of genome-scale datasets. Bioinformatics. 2009;25(20):2730–2731. doi: 10.1093/bioinformatics/btp472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.