Significance
The elemental sulfur aerosols are an important constituent in the atmospheres of Earth, Mars, and Venus. There is now evidence suggesting that these aerosols have also played a role in the evolution of early life on Earth. Traditionally, the photolysis of sulfur gases by UV light is thought to be the main mechanism for the formation of sulfur particles in these atmospheres. But, in the theoretical calculations reported here, we propose a nonphotochemical mechanism for the formation of elemental sulfur aerosols that takes advantage of the interaction between sulfur oxides and hydrogen sulfide under water or sulfuric acid catalysis. These results provide a chemical framework for understanding the formation mechanism of S0 aerosols in planetary atmospheres.
Keywords: sulfur aerosols, catalysis, planetary environment, nonphotochemical, hydrogen sulfide
Abstract
Elemental sulfur aerosols are ubiquitous in the atmospheres of Venus, ancient Earth, and Mars. There is now an evolving body of evidence suggesting that these aerosols have also played a role in the evolution of early life on Earth. However, the exact details of their formation mechanism remain an open question. The present theoretical calculations suggest a chemical mechanism that takes advantage of the interaction between sulfur oxides, SOn (n = 1, 2, 3) and hydrogen sulfide (nH2S), resulting in the efficient formation of a Sn+1 particle. Interestingly, the SOn + nH2S → Sn+1 + nH2O reactions occur via low-energy pathways under water or sulfuric acid catalysis. Once the Sn+1 particles are formed, they may further nucleate to form larger polysulfur aerosols, thus providing a chemical framework for understanding the formation mechanism of S0 aerosols in different environments.
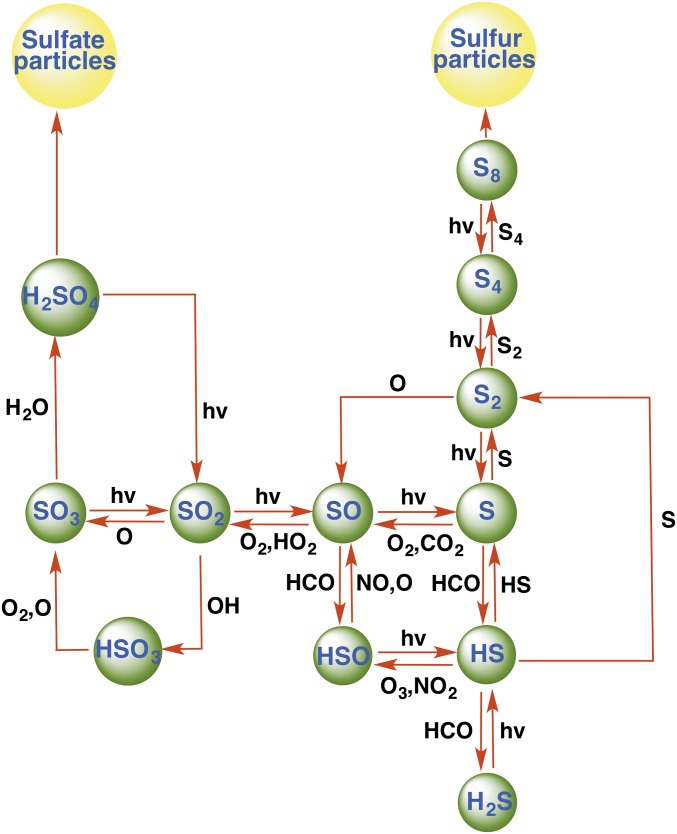
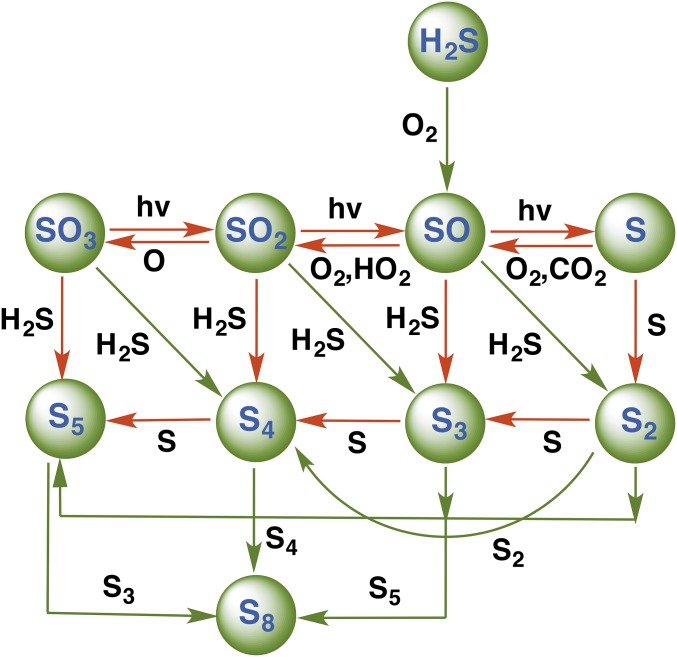
Sulfur chemistry is a ubiquitous component in the atmospheres of Venus, early Earth, and Mars (1). The different forms of sulfur (e.g., S2−, S−, S0, S2O32−, SO32−, SO42−) provide energy for different types of sulfur metabolisms in different environments. The sulfur cycle in the Archean atmosphere is also believed to have played a role in the early evolution of life on Earth (2–7). The emerging photochemical picture suggests that reduced elemental sulfur (S0) and sulfate (SO4) are the dominant sulfur species in the Archean (2–9). However, the latest isotope signatures of microscopic sulfides in marine sulfate deposits indicate that the ultimate source for this metabolic sulfur cycling was atmospherically derived S0 (10). One of the most important sources of sulfur into the atmosphere is from volcanoes, and the most abundant sulfur gases are SO2 and H2S. The photochemistry of these gases in the atmosphere yields elemental sulfur, sulfur particles, sulfuric acid, and oceanic sulfate. Scheme 1 illustrates the chemical processes suggested to be important in the photochemical oxidation of volcanic sulfur species in the early atmosphere of Earth.
Scheme 1.
Sulfur photochemistry in an anoxic early atmosphere. Sulfur is emitted to the atmosphere from volcanoes as sulfur dioxide and hydrogen sulfide, and is removed by rainout of soluble gases and by formation and deposition of sulfate and elemental sulfur particles.
The S0 aerosols are not only involved in the Archean life, but are also implicated in other environments (1, 11–19). For example, polysulfur (Sx = S2→8) aerosols are thought to exist in clouds of Venus and their role as the unknown UV absorber in its lower atmosphere has been discussed in the literature (14). The S8 particles are also observed in the marine troposphere (15). Finally, the role of S8 aerosols in explaining the early climate of Mars atmosphere has also been debated (16).
Despite being of broad appeal, the formation mechanism of S0 aerosols remains an open question. The photolysis of SO2 and SO by UV light with λ < 220 nm has generally been invoked to explain the mass-independent fractionation (MIF) of isotope effects in the sulfur cycle during the Archean (2–9). However, the contribution of other mass-independent chemical reactions to this geologic record remains unclear. To fully understand the sulfur cycle, it is necessary to identify all sources of sulfur compounds and account for all species which can occur in the atmosphere.
Results and Discussion
SOn (n = 1, 2, 3) + nH2S Potential Energy Surface.
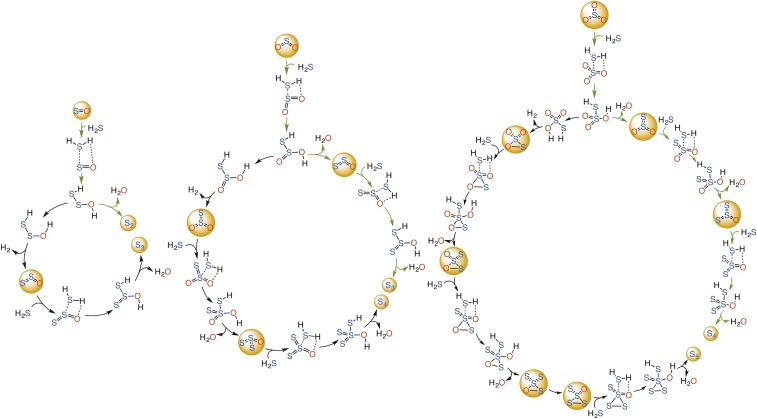
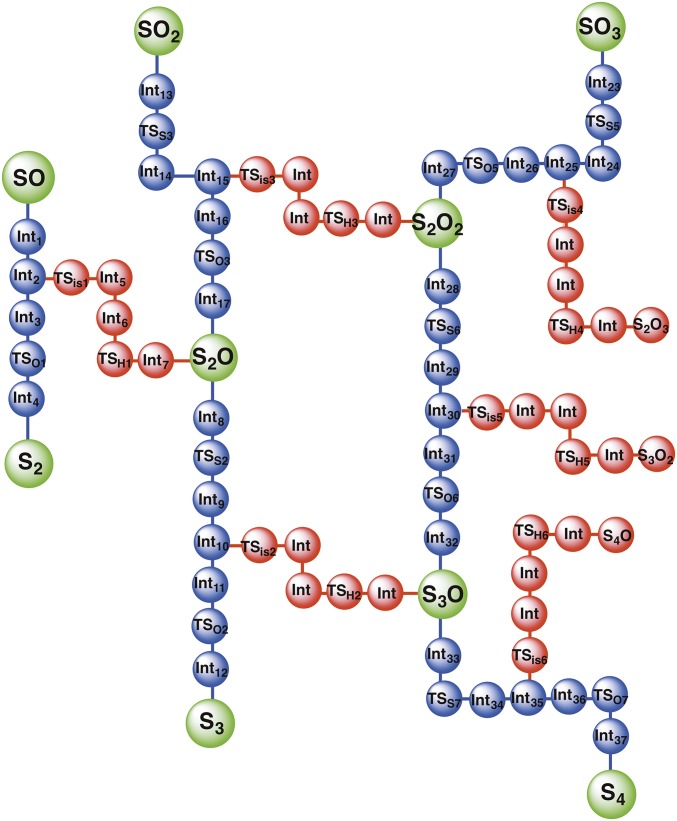
As can be seen from Scheme 1, which summarizes the current state of sulfur chemistry in the atmosphere, there is a gap in our understanding of the connection between sulfur oxide chemistry and sulfur aerosol formation. Herein, we describe a nonphotochemical reaction mechanism that may possibly convert the SOn + nH2S (n = 1, 2, 3) chemistries into the S8 aerosol in the gas phase (Scheme S1). It is the thermodynamics of these processes, and their catalysis by water and sulfuric acid, that we investigate here. This mechanism may not only help in better understanding the role of sulfur cycle involving SOn, S8, and H2S as the potential S MIF carrier from the atmosphere to the ocean surface, but may also provide deeper insight into the formation mechanism of S0 aerosols in various other environments.
Scheme S1.
Proposed nonphotochemical mechanisms for the conversion of SOn (n = 1, 2, 3) + nH2S chemistries into the elemental sulfur aerosols, respectively. The green arrowed pathways are the most probable ones whereas the black pathways may also involve high-barrier steps.
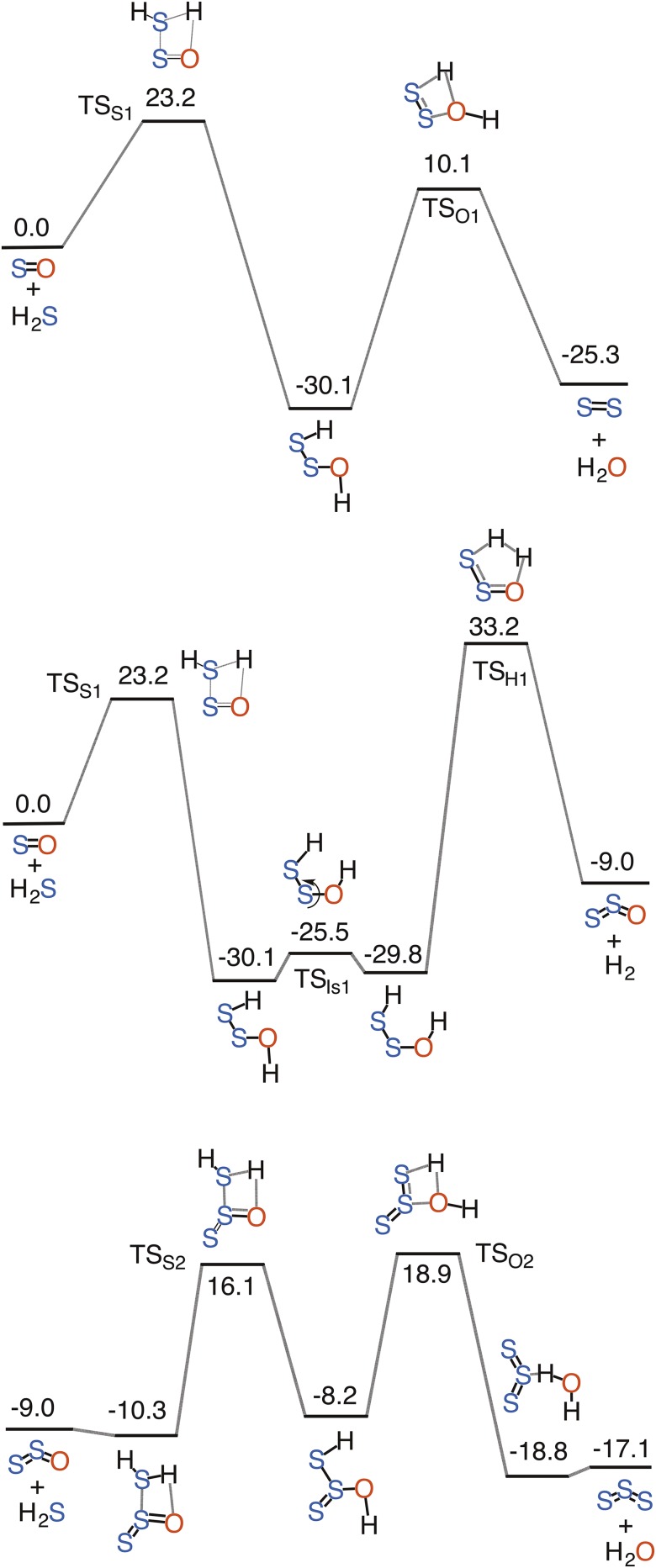
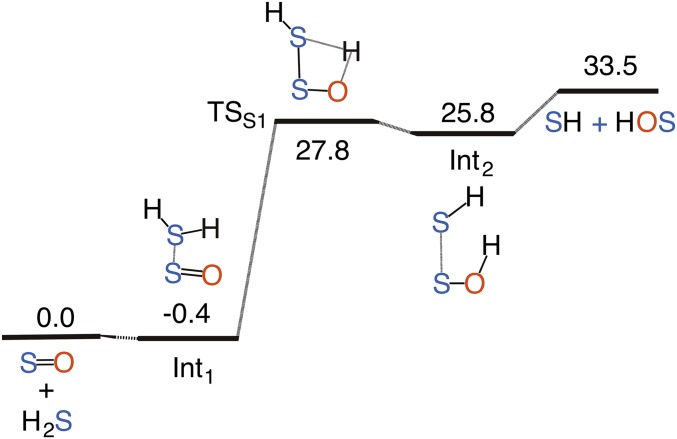
We first explored the uncatalyzed gas-phase reactions of SOn with nH2S using quantum-chemical calculations at the coupled cluster single and double substitution method with a perturbative treatment of triple excitations [CCSD(T)]/aug-cc-pVTZ//M06-2X/aug-cc-pVTZ level of theory. We considered both singlet and triplet states for SO. Although the triplet ground state of SO is more stable than its singlet state, the calculations suggest that the 3SO + H2S reaction leads to the formation of HS and HOS radicals, and is endothermic by 33.5 kcal/mol (Fig. S1). By contrast, the 1SO + H2S reaction is highly exothermic (Fig. S2). The relative energies of the computed transition-state structures and minima for the uncatalyzed 1SO + H2S reaction are shown in Fig. S2. The possible source of 1SO is either the photolysis of SO2 at λ < 220 nm or the partial oxidation of H2S. There have also been reports that 1SO could be ejected directly from the volcanic vent (20). However, the 1SO + H2S reaction would face competition from the 1SO + O2 → SO2 + O in atmosphere, suggesting that the 1SO + H2S is more likely to happen locally where the concentration of sulfur gases is expected to be high.
Fig. S1.
Calculated reaction profiles for the uncatalyzed gas-phase reaction of H2S with 1SO. A shows a S2-forming reaction pathway, whereas B and C show S3-forming reaction pathways. Relative energies (kcal mol−1) of minima and transition-state structures are calculated at the uCCSD(T)/aug-cc-pVTZ//uM06-2X/aug-cc-pVTZ level of theory.
Fig. S2.
Calculated potential energy surface for the uncatalyzed gas-phase reaction of H2S with 3SO. Relative energies (kcal mol−1) of minima and transition-state structures are calculated at the CCSD(T)/aug-cc-pVTZ//M06-2X/aug-cc-pVTZ level of theory.
The 1SO + H2S reaction results in the stepwise formation of H2S2O, which involves a barrier of 23.2 kcal/mol and has an exothermicity of 30.1 kcal/mol. The comparative analysis of the potential energy surfaces for the 1SO and SO2 (Fig. S3) reactions reveals that the 1SO reaction is relatively more favorable. Although the uncatalyzed SO2 + H2S reaction has been previously calculated (13), we reexamined the reaction here in greater detail at the same level of theory to facilitate the comparison between the 1SO and SO2 reactions. The effective barrier for the 1SO reaction is 7.3 kcal/mol lower than that for the SO2 reaction. The exothermicity of the H2S2O formation is dramatically higher than that for the H2S2O2 formation via the SO2 + H2S reaction. The higher reactivity of 1SO may provide an alternate mechanistic explanation as to why SO is rarely observed in the troposphere.
Fig. S3.
Calculated potential energy surface for the uncatalyzed gas-phase reaction of H2S with SO2. Relative energies (kcal mol−1) of minima and transition-state structures are calculated at the CCSD(T)/aug-cc-pVTZ//M06-2X/aug-cc-pVTZ level of theory.
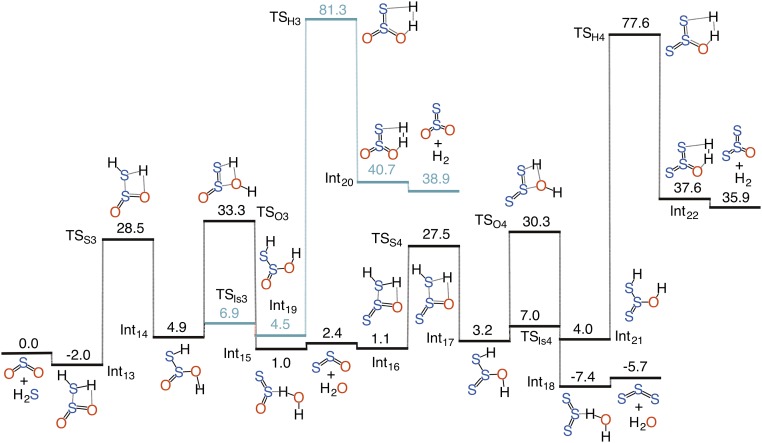
Once H2S2O is formed, it can either dehydrate to S2 or dehydrogenate to S2O that can subsequently react with H2S, resulting in the formation of S3. Thus, the overall 1SO + H2S reaction leads to the formation of a S2 or S3 particle. Analogously, the SO2 + 2H2S and SO3 + 3H2S (Fig. S4) chemistries produce S3 or S4 and S4 or S5 particles, respectively. The SO3 reaction is predicted to be more favorable than the SO2 one. These mechanistic outcomes point toward a more generalized interaction between a sulfur oxide, SOn and H2S, which can be qualitatively summarized in the form of a reaction, SOn + n/(n+1)H2S → Sn+1 + Sn+2 + n/(n+1)H2O. The formation of Sn+1 particle is predicted to be energetically more viable because it only involves low-barrier dehydration whereas the Sn+2 pathway must also go through high-barrier dehydrogenation in addition to low-barrier dehydration. Thus, it is reasonable to suggest that the SOn + nH2S → Sn+1 + nH2O is the most efficient reaction except for n = 1, where the Sn+2 formation may also become feasible under certain conditions.
Fig. S4.
Calculated potential energy surface for the uncatalyzed gas-phase reaction of H2S with SO3. Relative energies (kcal mol−1) of minima and transition-state structures are calculated at the CCSD(T)/aug-cc-pVTZ//M06-2X/aug-cc-pVTZ level of theory.
Reactivity Under Catalysis.
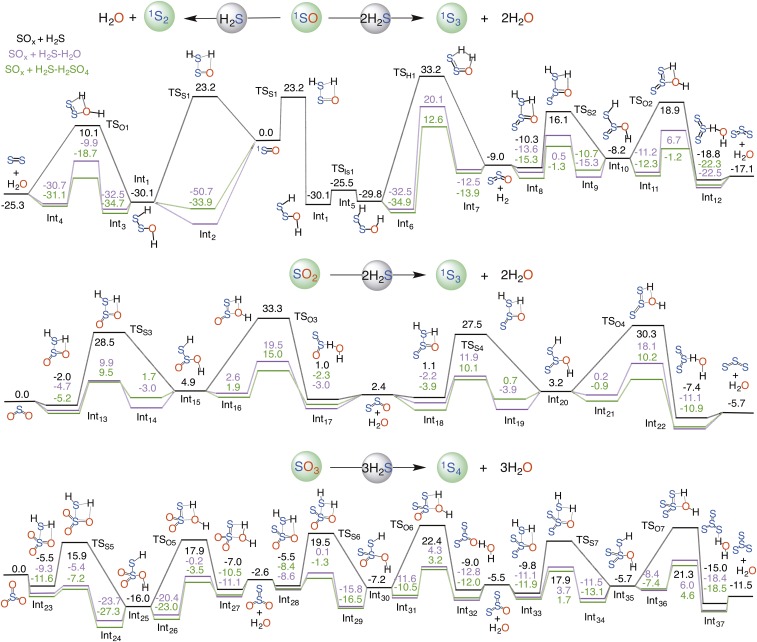
Although the formation of a Sn+1 particle via the SOn + nH2S reaction is more favorable, it still involves an appreciable thermal barrier that seems insurmountable under atmospheric conditions. However, recent studies (21–24) suggest that there are certain species in the atmospheres of Earth and Venus that may be able to catalyze these chemistries to such an extent that these processes become accessible. For example, H2O is the most dominant species in the troposphere and has been shown to catalyze hydrogen atom transfer (HAT)-based addition reactions (21). Sulfuric acid (H2SO4) is an important constituent in the Venus atmosphere (25) and has been predicted to be one of the most efficient catalysts available for the HAT-based reactions (22–24). Building upon these recent developments, we next examined the SOn + nH2S reaction, which also involves an HAT reaction, in the presence of H2O and H2SO4.
The formation of H2S2O from the 1SO + H2S reaction and its subsequent dehydration to S2 becomes facile under H2O or H2SO4 catalysis (Fig. 1). H2SO4 turns out to be a better catalyst than water because of its ability to stabilize reactants and products by forming sterically more favorable double hydrogen-bonding interactions. The alternate decomposition pathway for H2S2O, which leads to S2O, is also significantly impacted under catalysis. However, the barriers for the S2O-forming decomposition under catalysis are relatively higher than the dehydration one, suggesting that the probability of this decomposition pathway in water-rich surfaces or acidic environments may be quite low. H2S2O in the 1SO + H2S reaction is formed with an excess energy of 30.1 kcal/mol, which may play a role in making a S2O-based S3 channel accessible under catalytic conditions.
Fig. 1.
Calculated reaction profiles for the gas-phase reactions of SOn (n = 1, 2, 3) with H2S, (black), H2S–H2O (magenta) and H2S–H2SO4 (green), respectively. Relative energies (kcal mol−1) of minima and transition-state structures are calculated at the CCSD(T)/aug-cc-pVTZ//M06-2X/aug-cc-pVTZ level of theory. Note that the SO reactions have been calculated at the uCCSD(T)/aug-cc-pVTZ//uM06-2X/aug-cc-pVTZ level.
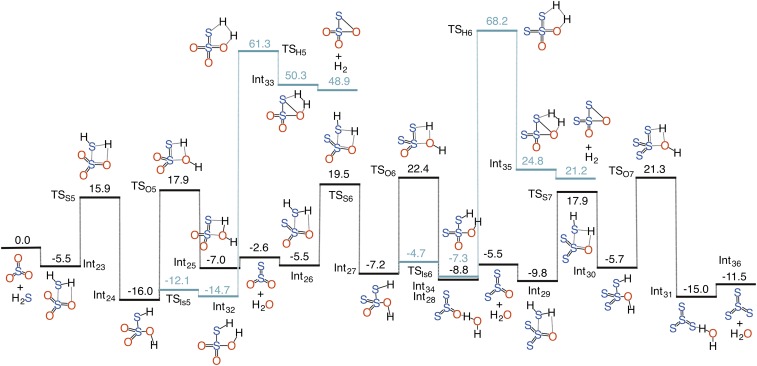
On the other hand, we only examined the SO2 + 2H2S → S3 + 2H2O-forming pathway in the presence of a single H2O and H2SO4 molecule (Fig. 1). This is because the S4-particle-forming pathways are mediated by very high-lying transition states (Fig. S3) and are not expected to become accessible even under H2O or H2SO4 catalysis. The overall SO2 + 2H2S → S3 + 2H2O reaction is calculated to be 5.7 kcal/mol exothermic. The uncatalyzed H2S2O2 formation involves an effective barrier of 30.5 kcal/mol. Under H2O and H2SO4 catalysis, the reaction barrier is appreciably lowered to 14.6 and 14.7 kcal/mol, respectively. The subsequent dehydration of H2S2O2, which produces S2O, has a barrier of 28.4 kcal/mol and an exothermicity of 2.5 kcal/mol that are significantly impacted under catalysis. H2SO4 produces more catalytic effect than water in this case; the dehydration barriers for the H2SO4- and H2O-catalyzed reactions are lowered to 12.1 and 15.8 kcal/mol, respectively. The reaction of S2O with H2S and the eventual decomposition of H2S3O into S3 + 2H2O are significantly influenced under catalysis. Although the S3 formation via the SO2 + 2H2S reaction is 11.4 kcal/mol less exothermic than that in the 1SO + 2H2S reaction, it is still expected to be the favored pathway because it bypasses the high-barrier dehydrogenation step, which significantly lowers the energetics of the overall reaction. The effect of catalysis on the S4-forming pathway from the SO3 + 3H2S reaction is also appreciable; all of the transition states are submerged below the separated reactants and the overall reaction occurs in a barrierless manner (Fig. 1). Moreover, the complexed S4 particle, which is hydrogen-bonded with catalyst, H2O or H2SO4, is at least 18.0 kcal/mol more stable than SO3 and ∼7.0 kcal/mol more stable than free S4 and catalyst. Among all of the sulfur gases considered, the sulfur particle formation via the SO3 reactions is the most favorable. Fig. 2 summarizes the optimum path for the Sn+1 formation from the SOn + nH2S reaction under H2SO4 catalysis. This qualitative profile reveals an interesting reactivity pattern; for a given interaction between SOn and nH2S, there are n high-energy dehydrogenation channels that may or may not open up depending upon reaction conditions.
Fig. 2.
Reaction scheme network showing the optimum path for the sulfuric acid-assisted formation of elemental Sn+1 aerosols from the SOn (n = 1, 2, 3) + nH2S reaction. The blue and green connections represent the favorable pathways whereas the red connections represent high-energy less likely pathways.
Redefining the Formation Mechanism of S0 Aerosols.
The S0 aerosols in the anoxic atmosphere of Archean are suggested to be produced by the UV photolysis of SO2 at λ< 220 nm (2–9). However, the present results suggest a nonphotochemical mechanism for the formation of these S0 aerosols, in which the Sn+1 particle is formed from the interaction of H2O- or H2SO4-bound H2S with SOn (Fig. 3). This indicates that once S2, S3, or S4 is formed, it could initiate a self-reaction that would build larger S0 particles. The mechanistic beauty of this aerosol-forming chemical process is that it does not require the conventional gas-phase three-body sulfur atom recombination, S + S, required for forming S2 (5, 26, 27) or the UV photon-induced reaction between S and SH to form S2 and H (28). It should be noted that several S2–4 allotropes generally have very low vapor pressures in planetary atmospheres (7) However, the consideration of the kinetics of condensation may make these gas-phase sulfur self-reactions important under certain conditions.
Fig. 3.
Reaction scheme showing the optimum path for the formation of elemental S8 aerosols from the SOn (n = 1, 2, 3) + nH2S reaction. The green connections represent the probable pathways whereas the red connections represent less likely pathways.
This mechanism has broad implications. For example, in Venus clouds, the exact source of polysulfur particle, which absorbs UV light, is unknown (14). The present calculations suggest that the Sn+1 formation is the most favorable under H2SO4 catalysis. The Sn+1 particle may then nucleate into the polysulfur particle. Sulfur species are abundantly available in the Venus atmosphere. The estimated concentration of SO2 lies in the range of 180 ± 50 ppm (25) and 130 ± 35 ppm (29), whereas the H2S concentration has been predicted to be 80 ± 40 ppm (30). H2SO4 and H2O are present in ∼5- and ∼30-ppm amounts, respectively (31), which lends support to such a mechanism. In a recently studied kinetic model of Venus chemistry (1), the SO3 mixing ratio in the 40–45-km altitude inversely correlates with the sulfur particle mixing ratio. The reaction of SO3 with 3H2S to form S4 may explain this inverse correlation. The SO3 + H2S reaction involves a relatively smaller barrier than that of SO2 + H2S. In 35–45-km altitude, the H2SO4 mixing ratio is ∼5 ppm, implying that a significant fraction of H2SO4 may exist as a van der Waals complex with H2S, and thus may catalyze the SO3 + H2S chemistry by invoking a bimolecular reaction between SO3 and H2SO4••H2S.
The CCSD(T) calculated binding energy of H2SO4••H2S complex is 5.3 kcal/mol. The equilibrium constants for the H2SO4••H2S complex calculated at various temperatures are collected in Table 1. The SO3 + H2SO4••H2S reaction occurs in a low-energy manner and is strongly exothermic. Note that the SO2 mixing ratio in the middle atmosphere is the highest among the sulfur gases (17, 18) implying that the SO2 + H2SO4••H2S reaction would also make an important contribution toward the overall elemental sulfur production via SOn + nH2S reaction. These conclusions are consistent with the fact that the sulfur cycles in the middle atmosphere are fast. Although chlorosulfane chemistry has been previously proposed to explain the elemental sulfur aerosols (30), the present results suggest a direct connection between the chemistries of sulfur gases and elemental sulfur particles.
Table 1.
Calculated equilibrium constants for the complexes of hydrogen sulfide (H2S) with water (H2O), thiosulfurous acid (H2S2O2), sulfuric acid (H2SO4), and carbonic acid (H2CO3) at various temperatures
| Equilibrium constant, Keq, cm3•molecule−1 | ||||
| Temperature, K | H2O••H2S (∆E = −1.36) | H2S2O2••H2S(∆E = −5.32) | H2CO3••H2S(∆E = −5.07) | H2SO4••H2S(∆E = −6.69) |
| 200 | 4.35 × 10−22 | 1.73 × 10−20 | 6.21 × 10−20 | 2.79 × 10−18 |
| 210 | 3.71 × 10−22 | 8.99 × 10−21 | 3.42 × 10−20 | 1.26 × 10−18 |
| 220 | 3.23 × 10−22 | 4.97 × 10−21 | 1.99 × 10−20 | 6.17 × 10−19 |
| 230 | 2.85 × 10−22 | 2.90 × 10−21 | 1.23 × 10−20 | 3.22 × 10−19 |
| 240 | 2.56 × 10−22 | 1.78 × 10−21 | 7.87 × 10−21 | 1.78 × 10−19 |
| 250 | 2.32 × 10−22 | 1.14 × 10−21 | 5.25 × 10−21 | 1.03 × 10−19 |
| 260 | 2.13 × 10−22 | 7.54 × 10−22 | 3.63 × 10−21 | 6.28 × 10−20 |
| 270 | 1.98 × 10−22 | 5.17 × 10−22 | 2.58 × 10−21 | 3.97 × 10−20 |
| 280 | 1.85 × 10−22 | 3.65 × 10−22 | 1.89 × 10−21 | 2.60 × 10−20 |
| 290 | 1.74 × 10−22 | 2.64 × 10−22 | 1.42 × 10−21 | 1.76 × 10−20 |
| 298.15 | 1.67 × 10−22 | 2.07 × 10−22 | 1.14 × 10−21 | 1.31 × 10−20 |
| 300 | 1.65 × 10−22 | 1.96 × 10−22 | 1.09 × 10−21 | 1.23 × 10−20 |
CCSD(T)/aug-cc-pVTZ//M06-2X/aug-cc-pVTZ calculated binding energies of the H2S complexes are given in parentheses.
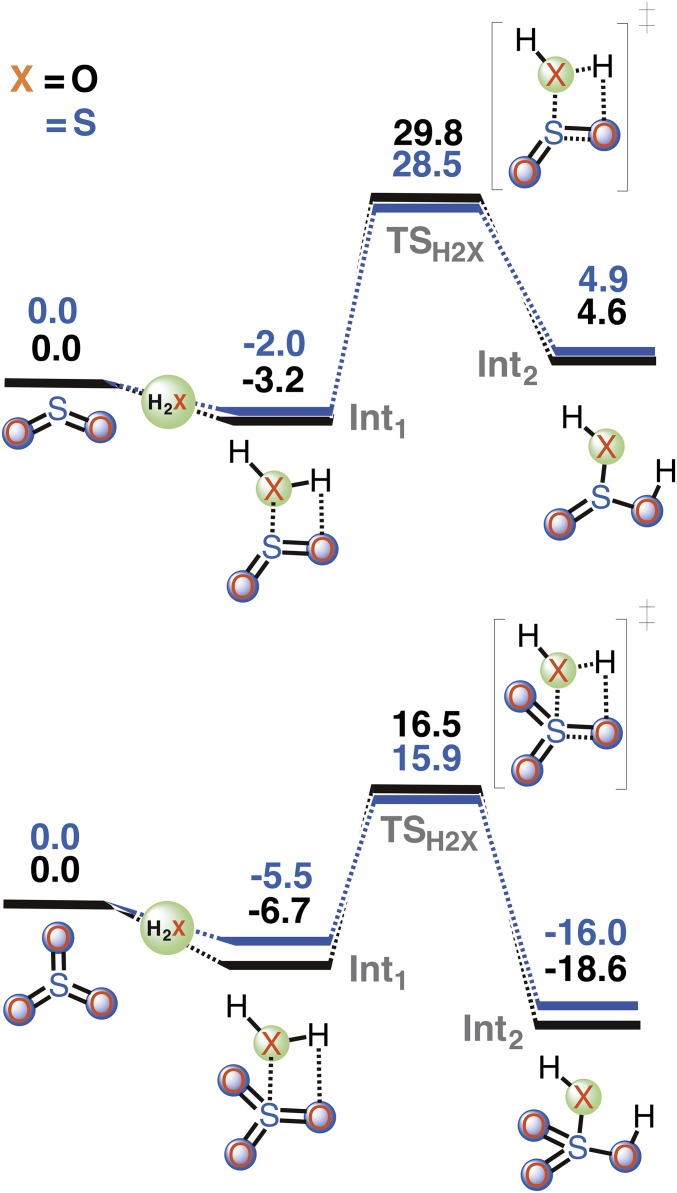
In a recent Venus model by Zhang et al. (18), a strong anticorrelation between the sulfur gases (SO3 and SO) and the elemental sulfur aerosol profiles below 65 km has also been observed. The thermal reactions of SO3 and SO with H2SO4••H2S clearly explain this anticorrelation. These thermal reactions not only provide useful mechanistic insights into an important sink of sulfur gases below 90 km in the Venus atmosphere, but may also help in understanding the mixing profiles of SO and SO2 at higher altitudes (1, 17, 18, 32–36). At 96-km Venus atmosphere, the rates of the SO3 hydration and the SO3 photolysis are found to be comparable (17), which is suggestive of the fact that nearly half of the sulfur in H2SO4 goes into SO3 and produces the inversion layers of SO2 and SO. However, our calculations on the reactions of SO2 and SO3 with H2X (X = O, and S) suggest that the bimolecular reactions of sulfur gases with H2S involve smaller barriers than the analogous reactions involving H2O (Fig. 4). This indicates that the SO3 + H2S reaction under H2SO4 catalysis may occur even at higher altitude, leading to the formation of elemental sulfur aerosol.
Fig. 4.
CCSD(T)/aug-cc-pVTZ//M06-2X/aug-cc-pVTZ calculated zero-point–corrected reaction profiles for the reactions of sulfur dioxide (Top) and sulfur trioxide (Bottom) with chalcogen hydrides, H2X (X = O, and S). The energies are given in kcal/mol units.
In the Venus photochemistry model analyzed by Mills (37), the SO and S2O sulfur gases, in addition to SO2, are present in significant amounts in the middle atmosphere, which makes the low-barrier SO + H2SO4••H2S and S2O + H2SO4••H2S chemistries important for the S2- and S3-formation mechanisms. A recently observed correlation between SO2 depletion and enhancement in UV absorber also endorses this nonphotochemical mechanism (33). This mechanism also opens up a chemical channel for the formation of S8 aerosols in the Mars atmosphere, which have been shown to play a role in Mars’ early climate (16).
Elemental sulfur deposits are also common at volcanic vents and fumaroles where high-temperature discharge gas is supersaturated in sulfur (38) as a result of equilibrium chemical reactions involving a variety of magmatic sulfur gases. The reaction between SO2 and H2S has been used to explain these sulfur sediments (39). It is important to note that in Kawah Ijen fumarole discharges (38), water is the most abundant gas species, followed by carbon dioxide (CO2) and sulfur gases, SO2 and H2S. As a result, an appreciable fraction of H2O may exist in a complexed form with H2S. This may alter the energetics of the overall elemental sulfur formation by invoking the bimolecular reaction between SO2 and H2O••H2S. Our results indicate that the barrier for the rate-determining step of the reaction between SO2 and H2O••H2S is lowered by ∼40% compared with the uncatalyzed SO2 + H2S reaction. Alternatively, H2O may add across either CO2 to form carbonic acid (H2CO3) or SO2 to form thiosulfurous acid (H2S2O2), which may subsequently influence the SO2 + H2S reaction. Both H2CO3 (–C=O, OH) and H2S2O2 (–S=O, OH) form strong complexes with H2S (Table 1) and possess mandatory functionalities to allow the SO2 + H2S reaction to occur under acid catalysis. Considering that these proposed chemical processes are all H2S-based, and no elemental sulfur formation has been seen in the absence of H2S (40), the results could help in better understanding the geochemistry of the magmatic–hydrothermal systems.
In summary, we have used electronic structure calculations to suggest a nonphotochemical mechanism for the formation of elemental sulfur aerosols in planetary atmospheres. The mechanistic beauty of this proposal is that the reactions of sulfur oxides and hydrogen sulfide under water or sulfuric acid catalysis provide low-energy pathways for the formation of S2–S4 particles. Interestingly, the uncatalyzed reactions of sulfur oxides and hydrogen sulfide result in the intermediates that are functionally similar to sulfuric acid, which points to the fact that these sulfur chemistries could be autocatalyzed.
Methods
The SOn (n = 1, 2, 3) + nH2S reactions in the gas phase have been examined in the absence and presence of H2O and H2SO4 catalysts. The uncatalyzed reactions have been briefly explored whereas the effect of catalysis on the most probable reactions has been examined in detail. In particular, the effect of H2O or H2SO4 catalysis on the H2S addition reactions and the subsequent H2O elimination reactions have been explored. The effect of catalysis on the dehydrogenation reactions has not been examined because these reactions involve very high barriers and are less likely to be important in atmosphere. The impact of catalysis was quantified by calculating the reaction profiles for the bimolecular reactions between the H2O- or H2SO4-bound H2S and SOn. All of the reactions have been calculated assuming the singlet ground state except for the SO reactions, which have been explored for both the singlet and triplet states. The singlet SO reactions have been examined because the spectroscopic signatures of the direct singlet SO ejection from the volcanic vent have been detected (20). All calculations were performed with Gaussian 09 (41). All geometries were optimized using the density-functional theory method, M06-2X (42), and the augmented correlation-consistent basis set, aug-cc-pVTZ (43). Because the open-shell 1SO is more stable than the closed-shell one, the calculations involving 1SO have been done using the uM06-2X/aug-cc-pVTZ level of theory. The energetics were further improved by performing single-point calculations at the CCSD(T) (44) and the aug-cc-pVTZ basis set. This level of theory has been found to provide an accurate description of hydrogen atom transfer-based chemistries in the recent past (23, 24). All stationary points were characterized by frequency calculations and reported energies include zero-point energy corrections (unscaled) from the method used for geometry optimization.
Acknowledgments
We are grateful to the Holland Computing Center, University of Nebraska-Lincoln for computational support of this work.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1620870114/-/DCSupplemental.
References
- 1.Krasnopolsky VA. Atmospheric chemistry on Venus, Earth, and Mars: Main features and comparison. Planet Space Sci. 2011;59:952–964. [Google Scholar]
- 2.Farquhar J, Bao H, Thiemens M. Atmospheric influence of Earth’s earliest sulfur cycle. Science. 2000;289(5480):756–759. doi: 10.1126/science.289.5480.756. [DOI] [PubMed] [Google Scholar]
- 3.Farquhar J, Wing BA. Multiple sulfur isotopes and the evolution of the atmosphere. Earth Planet Sci Lett. 2003;213:1–13. [Google Scholar]
- 4.Farquhar J, Savarino J, Airieau S, Thiemens MH. Observation of wavelength-sensitive mass-independent sulfur isotope effects during SO2 photolysis: Implications for the early atmosphere. J Geophys Res. 2001;106:32829–32839. [Google Scholar]
- 5.Pavlov AA, Kasting JF. Mass-independent fractionation of sulfur isotopes in Archean sediments: Strong evidence for an anoxic Archean atmosphere. Astrobiology. 2002;2(1):27–41. doi: 10.1089/153110702753621321. [DOI] [PubMed] [Google Scholar]
- 6.Ono S, et al. New insights into Archean sulfur cycle from mass-independent sulfur isotope records from the Hamersley Basin, Australia. Earth Planet Sci Lett. 2003;213:15–30. [Google Scholar]
- 7.Lyons JR. An estimate of the equilibrium speciation of sulfur vapor over solid sulfur and implications for planetary atmospheres. J Sulfur Chem. 2008;29:269–279. [Google Scholar]
- 8.Kamber BS, Whitehouse M. Micro-scale sulphur isotope evidence for sulphur cycling in the late Archean shallow ocean. Geobiology. 2007;5:5. doi: 10.1111/j.1472-4669.2006.00091.x. [DOI] [PubMed] [Google Scholar]
- 9.Halevy I. Production, preservation, and biological processing of mass-independent sulfur isotope fractionation in the Archean surface environment. Proc Natl Acad Sci USA. 2013;110(44):17644–17649. doi: 10.1073/pnas.1213148110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Philippot P, et al. Early Archaean microorganisms preferred elemental sulfur, not sulfate. Science. 2007;317(5844):1534–1537. doi: 10.1126/science.1145861. [DOI] [PubMed] [Google Scholar]
- 11.Glarborg P, Kubel D, Dam-Johansen K, Chiang H-M, Bozzelli JW. Impact of SO2 and NO on CO oxidation under post-flame conditions. Int J Chem Kinet. 1996;28:773–790. [Google Scholar]
- 12.Sendt K, Jazbec M, Haynes BS. Chemical kinetic modeling of the H/S system: H2S thermolysis and H2 sulfidation. Proc Combust Inst. 2002;29:2439–2446. [Google Scholar]
- 13.Sendt K, Haynes BS. Role of the direct reaction H2S + SO2 in the homogeneous Claus reaction. J Phys Chem A. 2005;109(36):8180–8186. doi: 10.1021/jp052622i. [DOI] [PubMed] [Google Scholar]
- 14.Toon OB, Turco RP, Pollack JB. The ultraviolet absorber on Venus-amorphous sulfur. Icarus. 1982;51:358–373. [Google Scholar]
- 15.Atlas E. Observation of possible elemental sulfur in the marine atmosphere and speculation on its origin. Atmos Environ. 1991;25A:2701–2705. [Google Scholar]
- 16.Tian F, et al. Photochemical and climate consequences of sulfur outgassing on early Mars. Earth Planet Sci Lett. 2010;295:412–418. [Google Scholar]
- 17.Krasnopolsky VA. Chemical kinetic model for the lower atmosphere of Venus. Icarus. 2007;191:25–37. [Google Scholar]
- 18.Zhang X, Liang MC, Mills FP, Belyaev DA, Yung YL. Sulfur chemistry in the middle atmosphere of Venus. Icarus. 2012;217:714–739. [Google Scholar]
- 19.Kerber L, Forget F. Wordsworth R (2015) Sulfur in the early martian atmosphere revisited: Experiments with a 3-D Global Climate Model. Icarus. 261:133–148. [Google Scholar]
- 20.de Pater I, Roe H, Graham JR, Strobel DF, Bernath P. Detection of the forbidden SO a1 ∆→ X3 ∑ rovibronic transition on I0 at 1.7 µm. Icarus. 2002;156:296–301. [Google Scholar]
- 21.Vöhringer-Martinez E, et al. Water catalysis of a radical-molecule gas-phase reaction. Science. 2007;315(5811):497–501. doi: 10.1126/science.1134494. [DOI] [PubMed] [Google Scholar]
- 22.Torrent-Sucarrat M, Francisco JS, Anglada JM. Sulfuric acid as autocatalyst in the formation of sulfuric acid. J Am Chem Soc. 2012;134(51):20632–20644. doi: 10.1021/ja307523b. [DOI] [PubMed] [Google Scholar]
- 23.Kumar M, Francisco JS. Red light-induced decomposition of an organic peroxy radical: A new source of the HO2 radical. Angew Chem Int Ed Engl. 2015;54(52):15711–15714. doi: 10.1002/anie.201509311. [DOI] [PubMed] [Google Scholar]
- 24.Kumar M, Sinha A, Francisco JS. Role of double hydrogen atom transfer reactions in atmospheric chemistry. Acc Chem Res. 2016;49(5):877–883. doi: 10.1021/acs.accounts.6b00040. [DOI] [PubMed] [Google Scholar]
- 25.Hoffman JH, Hodges RR, Jr, Donahue TM, Mcelroy MB. Composition of the Venus lower atmosphere from the Pioneer Venus mass spectrometer. J Geophys Res. 1980;85:7882–7890. [Google Scholar]
- 26.Peterson KA, Lyons JR, Francisco JS. An ab initio study of the low-lying electronic states of S3. J Chem Phys. 2006;125(8):084314. doi: 10.1063/1.2222367. [DOI] [PubMed] [Google Scholar]
- 27.Du S, et al. The kinetics study of the S + S2 → S3 reaction by the chaperone mechanism. J Chem Phys. 2011;134(15):154508. doi: 10.1063/1.3572226. [DOI] [PubMed] [Google Scholar]
- 28.Woodall J, Agundez M, Markwick-Kemper AJ, Millar TJ. The UMIST database for astrochemistry 2006. Astron Astrophys. 2007;466:1197–1204. [Google Scholar]
- 29.Golovin YM, Moshkin BE, Ekonomov AP. Aerosol component properties as measured by the Venera 11 and 12 spectrophotometer. Cosm Res. 1981;19:295–302. [Google Scholar]
- 30.Mukhin LM, et al. Gas chromatographic analysis of the Venus atmospheric chemical composition by the Vanera 13 and 14 descent probes. Cosm Res. 1983;21:168–172. [Google Scholar]
- 31.Krasnopolsky VA, Pollack JB. H2O-H2SO4 system in Venus’ clouds and OCS, CO, and H2SO4 profiles in Venus’ troposphere. Icarus. 1994;109(1):58–78. doi: 10.1006/icar.1994.1077. [DOI] [PubMed] [Google Scholar]
- 32.Mills FP, Allen M. A review of selected issues concerning the chemistry in Venus’ middle atmosphere. Planet Space Sci. 2007;55:1729–1740. [Google Scholar]
- 33.Marcq E, Belyaev D, Bertaux J-L, Fedorova A, Montmessin F. Long-term monitoring SO2 above the clouds of Venus using SPICAV-UV in nadir mode. EGU Gen Assem. 2011b;13:EGU2011–EGU2003. [Google Scholar]
- 34.Marcq E, et al. An investigation of the SO2 content of the Venusian mesosphere using SPICAV-UV in nadir mode. Icarus. 2011a;211:58–69. [Google Scholar]
- 35.Sandor BJ, Clancy RT, Moriarty-Schieven G, Mills FP. Sulfur chemistry in the Venus mesosphere from SO2 and SO microwave spectra. Icarus. 2010;208:49–60. [Google Scholar]
- 36.Krasnopolsky VA. Spatially-resolved high-resolution spectroscopy of Venus 2. Variations of HDO, OCS and SO2 at the cloud tops. Icarus. 2010b;209:314–322. [Google Scholar]
- 37.Mills FP. 1998. I. Observations and photochemical modeling of the Venus middle atmosphere. II. Thermal infrared spectroscopy of Europa and Callistro. PhD thesis (California Institute of Technology, Pasadena, CA)
- 38.Delmelle P, Bernard A, Kusakabe M, Fischer TP, Takano B. Geochemistry of the magmatic-hydrothermal system of Kawah Ijen volcano, East Java, Indonesia. J Volcanol Geotherm Res. 2000;97:31–53. [Google Scholar]
- 39.Rowe GL. Oxygen, hydrogen, and sulfur isotope systematics of the crater lake system of Poas volcano, Costa Rica. Geochem J. 1994;28:263–287. [Google Scholar]
- 40.Delmelle P, Bernard A. Geochemistry, mineralogy, and chemical modeling of the acid crater lake of Kawah Ijen volcano, Indonesia. Geochim Cosmochim Acta. 1994;58:2445–2460. [Google Scholar]
- 41.Frisch MJ, et al. 2009. Gaussian 09, revision D.01 (Gaussian, Pittsburgh)
- 42.Zhao Y, Truhlar DG. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc. 2008;120:215–241. [Google Scholar]
- 43.Kendall RA, Dunning TH, Jr, Harrison RJ. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J Chem Phys. 1992;96:6796–6806. [Google Scholar]
- 44.Noga J, Bartlett RJ. The full CCSDT model for molecular electronic structure. J Chem Phys. 1987;86:7041–7050. [Google Scholar]