Significance
AvrHah1 [avirulence (avr) gene homologous to avrBs3 and hax2, no. 1] is a transcription activator-like (TAL) effector (TALE) in Xanthomonas gardneri that enhances water soaking in its known hosts tomato, pepper, and Nicotiana benthamiana. We observe that the water soaking conferred by AvrHah1 is due to the movement of water into the infected apoplast from a wet environment. RNA sequencing identified two basic helix–loop–helix (bHLH) transcription factors that we confirmed as targets of AvrHah1. We discovered that a pectate lyase was upregulated by both of the bHLH transcription factors. Designer TALEs (dTALEs) for both bHLH transcription factors and the pectate lyase complemented the water-soaking phenotype of X. gardneriΔavrHah1. This report demonstrates virulence activity from an indirect TALE target.
Keywords: TAL effector, Xanthomonas bacterial spot, water soaking
Abstract
AvrHah1 [avirulence (avr) gene homologous to avrBs3 and hax2, no. 1] is a transcription activator-like (TAL) effector (TALE) in Xanthomonas gardneri that induces water-soaked disease lesions on fruits and leaves during bacterial spot of tomato. We observe that water from outside the leaf is drawn into the apoplast in X. gardneri-infected, but not X. gardneriΔavrHah1 (XgΔavrHah1)-infected, plants, conferring a dark, water-soaked appearance. The pull of water can facilitate entry of additional bacterial cells into the apoplast. Comparing the transcriptomes of tomato infected with X. gardneri vs. XgΔavrHah1 revealed the differential up-regulation of two basic helix–loop–helix (bHLH) transcription factors with predicted effector binding elements (EBEs) for AvrHah1. We mined our RNA-sequencing data for differentially up-regulated genes that could be direct targets of the bHLH transcription factors and therefore indirect targets of AvrHah1. We show that two pectin modification genes, a pectate lyase and pectinesterase, are targets of both bHLH transcription factors. Designer TALEs (dTALEs) for the bHLH transcription factors and the pectate lyase, but not for the pectinesterase, complement water soaking when delivered by XgΔavrHah1. By perturbing transcriptional networks and/or modifying the plant cell wall, AvrHah1 may promote water uptake to enhance tissue damage and eventual bacterial egression from the apoplast to the leaf surface. Understanding how disease symptoms develop may be a useful tool for improving the tolerance of crops from damaging disease lesions.
Bacterial spot disease caused by Xanthomonas sp. is a major limiting factor of agricultural yield (1). Xanthomonas gardneri has emerged recently as the dominant tomato pathogen in the midwestern United States and causes significant spotting on fruits (2). X. gardneri is also responsible for severe crop losses in Brazil (3) and appears to be spreading globally (4).
Among its repertoire of type III effectors, X. gardneri possesses a single transcription activator-like (TAL) effector (TALE) protein, AvrHah1 [avirulence (avr) gene homologous to avrBs3 and hax2, no. 1], which has been shown to confer enhanced water-soaked lesions in pepper (5). TALEs are secreted into host plant cells via the bacterial type III secretion apparatus and are delivered into the nucleus, where they activate the expression of target host genes (6). TALE binding specificity for host target DNA depends on the amino acid sequence of the central DNA-binding domain (DBD), which is composed of several repeats of 34–35 aa, nearly identical except for the 12th and 13th amino acids of each repeat, termed the repeat-variable diresidue (RVD) (7). Each RVD confers binding specificity to a particular nucleotide, and in combination the targeted host sequence, termed the “effector binding element” (EBE), can be predicted (7, 8). Once bound to a DNA target, the acidic activation domain (AD) of the TALE recruits the host’s transcriptional machinery to activate gene expression (6).
Plants have evolved diverse resistance mechanisms in response to TALEs (9). Some plants have strategically placed “EBE traps” in the promoters of resistance genes, as in the case of the pepper Bs3 resistance gene, which is transcriptionally activated at partially overlapping EBEs by the TALEs AvrBs3 and AvrHah1 (5, 10). Plants have developed mutations in promoter EBE regions that prevent successful activation of gene targets by TALEs, as in the case for rice Os8N3 (11). Tomato plants use Bs4, a nucleotide-binding leucine-rich repeat (NB-LRR) resistance (R) protein, to induce a cell death, or hypersensitive response (HR), in the presence of certain TALEs (12, 13).
If a plant gene targeted by the TALE promotes pathogen growth or spread, the gene is designated as a susceptibility (S) gene. Identifying and characterizing S genes reveals pathogen strategies and is useful in the design of disease resistant plants, for example through the removal of relevant EBEs via DNA editing technologies (9). For TALEs that activate multiple host gene targets, such as those with EBEs that partially span a TATA box, it becomes increasingly challenging to identify the bona fide S gene(s) (14, 15). To probe single genes for pathogenicity functions, designer TALEs (dTALEs) can be constructed and tested in planta for virulence contributions (16).
Several examples connecting lesion development and TALE S gene targets have been reported in diverse plant–xanthomonad pairs. In rice, Tal2g from Xanthomonas oryzae pv. oryzicoa activates expression of OsSULTR3;6, which encodes a sulfate transporter (17). Mutations in Tal2g reduced lesion expansion and X. oryzae pv. oryzicoa surface population, but not in planta growth, and dTALE activation of OsSULTR3;6 expression restored lesion expansion and surface growth to wild type levels (17). CsLOB1, a member of the lateral organ boundaries family from citrus, is activated by the PthA family of TALEs from xanthomonad pathogens of citrus. Loss of PthA reduces in planta bacterial growth and pustule formation (18, 19). TAL20 from the vascular cassava pathogen Xanthomonas axonopodis pv. manihotis activates expression of MeSWEET10a, which encodes a sugar transporter (20). dTALEs activating MeSWEET10a complemented the reduction in water soaking and midvein bacterial growth displayed by an X. axonopodis pv. manihotis TAL20 deletion strain in cassava (20). Avrb6 in Xanthomonas campestris pv. malvacearum correlates with increased water soaking and bacterial surface population in cotton (21). AvrBs3 activates the expression of pepper UPA20, which encodes a basic helix–loop–helix (bHLH) transcription factor that induces cell hypertrophy (22). Loss of AvrBs3 has been demonstrated to incur a bacterial fitness cost in the field (23).
AvrHah1 was found in a forward screen in search for a factor from X. gardneri that promotes the development of water-soaked lesions in pepper (5). We observed that AvrHah1 enables the absorption of water into the apoplast of X. gardneri-infected leaves, conferring a dark, water-soaked appearance. The AvrHah1-mediated intake of water can be observed in real time and can also be measured quantitatively by collection and weighting of apoplastic fluid. Furthermore, bacterial cells can be introduced into the apoplast during water soaking. We performed comparative RNA sequencing analysis of tomato leaves infected with X. gardneri or X. gardneriΔavrHah1 (XgΔavrHah1) and identified two bHLH transcription factors that were highly up-regulated in the presence of AvrHah1. We show that two pectin-modification genes—a pectate lyase (PL) and a pectinesterase (PE)—are downstream targets of both bHLH transcription factors and are therefore indirect targets of AvrHah1. We constructed dTALEs targeting the bHLH transcription factors and the pectin modification genes for gene activation and show that dTALEs activating the bHLH transcription factor or the PL encoding genes complemented the water-soaking defect of XgΔavrHah1.
Materials and Methods
X. gardneri Mutant Construction.
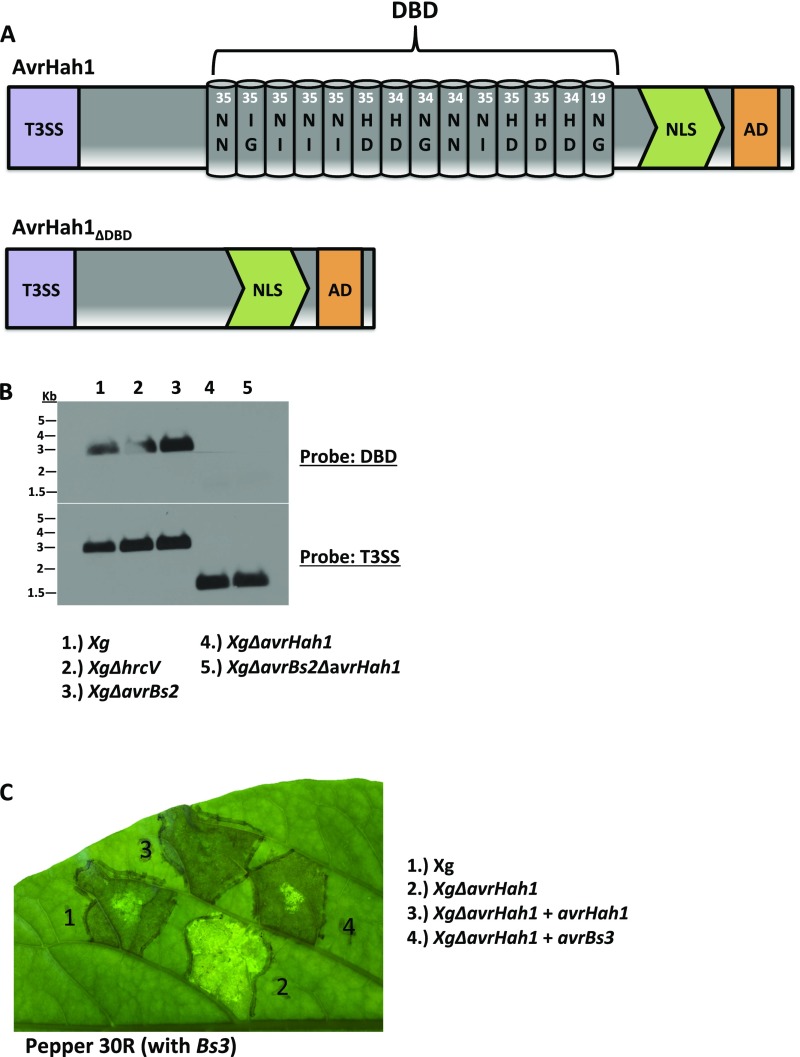
Xg153 (SM194-10) is from a collection of X. gardneri strains isolated between 2010 and 2012 in diseased tomato fields in Ohio and Michigan. Activation of Bs3 in pepper 30R, Southern blot analysis, and Sanger sequencing confirmed the identity of the single TALE in Xg153 as avrHah1 (24). All X. gardneri mutants were constructed in the Xg153 background. Xg∆avrHah1 was created by double homologous recombination using the suicide vector pLVC18 (25), such that the 13.5 repeats of the DBD were deleted in-frame (AvrHah1ΔDBD) (Fig. S1A). Deletion of the DBD was confirmed by Southern blot analysis, using 5 μg of bacterial genomic DNA restriction digested for 2 h with BamHI and run on a 0.7% agarose gel for 2 h at 100 V. DNA was transferred overnight to a Hybond-N+ membrane, hybridized with a digoxigenin (DIG)-labeled probe for the first 705 bp of AvrHah1 [which includes the type 3 secretion signal (T3SS)], and imaged with chemiluminescence. The blot was stripped and rehybridized with a DIG-labeled probe for the central DBD and reimaged (Fig. S1B). Xg∆avrHah1 was tested on pepper 30R for loss of Bs3 activation (Fig. S1C).
Fig. S1.
XgΔavrHah1 has an in-frame deletion of the DBD. (A) Schematic of the in-frame deletion that removes the DBD from avrHah1 to create avrHah1ΔDBD. NLS, nuclear localization signal. (B) Southern blot analysis of BamHI-digested genomic DNA from X. gardneri strains listed probed for the DBD (Top) or the T3SS (Bottom). The size of BamHI-digested avrHah1 is 2,964 bp, and the size of BamHI-digested DBD deletion of avrHah1 is 1,491 bp. (C) Cell death (or HR) occurs in response to activation of the Bs3 resistance gene in pepper 30R and appears here as a dark, opaque area. XgΔavrHah1 does not activate Bs3 and is pathogenic on pepper 30R. Complementation of XgΔavrHah1 with avrHah1 or avrBs3 restores activation of Bs3 resistance and HR in pepper 30R.
Complementation of Xg∆avrHah1.
All complementation constructs are driven by the X. axonopodis pv. manihotis strain 668 TAL20 promoter (proTAL20) (20). Gibson assembly (New England Biosciences) was used to clone proTAL20 upstream of avrHah1, avrBs3, or dTALEs into a gentamycinR entry vector using SalI and XbaI sites (26). Entry clones were then Gateway cloned (Invitrogen) into the broad host-range vector pVSP61 using LR Clonase (Invitrogen). dTALEs targeting the promoters of Solyc03g097820 (dT bHLH3), Solyc06g072520 (dT bHLH6), Solyc05g014000 (dT PL), and Solyc11g019910 (dT PE) were constructed as previously described (16). Information on the dTALEs including RVD sequences and binding scores as predicted by TALE-NT (27) can be found in Table S1. Triparental matings of pVSP61 complementation plasmids into Xg∆avrHah1 or Xanthomonas euvesicatoria 85-10 (Xe85-10) (28) were performed with the Escherichia coli helper strain pRK600, selected on rifampicin (Rif) and kanamycin, and confirmed with PCR.
Table S1.
Information on dTALE construction to activate expression of bHLH3, bHLH6, PL, and PE
| dTALE ID | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | Score | bp from ATG |
| dT bHLH3 | A | T | A | G | A | T | A | T | A | A | G | C | T | A | C | C | A | G | 5.45 | 274 |
| NI | NG | NI | NN | NI | NG | NI | NG | NI | NI | NN | HD | NG | NI | HD | HD | NI | NN | |||
| dT bHLH6 | A | T | A | C | A | G | G | A | T | A | T | C | C | C | T | T | T | C | 4.96 | 166 |
| NI | NG | NI | HD | NI | NN | NN | NI | NG | NI | NG | HD | HD | HD | NG | NG | NG | HD | |||
| dT PL | A | C | T | A | A | A | A | G | T | A | T | T | C | A | C | A | T | C | 4.22 | 381 |
| NI | HD | NG | NI | NI | NI | NI | NN | NG | NI | NG | NG | HD | NI | HD | NI | NG | HD | |||
| dT PE | A | T | T | T | C | C | C | T | C | A | C | T | A | A | T | A | C | T | 3.73 | 420 |
| NI | NG | NG | NG | HD | HD | HD | NG | HD | NI | HD | NG | NI | NI | NG | NI | HD | NG |
Binding scores were calculated using TALE-NT (27).
Bacterial-Growth Conditions.
Xanthomonas strains were grown on nutrient yeast glycerol agar (NYGA) supplemented, as appropriate, with 100 μg/mL Rif (all strains), 25 μg/mL kanamycin (Xg∆avrHah1 or Xe85-10 complemented with pVSP61), and 10 μg/mL tetracycline (Tet) (X. gardneri TetR used for water-soaking inoculum). Strains were incubated at 28 °C for 48 h. Cells were adjusted to appropriate concentrations with 10 mM MgCl2.
Assays for Water Soaking and in Planta Bacterial-Growth Assays.
For water-soaking assays in Nicotiana benthamiana, leaves were syringe-infiltrated with a bacterial suspension adjusted to OD600 = 0.1 (∼108 CFU/mL). At 48 hours postinfection (hpi), water soaking was induced in N. benthamiana by creating a small epidermal wound in the infected area and pipetting a 30 μL drop of water or 105 CFU/mL X. gardneri TetR on top of the wound. Total in planta bacteria were selected for on NYGA with Rif. X. gardneri TetR internalized during water soaking were selected by plating on Rif and Tet (Rif + Tet). All tomato experiments were performed in Heinz 1706 (Tomato Genetics Resource Center). For tomato water-soaking assays, leaves were infiltrated at OD600 = 0.1. At 48 hpi, infected leaves were submerged in water for 20 min without the introduction of surface wounds. For tomato in planta growth assays, six 0.5 cm2 leaf discs were collected at time 0 (t = 0) and at 6 d postinfection (dpi). Tomato leaves were infiltrated with bacterial suspensions at 104 CFU/mL for the development of discrete lesions and photographed 6 dpi.
Apoplastic Fluid Measurements.
For quantitative water-soaking assays, tomato leaves were syringe-infiltrated with a bacterial inoculum adjusted to OD600 = 0.1. At 48 hpi, leaves were submerged in water for 20 min and blotted with a Kimwipe to remove water from the leaf surface. Two 0.5 cm2 leaf discs were collected and placed in a 0.5 mL tube with a small hole cut in the bottom. This tube was placed in a preweighed 1.5-mL tube. The tubes were centrifuged at 3,000 × g for 5 min to collect the apoplastic fluid. Weights of the 1.5-mL tubes postspin were subtracted from the prespin weights to obtain a quantitative measurement of apoplastic fluid.
RNA Sequencing and TALE Prediction.
X. gardneri and XgΔavrHah1 were syringe-infiltrated into tomato Heinz 1706 leaves at OD600 = 0.25 and tissue was collected and frozen in liquid nitrogen at 24 hpi and 48 hpi. RNA from three biological replicates per time point were prepared using the Spectrum Total Plant RNA Kit (Sigma-Aldrich) and RNA sequencing libraries were prepared using the Illumina TruSeq RNA Library Prep Kit v2. Sequencing of 100-bp read length with Paired Ends was performed on a single lane of an Illumina HiSeq2000. Data analysis was performed using the CLC Genomics Workbench software to identify differentially expressed genes (at least twofold different with P < 0.05). Computational predictions for AvrHah1 EBEs were performed using the TALE-NT 2.0 algorithm (27) with RVDs for AvrHah1 (NN_IG_NI_ NI_NI_HD_HD_NG_NN_NI_HD_HD_HD_NG) and a cutoff of 4 times the best possible score of 3.76. Up-regulation of genes of interest were confirmed with semiquantitative RT-PCR. X. gardneri strains were infiltrated into tomato leaves at OD600 = 0.25 and tissue was collected at 24 hpi. The Spectrum Plant RNA kit (Sigma-Aldrich) (with on-column DNaseI treatment) and the SuperScript III First-Strand Synthesis System were used to make cDNA from 1.5 µg of RNA. Five microliters of 1:10 diluted cDNA were used for 24 cycles of amplification using Phusion HF polymerase (New England Biolabs).
Transient Promoter::Luciferase Assays in N. benthamiana.
Promoter sequences of 1 kb upstream of the start codon for bHLH3 (probHLH3), bHLH6 (probHLH6), PL (proPL), and PE (proPE) were Gateway (Invitrogen) cloned into the binary luciferase reporter construct pGWB35 (29). avrHah1 and the coding regions of bHLH3 and bHLH6 were cloned into the binary expression vector p1776. All constructs were conjugated into Agrobacterium GV3101 via triparental matings. N. benthamiana leaves were coinfiltrated with Agrobacterium (OD600 = 0.4 for each strain) for the combinations of promoter and transcriptional activator indicated. An empty Agrobacterium strain was coinfiltrated with each promoter::luciferase reporter for background promoter activation measurements. At 24 hpi, the leaves were syringe-infiltrated with 1 mM luciferin. Six 0.28 cm2 leaf punches per condition were taken and placed in separate wells of a black microtiter plate, suspended on 100 µL of water. Luciferase activity was read using a Wallace Envision plate reader.
Results
AvrHah1 Enables the Absorption of Water into the Apoplast of X. gardneri-Infected Leaves.
We observed a dark, apoplastic water soaking when X. gardneri, but not XgΔavrHah1, was infiltrated into tomato, pepper, and Nicotiana benthamiana, the known hosts of X. gardneri (24). This symptom was enhanced when infected plants were placed in a mist chamber. We could also obtain enhanced water soaking by growing infected plants at ambient humidity and then submerging leaves in water immediately before observation.
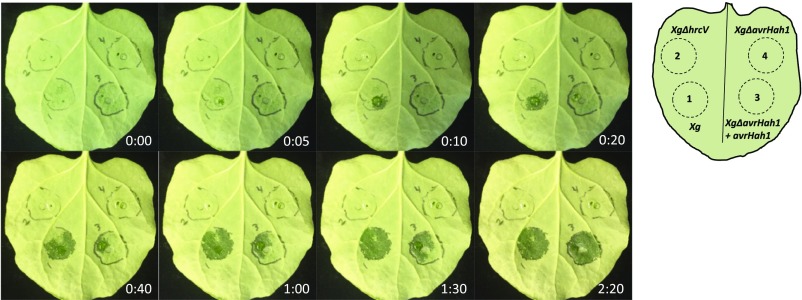
The AvrHah1-mediated intake of water into the apoplast was observed in real time in N. benthamiana (Fig. 1 and Movie S1). X. gardneri, XgΔhrcV (a mutant in type III effector secretion), XgΔavrHah1, and XgΔavrHah1 + avrHah1 were infiltrated into N. benthamiana. At 48 hpi, a pipette tip was used to make a small epidermal wound on the infiltrated areas. Immediately after introduction of the wound, a 30 μL drop of water was pipetted on top of each wound. In the zones infiltrated with X. gardneri or XgΔavrHah1 + avrHah1, the drop of water gradually shrunk as it was pulled into the leaf, darkening the apoplast as the advancing front proceeded away from the wound site within the boundaries of the infiltrated zone. The drop of water was completely absorbed by 2 min. In contrast, the water drop remained on top of the wound in zones infiltrated with XgΔhrcV or XgΔavrHah1.
Fig. 1.
AvrHah1 promotes the intake of water into the apoplast of X. gardneri (Xg)-infected plants. N. benthamiana was syringe-infiltrated with X. gardneri (1), XgΔhrcV (2), XgΔavrHah1 + avrHah1 (3), and XgΔavrHah1 (4) at OD600 = 0.1 [depicted in the diagram as the bottom left area of the leaf, top left area of the leaf (2), bottom right area of the leaf (3), and top right area of the leaf (4), respectively]. At 48 hpi, a 30-μL drop of water was pipetted on top of a small epidermal wound in the infiltrated areas. Pictures were taken at the times indicated in each frame until 2 min and 20 s after application of the first water drop.
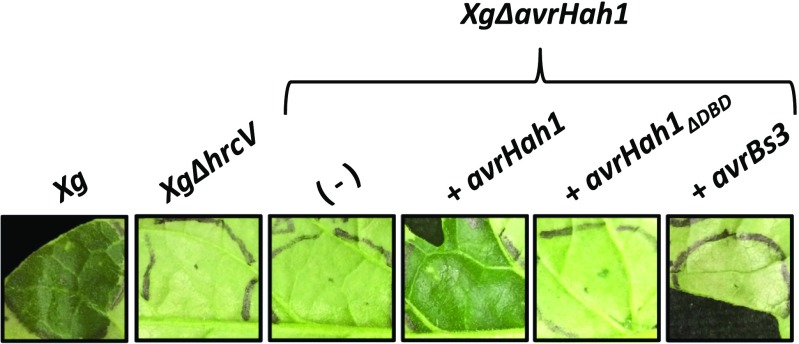
In tomato, the water-soaking effect of X. gardneri was similarly dramatic and enhanced by external water, yet progressed more slowly (20 min compared with 2 min in N. benthamiana) (Fig. 2). Leaves infiltrated with X. gardneri showed dark water soaking in the infected area, whereas leaves infiltrated with XgΔhrcV or XgΔavrHah1 did not develop water soaking. Complementation of full length avrHah1 into XgΔavrHah1 fully restored water soaking, but a mutant version of avrHah1 with an in-frame deletion of the DBD, AvrHah1ΔDBD, did not enhance water soaking. Complementation of XgΔavrHah1 with avrBs3 induced an immune reaction, or HR, likely due to recognition of AvrBs3 by the tomato R protein Bs4.
Fig. 2.
AvrHah1 induces water soaking in tomato, whereas AvrBs3 activates a hypersensitive response. Tomato Heinz 1706 leaves were syringe-infiltrated with the X. gardneri (Xg) strains indicated (OD600 = 0.1). At 48 hpi, leaves were submerged in water for 20 min, blotted with a Kimwipe, and photographed.
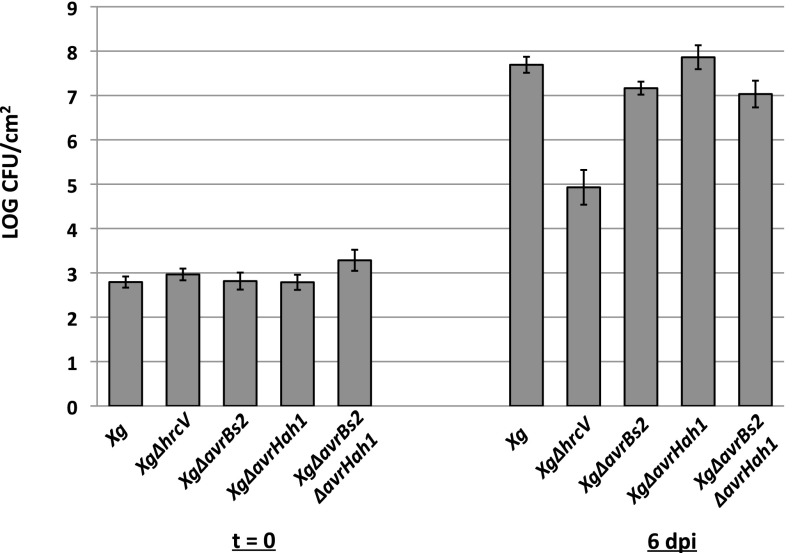
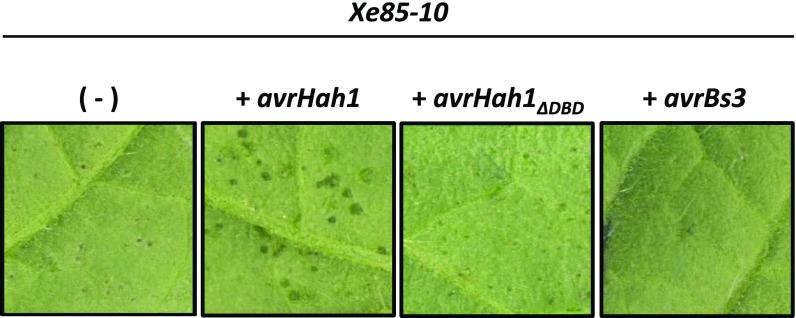
Previous work did not detect a difference in apoplastic growth between X. gardneri and an avrHah1 deletion mutant in pepper (5). We found these results to be consistent in tomato (Fig. S2). We did not detect a growth defect for avrHah1 in the background of an XgΔavrBs2 mutant. AvrBs2 is a type III effector protein that contributes measurably to in planta growth in many xanthomonads (30). Thus, it is unlikely that bacterial cell number accounts for the differential water soaking between X. gardneri and XgΔavrHah1. AvrHah1 was shown to confer enhanced water soaking to Xe85-10 in pepper (5), and we found that result was consistent in tomato (Fig. 3). Six days after infiltration into tomato leaves, X. euvesicatoria + avrHah1 developed water-soaked lesions, whereas X. euvesicatoria and X. euvesicatoria + avrHah1ΔDBD developed dry, flecked lesions (AvrHah1 is able to confer enhanced water soaking to X. euvesicatoria at an earlier time in infection compared with the endogenous water-soaking mechanisms in X. euvesicatoria). Tomato infiltrated with X. euvesicatoria + avrBs3 did not develop any visible lesions (likely due to activation of Bs4 resistance). Taken together, these results show that AvrHah1 enhances the intake of external water by the leaf during xanthomonad infection.
Fig. S2.
XgΔavrHah1 does not experience an in planta growth defect in tomato. Tomato leaves were infiltrated with X. gardneri strains at 104 CFU/mL. At 6 dpi, six leaf discs per strain of X. gardneri were sampled for in planta bacterial growth.
Fig. 3.
AvrHah1 enhances water soaked lesions in Xe85-10. Tomato leaves were infiltrated at 104 CFU/mL with Xe85-10 alone (−) or Xe85-10 complemented as indicated and observed at 6 dpi. Plants were grown at ambient humidity and were not submerged in water before observation.
Bacteria Can Be Introduced into the Apoplast During Water Soaking.
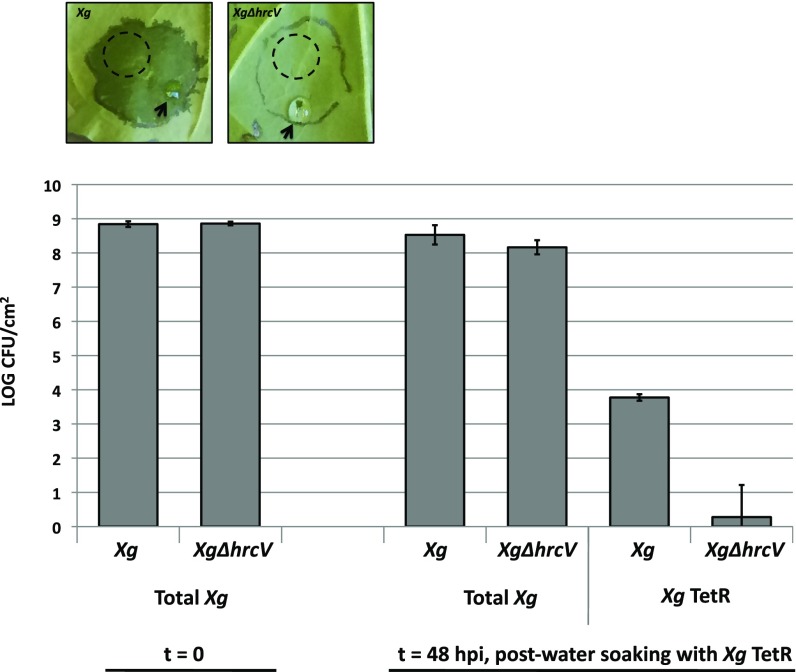
We wondered if water soaking could be a mechanism that introduces new bacteria into the apoplast. We set up a water-soaking assay in N. benthamiana with X. gardneri and XgΔhrcV as previously described, except we supplemented the drop of water on the wound site with Tet-resistant X. gardneri (X. gardneri TetR). We plated a dilution series from macerated leaf discs on Rif to measure all X. gardneri, or Rif + Tet to select for any newly introduced X. gardneri TetR (Fig. 4). We sampled leaf discs away from the wound site to avoid any X. gardneri TetR cells left on the leaf surface. We found that water soaking could ferry the Tet-resistant X. gardneri into the apoplast away from the initial wound site. No Tet-resistant X. gardneri were detected in the apoplasts of leaves infected with XgΔhrcV, which did not develop any water soaking.
Fig. 4.
Water soaking can introduce bacterial cells into the apoplast. N. benthamiana was syringe-infiltrated with either X. gardneri (Xg) or XgΔhrcV at OD600 = 0.1. Water soaking was induced at 48 hpi with a 30-μL drop of Tet-resistant X. gardneri (X. gardneri TetR, 105 CFU/mL in 1 0 mM MgCl2) on a wound (arrow). Leaf discs were collected away from the wound site after 5 min (dashed circle), ground in 10 mM MgCl2, and dilutions were plated on either Rif or Rif + Tet to select for the growth of all X. gardneri or the X. gardneri TetR from the water-soaking inoculum, respectively.
AvrBs3, but Not AvrHah1, Triggers Bs4-Mediated Resistance.
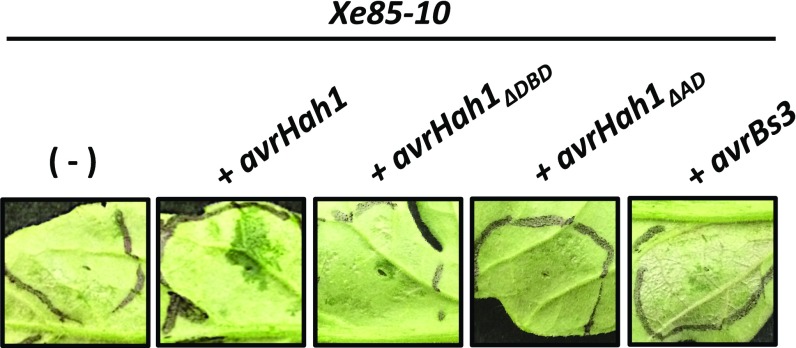
Bs4 is a TIR-NB-LRR type resistance protein in tomato that recognizes the TALEs AvrBs4, AvrBs3, Hax3, Hax4, but not Hax2 (12, 13, 31). Hax2 has a DBD composed of 35 amino acid repeats in contrast to its counterparts with 34 amino acid repeats (13). Although previous results showed that activation of Bs4 in response to AvrBs3 required strong expression in a transient system (31), we observed a HR in tomato in response to delivery of avrBs3 by XgΔavrHah1 and Xe85-10. Because AvrHah1 activates water soaking in tomato and not HR, we tested the possibility that water soaking was disrupting a potential Bs4-mediated cell death response. Evidence points to a direct recognition model between Bs4 and its recognized TALEs, likely involving the repeats of the DBD (12). We truncated the last 46 amino acids of avrHah1 to delete the AD (avrHah1ΔAD), which removed any water-soaking ability while maintaining the structure of the DBD. Delivery of avrHah1ΔAD did not induce a cell death response in tomato (Fig. 5), suggesting that Bs4 differentially recognizes AvrHah1 and AvrBs3. AvrHah1 is a structurally unique TALE as its DBD is composed of both 34 and 35 amino acid repeats (5). An intriguing hypothesis is that selection pressure caused AvrHah1 to adopt its 35 amino acid repeats to evade recognition by tomato Bs4.
Fig. 5.
AvrHah1 and AvrBs3 are differentially recognized by tomato Bs4. Tomato leaves were infiltrated with the Xe85-10 strains indicated at OD600 = 0.1 and observed 48 hpi. Cell death is visible in response to delivery of avrBs3, whereas the beginnings of water soaking are apparent in response to avrHah1. Leaves were left at ambient humidity and were not submerged in water before observation.
RNA Sequencing Reveals Direct and Indirect Targets of AvrHah1.
To study the tomato genes responsible for AvrHah1-mediated water soaking, we used RNA sequencing (RNA-seq) to identify the differentially expressed genes between tomato infected with X. gardneri or XgΔavrHah1. We found that 6,292 genes were differentially up-regulated in X. gardneri-infected tomato leaves at 48 hpi (greater than twofold change; P ≤ 0.05).
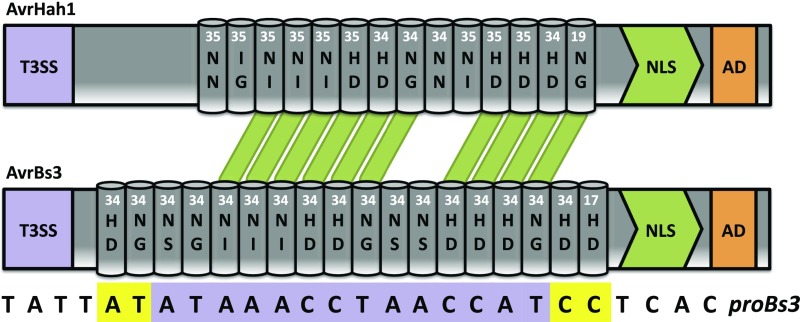
Of particular interest were genes that were highly differentially expressed and contained a predicted promoter EBE for AvrHah1. We used the TALE-NT 2.0 algorithm (27) to computationally predict the AvrHah1 EBEs in the tomato promoterome, defined here as the set of sequences 300 bp upstream of the start codon for annotated genes in the Heinz 1706 genome (32). This prediction resulted in 4,106 possible binding sites (on both the plus and minus strands). Among the most highly up-regulated genes in X. gardneri-, but not XgΔavrHah1-infected, tomato leaves, we identified two bHLH transcription factors, Solyc03g097820 (bHLH3) and Solyc06g072520 (bHLH6), that possessed EBEs for AvrHah1 (Table 1). Given that AvrBs3 targets UPA20, a pepper bHLH transcription factor (22), and that AvrBs3 and AvrHah1 share some binding specificity (both are capable of activating expression of the Bs3 resistance gene at overlapping EBEs) (Fig. S3) (5), we selected the two tomato bHLH transcription factors for further study.
Table 1.
Differentially up-regulated genes from an RNA-seq experiment comparing X. gardneri- and XgΔavrHah1-infected tomato
| Soly Gene ID | EBE score/bp from ATG | Mean RPKM | Predicted protein function | |
| X. gardneri | XgΔavrHah1 | |||
| Solyc02g070210 | 15.01/84 | 36.11 | 0.06 | Phosphatidylinositol transferase |
| Solyc02g084010 | 99.00 | 0.14 | Auxin-induced SAUR-like | |
| Solyc02g089350 | 997.81 | 0.78 | Gibberellin regulated | |
| Solyc03g033590 | 55.85 | 0.14 | Auxin-induced SAUR-like | |
| Solyc03g097820 | 3.96/108 | 1,267.09 | 0.75 | bHLH Transcription Factor |
| Solyc03g114430 | 133.86 | 0.27 | Unknown Protein | |
| Solyc03g116060 | 155.13 | 0.14 | Gibberellin-regulated | |
| Solyc04g017720 | 550.74 | 0.89 | Gibberellin regulated | |
| Solyc04g079700 | 13.26/173 | 16.26 | 0.07 | WD-40 repeat family |
| Solyc04g079860 | 39.32 | 0.13 | Glycosyltransferase family GT8 | |
| Solyc04g081870 | 442.81 | 1.38 | Expansin | |
| Solyc05g014000 | 206.02 | 0.25 | Pectate lyase | |
| Solyc06g067910 | 95.26 | 0.28 | Unknown function DUF642 | |
| Solyc06g068360 | 99.04 | 0.4 | Ethylene-resp. transcription factor 7 | |
| Solyc06g071930 | 461.79 | 2.81 | Unknown Protein | |
| Solyc06g072520 | 9.21/139 | 568.68 | 0.38 | bHLH Transcription Factor |
| Solyc07g006310 | 129.78 | 0.24 | Transcription factor | |
| Solyc08g062450 | 8.2/105 | 14.70 | 0.14 | class II heat shock |
| Solyc08g068720 | 13.74/220 | 196.13 | 0.01 | Tyramine hydroxycinnamoyl transferase |
| Solyc08g079780 | 13.96 | 0.08 | Blue copper protein | |
| Solyc11g011210 | 1,436.40 | 8.86 | Gibberellin regulated | |
| Solyc11g019910 | 89.27 | 0.22 | Pectinesterase | |
| Solyc11g067180 | 94.81 | 0.15 | Fatty acyl CoA reductase | |
| Solyc12g009840 | 156.10 | 0.64 | Pyrophosphate-energized proton pump | |
Mean reads per kilobase of transcript per million mapped reads (RPKM) of three biological replicates at 48 hpi are displayed. Genes are organized by Soly gene ID. Genes with predicted promoter EBEs for AvrHah1 are indicated with the score (best possible is 3.76) and distance from the start codon.
Fig. S3.
AvrHah1 has unique and overlapping targets with AvrBs3. AvrHah1 (Top) can activate expression from the Bs3 promoter (proBs3) using an overlapping EBE with that of AvrBs3 (Bottom). The predicted AvrHah1 EBE is in purple, whereas the AvrBs3 EBE has two nucleotide extensions on either side marked in yellow. Similar RVDs between AvrHah1 and AvrBs3 are depicted by green highlighting.
AvrHah1 Activates Expression from the Promoters of the bHLH Transcription Factors.
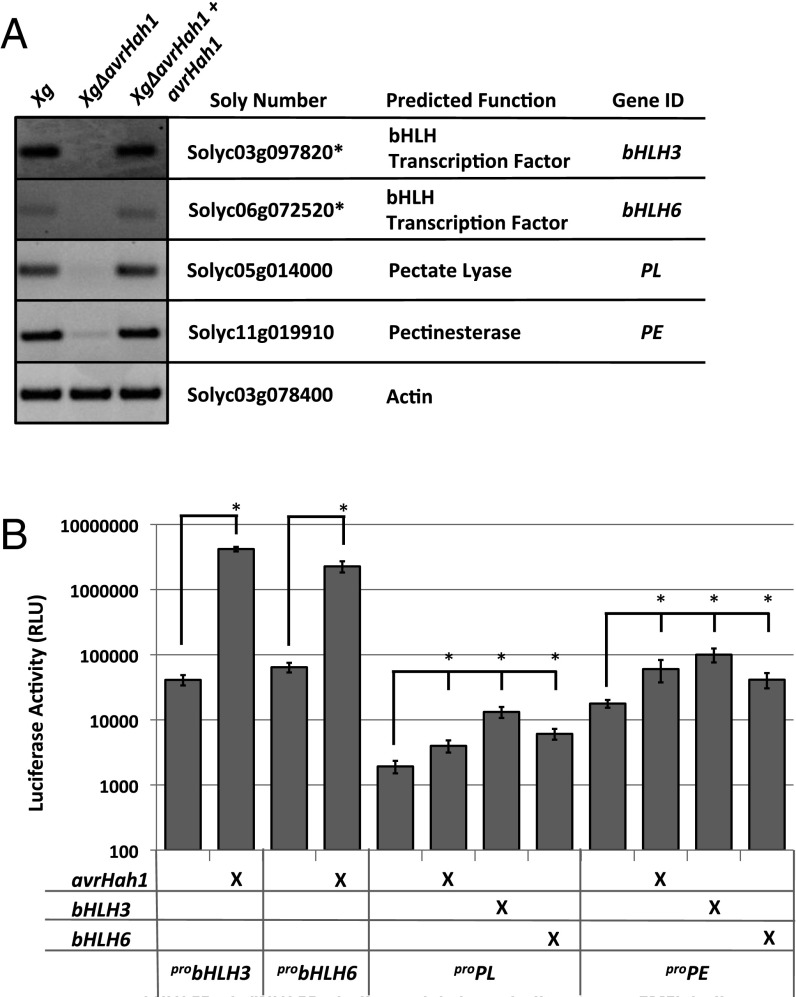
We confirmed by semiquantitative RT-PCR that gene activation of bHLH3 and bHLH6 occurred in X. gardneri-infected, but not XgΔavrHah1-infected, tomato leaves. Additionally, expression of bHLH3 and bHLH6 was rescued when XgΔavrHah1 was complemented with avrHah1 (Fig. 6A). To confirm that AvrHah1 was able to activate gene expression from the promoters of bHLH3 and bHLH6, we used an Agrobacterium tumefaciens luciferase reporter assay in N. benthamiana. Luciferase activity driven by probHLH3 or probHLH6 was significantly higher when codelivered with AvrHah1, indicating that AvrHah1 is capable of activating expression of bHLH3 and bHLH6 (Fig. 6B).
Fig. 6.
Tomato gene activation in response to AvrHah1. (A) Semiquantitative RT-PCR in tomato inoculated with the indicated X. gardneri (Xg) strains (24 hpi; OD600 = 0.25); * indicates genes with predicted promoter EBEs for AvrHah1 (e.g., proposed direct targets). (B) Transient luciferase reporter assay in N. benthamiana. Promoter::luciferase constructs are displayed along the x axis. Expression binary vectors carrying AvrHah1 or the bHLH transcription factors are listed on the left, and an “X” signifies codelivery with a promoter::luciferase reporter; * indicates significantly different luciferase activity from promoter background (codelivered with empty Agrobacterium) (P < 0.001).
The bHLH Transcription Factors Activate Expression of Two Pectin-Modification Genes.
We hypothesized that genes up-regulated by bHLH3 and bHLH6 would be among the highly up-regulated genes we identified in our RNA-seq experiment but without predicted EBEs for AvrHah1 (Table 1). Using semiquantitative RT-PCR, we observed AvrHah1-specific up-regulation of two genes involved in pectin modification: Solyc05g014000, a PL, and Solyc11g019910, a PE (Fig. 6A). We hypothesized that PL and PE were direct gene activation targets of bHLH3 and bHLH6, and therefore indirect targets of AvrHah1. We tested the promoters of PL and PE in the transient luciferase reporter assay for activation by bHLH3 and bHLH6. Luciferase activity driven by proPL and proPE was significantly higher when codelivered with avrHah1, bHLH3, or bHLH6 compared with an empty Agrobacterium strain (Fig. 6B), indicating that the bHLH transcription factors can activate expression of the pectin modification genes.
Because we observed activation of proPL and proPE in response to AvrHah1 in N. benthamiana, it is possible that AvrHah1 is activating endogenous bHLH transcription factors that are then activating the pectin modification promoters. Consistent with this prediction, two bHLH transcription factors in N. benthamiana (Niben101Scf00376g01004.1 and Niben101Scf01182g03011.1) have predicted AvrHah1 EBEs within 300 bp upstream of the start codon.
Delivery of dTALEs for the bHLH Transcription Factors Results in Activation of both Pectin-Modification Genes.
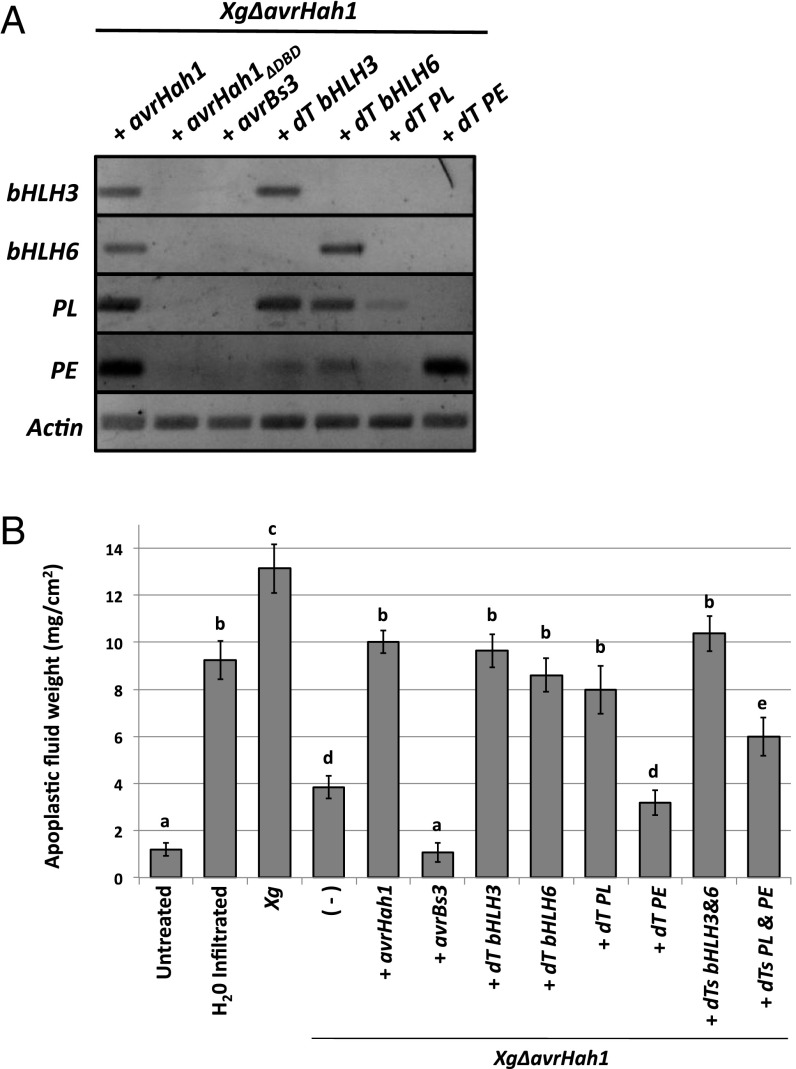
We constructed dTALEs to activate expression of bHLH3, bHLH6, PL, and PE to study their contributions to water soaking when delivered by XgΔavrHah1 in tomato. We first used semiquantitative PCR to confirm target gene expression. Tomato leaves infiltrated with XgΔavrHah1 + dT bHLH3 or dT bHLH6 showed activation of the corresponding bHLH transcription factor gene (Fig. 7A). Activation of PL and PE gene expression occurred in response to dT PL and dT PE, respectively. Importantly, we also observed strong activation of PL and weaker activation of PE in response to both dT bHLH3 and dT bHLH6, supporting the hypothesis that the PL and PE are downstream gene activation targets of both bHLH transcription factors. We did not observe activation of bHLH3, bHLH6, PL, or PE in response to AvrBs3, demonstrating that AvrHah1 has unique gene activation targets from AvrBs3 (AvrBs3 was reported to not cause water soaking in pepper) (5).
Fig. 7.
dTALEs demonstrate that the bHLH transcription factors and the PL are S genes of AvrHah1. (A) Semiquantitative RT-PCR in tomato infected with XgΔavrHah1 complemented with dTALEs for the bHLH transcription factors (dT bHLH3 and dT bHLH6), the PL (dT PL), and the PE (dT PE) (24 hpi; OD600 = 0.25). (B) Quantitative water-soaking measurements were obtained by centrifugation and weighing of apoplastic fluid from infected tomato leaves (48 hpi; OD600 = 0.1; 20 min water bath). Average weights and SEs from 12 samples (each consisting of two 0.5-cm2 leaf discs) are shown.
dTALEs for the bHLH Transcription Factors and the Pectate Lyase Complement Water Soaking in XgΔavrHah1.
We collected and weighed the apoplastic fluid from tomato leaves infiltrated with X. gardneri, XgΔavrHah1, and XgΔavrHah1 complemented with dTALEs to determine if the dTALE targeted genes could contribute to water soaking. Leaves were submerged in water to enhance water soaking. For comparison, we also measured the apoplastic fluid from untreated tomato leaves (also submerged in water) and leaves syringe-infiltrated with water. Little apoplastic fluid was collected from untreated leaves, whereas water-infiltrated leaves showed an approximate eightfold increase in apoplastic fluid (Fig. 7B). Leaves infiltrated with X. gardneri experienced the largest amount of water uptake, about a 12-fold increase from untreated leaves. Apoplastic fluid in XgΔavrHah1 was significantly reduced compared with X. gardneri and threefold higher than untreated leaves. XgΔavrHah1 was not fully complemented by AvrHah1 to wild type levels, however the apoplastic fluid was comparable to water-infiltrated leaves. XgΔavrHah1 complemented with AvrBs3 showed apoplastic fluid levels similar to untreated leaves.
dT bHLH3 and dT bHLH6 complemented water soaking back to XgΔavrHah1 + avrHah1 levels. dT PL also fully complemented water soaking back to XgΔavrHah1 + avrHah1 levels. We did not observe a difference in apoplastic fluid between XgΔavrHah1 + dT PE and XgΔavrHah1. A 1:1 mixture of dT bHLH3 and dT bHLH6 did not further enhance water soaking above the levels of the individual dTALEs, whereas a 1:1 mixture of dT PL and dT PE displayed an apoplastic fluid level intermediate of the two individual dTALEs. These results indicate that the bHLH transcription factors and their proposed PL target contribute to AvrHah1-mediated water soaking and are therefore S genes of AvrHah1.
Discussion
External Water Plays a Major Role in Water-Soaked Lesions of Bacterial Spot.
Disease outbreaks of bacterial spot are favored in periods of high humidity (33). Given our initial observation that the water soaking caused by X. gardneri was enhanced when the infected plants were placed in a mist chamber, we wondered how water external to the leaf was exacerbating Xg/AvrHah1-induced water soaking. In an agricultural setting, the external water could be from high humidity, rain, or sprinkler (overhead) watering systems. In the laboratory, we chose to submerge leaves in water before observation. We observed a relatively rapid intake of water into the apoplast, indicating that AvrHah1 may be priming the in planta environment such that X. gardneri can take advantage of sudden appearances of external water. The role of AvrHah1 and water soaking is likely to benefit the bacteria after the apoplastic growth phase of the pathogen’s life cycle, such as during bacterial egression or transmission. Further tests will need to be performed to determine the role of AvrHah1-induced water soaking on pathogen fitness and the epiphytic growth phase of the pathogen.
Two bHLH Transcription Factors Are S Gene Targets of AvrHah1.
To find the downstream targets of AvrHah1 responsible for water soaking, we used RNA-seq to study the gene expression profiles of X. gardneri- and XgΔavrHah1-infected tomato leaves. We identified two highly up-regulated bHLH transcription factors, Solyc03g097820 (bHLH3) and Solyc06g072520 (bHLH6), which encode proteins that share 66% amino acid identity and are 86% and 64% similar, respectively, to UPA20—a bHLH transcription factor target of AvrBs3 in pepper (22). bHLH3 and bHLH6 are in a sister clade and part of bHLH subfamily 1 (34). bHLH3 was found to be expressed preferentially in young fruits (annotated as bHLH022) (34). bHLH6 was identified as a drought responsive gene in drought-tolerant tomato (annotated as bHLH048) (34, 35). We found that dTALEs activating gene expression of the identified bHLH transcription factors restored water soaking in XgΔavrHah1, indicating that the AvrHah1-activated bHLH genes encode proteins with overlapping roles in water soaking and are bona fide S gene products of AvrHah1.
A Pectate Lyase Is an Indirect S Gene Target of AvrHah1.
To find targets of the bHLH transcription factors we selected genes in our RNA-seq dataset that were up-regulated in the presence of AvrHah1 but without predicted AvrHah1 EBEs. After confirming AvrHah1-specific activation using semiquantitative RT-PCR, we selected two pectin modification genes for further study: a PL, Solyc05g014000, and a PE, Solyc11g019910. We were interested in the pectin modification genes because several examples have implicated pectin as an important factor in plant-pathogen interactions (36, 37). We demonstrated that the promoters of PL and PE could be activated by either bHLH transcription factor using a transient luciferase reporter assay. Semiquantitative RT-PCR for PL and PE showed gene activation in response to delivery of dT bHLH3 and dT bHLH6 by XgΔavrHah1.
We constructed dTALEs to activate the transcription of the PL and PE genes. When delivered into tomato by XgΔavrHah1, the PL-specific, but not the PE-specific, dTALE was able to complement water soaking, suggesting that this PL is an indirect S gene target of AvrHah1. We hypothesize that PL activity increases the hygroscopicity of the cell wall, which—in the larger context of X. gardneri infection—conditions the apoplast to absorb water through breaks in the epidermis (likely caused by the lesion itself). As shown in Fig. 1, at time 0 (before the introduction of surface water) zones infiltrated with X. gardneri or XgΔavrHah1 + avrHah1 appear noticeably darker and damaged compared with zones infiltrated with XgΔhrcV or XgΔavrHah1. This result is perhaps a consequence of increased tissue maceration from PL activity. Future experiments exploring the composition of the cell wall in response to X. gardneri infection may reveal a mechanism by which PL maceration of plant tissue promotes water soaking.
Diverse Strategies and Implications of Water-Soaked Lesions.
The relatively fast absorption of water into the leaf apoplast due to AvrHah1 is a striking addition to the diverse mechanisms by which pathogens promote water soaking. We designed an experiment to show that bacterial cells can be introduced into the apoplast during AvrHah1-induced water soaking. Whether or not bacteria such as human pathogens can survive in the plant apoplast as a result of AvrHah1-induced water soaking will need to be determined. This finding is particularly important in the light of previous work that described how increased water soaking and foliar damage from xanthomonad pathogens facilitated the growth of the human pathogen Salmonella enterica on tomato leaves (38). Because bacterial manipulation of the leaf apoplast to promote an aqueous environment is required for pathogenesis (39), improving the tolerance of food crops from water-soaked lesion development as part of a multilayered disease management strategy may help reduce yield losses and even prevent the colonization of human pathogens on diseased crops.
Supplementary Material
Acknowledgments
We thank Megan Cohn for reading and critical review of the manuscript. A.R.S. was supported by the National Science Foundation Graduate Research Fellowship Program.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1620407114/-/DCSupplemental.
References
- 1.Potnis N, et al. Bacterial spot of tomato and pepper: diverse Xanthomonas species with a wide variety of virulence factors posing a worldwide challenge. Mol Plant Pathol. 2015;16(9):907–920. doi: 10.1111/mpp.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma X, Lewis Ivey ML, Miller SA. First report of Xanthomonas gardneri causing bacterial spot of tomato in Ohio and Michigan. Plant Dis. 2011;95(12):1584. doi: 10.1094/PDIS-05-11-0448. [DOI] [PubMed] [Google Scholar]
- 3.Quezado-Duval AM, Leite RP, Jr, Truffi D, Camargo LEA. Outbreaks of bacterial spot caused by Xanthomonas gardneri on processing tomato in central-west Brazil. Plant Dis. 2004;88(2):157–161. doi: 10.1094/PDIS.2004.88.2.157. [DOI] [PubMed] [Google Scholar]
- 4.Timilsina S, et al. Multilocus sequence analysis of xanthomonads causing bacterial spot of tomato and pepper plants reveals strains generated by recombination among species and recent global spread of Xanthomonas gardneri. Appl Environ Microbiol. 2015;81(4):1520–1529. doi: 10.1128/AEM.03000-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schornack S, Minsavage GV, Stall RE, Jones JB, Lahaye T. Characterization of AvrHah1, a novel AvrBs3-like effector from Xanthomonas gardneri with virulence and avirulence activity. New Phytol. 2008;179(2):546–556. doi: 10.1111/j.1469-8137.2008.02487.x. [DOI] [PubMed] [Google Scholar]
- 6.Boch J, Bonas U. Xanthomonas AvrBs3 family-type III effectors: discovery and function. Annu Rev Phytopathol. 2010;48:419–436. doi: 10.1146/annurev-phyto-080508-081936. [DOI] [PubMed] [Google Scholar]
- 7.Moscou MJ, Bogdanove AJ. A simple cipher governs DNA recognition by TAL effectors. Science. 2009;326(5959):1501. doi: 10.1126/science.1178817. [DOI] [PubMed] [Google Scholar]
- 8.Boch J, et al. Breaking the code of DNA binding specificity of TAL-type III effectors. Science. 2009;326(5959):1509–1512. doi: 10.1126/science.1178811. [DOI] [PubMed] [Google Scholar]
- 9.Schornack S, Moscou MJ, Ward ER, Horvath DM. Engineering plant disease resistance based on TAL effectors. Annu Rev Phytopathol. 2013;51(1):383–406. doi: 10.1146/annurev-phyto-082712-102255. [DOI] [PubMed] [Google Scholar]
- 10.Römer P, et al. Plant pathogen recognition mediated by promoter activation of the pepper Bs3 resistance gene. Science. 2007;318(5850):645–648. doi: 10.1126/science.1144958. [DOI] [PubMed] [Google Scholar]
- 11.Yang B, Sugio A, White FF. Os8N3 is a host disease-susceptibility gene for bacterial blight of rice. Proc Natl Acad Sci USA. 2006;103(27):10503–10508. doi: 10.1073/pnas.0604088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schornack S, et al. The tomato resistance protein Bs4 is a predicted non-nuclear TIR-NB-LRR protein that mediates defense responses to severely truncated derivatives of AvrBs4 and overexpressed AvrBs3. Plant J. 2004;37(1):46–60. doi: 10.1046/j.1365-313x.2003.01937.x. [DOI] [PubMed] [Google Scholar]
- 13.Kay S, Boch J, Bonas U. Characterization of AvrBs3-like effectors from a Brassicaceae pathogen reveals virulence and avirulence activities and a protein with a novel repeat architecture. Mol Plant Microbe Interact. 2005;18(8):838–848. doi: 10.1094/MPMI-18-0838. [DOI] [PubMed] [Google Scholar]
- 14.White F. Xanthomonas and the TAL effectors: Nature’s molecular biologist. Methods Mol Biol. 2016;1338:1–8. doi: 10.1007/978-1-4939-2932-0_1. [DOI] [PubMed] [Google Scholar]
- 15.Cohn M, Morbitzer R, Lahaye T, Staskawicz BJ. Comparison of gene activation by two TAL effectors from Xanthomonas axonopodis pv. manihotis reveals candidate host susceptibility genes in cassava. Mol Plant Pathol. 2016;17(6):875–889. doi: 10.1111/mpp.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morbitzer R, Elsaesser J, Hausner J, Lahaye T. Assembly of custom TALE-type DNA binding domains by modular cloning. Nucleic Acids Res. 2011;39(13):5790–5799. doi: 10.1093/nar/gkr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cernadas RA, et al. Code-assisted discovery of TAL effector targets in bacterial leaf streak of rice reveals contrast with bacterial blight and a novel susceptibility gene. PLoS Pathog. 2014;10(2):e1003972. doi: 10.1371/journal.ppat.1003972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z, et al. A potential disease susceptibility gene CsLOB of citrus is targeted by a major virulence effector PthA of Xanthomonas citri subsp. citri. Mol Plant. 2014;7(5):912–915. doi: 10.1093/mp/sst176. [DOI] [PubMed] [Google Scholar]
- 19.White FF, Potnis N, Jones JB, Koebnik R. The type III effectors of Xanthomonas. Mol Plant Pathol. 2009;10(6):749–766. doi: 10.1111/j.1364-3703.2009.00590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohn M, et al. Xanthomonas axonopodis virulence is promoted by a transcription activator-like effector-mediated induction of a SWEET sugar transporter in cassava. Mol Plant Microbe Interact. 2014;27(11):1186–1198. doi: 10.1094/MPMI-06-14-0161-R. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, De Feyter R, Gabriel DW. Host-specific symptoms and increased release of Xanthomonas citri and X. campestris pv. malvacearum from leaves are determined by the 102-bp tandem repeats of PthA and avrb6, respectively. Mol Plant Microbe Interact. 1994;7(3):345–355. [Google Scholar]
- 22.Kay S, Hahn S, Marois E, Hause G, Bonas U. A bacterial effector acts as a plant transcription factor and induces a cell size regulator. Science. 2007;318(5850):648–651. doi: 10.1126/science.1144956. [DOI] [PubMed] [Google Scholar]
- 23.Wichmann G, Bergelson J. Effector genes of Xanthomonas axonopodis pv. vesicatoria promote transmission and enhance other fitness traits in the field. Genetics. 2004;166(2):693–706. doi: 10.1534/genetics.166.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz AR, et al. Phylogenomics of Xanthomonas field strains infecting pepper and tomato reveals diversity in effector repertoires and identifies determinants of host specificity. Front Microbiol. 2015;6:535. doi: 10.3389/fmicb.2015.00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindgren PB, Peet RC, Panopoulos NJ. Gene cluster of Pseudomonas syringae pv. phaseolicola controls pathogenicity of bean plants and hypersensitivity of nonhost plants. J Bacteriol. 1986;168(2):512–522. doi: 10.1128/jb.168.2.512-522.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goritschnig S, Steinbrenner AD, Grunwald DJ, Staskawicz BJ. Structurally distinct Arabidopsis thaliana NLR immune receptors recognize tandem WY domains of an oomycete effector. New Phytol. 2016;210(3):984–996. doi: 10.1111/nph.13823. [DOI] [PubMed] [Google Scholar]
- 27.Doyle EL, et al. TAL Effector-Nucleotide Targeter (TALE-NT) 2.0: tools for TAL effector design and target prediction. Nucleic Acids Res. 2012;40(Web Server issue):W117–W122. doi: 10.1093/nar/gks608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thieme F, et al. Insights into genome plasticity and pathogenicity of the plant pathogenic bacterium Xanthomonas campestris pv. vesicatoria revealed by the complete genome sequence. J Bacteriol. 2005;187(21):7254–7266. doi: 10.1128/JB.187.21.7254-7266.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakagawa T, et al. Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J Biosci Bioeng. 2007;104(1):34–41. doi: 10.1263/jbb.104.34. [DOI] [PubMed] [Google Scholar]
- 30.Kearney B, Staskawicz B. Widespread distribution and fitness contribution of Xanthomonas campestris avirulence gene avrBs2. 1990;346(6282):385–386. doi: 10.1038/346385a0. [DOI] [PubMed] [Google Scholar]
- 31.Schornack S, Peter K, Bonas U, Lahaye T. Expression levels of avrBs3-like genes affect recognition specificity in tomato Bs4- but not in pepper Bs3-mediated perception. Mol Plant Microbe Interact. 2005;18(11):1215–1225. doi: 10.1094/MPMI-18-1215. [DOI] [PubMed] [Google Scholar]
- 32.Consortium TTG. Tomato Genome Consortium The tomato genome sequence provides insights into fleshy fruit evolution. Nature. 2012;485(7400):635–641. doi: 10.1038/nature11119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Obradovic A, Jones JB, Balogh B, Momol MT. 2008. Integrated management of tomato bacterial spot. Integrated Management of Disease Caused by Fungi, Phytoplasma, and Bacteria, Integrated Management of Plant Pests and Diseases, eds Ciancio A, Mukerji KG (Springer, Dordrecht, The Netherlands), Vol 3, pp 211–224.
- 34.Sun H, Fan H-J, Ling H-Q. Genome-wide identification and characterization of the bHLH gene family in tomato. BMC Genomics. 2015;16:9. doi: 10.1186/s12864-014-1209-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gong P, et al. Transcriptional profiles of drought-responsive genes in modulating transcription signal transduction, and biochemical pathways in tomato. J Exp Bot. 2010;61(13):3563–3575. doi: 10.1093/jxb/erq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lionetti V, Cervone F, Bellincampi D. Methyl esterification of pectin plays a role during plant-pathogen interactions and affects plant resistance to diseases. J Plant Physiol. 2012;169(16):1623–1630. doi: 10.1016/j.jplph.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Hématy K, Cherk C, Somerville S. Host-pathogen warfare at the plant cell wall. Curr Opin Plant Biol. 2009;12(4):406–413. doi: 10.1016/j.pbi.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Potnis N, Colee J, Jones JB, Barak JD. Plant pathogen-induced water-soaking promotes Salmonella enterica growth on tomato leaves. Appl Environ Microbiol. 2015;81(23):8126–8134. doi: 10.1128/AEM.01926-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xin X-F, et al. Bacteria establish an aqueous living space in plants crucial for virulence. Nature. 2016;539(7630):524–529. doi: 10.1038/nature20166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.