Significance
The kainate receptor (KAR) is a subfamily of glutamate receptors that mediates excitatory synaptic transmission in the central nervous system. GluK1 and GluK2 are two obligatory KAR subunits and their trafficking properties are quite different; however, the underlying molecular mechanism remains a mystery. Here we show that GluK2 receptor trafficking is independent on Neto auxiliary subunits, whereas the regulation of its biophysical properties by Neto proteins is the same as that of GluK1. Moreover, we show that it is the amino-terminal domains (ATDs) of GluK1 and GluK2 that determine their distinct trafficking performance and mediate their different dependence on Neto proteins for trafficking.
Keywords: kainate receptor, amino-terminal domain, Neto proteins, synaptic trafficking
Abstract
The kainate receptor (KAR), a subtype of glutamate receptor, mediates excitatory synaptic responses at a subset of glutamatergic synapses. However, the molecular mechanisms underlying the trafficking of its different subunits are poorly understood. Here we use the CA1 hippocampal pyramidal cell, which lacks KAR-mediated synaptic currents, as a null background to determine the minimal requirements for the extrasynaptic and synaptic expression of the GluK2 subunit. We find that the GluK2 receptor itself, in contrast to GluK1, traffics to the neuronal surface and synapse efficiently and the auxiliary subunits Neto1 and Neto2 caused no further enhancement of these two trafficking processes. However, the regulation of GluK2 biophysical properties by Neto proteins is the same as that of GluK1. We further determine that it is the amino-terminal domains (ATDs) of GluK1 and GluK2 that control the strikingly different trafficking properties between these two receptors. Moreover, the ATDs are critical for synaptic expression of heteromeric receptors at mossy fiber–CA3 synapses and also mediate the differential dependence on Neto proteins for surface and synaptic trafficking of GluK1 and GluK2. These results highlight the fundamental differences between the two major KAR subunits and their interplay with Neto auxiliary proteins.
Excitatory synaptic transmission in the brain is mediated primarily by glutamate, which acts on three types of glutamate receptors, AMPA receptors (AMPARs), NMDA receptors (NMDARs), and kainate receptors (KARs). AMPARs and NMDARs are expressed at most glutamatergic synapses, whereas KARs are expressed much more selectively at a subset of synapses. KARs are composed of low-affinity GluK1–3 subunits and high-affinity GluK4/5 subunits (1–3). Most of our knowledge of KARs comes from studies of excitatory mossy fiber synapses onto CA3 pyramidal cells (4). These receptors are expressed postsynaptically and are responsible for a slow excitatory postsynaptic current (EPSC) (5, 6). They are also expressed presynaptically and contribute to the profound frequency facilitation of these synapses (7–10). In the CA1 region, excitatory synapses onto pyramidal cells do not generate KAR-mediated currents (5, 11, 12), but, interestingly, functional GluK2-containing receptors are expressed on the surface of CA1 pyramidal cells (11). These findings raise the question as to what determines the synaptic expression of KARs.
Recently it has been discovered that Neto proteins serve as auxiliary subunits for KARs (13–15). They are neuropilin and tolloid-like proteins that are single pass transmembrane CUB domain-containing proteins. Although Neto1 and Neto2 have profound effects on the trafficking and kinetics of GluK1 (16–20), their effects on GluK2 are less clear. Neto2 slows deactivation (14) as well as desensitization of GluK2 (14, 18) and has similar effects on GluK2/5 receptors (19). Neto1 also slows desensitization of GluK2 receptors (18) and slows desensitization of GluK2/5 receptors (20, 21) as well as deactivation (21). In contrast to the dramatic effects that Neto proteins have on the kinetics of GluK2-containing receptors, their roles in trafficking of these receptors are less clear. Most studies find no effect of Neto1 or Neto2 on the surface expression of GluK2 (14, 21). On the other hand, Palacios-Filardo et al. reported that both Neto1 and Neto2 increased surface expression (18).
In the present study, we have used the excitatory synapses onto CA1 pyramidal cells, which do not express KARs, to study the properties of the GluK2 receptor and the effects of Neto proteins on this receptor. We recently found that GluK1, in the absence of Neto proteins, is poorly expressed on the neuronal surface and does not appear at synapses (16). However, in the presence of either Neto1 or Neto2, the surface expression of GluK1 is extremely high and the receptor is targeted to the synapse. Neto proteins also affect the rate of GluK1’s desensitization, and Neto1, but not Neto2, slows the rate of its deactivation. Here we show that GluK2, on its own, is expressed on the surface at high levels, equivalent to GluK1 coexpressed with Neto proteins. Neto proteins have no effect on surface or synaptic expression of GluK2. However, in outside-out patches, Neto1 and Neto2 have the identical effects on deactivation and desensitization of GluK2, as they do on GluK1. We find that the profound difference in the surface trafficking of GluK1 and GluK2 resides in the amino-terminal domain (ATD) and the GluK2 ATD is also critical for the targeting of GluK2/5 heteromeric receptors to mossy fiber–CA3 synapses. Moreover, the ATDs of GluK1 and GluK2 control their differential dependence on Neto proteins for extrasynaptic and synaptic trafficking. Taken together, these results demonstrate a role of the ATD and its interplay with auxiliary subunits in glutamate receptor trafficking.
Results
GluK2 Receptor Targets to Excitatory Synapse on Its Own.
For this study, we used organotypic rat hippocampal slices and exogenously expressed KARs and Neto proteins through biolistic transfection in CA1 neurons, which are devoid of KAR-mediated synaptic responses (5, 11, 12), and then measured the synaptic responses of transfected and neighboring wild-type CA1 neurons by dual whole-cell recordings (Fig. S1A). In agreement with our previous results (16), expression of GluK1, by itself, failed to affect synaptic currents (Fig. 1A). However, expression of GluK2, by itself, caused a profound enhancement of the evoked EPSC (eEPSC) recorded at −70 mV and some of this current remained in the presence of the AMPAR selective antagonist GYKI53655 (Fig. 1B). It should be noted that a high concentration of GYKI53655 (100 μM), which blocks ∼20–30% of KAR-mediated responses (22), was used to ensure that the remaining currents are, indeed, solely KAR mediated. And in the absence of a specific GluK2 antagonist, currently, we could not rule out the possibility that GluK2 expression leads to additional recruitment of synaptic AMPARs. This result suggests that the lack of KAR-mediated synaptic response in CA1 neurons might be due to the limited endogenous expression of GluK2 receptor, although some functional GluK2-containing receptors are found on the cell surface (11). Interestingly, there was also a significant enhancement of the NMDAR currents (Fig. S2B) in GluK2-expressing cells, which raises the possibility of a synaptogenic effect. Although presynaptic KARs are known to regulate glutamate release at mossy fibers, sparse expression of GluK1 or GluK2 receptors in CA1 neurons had no effect on presynaptic release probability as there were no significant changes of paired-pulse ratios (GluK1 vs. control: 1.51 ± 0.12 vs. 1.50 ± 0.09, P > 0.05; GluK2 vs. control: 1.28 ± 0.13 vs. 1.11 ± 0.05, P > 0.05). We next examined the possibility that activity played a role in the GluK2-induced enhancement. The experiments were repeated in the presence of the following drugs during culture: the AMPAR antagonist NBQX and the NMDAR antagonist APV (Fig. S3A), the sodium channel blocker tetrodotoxin (TTX) (Fig. S3B) or the calcium channel blocker nifedipine (Fig. S3C). None of these manipulations prevented the enhancement, either of the AMPAR/KAR or NMDAR responses.
Fig. S1.
The simplified diagram of hippocampal structure including dentate gyrus, CA3 and CA1 regions, and the dual whole-cell electrophysiological recording. The evoked EPSCs of CA1 (A) or CA3 (B) pyramidal neurons were recorded in voltage clamp. The stimulation electrode was put on the afferents of the Schaffer collateral pathways (A) or the mossy fibers (B). Then an experimental cell (identified by GFP fluorescence) and a neighboring wild-type one were patched with the respective recording electrodes.
Fig. 1.
Synaptic responses are potentiated by GluK2 but not GluK1 receptors in CA1 pyramidal neurons. Rat hippocampal slice cultures were biolistically transfected with GluK1 (A, n = 12) or GluK2 (B, n = 12). Simultaneous dual whole-cell recordings from a transfected CA1 pyramidal neuron (green trace) and a neighboring wild-type one (black trace) were performed. The evoked EPSCs (eEPSCs) were measured at −70 mV before and after GYKI53655 (100 μM) treatment. Open and filled circles represent amplitudes for single pairs and mean ± SEM, respectively. Insets show sample current traces from control (black) and experimental (green) cells. (Scale bars, 100 pA and 25 ms.) Bar graphs show normalized eEPSC amplitudes (mean ± SEM) of −70 mV pretreatment (A, 89.38 ± 20.24% control, P > 0.05 and B, 1721.09 ± 341.63% control, ***P < 0.001), GYKI53655 treatment (B, 487.70 ± 51.64% control pretreatment, ***P < 0.001) presented in scatter plots. The eEPSC amplitudes measured at −70 mV after GYKI53655 wash-in in A and B were normalized according to respective pretreated control neurons. All of the statistical analyses are compared with respective control neurons with two-tailed Wilcoxon signed-rank sum test.
Fig. S2.
The NMDAR EPSC and the spines morphology are modified by synaptic localized GluK2 receptors. (A and B) The same experimental design as in Fig. 1 except that the NMDAR eEPSCs were measured at +40 mV (the current amplitudes were calculated 100 ms after stimulation). Open and filled circles represent amplitudes for single pairs and mean ± SEM, respectively. Insets show sample current traces from control (black) and experimental (green) cells. (Scale bars, 50 pA and 25 ms.) Bar graphs show normalized NMDAR eEPSC amplitudes (mean ± SEM) (A, 82.77 ± 20.72% control, P > 0.05 and B, 238.76 ± 51.64% control, *P < 0.05) presented in scatter plots. All of the statistical analyses are compared with respective control neurons with two-tailed Wilcoxon signed-rank sum test. (C) Sample images of primary apical dendrite from neurons expressing FUGW-EGFP and FUGW-EGFP + GluK2 imaged using superresolution structured illumination microscopy (SIM). For all morphological analysis, EGFP expression control neurons, n = 10, 297 spines; GluK2 overexpression neurons, n = 7; 212 spines. (Scale bar, 5 μm.) (D) Spine density on primary apical dendrite is unchanged following GluK2 overexpression (EGFP, mean ± SEM number = 0.56 ± 0.04/μm; GluK2, mean ± SEM number = 0.58 ± 0.05/μm). (E, 1) Spine neck diameter is decreased by GluK2 overexpression (EGFP, mean ± SEM diameter = 344.37 ± 13.62 nm; GluK2, diameter = 232.71 ± 11.20 nm; ***P < 0.0001). (E, 2) Distribution of spine neck diamaters. (F, 1) Spine shaft length is increased by GluK2 overexpression (EGFP, length = 618.12 ± 23.51 nm; GluK2, length = 1096.28 ± 60.96 nm; ***P < 0.0001). (F, 2) Distribution of spine shaft lengths. (G, 1) Spine head diameter is reduced by GluK2 overexpression (EGFP, diameter = 502.13 ± 20.74 nm; GluK2, diameter = 340.92 ± 13.59 nm; ***P < 0.001). (G, 2) Distribution of spine head diameters.
Fig. S3.
Synaptic targeting of GluK2 receptor is independent of synaptic activity, neuronal excitation, and l-VGCC activation. Rat hippocampal slices were biolistically transfected with GluK2 plasmids and then treated with 25 μM NBQX and 100 μM AP5 (A), 0.5 μM TTX (B), and 20 μM nifedipine (C) during the incubation culture. Scatter plots show eEPSC amplitudes measured at −70 mV (A, 1; B, 1; and C, 1) and +40 mV (A, 2; B, 2; and C, 2, 100 ms after stimulation). Filled circles show mean ± SEM. Insets show sample current traces from control (black) and experimental (green) cells. [Scale bars for representative eEPSC trace, 100 pA and 25 ms (A, 1; B, 1; and C, 1) and 50 pA and 25 ms (A, 2; B, 2; and C, 2).] Bar graph shows normalized eEPSC amplitudes (mean ± SEM) (EPSCs at −70 mV: A, 1, n = 17, 1257.73 ± 171.29% control, ***P < 0.0005; B, 1, n = 16, 730.43 ± 143.43% control, ***P < 0.001; C, 1, n = 16, 927.21 ± 115.99% control, ***P < 0.001; NMDAR EPSCs: A, 2, n = 17, 229.67 ± 31.64% control, ***P < 0.001; B, 2, n = 12, 186.62 ± 69.57% control, *P < 0.05; C, 2, n = 16, 314.02 ± 50.42% control, ***P < 0.001) presented in scatter plots. All of the statistical analyses are compared with respective control neurons with two-tailed Wilcoxon signed-rank sum test.
There are a number of possible mechanisms by which GluK2 enhances synaptic transmission. First, receptors could selectively populate silent synapses as is the case for the expression of GluK1 (16). Second, they could colocalize with synaptic AMPARs. However, it seems unlikely that these two mechanisms could fully account for the magnitude of the enhancement. One additional possibility is that new synapses are formed. To test these various possibilities, we examined the effect of GluK2 expression on the properties of miniature EPSCs (mEPSCs) (Fig. S4A). Both the amplitude (Fig. S4 B, 1 and 2) and the frequency (Fig. S4 C, 1 and 2) of mEPSCs were increased in cells expressing GluK2 compared with neighboring control cells. In addition, there was a dramatic speeding in the decay of the mEPSCs (Fig. S4 D, 1 and 2). Application of GYKI53655 (30 μM) completely blocked the AMPAR-mediated mEPSCs in the control cells, as expected (Fig. S4 A, E, and F), but had a minor effect on the mEPSCs in the GluK2-expressing cells (Fig. S4 E and F), indicating that a large majority of events in the expressing cells are mediated by KARs. We selected a concentration of GYKI53655 (30 μM) that was just sufficient to entirely block responses in control cells and thus minimize the blocking of KAR-mediated mEPSCs. We next examined whether the large GluK2-mediated mEPSCs also contained an AMPAR component. For this analysis, we used a detection threshold of 40 pAs (Fig. S4 G, 1) so we were assured that the events were larger than the AMPAR-mediated mEPSCs in the control cells. If the large events contained an AMPAR component, then we would expect that GYKI53655 should cause a substantial reduction in the amplitude of these large events and an acceleration of the decay of the mEPSCs. Surprisingly, we saw minimal effect on the amplitude (Fig. S4E) and no effect on the decay (Fig. S4 G, 2), suggesting that there is no colocalization of AMPARs at the GluK2-expressing synapses.
Fig. S4.
Both mEPSC amplitude and frequency are increased by synaptic targeted GluK2. (A) Representative sample traces of simultaneously recorded spontaneous mEPSCs from wild-type control (black) and GluK2 overexpressed (green) CA1 neurons before (Left) and after (Right) GYKI53655 (30 μM) treatment. (Scale bars for single representative mEPSC traces, 5 pA and 20 ms.) (B, 1) mEPSC amplitude is significantly increased in GluK2-expressing neurons (n = 17, control: 15.52 ± 1.02 pA, GluK2: 22.18 ± 0.99 pA, ***P < 0.0005). Plot shows single pairs (open circles) and mean ± SEM (filled circles). (B, 2) Cumulative distribution plots of mEPSC amplitude from control (black) and GluK2-expressing (green) neurons. Cumulative distribution functions show no irregularities. (C, 1) mEPSC frequency is significantly increased in GluK2-expressing neurons (n = 17, control: 0.32 ± 0.09 Hz, GluK2: 1.66 ± 0.27 Hz, ***P < 0.0005). Plot shows single pairs (open circles) and mean ± SEM (filled circles). (C, 2) Cumulative distribution plots of mEPSC frequency from control (black) and GluK2-expressing (green) neurons. Cumulative distribution functions show no irregularities. (D, 1) The decay kinetics of mEPSC is significantly faster from GluK2-expressing than control neurons (n = 17, control: 9.13 ± 0.56 ms, GluK2: 4.84 ± 0.37 ms, ***P < 0.0005). Plot shows single pairs (open circles) and mean ± SEM (filled circles). Inset shows peak normalized sample traces. (D, 2) Cumulative distribution plots of mEPSC decay tau from control (black) and GluK2-expressing (green) neurons. Cumulative distribution functions show no irregularities. (E and F) Plots show single paired (open circles) and mean ± SEM (filled circles) of mEPSC amplitude (E) and frequency (F) from control and GluK2-expressing neurons before (black and green, n = 10; amplitude: control: 14.45 ± 1.49 pA, GluK2: 22.32 ± 1.38 pA, ***P < 0.005; frequency: control: 0.22 ± 0.09 Hz, GluK2: 1.23 ± 0.17 Hz, ***P < 0.005) and after 30-μM GYKI53655 treatment (blue and red, amplitude: GluK2: 19.93 ± 1.05 pA; frequency: GluK2: 0.99 ± 0.24 Hz). All of the statistical analyses above are compared with respective control neurons with two-tailed Wilcoxon signed-rank sum test. (G, 1) Distribution of mEPSCs amplitude of control and GluK2-expressing neurons. The dashed line and arrowhead indicate the extra large mEPSCs (>40 pA) from the experimental cells. (G, 2) The decay kinetics of >40 pA mEPSC from GluK2-expressing neurons is not significantly changed before (black, n = 10, 5.01 ± 0.32 ms) and after GYKI53655 treatment (green, 4.72 ± 0.44 ms), P > 0.05. Plot shows single pairs (open circles) and mean ± SEM (filled circles). Inset shows peak normalized sample traces. The statistical analysis between these two groups was tested using Mann–Whitney u test.
To determine whether there were any neuronal structural modifications that accompanied the profound effects of GluK2 expression, we imaged the dendritic spines as a proxy for the number of excitatory synapses. Remarkably, expression of GluK2 had no effect on spine density (Fig. S2D). It did reduce the diameter of the spine neck (Fig. S2 E, 1 and 2) and spine head (Fig. S2 G, 1 and 2), whereas increasing the length of the spine shaft (Fig. S2 F, 1 and 2). However, none of these anatomical changes can account for the large GluK2 effects. To account for the large increase in mEPSC frequency (Fig. S4 C, 1 and 2) and the increase in the NMDAR comment of the eEPSC (Fig. S4B), we suggest that the additional synapses are preferentially made on dendritic shafts.
Neto Proteins Do Not Regulate GluK2 Receptor Extrasynaptic and Synaptic Trafficking.
The coexpression of Neto1 or Neto2 with GluK1 has a profound effect on surface and synaptic expression, as well as the kinetics of GluK1-mediated responses (16). In striking contrast, neither Neto1 nor Neto2 had any effect on the enhancement of synaptic responses caused by GluK2 (Fig. S5). However, we were concerned that the massive enhancement caused by the expression of GluK2 might obscure any effects that the Neto proteins might have on GluK2. We therefore lowered the expression of GluK2 by expressing it after an internal ribosome entry site (IRES). This substantially reduced the magnitude of the enhancement (Fig. 2A). However, neither the coexpression of Neto1 (Fig. 2 B and D) nor the coexpression of Neto2 (Fig. 2 C and D) had any effect on the enhancement of the AMPAR/KAR-mediated eEPSCs and the NMDAR-mediated eEPSCs (Fig. S6). Because the trafficking of GluK2 is independent of Neto proteins, the lack of synaptic targeting of endogenous GluK2 cannot be related to these auxiliary proteins. Furthermore, unlike GluK1 (16), Neto1 and Neto2 had no effect on the size of the GluK2-mediated currents in outside-out patches (Fig. 2 E, 1). Neto1 and Neto2 did affect the kinetics of GluK2 currents obtained from outside-out patches and ultrafast glutamate application. These experiments were done in the presence of GYKI53655 (100 μM) to ensure that GluK2 was fully responsible for the recorded currents. Neto1 had no effect on deactivation, whereas Neto2 slowed deactivation (Fig. 2 E, 2). Neto1 hastened the onset of desensitization in contrast to Neto2, which slowed the rate of desensitization (Fig. 2 E, 3). All of the effects of Neto1 and Neto2 on the kinetics of GluK2 are the same as that for GluK1 receptor (16). We also recorded the effects of Neto proteins on the decay of isolated KAR-mediated mEPSCs (Fig. 2F) in the presence of GYKI53655 (30 μM). Neto1 had no effect, as would be expected, because it did not affect deactivation, whereas Neto2 slowed the decay of mEPSCs, presumably by its slowing of deactivation. All of the results indicate that Neto auxiliary subunits have no regulation of the surface and synaptic trafficking of GluK2, but regulate the receptor’s biophysical properties.
Fig. S5.
Neto auxiliary proteins have no effect on GluK2 synaptic trafficking. (A and B) Scatter plots show eEPSC amplitudes of GluK2/Neto1 or GluK2/Neto2-cotransfected CA1 neurons and the respective neighboring control ones in rat hippocampal slice cultures measured at −70 mV in the absence or presence of GYKI53655 (100 μM). Filled circles show mean ± SEM. Insets show sample current traces from control (black) and GluK2-expressing (green) cells. (Scale bars for representative eEPSC traces, 100 pA and 25 ms.) Bar graphs show normalized eEPSC amplitudes (mean ± SEM) of pretreated (GluK2/Neto1, n = 9, 2090.45 ± 714.04% control, **P < 0.005; GluK2/Neto2, n = 9, 1234.04 ± 271.01% control, **P < 0.005), and GYKI53655-treated (GluK2/Neto1, n = 9, 496.92 ± 93.63% control pretreatment, **P < 0.005; GluK2/Neto2, n = 9, 502.72 ± 128.99% control pretreatment, *P < 0.05) cells. All of the statistical analyses are compared with respective control neurons with two-tailed Wilcoxon signed-rank sum test. (C) Logarithm summary of the AMPAR/KAR eEPSC amplitude ratios between the experimental and respective control neurons (mean ± SEM) for the indicated transfections [(I) GluK2: 1.25 ± 0.07; (II) GluK2/Neto1: 1.19 ± 0.15; (III) GluK2/Neto2: 1.10 ± 0.15; I vs. II, P > 0.05; I vs. III, P > 0.05; II vs. III, P > 0.05]. All of the statistical analyses were tested with Mann–Whitney u test. (D and E) The same experiments were performed as in A and B except that NMDAR eEPSCs were measured at +40 mV (the current amplitudes were calculated 100 ms after stimulation). (Scale bars, 100 pA and 25 ms.) Bar graphs show normalized NMDAR eEPSC amplitudes (mean ± SEM) (E, n = 9, 360.39 ± 67.10% control, **P < 0.005 and F, n = 9, 244.42 ± 58.54% control, *P < 0.05) presented in scatter plots. All of the statistical analyses are compared with respective control neurons with two-tailed Wilcoxon signed-rank sum test. (F) Logarithm summary of the NMDAR eEPSC amplitudes ratio between the experimental and respective control neurons (mean ± SEM) for the indicated transfections [(I) GluK2: 0.40 ± 0.15; (II) GluK2/Neto1: 0.53 ± 0.07; (III) GluK2/Neto2: 0.49 ± 0.16; I vs. II, P > 0.05; I vs. III, P > 0.05; II vs. III, P > 0.05]. All of the statistical analyses were tested with Mann–Whitney u test.
Fig. 2.
The auxiliary Neto1 and Neto2 proteins regulate the biophysical properties of GluK2 receptor but have no effect on GluK2 surface and synaptic trafficking. (A–C) The same experimental design as in Fig. 1 except that EGFP-IRES-GluK2 (A), Neto1-IRES-GluK2 (B), and Neto2-IRES-GluK2 (C) were used for transfection. (Scale bars for representative eEPSC traces, 100 pA and 25 ms.) Bar graphs show normalized amplitudes of eEPSC (mean ± SEM) (A, n = 15, 468.54 ± 78.94% control, ***P < 0.0001; B, n = 12, 331.02 ± 66.03% control, ***P < 0.001; and C, n = 13, 379.13 ± 59.88% control, ***P < 0.001) presented in scatter plots. All of the statistical analyses are compared with respective control neurons with two-tailed Wilcoxon signed-rank sum test. (D) Logarithm summary of the eEPSC amplitude ratios between the experimental and respective control neurons (mean ± SEM) for the above three transfections [(I) EGFP-IRES-GluK2: 0.71 ± 0.08; (II) Neto1-IRES-GluK2: 0.52 ± 0.08; (III) Neto2-IRES-GluK2: 0.62 ± 0.11; I vs. II, P > 0.05; I vs. III, P > 0.05; II vs. III, P > 0.05]. All statistical analyses of the different groups are tested using Mann–Whitney u test. (E, 1) Bar graphs show the amplitude of GluK2 currents (mean ± SEM) from outside-out patches pulled from transfected CA1 neurons with indicated plasmids and exposed to applications of 10 mM glutamate and 100 μM GYKI53655 [(I) GluK2/EGFP: n = 8, 1831.72 ± 165.44 pA; (II) GluK2/Neto1: n = 8, 1,414.01 ± 288.70 pA; (III) GluK2/Neto2: n = 9, 1,788.50 ± 336.94 pA; I vs. II, P > 0.05; I vs. III, P > 0.05; II vs. III, P > 0.05]. Colored sample traces are shown above. (Scale bars, 500 pA and 10 ms.) (E, 2 and E, 3) Bar graphs show mean ± SEM. GluK2 deactivation [E, 2, (I) GluK2/EGFP: n = 9, 3.77 ± 0.25 ms; (II) GluK2/Neto1: n = 8, 3.85 ± 0.22 ms; (III) GluK2/Neto2: n = 9, 4.95 ± 0.39 ms; I vs. II, P > 0.05; I vs. II, *P < 0.05; II vs. III, *P < 0.05] and desensitization [E, 3, (I) GluK2/EGFP: n = 6, 9.62 ± 0.83 ms; (II) GluK2/Neto1: n = 7, 6.88 ± 0.45 ms; (III) GluK2/Neto2: n = 6, 13.78 ± 1.08 ms; I vs. II, *P < 0.05; I vs. III, *P < 0.05; II vs. III, ***P < 0.0001] from outside-out patches pulled from indicated transfected CA1 neurons and exposed to 1-ms or 100-ms applications of 10 mM glutamate and 100 μM GYKI53655, respectively. (F) Bar graphs show decay kinetics (mean ± SEM) of isolated GluK2 mEPSCs (in 30 μM GYKI53655) from transfected CA1 neurons with indicated plasmids [(I) GluK2/EGFP: n = 10, 4.72 ± 0.44 ms; (II) GluK2/Neto1: n = 10, 4.51 ± 0.43 ms; (III) GluK2/Neto2: n = 13, 7.75 ± 0.89 ms; I vs. II, P > 0.05; I vs. III, *P < 0.05; II vs. III, **P < 0.01]. Peak-normalized sample traces of E, 2, E, 3, and F are shown above. (Scale bar, 5 ms.) All of the statistical analyses were tested using Mann–Whitney u test.
Fig. S6.
Neto proteins have no effect on GluK2-enhanced NMDAR synaptic responses. (A–C) The same experimental design as in Fig. 2 A–C except that NMDAR eEPSCs were measured at +40 mV (the current amplitudes were calculated 100 ms after stimulation). (Scale bars, 50 pA and 25 ms.) Bar graphs show normalized NMDAR eEPSC amplitudes (mean ± SEM) (A, n = 15, 175.36 ± 29.62% control, **P < 0.005; B, n = 12, 175.38 ± 45.94% control, **P < 0.005; and C, n = 13, 191.51 ± 32.77% control, **P < 0.005) presented in scatter plots. All of the statistical analyses are compared with respective control neurons with two-tailed Wilcoxon signed-rank sum test. (D) Logarithm summary of the NMDAR eEPSC amplitude ratios between the experimental and respective control neurons (mean ± SEM) for the indicated transfections [(I) EGFP-IRES-GluK2: 0.26 ± 0.08; (II) Neto1-IRES-GluK2: 0.23 ± 0.05; (III) Neto2-IRES-GluK2: 0.31 ± 0.07; I vs. II, P > 0.05; I vs. III, P > 0.05; II vs. III, P > 0.05]. All of the statistical analyses were tested with Mann–Whitney u test.
The ATDs Are the Critical Determinants for GluK1 and GluK2 Differential Synaptic Trafficking.
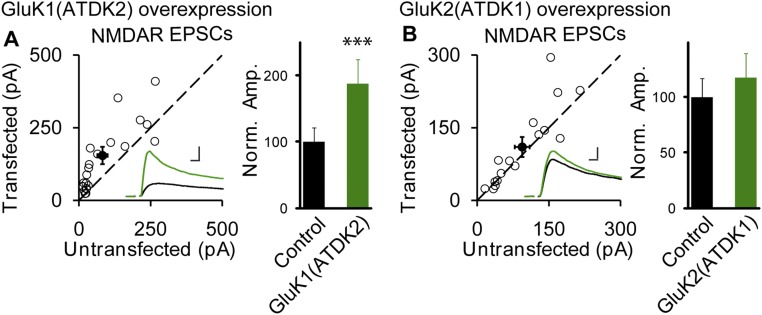
What could account for the finding that expression of GluK1 had no effect on synaptic transmission, whereas GluK2 caused a more than 10-fold increase? Previous studies have already indicated the importance of the intracellular C-terminal domain (CTD) for KARs trafficking. It has been found that there is an ER retention and retrieval motif in GluK1 CTD, thereby limiting the surface expression of this homomeric receptor (23, 24) and GluK2 CTD possesses a forward-trafficking signal for its ER exit (25). Therefore, we first examined the effects of the KAR CTD on synaptic expression by swapping the CTDs between GluK1 and GluK2 (Fig. 3). The GluK2 CTD had a modest but significant effect on GluK1 synaptic expression (Fig. 3 A and G). Although the enhancement of synaptic responses by the GluK2 mutants, either GluK2(CTDK1) (GluK2 replaced with the CTD of GluK1) or GluK2(ΔCTD) (GluK2 lacking its CTD), were slightly but significant less than wild-type GluK2, the two mutants still trafficked to synapses very efficiently (Fig. 3 B and G). All these results suggest that GluK2 CTD might not be the major determinant for the receptor’s synaptic targeting. We next replaced the entire extracellular domain of GluK1 with that of GluK2, and surprisingly the GluK2 extracellular domain delivered GluK1 receptors to synapses very efficiently and this GluK1(NK2) mutant almost phenocopied wild-type GluK2 (Fig. 3 C and G). More specifically, the enhancement caused by the extracellular domain of GluK2 resides in the ATD (Fig. 3 E and G). Consistent with these results, the presence of the extracellular domain [GluK2(NK1), Fig. 3 D and G] or the ATD [GluK2(ATDK1), Fig. 3 F and G] of GluK1 on GluK2 failed to increase synaptic transmission and phenocopied wild-type GluK1, suggesting it is the ATDs that account for the differential GluK1 and GluK2 synaptic trafficking. Moreover, we also found that the GluK1(ATDK2) indeed significantly increased the synaptic currents mediated by NMDARs, the same as GluK2. However, GluK2(ATDK1) had no effect on NMDAR-mediated synaptic responses, the same as GluK1 (Fig. S7), indicating that the GluK2-regulated enhancement of synaptic responses mediated by NMDARs is dependent on the ATD of GluK2.
Fig. 3.
The synaptic targeting of GluK2 is dependent on its extracellular ATD region. (A–F) The same experimental design as in Fig. 1 except that indicated various GluK1 and GluK2 mutants were used for transfection. (Scale bars for representative eEPSC traces, 100 pA and 25 ms.) (G) Logarithm summary of the eEPSC amplitude ratios between the experimental and respective control neurons (mean ± SEM) for the indicated transfections [(I) GluK1: n = 12, 0.03 ± 0.1; (II) GluK1(CTDK2): n = 19, 0.28 ± 0.08; (III) GluK1(NK2): n = 23, 0.86 ± 0.07; (IV) GluK1(ATDK2): n = 14, 0.84 ± 0.09; (V) GluK2: n = 12, 1.25 ± 0.07; (VI) GluK2(CTDK1): n = 21, 0.96 ± 0.06; (VII) GluK2(ΔCTD): n = 14, 0.87 ± 0.09; (VIII) GluK2(NK1): n = 16, 0.05 ± 0.05; (IX) GluK2(ATDK1): n = 17, 0.06 ± 0.06; I vs. II, *P < 0.05; I vs. III, ***P < 0.0001; I vs. IV, ***P < 0.0001; V vs. VI, #P < 0.05; V vs. VII, #P < 0.05; V vs. VIII, ###P < 0.0001; V vs. IX, ###P < 0.0001]. Below are the represented cartoons for the swapped domains between GluK1 (blue) and GluK2 (red) proteins. All of the statistical analyses were tested with Mann–Whitney u test.
Fig. S7.
The inceased NMDAR EPSCs by GluK2 is dependent on the ATD. (A and B) The same experiments as in Fig. 3 E and F except that the NMDAR eEPSCs were measured at +40 mV (the current amplitudes were calculated 100 ms after stimulation). Open and filled circles represent amplitudes for single pairs and mean ± SEM, respectively. Insets show sample current traces from control (black) and experimental (green) cells. (Scale bars, 50 pA and 25 ms.) Bar graphs show normalized NMDAR eEPSC amplitudes (mean ± SEM) (A, 188.16 ± 36.07% control, ***P < 0.0005 and B, 117.17 ± 21.95% control, P > 0.05) presented in scatter plots. All of the statistical analyses are compared with respective control neurons with two-tailed Wilcoxon signed-rank sum test.
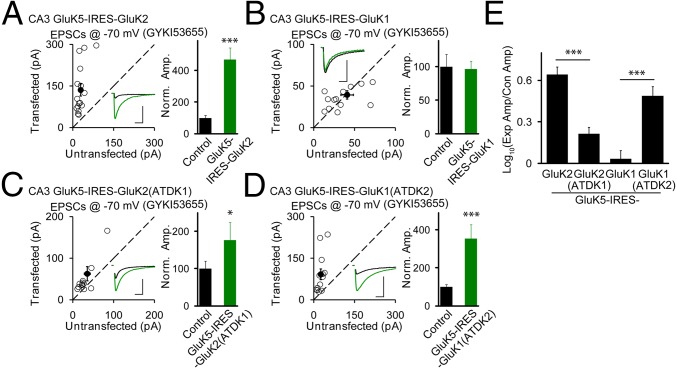
For our above studies, we have taken advantage of the absence of KAR synaptic currents at the Schaffer collateral–CA1 synapse to characterize the basic properties of KARs. Can we apply these findings to a synapse that normally expresses KAR-mediated currents? We were specifically interested in the role of the ATD of low-affinity subunits GluK1 and GluK2 for the synaptic trafficking of native KARs. For this purpose we turned to the mossy fiber–CA3 synapse. Based on an elegant series of genetic studies, it has been found that KARs at mossy fiber synapses are heteromers consisting of GluK2 (26) and the high-affinity subunits GluK4/5 (27). For these experiments, we expressed GluK5 before the IRES and GluK2 after the IRES. This would result in an excess of GluK5 expression compared with GluK2. Because GluK5 does not form functional homomeric receptors, it ensures that any increased currents recorded will consist of GluK2/5 heteromers. We carried out simultaneous whole-cell recordings from experimental and neighboring wild-type CA3 neurons in the presence of GYKI53655 and stimulated the mossy fibers locally (Fig. S1B). Expression of GluK2/5 resulted in a large enhancement of the slow EPSCs (Fig. 4 A and E), indicating that the mossy fiber synapses can accommodate additional GluK2/5 receptors. In contrast, expression of GluK1 with GluK5, which can form heteromeric receptors (28, 29), had no effect on the slow EPSCs (Fig. 4 B and E). When the ATD of GluK1 was placed on the GluK2 subunit the slow EPSCs was only modestly increased (Fig. 4 C and E), suggesting that the ATD of GluK2 is important for the synaptic targeting of heteromeric KARs. Indeed, the chimera mutant GluK1 containing the ATD of GluK2 [GluK1(ATDK2)] substantially increased the slow EPSCs (Fig. 4 D and E).
Fig. 4.
The ATD region of GluK2 is critical for synaptic trafficking of GluK2/GluK5 heteromeric kainate receptors in CA3 pyramidal neurons. (A–D) The same experimental design as in Fig. 1 except that GluK5-IRES-GluK2 (A), GluK5-IRES-GluK1 (B), GluK5-IRES-GluK2(ATDK1) (C), and GluK5-IRES-GluK1(ATDK2) (D) were used for transfection and CA3 pyramidal neurons were recorded in the presence of GYKI53655 (30 μM). (Scale bars for representative eEPSC traces, 100 pA and 50 ms for A, C, and D and 50 pA and 50 ms for B.) Bar graphs show normalized eEPSC amplitudes (mean ± SEM) (A, n = 13, 354.09 ± 74.23% control, ***P < 0.0001; B, n = 14, 96.21 ± 12.29% control, P > 0.05; C, n = 13, 176.38 ± 48.10% control, *P < 0.05; and D, n = 13, 354.09 ± 74.23% control, ***P < 0.0001) presented in scatter plots. All of the statistical analyses are compared with respective control neurons with two-tailed Wilcoxon signed-rank sum test. (E) Logarithm summary of the eEPSC amplitude ratios between the experimental and respective control neurons (mean ± SEM) for the indicated transfections [(I) GluK5-IRES-GluK2: 0.64± 0.06; (II) GluK5-IRES-GluK2TADK1: 0.21 ± 0.05; (III) GluK5-IRES-GluK1: 0.03 ± 0.06; (IV) GluK5-IRES-GluK1(ATDK2): 0.49 ± 0.07; I vs. II, ***P < 0.0001; III vs. IV, ***P < 0.0005]. All of the statistical analyses are tested with Mann–Whitney u test.
The ATDs of GluK1 and GluK2 Are Critical for Their Differential Trafficking Regulated by Neto Proteins.
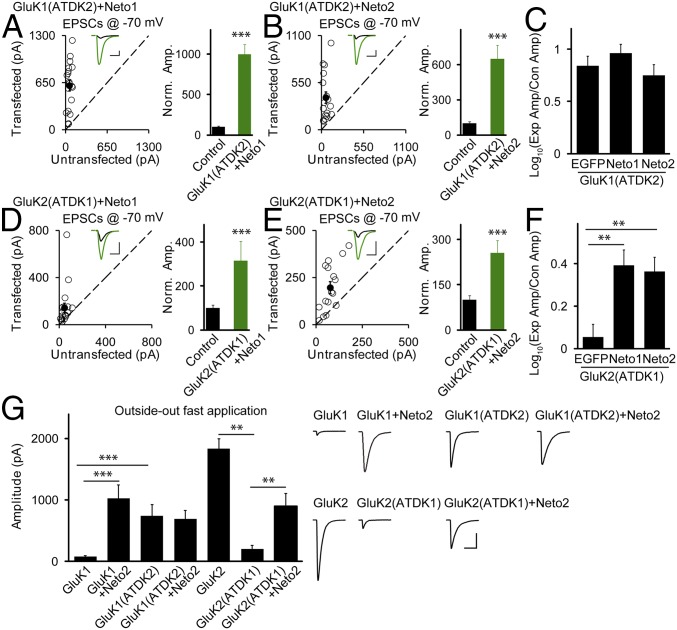
We were curious as to how the coexpression of Neto proteins with GluK1 phenocopied the wild-type GluK2 on its own (16), whereas these auxiliary subunits cause no enhancement of GluK2 synaptic trafficking (Fig. 2 and Fig. S5). To test the possibility that the ATDs of GluK1 and GluK2 might be involved in this differential regulation, we coexpressed either Neto1 or Neto2 with the GluK1 chimera in which the ATD is replaced with the GluK2’s, named GluK1(ATDK2), which traffics to synapses efficiently (Fig. 3 E and G), and found that neither caused any further enhancement of synaptic currents (Fig. 5 A–C). However, coexpression of Neto1 or Neto2 with the GluK2 chimera containing the GluK1 ATD [GluK2(ATDK1)], which failed to target to the CA1 synapses on its own (Fig. 3 F and G), significantly rescued synaptic potentiation (Fig. 5 D–F). These results indicate that it is the ATDs of GluK1 and GluK2 that control their differential dependence on Neto proteins for synaptic trafficking.
Fig. 5.
The ATD region mediates the differential dependence of GluK1 and GluK2 synaptic and surface trafficking by Neto proteins. (A and B and D and E) The same experimental design as in Fig. 1 except that GluK1(ATDK2)/Neto1 (A), GluK1(ATDK2)/Neto2 (B), GluK2(ATDK1)/Neto1 (D), and GluK2(ATDK1)/Neto2 (E) were used for transfection. (Scale bars for representative eEPSC traces, 100 pA and 25 ms.) Bar graphs show normalized eEPSC amplitudes (mean ± SEM) (A, n = 19, 996.83 ± 122.09% control, ***P < 0.0005; B, n = 17, 650.92 ± 116.38% control, ***P < 0.0001; D, n = 19, 315.93 ± 87.69% control, ***P < 0.0005; and E, n = 16, 255.71 ± 41.25% control, **P < 0.001) presented in scatter plots. All of the statistical analyses are compared with respective control neurons with two-tailed Wilcoxon signed-rank sum test. (C and F) Logarithm summary of the eEPSC amplitude ratios between the experimental and respective control neurons (mean ± SEM) for the indicated transfections [(I) GluK1(ATDK2): 0.84± 0.09; (II) GluK1(ATDK2)/Neto1: 0.96 ± 0.08; (III) GluK1(ATDK2)/Neto2: 0.75 ± 0.10; I vs. II, P > 0.05; I vs. III, P > 0.05; II vs. III, P > 0.05] (C) and [(IV) GluK2(ATDK1): 0.06 ± 0.06; (V) GluK2(ATDK1)/Neto1: 0.39 ± 0.07; (VI) GluK2(ATDK1)/Neto2: 0.36 ± 0.07; IV vs. V, **P < 0.005; IV vs. VI, **P < 0.005; V vs. VI, P > 0.05] (F). All of the statistical analyses were tested with Mann–Whitney u test. (G) Bar graphs show the amplitude of wild-type and mutated GluK1 and GluK2 receptor-mediated currents (mean ± SEM) from outside-out patches pulled from transfected CA1 neurons with indicated plasmids and exposed to applications of 10 mM glutamate and 100 μM GYKI53655 [(I) GluK1: n = 10, 81.65 ± 11.26 pA; (II) GluK1/Neto2: n = 1022.84 ± 220.74 pA; (III) GluK1(ATDK2): n = 7, 745.43 ± 176.42 pA; (IV) GluK1(ATDK2)/Neto2: n = 6, 687.67 ± 138.38 pA; (V) GluK2: n = 8, 1,831.72 ± 165.44 pA; (VI) GluK2(ATDK1): n = 5, 200.60 ± 58.33 pA; (VII) GluK2(ATDK1)/Neto2: n = 7, 901.57 ± 208.07 pA; I vs. II, ***P < 0.0005; I vs. III, ***P < 0.0005; III vs. IV, P > 0.05; V vs. VI, **P < 0.005; VI vs. VII, **P < 0.005]. It should be noted that the raw data of GluK1 and GluK1/Neto2 are reused from our previous study (16). All of the statistical analyses were tested with Mann–Whitney u test. Sample traces are shown at Right. (Scale bars, 400 pA and 10 ms.)
Is the ATD of GluK2 necessary for delivery of the receptor to the surface and/or is it responsible for the targeting of surface receptors to the synapse? To address this question, we recorded extrasynaptic currents in the presence of GYKI53655 (100 μM) from outside-out somatic patches and used ultrafast glutamate application, because KARs desensitize extremely rapidly. The magnitude of the currents for the various chimeras generally correlated with the magnitude of their synaptic currents. Specifically, the marginal surface expression of GluK2(ATDK1) on its own was similar to that of GluK1, whereas the ATD of GluK2 delivered the GluK1(ATDK2) mutated receptors to the surface efficiently without Neto proteins (Fig. 5G), suggesting that the ATDs of GluK1 and GluK2 are also critical for these receptors’ surface expression. Moreover, coexpression of Neto2 had virtually the same enhancing effect on GluK1 and GluK2(ATDK1) (Fig. 5G), implying that the Neto interaction with the GluK1 ATD is essential for the surface expression of GluK1. On the other hand, Neto2 failed to further increase the magnitude of GluK1(ATDK2)-mediated enhancement (Fig. 5G), which is similar to the regulation of GluK2 by Neto proteins (Fig. 2 E, 1). Taken together, these results suggest that the ATDs of GluK1 and GluK2 interplay with auxiliary Neto proteins, regulating the KARs’ surface and synaptic trafficking.
Discussion
In this study, we first used the hippocampal CA1 pyramidal neuron as a null background system to study the mechanism regulating the trafficking of KARs because the Schaffer collateral–CA1 synapses lack KAR expression. We find that unlike GluK1, which requires the auxiliary subunits Neto1 and Neto2 for surface and synaptic trafficking (16, 17), GluK2 extrasynaptic and synaptic trafficking is independent of Neto proteins. However, its proper synaptic targeting requires the extracellular ATD region. At the mossy fiber–CA3 synapses, which do express KARs, we find that the GluK2 ATD is also critical for the synaptic targeting of GluK2/GluK5 heteromeric receptor complexes. Furthermore, we determine that it is the ATDs of GluK1 and GluK2 contributing their differential dependence on Neto proteins for surface and synaptic trafficking. Our results demonstrate the important role of the interplay of the KAR ATDs and the Neto auxiliary subunits in controlling the surface expression and synaptic incorporation of kainate receptors.
Consistent with our previous study (16), expressing GluK1 in CA1 pyramidal neurons results in very few KAR synaptic and surface currents. We interpret this finding as a defect in the forward trafficking of GluK1, although it is formally possible that the removal of surface receptors is greatly enhanced. By contrast, the expression of GluK2 generates huge responses from outside-out patches from the cell body membrane as well as the evoked EPSCs. These differences cannot be explained by the biophysical properties between GluK1 and GluK2 receptors because the rates of their desensitization or deactivation kinetics are very similar (16). Thus, the trafficking properties of GluK1 and GluK2 are fundamentally different. This finding is consistent with a previous study showing that the surface staining intensity of GluK2 is much higher than GluK1 when expressed in COS-7 or primary cultured hippocampal neurons (23). We also find that when coexpressing GluK5 with GluK1 or GluK2 in CA3 cells, the GluK2/GluK5 but not the GluK1/GluK5 heteromeric receptors can target to the mossy fiber–CA3 synapses, suggesting that the low-affinity KARs may also be critical for the expression and localization of the heteromeric receptors. The different trafficking abilities of KARs are presumably due to their different amino acid sequences (30, 31). Several studies have already shown the importance of the CTDs of KARs for surface trafficking (23–25, 32, 33), and thus we first examined the effects on synaptic expression by swapping the CTDs between GluK1 and GluK2. Much to our surprise, we found that the enhancement of synaptic responses mediated by the GluK2(CTDK1) or GluK2(ΔCTD) mutated receptor is similar to that seen with the wild-type GluK2 receptor. There are several possible reasons underlying the difference between our present observation and previous studies. First, many of the previous experiments (25, 33) were carried out in heterologous expression systems in which the regulation of GluK2 trafficking may differ from that in neurons. Consistent with this proposal, it has been reported that in COS-7 cells GluK2a, the splicing isoform with a long CTD and used in our current studies, traffics to the cell membrane more effectively than the shorter isoform GluK2b (23). However, the same study reported that the surface expression of the two isoforms is similar in primary cultured neurons (23). Second, Yan et al. (25) used a GluK2 mutant containing a GFP tag in the ATD. Given the critical role we have found for the ATD of GluK2 in trafficking, it is possible that the tag may have masked the role of the ATD of GluK2, allowing the role of the CTD to be dissected. Consistent with this hypothesis, we previously found that the synaptic expression of N-terminal HA or Myc tagged-GluK1 is impaired even in the presence of Neto proteins, although they could traffic to the surface successfully (16). The knockin studies by Straub et al. (33) suggest that the GluK2 CTD stabilizes the receptor at synapses and in agreement we also find that the GluK2 CTD endows GluK1 with the ability to express at synapses. Given that the CTD is not critical for GluK2 synaptic trafficking, we focused on the extracellular domains of GluK2. Surprisingly, swapping the entire extracellular domains or just ATDs between GluK1 and GluK2 fully switches their surface and synaptic trafficking abilities. Furthermore, we show here that the ATDs of GluK1 and GluK2 determine their differential dependence on auxiliary Neto proteins for trafficking. All these results indicate that the ATDs of GluK1 and GluK2 receptors are the major determinant for their trafficking. Recently two studies have revealed that C1q-like proteins interact with the ATD of GluK2 or GluK4 to organize the KARs at mossy fiber–CA3 synapses (33, 34), and in accordance, we also find that the ATD of GluK2 is critical for the synaptic expression of GluK2/GluK5 heteromeric receptors at mossy fiber–CA3 synapses.
In contrast to GluK1 (16), GluK2 surface and synaptic expression are independent on the auxiliary subunits Neto1 and Neto2. A similar conclusion was reached in previous studies showing that Neto2 has no effect on GluK2 trafficking in oocytes (14) but does promote GluK1 surface expression in HEK cells and primary cultured neurons (17). However, the underlying molecular mechanism remains unknown. This differential dependence on auxiliary subunits for trafficking could not be explained by any specific interaction between GluK1 and Neto proteins because we find that the biophysical effects of Neto proteins on GluK1 and GluK2 are the same (16). Moreover, the decay of GluK2 mEPSCs is increased by coexpressed Neto2. All these results indicate that GluK2 and Neto proteins indeed interact at both the surface and the synapse. It has been reported that it is the extracellular CUB domains of Neto1 and Neto2 that mediate their interaction with GluK2 (35), but it is unknown how they bind to GluK1 or which domains of GluK1 and GluK2 mediate their interactions with Neto proteins. Additionally, it has been reported that the extracellular LDLa domain of the Neto proteins is critical for their effects on GluK2 desensitization, but the intracellular C-terminal domain is critical for their regulation of GluK2 rectification (36), indicating that Neto proteins can regulate KAR function through different domains. We report here that the ATDs of GluK1 and GluK2 mediate the differential dependence on Neto proteins for trafficking. It will be of interest to identify the detailed structural and molecular basis for the difference. Importantly, in the central nervous system, most native KARs are heteromeric complexes and the presence of high-affinity subunits could affect the function of Neto proteins. Such a scenario might explain our finding that Neto2 but not Neto1 slows homomeric GluK2 mEPSC decay time, whereas previous studies indicate that Neto1 but not Neto2 is critical for the decay of the slow EPSC at mossy fiber–CA3 synapses (21, 35). It would be of interest to study further the effects of Neto proteins on the trafficking and biophysical properties of specific heteromeric KAR complexes.
In summary, this study has characterized the critical role of the ATD for KAR trafficking in hippocampal neurons as well as their interplay with auxiliary subunit Neto proteins in this process. We first selected the Schaffer collateral–CA1 synapse that normally does not express KARs, to determine the minimal requirements that govern the insertion of KARs into excitatory synapses, and further confirm the findings at mossy fiber–CA3 synapses that express heteromeric KARs. Our results demonstrate the critical role of the extracellular ATD for KAR surface and synaptic expression as well as their contribution to KARs’ differential dependence on Neto proteins for trafficking.
Materials and Methods
Experimental Constructs.
The cDNAs of rat GluK1, rat GluK2, mouse Neto1, and rat Neto2 as well as the GluK1 and GluK2 mutants were subcloned into pCAGGS vector for biolistic transfection.
Electrophysiology in Slice Cultures.
All experiments were performed in accordance with established protocols approved by the University of California San Francisco Institutional Animal Care and Use Committee. The methods of electrophysiology in slice culture in this study are described in SI Text.
Anatomy Imaging.
The method of anatomy imaging in this study is described in SI Text.
Statistical Analysis.
Significance of evoked dual whole-cell recordings and mEPSC recordings compared with controls was determined using the two-tailed Wilcoxon signed-rank sum test. For all experiments involving unpaired data, including all outside-out patch data, a Mann–Whitney u test with Bonferonni correction for multiple comparisons was used. Paired-pulse ratios and spine densities were analyzed with unpaired t test. Data analysis was carried out in Igor Pro (Wavemetrics), Excel (Microsoft), and GraphPad Prism (GraphPad Software).
SI Text
Electrophysiology in Slice Cultures.
Organotypic hippocampal slice cultures were made from P6–P8 rats. Transfections were carried out on DIV 2 after culturing using a Helios Gene Gun (Bio-Rad) with 1 μm DNA-coated gold particles. When biolistically expressing two plasmids, gold particles were coated with equal amounts of each plasmid and plasmids always expressed different fluorescent markers. Observed frequency of coexpression was nearly 100%. Slices were maintained at 34 °C with media changes every other day. Then on DIV 8, dual whole-cell recordings in area CA1 or CA3 were done by simultaneously recording responses from a fluorescent transfected neuron and neighboring untransfected control neuron. Pyramidal neurons were identified by morphology and location. Series resistance was monitored on-line, and recordings in which series increased to >30 MOhm or varied by >50% between neurons were discarded. Dual whole-cell recordings measuring evoked EPSCs used artificial cerebrospinal fluid (ACSF) bubbled with 95% O2/5% CO2 consisting of (in millimoles) 119 NaCl, 2.5 KCl, 4 CaCl2, 4 MgSO4, 1 NaH2PO4, 26.2 NaHCO3, 11 glucose. A total of 100 μM picrotoxin was added to block inhibitory currents and 4 μM 2-chloroadenosine was used to control epileptiform activity. Intracellular solution contained (in millimoles) 135 CsMeSO4, 8 NaCl, 10 Hepes, 0.3 EGTA, 5 QX314-Cl, 4 MgATP, 0.3 Na3GTP, 0.1 spermine. A bipolar stimulation electrode was placed in stratum radiatum, and responses were evoked at 0.2 Hz. Peak AMPAR and KAR currents were recorded at −70 mV, and NMDAR current amplitudes 100 ms following the stimulus were recorded at +40 mV. Paired-pulse ratio was determined by delivering two stimuli 40 ms apart and dividing the peak response to stimulus 2 by the peak response to stimulus 1. All these data were analyzed off-line with custom software (IGOR Pro). For miniature EPSC recording, the ACSF was the same as above, and 100 μM picrotoxin and 0.5 μM TTX was also included but without 2-chloroadenosine. mEPSCs with an amplitude of ≥5 pA and a rate of rise of ≥4 pA/ms were automatically detected and analyzed off-line with customized software in IGOR. For fast application, somatic out-side out patches were excised from wild-type or transfected CA1 pyramidal neurons using 3- to 5-MΩ pipettes. The fast responses to glutamate were recorded at −70 mV. Glutamate pulses of 1 or 100 ms were applied to patches by a theta-glass pipette every 10–20 s using a piezoelectric controller (Siskiyou). Glutamate (10 mM) was dissolved in the Hepes ACSF consisting of (in millimoles): 140 NaCl, 5 KCl, 1.4 MgCl2, 1 CaCl2, 5 EGTA, 10 Hepes, 1 NaH2PO4, 10 d-glucose, with pH adjusted to 7.4, with the addition of 100 μM d-APV, 0.5 μM tetrodotoxin and 100 μM GYKI53655 to isolate KAR-mediated currents. The control barrel contained the same Hepes ACSF with all of the inhibitors and 1 mM sucrose but not glutamate. The open-tip response had a switch on and off time of less than 200 μs. Responses were collected with a Multiclamp 700A amplifier (Axon Instruments), filtered at 2 kHz, and digitized at 10 kHz.
Anatomy Imaging.
Slice cultures were maintained as described above and transfected with GluK2 and FUGW-EGFP or just FUGW-EGFP itself (as wild-type control). On DIV 8, the transfected CA1 pyramidal neurons were identified under microscope then slices were fixed in 4% PFA/4% sucrose in PBS and washed three times with PBS. To amplify the GFP signal, slices were then blocked and permeabalized in 3% BSA in PBS containing 0.1% Triton-X and stained with primary antibody against GFP (2 μg/mL, Life Technologies A-11122) followed by washes in PBST five times and staining with Alexa 488-conjugated secondary (4 μg/mL, Life Technologies A-11034) as well as five times PBST washing. Slices were mounted in SlowFade Gold (Life Technologies) for imaging. For spine density and morphology measurements, images were acquired using superresolution microscopy (N-SIM Microscope System, Nikon). Only dendrites in the top 20 μm of the slice were imaged. Images were acquired with a 100× oil objective in 3D-SIM mode using supplied SIM grating (3D EX V-R 100×/1.49) and processed and reconstructed using supplied software (NIS-Elements, Nikon). Morphological analysis was done on individual sections using ImageJ to perform geometric measurements on spines extending laterally from the dendrite. Spine neck diameter was obtained from full width half maximum (FWHM) measurements based on Gaussian fits of line profile plots. Shaft length was measured from the base of the spine to the end of the head. Head diameter was measured perpendicular to the spine neck axis through the thickest part of the spine head. Head diameter was obtained using full width tenth maximum (FWTM) measurements based on Gaussian fits to approximate manual head measurement.
Acknowledgments
We thank K. Bjorgan, M. Cerpas, and D. Qin for technical assistance and all members of the R.A.N. laboratory for discussion of and comments on the manuscript. This work was funded by grants from the National Institute of Mental Health (to R.A.N.). Y.S.S. is supported by grants from Natural Science Foundation of China (31371061 and 31571060) and the Ministry of Science and Technology of China (2014CB942804).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1619253114/-/DCSupplemental.
References
- 1.Contractor A, Mulle C, Swanson GT. Kainate receptors coming of age: Milestones of two decades of research. Trends Neurosci. 2011;34(3):154–163. doi: 10.1016/j.tins.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jane DE, Lodge D, Collingridge GL. Kainate receptors: Pharmacology, function and therapeutic potential. Neuropharmacology. 2009;56(1):90–113. doi: 10.1016/j.neuropharm.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 3.Lerma J, Marques JM. Kainate receptors in health and disease. Neuron. 2013;80(2):292–311. doi: 10.1016/j.neuron.2013.09.045. [DOI] [PubMed] [Google Scholar]
- 4.Nicoll RA, Schmitz D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat Rev Neurosci. 2005;6(11):863–876. doi: 10.1038/nrn1786. [DOI] [PubMed] [Google Scholar]
- 5.Castillo PE, Malenka RC, Nicoll RA. Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature. 1997;388(6638):182–186. doi: 10.1038/40645. [DOI] [PubMed] [Google Scholar]
- 6.Vignes M, Collingridge GL. The synaptic activation of kainate receptors. Nature. 1997;388(6638):179–182. doi: 10.1038/40639. [DOI] [PubMed] [Google Scholar]
- 7.Schmitz D, Frerking M, Nicoll RA. Synaptic activation of presynaptic kainate receptors on hippocampal mossy fiber synapses. Neuron. 2000;27(2):327–338. doi: 10.1016/s0896-6273(00)00040-4. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz D, Mellor J, Nicoll RA. Presynaptic kainate receptor mediation of frequency facilitation at hippocampal mossy fiber synapses. Science. 2001;291(5510):1972–1976. doi: 10.1126/science.1057105. [DOI] [PubMed] [Google Scholar]
- 9.Lauri SE, et al. A critical role of a facilitatory presynaptic kainate receptor in mossy fiber LTP. Neuron. 2001;32(4):697–709. doi: 10.1016/s0896-6273(01)00511-6. [DOI] [PubMed] [Google Scholar]
- 10.Contractor A, Swanson G, Heinemann SF. Kainate receptors are involved in short- and long-term plasticity at mossy fiber synapses in the hippocampus. Neuron. 2001;29(1):209–216. doi: 10.1016/s0896-6273(01)00191-x. [DOI] [PubMed] [Google Scholar]
- 11.Bureau I, Bischoff S, Heinemann SF, Mulle C. Kainate receptor-mediated responses in the CA1 field of wild-type and GluR6-deficient mice. J Neurosci. 1999;19(2):653–663. doi: 10.1523/JNEUROSCI.19-02-00653.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Granger AJ, Shi Y, Lu W, Cerpas M, Nicoll RA. LTP requires a reserve pool of glutamate receptors independent of subunit type. Nature. 2013;493(7433):495–500. doi: 10.1038/nature11775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Copits BA, Swanson GT. Dancing partners at the synapse: Auxiliary subunits that shape kainate receptor function. Nat Rev Neurosci. 2012;13(10):675–686. doi: 10.1038/nrn3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang W, et al. A transmembrane accessory subunit that modulates kainate-type glutamate receptors. Neuron. 2009;61(3):385–396. doi: 10.1016/j.neuron.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Straub C, Tomita S. The regulation of glutamate receptor trafficking and function by TARPs and other transmembrane auxiliary subunits. Curr Opin Neurobiol. 2012;22(3):488–495. doi: 10.1016/j.conb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheng N, Shi YS, Lomash RM, Roche KW, Nicoll RA. Neto auxiliary proteins control both the trafficking and biophysical properties of the kainate receptor GluK1. Elife. 2015;4:e11682. doi: 10.7554/eLife.11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Copits BA, Robbins JS, Frausto S, Swanson GT. Synaptic targeting and functional modulation of GluK1 kainate receptors by the auxiliary neuropilin and tolloid-like (NETO) proteins. J Neurosci. 2011;31(20):7334–7340. doi: 10.1523/JNEUROSCI.0100-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palacios-Filardo J, Aller MI, Lerma J. Synaptic targeting of kainate receptors. Cereb Cortex. 2016;26(4):1464–1472. doi: 10.1093/cercor/bhu244. [DOI] [PubMed] [Google Scholar]
- 19.Straub C, Zhang W, Howe JR. Neto2 modulation of kainate receptors with different subunit compositions. J Neurosci. 2011;31(22):8078–8082. doi: 10.1523/JNEUROSCI.0024-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher JL, Mott DD. Modulation of homomeric and heteromeric kainate receptors by the auxiliary subunit Neto1. J Physiol. 2013;591(19):4711–4724. doi: 10.1113/jphysiol.2013.256776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Straub C, et al. Distinct functions of kainate receptors in the brain are determined by the auxiliary subunit Neto1. Nat Neurosci. 2011;14(7):866–873. doi: 10.1038/nn.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bleakman D, et al. Activity of 2,3-benzodiazepines at native rat and recombinant human glutamate receptors in vitro: Stereospecificity and selectivity profiles. Neuropharmacology. 1996;35(12):1689–1702. doi: 10.1016/s0028-3908(96)00156-6. [DOI] [PubMed] [Google Scholar]
- 23.Jaskolski F, et al. Subunit composition and alternative splicing regulate membrane delivery of kainate receptors. J Neurosci. 2004;24(10):2506–2515. doi: 10.1523/JNEUROSCI.5116-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren Z, et al. Cell surface expression of GluR5 kainate receptors is regulated by an endoplasmic reticulum retention signal. J Biol Chem. 2003;278(52):52700–52709. doi: 10.1074/jbc.M309585200. [DOI] [PubMed] [Google Scholar]
- 25.Yan S, et al. A C-terminal determinant of GluR6 kainate receptor trafficking. J Neurosci. 2004;24(3):679–691. doi: 10.1523/JNEUROSCI.4985-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mulle C, et al. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature. 1998;392(6676):601–605. doi: 10.1038/33408. [DOI] [PubMed] [Google Scholar]
- 27.Fernandes HB, et al. High-affinity kainate receptor subunits are necessary for ionotropic but not metabotropic signaling. Neuron. 2009;63(6):818–829. doi: 10.1016/j.neuron.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christensen JK, Paternain AV, Selak S, Ahring PK, Lerma J. A mosaic of functional kainate receptors in hippocampal interneurons. J Neurosci. 2004;24(41):8986–8993. doi: 10.1523/JNEUROSCI.2156-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher MT, Fisher JL. Contributions of different kainate receptor subunits to the properties of recombinant homomeric and heteromeric receptors. Neuroscience. 2014;278:70–80. doi: 10.1016/j.neuroscience.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coussen F. Molecular determinants of kainate receptor trafficking. Neuroscience. 2009;158(1):25–35. doi: 10.1016/j.neuroscience.2007.12.052. [DOI] [PubMed] [Google Scholar]
- 31.Jaskolski F, Coussen F, Mulle C. Subcellular localization and trafficking of kainate receptors. Trends Pharmacol Sci. 2005;26(1):20–26. doi: 10.1016/j.tips.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Ren Z, et al. Multiple trafficking signals regulate kainate receptor KA2 subunit surface expression. J Neurosci. 2003;23(16):6608–6616. doi: 10.1523/JNEUROSCI.23-16-06608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Straub C, et al. Distinct subunit domains govern synaptic stability and specificity of the kainate receptor. Cell Reports. 2016;16(2):531–544. doi: 10.1016/j.celrep.2016.05.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsuda K, et al. Transsynaptic modulation of kainate receptor functions by C1q-like proteins. Neuron. 2016;90(4):752–767. doi: 10.1016/j.neuron.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Tang M, et al. Neto1 is an auxiliary subunit of native synaptic kainate receptors. J Neurosci. 2011;31(27):10009–10018. doi: 10.1523/JNEUROSCI.6617-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fisher JL, Mott DD. The auxiliary subunits Neto1 and Neto2 reduce voltage-dependent inhibition of recombinant kainate receptors. J Neurosci. 2012;32(37):12928–12933. doi: 10.1523/JNEUROSCI.2211-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]