Significance
Quality control of ribosomal RNA (rRNA) processing is critical for ribosome biogenesis, nucleolar homeostasis, and cell survival; however, the molecular mechanisms governing rRNA processing under stress conditions are poorly understood. This study identifies human NF-κB repressing factor (NKRF) as a HSF1 target gene essential for nucleolar homeostasis during proteotoxic stress. Rather than preventing protein misfolding and/or aggregation, this unconventional stress protein has a critical role in preventing aberrant rRNA precursors and discarded fragment accumulation and directing rRNA processing dynamics. The findings highlight a key aspect of the human cell response to proteotoxic stress, opening new scenarios on ribosome biogenesis regulation.
Keywords: heat shock factor 1, NF-kappaB, nucleolus, proteotoxic stress, rRNA processing
Abstract
The nucleolus, a dynamic nuclear compartment long regarded as the cell ribosome factory, is emerging as an important player in the regulation of cell survival and recovery from stress. In larger eukaryotes, the stress-induced transcriptional response is mediated by a family of heat-shock transcription factors. Among these, HSF1, considered the master regulator of stress-induced transcriptional responses, controls the expression of cytoprotective heat shock proteins (HSPs), molecular chaperones/cochaperones constituting a major component of the cell protein quality control machinery essential to circumvent stress-induced degradation and aggregation of misfolded proteins. Herein we identify human NF-κB repressing factor (NKRF) as a nucleolar HSP essential for nucleolus homeostasis and cell survival under proteotoxic stress. NKRF acts as a thermosensor translocating from the nucleolus to the nucleoplasm during heat stress; nucleolar pools are replenished during recovery upon HSF1-mediated NKRF resynthesis. Silencing experiments demonstrate that NKRF is an unconventional HSP crucial for correct ribosomal RNA (rRNA) processing and preventing aberrant rRNA precursors and discarded fragment accumulation. These effects are mediated by NKRF interaction with the 5′-to-3′ exoribonuclease XRN2, a key coordinator of multiple pre-rRNA cleavages, driving mature rRNA formation and discarded rRNA decay. Under stress conditions, NKRF directs XRN2 nucleolus/nucleoplasm trafficking, controlling 5′-to-3′ exoribonuclease nucleolar levels and regulating rRNA processing. Our study reveals a different aspect of rRNA biogenesis control in human cells and sheds light on a sophisticated mechanism of nucleolar homeostasis surveillance during stress.
Protein homeostasis is essential for life in eukaryotes (1). A critical consequence of proteotoxic stress is the activation of the heat shock response (HSR), a fundamental cell defense mechanism, regulated by a family of heat shock transcription factors (HSFs) that are expressed and maintained in an inactive state under nonstress conditions (1–3). Mammalian genomes encode three homologs of HSF (HSF1, HSF2, and HSF4); among these, HSF1 is considered the paralog responsible for regulating stress-induced transcriptional responses (3, 4).
HSF1 is generally found as an inert monomer in unstressed cells (4). Upon exposure to proteotoxic stress, HSF1 is derepressed in a stepwise process that involves HSF1 trimerization, nuclear translocation, phosphorylation/sumoylation, and binding to DNA sequences (heat shock elements, HSEs), characterized by inverted repeats of a “nGAAn”-pentameric motif (4). Upon stress-signal removal, the response attenuates rapidly with HSF1 reconversion to monomers (4). HSF1 binding to HSE triggers a rapid shift in the transcriptional program, resulting in the expression of cytoprotective heat shock proteins (HSPs), which include molecular chaperones of the HSP70 and HSP90 families, HSP27, and other proteins of the network (2, 3). HSF1-binding sites have been described also in genes encoding proteins with nonchaperone function (5). We have recently identified the human zinc-finger AN1-type domain-2a gene as a canonical HSF-1 target gene (6). During these studies, gene expression profile analysis of HSF1 knockdown (HeLa-HSF1i) (7) versus wild-type HeLa cells under stress conditions revealed an increase in the expression of NF-κB repressing factor (NKRF) selectively in wild-type cells.
NF-κB transcription factors comprise a family of critical regulators of the innate and adaptive immune response, playing an important role in promoting inflammation and in the control of cell proliferation and survival (8). NF-κB normally exists as an inactive cytoplasmic complex, whose predominant form is a heterodimer composed of p50 and p65 (RelA) subunits, bound to inhibitory proteins of the IκB family, and is induced in response to a variety of pathogenic stimuli, including exposure to proinflammatory cytokines, mitogens, and viral infection (8, 9). NKRF is known as a silencer protein binding negative regulatory elements (NRE) specific for suppression of NF-κB/Rel-binding element basal activity in several NF-κB–regulated genes (10–13). NKRF has also been shown to interact with NF-κB/p65 through a minimal-core sequence, differentially controlling NF-κB–driven transcription under basal and/or stimulated conditions (14). The fact that we previously described a cross-talk between HSF1 and NF-κB (15–17) prompted us to investigate whether NKRF could be HSF1-regulated and induced by heat exposure. The results unexpectedly show that human NKRF is an unconventional HSP strictly controlled by HSF1, essential for correct ribosomal RNA (rRNA) processing and nucleolar homeostasis under proteotoxic stress conditions.
Results and Discussion
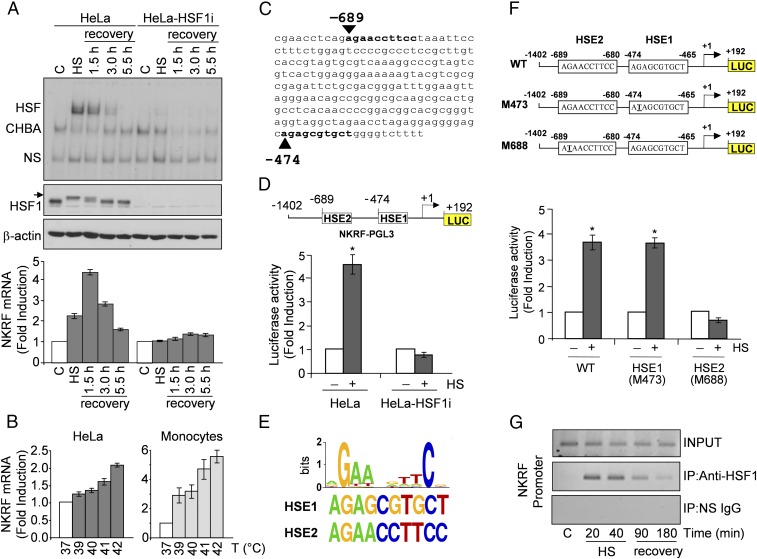
To investigate whether HSF1 is implicated in NKRF gene regulation, we first analyzed the effect of heat treatment on NKRF expression. Heat-induced HSF1 activation is strictly dependent on both the temperature increase above physiological conditions and exposure duration. When HeLa cells were exposed to 43 °C for 40 min, HSF1 phosphorylation and DNA-binding activity were detected during heat stress, continuing during recovery at 37 °C for 1.5 h and declining rapidly thereafter; under these conditions, heat stress induced NKRF-mRNA expression with a kinetics parallel to HSF1 activation (Fig. 1A). A temperature-dependent increase in NKRF-mRNA levels after short (2 h) heat exposure was detected starting at 39 °C (Fig. 1B), indicating that NKRF expression can be induced also under febrile temperature conditions.
Fig. 1.
HSF1 directly regulates NKRF expression during temperature increase. (A) HSF1 DNA-binding activity (gel-shift analysis, Top) and phosphorylation (immunoblot, IB, Middle) and NKRF-mRNA levels (qPCR, Bottom) of whole-cell protein or RNA extracts from HeLa and HeLa-HSF1i cells exposed to heat stress (HS, 43 °C, 40 min) and allowed to recover at 37 °C for the indicated times. CHBA, constitutive HSE-binding activity; HSF, HSF/DNA complexes; NS, nonspecific protein–DNA interactions. Arrow indicates hyperphosphorylated HSF1. (B) NKRF mRNA levels in HeLa cells or human peripheral-blood monocytes incubated at febrile temperatures (2 h). (C) NKRF promoter putative HSF1-binding sites (bold) identified by TFSearch. (D) NKRF promoter reporter analysis in HeLa and HeLa-HSF1i cells transfected with NKRF–PGL3 (Top) and heat stressed (6 h recovery). Putative HSF1-binding sites (HSE1, HSE2) identified in C are shown; numbers indicate positions relative to TSS (+1). (E, Top) WebLogo-generated HSF1 consensus motif. (Bottom) NKRF promoter HSE1 and HSE2 sequences. (F) Wild-type (WT) and point-mutated (G-to-T) (M473, M688) constructs (Top) were used for reporter analysis in HeLa cells treated as in D (Bottom). (G) ChIP analysis of HSF1 binding to the NKRF promoter in HeLa cells treated as in A. ChIP-enriched DNAs using preimmune (IP:NS-IgG) or anti-HSF1 (IP:anti-HSF1) serum and input DNAs are shown. Error bars indicate ±SD. *P < 0.05.
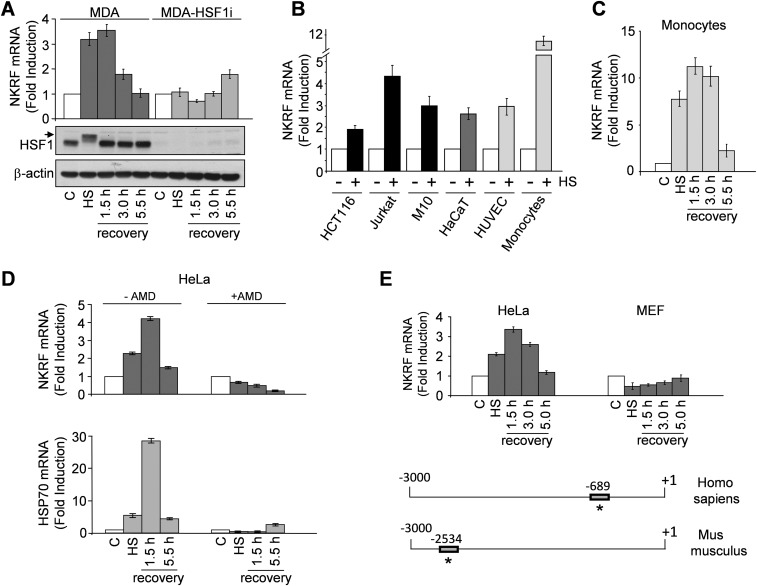
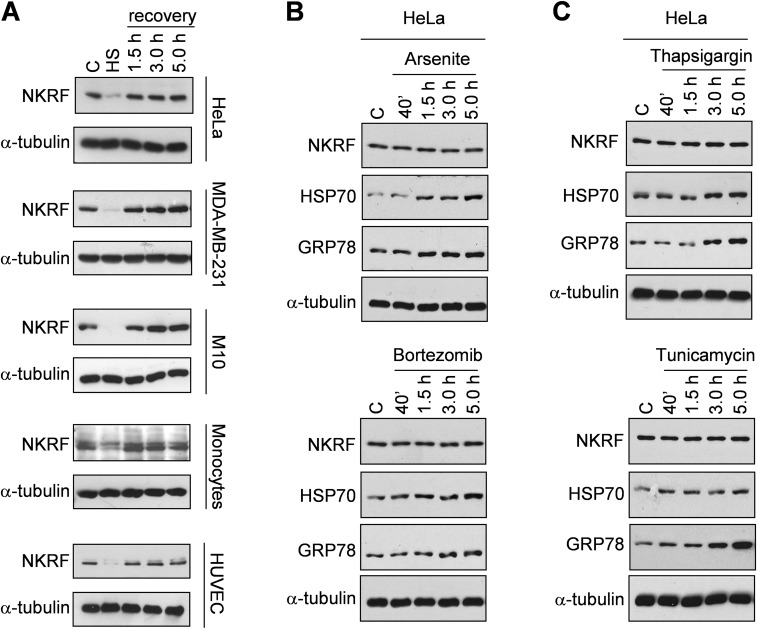
NKRF induction represents a general response of human cells to temperature increase. In addition to HeLa cells, heat exposure induced NKRF expression in human cancer cells of different origin, including breast adenocarcinoma, colon carcinoma, T-cell lymphoma and melanoma, and primary cells, including peripheral-blood monocytes, endothelial cells, and keratinocytes (Fig. S1 A–C). Interestingly, human monocytes showed the highest level of heat-induced NKRF expression also at febrile temperatures (Fig. 1B).
Fig. S1.
Temperature-dependent induction of NKRF-mRNA expression in human primary and cancer cells. (A) NKRF-mRNA levels (qPCR, Top) and HSF1 phosphorylation (IB, Bottom) in RNA or whole-cell protein extracts from human breast adenocarcinoma MDA-MB-231 cells (MDA) and MDA-MB-231 cells transiently transfected with HSF1-siRNA (MDA-HSF1i) subjected to heat stress (HS, 43 °C, 40 min) and allowed to recover at 37 °C for the indicated times. Arrow indicates the hyperphosphorylated HSF1 form. (B) NKRF-mRNA levels (qPCR) in human colon carcinoma (HCT116), T-lymphoma (Jurkat) and melanoma (M10) cancer cell lines, immortalized keratinocytes (HaCaT), primary HUVECs, and peripheral blood monocytes untreated (–) or subjected to HS as in A and allowed to recover for 1.5 h (+). (C) Kinetics of NKRF-mRNA expression (qPCR) in extracts from human monocytes treated as in A. (D) NKRF- and HSP70-mRNA levels (qPCR) in HeLa cells treated as in A in the presence (+) or absence (–) of AMD (5 μg/mL). (E, Top) NKRF expression in HeLa cells versus MEFs treated as in A. (Bottom) Schematic representation of human and mouse NKRF promoter. Positions of functional human HSE and putative mouse HSE are indicated by asterisks. Fold induction was calculated by comparing the induction of the indicated genes in the treated samples to the relative control, which was arbitrarily set to 1.
Notably, heat stress did not affect NKRF expression in HSF1i-silenced cells (Fig. 1A and Fig. S1A).
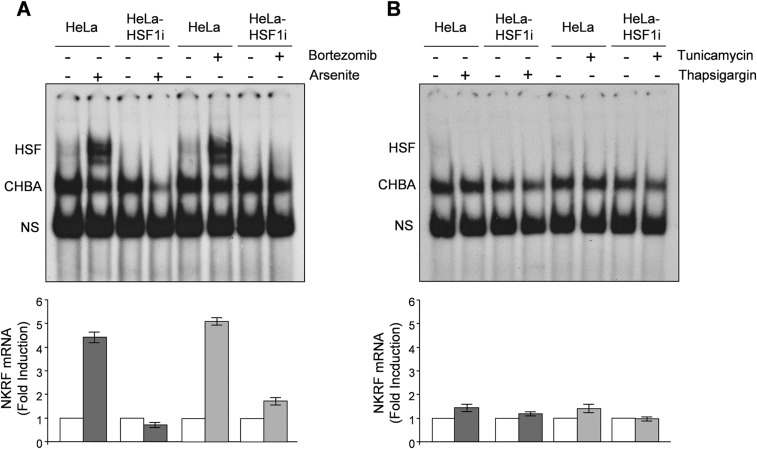
Heat-induced NKRF-mRNA increase is abolished by actinomycin-D (Fig. S1D), suggesting de novo gene transcription. NKRF promoter nucleotide sequence analysis revealed two putative HSEs located at –474 (HSE1) and –689 (HSE2) from the transcription start site (TSS) (Fig. 1C). The NKRF promoter HSE-comprising region was cloned and used for reporter analysis, which confirmed heat-induced NKRF promoter transcription in wild-type but not in HSF1-silenced cells (Fig. 1D). Next, a G-to-T mutation in the second nucleotide of the HSE nGAAn unit, known to reduce HSF1 DNA binding (6), was inserted in HSE1 or HSE2 (Fig. 1 E and F). Mutating HSE1 did not alter heat-induced NKRF promoter activity, whereas mutating HSE2 prevented transcription (Fig. 1F), identifying HSE2 as the critical element for heat-induced NKRF transcription. Finally, ChIP analysis revealed that HSF1 binds directly to the NKRF promoter in vivo starting 20 min after heat stress (Fig. 1G), confirming a critical role of HSF1 in heat-regulated NKRF transcription. Analysis of genome-wide ChIP-seq data (Gene Expression Omnibus accession no. GSE43579) also confirmed that NKRF is included in the 1,242 HSF1 target genes identified during heat stress in a different type of human cell (K562 erythroleukemia) (18). Interestingly, in addition to heat, HSF1 activation during proteotoxic stress induced by arsenite or proteasome inhibition also triggered NKRF expression, whereas endoplasmic reticulum (ER) stress inducers thapsigargin and tunicamycin had no effect (Fig. S2). Differently from human cells, heat stress did not induce NKRF expression in murine fibroblasts; in silico analysis revealed substantial differences in murine versus human NKRF promoter structure, including lack of HSEs at position –689, which may account for the differential responses observed (Fig. S1E).
Fig. S2.
Differential effect of proteotoxic stress and ER stress on NKRF gene expression. (A) HSF1 DNA-binding activity (gel-shift analysis, Top) and NKRF-mRNA levels (qPCR, Bottom) of whole-cell protein or RNA extracts from HeLa and HeLa-HSF1i cells treated with 25 nM bortezomib (+), 80 μM arsenite (+), or vehicle (–) for 8 h. (B) Extracts from HeLa and HeLa-HSF1i cells treated with 0.5 μM thapsigargin (+), 1.8 μM tunicamycin (+), or vehicle (–) for 8 h were processed as in A. HSF/DNA complexes (HSF), constitutive HSE-binding activity (CHBA), and nonspecific protein–DNA interactions (NS) are indicated. Fold induction was calculated by comparing the induction of the NKRF gene in treated samples to the relative control, which was arbitrarily set to 1.
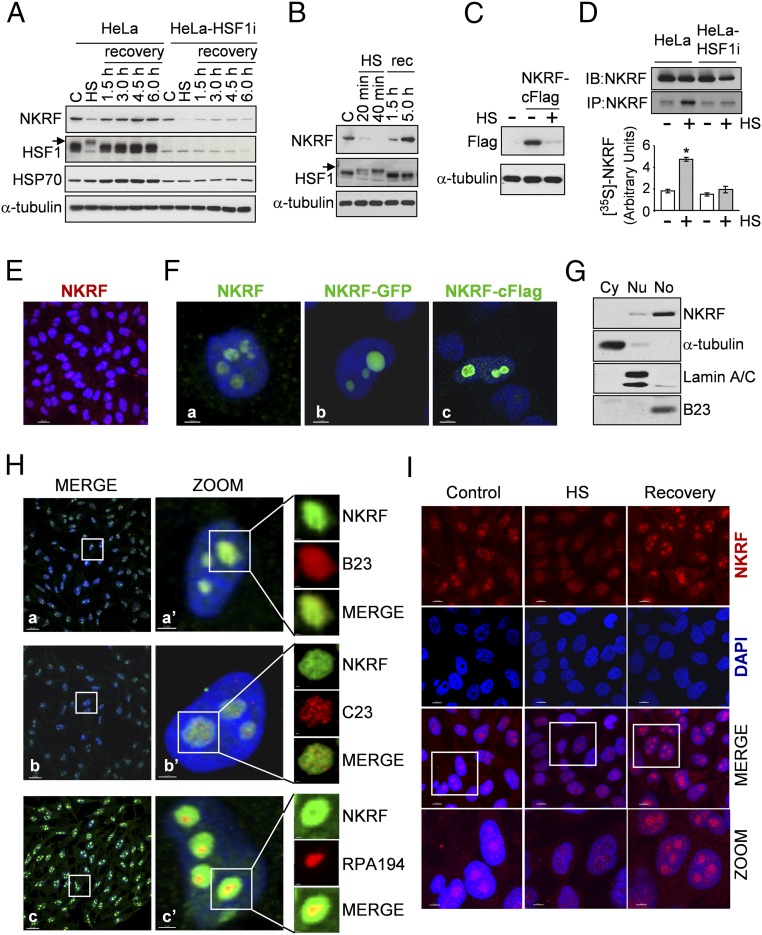
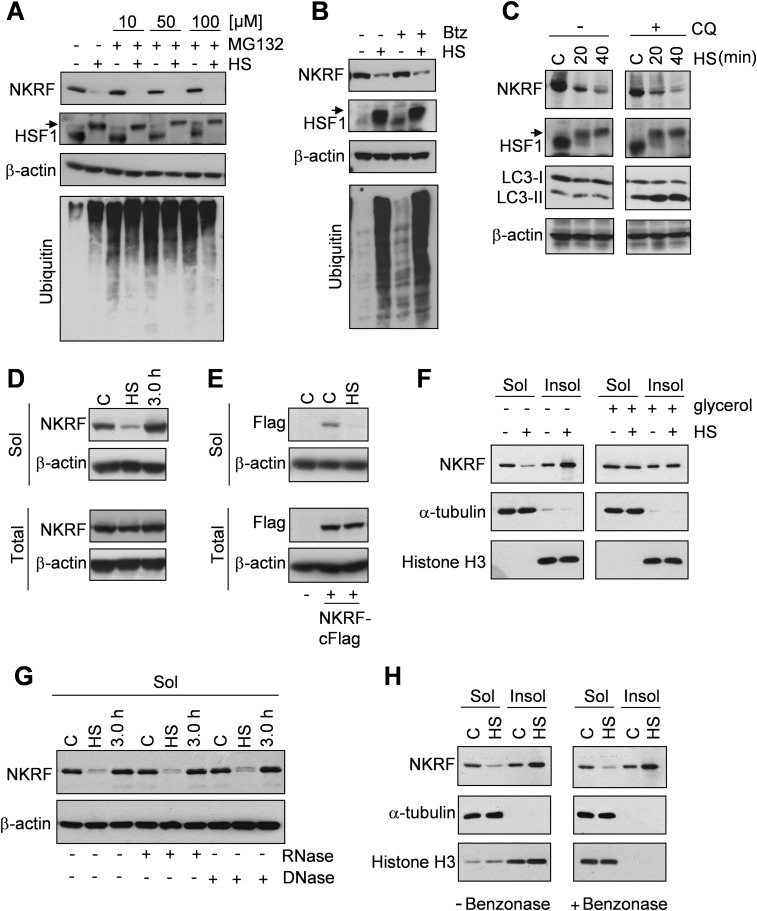
Endogenous or exogenous NKRF protein analysis surprisingly revealed that NKRF levels decreased during heat stress (Fig. 2 A–C and Fig. S3A) to recover thereafter, an effect largely due to newly synthesized NKRF accumulation (Fig. 2D). Notably, NKRF-mRNA contains a type-I Internal Ribosome Entry Site (IRES), allowing cap-independent translation under stress conditions (19). NKRF synthesis was not detected in HSF1i-silenced cells (Fig. 2 A and D). NKRF reduction during heat stress was not prevented by proteasome or autophagy inhibitors (Fig. S4 A–C), suggesting an effect independent of proteasome- or autophagy-mediated degradation; instead, we found that NKRF is heat-sensitive, converting from a soluble to insoluble state selectively during heat stress (Fig. S4 D and E) but not after arsenite- or bortezomib-induced proteotoxic stress or ER stress (Fig. S3 B and C) and was rescued by tertiary-structure stabilizer glycerol during heat exposure (Fig. S4F). One explanation for these observations is that NKRF may undergo temperature-induced conformational changes and/or become associated with specific structures following heat treatment. NKRF binds to DNA (10) and RNA (20); however, RNase, DNase, and benzonase treatment did not restore soluble NKRF levels (Fig. S4 G and H).
Fig. 2.
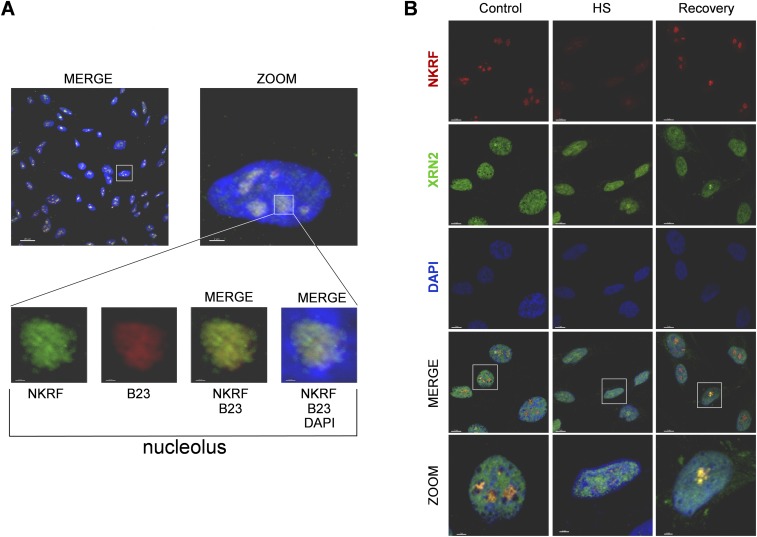
NKRF dynamic nucleolar–nuclear trafficking and synthesis in response to heat. (A and B) NKRF, HSF1, HSP70, and α-tubulin levels from HeLa and HeLa-HSF1i cells exposed to HS (43 °C, 40 min) and recovered (rec) at 37 °C for the indicated times. Arrows indicate hyperphosphorylated HSF1. NKRF levels decrease during HS, due to protein insolubilization (described in Fig. S4). (C) Flag-tagged NKRF levels from NKRF-cFlag–expressing HeLa cells after HS (40 min). (D, Top) IB (IB:NKRF) and autoradiography of immunoprecipitated endogenous NKRF (IP:NKRF) from HeLa and HeLa-HSF1i cells [35S]methionine-labeled (3 h pulse) during recovery after HS. (Bottom) [35S]-NKRF quantification. Error bars indicate ±SD. *P < 0.05. (E and F) NKRF nucleolar localization detected by immunoconfocal microscopy in nontransfected (E and F, a), NKRF-GFP–transfected (F, b), or NKRF-cFlag–transfected (F, c) HeLa cells. Nuclei are stained with DAPI (blue). [Scale bars, 20 μm (E) and 2 μm (F).] (G) IB of NKRF in HeLa cytoplasmic (Cy), nuclear (Nu), and nucleolar (No) fractions. α-Tubulin, lamin-A/C, and nucleophosmin/B23 proteins were used as cytoplasm, nucleus, and nucleolus markers. (H) Confocal images of NKRF (green) distribution in nucleolar compartments. Merge (a, b, and c) and zoom (a’, b’, and c’) images are shown. Markers for granular (nucleophosmin/B23), dense-fibrillar (nucleolin/C23), and fibrillar-center (Pol-I RPA194 subunit) compartments are indicated. [Scale bars, 20 μm (zoom, 2 μm; nucleoli, 0.5 μm).] (I) Confocal images of NKRF (red) localization in HeLa cells untreated (control), heat stressed (40 min, HS), or recovered 3 h at 37 °C. [Scale bar, 10 μm (zoom, 5 μm).]
Fig. S3.
NKRF is a thermolabile protein in human primary and cancer cells. (A) IB for NKRF and α-tubulin of WCEs from human HeLa, MDA-MB-231 breast adenocarcinoma and M10 melanoma cell lines, and primary HUVECs and peripheral blood monocytes untreated (C) or subjected to heat stress (HS, 43 °C, 40 min) and allowed to recover at 37 °C for the indicated times. (B and C) IB analysis for NKRF, HSP70, GRP78, and α-tubulin of WCEs from HeLa cells untreated (C) or treated with proteotoxic stress inducers arsenite (80 μM) or bortezomib (25 nM) (B) or ER stress inducers thapsigargin (0.5 μM) or tunicamycin (1.8 μM) (C) for the indicated times.
Fig. S4.
Heat stress causes NKRF protein insolubilization. (A and B) IB analysis for NKRF, HSF1, ubiquitin, and β-actin in WCEs in buffer B from HeLa cells treated with proteasome inhibitor MG132 at different concentrations (A) and bortezomib 25 nM (Btz, B) or vehicle (–) and heat stressed at 43 °C for 40 min (+HS) or kept at 37 °C (–HS). Proteasome inhibitor treatment was started 30 min before HS. (C) IB analysis for NKRF, HSF1, LC3 I/II, and β-actin of WCEs from HeLa cells treated with autophagy inhibitor chloroquine (CQ) 20 μM and heat stressed at 43 °C for 20 or 40 min (HS) or left untreated (C). CQ treatment was started 45 min before HS. (A–C) Arrows indicate the hyperphosphorylated form of HSF1. (D and E) IB analysis for NKRF, Flag-NKRF, and β-actin in HeLa cells (D) or HeLa cells transiently (24 h) transfected with NKRF-cFlag (E) heat stressed at 43 °C for 40 min (HS) and allowed to recover for 3 h (D). Soluble fractions of cell lysates extracted with buffer B (Sol) or total cell lysates from parallel samples extracted with Laemmli sample buffer (Total) are indicated. (F) IB for NKRF, α-tubulin, and histone H3 of soluble (Sol) and insoluble (Insol) fractions of WCEs extracted with buffer B from HeLa cells treated with 1 M glycerol (+) or vehicle (–) and heat stressed at 43 °C for 40 min (+HS) or left untreated (–HS). Insoluble fractions were processed as described in Materials and Methods. Treatment with glycerol was started 45 min before HS. (G) IB analysis for NKRF of HeLa cells treated as in D. Soluble fractions of cell lysates extracted with buffer B were subjected to dialysis and then digested with DNase or RNase (+) for 1 h. (H) IB for NKRF, α-tubulin, and histone H3 from HeLa cells untreated (C) or heat stressed (HS) as in A. Cell lysates extracted with buffer B were diluted, digested with benzonase, and processed as described in Materials and Methods. Soluble (Sol) and insoluble (Insol) fractions were separated and analyzed.
To investigate whether heat causes intracellular redistribution of the factor, NKRF localization was determined by cell fractionation and confocal immunomicroscopy studies. Under normal conditions, endogenous or exogenous NKRF is predominantly localized in nucleoli of human cells (Fig. 2 E–G and Fig. S5), as described in murine cells (20). In HeLa cells, NKRF is distributed throughout the three structural nucleolar compartments: fibrillar and dense-fibrillar centers, where rRNA transcription and posttranscriptional maturation occurs, and granular component, the site of final rRNA processing and preribosomes assembly (21) (Fig. 2H). Interestingly, heat exposure caused NKRF relocalization to the nucleoplasm; however, NKRF nucleolar levels were completely restored after 3 h of recovery (Figs. 2I and 3A and Fig. S5B).
Fig. S5.
NKRF/XRN2 nucleolar–nuclear trafficking in response to heat in human breast cancer cells. (A) NKRF nucleolar localization in MDA-MB-231 cells. Confocal images of NKRF (green) and nucleolar marker nucleophosmin/B23 (red) distribution in MDA-MB-231 cells. Nuclei are stained with DAPI (blue). Merge and zoom images are shown. [Scale bar, 20 μm (zoom, 2 μm; nucleoli, 0.5 μm).] (B) Confocal images of MDA-MB-231 cells untreated (Control), heat stressed for 40 min (HS), or allowed to recover at 37 °C for 3 h, stained for NKRF (red), XRN2 (green), and DNA (DAPI, blue). Colocalization is shown in merge and zoom. [Scale bar, 7 μm (zoom, 2 μm).]
Fig. 3.
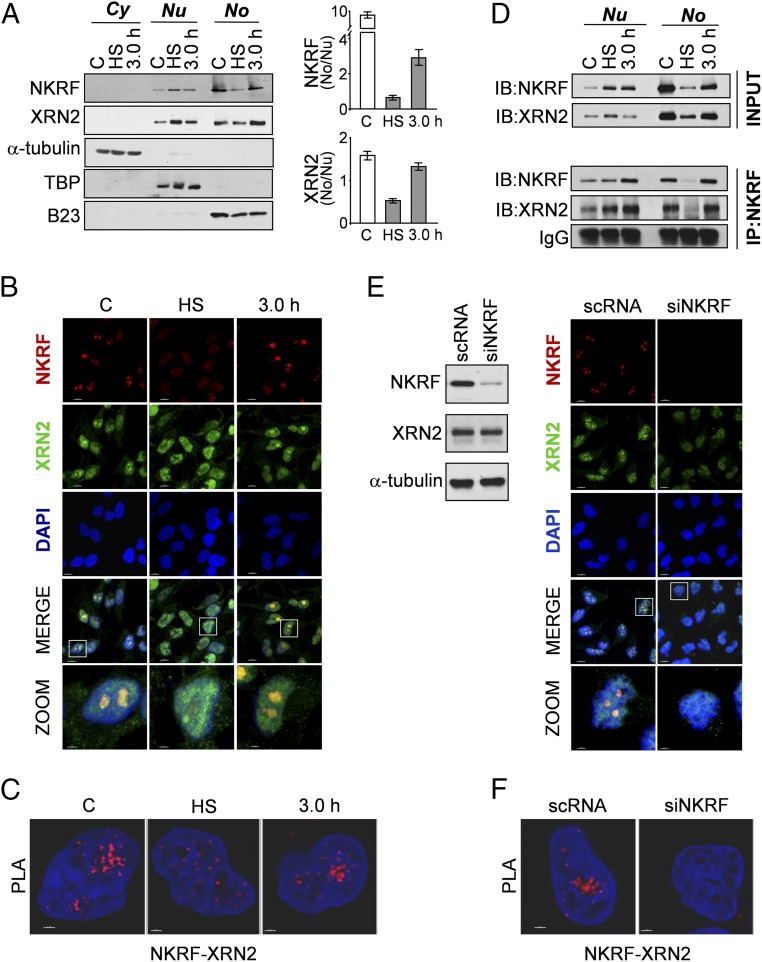
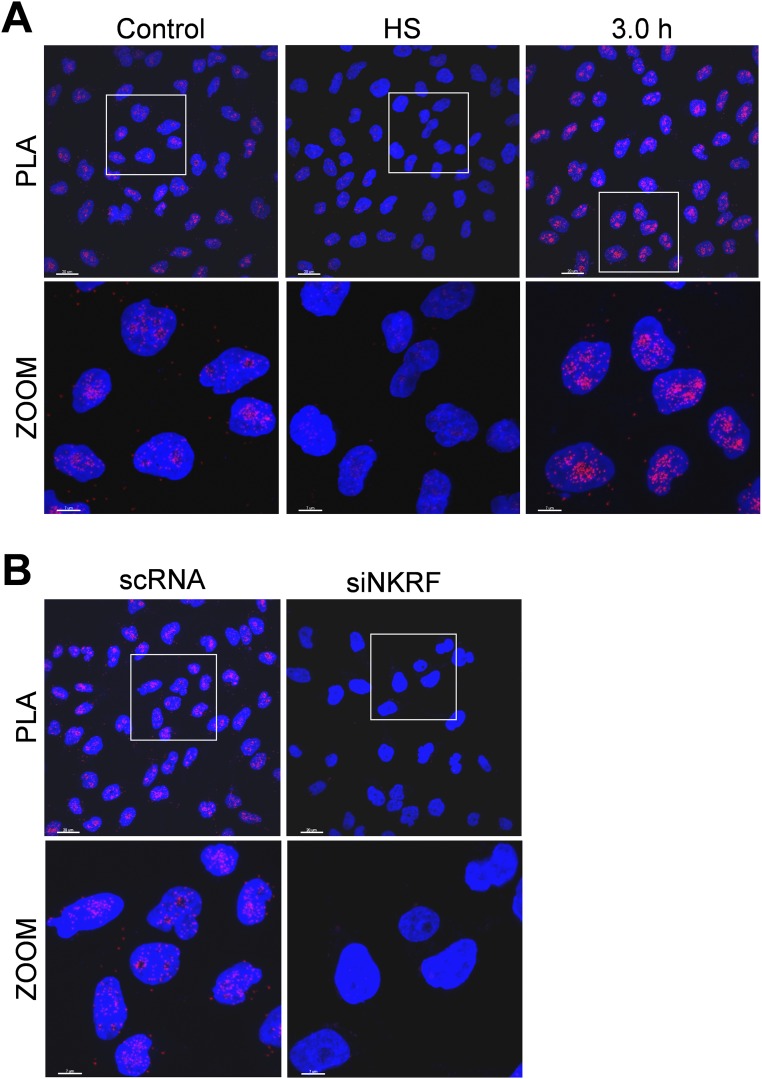
NKRF interacts with 5′-to-3′ exoribonuclease XRN2 and controls XRN2 nucleolar localization. (A) IB of NKRF and XRN2 levels in cytoplasmic (Cy), nuclear (Nu), and nucleolar (No) fractions of HeLa cells untreated (C), heat stressed (40 min, HS), or recovered 3 h at 37 °C (Left). Cytoplasm (α-tubulin), nucleus (TATA-binding protein, TBP), and nucleolus (B23) markers are indicated. (Right) Quantification of NKRF and XRN2 No/Nu ratios in the same samples, as described in Materials and Methods. (B) Confocal images of HeLa cells treated as in A stained for NKRF, XRN2, and DNA (DAPI). Colocalization is shown in merge and zoom. [Scale bar, 7 μm (zoom, 2 μm).] (C) NKRF–XRN2 interactions (visualized as red spots) detected by PLA in samples treated as in B. (Scale bar, 2 μm.) (D, Bottom) Co-IP of NKRF and XRN2 in nuclear (Nu) and nucleolar (No) fractions of HeLa cells treated as in A. IgG are shown as control. Antibodies for IP and IB analyses are indicated. Relative inputs are shown (Top). (E, Right) Confocal images of HeLa cells transfected (48 h) with scramble-RNA (scRNA) or NKRF-siRNA (siNKRF) and stained as in B. (Left) IB for NKRF, XRN2, and α-tubulin of parallel samples. [Scale bar, 7 μm (zoom, 2 μm).] (F) NKRF–XRN2 interactions detected by PLA in samples described in E. (Scale bar, 2 μm.)
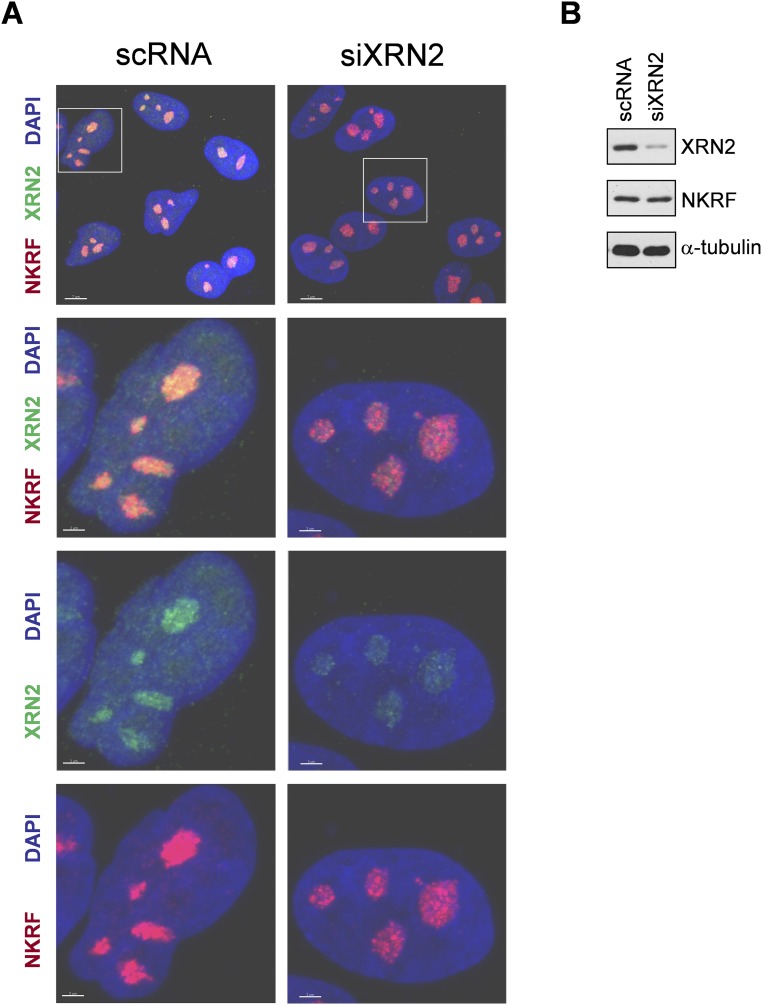
NKRF is known to bind specific DNA sequences in several NF-κB–regulated genes and to interact with NF-κB/p65, differentially controlling NF-κB–driven transcription under basal and/or stimulated conditions (10–14). Therefore, proteotoxic stress-induced NKRF expression and dynamic nucleolar–nuclear movement may contribute to NF-κB–driven transcription regulation, with important implications in inflammation and cancer. However, as NKRF was mainly localized in human nucleoli, organelles emerging as important players in the recovery from stress (22), we focused our attention on its nucleolar function during heat stress. The recently described sequence homology of NKRF with Caenorhabditis elegans PAXT-1 protein (23), known to interact and stabilize the 5′-to-3′ exoribonuclease XRN2 (24, 25), prompted us to investigate a possible NKRF/XRN2 interaction in human nucleoli. Cell fractionation, immunoprecipitation (IP), proximity ligation assay (PLA), and confocal immunomicroscopy colocalization studies clearly show that NKRF interacts with XRN2 in human nucleoli under normal conditions (Fig. 3). During heat stress XRN2 follows NKRF fate, transiently redistributing to the nucleoplasm during heat exposure and returning to nucleoli after 3 h of recovery (Fig. 3 A–D and Figs. S5B and S6A). Interestingly, XRN2 was unable to relocalize to the nucleolus in NKRF-silenced cells (Fig. 3 E and F and Fig. S6B), whereas NKRF nucleolar localization was not affected by XRN2 silencing (Fig. S7), indicating that NKRF directs XRN2 nucleolus/nucleoplasm trafficking.
Fig. S6.
NKRF interacts with 5′-to-3′ exoribonuclease XRN2. (A) NKRF–XRN2 interactions (visualized as red spots) detected by PLA in HeLa cells untreated (Control), heat stressed at 43 °C for 40 min (HS), or recovered 3 h at 37 °C (3.0 h). [Scale bar, 20 μm (zoom, 7 μm).] (B) NKRF–XRN2 interactions (visualized as red spots) detected by PLA in HeLa cells transfected (48 h) with scRNA or NKRF-siRNA (siNKRF). [Scale bar, 20 μm (zoom, 7 μm).]
Fig. S7.
XRN2 silencing does not affect NKRF nucleolar localization. (A) Confocal images of HeLa cells transfected (48 h) with scRNA or XRN2-siRNA (siXRN2) and stained for NKRF (red), XRN2 (green), and DNA (DAPI, blue). Merge and zoom images are shown. [Scale bar, 7 μm (zoom, 2 μm).] (B) IB for XRN2, NKRF, and α-tubulin of parallel samples.
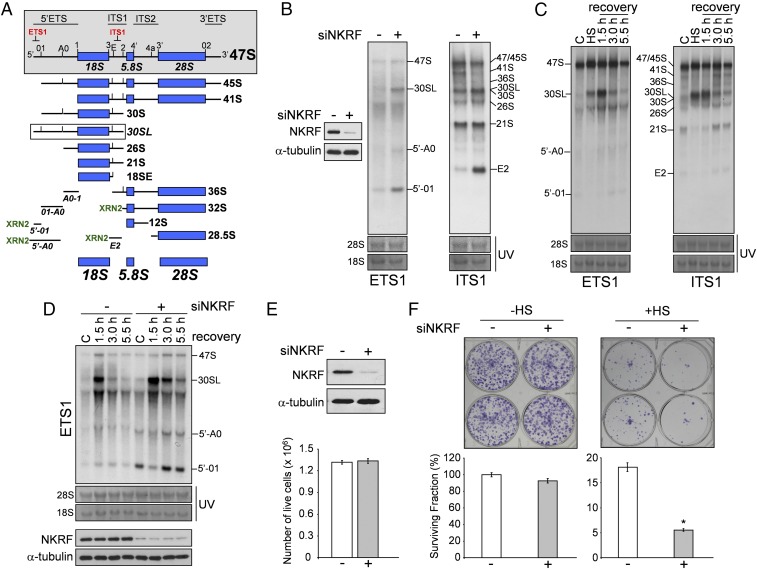
In mammalian cells, the main structural ribosome components (small-subunit 18S rRNA and large-subunit 5.8/28S rRNAs) are transcribed by RNA polymerase-I (Pol-I) from 300 to 400 rDNA head-to-tail tandem repeats as a single large polycistronic precursor, 47S pre-rRNA, that contains internal (ITS1, ITS2) and external (5′ETS, 3′ETS) transcribed spacers (Fig. 4A) (26–28). rRNA biogenesis is a highly energy-consuming process requiring a complex series of endonucleolytic cleavages within spacers–regions followed by exonucleolytic trimming to form mature rRNA 5′ and 3′ ends (26). This process was mainly characterized in yeast and, surprisingly, only recently investigated in human cells, revealing that pre-rRNA processing pathways are notably different in metazoan: ∼27% of human factors have distinct or additional functions in pre-rRNA processing compared with their yeast orthologs, and several pre-RNA processing factors have no yeast homolog (26, 29).
Fig. 4.
NKRF is essential for XRN2-driven pre-rRNA processing and aberrant fragment turnover. (A) Schematic representation of human 47S pre-rRNA processing. ETS,ITS: external- and internal-transcribed spacers. The 47S pre-rRNA cleavage sites and oligonucleotides for Northern hybridization (red) are indicated (gray box). Aberrant 30SL pre-rRNA, accumulated during 01-processing inhibition, is shown (white box). Steps requiring XRN2 exonuclease activity are shown (XRN2); discarded fragments 5′-01, 5′-A0, and E2 are indicated. (B) Northern blot of HeLa cells transfected (48 h) with scRNA (–) or NKRF-siRNA (+). rRNA intermediates and discarded fragments recognized by ETS1 or ITS1 probes are indicated. Hybridizations were performed on the same membrane after stripping and reprobing. The same 28S and 18S loading control is shown for ETS1 and ITS1 probes. IB of NKRF in the same samples is shown. (C) Northern blot (ETS1,ITS1 probes) of HeLa cells heat stressed (HS, 43 °C, 40 min) and recovered at 37 °C for the indicated times. Hybridizations were performed on the same membrane after stripping and reprobing. The same 28S and 18S loading control is shown for ETS1 and ITS1 probes. (D, Top) Northern blot (ETS1 probe) of cells transfected (48 h) with scRNA or NKRF-siRNA and treated as in C. (Bottom) IB of NKRF in the same samples. (E and F) NKRF silencing enhances cell sensitivity to heat. IB for NKRF and α-tubulin in HeLa cells transfected for 48 h with scramble RNA (–) or NKRF-siRNA (+); live cell numbers in the same samples are shown (E). HeLa cells described in E were left untreated (–HS) or subjected to sublethal hyperthermic treatment (43 °C, 120 min) (+HS); cell survival was analyzed by clonogenic assay after 10 d (F). Representative images (Top) and quantification of clonogenic assay (Bottom) are shown. Data are expressed as percentage of surviving fractions. Error bars indicate ±SD. *P < 0.01.
XRN2 (homolog of yeast XRN2/Rat1) plays a major role in rRNA maturation, coordinating the optimal order of multiple pre-rRNA cleavages (25); it is essential for degradation of 5′-extended 45.5S and 34.5S pre-rRNAs forms, it removes ITS-1–derived extensions to generate 32S from 32.5S pre-rRNA, and it promotes decay of 5′-01, 5′-A0, and E2 fragments, generated by endonucleolytic cleavages in the 5′ETS/ITS1 region in human cells (27–29) (Fig. 4A). As the pre-rRNA transcription rate is high, discarded fragment decay is important for nucleolar homeostasis; in addition to nucleolar toxicity, aberrant pre-rRNA and excised spacer-region turnover is also important for cellular nucleotide level maintenance (25).
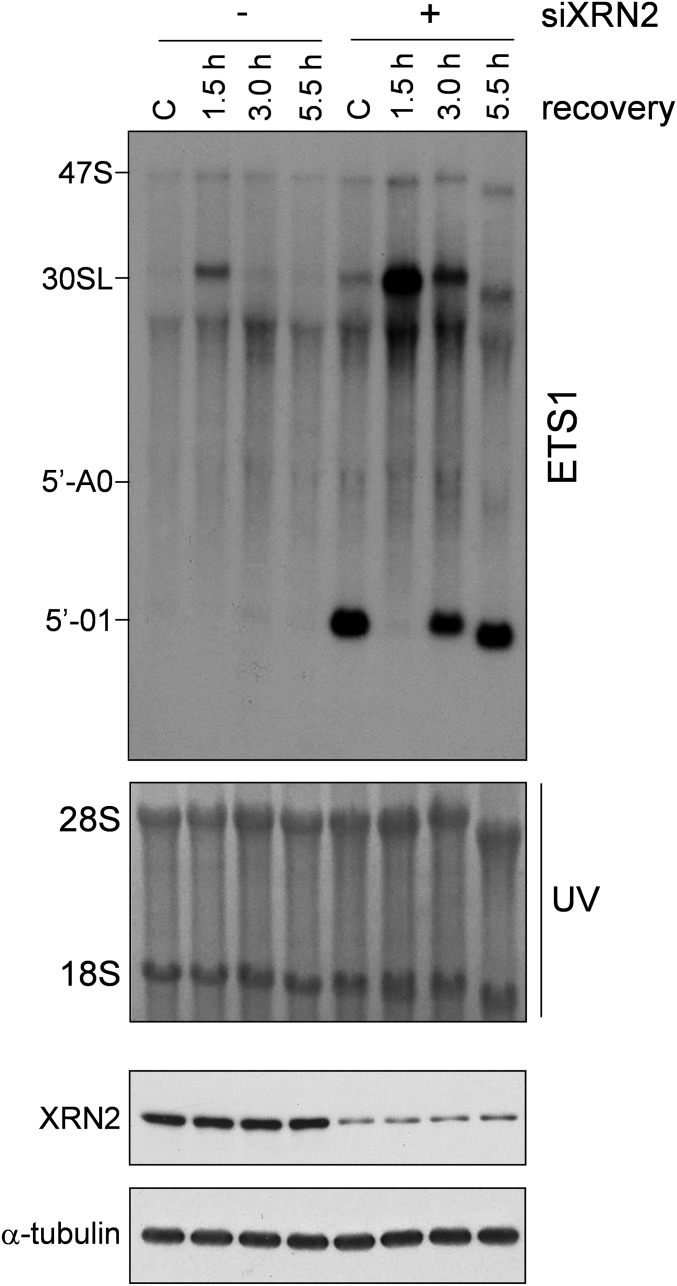
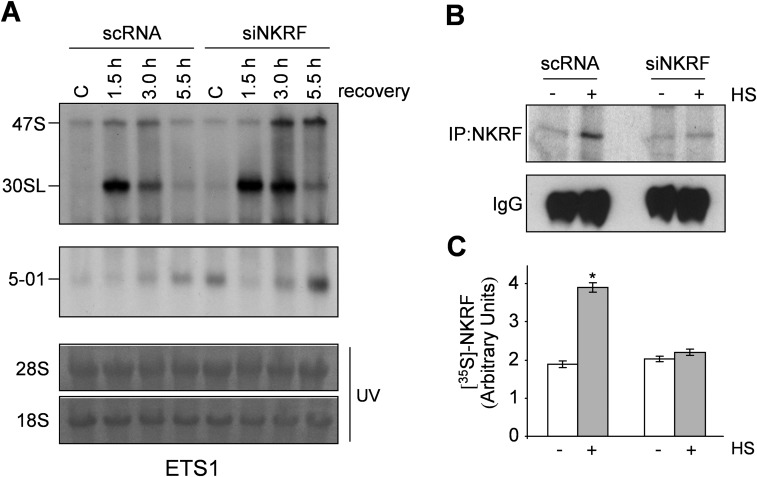
We postulated that NKRF may participate in rRNA processing via XRN2 interaction and investigated the effect of NKRF silencing on 5′-ETS and ITS1 pre-rRNA regions. NKRF silencing suppressed 45S/47S pre-rRNA processing and resulted in accumulation of aberrant 30SL pre-rRNA and 5′-01, 5′-A0, and E2 fragments (Fig. 4B). Next we investigated rRNA processing during heat stress, a phenomenon still poorly characterized in human cells. Heat exposure (43 °C, 40 min) caused transient halting of rRNA processing characterized by accumulation of 30SL precursor and XRN2 target fragments, with rRNA processing returning to normal functions at 3 h of recovery (Fig. 4 C and D), parallel to the kinetics of NKRF-driven XRN2 nucleolus–nucleoplasm trafficking (Fig. 3). Interestingly, recovery was impaired in NKRF-silenced cells, where accumulation of 30SL precursor and 5′-01 and 5′-A0 discarded fragments was evident for several hours after stress (Fig. 4D). XRN2 silencing resulted in rRNA processing alterations comparable to NKRF silencing during heat stress (Fig. S8). In addition, transient (14 h) NKRF silencing, preventing NKRF resynthesis after heat stress, was sufficient to impair rRNA processing recovery (Fig. S9), indicating an important role of newly synthesized NKRF for reestablishment of rRNA metabolism. These results are summarized in the NKRF cycle model proposed in Fig. S10.
Fig. S8.
XRN2 silencing results in pre-rRNA processing alterations during heat stress. (Top) Northern blot (ETS1-probe) of HeLa cells transfected (48 h) with scRNA (–) or XRN2-siRNA (+), kept at 37 °C (C) or heat stressed (43 °C, 40 min) and recovered at 37 °C for the indicated times. The 30SL rRNA intermediate and discarded fragments 5′-01 and 5′-A0 recognized by the ETS1 probe are indicated. (Bottom) IB of XRN2 in the same samples is shown.
Fig. S9.
Heat-induced NKRF expression is necessary to restore pre-rRNA processing after stress. (A) Northern blot (ETS1-probe) of HeLa cells transfected for 14 h with scRNA or NKRF-siRNA (siNKRF) and kept at 37 °C (C) or subjected to heat stress (43 °C, 40 min) and allowed to recover at 37 °C for the indicated times. (B) Autoradiography of immunoprecipitated endogenous NKRF (IP:NKRF) labeled with [35S]methionine for 3 h during recovery after HS (43 °C, 40 min) in parallel samples. IB of IgG in the same samples is shown. (C) Quantification of neo-synthesized NKRF. Error bars indicate ±SD. *P < 0.01.
Fig. S10.
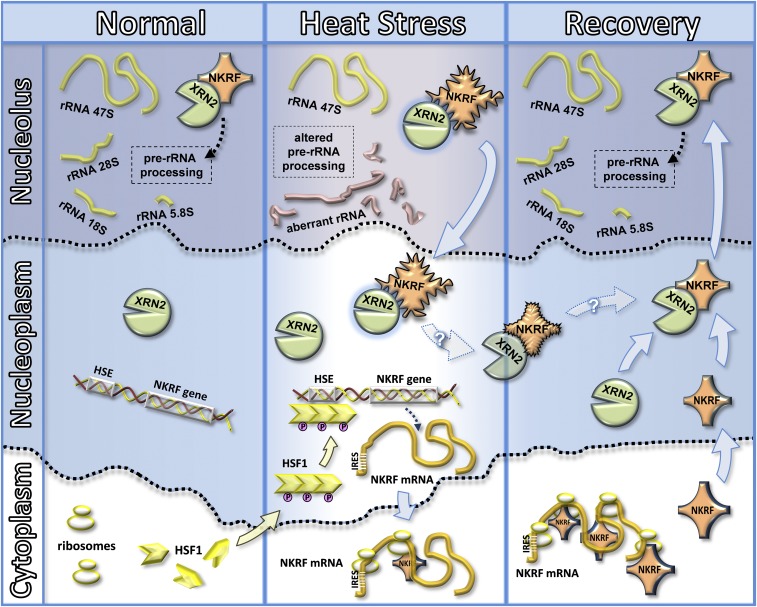
The NKRF cycle. Shown is the proposed model depicting an HSF1-dependent strategy to restore nucleolus homeostasis after heat stress in human cells. Under normal conditions NKRF is mainly localized in the nucleolus of human cells, where it regulates the processing of the rRNA 47S-precursor leading to mature 28S, 18S, and 5.8S rRNA formation by interacting with 5′-to-3′ exoribonuclease XRN2. Upon heat stress, NKRF undergoes conformational changes and translocates to the nucleoplasm. The NKRF–XRN2 interaction is maintained under stress conditions, leading to XRN2 exit from the nucleolus, consequently halting rRNA processing and provoking aberrant rRNA precursors and discarded fragment accumulation. During stress, HSF1, which is present as an inactive monomer in the cytoplasm under normal conditions, oligomerizes to a trimeric state, undergoes inducible phosphorylation, translocates to the nucleoplasm, and binds to HSEs in the NKRF promoter, rapidly triggering NKRF-mRNA transcription. The presence of a type-I IRES in NKRF-mRNA 5′UTR allows cap-independent translation under stress conditions. During recovery, the newly synthesized NKRF recruits nucleoplasmic XRN2, and the complex relocates to the nucleolus, restoring normal rRNA processing and digestion of discarded fragments. Refolded NKRF may also contribute to restoring nucleolar homeostasis (dotted arrows with question mark).
Notably, mammalian XRN2 is also present in the nucleoplasm and is implicated in Pol-II transcription termination, intron degradation, and pre-RNA and microRNA metabolism (24). Because NKRF was lately implicated in XRN2 function in the nucleoplasm (30), our results suggest the intriguing possibility that proteotoxic stress may also affect these other aspects of RNA metabolism via NKRF–XRN2 trafficking control.
As ribosome biogenesis is directly linked to protein synthesis and therefore to cell proliferation and survival control (31), we postulated that NKRF silencing may sensitize cancer cells to heat stress. In fact, whereas transient (48 h) NKRF silencing did not affect the viability of HeLa cells under nonstress conditions, it greatly hindered their ability to recover from sublethal heat stress (Fig. 4 E and F), indicating an important role of NKRF in cell survival after proteotoxic stress. NKRF was reported to participate in pancreatic cancer growth control via NF-κB regulation (32); however, because dysregulated rRNA synthesis is common in cancer cells (31), the NKRF rRNA controlling function described in this study may inspire novel therapeutic strategies against cancers addicted to accelerated ribosome biogenesis.
Our study identifies NKRF as a stress protein acting as a guardian of rRNA biogenesis and nucleolus homeostasis in human cells. We propose that NKRF is part of a dynamic nucleolar multitasking protein network contributing to orchestrate nuclear functions under stress conditions.
Materials and Methods
The establishment of HeLa cells stably transfected with pSUPER-HSF1i/pcDNA (HeLa-HSF1i) or control (HeLa wild-type) plasmids was described previously (7). All cell lines; culture conditions; source of antibodies, reagents, and plasmids; and methods are described in SI Materials and Methods and Table S1.
Table S1.
Oligonucleotide sequences used
| Primers used for constructs generation | ||
| Construct | Primer direction | Primer sequence |
| cFlag-NKRF-pcDNA3 | Forward | 5′-TCCCAAGCTTCCACCATGGAAAAAATTCTCCAAATGCCT-3′ |
| Reverse | 5′-CGCGGATCCCTACTTATCGTCGTCATCCTTGTAATCATTTGCTTGAGGCATAACAAGCTCG-3′ | |
| NKRF-pGL3-WT | Forward | 5′-CGGGGTACCGGCTATTTCTCCCCACCAAG-3′ |
| Reverse | 5′-TCCCAAGCTTCTAACCCGACACCCACACCT-3′ | |
| NKRF-PGL3-M473-HSE1 | Forward | 5′-AGAGGAGGGGAGCATAGCGTGCTGGGGTC-3′ |
| Reverse | 5′-GACCCCAGCACGCTATGCTCCCCTCCTCT-3′ | |
| NKRF-PGL3-M688-HSE2 | Forward | 5′-TCTCGAACCTCAGATAACCTTCCTAAATT-3′ |
| Reverse | 5′-AATTTAGGAAGGTTATCTGAGGTTCGAGA-3′ | |
| Primers used for mRNA gene expression | ||
| Gene | Primer direction | Primer sequence |
| Human NKRF | Forward | 5′-CCAAACCTTCCAAAGGTCAA-3′ |
| Reverse | 5′-CAGGGTTCCCACTGTCAAAA-3′ | |
| Human HSP701A | Forward | 5′-TGGAGTCCTACGCCTTCAAC-3′ |
| Reverse | 5′-TGAATTCTCAGCCCTCTTCAA-3′ | |
| Human β-actin | Forward | 5′-GCGCTCAGGAGGAGCAAT-3′ |
| Reverse | 5′-GCACTCTTCCAGCCTTCC-3′ | |
| Human GAPDH | Forward | 5′-GTCATCAATGGAAATCCC-3′ |
| Reverse | 5′-GGTGGTGCAGGAGG-3′ | |
| Mouse β-actin | Forward | 5′-ACTGGGACGACATGGAGAAG-3′ |
| Reverse | 5′-TTTGATGTCACGCACGATTT-3′ | |
| Mouse NKRF | Forward | 5′-GCCAAAAACGCTACCTTTCA-3′ |
| Reverse | 5′-GCAACCAAGGACTCAGGGTA-3′ | |
| Primers used for ChIP | ||
| Gene/promoter | Primer direction | Primer sequence |
| NKRF | Forward | 5′-CGCTTAAAAATCCAGGGAAA-3′ |
| Reverse | 5′-TTTTTCCCTCCAGTGACGAC-3′ | |
| HSP701A | Forward | 5′-CACTCCCCCTTCCTCTCAG-3′ |
| Reverse | 5′-TTCCCTTCTGAGCCAATCAC-3′ | |
| siRNA sequences | ||
| siRNA | Source | Target sequence |
| si-NKRF1 | This study | 5′-AACCGAATGACAGTTGAGTAT-3′ |
| si-NKRF2 | This study | 5′-GTGCTGTCCAAACCTTCCAAA-3′ |
| si-XRN2 | 27 | 5′-CAGGGAAGAAATATTGGCAAA-3′ |
| Northern blot probe sequences | ||
| Probe | Source | Sequence |
| ITS1 | 27 | 5′-AGGGGTCTTTAAACCTCCGCGCCGGAACGCGCTAGGTAC-3′ |
| ETS1 | 39 | 5′-TCGGACGCGCGAGAGAACAGCAGG-3′ |
SI Materials and Methods
Cell Culture and Treatments.
HeLa cells stably transfected with pSUPER-HSF1i/pcDNA (HeLa-HSF1i) or control (HeLa wild-type) plasmids were described previously (7). Human melanoma derived from metastatic nodules (M10) (kindly provided by G. Zupi, Regina Elena Cancer Institute, Rome, Italy), breast adenocarcinoma (MDA-MB-231), colorectal carcinoma (HCT116) and T-lymphoma (Jurkat) cells, and human keratinocytes (HaCaT) (American Type Culture Collection) were maintained in DMEM (MDA-MB-231 and HaCaT), McCoy’s 5 A (HCT116), or RPMI 1640 (M10, Jurkat) medium supplemented with 10% (vol/vol) FCS, 2 mM glutamine, and antibiotics. Primary human umbilical vein endothelial cells (HUVECs) (Cambrex Bio Science) were grown in EGM-2 complete medium (Cambrex Bio Science) according to the manufacturer’s instructions; all experiments were performed using HUVEC passages 2–5. Human peripheral blood monocytes, isolated and purified from buffy coats of healthy blood donors (kindly provided by G. Adorno, University of Rome Tor Vergata, Rome, Italy) as described elsewhere (33), were grown for 24 h in RPMI 1640 medium supplemented with 10% (vol/vol) FCS and antibiotics as indicated above. Murine embryonic fibroblasts (MEFs) (6) were cultured in DMEM supplemented with 10% (vol/vol) FCS, 2 mM glutamine, and antibiotics. For heating procedures, cells were subjected to heat shock at the indicated temperatures in a precision water bath W14 (Grant Instruments). Actinomycin D (AMD), proteasome inhibitors MG132 and bortezomib, chloroquine, tunicamycin, and thapsigargin (Sigma-Aldrich) were dissolved in DMSO; arsenite was dissolved in water, and glycerol was added directly to the culture medium. Control cells received the same amount of vehicles.
Promoter Cloning, Vector Construction, and Mutagenesis.
To generate the NKRF–PGL3 wild-type vector (NKRF-pGL3-WT), a pair of gene-specific primers listed in Table S1 was designed to amplify the NKRF gene promoter region (spanning from –1402 upstream of the gene TSS to +192) from human genomic DNA (Promega) by using Phusion High-Fidelity DNA Pol (Finnzymes). The reaction product was analyzed by agarose gel electrophoresis, digested with HindIII and KpnI, and inserted upstream of the luciferase gene of the pGL3-Basic vector (Promega) to generate the NKRF-pGL3-WT construct. The NKRF-PGL3-M473-HSE1 (M473) and NKRF-PGL3-M688-HSE2 (M688) mutant constructs were generated by using the QuikChange Site-Directed Mutagenesis kit following the manufacturer’s instructions (Stratagene) using the oligos listed in Table S1. To generate the cFlag-tagged NKRF-pcDNA3 vector, the human NKRF gene was amplified from the NKRF cDNA (human) clone ID 5228666 (Open Biosystems) by using primers described in Table S1. The PCR product was digested with HindIII and BamHI and inserted into a HindIII/BamHI-cut pcDNA3 vector. The nucleotide sequence of each construct was verified by DNA sequencing. Sequence logo of consensus motif for HSF1 (Fig. 1E) was generated by the program WebLogo (34) using previously known HSF1 binding sites as found in TRANSFAC (35).
Cell Transfection and Reporter Assays.
All transfections were performed using FuGENE HD Transfection Reagent (Roche) according to the manufacturer’s protocols. For reporter gene experiments, the different NKRF–PGL3 constructs were cotransfected with a control plasmid (pRL-TK encoding Renilla luciferase Promega) to normalize transfection efficiency. Transfected cells were grown for 16 h before heat treatment. Luciferase activity of quadruplicate samples was measured in a Microplate Luminometer (Wallac-Perkin-Elmer) using Dual-Luciferase kit (Promega). NKRF promoter firefly luciferase activity was normalized to Renilla luciferase activity in the same sample.
Protein Analysis, Metabolic Labeling, IP, and Western Blot.
Whole-cell extracts (WCEs) were prepared after lysis in high-salt extraction buffer (buffer B) [50 mM Tris·HCl, pH 7.5, 400 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 0.5% Nonidet P-40, and 10% (vol/vol) glycerol] supplemented with 2 mM DTT, 20 mM β-glycerolphosphate, 19 mM (p-Nitrophenyl Phosphate) PNPP, 2 mM Na3VO4, 1 mM PMSF, and protease inhibitor mixture (Roche) (17). Briefly, cells were washed twice with ice-cold PBS and then lysed in buffer B (90 μL/8 × 105 cells). After one cycle of freeze and thaw and centrifugation at 17,500 × g (15 min at 4 °C), supernatant (soluble) and pellet (insoluble) fractions were collected. Insoluble fractions were solubilized in 60 μL of buffer S (50 mM Tris·HCl, pH 8.5, 1% SDS, and protease inhibitors) by sonication on ice, using an ultrasonic UP50H processor (Hielscher) (40% amplitude; pulse mode, 6 × 10 s, 15 s pauses). Total extracts were obtained by lysing cells in Laemmli buffer followed by DNA shearing through a 28 1/2-gauge insulin syringe 10 times. For RNase and DNase digestion, after lysis in buffer B, extracts were dialyzed against buffer E [50 mM Tris·HCl, pH 7.5, 20 mM NaCl, 10 mM MgCl2, 0.3% Triton X-100, 0.15% Nonidet P-40, and 3% (vol/vol) glycerol] and digested with 10 μg/mL of RNase (Sigma-Aldrich) or with 100 U/mL DNase I (Invitrogen) for 1 h at 37 °C. After centrifugation at 17,500 × g (15 min at 4 °C), soluble fractions were used in Western blot analysis. For benzonase digestion, after lysis in buffer B, extracts were diluted 1:3 in buffer D (50 mM Tris·HCl, pH 7.5, 2 mM MgCl2) and digested with 100 U/mL benzonase (Sigma-Aldrich) for 1 h at 37 °C; after centrifugation, soluble and insoluble fractions were collected, as above. Cytoplasmic, nucleoplasmic, and nucleolar extracts were prepared from equal numbers of cells (50 × 106 per sample), as described (36); in parallel, total extracts from 106 cells per sample were prepared in Laemmli buffer, as described above, for loading control. For Western blot analysis, aliquots of WCEs (25 μg) or cytoplasmic, nuclear, and nucleolar extracts (15 μg) were separated by SDS/PAGE and blotted to nitrocellulose. Primary and secondary peroxidase-labeled antibodies used are listed below. Detection was performed using the Super Signal detection kit (Pierce).
For IP, WCEs (200 μg) or nucleoplasmic and nucleolar extracts (150 μg) were precleared at 4 °C for 1 h with 40 μL of protein A agarose beads in 300 μL of buffer B (WCEs) or in 250 μL RIPA buffer (nucleoplasm and nucleolar extracts) and incubated overnight with anti-NKRF antibodies (Bethyl Laboratories Inc. A304-016A), followed by 2 h of incubation at 4 °C with protein A agarose beads. For NKRF IP, 2 μg of anti-NKRF per 200 μg of cell lysate were used. After extensive washing, immunocomplexes were analyzed by Western blot analysis. Aliquots of WCEs (25 μg) or aliquots of nucleoplasmic and nucleolar (15 μg) extracts were used as input. Quantitative evaluation of proteins was determined by Versadoc 1000 (Bio-Rad) analysis using the Quantity One software program (Bio-Rad Laboratories). For cell fractionation studies, nucleolar versus nucleoplasmic NKRF and XRN2 ratios were determined for each sample after normalization of NKRF or XRN2 nucleolar/nucleoplasmic levels to the total level of NKRF and XRN2 proteins in WCEs of the same sample prepared in Laemmli buffer.
For metabolic labeling experiments, HeLa and HeLa-HSF1i cells subjected to heat shock (43 °C, 40 min) were labeled with 100 μCi/mL of [35S]-methionine/cysteine ([35S]-Met/Cys, Redivue Pro-Mix 35S in vitro cell-labeling mix; GE Healthcare) for 3 h during the recovery period in Met/Cys-free medium. IP of [35S]-NKRF was performed in 200 μg WCEs as described above. Quantitative evaluation of [35S]-NKRF was determined by Typhoon 8600 imager (Molecular Dynamics, GE Healthcare) with the use of ImageQuant (GE Healthcare).
Antibodies.
For Western blot, the following antibodies were used: NKRF (1:5,000, Bethyl Laboratories Inc. A304-016A), XRN2 (1:5,000, Bethyl Laboratories Inc. A301-103A), HSF1 (H-311) (1:200, Santa Cruz Biotechnology, Inc. sc-9144), HSP70 (C92F3A-5) (1:1,000, Enzo Life Sciences, Inc. ADI-SPA-810), B23 (1:500, Sigma-Aldrich, Inc. B 0556), Lamin A/C (1:1,000, BD Biosciences, Inc. 612162), Ub (P4D1) (1:200, Santa Cruz Biotechnology, Inc. sc-8017), LC3B (D11) (1:1,000, Cell Signaling Technology, Inc. 3868), KDEL (10C3) (1:1,000, Enzo Life Sciences, Inc. ADI-SPA-810), TBP (1:2,000, Abcam, Inc. ab818), Histone-H3 (1:1,000, Abcam, Inc. ab1791), DYKDDDDK Tag (1:1,000, Cell Signaling Technology, Inc. 2368), α-tubulin (1:1,000, Sigma-Aldrich, Inc. T5168), β-actin (1:5,000, Sigma-Aldrich, Inc. A 2066), goat anti-mouse IgG (H+L) (1:5,000, Jackson ImmunoResearch, Inc. 115–035-003), and goat anti-rabbit IgG (H+L) (1:2,500, Thermo Fisher Scientific, Inc. 31460).
EMSA.
WCEs (15 μg of protein) prepared after lysis in buffer B (17) were incubated with a [32P]-labeled HSE DNA probe followed by analysis of DNA binding activity by electrophoretic mobility shift assay. Binding reactions were performed as described (15). Complexes were analyzed by nondenaturing 4% (wt/vol) polyacrylamide gel electrophoresis. Quantitative evaluation of HSF–HSE complex formation was determined by Typhoon 8600 imager with the use of ImageQuant.
RNA Extraction and Real-Time PCR.
Total RNA was extracted using TRIzol Reagent (Invitrogen) according to the manufacturer’s protocol. For RT-PCR analysis, extracted RNA (1 μg) was digested with two units of DNase I (Invitrogen) for 30 min at 37 °C. Samples were reverse-transcribed to cDNA with 200 units of Moloney murine leukemia virus reverse transcriptase (M-MLV RT) (Invitrogen) using 5 μg of random primers (Invitrogen) for 1 h at 45 °C in a total volume of 20 μL. RT was inactivated at 95 °C for 5 min. For each sample, an aliquot of DNase I-digested RNA, without RT, was used as a negative control for PCR amplification. Real-time PCR analyses were performed with specific primers for each gene, on a CFX96 (Bio-Rad), using SsoAdvanced Universal SYBR Green Supermix (Bio-Rad). Relative quantities of selected mRNAs were normalized to β-actin or GAPDH in the same samples. Primers are listed in Table S1.
siRNA Interference.
siRNA duplex sequences (listed in Table S1) and their scrambled control (scRNA) sequences were purchased from QIAGEN. Two siRNAs (siNKRF1 and siNKRF2) were used for NKRF silencing, whereas one siRNA (siXRN2) was used for XRN2. Transfections were performed using jetPRIME Transfection Reagent (Polyplus-transfection) according to the manufacturer’s instructions. Briefly, for long-term (48 h) NKRF or XRN2 silencing, cells were plated on 35-mm wells (1.5 × 105 cells per well) and, after 18 h, were transfected with 60 nM of the indicated siRNA or scrambled control. After 24 h, cells were washed twice with culture medium and transfection was repeated as above. For short-term (14 h) NKRF silencing, cells were plated at 6 × 105 cells per well and, after 24 h, were transfected with 60 nM of siRNA or scRNA. For heat stress experiments, at 14 h (short term) or 48 h (long term) after transfection, siRNAs and scRNAs were removed, and cells were washed twice with culture medium and subjected to heat stress (43 °C, 40 min) after 2 h. For HSF1 down-regulation, MDA-MB-231 cells were transiently transfected with pSUPER-HSFi or empty pSUPER vectors as described (7).
rRNA Analysis.
Total RNA was extracted using TRIzol Reagent (Invitrogen) according to the manufacturer’s instructions. Oligonucleotides (listed in Table S1) were 5′-labeled with γ-[32P]ATP using T4 polynucleotide kinase (BioLabs). For Northern blot analysis, 15 μg of RNAs were electrophoresed in an agarose/formaldehyde gel as described (37) and transferred to a Hybond N+ membrane (GE Healthcare). Membranes were prehybridized for 1 h at 45 °C in 10% (vol/vol) formamide, 6× SSC, SDS 0.2%, 2× Denhardt’s Solution, and 15 μg/mL tRNA (Sigma-Aldrich). [32P]-labeled probes were added and incubated for 12 h at 45 °C. After washing twice with nonstringent wash [2× SSC, SDS 2% (wt/vol)] and once with stringent wash [0.2× SSC, SDS 2% (wt/vol)] solutions at 45 °C, membranes were processed for autoradiography.
ChIP Assay.
Cells were fixed by adding formaldehyde (Sigma) to the medium to a final concentration of 1%. After 15 min, cells were washed with ice-cold PBS containing 1 mM phenylmethylsulfonyl fluoride, shaken for 20 min at 4 °C in lysis buffer 1 [50 mM Hepes–KOH, pH 7.5, 140 mM NaCl, 1 mM EDTA, 10% (vol/vol) glycerol, 0.5% Nonidet P-40, 0.25% Triton X-100, containing protease inhibitors] and harvested using a cell scraper. After centrifugation at 1,500 × g for 10 min, the pellet was resuspended in lysis buffer 2 (10 mM Tris⋅HCl, pH 8.0, 200 mM NaCl, 1 mM EDTA, 0.5 mM EGTA, containing protease inhibitors) and shaken at room temperature for 10 min. After centrifugation, nuclei were resuspended in lysis buffer 3 (50 mM Tris, pH 8.0, 1% SDS, 5 mM EDTA), and chromatin was sheared by sonication. After removal of nuclear debris by centrifugation at 15,000 × g for 5 min at 8 °C, lysates were diluted 10-fold with DB buffer (50 mM Tris, pH 8.0, 5 mM EDTA, 200 mM NaCl, 0.5% Nonidet P-40) and then precleared for 3 h using 80 μL of 50% (vol/vol) salmon sperm DNA saturated protein A (ssproteinA) agarose beads. IP was carried out at 4 °C overnight, and immune complexes were collected with ssproteinA agarose beads. Antibodies used included anti-HSF1 (Santa Cruz Biotechnologies Inc. sc-9144) or preimmune rabbit serum as a control for nonspecific interaction. After washing three times with high-salt WB buffer (20 mM Tris, pH 8.0, 0.1% SDS, 1% Nonidet P-40, 2 mM EDTA, 0.5 M NaCl) and twice with low-salt TE buffer (10 mM Tris, pH 8.0, 1 mM EDTA), immunocomplexes were eluted with TE containing 1% SDS. Protein–DNA cross-links were reverted by incubating at 65 °C overnight. After proteinase K digestion, DNA was extracted with phenol-chloroform and precipitated with ethanol using 15 μg of tRNA as carrier. PCR was performed (30 cycles, denaturing at 94 °C for 45 s, annealing at 60 °C for 30 s, and extension at 72 °C for 45 s) using the primers listed in Table S1.
Immunofluorescence Microscopy and PLA.
Cells grown on coverslips were fixed with 4% (wt/vol) paraformaldehyde (PFA) in PBS and permeabilized with PBS-TRITON 0.2%. After incubation with a blocking solution containing 10% (vol/vol) normal goat serum (NGS), 3% (wt/vol) BSA for 1 h, to saturate unspecific binding sites, cells were incubated with primary antibodies listed below, followed by decoration with specific secondary antibodies. Control incubations demonstrated non–cross-reactivity between the anti-Ig conjugates or between the anti-Ig conjugate and the irrelevant primary antibody. Nuclei were stained with DAPI (Sigma-Aldrich).
For PLA, cells were grown on coverslips and processed as described above. After incubation with the primary antibodies, Duolink in situ PLA (Sigma-Aldrich) was performed according to the manufacturer’s protocol. Briefly, PLA probes were incubated for 1 h at 37 °C, followed by hybridization, ligation (30 min at 37 °C), and amplification (100 min at 37 °C). Nuclei were stained with DAPI in Duolink In Situ Mounting Medium (Sigma).
Images were captured using an Olympus Fluoview FV1000 confocal laser scanning system (Olympus America Inc.) based on an Olympus Ix81 inverted microscope equipped with Olympus Plan-Apochromat 60× oil-immersion objective. Images (800 × 800 pixel resolution) were analyzed using Imaris 6.2 software (Bitplane). Images shown in all figures are representative of at least five random fields (scale bars are indicated).
Antibodies.
The following antibodies were used: NKRF (1:500, Bethyl Laboratories Inc. A304-016A), NKRF (1:500, Santa Cruz Biotechnology, Inc. sc-365568), XRN2 (1:200, Bethyl Laboratories Inc. A301-103A), B23 (1:1,000, Sigma-Aldrich, Inc. B 0556), RPA194 (F-6) (1:500, Santa Cruz Biotechnology, Inc. sc-46699), C23 (MS3) (1:100, Santa Cruz Biotechnology, Inc. sc-8031), DYKDDDDK Tag (1:500 Cell Signaling Technology, Inc. 2368), and anti-mouse/rabbit Alexa Fluor 555/488 (1:200, Thermo Fisher Scientific).
Clonogenic Assay.
Cells transfected for 48 h with siNKRF or scRNA (see section siRNA Interference for detail) were trypsinized, counted, and plated at a density of 500 cells per well in six-well plates. After 3 h, cells were subjected to sublethal hyperthermic treatment (43 °C for 120 min) or left untreated and then incubated at 37 °C for 10 d. Adherent cells were fixed with 4% (wt/vol) paraformaldehyde at room temperature for 10 min and then stained with 0.1% crystal violet for 10 min. Colonies with more than 50 cells were considered positive and counted. The surviving fraction was calculated as described (38).
Statistical Analysis.
Statistical analysis was performed using the Student’s t test for unpaired data. Data are expressed as the mean ± SD of duplicate samples, and P values < 0.05 were considered significant. All of the results and images shown in this manuscript are representative of at least three independent experiments with similar results.
Acknowledgments
We thank G. Adorno (Transfusion Medicine Center, University of Rome Tor Vergata) for providing human buffy coats, H. Hauser (Helmholtz Centre for Infection Research) for providing the NKRF-GFP construct, E. Romano (Center for Advanced Microscopy, University of Rome Tor Vergata) for assistance with confocal microscopy, and F. Loreni (Department of Biology, University of Rome Tor Vergata) for helpful discussions. This work was supported by grants from the Italian Ministry of University and Scientific Research (PRIN Project 2010PHT9NF-006).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1616112114/-/DCSupplemental.
References
- 1.Labbadia J, Morimoto RI. The biology of proteostasis in aging and disease. Annu Rev Biochem. 2015;84:435–464. doi: 10.1146/annurev-biochem-060614-033955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shibata Y, Morimoto RI. How the nucleus copes with proteotoxic stress. Curr Biol. 2014;24(10):R463–R474. doi: 10.1016/j.cub.2014.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: Integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 2010;11(8):545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakai A. Molecular basis of HSF regulation. Nat Struct Mol Biol. 2016;23(2):93–95. doi: 10.1038/nsmb.3165. [DOI] [PubMed] [Google Scholar]
- 5.Trinklein ND, Murray JI, Hartman SJ, Botstein D, Myers RM. The role of heat shock transcription factor 1 in the genome-wide regulation of the mammalian heat shock response. Mol Biol Cell. 2004;15(3):1254–1261. doi: 10.1091/mbc.E03-10-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossi A, et al. AIRAP, a new human heat shock gene regulated by heat shock factor 1. J Biol Chem. 2010;285(18):13607–13615. doi: 10.1074/jbc.M109.082693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossi A, Ciafrè S, Balsamo M, Pierimarchi P, Santoro MG. Targeting the heat shock factor 1 by RNA interference: A potent tool to enhance hyperthermochemotherapy efficacy in cervical cancer. Cancer Res. 2006;66(15):7678–7685. doi: 10.1158/0008-5472.CAN-05-4282. [DOI] [PubMed] [Google Scholar]
- 8.DiDonato JA, Mercurio F, Karin M. NF-κB and the link between inflammation and cancer. Immunol Rev. 2012;246(1):379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 9.Santoro MG, Rossi A, Amici C. NF-kappaB and virus infection: Who controls whom. EMBO J. 2003;22(11):2552–2560. doi: 10.1093/emboj/cdg267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nourbakhsh M, Hauser H. Constitutive silencing of IFN-beta promoter is mediated by NRF (NF-kappaB-repressing factor), a nuclear inhibitor of NF-kappaB. EMBO J. 1999;18(22):6415–6425. doi: 10.1093/emboj/18.22.6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng X, et al. Identification of a negative response element in the human inducible nitric-oxide synthase (hiNOS) promoter: The role of NF-kappa B-repressing factor (NRF) in basal repression of the hiNOS gene. Proc Natl Acad Sci USA. 2002;99(22):14212–14217. doi: 10.1073/pnas.212306199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nourbakhsh M, et al. The NF-kappa b repressing factor is involved in basal repression and interleukin (IL)-1-induced activation of IL-8 transcription by binding to a conserved NF-kappa b-flanking sequence element. J Biol Chem. 2001;276(6):4501–4508. doi: 10.1074/jbc.M007532200. [DOI] [PubMed] [Google Scholar]
- 13.Dreikhausen U, Hiebenthal-Millow K, Bartels M, Resch K, Nourbakhsh M. NF-kappaB-repressing factor inhibits elongation of human immunodeficiency virus type 1 transcription by DRB sensitivity-inducing factor. Mol Cell Biol. 2005;25(17):7473–7483. doi: 10.1128/MCB.25.17.7473-7483.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reboll MR, et al. Mapping of NRF binding motifs of NF-kappaB p65 subunit. J Biochem. 2011;150(5):553–562. doi: 10.1093/jb/mvr099. [DOI] [PubMed] [Google Scholar]
- 15.Rossi A, Elia G, Santoro MG. Inhibition of nuclear factor kappa B by prostaglandin A1: An effect associated with heat shock transcription factor activation. Proc Natl Acad Sci USA. 1997;94(2):746–750. doi: 10.1073/pnas.94.2.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morimoto RI, Santoro MG. Stress-inducible responses and heat shock proteins: New pharmacologic targets for cytoprotection. Nat Biotechnol. 1998;16(9):833–838. doi: 10.1038/nbt0998-833. [DOI] [PubMed] [Google Scholar]
- 17.Rossi A, et al. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature. 2000;403(6765):103–108. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- 18.Vihervaara A, et al. Transcriptional response to stress in the dynamic chromatin environment of cycling and mitotic cells. Proc Natl Acad Sci USA. 2013;110(36):E3388–E3397. doi: 10.1073/pnas.1305275110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oumard A, Hennecke M, Hauser H, Nourbakhsh M. Translation of NRF mRNA is mediated by highly efficient internal ribosome entry. Mol Cell Biol. 2000;20(8):2755–2759. doi: 10.1128/mcb.20.8.2755-2759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niedick I, et al. Nucleolar localization and mobility analysis of the NF-kappaB repressing factor NRF. J Cell Sci. 2004;117(Pt 16):3447–3458. doi: 10.1242/jcs.01129. [DOI] [PubMed] [Google Scholar]
- 21.Boisvert FM, van Koningsbruggen S, Navascués J, Lamond AI. The multifunctional nucleolus. Nat Rev Mol Cell Biol. 2007;8(7):574–585. doi: 10.1038/nrm2184. [DOI] [PubMed] [Google Scholar]
- 22.Boulon S, Westman BJ, Hutten S, Boisvert FM, Lamond AI. The nucleolus under stress. Mol Cell. 2010;40(2):216–227. doi: 10.1016/j.molcel.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miki TS, Richter H, Rüegger S, Großhans H. PAXT-1 promotes XRN2 activity by stabilizing it through a conserved domain. Mol Cell. 2014;53(2):351–360. doi: 10.1016/j.molcel.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Nagarajan VK, Jones CI, Newbury SF, Green PJ. XRN 5′→3′ exoribonucleases: Structure, mechanisms and functions. Biochim Biophys Acta. 2013;1829(6-7):590–603. doi: 10.1016/j.bbagrm.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sloan KE, Bohnsack MT, Schneider C, Watkins NJ. The roles of SSU processome components and surveillance factors in the initial processing of human ribosomal RNA. RNA. 2014;20(4):540–550. doi: 10.1261/rna.043471.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tafforeau L, et al. The complexity of human ribosome biogenesis revealed by systematic nucleolar screening of pre-rRNA processing factors. Mol Cell. 2013;51(4):539–551. doi: 10.1016/j.molcel.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Sloan KE, et al. Both endonucleolytic and exonucleolytic cleavage mediate ITS1 removal during human ribosomal RNA processing. J Cell Biol. 2013;200(5):577–588. doi: 10.1083/jcb.201207131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Preti M, et al. Gradual processing of the ITS1 from the nucleolus to the cytoplasm during synthesis of the human 18S rRNA. Nucleic Acids Res. 2013;41(8):4709–4723. doi: 10.1093/nar/gkt160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang M, Pestov DG. 5′-end surveillance by Xrn2 acts as a shared mechanism for mammalian pre-rRNA maturation and decay. Nucleic Acids Res. 2011;39(5):1811–1822. doi: 10.1093/nar/gkq1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rother S, et al. NF-κB-repressing factor phosphorylation regulates transcription elongation via its interactions with 5′→3′ exoribonuclease 2 and negative elongation factor. FASEB J. 2016;30(1):174–185. doi: 10.1096/fj.15-270256. [DOI] [PubMed] [Google Scholar]
- 31.Hein N, Hannan KM, George AJ, Sanij E, Hannan RD. The nucleolus: An emerging target for cancer therapy. Trends Mol Med. 2013;19(11):643–654. doi: 10.1016/j.molmed.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Lu Z, et al. miR-301a as an NF-κB activator in pancreatic cancer cells. EMBO J. 2011;30(1):57–67. doi: 10.1038/emboj.2010.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elia G, Polla B, Rossi A, Santoro MG. Induction of ferritin and heat shock proteins by prostaglandin A1 in human monocytes. Evidence for transcriptional and post-transcriptional regulation. Eur J Biochem. 1999;264(3):736–745. doi: 10.1046/j.1432-1327.1999.00610.x. [DOI] [PubMed] [Google Scholar]
- 34.Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: A sequence logo generator. Genome Res. 2004;14(6):1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heinemeyer T, et al. Databases on transcriptional regulation: TRANSFAC, TRRD and COMPEL. Nucleic Acids Res. 1998;26(1):362–367. doi: 10.1093/nar/26.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chamousset D, Mamane S, Boisvert FM, Trinkle-Mulcahy L. Efficient extraction of nucleolar proteins for interactome analyses. Proteomics. 2010;10(16):3045–3050. doi: 10.1002/pmic.201000162. [DOI] [PubMed] [Google Scholar]
- 37.Mansour FH, Pestov DG. Separation of long RNA by agarose-formaldehyde gel electrophoresis. Anal Biochem. 2013;441(1):18–20. doi: 10.1016/j.ab.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1(5):2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 39.Miyazawa N, et al. Human cell growth regulator Ly-1 antibody reactive homologue accelerates processing of preribosomal RNA. Genes Cells. 2014;19(4):273–286. doi: 10.1111/gtc.12129. [DOI] [PubMed] [Google Scholar]