Abstract
Problem
Intrauterine inflammation is a frequent and significant factor associated with the pathogenesis of preterm labor/birth (PTL/PTB). It is unclear however, whether the intrauterine inflammatory responses activate the maternal peripheral circulation. We explored the association between PTL/PTB and the “activation” of the peripheral circulatory system by determining whether CD55 mRNA expression within peripheral WBC’s differed between PTL and control patients not in labor.
Method of Study
RNA was purified from white blood cells collected from pregnant women with preterm labor (n = 45), and from pregnant (n = 30) control women. CD55 gene expression was evaluated by quantitative PCR.
Results
The mean CD55 mRNA level within the PTL group (0.77 ± 0.03) was 1.48-fold higher then that observed (0.52 ± 0.02) within the control group (P < 0.0001); 71% of PTL patients and only 6.7% of control subjects expressed elevated CD55 mRNA. The receiver operating characteristics (with 95% CI) of CD55 as a marker for PTL were: Sensitivity; 69% (53–82%), Specificity; 93% (78–99%), Positive Predictive Value; 94% (80–99%) and Negative Predictive Value 67% (51–80%). In the patient population that delivered prematurely (before 37 weeks), 81% expressed elevated CD55 mRNA levels with a mean of 0.78 +/−0.03 and 95% CI of 0.71 to 0.84. The receiver operating characteristics were: Sensitivity; 73% (54–88%), Specificity; 86% (71–95%), Positive Predictive Value; 81.5% (62–94%) and Negative Predictive Value 80% (64–91%).
Conclusion
Here we report for the first time that CD55 mRNA expression was elevated in the peripheral WBC’s of subjects with preterm labor as compared to control gestationally-matched pregnant woman and that elevated leukocyte CD55 may be a useful predictor of subsequent PTB.
Keywords: Infection, preterm labor, preterm birth, maternal circulatory immune response
Introduction
The rate of spontaneous preterm birth (PTB), occurring before week 37 of gestation, has risen over the last decade in spite of intense efforts toward prevention, detection and treatment. Preterm births account for 75% of perinatal mortality, >50% of childhood and long-term morbidity, and remains a major socioeconomic burden (1–4). Infectious and non-infectious intrauterine inflammation is a frequent and significant factor associated with the pathogenesis of preterm labor (PTL)/ PTB (5–11). Subclinical intrauterine infections have been suspected to play a role in the onset of idiopathic preterm labor (12). Inflammatory processes outside the reproductive unit, such as in periodontal disease (13;14) and urinary tract infection (15;16) have also been associated with PTL.
Infiltration of gestational tissues by white blood cells and the ensuing inflammatory cascade appears to be the final common pathway that ultimately leads to the induction of preterm labor (9;17–19). While a plethora of studies have demonstrated that term and PTL is associated with an inflammatory signature within tissues of the reproductive unit, there is little evidence of inflammatory signaling within the peripheral blood stream (see (11;17;20) and references therein).
The innate immune system of the peripheral circulartory compartment, which is composed of circulating white blood cells and serum complement (21–23), has also been implicated in the pathophysiology of pregnancy (see (24) and references therein). As the allogeneic fetal tissue is directly exposed to the maternal blood, there is risk of complement-mediated cell lysis at the maternal/fetal interface (23;25–29). The complement regulatory protein, CD55 (also called decay accelerating factor) is a multifunctional cell surface receptor present on leukocytes and all tissues exposed to maternal serum (22;30;31). Elevated CD55 expression on the syncytiotrophoblast protects the fetus from injury by maternal serum complement (26). Low expression of CD55 in the endometrium in patients with luteal phase defect (LPD) has been implicated in infertility and/or recurrent pregnancy losses (32;33). Aberrant expression of CD55 is also associated with a number of other inflammatory/pathologic processes including paroxysmal nocturnal hemoglobinuria (34), endometrial adeno-carcinoma (35) and renal transplant rejection (36). Paradoxically, protective up-regulation of CD55 is exploited by various pathogens which recognize and utilize the CD55 receptor to invade and promote chronic inflammation of uro-genital tissue (37;38).
Of particular relevance to our study is that inflammatory stimuli associated with active ulcerative colitis have been shown to induce expression of CD55 on peripheral white blood cells, suggesting that localized tissue inflammation may activate the circulatory system (39). As inflammation within the reproductive unit is associated with the onset of PTL, we elected to explore the association between PTL/PTB and the “activation” of the peripheral circulatory system by determining whether CD55 mRNA expression within peripheral WBC’s differed between PTL and control patients not in labor.
MATERIALS AND METHODS
Subjects
The study was approved by the Human Research Committee at the University of Texas Medical Branch (UTMB) in Galveston, Texas and at Meharry Medical College, Nashville Tennessee. Written informed consent was obtained from all 75 recruited women. The following clinical groups were evaluated: pregnant controls without preterm labor (n = 30) and pregnant women with preterm labor (PTL) (n = 45).
The clinical criteria for preterm labor were those used by the American College of Obstetricians and included regular contractions, cervical dilation ≥ 2 cm and/or cervical effacement. Exclusion criteria included maternal illness, anemia, uterine malformations, cervical incompetence, placental abruption, placenta previa and steroid use. All women in preterm labor were clinically evaluated for symptoms of chorioamnionitis (40), bacterial vaginosis (BV) and urinary tract infection (UTI) using ACOG guidelines. The subpopulation of PTL patients (N=11) with infection-associated PTL were identified to have urinary tract or vaginal/cervical infections. Because the major goal of this investigation was to evaluate the CD55 mRNA levels in peripheral blood we collected blood samples only.
White blood cell and RNA isolation
Peripheral venous blood (5 mL) was drawn once from each woman into heparinized vacutainers prior to treatment. White blood cells were separated from erythrocytes by dextran sedimentation and pelleted by centrifugation; total RNA was isolated using Tri-Reagent (Sigma, St. Louis, Mo). Isolated RNA was quantified by optical density readings at 260 nm, and the purity was estimated by the ratio of 260/280 nm.
Reverse transcriptase-polymerase chain reaction
The Dual Gene Quantitative (Maxim Biotech Dp-10201) method was used to determine CD55 mRNA levels. Isolated RNA was treated with RNAse-free DNase (Ambion, Austin, Tex) to ensure no contamination with genomic DNA and 1 μg of RNA was reverse transcribed using Moloney-Murine Leukemia Virus reverse transcriptase (RT) and random decamer primers using the manufactures protocol (RETROscript Kit, Ambion). Dual quantitative polymerase chain reaction (PCR) amplification was performed using 5 μL of cDNA (~0.25 μg), 1.5 U of Taq polymerase (Life Technologies, Carlsbad, Calif.), and PCR thermal cycler (Thermo Hybrid, Franklin, Mass.). The PCR amplification reaction was optimized (96° C for 1 minute, 36 cycles of 94° C for 1 minute, 60° C for 90 seconds followed by a final extension at 72° C for 10 minutes) so that end-point analysis of the amplified CD55 amplicon was in the linear range. The observed differences between replicates from the same women were less than 10% and on average varied from 2% to 5%. Of several house keeping genes evaluated, we selected the 18S rRNA gene since its expression remained constant during pregnancy. As the number of 18S copies greatly exceeds that of CD55, the amount of 18S was determined using a 1:500 dilution of the rtRNA with linear range amplification at cycle 14.
Quantitation of RT-PCR products
The intensities of the PCR products were digitally captured and quantitated using the Alpha-imager image-scanning system (Alpha Innotech Corporation, San Fernando, Calif.). The expression level of CD55 was normalized based on the expression level of the 18S ribosomal gene.
After our analysis was complete, we had leftover RNA form PTL (N=17) and control (N=14) samples. We retrospectively determined CD55 mRNA levels (relative to 18S RNA) within these samples using the quantitative real time PCR approach. These data were consistent with those obtained using the Dual Gene approach.
Statistical analyses
An unpaired t-test was used to compare differences in CD55 expression levels between the control and PTL groups. To analyze differences in CD55 expression levels between various PTL subgroups (as a function of gestational age at time of blood draw, idiopathic vs. infection-associated PTL, and delivery date) a pair-wise analysis utilizing the nonparametric one-way ANOVA (Kruskal-Wallis) test was performed. The t-test and One-way ANOVA was performed using GraphPad Prism version 4.03 for Windows, GraphPad Software, San Diego California USA, www.graphpad.com. Post-hoc power analysis was performed using PS power and sample size program, version 2.1.31, 2004 (Dupont WD and Plummer WD, http://biostat.mc.vanderbilt.edu/twiki/bin/view/Main/PowerSampleSize).
Sensitivity/Specificity: Receiver Operating Characteristic (ROC) Curves
In order to obtain measures of sensitivity and specificity for the data set, receiver operating characteristics curve analysis was conducted using MedCalc for Windows, version 10.0.0.0 (MedCalc Software, Mariakerke, Belgium). This technique avoids using a single value as the classification threshold by graphing coordinate pairs of sensitivity versus 1-specificity across a range of thresholds.
Results
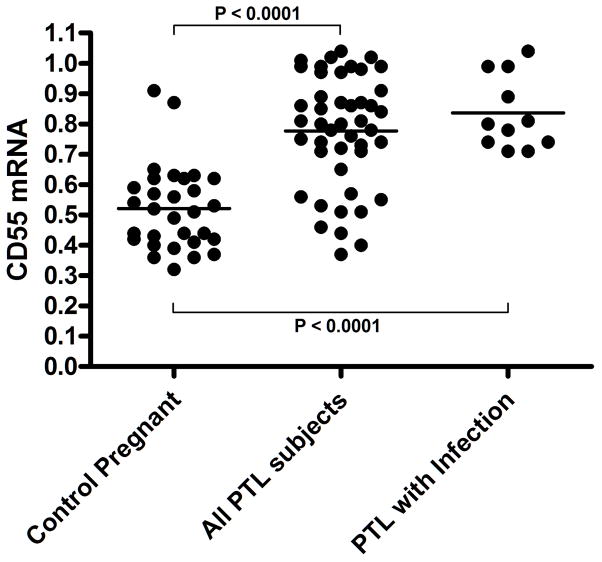
The mean CD55 mRNA level, represented as the normalized integrated density value (IDV), within the PTL group (0.77 ± 0.03 (SEM); N=45) was 1.48-fold higher then that observed (0.52 ± 0.02 N=30) within the control group ((P < 0.0001 (Fig. 1)). Using 0.72 IDV (lower 95% confidence interval) as a cutoff value for elevated CD55 mRNA levels, 32/45 (71.1%) of PTL patients expressed elevated CD55 mRNA as compared to 2/30 (6.7%) in control subjects. Post-hoc power analysis was conducted to determine the power of the study, assuming the effect size in the samples was equal to the effect size in the population. Given the sample sizes and observed standard deviations our study achieved more than 99% power with a level of significance of 0.05. A sample size of at least 12 in each group is sufficient to achieve 80% power at a 0.05 significance level. We also determined (as a function of true positive fraction (sensitivity) versus false positive fraction (1 - specificity)) the receiver operating characteristic (ROC) curve to explore the “diagnostic accuracy” of elevated CD55 levels as a marker for PTL (Figure 2). The area under the curve (AUC) was 0.844 +/− 0.04 (p<0.0001) with a 95%CI of 0.742 to 0.918. That is to say that 84.4% of the time a randomly chosen PTL subject would have a higher CD55 level than a randomly chosen individual for the negative group. Using elevated CD55 levels (0.72) as the decision threshold, the receiver operating characteristics (with 95% CI) of CD55 as a marker for PTL were: Sensitivity; 69% (53–82%), Specificity; 93% (78–99%), Positive Predictive Value; 94% (80–99%) and Negative Predictive Value 67% (51–80%).
Figure 1. CD55 mRNA is Elevated in the PWBC’fs of Infection-associated and Idiopathic PTL Subjects.
The normalized integrated density values for CD55 mRNA levels are plotted for PTL (weeks 24–37 of gestation) and gestationally-matched control subjects as indicated; the horizontal bar indicates the mean value. The P value was determined using a two-tailed t-test. The CD55 mRNA levels of infection-associated PTL subjects are plotted as indicated.
Figure 2. Receiver Operating Characteristics (ROC) of CD55 Levels as Indicator of PTL and Infection-associated PTL.
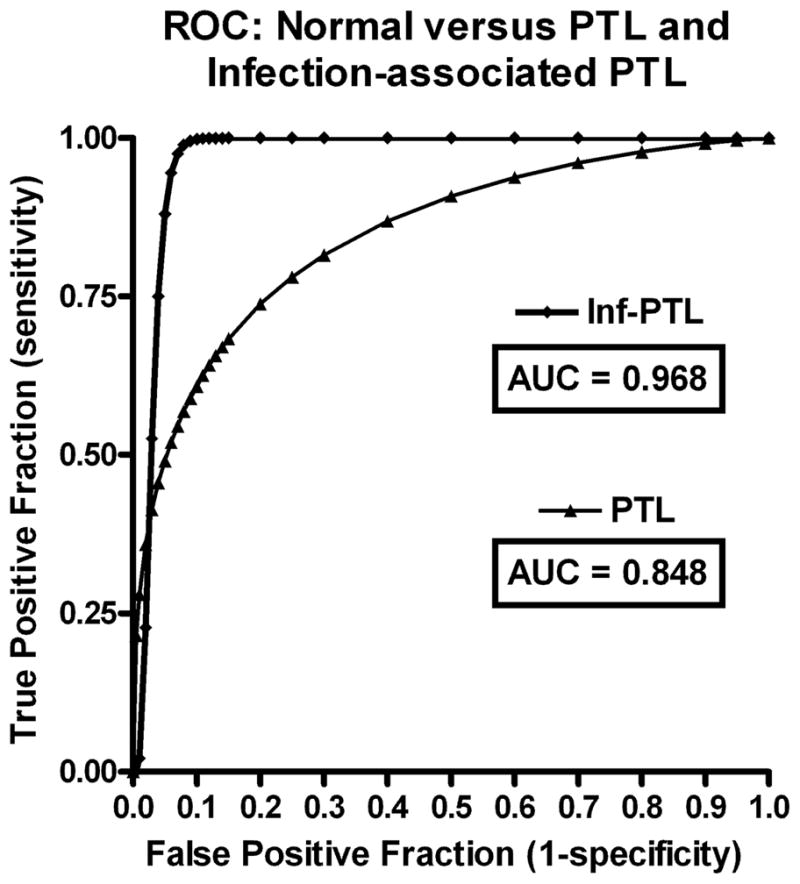
The true positive rate (Sensitivity) is plotted in function of the false positive rate (1- Specificity) for different cut-off points. Area Under ROC Curve (AUC) are indicated. The upper and lower confidence intervals @ 95% are included in the Results Section text.
As there is an association between urogenital infections and premature labor/delivery, we analyzed the subpopulation of PTL subjects (N=11) that presented with clinical evidence of infection. The mean CD55 expression level was (0.84 +/− 0.04) in this subpopulation of PTL subjects with an 95% CI from 0.75 to 0.92 (Figure 1), which was 1.6-fold higher then that observed within the control group (P < 0.0001). Strikingly, 100% of these patients expressed high levels (≥ 0.71) of CD55 mRNA. Given the sample sizes and observed standard deviations between the infection-associated and control subjects, this comparison achieved more than 95% power with a level of significance of 0.05. Even though meaningful qualitative conclusions cannot be drawn because of the limited nature of the data set, the receiver operating characteristics were engaging. The AUC value determined from the ROC curve (Figure 2) was 0.955 +/− 0.05 (p < 0.0001). Using 0.71 as the decision threshold for elevated CD55 as a marker for infection-associated PTL, the receiver operating characteristics were: Sensitivity; 81% (48–97%), Specificity; 93% (78–99%), Positive Predictive Value; 81% (43–97%) and Negative Predictive Value 93% (78–99%). The remaining PTL subjects, 68% (23/34) expressed elevated CD55 mRNA. We found no significant difference in the mean CD55 expression levels as a function of elevated risk factors associated with gestational age (20–29 vs 30–34 weeks).
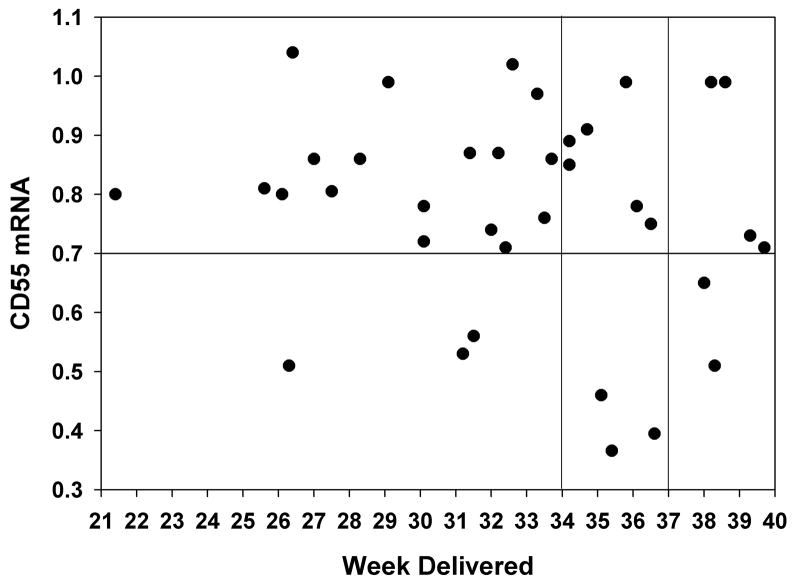
Finally, we evaluated the relationship between the CD55 expressions levels (obtained upon admission to hospital) and the ultimate date of delivery. The delivery date was available for 36 of 45 PTL patients. In Figure 3, the level of CD55 at the time of admission was plotted as a function of gestational age at the time of delivery. The horizontal line divides patients into high and low CD55 (>0.7 IDV) and the vertical lines divide patients according to high risk (before 34 weeks) and premature (before week 37) birth. In the patient population that delivered prematurely (before 37 weeks), 22 of 27 (81%) expressed elevated CD55 mRNA levels with a mean of 0.78 +/− 0/03 and 95% CI of 0.71 to 0.84. There was no statistical difference in CD55 expression within the high risk PTB subgroup, 17 of 20 (85%) patients had elevated CD55. The mean for this subgroup was 0.8 =+/− 0.03 with an 95% CI of 0.74 to 0.87.
Figure 3. CD55 Expression Levels and Preterm Birth.
CD55 expression level of each individual patient at the time of admission is plotted as a function of gestational age at delivery. Horizontal line indicates elevated CD55 threshold value and vertical line separates patients into high (<34 weeks) and low (34 – 37 weeks) risk PTL.
We also determined the receiver operating characteristic curve to explore the potential of elevated CD55 levels as a predictor for PTB. For this, the control and PTL subjects were analyzed as a function of premature (N=30) vs normal (N=36) delivery dates. The area under the curve (AUC) was 0.795 +/− 0.06 (p<0.0001) with a 95%CI of 0.687 to 0.885. Using elevated CD55 levels of 0.72 as the selection criterion, the receiver operating characteristics (with 95% CI) were: Sensitivity; 73% (54–88%), Specificity; 86% (71–95%), Positive Predictive Value; 81.5% (62–94%) and Negative Predictive Value 80% (64–91%).
Discussion
Here we report for the first time that CD55 mRNA expression was elevated in the peripheral WBC’s of subjects with preterm labor as compared to control gestationally-matched pregnant woman and that elevated leukocyte CD55 may be a useful predictor of subsequent PTB.
Infection/inflammation has been proposed to be a major contributing factor in idiopathic PTL (6;9). The detection of increased PWBC’s CD55 in PTL is reminiscent of the observed induction of CD55 expression on PWBC’s of patients with active ulcerative colitis (39). Our data set on patients with diagnosed urogenital infections (N=11, 95% power) supports the notion that elevated peripheral blood CD55 mRNA expression is a potential biomarker for infection/inflammation. Within the non-infection associated PTL population, there were 18 patients with no overt manifestations of obstetric complications (e.g. chronic hypertension, gestational diabetes). Remarkable, 18 out of 18 of these patients expressed elevated CD55 mRNA levels with a mean value of 0.90 +/− 0.02 and 95% CI from 0.85–0.95 (Figure not shown). We propose that in observed upregulation of CD55 within the PWBC’s of these idiopathic PTL subjects may be a manifestation of a subclinical inflammatory process within the uterus.
At present we do not know the mechanism(s) by which CD55 is upregulated in the peripheral blood system. In the case of microbial or viral infections, it could be in direct response to pathogen molecular pattern recognition involving Toll-receptor signaling. An alternative, but not mutually exclusive pathway might involve Th1 and/or Th2 signaling intermediates. Additional mediators are possible (41). Previous data published in our laboratory demonstrated regulation of endometrial CD55 expression was influenced by nitric oxide and progesterone of which both are significant factors in uterine/PTL pathophisiology (29;37;42;43). Further analysis will be required to resolve this issue.
It is also not clear if elevated CD55 precedes the initiation of PTL or is a consequence thereof. Our data suggests that elevated CD55 expression in peripheral WBC may serve as a useful marker for PTL. The receiver operating characteristic curve analysis appears promising in terms of sensitivity versus specificity. In order to provide a metric for the aggregate performance of the ROC across the range of sensitivity/specificity trade-offs, the Area Under the ROC Curve (AUC) was calculated. This allows for assessing the overall performance of the classifier rather than a single threshold point and further allows for direct comparisons to other systems. In general, an AUC of 0.5 means that the classifier or variable in question is no better than random guessing and therefore not useful for prediction or diagnosis. In contrast, an AUC of 1.0 means that the classifier is perfect and always gives the “right answer”. While there is no absolute consensus for what a “good” AUC number threshold is, conventions are that AUC from 0.9 to 1.0 are considered “excellent”, an AUC from 0.8 to 0.9 is “very good”, and an AUC from 0.5 to 0.6 are “failures”. The AUC for the all PTL (n=45) data set was 0.836 indicating “very good” performance in terms of sensitivity versus specificity. The AUC for the infection-associated PTL subgroup (AUC = 0.966, n=11) was in the excellent range. The AUC for the pre term birth group (AUC = 0.795, n=30) was at the upper limit of the good range One weakness of the ROC analysis is that sample sizes were small, as indicated by the 95%CI’s of the various receiver operating characteristics. Regardless, the results were statistically significant and support the notion that elevated peripheral blood CD55 mRNA levels may serve as a marker for infection and PTL and a predictor for PTB. Further studies with larger sample sizes and temporal expression patterns of CD55 will be required to more precisely determine the predictive value of CD55 expression on PTL and PTB.
In summary, our primary findings are that peripheral WBC CD55 expression is elevated in PTL and it may have potential predictive value for PTB. Studies are in progress to further delineate the cell-specific, temporal, and functional role of increased CD55 expression in PTL and to explore the diagnostic potential of CD55 as a biomarker for woman at risk of PTL/PTB in a larger patient population.
Acknowledgments
This work was supported by Public Health Service grant HD41687 from the National Institute of Child Health and Human Development to S. Nowicki and B.J. Nowicki, and in part by grant DK42029 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) to B.J. Nowicki.
Reference List
- 1.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haas JS, Fuentes-Afflick E, Stewart AL, Jackson RA, Dean ML, Brawarsky P, et al. Prepregnancy Health Status and the Risk of Preterm Delivery. Arch Pediatr Adolesc Med. 2005;159(1):58–63. doi: 10.1001/archpedi.159.1.58. [DOI] [PubMed] [Google Scholar]
- 3.Romero R, Espinoza J, Chaiworapongsa T, Kalache K. Infection and prematurity and the role of preventive strategies. Seminars in Neonatology. 2002;7(4):259–274. doi: 10.1016/s1084-2756(02)90121-1. [DOI] [PubMed] [Google Scholar]
- 4.Guinn D, Gibbs R. Infection-related Preterm Birth: A Review of the Evidence. NeoReviews. 2002;3(5):e86–e96. [Google Scholar]
- 5.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Seminars in Fetal and Neonatal Medicine. 2006;11(5):317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiGiulio DB, Romero R, Amogan HP, Kusanovic JP, Bik EM, Gotsch F, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS ONE. 2008;3(8):e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nemeth E, Millar LK, Bryant-Greenwood G. Fetal membrane distention: II. Differentially expressed genes regulated by acute distention in vitro. American journal of obstetrics and gynecology. 2000;182(1 Pt 1):60–67. doi: 10.1016/s0002-9378(00)70491-1. [DOI] [PubMed] [Google Scholar]
- 8.Nemeth E, Tashima LS, Yu Z, Bryant-Greenwood GD. Fetal membrane distention: I. Differentially expressed genes regulated by acute distention in amniotic epithelial (WISH) cells. American journal of obstetrics and gynecology. 2000;182(1 Pt 1):50–59. doi: 10.1016/s0002-9378(00)70490-x. [DOI] [PubMed] [Google Scholar]
- 9.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Seminars in fetal & neonatal medicine. 2006;11(5):317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salafia CM, Lopez-Zeno JA, Sherer DM, Whittington SS, Minior VK, Vintzileos AM. Histologic evidence of old intrauterine bleeding is more frequent in prematurity. American journal of obstetrics and gynecology. 1995;173(4):1065–1070. doi: 10.1016/0002-9378(95)91327-0. [DOI] [PubMed] [Google Scholar]
- 11.Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim YM, Mazor M, et al. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. American journal of obstetrics and gynecology. 2006;195(2):394. doi: 10.1016/j.ajog.2005.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. The New England journal of medicine. 2000;342(20):1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez AR, Bagniewski S, Weaver AL, Vallejos N. Correlations between maternal periodontal conditions and preterm low birth weight infants. Journal of the International Academy of Periodontology. 2007;9(2):34–41. [PubMed] [Google Scholar]
- 14.Jeffcoat MK, Geurs NC, Reddy MS, Cliver SP, Goldenberg RL, Hauth JC. Periodontal infection and preterm birth: Results of a prospective study. J Am Dent Assoc. 2001;132(7):875–880. doi: 10.14219/jada.archive.2001.0299. [DOI] [PubMed] [Google Scholar]
- 15.McCormick T, Ashe RG, Kearney PM. Urinary tract infection in pregnancy. The Obstetrician and Gynaecologist. 2008;10(3):156–162. [Google Scholar]
- 16.Banhidy F, Acs N, Puho EH, Czeizel AE. Pregnancy complications and birth outcomes of pregnant women with urinary tract infections and related drug treatments. Scandinavian journal of infectious diseases. 2007;39(5):390–397. doi: 10.1080/00365540601087566. [DOI] [PubMed] [Google Scholar]
- 17.Marvin KW, Keelan JA, Eykholt RL, Sato TA, Mitchell MD. Use of cDNA arrays to generate differential expression profiles for inflammatory genes in human gestational membranes delivered at term and preterm. Molecular human reproduction. 2002;8(4):399–408. doi: 10.1093/molehr/8.4.399. [DOI] [PubMed] [Google Scholar]
- 18.Gravett MG, Novy MJ, Rosenfeld RG, Reddy AP, Jacob T, Turner M, et al. Diagnosis of intra-amniotic infection by proteomic profiling and identification of novel biomarkers. Jama. 2004;292(4):462–469. doi: 10.1001/jama.292.4.462. [DOI] [PubMed] [Google Scholar]
- 19.Lockwood CJ, Kuczynski E. Markers of risk for preterm delivery. Journal of perinatal medicine. 1999;27(1):5–20. doi: 10.1515/JPM.1999.001. [DOI] [PubMed] [Google Scholar]
- 20.Norman J, Bollapragada S, Yuan M, Nelson S. Inflammatory pathways in the mechanism of parturition. BMC Pregnancy and Childbirth. 2007;7(Suppl 1):S7. doi: 10.1186/1471-2393-7-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nowicki S, Selvarangan R, Anderson G. Experimental transmission of Neisseria gonorrhoeae from pregnant rat to fetus. Infection and immunity. 1999;67(9):4974–4976. doi: 10.1128/iai.67.9.4974-4976.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lublin DM. Review: Cromer and DAF: role in health and disease. Immunohematology. 2005;21(2):39–47. [PubMed] [Google Scholar]
- 23.Brooimans RA, van W, van E, Daha MR. Relative roles of decay-accelerating factor, membrane cofactor protein, and CD59 in the protection of human endothelial cells against complement-mediated lysis. Eur J Immunol. 1992;22(12):3135–3140. doi: 10.1002/eji.1830221216. [DOI] [PubMed] [Google Scholar]
- 24.Girardi G, Bulla R, Salmon JE, Tedesco F. The complement system in the pathophysiology of pregnancy. Molecular Immunology. 2006;43(1–2):68–77. doi: 10.1016/j.molimm.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 25.Cunningham DS, Tichenor JR., Jr Decay-accelerating factor protects human trophoblast from complement-mediated attack. Clinical immunology and immunopathology. 1995;74(2):156–161. doi: 10.1006/clin.1995.1023. [DOI] [PubMed] [Google Scholar]
- 26.Zarkadis IK, Omigbodun A, Forson A, Ziolkiewicz P, Kinoshita T, Lambris JD, et al. Differentiation-dependent changes in human trophoblast expression of decay-accelerating factor are modulated by 3′,5′ cyclic adenosine monophosphate. Journal of the Society for Gynecologic Investigation. 1997;4(1):47–53. doi: 10.1016/S1071-5576(96)00061-5. [DOI] [PubMed] [Google Scholar]
- 27.Bulla R, Bossi F, Fischetti F, De S, Tedesco F. The complement system at the fetomaternal interface. Chem Immunol Allergy. 2005;89:149–157. doi: 10.1159/000087963. [DOI] [PubMed] [Google Scholar]
- 28.Holmes CH, Simpson KL, Wainwright SD, Tate CG, Houlihan JM, Sawyer IH, et al. Preferential expression of the complement regulatory protein decay accelerating factor at the fetomaternal interface during human pregnancy. J Immunol. 1990;144(8):3099–3105. [PubMed] [Google Scholar]
- 29.Kaul AK, Kumar D, Nagamani M, Goluszko P, Nowicki S, Nowicki BJ. Rapid cyclic changes in density and accessibility of endometrial ligands for Escherichia coli Dr fimbriae. Infection and immunity. 1996;64(2):611–615. doi: 10.1128/iai.64.2.611-615.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasan RJ, Pawelczyk E, Urvil PT, Venkatarajan MS, Goluszko P, Kur J, et al. Structure-Function Analysis of Decay-Accelerating Factor: Identification of Residues Important for Binding of the Escherichia coli Dr Adhesin and Complement Regulation. Infection and immunity. 2002;70(8):4485–4493. doi: 10.1128/IAI.70.8.4485-4493.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sayegh RA, Tao XJ, Awwad JT, Isaacson KB. Localization of the expression of complement component 3 in the human endometrium by in situ hybridization. J Clin Endocrinol Metab. 1996;81(4):1641–1649. doi: 10.1210/jcem.81.4.8636381. [DOI] [PubMed] [Google Scholar]
- 32.Francis J, Rai R, Sebire NJ, El Gaddal S, Fernandes MS, Jindal P, et al. Impaired expression of endometrial differentiation markers and complement regulatory proteins in patients with recurrent pregnancy loss associated with antiphospholipid syndrome. Molecular human reproduction. 2006;12(7):435–442. doi: 10.1093/molehr/gal048. [DOI] [PubMed] [Google Scholar]
- 33.Kaul A, Nagamani M, Nowicki B. Decreased expression of endometrial decay accelerating factor (DAF), a complement regulatory protein, in patients with luteal phase defect. Am J Reprod Immunol. 1995;34(4):236–240. doi: 10.1111/j.1600-0897.1995.tb00947.x. [DOI] [PubMed] [Google Scholar]
- 34.Nowicki B, Hull R, Moulds J. Use of the Dr hemagglutinin of uropathogenic Escherichia coli to differentiate normal from abnormal red cells in paroxysmal nocturnal hemoglobinuria. The New England journal of medicine. 1988;319(19):1289–1290. doi: 10.1056/NEJM198811103191916. [DOI] [PubMed] [Google Scholar]
- 35.Nowicki S, Nowicki B, Pham T, Hasan R, Nagamani M. Expression of decay accelerating factor in endometrial adenocarcinoma is inversely related to the stage of tumor. Am J Reprod Immunol. 2001;46(2):144–148. doi: 10.1111/j.8755-8920.2001.460205.x. [DOI] [PubMed] [Google Scholar]
- 36.Sun H, Chen G, Liu W, Kubelik D, Yang H, White DJ, et al. The influence of baseline expression of human decay accelerating factor transgene on graft survival and acute humoral xenograft rejection. Transplantation. 2005;80(9):1331–1339. doi: 10.1097/01.tp.0000177649.30721.31. [DOI] [PubMed] [Google Scholar]
- 37.Fang L, Nowicki BJ, Urvil P, Goluszko P, Nowicki S, Young SL, et al. Epithelial invasion by Escherichia coli bearing Dr fimbriae is controlled by nitric oxide-regulated expression of CD55. Infection and immunity. 2004;72(5):2907–2914. doi: 10.1128/IAI.72.5.2907-2914.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nowicki B, Selvarangan R, Nowicki S. Family of Escherichia coli Dr Adhesins: Decay-Accelerating Factor Receptor Recognition and Invasiveness. The Journal of Infectious Diseases. 2001;183(s1):S24–S27. doi: 10.1086/318846. [DOI] [PubMed] [Google Scholar]
- 39.Makidono C, Mizuno M, Nasu J, Hiraoka S, Okada H, Yamamoto K, et al. Increased serum concentrations and surface expression on peripheral white blood cells of decay-accelerating factor (cd55) in patients with active ulcerative colitis. Journal of Laboratory and Clinical Medicine. 2004;143(3):152–158. doi: 10.1016/j.lab.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Goldman AS, Schmalstieg FC. The pathogenesis of chorioamnionitis. The Journal of pediatrics. 2008;153(1):3–4. doi: 10.1016/j.jpeds.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 41.Louis NA, Hamilton KE, Kong T, Colgan SP. HIF-dependent induction of apical CD55 coordinates epithelial clearance of neutrophils. Faseb J. 2005;19(8):950–959. doi: 10.1096/fj.04-3251com. [DOI] [PubMed] [Google Scholar]
- 42.Nowicki B, Fang L, Singhal J, Nowicki S, Yallampalli C. Lethal outcome of uterine infection in pregnant but not in nonpregnant rats and increased death rate with inhibition of nitric oxide. Am J Reprod Immunol. 1997;38(4):309–312. doi: 10.1111/j.1600-0897.1997.tb00521.x. [DOI] [PubMed] [Google Scholar]
- 43.Young SL, Lessey BA, Fritz MA, Meyer WR, Murray MJ, Speckman PL, et al. In Vivo and in Vitro Evidence Suggest That HB-EGF Regulates Endometrial Expression of Human Decay-Accelerating Factor. J Clin Endocrinol Metab. 2002;87(3):1368–1375. doi: 10.1210/jcem.87.3.8350. [DOI] [PubMed] [Google Scholar]