Abstract
Objective
To clarify the role of oxidative stress and antioxidant activity in ADHD.
Method
We examined the association of ADHD and oxidative stress by applying random effects meta-analysis to studies of oxidative stress and antioxidant status in medication naive patients with ADHD and controls.
Results
Six studies of a total of 231 ADHD patients and 207 controls met our selection criteria. The association between ADHD and antioxidant status was not significant. We found a significant association between ADHD and oxidative stress that could not be accounted for by publication bias. The significant association lost significance after correcting for intrastudy clustering. No one observation accounted for the positive result.
Conclusion
These results are preliminary given the small number of studies. They suggest that patients with ADHD have normal levels of antioxidant production, but that their response to oxidative stress is insufficient, leading to oxidative damage.
Keywords: ADHD, meta-analysis
Prior research suggests that oxidative stress predisposes to a diverse range of psychiatric conditions, including schizophrenia, bipolar disorder, major depressive disorder, and anxiety disorders (Ng, Berk, Dean, & Bush, 2008). Humans face an “oxygen paradox” (Davies, 1995). We need oxygen to survive, but an increased quantity of free oxygen radicals causes cellular pathologies that lead to disease and aging. During cellular metabolism, the normal oxidation–reduction reactions that create energy lead to the formation of toxic metabolic by-products called oxidants or reactive oxygen species (ROS). These by-products of normal oxidation–reduction reactions are highly unstable and create oxidative stress, which damages cellular proteins, lipids, carbohydrates, and nucleic acids (Filomeni & Ciriolo, 2006). To counteract the harmful effect of oxidants, the organism defends itself with antioxidants. When antioxidants are not sufficient to deal with the effects of oxidants, oxidative stress can occur (Valko, Rhodes, Moncol, Izakovic, & Mazur, 2006). Brain tissue is highly susceptible to oxidative stress by free radicals due to its high level of oxygen utilization along with modest antioxidant defenses. The high lipid content of the brain worsens the problem because lipids act as a substrate for oxidation.
Although details about the pathogenesis of ADHD remain to be worked out, family, twin, and molecular genetic disorders suggest that it has a strong genetic component (Faraone & Mick, 2010). ADHD is also influenced by neurodevelopmental exposure to pregnancy and delivery complications, lead, pesticides, polychlorinated biphenyls, and other toxins (Banerjee, Middleton, & Faraone, 2007). Because either genetic or environmental risk factors could increase the likelihood of oxidative stress, several authors have studied peripheral measures of oxidative stress in patients with ADHD. Some of these studies concluded that measures of oxidative stress were elevated among ADHD patients (Archana et al., 2012; Bulut et al., 2007; M. Ceylan, Sener, Bayraktar, & Kavutcu, 2010; M. F. Ceylan, Sener, Bayraktar, & Kavutcu, 2012; Selek, Bulut, Ocak, Kalenderoglu, & Savas, 2012; Selek, Savas, Gergerlioglu, Bulut, & Yilmaz, 2008), but others could not confirm that finding (Oztop, Altun, Baskol, & Ozsoy, 2012).
Indirect evidence for oxidative stress in ADHD comes from studies showing some treatment efficacy for antioxidant compounds such as omega-3 fatty acids, pycnogenol, and N-acetylcysteine (NAC). Bloch et al.’s (2011) meta-analysis concluded that treatment with omega-3 fatty acids (especially formulations with higher doses of eicosapentaenoic acid [EPA]) significantly reduced symptoms of ADHD. Moreover, some evidence suggests that two antioxidants, pycnogenol (Chovanova et al., 2006) and NAC, are effective in treating ADHD symptoms (Garcia et al., 2013).
Confirming oxidative stress as a component of ADHD’s pathophysiology would have considerable research and clinical implications. From a research perspective, it would motivate efforts to determine how the oxidative stress pathway leads to ADHD. From a clinical perspective, it would suggest that antioxidants might be useful for the treatment of ADHD and would motivate the search for more effective antioxidant therapies. For these reasons, we decided to use meta-analysis to clarify the conflicting literature about oxidative stress and ADHD.
Method
We used the following search algorithm at pubmed.gov: (adhd[Title/Abstract] OR “attention deficit” [Title/Abstract] OR “hyperactive” [Title/Abstract]) AND (oxidative [Title/Abstract] OR “antioxidant” [Title/Abstract] “redox” [Title/Abstract] OR oxidation [Title/Abstract]). If the reference sections of any of these articles suggested additional articles, these were also examined. From the PubMed search, we selected studies that met the following criteria: (a) the study compared ADHD patients and controls on a measure of oxidative stress or antioxidant activity; (b) the ADHD diagnoses were based on the Diagnostic and Statistical Manual of Mental Disorders (3rd ed.; DSM-III; American Psychiatric Association [APA], 1980) or later, or its equivalent in the International Classification of Diseases (ICD); (c) the publication provided the numbers of ADHD and non-ADHD participants and the means and standard deviations of oxidative stress or antioxidant measures; and (d) the participants had not been treated with psychoactive medications.
We extracted the following methodological features of each article: the mean age of participants, the percentage of male participants, the diagnostic system used to make diagnoses, the method of diagnosis (structured interview vs. rating scale), the source of patients (clinic, community, or both), the fraction of the sample that was Caucasian, and the tissue used to assay measures of oxidative stress or antioxidant activity.
For each study, all dependent outcome measures reported were treated as a separate data point for entry into the analysis, with several studies providing data on more than one measure to permit comparison of measures as well as among drugs in this population. Because measures reported from the same study are not statistically independent of one another, standard statistical procedures will produce inaccurate p values. To address this intrastudy clustering, to compute accurate p values for estimates of effect sizes, variance estimates were adjusted using Huber’s (1967) formula as implemented in STATA (Stata Corporation, 2001). This formula is a “theoretical bootstrap” that produces robust statistical tests. The method works by entering the cluster scores (i.e., sum of scores within families) into the formula for the estimate of variance. The resulting p values are valid even when observations are not statistically independent.
We computed separate meta-analyses for measures of oxidative stress and antioxidant activity. These meta-analyses used the random effects model of DerSimonian and Laird (1986), which computes a pooled standardized mean difference (SMD) weighted by sample size. We used the I2 index to assess the heterogeneity of effect sizes (Higgins, Thompson, Deeks, & Altman, 2003). Its value lies between 0 and 100 and estimates the percentage of variation among effect sizes that can be attributed to heterogeneity. A significant I2 suggests that the effect sizes analyzed are not estimating the same population effect size. We used Egger at al.’s (1997) method to assess for publication biases. To determine whether any one observation was skewing the results, we reran the meta-analysis deleting one observation at a time to determine whether the statistical significance of the pooled effect could be accounted for by any one observation.
We used meta-analytic regression to assess the degree to which the effect sizes varied with the methodological features of each study (Hedges & Olkin, 1985; Hunter & Schmidt, 1990). We estimated a separate model for each feature. The meta-analyses and meta-analytic regressions were weighted by the reciprocal of the variance of the effect size. To address intrastudy clustering, variance estimates were adjusted using Huber’s (1967) formula as implemented in STATA (Stata Corporation, 2001).
Results
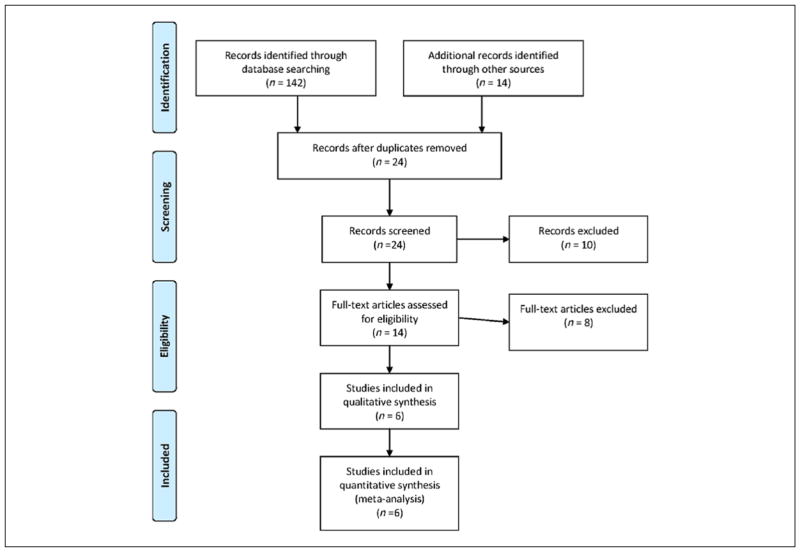
Figure 1 describes our selection of studies using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) format. Six studies met our selection criteria. Six of these provided 8 measures of antioxidant activity among 170 ADHD and 151 control participants. The six included studies provided 10 measures of oxidative stress among 231 ADHD and 207 control participants. Table 1 gives the characteristics of each study’s sample. The participants from most studies were predominantly male youth. All studies ascertained patients from clinics and all but one used Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM-IV; APA, 1994) diagnostic criteria. There was more variability in the diagnostic method used (structured interview vs. rating scale) and all but one study used blood as the basis for their assays.
Figure 1.
PRISMA flow diagram.
Note. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses (http://www.prisma-statement.org/).
Table 1.
Study Features.
| Study | Number of ADHD | Number of controls | % male | M age | Diagnostic system | Source of patients | Diagnostic method | Tissue used for assay |
|---|---|---|---|---|---|---|---|---|
| M. Ceylan, Sener, Bayraktar, and Kavutcu (2010) | 35 | 35 | 69 | 10 | DSM-IV | Clinic | SI | Blood |
| Archana et_al. (2012) | 20 | 20 | 70 | 9 | DSM-IV | Clinic | RS | Saliva |
| M. F. Ceylan, Sener, Bayraktar, and Kavutcu (2012) | 35 | 35 | 69 | 10 | DSM-IV | Clinic | SI | Blood |
| Selek, Bulut, Ocak, Kalenderoglu, and Savas (2012) | 50 | 31 | 68 | 26 | DSM-IV | Clinic | RS | Blood |
| Oztop, Altun, Baskol, and Ozsoy (2012) | 30 | 30 | 75 | 9 | DSM-IV | Clinic | SI | Blood |
| Chovanova et_al. (2006) | 61 | 56 | 82 | 12 | ICD-10 | Clinic | RS | Blood |
Note. DSM-IV = Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM-IV; American Psychiatric Association [APA], 1994); SI = structured interview; RS = rating scale; ICD-10 = International Classification of Diseases–10th Revision.
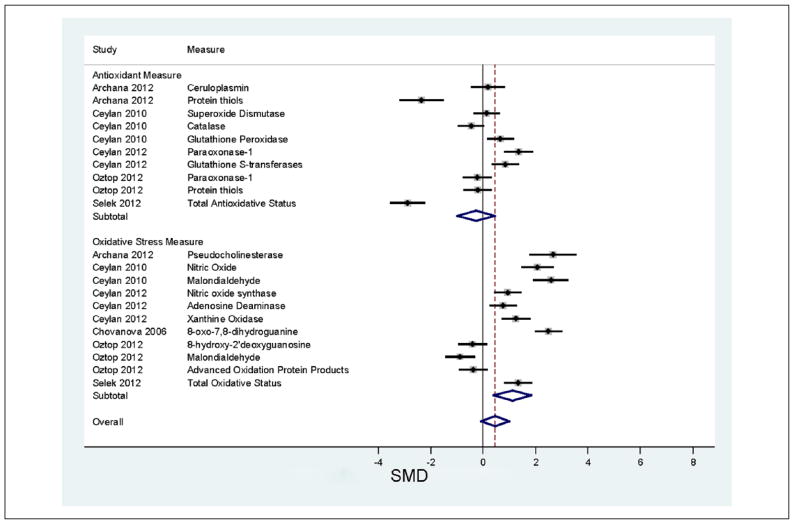
The results of the meta-analyses are depicted in Figure 2. The top panel of the figure gives the results for measures of antioxidant activity. SMDs greater than zero indicate that ADHD patients show lower antioxidant activity than controls, which would indicate a risk for oxidative stress due to less protection from antioxidants. For studies of antioxidant activity, the pooled SMD of −0.27 was not significant (z = 0.8, p = .5). Only two studies were individually significant (in the figure, their 95% confidence intervals exclude zero). Both of these studies suggest, contrary to our hypothesis, that ADHD patients have greater antioxidant activity than controls. Taken together, the antioxidant studies were highly and significantly heterogeneous (I2 = 94.4, p < .001). We also found significant evidence of publication bias (t = 2.3, p = .49).
Figure 2.
Meta-analysis comparing measures of oxidative stress and antioxidant activity in ADHD and control participants.
Note. SMDs greater than zero indicate increasing support for the hypothesis that oxidative stress, or low antioxidant activity are associated with ADHD. For each comparison, the dot gives the relative risk and the horizontal line gives the 95% confidence interval. The center of the diamond at the bottom gives the weighted relative risk across all studies and the width of the diamond gives its 95% confidence interval. The subtotals give the SMD stratified by type of measure (antioxidant activity vs. oxidative stress). SMD = standardized mean difference.
The results for oxidative stress measures are shown in the bottom panel of Figure 2. Across these measures, the pooled SMD of 1.1 was statistically significant (z = 3.1, p = .002) but lost significance after correcting for intrastudy clustering (t = 1.9, p = .1). Heterogeneity was high and statistically significant (I2 = 94.9, p < .001), and there was no evidence of publication bias (t = 1.2, p = .3). As Figure 2 (bottom panel) shows, 8 of 11 observations are consistent with oxidative stress being associated with ADHD because the lower bounds of their 95% confidence intervals exceed zero. Two observations provide no evidence for association and one found that ADHD was associated with lower levels of oxidative stress. When we reestimated the meta-analysis deleting one observation at a time, the pooled SMD remained significant, indicating that no one observation accounted for the positive result.
Meta-analysis regression found a statistically significant difference between the pooled SMD from the antioxidant activity measures and the pooled SMD from the oxidative stress measures (t = 2.5, p = .02). Larger SMDs were seen in studies of children compared with studies of adults (t = 3.6, p = .02) and with studies having an earlier year of publication (t = 3.7, p = .02). None of the other study features in Table 1 were predictive of the SMDs (all ps > .07). The features that were significant predictors were not significantly associated with one another (all ps > .5).
Discussion
Our results are preliminary given the small number of studies. From our two meta-analyses, we can draw two conclusions based on the literature comprising 231 ADHD and 207 control participants. These studies found no evidence for decreased antioxidant activity among ADHD patients but modest evidence for increased oxidative stress to be associated with ADHD. The results for oxidative stress measures could not be accounted for by publication biases, unusual results from any one observation, sample characteristics or study design features. There was substantial and significant heterogeneity among both the antioxidant and oxidative stress studies, some of which was accounted for by the year of publication and the age of the study participants. The finding of greater effect sizes for studies of children compared with studies of adults suggests that attempts to replicate these results should focus on the studies of children.
Some disorders, such as leprosy (Reddy, Murthy, Krishna, & Prabhakar, 2003) and hyperthyroidism (Bianchi et al., 1999), are characterized by high oxidative stress indices and low antioxidant activity. In such cases, one would suspect that the pathophysiology of disease involves defects in the body’s ability to maintain normal levels of antioxidant activity. Although we cannot rule out such a mechanism for ADHD, it seems unlikely given that, as a group, the studies reviewed suggest that ADHD patients show normal levels of antioxidant activity. Moreover, as is apparent from Figure 2, two studies found that ADHD patients had significantly higher antioxidant activity than normal (Archana et al., 2012; Selek et al., 2012). One possibility is that patients with ADHD have a normal baseline level of antioxidant production but that their response to oxidative stress is insufficient, leading to greater than expected levels of oxidative damage. This idea suggests that future studies should measure the balance between oxidative stress and antioxidant activity. One of the studies we reviewed attempted to do this by creating a ratio of two measures: total antioxidative status of plasma (TAS) and total oxidative status of plasma (TOS; Selek et al., 2012). As can be seen in Figure 2 (last line of each subgroup), their ADHD group showed significantly high TOS and significantly high TAS. The ratio of TOS to TAS was significantly greater in ADHD patients and controls, which supports the idea that, for the ADHD patients, the antioxidant response to oxidative stress was insufficient.
These data showing an increased ratio of oxidative to antioxidative status in ADHD suggest the possibility that ADHD people cannot mount a sufficient response to increased oxidative stress. It is the imbalance between ROS and antioxidant defenses that ultimately leads to oxidative damage to lipids, proteins, and nucleic acids. The oxidative damage to these targets can lead to altered protein structure, localization, activity and interaction networks, epigenetic modification to DNA chromatin structure, disrupted membrane lipid integrity, mitochondria dysfunction, and even apoptosis (Avery, 2011; Garcia-Gimenez et al., 2012; Martinez, Portero-Otin, Pamplona, & Ferrer, 2010; Wang, Yang, & Yi, 2012).
Oxidative stress has also been implicated in autism, schizophrenia, bipolar disorder, and depression (Ng et al., 2008; Tsaluchidu, Cocchi, Tonello, & Puri, 2008). Given that ADHD shares genetic underpinnings with these disorders (Faraone, Biederman, & Wozniak, 2012; Faraone & Mick, 2010; Mulligan et al., 2009; Nijmeijer et al., 2010), it is possible that some of this shared diathesis mediates abnormalities in oxidative stress response pathways. Moreover, obstetric complications have been implicated in these disorders and such complications are another source of oxidative stress and damage to the developing brain (Shim & Kim, 2013).
The brain is particularly vulnerable to oxidative stress because of its high lipid content and because neurons have an especially high demand for energy consumption (DiMauro & Schon, 2008; Mattson & Liu, 2002). Neurons use mitochondria to produce adenosine triphosphate (ATP) to power the ion pumps which are concentrated in the pre-synaptic terminals and required to restore the membrane potentials after depolarization and neurotransmitter release (Mattson & Liu, 2002). During the ATP production, the mitochondrial electron transport chain produces a proton gradient to drive phosphorylation of adenosine diphosphate (ADP), and transfers the electrons to oxygen to produce water with the addition of two protons (Rich, 2003). However, partial reduction of oxygen generates harmful “side products” such as superoxide anion ( ; Davies, 1995). Thus, the increased energy demand by neurons and synapses further exacerbates the risk of oxidative stress for brain disorders like ADHD.
ADHD is thought to be a disorder with deficiency of striatal-frontal dopamine circuits (Faraone, 2006; Faraone & Biederman, 1998). Stimulant medication is effective in treating ADHD symptoms by blocking the dopamine transporter, thus enhancing synaptic dopamine levels (Faraone, 2006; Faraone & Biederman, 1998). Interestingly, elevated hydrogen peroxide (H2O2), a major oxidant generated by dysfunctional mitochondria, suppresses striatal dopamine release (Avshalumov & Rice, 2003), which may be a potential mechanism underlying the dopamine deficiency in ADHD. Although dopamine and other catecholamines possess antioxidative and free radical scavenging properties (Cao, Sofic, & Prior, 1997; Yen & Hsieh, 1997), dopamine is easily oxidized and generates highly reactive metabolites such as dopamine quinone, which further lead to mitochondrial dysfunction and oxidative stress (Miyazaki & Asanuma, 2008). Thus, it is not surprising to see the “paradoxical effect” of stimulant treatment, which causes oxidative stress (El-Tawil, Abou-Hadeed, El-Bab, & Shalaby, 2011; Martins et al., 2006) but is also neuroprotective (Volz, 2008) and is an effective treatment for ADHD (Faraone & Buitelaar, 2010). In contrast, clonidine, which is also an effective treatment for ADHD, reduces oxidative stress in rats (Filos et al., 2012; Nik Yusoff, Mustapha, Govindasamy, & Sirajudeen, 2013). Whether this is an alternative mechanism of action for this drug is unknown.
If the imbalance of ROS production and antioxidant defense is a risk factor for ADHD, then antioxidant therapy should combat increased oxidative stress and reduce ADHD symptoms by modulating striatal dopamine release. This idea is consistent with the results of a meta-analysis, which reported modest improvements in ADHD symptoms after treatment with omega-3 fatty acids (Bloch & Qawasmi, 2011). The therapeutic effect was strongest for formulations having higher levels of EPA, which is known to reduce oxidative stress (Mori et al., 2003). In addition to the omega-3 fatty acid findings, emerging evidence suggests that NAC reduces ADHD symptoms in patients with systemic lupus erythematosus (Garcia et al., 2013). NAC is a precursor of glutathione, which is a potent antioxidant. Consistent with these data, nitric oxide increases oxidative stress, and several studies show that nitric oxide inhibitors decrease inattention and hyperactivity in rat models of ADHD (e.g., Aspide, Gironi Carnevale, Sergeant, & Sadile, 1998; Grammatikopoulos et al., 2002).
The idea that oxidative stress plays a role in the pathophysiology of ADHD is also consistent with meta-analysis results finding low levels of zinc in patients with ADHD (Scassellati, Bonvicini, Faraone, & Gennarelli, 2012), because zinc is a cofactor in many reactions that promote antioxidant defenses (Jomova & Valko, 2011). Meta-analysis also indicates that increased lead exposure is associated with ADHD and lead promotes oxidative stress by depleting glutathione (Jomova & Valko, 2011).
Another relevant line of evidence comes from studies of ADHD and allergic rhinitis. Several population studies have reported an association between ADHD and allergic rhinitis (Chen et al., 2013; Chou et al., 2013; Shyu, Lin, Lin, & Fu, 2012). This association is relevant to our findings, because increased levels of oxidative stress markers have been reported in patients with allergic rhinitis (Celik et al., 2012; Emin, Hasan, Aysegul, & Rusen, 2012; Sadowska-Woda, Bieszczad-Bedrejczuk, & Rachel, 2010). Of note, one of these studies found increases in both a measure of oxidative stress and a measure of antioxidant status (Emin et al., 2012), which is consistent with the results we have reported for ADHD.
Any discussion of ADHD and oxidative stress must consider the effects of iron, which catalyzes reactions that create oxidative stress (Valko et al., 2006). Meta-analysis shows that ADHD is associated with low levels of serum ferritin, the storage mechanism for iron that is used as a proxy for iron burden (Scassellati et al., 2012). And a brain imaging study found ADHD patients to have reduced iron levels in the thalamus (Cortese, Azoulay, et al., 2012). These data have been interpreted to mean that ADHD youth suffer from iron deficiency. However, studies of iron supplementation as a treatment for ADHD do not support this hypothesis as these have been equivocal (Cortese, Angriman, Lecendreux, & Konofal, 2012). It is possible that low serum ferritin, rather than signaling iron deficiency, is an indicator of oxidative stress in ADHD. Ferritin is not simply a storage vehicle for iron. It limits the availability of iron for participation in oxygen radical generation and is normally activated by iron regulatory proteins in response to oxidative stress (Orino et al., 2001; Tsuji et al., 2000). Thus, low levels of ferritin in ADHD could indicate that defects in iron regulatory proteins cause an abnormal ferritin response, which would be expected to increase oxidative stress.
We also must consider the possibility that the association between ADHD and oxidative stress is an epiphenomenon caused by an unmeasured third variable. For example, people with ADHD are at increased risk for obesity (Albayrak et al., 2013; Cortese et al., 2008) and cigarette smoking (Biederman, Petty, Hammerness, Batchelder, & Faraone, 2012; Biederman, Petty, Woodworth, et al., 2012), both of which are known to cause oxidative stress (Babizhayev, Savel’yeva, Moskvina, & Yegorov, 2011; Bondia-Pons, Ryan, & Martinez, 2012). Because most of the samples studied were from children, it is unlikely that smoking confounds our meta-analytic results, but effects of obesity on oxidative stress cannot be ruled out.
Our conclusions are limited by some methodological issues. Like all meta-analyses, our analyses of covariates were limited by the information provided in the papers we reviewed. More importantly, only six studies met our inclusion criteria and these provided a modest total sample size of 231 ADHD and 207 control participants, which is not large by meta-analysis standards. Nevertheless, because the power of meta-analysis derives from the total number of participants across studies, we had sufficient power for our primary analyses as is supported by the significant effect for oxidative stress measures. By necessity, all of the studies examined peripheral measures of oxidative stress, which may only have a tenuous link to events in the brain. However, other peripheral biomarkers have been significantly associated with ADHD (Scassellati et al., 2012), so our findings are not unusual in that respect. Also, as discussed above, the measures of oxidative stress could reflect peripheral sources of ADHD’s pathophysiology, such as immune system abnormalities.
Despite these limitations, our meta-analyses provide preliminary, suggestive evidence that oxidative stress plays a role in the pathophysiology of ADHD. These results could not be accounted for by medication effects, publication biases, sample characteristics, study design features, or unusual results for any one observation. Combined with the fact that some antioxidant therapies have shown efficacy for treating ADHD, our finding suggests that more work be done to identify a subgroup of ADHD youth at high risk for oxidative stress who may benefit from antioxidant treatments.
Acknowledgments
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Biographies
Nidhin Joseph completed his MBBS (Bachelor of Medicine and Surgery) from ACPM Medical College, Dhule, India in 2007. He graduated from the Masters Program for Public Health at the University of Massachusetts in 2012, and voluteered at SUNY Upstate for 2 months after gradution. During his visit to India, there was a flood disaster in north India and he volunteered his services to diagnose and treat people in need. Currently, he is working as a physician in the Himalayan region of India where the disaster occurred, and trying to improve the healthcare of the villagers by providing free health care.
Yanli Zhang-James, MD, PhD, is a Research Assistant Professor in the Department of Psychiatry & Behavioral Sciences at SUNY Upstate Medical University. The primary goal of her research is to understand the biological mechanisms of complex neuropsychiatric disorders, including ADHD, autism, and Parkinson’s disease, and to identify new biological pathways that may ultimately lead to novel and better treatments for these disorders.
Andras Perl, MD, PhD, is a Professor in the Departments of Medicine, Biochemistry and Molecular Biology, and Microbiology and Immunology at SUNY Upstate Medical University. He is the Chief of Rheumatology and Co-chair of the MD/PhD program. His clinical interests include: Arthritis, Rheumatic Diseases, Autoimmune Diseases, Systemic Lupus Erythematosus, and Transaldolase Deficiency. His research interests include: Genes and Viruses Predisposing to Autoimmunity, Genetics, Apoptosis, Endogenous Retroviruses, and Transaldolase.
Stephen V. Faraone, PhD, is Director of Medical Genetics Research at SUNY Upstate Medical University and Professor of Psychiatry and of Neuroscience & Physiology, and Director of Psychiatric Research. He has a long funding history and numerous publications in psychiatry, psychiatric genetics, pharmacogenetics, clinical and preclinical studies. His work has had substantial scientific impact. In 2011, he was the seventh most highly cited researcher in psychiatry and psychology for the preceding decade with a 2012 lifetime H-Index of 71 (http://academic.research.microsoft.com).
Footnotes
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: In the past year, Dr. Faraone received consulting income and/or research support from Shire, Akili Interactive Labs, and Alcobra, and research support from the National Institutes of Health (NIH). His institution is seeking a patent for the use of sodium–hydrogen exchange inhibitors in the treatment of ADHD. In previous years, he received consulting fees or was on Advisory Boards or participated in continuing medical education programs sponsored by Shire, Alcobra, Otsuka, McNeil, Janssen, Novartis, Pfizer, and Eli Lilly. Dr. Faraone receives royalties from books published by Guilford Press: Straight Talk about Your Child’s Mental Health and Oxford University Press: Schizophrenia: The Facts.
References
- Albayrak O, Putter C, Volckmar AL, Cichon S, Hoffmann P, Nothen MM, … Hinney A. Common obesity risk alleles in childhood attention-deficit/hyperactivity disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2013;162:295–305. doi: 10.1002/ajmg.b.32144. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: Author; 1980. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Archana E, Pai P, Prabhu BK, Shenoy RP, Prabhu K, Rao A. Altered biochemical parameters in saliva of pediatric attention deficit hyperactivity disorder. Neurochemical Research. 2012;37:330–334. doi: 10.1007/s11064-011-0616-x. [DOI] [PubMed] [Google Scholar]
- Aspide R, Gironi Carnevale UA, Sergeant JA, Sadile AG. Non-selective attention and nitric oxide in putative animal models of attention-deficit hyperactivity disorder. Behavioural Brain Research. 1998;95:123–133. doi: 10.1016/s0166-4328(97)00217-9. [DOI] [PubMed] [Google Scholar]
- Avery SV. Molecular targets of oxidative stress [Research Support, Non-U.S. Gov’t Review] Biochemical Journal. 2011;434:201–210. doi: 10.1042/BJ20101695. [DOI] [PubMed] [Google Scholar]
- Avshalumov MV, Rice ME. Activation of ATP-sensitive K+ (K(ATP)) channels by H2O2 underlies glutamate-dependent inhibition of striatal dopamine release [In Vitro Research Support, U.S. Gov’t, P.H.S.] Proceedings of the National Academy of Sciences. 2003;100:11729–11734. doi: 10.1073/pnas.1834314100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babizhayev MA, Savel’yeva EL, Moskvina SN, Yegorov YE. Telomere length is a biomarker of cumulative oxidative stress, biologic age, and an independent predictor of survival and therapeutic treatment requirement associated with smoking behavior [Research Support, Non-U.S. Gov’t Review] American Journal of Therapeutics. 2011;18:e209–e226. doi: 10.1097/MJT.0b013e3181cf8ebb. [DOI] [PubMed] [Google Scholar]
- Banerjee TD, Middleton F, Faraone SV. Environmental risk factors for attention-deficit hyperactivity disorder. Acta Pediatrica. 2007;96:1269–1274. doi: 10.1111/j.1651-2227.2007.00430.x. [DOI] [PubMed] [Google Scholar]
- Bianchi G, Solaroli E, Zaccheroni V, Grossi G, Bargossi AM, Melchionda N, Marchesini G. Oxidative stress and anti-oxidant metabolites in patients with hyperthyroidism: Effect of treatment [Clinical Trial] Hormone and Metabolic Research. 1999;31:620–624. doi: 10.1055/s-2007-978808. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Hammerness P, Batchelder H, Faraone SV. Cigarette smoking as a risk factor for other substance misuse: 10-year study of individuals with and without attention-deficit hyperactivity disorder. British Journal of Psychiatry. 2012;201:207–214. doi: 10.1192/bjp.bp.111.100339. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Woodworth KY, Lomedico A, Hyder LL, Faraone SV. Adult outcome of attention-deficit/hyperactivity disorder: A controlled 16-year follow-up study. Journal of Clinical Psychiatry. 2012;73:941–950. doi: 10.4088/JCP.11m07529. [DOI] [PubMed] [Google Scholar]
- Bloch MH, Qawasmi A. Omega-3 fatty acid supplementation for the treatment of children with attention-deficit/hyperactivity disorder symptomatology: Systematic review and meta-analysis. Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50:991–1000. doi: 10.1016/j.jaac.2011.06.008. S0890-8567(11)00484-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondia-Pons I, Ryan L, Martinez JA. Oxidative stress and inflammation interactions in human obesity [Research Support, Non-U.S. Gov’t Review] Journal of Physiology and Biochemistry. 2012;68:701–711. doi: 10.1007/s13105-012-0154-2. [DOI] [PubMed] [Google Scholar]
- Bulut M, Selek S, Gergerlioglu HS, Savas HA, Yilmaz HR, Yuce M, Ekici G. Malondialdehyde levels in adult attention-deficit hyperactivity disorder. Journal of Psychiatry & Neuroscience. 2007;32:435–438. [PMC free article] [PubMed] [Google Scholar]
- Cao G, Sofic E, Prior RL. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radical Biology & Medicine. 1997;22:749–760. doi: 10.1016/s0891-5849(96)00351-6. [DOI] [PubMed] [Google Scholar]
- Celik M, Tuncer A, Soyer OU, Sackesen C, Tanju Besler H, Kalayci O. Oxidative stress in the airways of children with asthma and allergic rhinitis. Pediatric Allergy and Immunology. 2012;23:556–561. doi: 10.1111/j.1399-3038.2012.01294.x. [DOI] [PubMed] [Google Scholar]
- Ceylan M, Sener S, Bayraktar AC, Kavutcu M. Oxidative imbalance in child and adolescent patients with attention-deficit/hyperactivity disorder. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2010;34:1491–1494. doi: 10.1016/j.pnpbp.2010.08.010. S0278-5846(10)00313-1 [pii] [DOI] [PubMed] [Google Scholar]
- Ceylan MF, Sener S, Bayraktar AC, Kavutcu M. Changes in oxidative stress and cellular immunity serum markers in attention-deficit/hyperactivity disorder. Psychiatry and Clinical Neurosciences. 2012;66:220–226. doi: 10.1111/j.1440-1819.2012.02330.x. [DOI] [PubMed] [Google Scholar]
- Chen MH, Su TP, Chen YS, Hsu JW, Huang KL, Chang WH, … Bai YM. Comorbidity of allergic and autoimmune diseases among patients with ADHD: A nationwide population-based study. Journal of Attention Disorders. 2013 doi: 10.1177/1087054712474686. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Chou PH, Lin CC, Lin CH, Loh el W, Chan CH, Lan TH. Prevalence of allergic rhinitis in patients with attention-deficit/hyperactivity disorder: A population-based study. European Child & Adolescent Psychiatry. 2013;22:301–307. doi: 10.1007/s00787-012-0369-3. [DOI] [PubMed] [Google Scholar]
- Chovanova Z, Muchova J, Sivonova M, Dvorakova M, Zitnanova I, Waczulikova I, … Durackova Z. Effect of polyphenolic extract, Pycnogenol, on the level of 8-oxoguanine in children suffering from attention deficit/hyperactivity disorder. Free Radical Research. 2006;40:1003–1010. doi: 10.1080/10715760600824902. WM44447X8027783L [pii] [DOI] [PubMed] [Google Scholar]
- Cortese S, Angriman M, Lecendreux M, Konofal E. Iron and attention deficit/hyperactivity disorder: What is the empirical evidence so far? A systematic review of the literature. Expert Review of Neurotherapeutics. 2012;12:1227–1240. doi: 10.1586/ern.12.116. [DOI] [PubMed] [Google Scholar]
- Cortese S, Angriman M, Maffeis C, Isnard P, Konofal E, Lecendreux M, … Mouren MC. Attention-deficit/hyperactivity disorder (ADHD) and obesity: A systematic review of the literatur. Critical Reviews in Food Science and Nutrition. 2008;48:524–537. doi: 10.1080/10408390701540124. 793843639 [pii] [DOI] [PubMed] [Google Scholar]
- Cortese S, Azoulay R, Castellanos FX, Chalard F, Lecendreux M, Chechin D, … Konofal E. Brain iron levels in attention-deficit/hyperactivity disorder: A pilot MRI study. The World Journal of Biological Psychiatry. 2012;13:223–231. doi: 10.3109/15622975.2011.570376. [DOI] [PubMed] [Google Scholar]
- Davies KJ. Oxidative stress: The paradox of aerobic life [Review] Biochemical Society Symposium. 1995;61:1–31. doi: 10.1042/bss0610001. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- DiMauro S, Schon EA. Mitochondrial disorders in the nervous system [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review] Annual Review of Neuroscience. 2008;31:91–123. doi: 10.1146/annurev.neuro.30.051606.094302. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Tawil OS, Abou-Hadeed AH, El-Bab MF, Shalaby AA. d-Amphetamine-induced cytotoxicity and oxidative stress in isolated rat hepatocytes. Pathophysiology. 2011;18:279–285. doi: 10.1016/j.pathophys.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Emin O, Hasan A, Aysegul D, Rusen D. Total antioxidant status and oxidative stress and their relationship to total IgE levels and eosinophil counts in children with allergic rhinitis. Journal of Investigational Allergology and Clinical Immunology. 2012;22:188–192. [PubMed] [Google Scholar]
- Faraone SV. Advances in the genetics and neurobiology of attention deficit hyperactivity disorder. Biological Psychiatry. 2006;60:1025–1027. doi: 10.1016/s0006-3223(98)00240-6. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J. Neurobiology of attention-deficit hyperactivity disorder. Biological Psychiatry. 1998;44:951–958. doi: 10.1016/s0006-3223(98)00240-6. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Wozniak J. Examining the comorbidity between attention deficit hyperactivity disorder and bipolar I disorder: A meta-analysis of family genetic studies. American Journal of Psychiatry. 2012;169:1256–1266. doi: 10.1176/appi.ajp.2012.12010087. 1461101 [pii] [DOI] [PubMed] [Google Scholar]
- Faraone SV, Buitelaar J. Comparing the efficacy of stimulants for ADHD in children and adolescents using meta-analysis. European Child & Adolescent Psychiatry. 2010;19:353–364. doi: 10.1007/s00787-009-0054-3. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Mick E. Molecular genetics of attention deficit hyperactivity disorder. Psychiatric Clinics of North America. 2010;33:159–180. doi: 10.1016/j.psc.2009.12.004. S0193-953X(09)00106-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filomeni G, Ciriolo MR. Redox control of apoptosis: An update [Research Support, Non-U.S. Gov’t Review] Antioxidants & Redox Signaling. 2006;8:2187–2192. doi: 10.1089/ars.2006.8.2187. [DOI] [PubMed] [Google Scholar]
- Filos KS, Panteli ES, Fligou F, Papamichail C, Papapostolou I, Zervoudakis G, … Georgiou C. Clonidine pre-treatment prevents hemorrhagic shock-induced endotoxemia and oxidative stress in the gut, liver, and lungs of the rat. Redox Report. 2012;17:246–251. doi: 10.1179/1351000212Y.0000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia RJ, Francis L, Dawood M, Lai ZW, Faraone SV, Perl A. Attention deficit and hyperactivity disorder scores are elevated and respond to NAC treatment in patients with SLE. Arthritis & Rheumatism. 2013;65:1313–1318. doi: 10.1002/art.37893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gimenez JL, Ledesma AM, Esmoris I, Roma-Mateo C, Sanz P, Vina J, Pallardo FV. Histone carbonylation occurs in proliferating cells [Research Support, Non-U.S. Gov’t] Free Radical Biology & Medicine. 2012;52:1453–1464. doi: 10.1016/j.freeradbiomed.2012.01.022. [DOI] [PubMed] [Google Scholar]
- Grammatikopoulos G, Pignatelli M, D’Amico F, Fiorillo C, Fresiello A, Sadile AG. Selective inhibition of neuronal nitric oxide synthesis reduces hyperactivity and increases non-selective attention in the Naples high-excitability rat. Behavioural Brain Research. 2002;130:127–132. doi: 10.1016/s0166-4328(01)00424-7. [DOI] [PubMed] [Google Scholar]
- Hedges LV, Olkin I. Statistical methods for meta-analysis. Orlando, FL: Academic Press; 1985. [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. British Medical Journal. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber PJ. The behavior of maximum likelihood estimates under non-standard conditions. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability. 1967;1:221–233. [Google Scholar]
- Hunter JE, Schmidt FL. Methods of meta-analysis: Correcting error and bias in research findings. Newbury Park, CA: Sage; 1990. [Google Scholar]
- Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65–87. doi: 10.1016/j.tox.2011.03.001. S0300-483X(11)00088-6 [pii] [DOI] [PubMed] [Google Scholar]
- Martinez A, Portero-Otin M, Pamplona R, Ferrer I. Protein targets of oxidative damage in human neurodegenerative diseases with abnormal protein aggregates [Research Support, Non-U.S. Gov’t Review] Brain Pathology. 2010;20:281–297. doi: 10.1111/j.1750-3639.2009.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins MR, Reinke A, Petronilho FC, Gomes KM, Dal-Pizzol F, Quevedo J. Methylphenidate treatment induces oxidative stress in young rat brain [Comparative Study Research Support, Non-U.S. Gov’t] Brain Research. 2006;1078:189–197. doi: 10.1016/j.brainres.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Liu D. Energetics and oxidative stress in synaptic plasticity and neurodegenerative disorders [Review] NeuroMolecular Medicine. 2002;2:215–231. doi: 10.1385/NMM:2:2:215. [DOI] [PubMed] [Google Scholar]
- Miyazaki I, Asanuma M. Dopaminergic neuron-specific oxidative stress caused by dopamine itself. Acta Medica Okayama. 2008;62:141–150. doi: 10.18926/AMO/30942. [DOI] [PubMed] [Google Scholar]
- Mori TA, Woodman RJ, Burke V, Puddey IB, Croft KD, Beilin LJ. Effect of eicosapentaenoic acid and docosahexaenoic acid on oxidative stress and inflammatory markers in treated-hypertensive type 2 diabetic subjects [Clinical Trial Comparative Study Randomized Controlled Trial Research Support, Non-U.S. Gov’t] Free Radical Biology & Medicine. 2003;35:772–781. doi: 10.1016/s0891-5849(03)00407-6. [DOI] [PubMed] [Google Scholar]
- Mulligan A, Anney RJ, O’Regan M, Chen W, Butler L, Fitzgerald M, … Gill M. Autism symptoms in attention-deficit/hyperactivity disorder: A familial trait which correlates with conduct, oppositional defiant, language and motor disorders. Journal of Autism and Developmental Disorders. 2009;39:197–209. doi: 10.1007/s10803-008-0621-3. [DOI] [PubMed] [Google Scholar]
- Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: Evidence base and therapeutic implications [Review] International Journal of Neuropsychopharmacology. 2008;11:851–876. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- Nijmeijer JS, Arias-Vasquez A, Rommelse NN, Altink ME, Anney RJ, Asherson P, … Hoekstra PJ. Identifying loci for the overlap between attention-deficit/hyperactivity disorder and autism spectrum disorder using a genome-wide QTL linkage approach. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49:675–685. doi: 10.1016/j.jaac.2010.03.015. S0890-8567(10)00296-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nik Yusoff NS, Mustapha Z, Govindasamy C, Sirajudeen KN. Effect of Clonidine (an antihypertensive drug) treatment on oxidative stress markers in the heart of spontaneously hypertensive rats. Oxidative Medicine and Cellular Longevity, 2013. 2013 doi: 10.1155/2013/927214. Article 927214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orino K, Lehman L, Tsuji Y, Ayaki H, Torti SV, Torti FM. Ferritin and the response to oxidative stress [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] Biochemical Journal. 2001;357(Pt. 1):241–247. doi: 10.1042/0264-6021:3570241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oztop D, Altun H, Baskol G, Ozsoy S. Oxidative stress in children with attention deficit hyperactivity disorder. Clinical Biochemistry. 2012;45:745–748. doi: 10.1016/j.clinbiochem.2012.03.027. S0009-9120(12)00158-0 [pii] [DOI] [PubMed] [Google Scholar]
- Reddy YN, Murthy SV, Krishna DR, Prabhakar MC. Oxidative stress and anti-oxidant status in leprosy patients [Research Support, Non-U.S. Gov’t] Indian Journal of Leprosy. 2003;75:307–316. [PubMed] [Google Scholar]
- Rich PR. The molecular machinery of Keilin’s respiratory chain [Lectures Research Support, Non-U.S. Gov’t] Biochemical Society Transactions. 2003;31(Pt. 6):1095–1105. doi: 10.1042/bst0311095. [DOI] [PubMed] [Google Scholar]
- Sadowska-Woda I, Bieszczad-Bedrejczuk E, Rachel M. Influence of desloratadine on selected oxidative stress markers in patients between 3 and 10 years of age with allergic perennial rhinitis [Clinical Trial] European Journal of Pharmacology. 2010;640:197–201. doi: 10.1016/j.ejphar.2010.04.060. [DOI] [PubMed] [Google Scholar]
- Scassellati C, Bonvicini C, Faraone SV, Gennarelli M. Biomarkers and attention-deficit/hyperactivity disorder: A systematic review and meta-analyses. Journal of the American Academy of Child & Adolescent Psychiatry. 2012;51:1003–1019. e20. doi: 10.1016/j.jaac.2012.08.015. S0890-8567(12)00605-3 [pii] [DOI] [PubMed] [Google Scholar]
- Selek S, Bulut M, Ocak AR, Kalenderoglu A, Savas HA. Evaluation of total oxidative status in adult attention deficit hyperactivity disorder and its diagnostic implications. Journal of Psychiatric Research. 2012;46:451–455. doi: 10.1016/j.jpsychires.2011.12.007. S0022-3956(11)00289-5 [pii] [DOI] [PubMed] [Google Scholar]
- Selek S, Savas HA, Gergerlioglu HS, Bulut M, Yilmaz HR. Oxidative imbalance in adult attention deficit/hyperactivity disorder. Biological Psychology. 2008;79:256–259. doi: 10.1016/j.biopsycho.2008.06.005. S0301-0511(08)00147-6 [pii] [DOI] [PubMed] [Google Scholar]
- Shim SY, Kim HS. Oxidative stress and the antioxidant enzyme system in the developing brain. Korean Journal of Pediatrics. 2013;56:107–111. doi: 10.3345/kjp.2013.56.3.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu CS, Lin HK, Lin CH, Fu LS. Prevalence of attention-deficit/hyperactivity disorder in patients with pediatric allergic disorders: A nationwide, population-based study [Research Support, Non-U.S. Gov’t] Journal of Microbiology, Immunology and Infection. 2012;45:237–242. doi: 10.1016/j.jmii.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Stata Corporation. Stata user’s guide: Release 7.0. College Station, TX: Author; 2001. [Google Scholar]
- Tsaluchidu S, Cocchi M, Tonello L, Puri BK. Fatty acids and oxidative stress in psychiatric disorders. BMC Psychiatry. 2008;8:S5. doi: 10.1186/1471-244X-8-S1-S5. 1471-244X-8-S1-S5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji Y, Ayaki H, Whitman SP, Morrow CS, Torti SV, Torti FM. Coordinate transcriptional and translational regulation of ferritin in response to oxidative stress [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] Molecular and Cellular Biology. 2000;20:5818–5827. doi: 10.1128/mcb.20.16.5818-5827.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer [Research Support, Non-U.S. Gov’t Review] Chemico-Biological Interactions. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Volz TJ. Neuropharmacological mechanisms underlying the neuroprotective effects of methylphenidate. Current Neuropharmacology. 2008;6:379–385. doi: 10.2174/157015908787386041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yang J, Yi J. Redox sensing by proteins: Oxidative modifications on cysteines and the consequent events [Research Support, Non-U.S. Gov’t Review] Antioxidants & Redox Signaling. 2012;16:649–657. doi: 10.1089/ars.2011.4313. [DOI] [PubMed] [Google Scholar]
- Yen GC, Hsieh CL. Antioxidant effects of dopamine and related compounds [Research Support, Non-U.S. Gov’t] Bioscience, Biotechnology, and Biochemistry. 1997;61:1646–1649. doi: 10.1271/bbb.61.1646. [DOI] [PubMed] [Google Scholar]