Abstract
IL-15 is a member of the gamma chain family of cytokines (γc – CD132). The IL-15 receptor (IL-15R) complex consists of 3 subunits: the ligand-binding IL-15Rα chain (CD215), the β chain (CD122; also used by IL-2), and the common γ chain. The biological activities of IL-15 are mostly mediated by the IL-15:IL-15Rα complex, produced by the same cell and ‘trans-presented’ to responder cells expressing the IL-15Rβγc. The peculiar and almost unique requirement for IL-15 to be trans-presented by IL-15Rα suggests that the biological effects of IL-15 signaling are tightly regulated even at the level of availability of IL-15. Tissue-specific deletion of IL-15Rα has shown macrophage-and dendritic cell-derived IL-15Rα mediate the homeostasis of different CD8+ T cell subsets. Here we show that hepatocyte and macrophage- specific expression of IL-15Rα is required to maintain the homeostasis of NK and NKT cells in the liver. Thus, homeostasis of IL-15-dependent lymphocyte subsets is also regulated by trans-presentation of IL-15 by non-hematopoietic cells in the tissue environment.
Keywords: Interleukin-15, Liver, NAFLD
1. Introduction
Interleukin-15 (IL-15), that was initially characterized as a cytokine required for the maintenance of memory CD8+ T cells, has a multi-faceted role in hematopoietic and non-hematopoietic system [1]. IL-15 plays a critical role in the growth and differentiation of T lymphocytes, NK cells, NKT cells and different subsets of innate lymphoid cells [1,2]. Notably, IL-15 promotes the survival of memory CD8+ and CD4+ T cells [3,4]. The IL-15 receptor (IL-15R) complex consists of 3 subunits: the ligand-binding IL-15Rα chain (CD215), the β chain (CD122; also used by IL-2), and the common γc chain. The biological activities of IL-15 are mostly mediated by the IL-15:IL-15Rα complex, produced by non-T cells and ‘transpresented’ to responder cells expressing the IL-2/15Rβγc [5–7]. This peculiar and almost unique requirement for trans-presentation by IL-15Rα suggests that the biological effects of IL-15 are tightly regulated even at the level of IL-15 availability. Most of the effects of IL-15 on lymphocytes can be attributed to the IL-15 that is transpresented by hematopoietic and non-hematopoietic cells [8]. Under steady-state even though transcripts for IL-15 are detected in different organs such as placenta, skeletal muscle, intestine and kidney [9], the expression of the protein is below the level of detection by conventional methods [10]. IL-15Rα is widely expressed in various tissues [11]. As IL-15 exists in the circulation mainly in complex with IL-15Rα, it may be already primed for trans-presentation to cells that express IL-2Rβ and γc [12]. The cytoplasmic domain of IL-15Rα, like that of IL-2Rα, appears to be dispensable for signalling. Tissue specific ablation of IL-15Rα has revealed specific, but distinct patterns of requirement for the source of the trans-presented IL-15. For example, NK cells require IL-15 to be trans-presented by both dendritic cells (DCs) and macrophages while central memory CD8+ T cells require DC-derived IL-15 [13–15]. However, maintenance of IL-15 dependent T cell subsets in the intestine requires the expression of IL-15Rα by the epithelial cells of the villi [14].
Liver is host to different subsets of lymphocytes such as CD8+ T cells, NK cells and NKT cells [16] that are dependent on IL-15 for survival and maintenance [17]. Transcripts for IL-15 have been detected in different cell types of both hematopoietic and non-hematopoietic origin in the liver. Liver resident macrophages called Kupffer cells are one of the major subsets that express IL-15 [10,18]. IL-15 from stellate cells has been shown to support the homeostasis of NKT cells [19], while hepatocytes are a significant source of IL-15:IL-15Rα complex [20]. Thus any of the above mentioned populations could support the survival of IL-15 dependent T cell subsets in the liver.
Hepatic accumulation of excess fat derived from diet results in a range of asymptomatic pathologies starting from fatty liver (steatosis) to non-alcoholic steatohepatitis (NASH) that eventually lead to cirrhosis and hepatocellular carcinoma [21]. NASH and insulin resistance are extensively associated with the increased presence of pro-inflammatory cytokines in circulation and in adipose tissues and liver. Activated CD8+ T and NKT cells have been implicated in the progression of NAFLD to HCC [22]. We have shown recently that absence of IL-15 protected from NAFLD (Cepero-Donates et al., manuscript in this issue). As the mice used in the above-mentioned study did not express IL-15 or IL-15Rα at all, it is difficult to identify the source of IL-15 that contributes to NAFLD. Using hepatocyte- and macrophage- specific deletion of IL-15Rα, we show here that both macrophage and hepatocyte mediated trans-presentation of IL-15 is required to support NK and NKT cell subsets in the liver. We further show that deletion of IL-15Rα in hepatocytes or macrophages is not sufficient to prevent the development of NAFLD.
2. Materials and methods
2.1. Mice
All the mice used were in C57BL/6 background. Il15−/− mice have been already described [23]. Il15ra−/− mice were purchased from Jackson Laboratory and bred into C57BL/6 (Charles River) background for more than ten generations. Conditional Il15ra-floxed mice have been described previously [14]. Tissue specific deletion of Il15ra was achieved by crossing these mice with Alb-Cre or LysM-Cre transgenic mice (Jackson Laboratory) to ablate the IL15ra gene specifically in hepatocytes (Il15rafl/fl Alb-Cre+) or macrophages (Il15rafl/fl LysM-Cre). Male mice were used in all the experiments. All the experiments were approved by the institutional Animal Ethics committee.
2.2. Isolation of intrahepatic lymphocytes (IHL)
Intrahepatic lymphocytes were isolated using gentleMACS Dissociator (Miltenyi Biotec) according to the instructions of the manufacturer. Briefly, mice were sacrificed and the livers were collected and digested in collagenase digestion buffer (300 CDU-casein digestion unit/ml collagenase IV) using gentleMACS Dissociator. Next, the homogenized liver samples were gently agitated on a rocking shaker for 30 min at room temperature and passed through 40 μm cell strainers. Cells were resuspended in 25 ml of cold 0.5% Bovine serum albumin and 2 mM EDTA in Phosphate buffered saline (PEB) and centrifuged at 50g for 5 min at 4 °C to eliminate contaminating hepatocytes. The supernatant was centrifuged at 300g for 10 min at 4 °C to collect the lymphocytes. Cells were resuspended in ACK to lyse the red blood cells and washed twice with PEB to prepare the single cell suspension for FACS analysis.
2.3. Flow cytometry
Flow cytometric analyses were performed using standard protocols. Abs against mouse CD3, CD8α, CD8β, CD4, CD44, CD62L, and NK1.1 conjugated to flurochromes were purchased from eBioscience (San Diego, CA), BD Biosciences (San Jose, CA) or Biolegend (San Diego, CA). Mouse CD1d tetramer pre-loaded with PBS57 (an α-GalCer analog; CD1d/PBS57) conjugated to PE was obtained from NIH tetramer Facility. Data was acquired on FACS Canto flow cytometer (BD Biosciences San Diego, CA) and was analyzed using FlowJo software from TreeStar Inc (Ashland, OR).
2.4. Induction of NAFLD in mice
To induce hepatic steatosis, 8-weeks old WT, Il15−/− and Il15ra−/− mice, were maintained on high fat diet (HFD) (Research Diets, New Brunswick, NJ, USA; D12492: 20%kcal protein, 20% carbohydrate and 60% fat). Mice fed with normal control diet (NCD) were used as controls. Mice were maintained on NCD or HFD for 12 weeks before sacrifice. The weight of livers was measured at sacrifice.
2.5. Histology
At sacrifice, livers were kept frozen in OCT for lipid staining. Hematoxylin and eosin or Sudan Black lipid staining was carried out on tissue samples fixed in OCT. The slides were washed in water and mounted with aqueous mounting media (Vecta-Mount™). Images were taken using automatic tissue slide scanning (Hamamatsu NanoZoomer Digital Pathology (NDP) system).
2.6. Statistical analysis
Statistical analyses were performed using GraphPad Prism 6 software. The values are presented as mean ± standard error of the mean. The statistical significance (p value) was calculated by non-parametric comparison between two groups (Mann Whitney test).
3. Results and discussion
3.1. Hepatocyte mediated trans-presentation of IL-15 by IL-15Rα is required to maintain NK cells in the liver
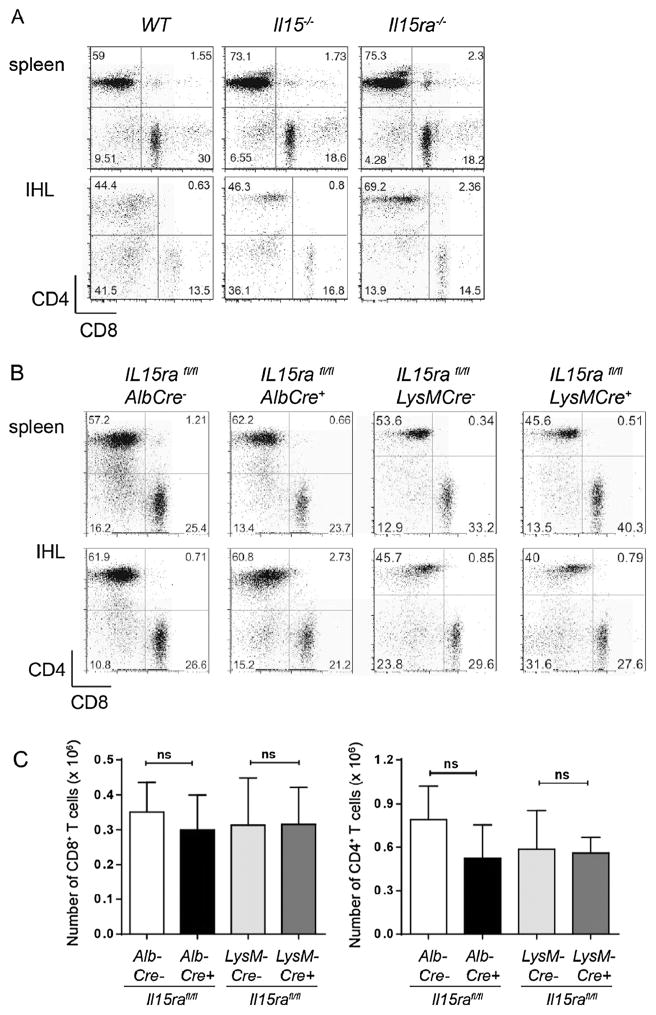
IL-15Rα-mediated trans-presentation of IL-15 is required for the homeostasis of CD8+ T cell subsets in the secondary lymphoid organs [14]. Even though the frequency of antigen-specific CD8+ T cells is reduced during primary and secondary immune responses in the liver [24], it is not clear whether IL-15 signalling is involved in their maintenance in the liver. We observed that the frequency of CD4+ and CD8+ T cells are comparable in the livers of WT, Il15−/− and Il15ra−/− mice (Fig. 1A). Similarly, hepatocyte- or macrophage-specific ablation of IL-15Rα did not change the frequency or the total numbers of CD4+ and CD8+ T cells in the liver (Fig. 1B and C). These observations suggest that basal the homeostasis of T cells in the liver is not influenced by IL-15 signaling.
Fig. 1.
Absence of IL-15 or IL-15Rα does not alter the frequency of CD8+ T cells in the liver. Lymphocytes were isolated from spleen and liver of age matched (A) WT, Il15−/− and Il15ra−/− mice or (B) hepatocyte- and macrophage-specific deletion of IL-15Rα and phenotyped for CD4+ and CD8+ T cells. Representative data from 4 individual mice per group is shown. (C) Absolute numbers of CD4+ and CD8+ T cells were calculated from 4 mice per group. Values are expressed as the means ± SEM. Mann Whitney test; ns – not significant.
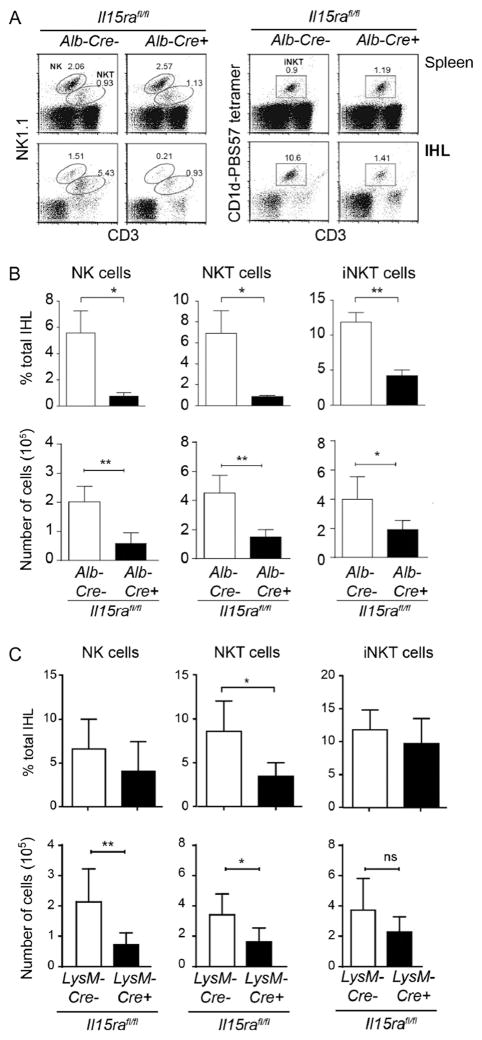
Studies using IL-15 and IL-15Rα deficient mice and adoptive transfer experiments have shown the requirement for IL-15 in the generation and maintenance of NK and NKT cells [25,26]. Thus in the absence of IL-15 or IL-15Rα these subsets were not detected in the intra hepatic lymphocyte population (IHLs) (Fig. 2A, lower panel). Lineage specific ablation of IL-15Rα have revealed the complexity of IL-15 trans-presentation. In the secondary lymphoid organs, IL-15Rα-mediated trans-presentation by DCs and macrophages contributes to the maintenance of NK cells [14]. In the absence of overt infection, NK cells are located in the sinusoidal regions along with the liver resident macrophages, Kupffer cells (KCs) [16]. Ablation of IL-15Rα expression in macrophages resulted in decreased numbers of NK cells in the liver [14]. Similarly, under conditions where IL-15Rα was not expressed by DCs, the numbers of NK cells was also decreased. However deletion of IL-15Rα expression in both macrophages and DCs did not synergistically bring down the NK cell numbers suggesting that IL-15Rα expressed by other cell types in the liver can trans-present IL-15 and contribute to the maintenance of NK cells.
Fig. 2. IL-15Rα expression in hepatocytes and macrophages is required for the maintenance of NK and NKT in the liver.
Lymphocytes were isolated from spleen and liver of age matched (A and B) hepatocyte- and (C) macrophage-specific deletion of IL-15Rα and phenotyped for NK and NKT cells. (A) Representative data from 4 individual mice per group is shown. (B and C) The frequency (upper panels) and the absolute numbers (lower panels) of NK, NKT and iNKT cells were calculated from 4 mice per group. Values are expressed as the means ± SEM. Mann Whitney test; p < 0.05(*); ns – not significant.
Hepatocytes constitute 80% of the cells in the liver and express IL-15 and IL-15Rα [20,27]. To determine whether the NK cell homeostasis is maintained by IL-15 from hepatocytes or macrophages, we analysed the intra-hepatic lymphocyte populations following deletion of IL-15Rα in hepatocytes or macrophages by crossing Il15rafl/fl mice with mice expressing the Cre-recombinase either in liver under the albumin promoter (Alb-Cre) or in macrophages under the lysozyme promoter ((LysM-Cre). We observed that the frequency and the total numbers of NK cells were significantly reduced in Il15rafl/flAlb-Cre+ when compared to Il15rafl/flAlb-Cre− littermate controls (Fig. 2B). As the expression of IL-15 in all other cell types is normal, the spleens of the same mice did not show any difference in the frequency of NK cells (Data not shown). In accordance with the previous report by Mortier et al. [14], the total numbers of NK cells was decreased significantly in Il15rafl/flLysM-Cre+ when compared to Il15rafl/flLysM-Cre− littermate controls (Fig. 2C). Thus, the above results identify hepatocytes as an important cell type that is required for the maintenance of NK cells in the liver.
NK cells develop in the bone marrow, mature in the periphery and reside predominantly in blood, splenic red pulp and in the sinusoidal regions of the liver [28]. Hepatocytes have been shown to be the major producers of IL-15/IL-15Rα complexes in the liver following viral infections through the production of the transcription factor, IRF-1 [20]. Following certain viral infections, NK cells undergo 1000-fold expansions in the liver [28]. Given the requirement of cell-to-cell contact for IL-15 signaling in NK cells, it is plausible that hepatocytes activate NK cells by upregulating IL-15Rα to support the activity of NK cells. NK cells express high levels of CD122, indicating that IL-15 signaling is required throughout their development [29]. IL-15 signals upregulate Bcl-2 family of anti-apoptotic proteins to maintain their survival as overexpression of Bcl-2 in NK cells can compensate for the absence of IL-15 [30,31]. IL-15/IL-15Rα complexes also support the expansion of NK cells [28]. Bone marrow chimera experiments have shown that IL-15 and IL-15Rα need to be expressed on the same cell to support NK cells [32]. Studies using tissue specific ablation of IL-15Rα expression has revealed further intricacies in the requirement for IL-15 in NK cells. Mortier et al. [14] showed that DC and macrophage specific trans-presentation of IL-15 is required to support NK cells in the spleen, liver and lungs. However, despite the absence of IL-15Rα expression on macrophages and DCs, the frequency of NK cells was diminished only by 50% in the liver [14]. Our results show that in addition to DCs and macrophages, survival of NK cells in the liver requires trans-presentation of IL-15 by IL-15Rα expressed on hepatocytes (Fig. 2B). It is probable that expression of IL-15Rα by the parenchymal non-hematopoietic cells of any given tissue contribute to the maintenance of NK cells.
3.2. Hepatocyte- and macrophage-mediated trans-presentation of IL-15 by IL-15Rα is required to maintain NKT cells in the liver
A subset of NK1.1+ cells also co-express CD3/TCR complex (NKT cells) and are selected in the thymus on CD1, a non-classical MHC class I like molecule [33]. Type 1 NKT cells (iNKT) express a restricted invariant T-cell receptor α-chain (TCRα; Vα14-Jα18 in mice) in combination with certain Vβ chains (Vβ8.2, Vβ7 or Vβ2 in mice) that recognize the CD1d restricted glycosphingolipid antigen, α-galectosylceramide. Type 1 NKT cells can be identified by staining with CD1d tetramer loaded with α-galectosylceramide or its analog, PBS57 [34]. Type II NKT cells are also restricted by CD1d but they express restricted, but diverse TCR repertoire. In mice, NKT cells are dependent on IL-15 for development and maintenance and are present in abundance in the liver where they have been observed to be associated with hepatic stellate cells [19,35]. Here we examined whether IL-15Rα expression by hepatocyte or macrophages is required to support NKT cells. To our surprise, NKT cell numbers were significantly reduced in Il15rafl/flAlb-Cre+ when compared to Il15rafl/flAlb-Cre− mice (Fig. 2B, middle panel). NKT cells also required the expression of IL-15Rα on macrophages for their maintenance in the liver (Fig. 2C, middle panel). On the other hand, survival of iNKT cell subset was dependent on hepatocyte-, but not macrophage mediated trans-presentation of IL-15Rα in the liver (Fig. 2B and C, right panels). These observations show that trans-presentation of IL-15 by parenchymal cells in the liver plays an important role in the homeostasis of NKT cells.
The requirement for IL-15 in NKT cell homeostasis appears to be different from that of NK cells (Fig. 2). In contrast to NK cells, NKT cell development can be sustained by IL-15Rα expressed on non-hematopoietic cells [36,37]. Similarly, IFN-γ production by NKT cells following α-galactosylceramide stimulation is deficient in Il15ra−/− mice suggesting that IL-15 signalling also regulates their activation [33,37]. In contrast to NK cells, IL-15 mediated survival of NKT cells is not mediated primarily through the upregulation of anti-apoptotic factors [30]. In the periphery, studies using bone marrow chimeras and transgenic models suggest that IL-15Rα expression is equally important in both hematopoietic and non-hematopoietic cells [36]. Our results show that hepatocyte- and macrophage- mediated presentation of IL-15Rα is essential for the maintenance of NKT cells in the liver (Fig. 2). However iNKT cells appear to be less dependent on IL-15Rα present on macrophages for their homeostasis.
3.3. Deletion of IL-15Rα in hepatocytes or macrophages does not prevent the development of non-alcoholic fatty liver disease (NAFLD)
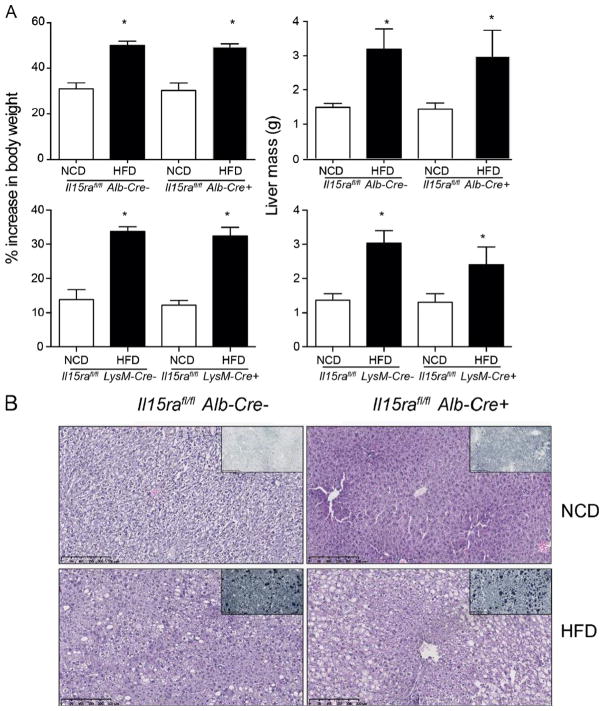
Given the important role of IL-15 in the activation of different immune subsets in the liver, we showed that the absence of IL-15 or IL-15Rα prevented the development of NAFLD in mice maintained on high fat diet (HFD) (Cepero-Donates et al., this issue). Macrophages play an important role in promoting the inflammatory cascade in metabolic syndrome. Deletion of Pparg in macrophages prevents the development of type 2 diabetes but not hepatic steatosis [38]. On the other hand, deletion of Tnfa in macrophages reduced steatosis and non-alcoholic steatohepatitis in mice maintained on methionine/choline-deficient diet [39]. As IL-15 induces Tnfa expression in rheumatoid arthritis [40], we assessed whether deficiency of IL-15Rα in macrophages or hepatocytes is sufficient to prevent the development of NAFLD in mice maintained on HFD. Body mass and livers of Il15rafl/flLysM-Cre+ and Il15rafl/flAlb-Cre+ mice maintained on HFD showed significant increase in weight compared to littermate controls (Fig. 3A). Furthermore, lipid accumulation was observed in the livers of these mice as seen from the H&E and Sudan Black staining (Fig. 3B, data not shown). These observations suggest that absence of IL15-Rα expression on hepatocyte or macrophages is not sufficient to prevent NAFLD.
Fig. 3. Hepatocyte- and macrophage-specific deletion of IL-15Rα does not prevent lipid accumulation in the liver.
(A) Body (left panels) and liver (right panels) weight was measured in mice of indicated genotype that were maintained on either normal control diet (NCD) or high fat diet (HFD) for 12 weeks. Values are expressed as mean ± SD. Mann Whitney test N = 4–8 per group; p < 0.05(*). (B) Sections of livers collected from the indicate mice maintained on NCD or HFD for 12 weeks were stained with hematoxylin and eosin or Sudan Black. Representative images from at least 4 mice for each group are shown. Magnification 10×.
We have observed that the accumulation of CD8+ T, NK and NKT cells is increased in the livers of mice maintained on HFD. However, whole body deficiency in IL-15 or IL-15Rα protected mice from NAFLD (Cepero-Donates et al., this issue). These mice were characterized by reduction in inflammation as well as alterations in fatty acid oxidation in the liver. Even though NK and NKT cells are significantly reduced in both groups of mice (total body ablation of IL-15Rα and hepatocyte or macrophage restricted deletion) the outcome of the disease phenotype is completely different. These observations rather indicate that the presence or absence of NK or NKT cells have minimal impact on NAFLD. The role of NKT cells in NAFLD is controversial [41,42]. Our observations suggest that inflammation, rather than any given cell type, combined with modulation of metabolic characteristics of the liver by IL-15 determines the progression of NAFLD. Detailed analysis of the role of IL-15 in metabolism and diabetes will help designing rational immunotherapeutic approaches to treat NAFLD.
Acknowledgments
This work was funded by CIHR operating grant to SR. YCD is a recipient of studentship ‘Professeur Cossette’ from the Faculty of Medicine. YCD, VR and MM carried out the experiments. YCD and SR planned the experiments. YGC provided expertise on NKT cells. YCD and SR wrote the manuscript.
Abbreviations
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- IHL
intrahepatic lymphocytes
References
- 1.Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, Newberry RD, et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-gamma-producing cells. Immunity. 2013;38:769–781. doi: 10.1016/j.immuni.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 4.Purton JF, Tan JT, Rubinstein MP, Kim DM, Sprent J, Surh CD. Antiviral CD4+ memory T cells are IL-15 dependent. J Exp Med. 2007;204:951–961. doi: 10.1084/jem.20061805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 6.Lodolce JP, Burkett PR, Boone DL, Chien M, Ma A. T cell-independent interleukin 15Ralpha signals are required for bystander proliferation. J Exp Med. 2001;194:1187–1194. doi: 10.1084/jem.194.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 8.Colpitts SL, Stonier SW, Stoklasek TA, Root SH, Aguila HL, Schluns KS, et al. Transcriptional regulation of IL-15 expression during hematopoiesis. J Immunol. 2013;191:3017–3024. doi: 10.4049/jimmunol.1301389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 10.Cui G, Hara T, Simmons S, Wagatsuma K, Abe A, Miyachi H, et al. Characterization of the IL-15 niche in primary and secondary lymphoid organs in vivo. Proc Natl Acad Sci USA. 2014;111:1915–1920. doi: 10.1073/pnas.1318281111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson DM, Kumaki S, Ahdieh M, Bertles J, Tometsko M, Loomis A, et al. Functional characterization of the human interleukin-15 receptor alpha chain and close linkage of IL15RA and IL2RA genes. J Biol Chem. 1995;270:29862–29869. doi: 10.1074/jbc.270.50.29862. [DOI] [PubMed] [Google Scholar]
- 12.Stonier SW, Schluns KS. Trans-presentation: a novel mechanism regulating IL-15 delivery and responses. Immunol Lett. 2010;127:85–92. doi: 10.1016/j.imlet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schluns KS, Stoklasek T, Lefrancois L. The roles of interleukin-15 receptor alpha: trans-presentation, receptor component, or both? Int J Biochem Cell Biol. 2005;37:1567–1571. doi: 10.1016/j.biocel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Mortier E, Advincula R, Kim L, Chmura S, Barrera J, Reizis B, et al. Macrophage- and dendritic-cell-derived interleukin-15 receptor alpha supports homeostasis of distinct CD8+ T cell subsets. Immunity. 2009;31:811–822. doi: 10.1016/j.immuni.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 15.Castillo EF, Stonier SW, Frasca L, Schluns KS. Dendritic cells support the in vivo development and maintenance of NK cells via IL-15 trans-presentation. J Immunol. 2009;183:4948–4956. doi: 10.4049/jimmunol.0900719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 17.Lodolce J, Burkett P, Koka R, Boone D, Chien M, Chan F, et al. Interleukin-15 and the regulation of lymphoid homeostasis. Mol Immunol. 2002;39:537–544. doi: 10.1016/s0161-5890(02)00211-0. [DOI] [PubMed] [Google Scholar]
- 18.Stienstra R, Saudale F, Duval C, Keshtkar S, Groener JE, van Rooijen N, et al. Kupffer cells promote hepatic steatosis via interleukin-1beta-dependent suppression of peroxisome proliferator-activated receptor alpha activity. Hepatology. 2010;51:511–522. doi: 10.1002/hep.23337. [DOI] [PubMed] [Google Scholar]
- 19.Winau F, Hegasy G, Weiskirchen R, Weber S, Cassan C, Sieling PA, et al. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26:117–129. doi: 10.1016/j.immuni.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Yokota S, Yoshida O, Dou L, Spadaro AV, Isse K, Ross MA, et al. IRF-1 promotes liver transplant ischemia/reperfusion injury via hepatocyte IL-15/IL-15Ralpha production. J Immunol. 2015;194:6045–6056. doi: 10.4049/jimmunol.1402505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed M. Non-alcoholic fatty liver disease in 2015. World J Hepatol. 2015;7:1450–1459. doi: 10.4254/wjh.v7.i11.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolf MJ, Adili A, Piotrowitz K, Abdullah Z, Boege Y, Stemmer K, et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell. 2014;26:549–564. doi: 10.1016/j.ccell.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Ramanathan S, Gagnon J, Leblanc C, Rottapel R, Ilangumaran S. Suppressor of cytokine signaling 1 stringently regulates distinct functions of IL-7 and IL-15 in vivo during T lymphocyte development and homeostasis. J Immunol. 2006;176:4029–4041. doi: 10.4049/jimmunol.176.7.4029. [DOI] [PubMed] [Google Scholar]
- 24.Schluns KS, Williams K, Ma A, Zheng XX, Lefrancois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 25.Koka R, Burkett PR, Chien M, Chai S, Chan F, Lodolce JP, et al. Interleukin (IL)-15R[alpha]-deficient natural killer cells survive in normal but not IL-15R [alpha]-deficient mice. J Exp Med. 2003;197:977–984. doi: 10.1084/jem.20021836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marcais A, Cherfils-Vicini J, Viant C, Degouve S, Viel S, Fenis A, et al. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat Immunol. 2014;15:749–757. doi: 10.1038/ni.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54–S62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 28.Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8(+) T cells. Nat Rev Immunol. 2011;11:645–657. doi: 10.1038/nri3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vosshenrich CA, Ranson T, Samson SI, Corcuff E, Colucci F, Rosmaraki EE, et al. Roles for common cytokine receptor gamma-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J Immunol. 2005;174:1213–1221. doi: 10.4049/jimmunol.174.3.1213. [DOI] [PubMed] [Google Scholar]
- 30.Huntington ND, Puthalakath H, Gunn P, Naik E, Michalak EM, Smyth MJ, et al. Interleukin 15-mediated survival of natural killer cells is determined by interactions among Bim, Noxa and Mcl-1. Nat Immunol. 2007;8:856–863. doi: 10.1038/ni1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minagawa M, Watanabe H, Miyaji C, Tomiyama K, Shimura H, Ito A, et al. Enforced expression of Bcl-2 restores the number of NK cells, but does not rescue the impaired development of NKT cells or intraepithelial lymphocytes, in IL-2/IL-15 receptor beta-chain-deficient mice. J Immunol. 2002;169:4153–4160. doi: 10.4049/jimmunol.169.8.4153. [DOI] [PubMed] [Google Scholar]
- 32.Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J Exp Med. 2004;200:825–834. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordy LE, Bezbradica JS, Flyak AI, Spencer CT, Dunkle A, Sun J, et al. IL-15 regulates homeostasis and terminal maturation of NKT cells. J Immunol. 2011;187:6335–6345. doi: 10.4049/jimmunol.1003965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Godfrey DI, Berzins SP. Control points in NKT-cell development. Nat Rev Immunol. 2007;7:505–518. doi: 10.1038/nri2116. [DOI] [PubMed] [Google Scholar]
- 35.Exley MA, Koziel MJ. To be or not to be NKT: natural killer T cells in the liver. Hepatology. 2004;40:1033–1040. doi: 10.1002/hep.20433. [DOI] [PubMed] [Google Scholar]
- 36.Castillo EF, Acero LF, Stonier SW, Zhou D, Schluns KS. Thymic and peripheral microenvironments differentially mediate development and maturation of iNKT cells by IL-15 transpresentation. Blood. 2010;116:2494–2503. doi: 10.1182/blood-2010-03-277103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biswas PS, Gupta S, Chang E, Bhagat G, Pernis AB. Aberrant ROCK activation promotes the development of type I diabetes in NOD mice. Cell Immunol. 2011;266:111–115. doi: 10.1016/j.cellimm.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moran-Salvador E, Lopez-Parra M, Garcia-Alonso V, Titos E, Martinez-Clemente M, Gonzalez-Periz A, et al. Role for PPARgamma in obesity-induced hepatic steatosis as determined by hepatocyte- and macrophage-specific conditional knockouts. FASEB J. 2011;25:2538–2550. doi: 10.1096/fj.10-173716. [DOI] [PubMed] [Google Scholar]
- 39.Tosello-Trampont AC, Landes SG, Nguyen V, Novobrantseva TI, Hahn YS. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-alpha production. J Biol Chem. 2012;287:40161–40172. doi: 10.1074/jbc.M112.417014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McInnes IB, Leung BP, Sturrock RD, Field M, Liew FY. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-alpha production in rheumatoid arthritis. Nat Med. 1997;3:189–195. doi: 10.1038/nm0297-189. [DOI] [PubMed] [Google Scholar]
- 41.Mantell BS, Stefanovic-Racic M, Yang X, Dedousis N, Sipula IJ, O’Doherty RM. Mice lacking NKT cells but with a complete complement of CD8+ T-cells are not protected against the metabolic abnormalities of diet-induced obesity. PLoS ONE. 2011;6:e19831. doi: 10.1371/journal.pone.0019831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin-Murphy BV, You Q, Wang H, De La Houssaye BA, Reilly TP, Friedman JE, et al. Mice lacking natural killer T cells are more susceptible to metabolic alterations following high fat diet feeding. PLoS ONE. 2014;9:e80949. doi: 10.1371/journal.pone.0080949. [DOI] [PMC free article] [PubMed] [Google Scholar]