Supplemental Digital Content is available in the text.
Key Words: human papillomavirus, DNA vaccine, E6 and E7 oncogenes, tumor, immune checkpoint blockade, PD-L1
Abstract
We have previously shown that a novel DNA vaccine technology of codon optimization and the addition of ubiquitin sequences enhanced immunogenicity of a herpes simplex virus 2 polynucleotide vaccine in mice, and induced cell-mediated immunity when administered in humans at relatively low doses of naked DNA. We here show that a new polynucleotide vaccine using the same technology and encoding a fusion protein of the E6 and E7 oncogenes of high-risk human papillomavirus type 16 (HPV16) is immunogenic in mice. This vaccine induces long-lasting humoral and cell-mediated immunity and protects mice from establishment of HPV16-E7-expressing tumors. In addition, it suppresses growth of readily established tumors and shows enhanced efficacy when combined with immune checkpoint blockade targeted at PD-L1. This vaccine also facilitates rejection of HPV16-E7-expressing skin grafts that demonstrate epidermal hyperplasia with characteristics of cervical and vulvar intraepithelial neoplasia. Clinical studies evaluating the efficacy of this vaccine in patients with HPV16+ premalignancies are planned.
High-risk human papillomaviruses (HPV), especially genotypes HPV16 and 18, are associated with a range of epithelial diseases that can lead to intraepithelial neoplasia and invasive cancer of various body parts including genitals and oropharynx.1 Although prophylactic vaccines prevent HPV spread and persistence which can cause malignant transformation, HPV-related cancers still cause >250.000 deaths annually worldwide. Therefore the need to develop therapeutic treatments prevails.
Although prophylactic HPV vaccination aims to prevent viral infection and spread and dominantly functions by inducing a strong virus-neutralizing antibody response against capsid proteins L1 and L2, the objective of therapeutic vaccination is to reverse premalignant neoplasia or even eliminate already established invasive cancers. Two preventive HPV vaccines are currently commercially available (Gardasil and Cervarix) and have proven efficacy in strongly reducing the occurrence of high-grade histologically proven cervical abnormalities.2 However, because the expression of L1 and L2 viral proteins is lost in infected cells in which the HPV genome has been integrated into the host DNA, these proteins are no longer expressed by transformed cells, which makes the existing vaccines noneffective at this stage of disease.1
An effective therapeutic HPV vaccine must not only neutralize infectious particles but also eliminate transformed cells, which display HPV proteins. The oncoproteins E6 and E7 are not only required for cell transformation, but are also continually expressed by HPV-associated premalignant neoplastic epithelial and cancer cells, which makes them optimal antigen targets for immunotherapy. The killing and elimination of HPV transformed cells requires an adaptive cellular immune response. Hence, therapeutic vaccines need to be designed to favor such a response. A variety of therapeutic vaccines targeting HPV-associated malignancies have been tested in clinical trials with only partial success. These vaccines include live vectors, adjuvanted peptides or proteins, or DNA or are dendritic cell-based.1
Polynucleotide or DNA vaccines harbor the potential to tailor the outcome of the immune response, for example, by choosing multiple antigens of interest or fusing immune-modulatory elements or immune targets to the antigen.3 In addition, polynucleotide vaccines are heat stable and very cost-effective in production, making them a globally desired vaccine option. However, polynucleotide vaccines have demonstrated rather low immunogenicity in human studies. In an attempt to overcome this, we developed a polynucleotide vaccine technology which combines constructs that are codon-optimized and either soluble or proteasome targeted by ubiquitination, resulting in both adaptive humoral and cell-mediated immunity.4 We have previously shown that a herpes simplex virus 2 (HSV-2) polynucleotide vaccine using this technology protects mice against lethal HSV-2 infection.5 This vaccine, COR-1, when administered intradermally to healthy HSV-1/2 seronegative individuals was further shown to induce cell-mediated immune responses in the majority of subjects6 and is currently being evaluated for clinical efficacy in HSV-2+ symptomatic patients (clinical trial number ACTRN 12615000094572).
Using a comparable technology, we engineered polynucleotide constructs encoding a codon-optimized HPV16-derived E6 and E7 fusion protein, which was either secreted or ubiquitinated. We show here that a vaccine comprising these constructs induced long-lasting antibody and CD8+ T-cell responses in mice, and protected against growth of HPV16-E7-expressing tumors. When delivered in combination with PD-L1 immune checkpoint blockade it further enhanced antitumor immunity, and induced rejection of otherwise tolerated E7-expressing skin grafts.
MATERIALS AND METHODS
Mice
C57BL/6J and C57/6J.K14E7 (K14E7) mice were provided by the animal research center in Perth, Australia. E7TCR269 mice were obtained from Leggatt7 and were bred in house (Biological Research Facility of Translational Research Institute Brisbane). All mice were kept under specific pathogen-free conditions at the Biological Research Facility of the Translational Research Institute (Brisbane, Australia), are female and were used at 6–12 weeks of age. All animal procedures and experiments were performed in compliance with the ethical guidelines of the National Health and Medical Research Council of Australia, with approval from the IMVS Animal Ethics Committee and the University of Queensland Animal Ethics Committee (#093/15 and #351/15)
Plasmids
A DNA sequence encoding a fusion protein made up of HPV16-E6[C70G, I135T] linked by Ala-Gly-Ala to HPV16-E7[C24G, E26G] was designed. C24G and E26G mutations were introduced as they have been shown to independently render the E7 protein nontransforming.8 For E6, C70G, and I135T mutations were introduced as these mutations have been shown to eliminate its ability to degrade p53.9–12 Two variants of this sequence were made: 1 incorporating a single ubiquitin repeat upstream and in-frame with the E6–E7 fusion protein and the other incorporating a murine IgK secretory sequence (GenBank: AAH80787.1). These sequences were codon modified according to our codon preference table (refer to patent: St Lucia US 2011/0287039 A1.),13 synthesized and cloned into pcDNA3.1 (GeneArt, Life Technologies). The inserts were then subcloned into NTC8485 and near-GMP grade plasmid DNA manufactured (Nature Technology Corporation, Lincoln, NE). The resulting plasmids were denoted (NTC8485-O-U-E6[C70G, I135T]-AGA-E7[C24G, E26G] and NTC-O-s-E6[C70G, I135T]-AGA-E7[C24G, E26G]), where O indicates that the sequences were codon modified, U indicates the presence of the ubiquitin-encoding sequence, and s indicates the presence of a secretory sequence (GenBank accession numbers KY449456 and KY449457, respectively). NTC-gD2 control vaccine was prepared as described previously.5
Immunizations and Antibody Treatment
For DNA vaccine immunizations, mice were immunized intradermally in the pinna of each ear with a total of 30 μg HPV DNA vaccine (NTC-HPV16-E6/E7, secreted and ubiquitinated constructs pooled 1:1 by weight) or irrelevant vector vaccine (NTC-gD2, secreted constructs).
Mice were injected intraperitoneal with 200 μg of monoclonal antibodies targeting PD-L1 (clone B7-H1, BioXcell) twice weekly for 3 consecutive weeks following the first vaccination.
Tumor Challenge
Tissue culture line 1 (TC-1) cells were kindly provided by John Hopkins. TC-1 cells were cultured in RPMI-1640 media supplemented with 10% fetal bovine serum, nonessential amino acids, l-glutamine, sodium pyruvate, penicillin/streptomycin, and 0.4 mg/mL geneticin (Life Technologies). For tumor experiments, freshly thawed TC-1 cells were expanded for 1 week before injection. Cells were injected subcutaneously to the back of mice in a volume of 100 μL.
Skin Transplantation
E7TCR269 mice received double skin grafts of C57BL/6J mice and C57/6J.K14E7 mice 7 days after the second immunization. Skin transplantation has been described previously.14 Briefly, donor ear skin was split into dorsal and ventral surfaces (∼1 cm2). Dorsal ear surfaces were placed onto the thoracic flank region of anesthetized E7TCR269 recipients. Grafts were covered with antibiotic-permeated gauze (Bactigras, Smith and Nephew, London, UK) and bandaged with micropore tape and Flex-wrap (Lyppard, Qld, Australia). Bandages were removed 7 days later and grafts were monitored for 7–8 weeks. Photographs including a ruler were taken weekly and graft size was analyzed using Fiji Imaging software. Graft rejection was defined as loss of distinct border, signs of ulceration, and necrosis.
ELISPOT
HPV16-E6 and E7-specific CD8+ T-cell responses were measured using IFNγ ELISPOT. The ELISPOT protocol was similar to our previously described method for HSV gD2.5 Briefly, 1×106 cells were plated in triplicates in complete Dulbecco’s Modified Eagle's Medium containing 10% FCS in 96-well ELISPOT plates (Millipore) coated with 5 μg capture monoclonal antibody against IFNγ (AN18, Mabtech AB, Stockholm, Sweden). Cells were restimulated with 20 μg/mL HPV16-E6/E7 peptides (Mimotopes) for 16–20 hours at 37°C. After washing, plates were incubated for 2 hours at room temperature with a biotinylated monoclonal antibody against IFNγ (R4-6A2, Mabtech AB). For detection, horseradish peroxidase-conjugated strepavidin (Mabtech AB) and DAB tablets (Sigma-Aldrich, St Louis, MO) were used. Spots were counted using an automated ELISPOT Classic reader system (Autoimmun Diagnostika GmbH, Strassberg, Germany).
ELISA
To determine antibody responses, serum was collected from animals at different time points. Maxisorp microtiter plates (Nunc, Roskilde, Denmark) were coated overnight at 4°C with 50 μL of 0.25 μg/mL HPV16-E7 recombinant protein (Bioclone). After coating, plates were washed 3 times with phosphate buffered saline (PBS)/0.1% Tween (PBS-T) and blocked for 2 hours at 37°C with 100 μL of 5% skim milk powder in PBS-T. Plates were washed with PBS-T and 50 μL of mouse sera at a dilution of 1:100 were incubated for 1 hour at 37°C in duplicates. After washing 3 times, 50 μL of antimouse IgG peroxidase conjugate (Sigma-Aldrich) was incubated for 1 hour at 37°C. Plates were washed again 3 times and incubated with o-phenylenediamine dihydrochloride (OPD) substrate (Sigma-Aldrich). Absorbance was measured after 30 minutes and following addition of 25 μL of 3N HCl, at 492 nm in a Multiskan EX plate reader (Pathtech, Melbourne, Vic., Australia).
Flow Cytometry
TC-1 cells were harvested by trypsinization for 5 minutes. TC-1 cells were resuspended in tumor medium and incubated in a Falcon tube (BD) for 90 minutes at 37°C to allow reexpression of surface molecules. Antibody staining for flow cytometry has been described previously.15 TC-1 cells were stained with isotype or α-PD-L1 antibody (clone 10F.9G2, BioLegend, San Diego, CA), acquired using a BD LSR Fortessa X20 cytometer and analyzed using Kaluza software (Beckman Coulter).
Statistical Analysis
Statistical analysis was performed with GraphPad Prism version 5.03 for Windows (GraphPad Software, San Diego, CA). Unpaired 2-tailed t tests were performed to determine statistical significance in T-cell responses, antibody responses, tumor growth, and skin transplant studies. Survival curves were compared by a log-rank (Mantel Cox) test. Differences were considered significant if P<0.05.
RESULTS
Mixed Codon-optimized Secreted and Ubiquitinated Constructs Encoding HPV16-E6 and E7 Fusion Proteins Induce Long-Lasting Humoral and Cell-mediated Immunity
We have previously shown that codon optimization can overcome poor immunogenicity of polynucleotide vaccines and that conjugation of an ubiquitin gene to a target gene can enhance cellular immunity.4 Further, we illustrated that immunization with a 1:1 mixture of codon-optimized secreted and ubiquitinated constructs encoding the glycoprotein D of HSV-2 (gD2) induced both humoral and cellular immune responses and protected mice against lethal HSV infection.5 Here, we tested the immunogenicity of a polynucleotide D vaccine consisting of a 1:1 mixture of codon-optimized secreted and ubiquitinated constructs encoding a HPV16-derived E6 and E7 fusion protein (NTC-HPV16-E6/E7). Intradermal delivery of NTC-HPV16-E6/E7 resulted in high E7-specific antibody responses, which were still detectable 5 months after immunization (Figs. 1A, C). NTC-HPV16-E6/E7 also induced an E6-specific and E7-specific T-cell response, which we could still recall after 5 months (Figs. 1B, D).
FIGURE 1.
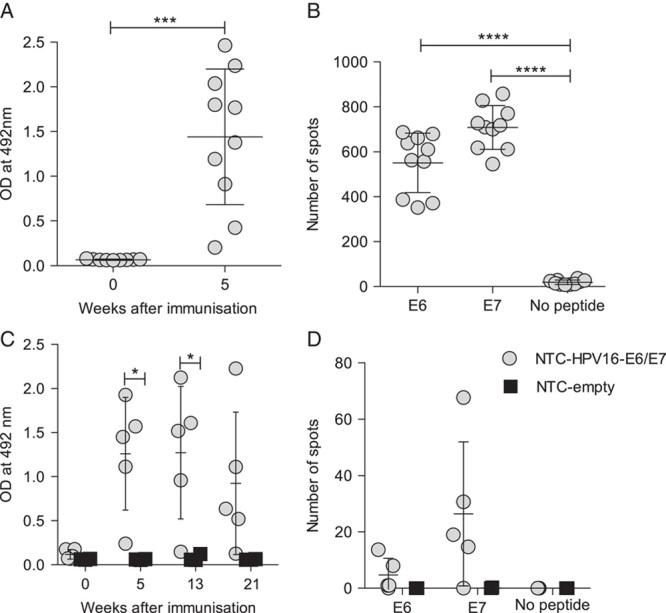
Immunization with codon-optimized polynucleotides encoding HPV16-E6/E7 induce acute and long-lasting humoral and cytotoxic immune responses. Mice were immunized intradermally into the ear twice 3 weeks apart with HPV16-E6/E7 DNA vaccine (NTC-HPV16-E6/E7) or empty vector (NTC-empty). A and B, E7-specific IgG antibody responses of NTC-HPV16-E6/E7 immunized mice (grey circles) were determined in serum at week 0 and 5 by ELISA (A). E6-specific and E7-specific cytotoxic T-cell responses were measured by ELISPOT in splenocytes at week 5 after immunization (B) (n=10). C and D, E7-specific IgG antibody responses of NTC-HPV16-E6/E7 immunized mice (grey circles, n=5) or NTC-empty immunized mice (black squares, n=3) were determined in serum at week 0 and 5, 13, and 21 by ELISA (C). E6-specific and E7-specific T-cell responses were measured in splenocytes at week 21 by IFNγ ELISPOT (D). Each data point represents means of technical replicates of 1 individual animal. Mean values of averaged individuals and SDs are indicated. *P≤0.05, ***P≤0.001, ****P≤0.0001.
Immunization With NTC-HPV16-E6/E7 Prevents TC-1 Tumor Growth and Suppresses Progression of Established TC-1 Tumors
To test the efficacy of NTC-HPV16-E6/E7, we utilized the TC-1 solid tumor model which expresses HPV16-E6 and E7 as a tumor antigens.16 We immunized mice twice with a 3-week interval and inoculated with 5×105 TC-1 cells 1 week after the second immunization when palpable tumor was present. Although immunization with an irrelevant vector encoding HSV gD2 had no effect on tumor growth compared with unimmunized mice, tumor growth was completely prevented in mice that were immunized with NTC-HPV16-E6/E7 (Figs. 2A, B), demonstrating that NTC-HPV16-E6/E7 immunization induces strong antitumor effects. Furthermore, NTC-HPV16-E6/E7 immunization starting 3 or 7 days after tumor inoculation significantly suppressed the progression of established TC-1 tumors (Figs. 2C, D), indicating a therapeutic potential of the vaccine.
FIGURE 2.
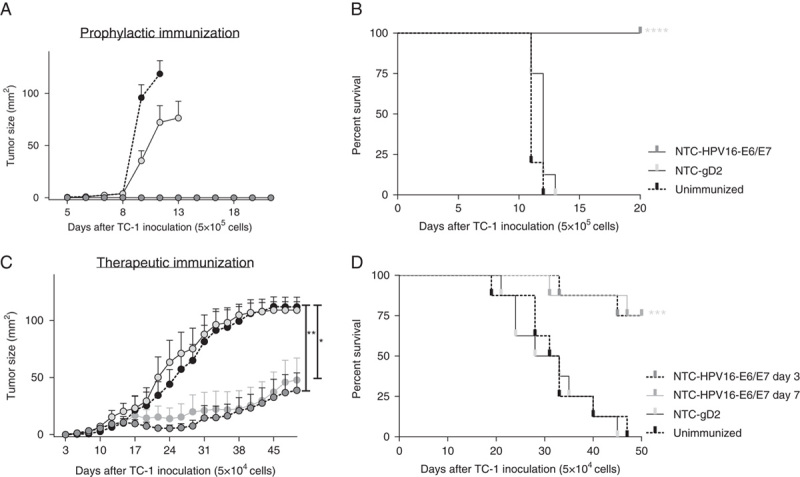
NTC-HPV16-E6/E7 immunization prevents TC-1 tumor growth and suppresses progression of established tumors. A and B, Mice were immunized intradermally twice 3 weeks apart with NTC-HPV16-E6/E7 or a vector expressing an irrelevant protein of herpes simplex virus 2 (NTC-gD2). One week later, mice were injected subcutaneously with 5×105 HPV16-E7-expressing TC-1 tumor cells. Unimmunized mice were used as positive control for tumor growth. Mice were killed when tumor size reached 1 cm3. Shown are mean values of individual tumor sizes (n=10) with SE of mean) (A) and percentage of survival (B) over a period of 20 days. C and D, Mice were injected subcutaneously with 5×104 HPV16-E7-expressing TC-1 tumor cells. Three days after tumor inoculation, mice were immunized 3 times in weekly intervals with NTC-HPV16-E6/E7 or NTC-gD2. One additional group was immunized 3 times in weekly intervals with NTC-HPV16-E6/E7 starting 7 days after tumor inoculation. Tumor size was measured and mice were killed when tumor size reached 1 cm3. Shown are mean values of individual tumor sizes (n=8) with SE of mean indicated (C) and percentage of survival (D) over a period of 50 days. *P≤0.01, **P≤0.01, ***P<0.001, ****P<0.0001.
Combination Immunotherapy Targeting PD-L1 Adjacent to Vaccination With NTC-HPV16-E6/E7 Further Enhances Antitumor Immunity
It has been established that blockade of immune checkpoint inhibitors is beneficial in a variety of malignancies and first antibodies targeting immune checkpoints are now used in humans for treatment of melanoma or are clinically evaluated in a range of other malignancies.17 In particular, blockade of the programmed cell death receptor 1 (PD-1) has proven clinical efficacy in a variety of cancers.18 However, targeting PD-1, which is expressed by effector T cells to limit an immune response and therewith T-cell induced pathology, also causes a wide range of side effects including colitis, hepatitis, and pneumonitis.19 We therefore targeted its ligand PD-L1, which is often expressed by tumor cells to suppress tumor-reactive cytotoxic T cells.18 To investigate if targeting PD-L1 adjacent to vaccination with NTC-HPV16-E6/E7 enhances antitumor immunity against TC-1 cells, we inoculated mice with a higher number of TC-1 cells (1×105) compared with previous therapeutic experiments. Seven days later, when tumors were palpable, we immunized mice with 3 doses of NTC-HPV16-E6/E7 in weekly intervals, and systemically treated mice twice weekly with monoclonal antibodies targeting PD-L1, for 3 consecutive weeks. We found that targeting PD-L1 alone without vaccination had no effect on tumor growth and survival (Figs. 3A, B). Vaccination with NTC-HPV16-E6/E7 significantly suppressed progression of TC-1 tumors. Combination immunotherapy of NTC-HPV16-E6/E7 vaccination and PD-L1 targeting further enhanced the antitumor immunity against TC-1. Impressively, 6 of 8 mice (75%) survived tumor burden up to 60 days when treated with DNA vaccination and α-PD-L1 combined, with 3 of 8 mice completely clearing the tumor. We further confirmed PD-L1 expression on TC-1 cells (Fig. 3C). These results indicate that combination immunotherapy of antigen-specific vaccination together with blockade of PD-L1 could be beneficial for patients with HPV16+ PD-L1+ malignancies.
FIGURE 3.
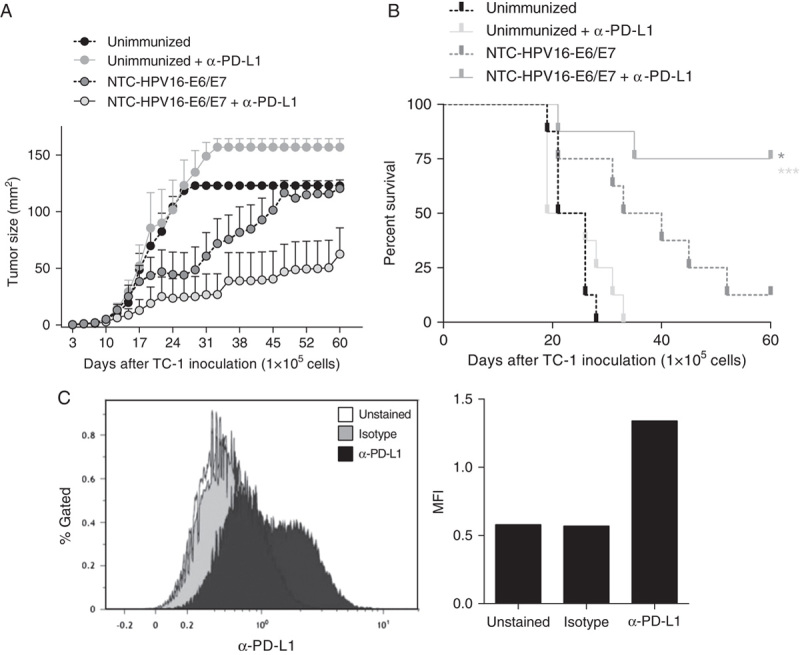
Combination immunotherapy of HPV16-E6/E7 DNA vaccination adjacent to targeting PD-L1 further increases antitumor immunity. A and B, Mice were injected subcutaneously with 1×105 HPV16-E7-expressing TC-1 tumor cells. Seven days after tumor inoculation, mice were immunized 3 times in weekly intervals with NTC-HPV16-E6/E7. Mice received monoclonal antibodies targeting PD-L1 twice weekly after each vaccination, for 3 constitutive weeks. Tumor size was measured and mice were killed when tumor size reached 1 cm3. Shown are mean values of individual tumor sizes (n=8) with SE of mean indicated (A) and percentage of survival (B) over a period of 50 days. C, TC-1 cells were analyzed by flow cytometry for the expression of PD-L1. Shown are histogram and median fluorescent intensity (MFI) of unstained, isotype stained, and α-PD-L1 stained TC-1 cells. *P<0.05, ***P<0.001.
Immunization With NTC-HPV16-E6/E7 Induced Rejection of E7-expressing Skin Grafts
We used a model of transgenic mice expressing HPV16-E7 under the control of a keratin promotor (K14E7), resulting in epithelial hyperplasia and local immune suppression and therewith modeling precancerous HPV16-associated skin lesions.20 We have previously shown that K14E7 ear skin transplanted to immune-competent hosts is tolerated despite the expression of HPV16-E7 as a neo-antigen,21 and have identified a series of immune factors that contribute to immune suppression in K14E7 skin.14,15,22–24 We have also shown that rejection of K14E7 skin grafts from immune-compromised mice depends on a cytotoxic T-cell response,25 which makes this an optimal model to examine clinical efficacy of our DNA vaccine. Previously, we found that grafts on normal C57BL/6 recipient mice immunized with the HPV DNA vaccine were not rejected (see Table, Supplemental Digital Content 1, http://links.lww.com/JIT/A457 which shows that the graft area did not reduce with time) which was unsurprising as the nontransgenic mice lack the necessary E7-specific T-cell repertoire to induce rejection. We here show that when K14E7 ear skin is transplanted to mice which transgenically express a HPV16-E7-specific major histocompatibility complex (MHC) class I-restricted T-cell receptor (E7TCR269 mice) we find minor graft size reduction in otherwise unmanipulated mice (Figs. 4A, B). Prophylactic immunization with NTC-HPV16-E6/E7 resulted in significantly enhanced graft size reduction of K14E7 skin from E7TCR269 mice, whereas an irrelevant vector encoding HSV gD2 had no effect on graft rejection compared with unimmunized mice (Figs. 4A, B). We further detected significantly elevated E6-specific and E7-specific T-cell responses in E7TCR269 mice immunized with NTC-HPV16-E6/E7 vaccine compared with unimmunized mice and mice receiving irrelevant vector (Fig. 4C), but no significant increase in E7-specific antibody production (Fig. 4D).
FIGURE 4.
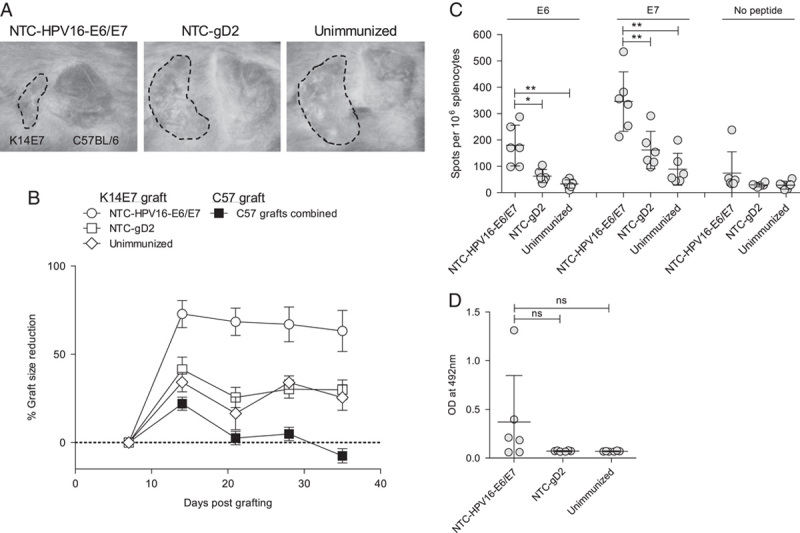
E7TCR269 mice immunized with HPV16-E6/E7 DNA vaccine reject HPV16-E7-expressing skin grafts. E7TCR269 mice were immunized with HPV16-E6/E7 DNA vaccine or vector expressing an irrelevant protein (HSV gD2) intradermally into the ear twice 3 weeks apart. One week after the second immunization, mice received skin transplants from C57BL/6 and K14E7 animals. Grafts were measured weekly (n=4–6). A, Photographs taken 28 days after grafting. Shown is 1 representative per group. B, Graft size reduction was calculated based on graft size on day 7 after grafting, when bandages were removed. C, E6-specific and E7-specific T-cell responses were measured in splenocytes at the end of the grafting study by IFNγ ELISPOT. Each data point represents means of technical replicates of 1 individual animal. Mean values of averaged individuals and SDs are indicated. D, E7-specific IgG antibody responses were determined in serum at the end of the grafting study by ELISA. Each data point represents means of technical replicates of 1 individual animal. Mean values of averaged individuals and SDs are indicated. *P≤0.05, **P≤0.01.
DISCUSSION
Current treatment of HPV-associated malignancies are limited to surgery, radiation, and chemotherapy. These can be effective when the malignancy is diagnosed early, and easily accessible for removal. However, the treatment of advanced and metastatic malignancies is more difficult. A wide variety of different cancer immunotherapy approaches are currently being evaluated in a high number of ongoing clinical trials,1,26 with the aim to harness the body’s own ability to eliminate solid cancers and malignant cells by immune cell activation. Tumor antigen-specific immune activation can be achieved by immunization, if tumor antigens are known and stable. Tumors induced by viruses such as HPV have the advantage that the tumor antigens are known and virus-derived, allowing for the creation of a universal vaccine against HPV-associated malignancies. HPV-associated tumors such as cervical cancer or HPV+ oropharyngeal squamous cell carcinima are mostly related to high-risk HPV type 16, and express the tumor antigens E6 and E7, making these favorable targets for immunization. Here, we chose a polynucleotide vaccine platform, which allowed us to improve immunogenicity by codon-modification for enhanced antigen expression and fusion of an ubiquitin sequence to the antigen to target it to MHC class I molecules and subsequent presentation to and activation of CD8+ cytotoxic T cells with killing ability.27,28 A variety of other studies indicated that ubiquitinated antigen-encoding DNA vaccines drive strong CD8+ T-cell responses.4,29–32 We demonstrated previously that mixing codon-optimized DNA constructs which encode ubiquitinated and secreted forms of HPV antigens results in strong humoral and CD8 T-cell responses.4 Furthermore, one other study demonstrated that the combination of ubiquitinated and nonubiquitinated Wilms tumor 1 epitopes is more effective in a mouse tumor model than either construct alone.33 We have previously applied this strategy of mixing DNA constructs to the development of a HSV-2 vaccine, which conferred high rates of survival in a murine HSV-2 challenge model and prevented latency in the dorsal root ganglia in up to 70% of mice surviving 50×LD50 challenge.5 In a phase I clinical trial we demonstrated the safety and tolerability of the vaccine in healthy volunteers with an indication that cell-mediated immunity was induced.6 A double-blinded, placebo-controlled phase II clinical trial in symptomatic HSV-2+ subjects is ongoing, in which the interim analysis indicates a reduction in viral shedding rate and induction of cell-mediated immunity (www.proactiveinvestors.com.au/companies/news/167602/admedus-ltd-receives-some-more-positive-results-from-hsv-2-study-167602.html).
HPV wildtype E6 and E7 proteins are oncoproteins which are involved in transformation.34,35 We have therefore introduced mutations into the sequences that are responsible for the E7 interaction with retinoblastoma protein (pRB) and the E6 interaction with p53, which are responsible for their transforming potential.8–12 In addition, we generated a fusion of E6 and E7 coding sequences which should further reduce their oncogenic potential. Furthermore, codon-modification, which was primarily applied to enhance protein expression, should also limit the possibility of recombination of the vaccine sequences with wildtype HPV viruses.
We here show that our HPV DNA vaccine expressing an ubiquitinated and secreted form of a E6/E7 fusion protein is immunogenic and able to induce both humoral and cell-mediated immunity, which protected mice in tumor challenge. Furthermore, the vaccine suppressed growth of established, palpable tumors. In a mouse skin grafting model which mimics epithelial neoplasia similar to HPV-induced cervical intraepithelial neoplasia and which exhibits a variety of immune tolerance mechanisms,14,15,22–24 we also showed that the vaccine was able to induce rejection of E7-expressing skin grafts.
A number of therapeutic HPV vaccines have been developed, ranging from live vectors, peptide and protein vaccines, DNA vaccines and dendritic cell-based vaccines, with only modest responses in clinical trials.1 After promising preclinical results in the mouse TC-1 tumor model,36,37 several HPV DNA vaccines have undergone clinical trials demonstrating their safety and tolerability. In a phase I trial, VGX-3100, encoding E6/E7 fusion proteins of HPV16 and HPV18, and delivered intramuscularly in combination with electroporation to patients previously treated for CIN2/3, indicated induction of robust immune responses including antibody and cytotoxic CD8+ T cells.38 However, in a phase II placebo-controlled trial, VGX-3100 only led to ∼40% complete regression of CIN2/3 compared with 20% observed in the placebo group.39 GX-188E, another HPV DNA vaccine encoding shuffled sequences of E6 and E7 of HPV16 and 18, and fused to the extracellular domain of Flt3L for dendritic cell targeting, induced cell-mediated immunity in CIN3 patients and remarkably led to complete regression and viral clearance in 7 of 9 patients after delivery together with electroporation.40 A different HPV DNA vaccine, in which E7 is fused to calreticulin (pNGVL4a-CRT/E7), a member of heat shock proteins, which facilitate transportation of MHC class I complexes to the endoplasmic reticulum and therewith enhance CD8+ T-cell responses, was shown to induce robust CD8+ responses and protection in TC-1 tumor challenges,41,42 but lead to only 30% of regression from CIN2/3 to CIN1 in patients.43 A new animal study using different heat shock proteins–fused E7 constructs reveals that a DNA vaccine-prime, and protein-vaccine boost regimen is more efficient in inducing humoral and cell-mediated immune responses which protect in TC-1 tumor challenge.42 Further exploration of such vaccination regime is warranted.
One reason why promising preclinical observations in murine HPV models often do not translate into comparable efficacy in patients might be that patients are chronically exposed to the virus for years before antitumor therapy is required, and have ultimately developed immune tolerance to the antigens. Hence, to break this established immune tolerance and skew the immune response toward effector function requires a stronger input compared with inducing immunogenicity in naïve mice. Furthermore, limitations of cancer immunotherapy arise from the tumor’s ability to suppress the intrinsic immune fight machinery by mechanisms such as induction of immune tolerance and immune checkpoint inhibition. Hence, we learn to understand that successful immunotherapy will require a boost of antitumor immunity (eg, by antigen-specific immunization), but also a blockade of virus and tumor-established immune tolerance (eg, by immune checkpoint inhibition). HPV-associated malignancies have been shown to express a variety of immune-suppressive mechanisms such as the PD-1/PD-L1 axis and expression of Tim-3 on tumor-infiltrating T cells.44–46 Here we demonstrated that the vaccine in combination with immune checkpoint blockade targeting PD-L1 enhanced rejection of readily established tumors. Knowing that HPV-associated cancers express PD-L1,44 this immunotherapy strategy might be of relevance to treat patients.
In summary, we developed a novel therapeutic DNA-based HPV16 vaccine, encoding a E6/E7 fusion protein, which was codon-optimized for enhanced expression, and combines sequences which are secreted and ubiquitinated to induce a balanced humoral and cell-mediated immune response. We showed that this vaccine was immunogenic, and protective in the TC-1 tumor model. Furthermore, we applied our vaccine in an E7-expressing skin grafting model, which resembles intraepithelial neoplasia in that it displays epithelial hyperplasia and immune tolerance mechanisms similar as seen in human HPV+ lesions. In this model, we demonstrated that the vaccine was able to induce skin graft rejection and this result is a promising indicator for clinical success. We further combined our vaccine with immune checkpoint inhibition targeting PD-L1 and demonstrated enhanced antitumor responses when vaccinating therapeutically. Exploration of the safety, tolerability, and clinical efficacy of this vaccine in humans displaying HPV-associated malignancies is desired.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.immunotherapy-journal.com.
ACKNOWLEDGMENTS
The authors thank the staff of the Biological Research Facility of the Translational Research Institute for excellent care of animals and technical assistance and Jennifer Bridge for assisting in skin grafting of animals.
CONFLICT OF INTEREST/FINANCIAL DISCLOSURES
J.L.D., B.L., W.-P.W., N.F., and Y.X. have share options in Admedus Vaccines Pty Ltd. I.H.F. is an inventor on the patent US 2011/0287039 A1, “Expression system for modulating an immune response” and WO 02/083181 A1, “Novel compositions and uses therefor” and is a director and shareholder in Admedus Vaccines Pty Ltd to which these patents have been assigned. J.L.D. is also an inventor on patent US 2011/0287039 A1.
All remaining authors have declared there are no financial conflicts of interest with regard to this work.
REFERENCES
- 1.Lee SJ, Yang A, Wu TC, et al. Immunotherapy for human papillomavirus-associated disease and cervical cancer: review of clinical and translational research. J Gynecol Oncol. 2016;27:e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castle PE, Maza M. Prophylactic HPV vaccination: past, present, and future—CORRIGENDUM. Epidemiol Infect. 2016;144:2472 10.1017/S0950268816000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L, Saade F, Petrovsky N. The future of human DNA vaccines. J Biotechnol. 2012;162:171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu WJ, Zhao KN, Gao FG, et al. Polynucleotide viral vaccines: codon optimisation and ubiquitin conjugation enhances prophylactic and therapeutic efficacy. Vaccine. 2001;20:862–869. [DOI] [PubMed] [Google Scholar]
- 5.Dutton JL, Li B, Woo WP, et al. A novel DNA vaccine technology conveying protection against a lethal herpes simplex viral challenge in mice. PLoS One. 2013;8:e76407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dutton JL, Woo WP, Chandra J, et al. An escalating dose study to assess the safety, tolerability and immunogenicity of a Herpes Simplex Virus DNA vaccine, COR-1. Hum Vaccin Immunother. 2016;12:3079–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narayan N, Choyce A, Linedale R, et al. Epithelial expression of human papillomavirus type 16 E7 protein results in peripheral CD8 T-cell suppression mediated by CD4+CD25+ T cells. Eur J Immunol. 2009;39:481–490. [DOI] [PubMed] [Google Scholar]
- 8.Edmonds C, Vousden KH. A point mutational analysis of human papillomavirus type 16 E7 protein. J Virol. 1989;63:2650–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalal S, Gao Q, Androphy EJ, et al. Mutational analysis of human papillomavirus type 16 E6 demonstrates that p53 degradation is necessary for immortalization of mammary epithelial cells. J Virol. 1996;70:683–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen M, Song S, Liem A, et al. A mutant of human papillomavirus type 16 E6 deficient in binding alpha-helix partners displays reduced oncogenic potential in vivo. J Virol. 2002;76:13039–13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polakova I, Pokorna D, Duskova M, et al. DNA vaccine against human papillomavirus type 16: modifications of the E6 oncogene. Vaccine. 2010;28:1506–1513. [DOI] [PubMed] [Google Scholar]
- 12.Mesplede T, Gagnon D, Bergeron-Labrecque F, et al. p53 degradation activity, expression, and subcellular localization of E6 proteins from 29 human papillomavirus genotypes. J Virol. 2012;86:94–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frazer IH, Dutton JL.USPTO Expression system for modulating an immune response. USPTO. Australia: The University of Queensland; 2011; US20110287039 A1. [Google Scholar]
- 14.Mittal D, Kassianos AJ, Tran LS, et al. Indoleamine 2,3-dioxygenase activity contributes to local immune suppression in the skin expressing human papillomavirus oncoprotein E7. J Invest Dermatol. 2013;133:2686–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandra J, Miao Y, Romoff N, et al. Epithelium expressing the E7 oncoprotein of HPV16 attracts immune-modulatory dendritic cells to the skin and suppresses their antigen-processing capacity. PLoS One. 2016;11:e0152886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu R, Coleman N, Stanley M. Different susceptibility of cervical keratinocytes containing human papillomavirus to cell-mediated cytotoxicity. Chin Med J (Engl). 1996;109:854–858. [PubMed] [Google Scholar]
- 17.Robert L, Ribas A, Hu-Lieskovan S. Combining targeted therapy with immunotherapy. Can 1+1 equal more than 2? Semin Immunol. 2016;28:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng X, Huang Z, Teng F, et al. Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treat Rev. 2015;41:868–876. [DOI] [PubMed] [Google Scholar]
- 19.Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;10:1346–1353. [DOI] [PubMed] [Google Scholar]
- 20.Herber R, Liem A, Pitot H, et al. Squamous epithelial hyperplasia and carcinoma in mice transgenic for the human papillomavirus type 16 E7 oncogene. J Virol. 1996;70:1873–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunn LA, Evander M, Tindle RW, et al. Presentation of the HPV16E7 protein by skin grafts is insufficient to allow graft rejection in an E7-primed animal. Virology. 1997;235:94–103. [DOI] [PubMed] [Google Scholar]
- 22.Mattarollo SR, Rahimpour A, Choyce A, et al. Invariant NKT cells in hyperplastic skin induce a local immune suppressive environment by IFN-gamma production. J Immunol. 2010;184:1242–1250. [DOI] [PubMed] [Google Scholar]
- 23.Gosmann C, Mattarollo SR, Bridge JA, et al. IL-17 suppresses immune effector functions in human papillomavirus-associated epithelial hyperplasia. J Immunol. 2014;193:2248–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergot AS, Ford N, Leggatt GR, et al. HPV16-E7 expression in squamous epithelium creates a local immune suppressive environment via CCL2- and CCL5- mediated recruitment of mast cells. PLoS Pathog. 2014;10:e1004466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattarollo SR, Yong M, Gosmann C, et al. NKT cells inhibit antigen-specific effector CD8 T cell induction to skin viral proteins. J Immunol. 2011;187:1601–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen CT, Clavijo PE, Van Waes C, et al. Anti-tumor immunity in head and neck cancer: understanding the evidence, how tumors escape and immunotherapeutic approaches. Cancers. 2015;7:2397–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant EP, Michalek MT, Goldberg AL, et al. Rate of antigen degradation by the ubiquitin-proteasome pathway influences MHC class I presentation. J Immunol. 1995;155:3750–3758. [PubMed] [Google Scholar]
- 28.Michalek MT, Grant EP, Gramm C, et al. A role for the ubiquitin-dependent proteolytic pathway in MHC class I-restricted antigen presentation. Nature. 1993;363:552–554. [DOI] [PubMed] [Google Scholar]
- 29.Chou B, Hiromatsu K, Hisaeda H, et al. Genetic immunization based on the ubiquitin-fusion degradation pathway against Trypanosoma cruzi. Biochem Biophys Res Commun. 2010;392:277–282. [DOI] [PubMed] [Google Scholar]
- 30.Delogu G, Howard A, Collins FM, et al. DNA vaccination against tuberculosis: expression of a ubiquitin-conjugated tuberculosis protein enhances antimycobacterial immunity. Infect Immun. 2000;68:3097–3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma A, Madhubala R. Ubiquitin conjugation of open reading frame F DNA vaccine leads to enhanced cell-mediated immune response and induces protection against both antimony-susceptible and -resistant strains of Leishmania donovani. J Immunol. 2009;183:7719–7731. [DOI] [PubMed] [Google Scholar]
- 32.Wang QM, Tang Y, Lei Ch X, et al. Enhanced cellular immune response elicited by a DNA vaccine fused with Ub against Mycobacterium tuberculosis. Scand J Immunol. 2012;76:123–130. [DOI] [PubMed] [Google Scholar]
- 33.Eslami NS, Shokrgozar MA, Mousavi A, et al. Simultaneous immunisation with a Wilms’ tumour 1 epitope and its ubiquitin fusions results in enhanced cell mediated immunity and tumour rejection in C57BL/6 mice. Mol Immunol. 2012;51:325–331. [DOI] [PubMed] [Google Scholar]
- 34.Lehoux M, D’Abramo CM, Archambault J. Molecular mechanisms of human papillomavirus-induced carcinogenesis. Public Health Genomics. 2009;12:268–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghittoni R, Accardi R, Hasan U, et al. The biological properties of E6 and E7 oncoproteins from human papillomaviruses. Virus Genes. 2010;40:1–13. [DOI] [PubMed] [Google Scholar]
- 36.Yan J, Reichenbach DK, Corbitt N, et al. Induction of antitumor immunity in vivo following delivery of a novel HPV-16 DNA vaccine encoding an E6/E7 fusion antigen. Vaccine. 2009;27:431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan J, Harris K, Khan AS, et al. Cellular immunity induced by a novel HPV18 DNA vaccine encoding an E6/E7 fusion consensus protein in mice and rhesus macaques. Vaccine. 2008;26:5210–5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bagarazzi ML, Yan J, Morrow MP, et al. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci Transl Med. 2012;4:155ra138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trimble CL, Frazer IH. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet. 2015;386:2078–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim TJ, Jin HT, Hur SY, et al. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nat Commun. 2014;5:5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim JW, Hung CF, Juang J, et al. Comparison of HPV DNA vaccines employing intracellular targeting strategies. Gene Ther. 2004;11:1011–1018. [DOI] [PubMed] [Google Scholar]
- 42.Peng S, Qiu J, Yang A, et al. Optimization of heterologous DNA-prime, protein boost regimens and site of vaccination to enhance therapeutic immunity against human papillomavirus-associated disease. Cell Biosci. 2016;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alvarez RD, Huh WK, Bae S, et al. A pilot study of pNGVL4a-CRT/E7(detox) for the treatment of patients with HPV16+ cervical intraepithelial neoplasia 2/3 (CIN2/3). Gynecol Oncol. 2016;140:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mezache L, Paniccia B, Nyinawabera A, et al. Enhanced expression of PD L1 in cervical intraepithelial neoplasia and cervical cancers. Mod Pathol. 2015;28:1594–1602. [DOI] [PubMed] [Google Scholar]
- 45.Badoual C, Hans S, Merillon N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73:128–138. [DOI] [PubMed] [Google Scholar]
- 46.Baruah P, Lee M, Odutoye T, et al. Decreased levels of alternative co-stimulatory receptors OX40 and 4-1BB characterise T cells from head and neck cancer patients. Immunobiology. 2012;217:669–675. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.immunotherapy-journal.com.


