ABSTRACT
Corilagin, a component of Phyllanthus urinaria extract, possesses antioxidant, thrombolytic, antiatherogenic, and hepatoprotective properties, but the mechanism underlying these effects remains unclear. Previous studies showed that the Akt (protein kinase B) signaling pathway exerts anti-inflammatory and organ protective effects. The aim of this study was to investigate the mechanism of action of corilagin and determine whether these effects are mediated through the Akt-dependent pathway in a trauma-hemorrhagic shock-induced liver injury rodent model. Hemorrhagic shock was induced in male Sprague–Dawley rats; mean blood pressure was maintained at 35 mm Hg to 40 mm Hg for 90 min, followed by fluid resuscitation. During resuscitation, three doses of corilagin alone (1 mg/kg, 5 mg/kg, or 10 mg/kg, intravenously) were administered. Furthermore, a single dose of corilagin (5 mg/kg) with and without Wortmannin (1 mg/kg, PI3K inhibitor), Wortmannin alone, or vehicle was administered. Twenty-four hours after resuscitation, plasma alanine aminotransferase and aspartate aminotransferase concentration and hepatic parameters were measured. One-way ANOVA was used for statistical analysis. Hepatic myeloperoxidase activity and the concentrations of plasma alanine aminotransferase and aspartate aminotransferase, interleukin-6, tumor necrosis factor-α, intercellular adhesion molecule-1, and cytokine-induced neutrophil chemoattractant-1 (CINC-1) and CINC-3 increased following hemorrhagic shock. These parameters were significantly attenuated in corilagin-treated rats following hemorrhagic shock. Hepatic phospho-Akt expression was also higher in corilagin-treated rats than in vehicle-treated rats. The elevation of phospho-Akt was abolished by combined treatment with Wortmannin and corilagin. Our results suggest that corilagin exerts its protective effects on hemorrhagic shock-induced liver injury, at least, via the Akt-dependent pathway.
Keywords: Akt, corilagin, hepatic injury, trauma-hemorrhage
INTRODUCTION
Traumatic injury with hemorrhagic shock is an important clinical issue (1). Severe hemorrhagic shock results in circulating blood supply insufficiency, leading to tissue damage and vital organ dysfunction (2). The liver is a major solid intra-abdominal organ that is supplied with rich blood, and can cause severe hepatic injury following hemorrhagic shock (3). Previous studies showed that hemorrhagic shock results in high production of pro-inflammatory mediators and cytokines, such as interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), cytokine-induced neutrophil chemoattractant-1 (CINC-1), and CINC-3 (4).
Following hemorrhagic shock, neutrophils are activated by pro-inflammatory chemokines/cytokines (4). The activated neutrophils are recruited to adhesion molecules and induce severe organ injury (4). Intercellular adhesion molecule-1 (ICAM-1) is upregulated after trauma-hemorrhage, which may enhance neutrophil adhesion to the vascular endothelium (5). IL-6 and TNF-α also play important roles in neutrophil infiltration and hepatic injury (4).
The phosphoinositide-3-kinase (PI3K)/Akt (protein kinase B) signaling pathway regulates cell survival in response to various injuries (6, 7). Previous studies showed that activation of the PI3K/Akt pathway decreases the overproduction of trauma-hemorrhage-induced proinflammatory mediators and adhesion molecules to protect organs from injury (5).
Phyllanthus urinaria is a popular herb belonging to the Phyllanthaceae family and has been used in traditional antidiabetic, antiviral, and gastrointestinal treatments (8). In addition, P. urinaria extracts also possess antioxidant and anti-inflammatory activities (9). Previous studies reported that P. urinaria extracts can inhibit the phagocytic activity of neutrophils, particularly by suppressing reactive oxygen species production (10) and reducing TNF-α expression in radiation-induced brain inflammation (11). In addition, P. urinaria has shown antiviral potential against A16, enterovirus 71, and coxsackievirus A16 infections (12). Corilagin is an important component of P. urinaria extract. Previous reports indicated that corilagin has many medical benefits, including antihypertensive, anticancer, antihyperalgesic, thrombolytic, and hepatoprotective properties (13–16).
Although various protective effects of corlagin have been reported, it has not been examined if this herb has any salutary effects following hemorrhage. Furthermore, the precise mechanism by which this agent produces organ protection effects remains unknown. We hypothesized that the protective effects of corilagin after hemorrhagic shock are exerted through the Akt-dependent pathway. Experimental animals were treated with corilagin alone and in combination with the PI3K inhibitor Wortmannin following hemorrhagic shock. The effects of these treatments were examined and the plasma concentrations of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), hepatic tissue myeloperoxidase (MPO) activity, pro-inflammatory mediator levels, and Akt expression were determined following hemorrhagic shock.
MATERIALS AND METHODS
Animals
Male Sprague–Dawley rats were used in this study. Rats were obtained from the National Science Council Experimental Animal Center of Taiwan. All experimental procedures and protocols were approved by the Institutional Animal Care and Use Committee of Chang Gung Memorial Hospital. The animal experiments followed the guidelines of the Animal Welfare Act and The Guide for Care and Use of Laboratory Animals from the National Institutes of Health.
Experimental groups
Thirty-six male Sprague–Dawley rats (275–325 g) were randomly assigned to six groups (n = 6/group) for evaluation of the dose–response effect of corilagin on hepatic injury following hemorrhagic shock. Groups receiving corilagin (0 mg/kg, 1 mg/kg, 5 mg/kg, or 10 mg/kg) and sham groups were also included in the initial studies. An additional 36 male Sprague–Dawley rats were also randomly divided into six groups (n = 6/group). Rats in the hemorrhagic shock groups were divided into four groups and treated with corilagin (5 mg/kg), corilagin and Wortmannin (1 mg/kg, intravenously), Wortmannin alone, or vehicle; sham groups were also included.
Rat hemorrhagic shock and resuscitation model
All animals were placed in individual air-conditioned cages (humidity: 70–75%) with controlled temperature (24–25oC) and lighting (12:12 light–dark cycle: lights on from 06:00 to 18:00) in an animal house. One week before the experiments, all experimental animals were sent to the animal house and allowed to adapt to the environment. Basal feed and water was provided. Before the experiment, male Sprague–Dawley rats were fasted overnight but allowed free access to water. Hemorrhagic shock and resuscitation was performed as described previously (4).
Briefly, a 5-cm midline abdominal laparotomy was performed on anesthetized rats by isoflurane inhalation to induce tissue trauma, and then the abdominal wound layers were sutured in layers. Under anesthesia, polyethylene catheters (PE-50; BD Biosciences, Franklin Lakes, NJ) were placed in the right femoral vein and bilateral femoral arteries, fixed, and the incision sites were closed. To reduce postoperative pain, the wounds were infiltrated with 1% xylocaine (Elkins-Sinn Inc, Cherry Hill, NJ) during the operative procedure. The rats were then allowed to awaken and bled rapidly within 10 min to a mean arterial pressure of 35 mm Hg to 40 mm Hg within 10 min. This level of hypotension was maintained until the animals could no longer maintain a 40 mm Hg mean arterial pressure. This time was defined as the maximum bleed-out. After the maximal bleed-out, mean arterial pressure was also maintained at 35 mm Hg to 40 mm Hg until 40% of the maximal bleed-out volume was returned in the form of Ringer lactate solution (approximately 90 min from the onset of bleeding). The rats were then resuscitated with a 4-fold volume of the shed blood with Ringer lactate over 60 min. Thirty minutes before the end of the resuscitation period, the rats received corilagin (5 mg/kg, intravenously), corilagin plus the PI3K inhibitor Wortmannin (1 mg/kg, intravenously at the beginning of resuscitation), Wortmannin, or an equal volume of vehicle (approximately 0.2 mL, 10% dimethyl sulfoxide). The insertion catheters were removed, vessels were ligated, and skin incision wounds were closed with sutures after resuscitation. In sham-operated rats, vehicle or corilagin was also administered after the catheters were placed. Sham-operated rats underwent the same operative procedures, but neither hemorrhage nor resuscitation was carried out. All animals were humanely sacrificed at 24 h after the end of resuscitation or sham operation.
Assessment of hepatic injury
Blood samples were collected in a heparin tube and the plasma was separated by centrifugation at 24 h following trauma-hemorrhage or sham surgery. Hepatic injury was determined by measuring plasma ALT and AST levels using a colorimetric analyzer (Dri-Chem 3000; Fuji Photo Film Co, Tokyo, Japan).
Determination of MPO activity
The MPO activity assay in homogenates of rat liver tissues was measured as described previously (4). Frozen liver tissue samples were thawed and suspended in pH 6.0 phosphate buffer containing 0.5% hexadecyltrimethylammonium bromide (Sigma, St. Louis, Mo). All samples were sonicated on ice, centrifuged at 12,000 × g for 15 min at 4°C, and then aliquots were transferred into phosphate buffer (pH 6.0) containing 0.167 mg/mL O-dianisidine hydrochloride and 0.0005% hydrogen peroxide (Sigma). The change in absorbance was measured at a wavelength of 460 nm with a spectrophotometer for 5 min. MPO activity was calculated using a standard curve generated using human MPO (Sigma), and values were normalized according to the protein concentration.
Measurement of CINC-1, CINC-3, ICAM-1, IL-6, and TNF-α levels
Liver tissues were homogenized in potassium phosphate buffer (1:10/weight:volume; pH 7.4) containing protease inhibitors (Complete Protease Inhibitor Cocktail; Boehringer, Mannheim, Germany). The homogenates were centrifuged at 2,000 × g (4°C) for 20 min and the supernatants were analyzed for the presence of IL-6, TNF-α, ICAM-1, CINC-1, and CINC-3 using ELISA kits (R&D Systems, Minneapolis, Minn) according to the manufacturer's instructions, and as described previously (4). Aliquots of the supernatant were used to determine protein concentration using the Bio-Rad DC Protein Assay (Bio-Rad, Hercules, Calif).
Western blot assay
Rat liver tissues were homogenized in buffer as described previously (4). Protein aliquots were used to determine protein concentration using the Bio-Rad DC Protein Assay (Bio-Rad). Samples were mixed with ×4 sample buffer, electrophoresed on sodium dodecyl sulfate-polyacrylamide gels, and electrophoretically transferred onto nitrocellulose membranes. The membranes were incubated with antibodies for total Akt protein, phosphorylate-Akt (Ser473) (Cell Signaling Technology, Beverly, Mass), or GAPDH (Abcam, Cambridge, UK) overnight at 4°C. The membranes were then incubated with horseradish peroxidase-conjugated goat antimouse antibody or goat antirabbit antibody for 1.5 h at room temperature. After the final wash, the membranes were probed by enhanced chemiluminescence (Amersham, Amersham, UK) and exposed to film for autoradiography.
Statistical analysis
The InStat 3.0 biostatistics program (Graph Pad Software Inc, San Diego, Calif) was used for statistical analysis. The results are presented as the mean ± standard error of the mean (SEM). The data were analyzed using one-way analysis of variance and the Tukey test, and differences were considered significant when P ≤ 0.05.
RESULTS
Dose–response effects of corilagin on plasma AST and ALT levels
Hemorrhagic shock caused a significant increase in plasma AST and ALT levels at 24 h after resuscitation (Fig. 1, A and B). Treatment with corilagin at 1 mg/kg, 5 mg/kg, or 10 mg/kg was used to evaluate the beneficial effects of corilagin on the attenuation of liver injury following hemorrhagic shock. As shown in Figure 1, 1 mg/kg, 5 mg/kg, or 10 mg/kg corilagin administration had beneficial effects. However, the effects of corilagin were equivalent when administered at a dose of 5 mg/kg or 10 mg/kg.
Fig. 1.
Dose-dependent responses to corilagin treatment of plasma AST (A) and ALT (B) in rats at 24 h after sham operation (sham) or hemorrhagic shock (H-S).
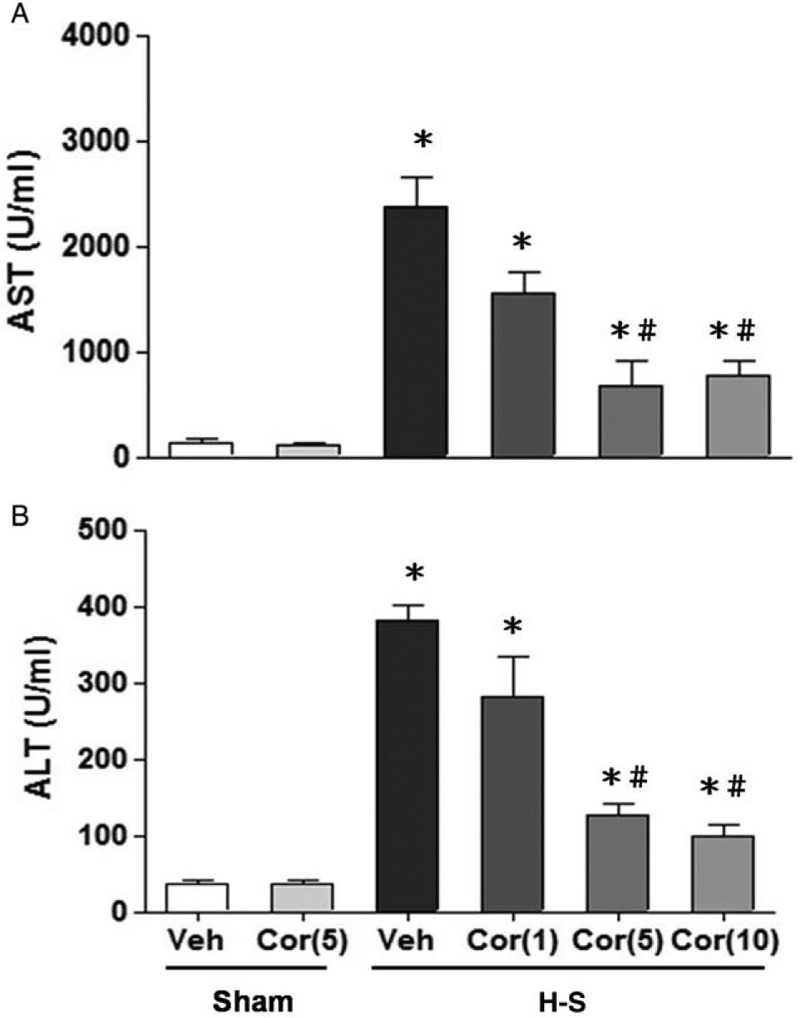
Animals were treated with vehicle (Veh), corilagin (Cor: 5 mg/kg), or corilagin at doses of 0 mg/kg, 1 mg/kg, 5 mg/kg, or 10 mg/kg. Data are shown as the mean ± SEM. n = 6 rats in each group. ∗P < 0.05 compared with sham; #P < 0.05 compared with H-S + Cor (0 mg/kg). ALT indicates alanine aminotransferase; AST, aspartate aminotransferase.
Alteration in plasma AST and ALT levels
At 24 h after sham operation, there was no significant difference in plasma AST and ALT levels between vehicle- and corilagin-treated groups (Fig. 2, A and B). Plasma AST and ALT levels were significantly increased in trauma-hemorrhaged rats. Treatment with corilagin (5 mg/kg) attenuated the hemorrhagic shock-induced increase in plasma AST and ALT levels. We next investigated whether the protective effects of corilagin in attenuating hepatic injury following hemorrhagic shock were mediated via Akt-mediated activity. In the group of corilagin-treated trauma-hemorrhaged rats cotreated with the PI3K inhibitor Wortmannin, the corilagin-induced reduction in plasma AST and ALT levels was abolished (Fig. 2).
Fig. 2.
Effect of corilagin treatment on plasma AST (A) and ALT (B) in rats at 24 h after sham operation (Sham) or hemorrhagic shock (H-S).
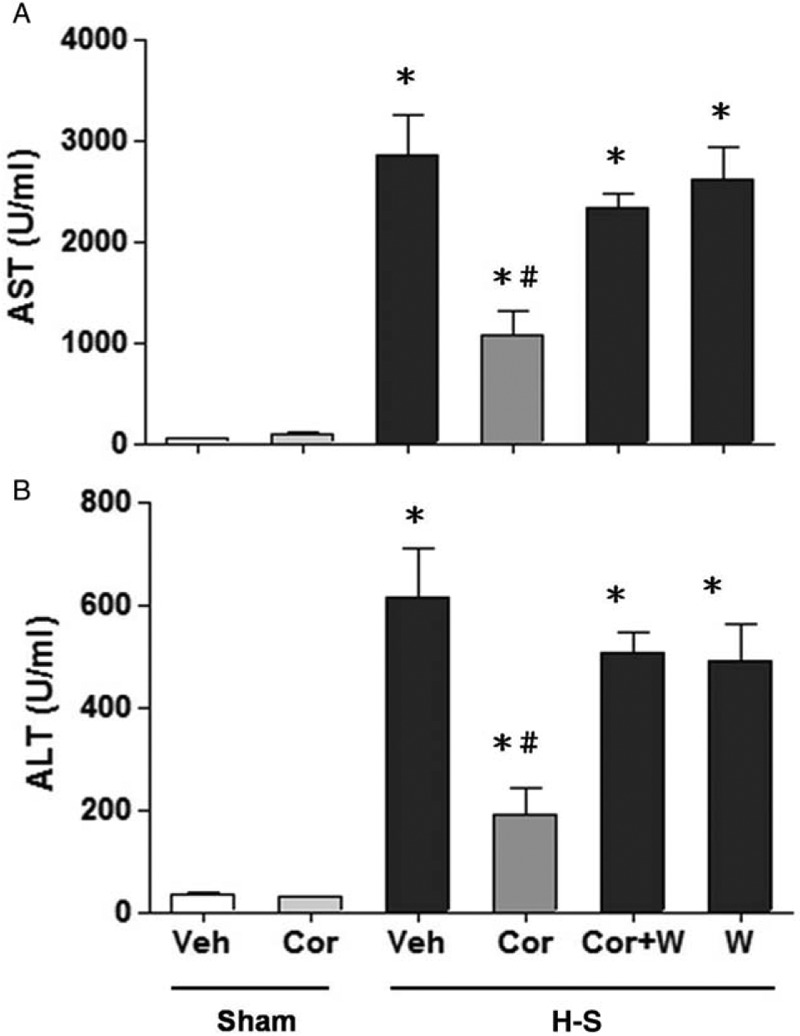
Animals were treated with either vehicle (Veh), corilagin (Cor), corilagin in combination with Wortmannin (Cor+W), or Wortmannin (W). Data are shown as the mean ± SEM of six rats in each group. ∗P < 0.05 compared with Sham; #P < 0.05 compared with H-S +Veh, H-S +Cor+W, and H-S +W. ALT indicates alanine aminotransferase; AST, aspartate aminotransferase.
Alteration in hepatic MPO activity
As shown in Figure 3, hepatic MPO activity was observed in sham-operated or trauma-hemorrhaged animals with and without corilagin treatment. In sham-operated rats, corilagin did not alter hepatic MPO activity. Hemorrhagic shock significantly increased hepatic MPO activity in vehicle-treated animals. Furthermore, treatment with corilagin attenuated the increase in hepatic MPO activity. Co-administration of the PI3K inhibitor Wortmannin with corilagin prevented corilagin-mediated attenuation of hepatic MPO activity after hemorrhagic shock.
Fig. 3.
Effect of corilagin treatment on hepatic MPO activity in rats at 24 h after sham operation (Sham) or hemorrhagic shock (H-S).
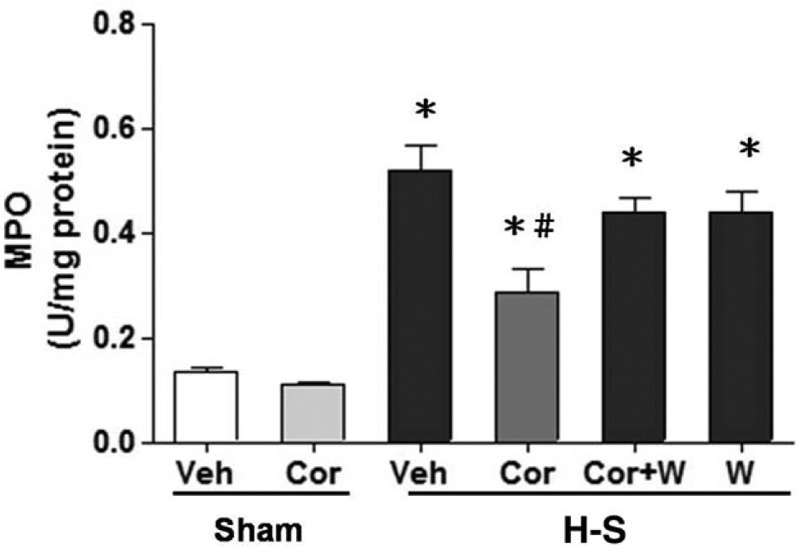
Animals were treated with either vehicle (Veh), corilagin (Cor), corilagin in combination with Wortmannin (Cor+W), or Wortmannin (W). Data are shown as the mean ± SEM of six rats in each group. ∗P < 0.05 compared with Sham; #P < 0.05 compared with H-S +Veh, H-S +Cor+W, and H-S +W. MPO indicates myeloperoxidase.
Alteration of hepatic CINC-1, CINC-3, and ICAM-1 levels
As shown in Figures 4A, B and 5, no significant change in hepatic CINC-1, CINC-3, and ICAM-1 levels between the vehicle- and corilagin-treated sham groups were observed. Hemorrhagic shock significantly elevated hepatic CINC-1, CINC-3, and ICAM-1 levels. The increase in hepatic CINC-1, CINC-3, and ICAM-1 levels was reduced by corilagin treatment. The corilagin-mediated reduction in CINC-1, CINC-3, and ICAM-1 levels was abolished by co-administration of the PI3K inhibitor Wortmannin.
Fig. 4.
Effect of corilagin treatment on hepatic CINC-1 (A) and CINC-1 (B) levels in rats at 24 h after sham operation (Sham) or hemorrhagic shock (H-S).
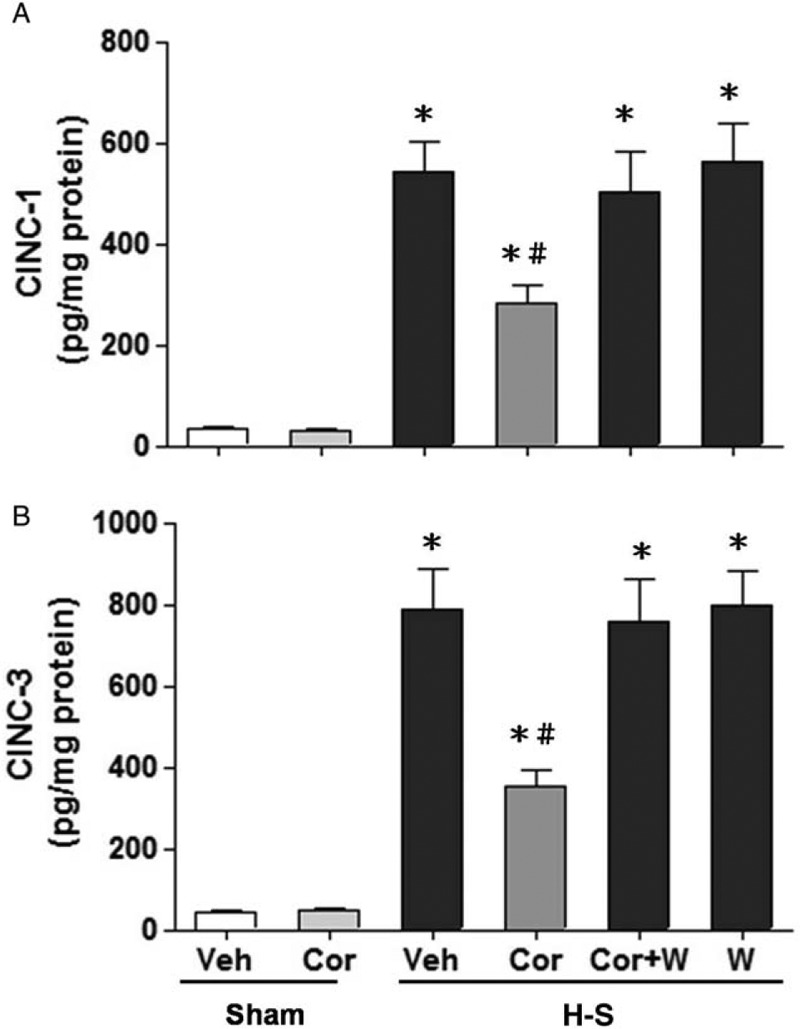
Animals were treated with either vehicle (Veh), corilagin (Cor), corilagin in combination with Wortmannin (Cor+W), or Wortmannin (W). Data are shown as the mean ± SEM of six rats in each group. ∗P < 0.05 compared with Sham; #P < 0.05 compared with H-S +Veh, H-S +Cor+W, and H-S +W. CINC-1 indicates cytokine-induced neutrophil chemoattractant-1.
Fig. 5.
ICAM-1 levels in the liver in rats after sham operation (Sham) or hemorrhagic shock (H-S).
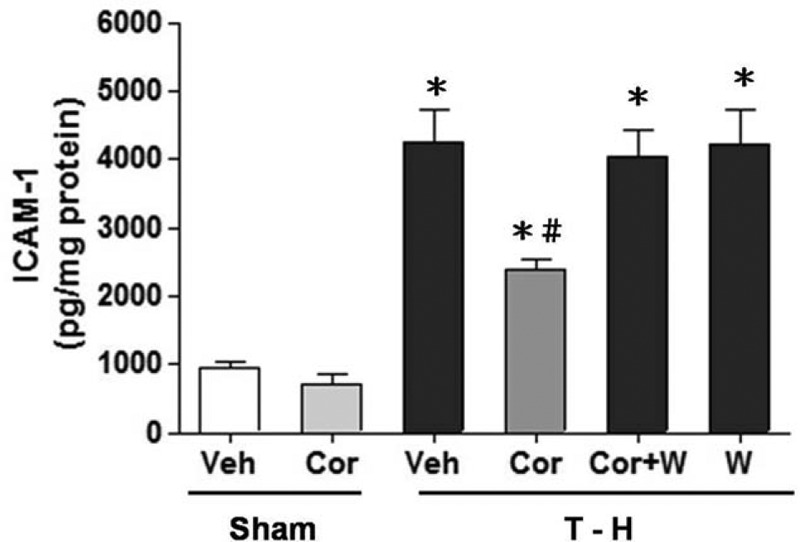
Animals were treated with either vehicle (Veh), corilagin (Cor), corilagin in combination with Wortmannin (Cor+W), or Wortmannin (W). Data are shown as the mean ± SEM of six rats in each group. ∗P < 0.05 compared with Sham; #P < 0.05 compared with H-S +Veh, H-S +Cor+W, and H-S +W. ICAM-1 indicates intercellular adhesion molecule-1.
Alteration in hepatic IL-6 and TNF-α expression
There was no significant difference in hepatic IL-6 and TNF-α expression between the sham groups (Fig. 6). Following hemorrhagic shock, hepatic IL-6 and TNF-α concentrations were significantly higher in vehicle-treated rats than in sham animals (Fig. 6, A and B). Treatment with corilagin (5 mg/kg) attenuated the hemorrhagic shock-induced increase in IL-6 and TNF-α expression. Co-administration of corilagin with the PI3K inhibitor Wortmannin prevented the corilagin-induced decrease in IL-6 and TNF-α concentrations.
Fig. 6.
Effect of corilagin treatment on hepatic IL-6 (A) and TNF-α (B) levels in rats at 24 h after sham operation (Sham) or hemorrhagic shock (H-S).
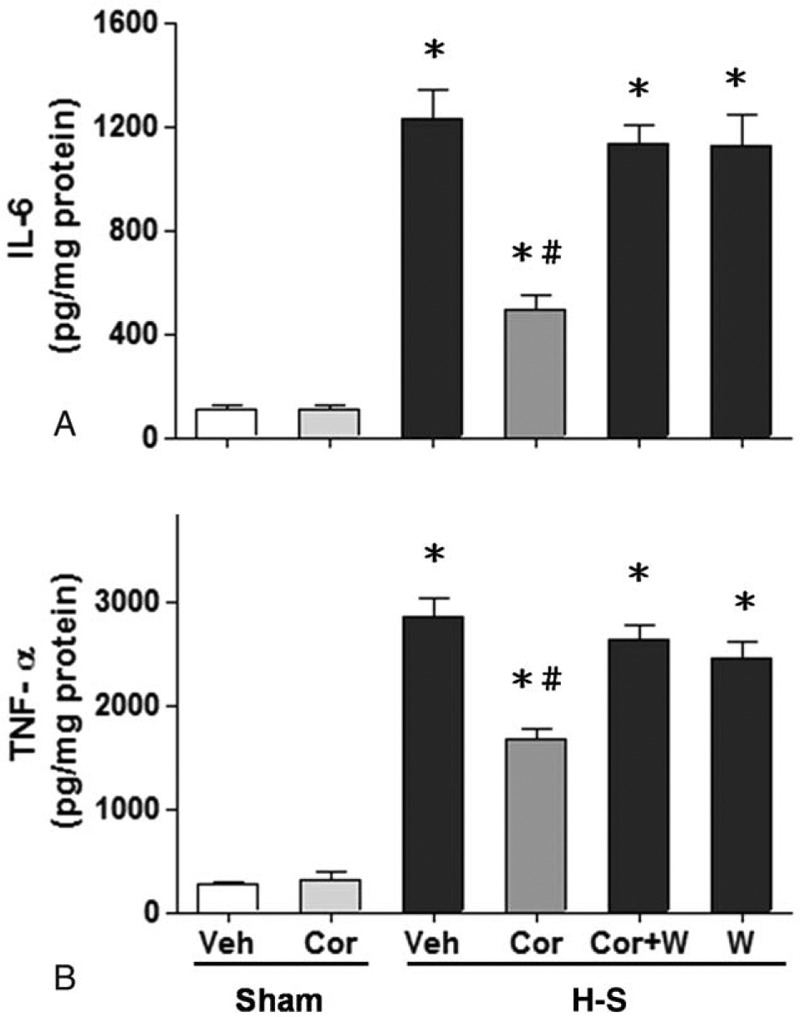
Animals were treated with either vehicle (Veh), corilagin (Cor), corilagin in combination with Wortmannin (Cor+W), or Wortmannin (W). Data are shown as the mean ± SEM of six rats in each group. ∗P < 0.05 compared with Sham; #P < 0.05 compared with H-S +Veh, H-S +Cor+W, and H-S +W. IL-6 indicates interleukin-6; TNF-α, tumor necrosis factor-α.
Activity of hepatic Akt protein
As shown in Figure 7, hepatic Akt protein expression between the sham and trauma-hemorrhaged rats was not significantly different. Phosphorylation of Akt is typically used to determine Akt activation. The Akt phosphorylation status was significantly decreased after hemorrhagic shock, and treatment of corilagin restored Akt phosphorylation to the observed levels in sham animals. The increase in phosphorylated-Akt induced by corilagin was abolished when the PI3K inhibitor Wortmannin was co-administered with corilagin.
Fig. 7.
Hepatic p-Akt and Akt protein expressions from sham-operated animals receiving vehicle (Sham+Veh; lane 1) or corilagin (Sham+Cor; lane 2), trauma-hemorrhaged animals receiving vehicle (H-S+Veh; lane 3), corilagin (H-S+Cor; lane 4), corilagin and wortmannin (H-S+Cor+W; lane 5), or wortmannin (H-S+W; lane 6).
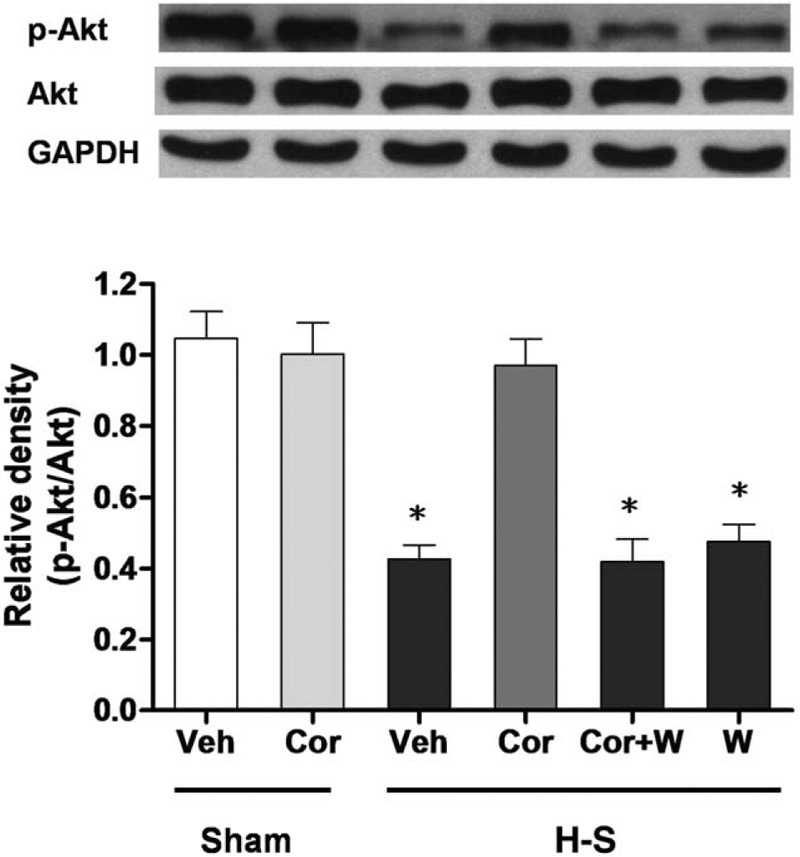
Blots were reprobed for GAPDH as a control for equal protein loading in all lanes. The bands were analyzed using densitometry, and the values are presented as mean ± SEM for six rats in each group. ∗P < 0.05 versus all other groups.
DISCUSSION
In the present study, we investigated the protective effects of corilagin on rodent hepatic injury following hemorrhagic shock and evaluated the role of the Akt-dependent pathways in corilagin-mediated hepatoprotection. Hepatic injury-related parameters, including plasma AST and ALT concentrations, hepatic MPO activity, and CINC-1, CINC-3, ICAM-1, IL-6, and TNF-α levels were significantly elevated at 24 h after trauma-hemorrhagic shock. The hepatoprotective effects of corilagin at doses of 1 mg/kg, 5 mg/kg, and 10 mg/kg were evaluated following hemorrhagic shock. Similar beneficial effects of corilagin treatment at doses of 5 mg/kg or 10 mg/kg were observed, and thus the dosage of 5 mg/kg was used in subsequent analyses. Treatment with corilagin (5 mg/kg) during resuscitation attenuated exacerbation of liver injury and inflammatory parameters. Such a treatment also prevented the trauma-hemorrhagic shock-induced reduction in phosphorylated-Akt expression. When the PI3K inhibitor Wortmannin was co-administered with corilagin, the corilagin-induced liver protective effects were abolished. Collectively, these results suggest that the protective effects of corilagin are exerted through an Akt-related pathway.
The liver is an important internal organ in humans and is easily damaged by hemorrhagic shock (3). Previous studies have indicated that hemorrhagic shock-induced hepatic injury is associated with neutrophil activation and accumulation (5). The activated neutrophils infiltrate the liver and lead to massive release of cytokines and proinflammatory mediators (5).
CINC-1 and CINC-3 are potent chemoattractants for neutrophils and have been correlated with tissue MPO activity in injured tissue (17). Our results showed that hemorrhagic shock induced a marked increase in hepatic MPO activity and CINC-1 and CINC-3 levels, which were attenuated by corilagin treatment. In addition, neutrophil migration requires the interaction with multiple adhesion molecules. ICAM-1 is an important adhesion molecule in neutrophils for adhesion onto the vascular endothelium after organ injury (5). In this study, hemorrhagic shock resulted in a significant increase in hepatic ICAM-1 levels, which was accompanied by hepatic MPO activity elevation. Furthermore, MPO activity and ICAM-1 levels were alleviated in corilagin-treated hemorrhagic shock rats.
IL-6 and TNF-α are important pro-inflammatory mediators and their expression reflects the severity of tissue injury and influence on organ function (4). A previous study showed that liver injury leads to marked increases in hepatic IL-6 and TNF-α (4). Furthermore, adhesion molecule expression and cytokine release are related to IL-6 and TNF-α stimuli (4). In this study, we found that the hepatic IL-6 and TNF-α expression in rats was elevated following hemorrhagic shock. Treatment of the animals with corilagin significantly decreased hepatic IL-6 and TNF-α concentrations. These findings suggest that corilagin reduces inflammatory cytokine and adhesion molecules expression to regulate hepatic inflammation.
The PI3K/Akt pathway is an important intracellular signaling pathway involved in cell survival and tissue function maintenance (7). Previous studies have reported that activation of the PI3K/Akt pathway protects organs against hypoxia or ischemia injury (6). Additionally, PI3K influences neutrophil function and infiltration in the injured liver (18). Previous reports have also indicated that up-regulation of the PI3K/Akt signaling pathway decreased neutrophil accumulation and cytokine/adhesion molecule overproduction after hemorrhagic shock (19). Our previous studies also suggested that the PI3K/Akt pathway plays a critical role in organ protection following hemorrhagic shock (5).
Phyllanthus urinaria, a traditional herbal medicine, has been reported to possess different biomedical characteristics including antipathogenic, anticancer, and antihepatotoxicity effects (12, 14, 16). Studies showed that P. urinaria extracts have numerous biological activities including anti-oxidative, antiinflammatory, antiapoptotic, and hepatoprotective effects (16). Corilagin, an important P. urinaria extract, has been reported to exhibit antiviral activity against hepatitis B virus infection (20) and antitumor activity against Hep3B hepatoma in mice (21). Corilagin was also shown to alleviate post-parasiticide liver fibrosis in a Schistosomiasis-infected animal model (22). In addition, corilagin protected against galactosamine/lipopolysaccharide-induced liver injuries through anti-oxidative stress and antilipid peroxidation effects, as well as by inhibiting apoptosis (23). In view of the above reported effects, we evaluated whether the PI3K/Akt pathway is involved in mediating the hepatoprotective effects following corilagin treatment. Our results demonstrated that the corilagin-induced attenuation of hepatic enzymes and pro-inflammatory mediators were blocked by the PI3K inhibitor Wortmannin. The elevation in Akt phosphorylation by corilagin following trauma-hemorrhagic shock was abolished by co-administration of Wortmannin. The current results indicate that the hepatic protective effects of corilagin are in part mediated by the Akt-dependent pathway.
It should be noted that the present study examined the mechanism of the salutary effects of corlagin following hemorrhagic shock at a single time, i.e., 24 h after treatment. Thus, it remains unclear if the same or different mechanisms are involved in the early or late protective effects by this agent. Furthermore, although we examined the mechanism of corlagin following hemorrhagic shock, we did not examine if this agent had any protective effects on survival in this study not did we examine if it had protective effects on hepatic architecture, function of other organs such as the heart, intestine and brain, or functional aspects of the liver following hemorrhagic shock. However, since the liver enzymes did decreasing with corlagin it would suggest that there were some protective effects on hepatic architecture. Nonetheless, we did not measure any functional aspects such as synthesis of acute phase synthesis of the liver under those conditions and thus it remains unclear if this agent also had salutary effects of the functional aspects of the liver. Our primary focus in this study was on examining the potential mechanism of hepatoprotection by corilagin and we hope to examine the other aspects listed above in our future studies. Wortmannin can have effects on other signaling pathways such as mammalian target of rapamycin, p38 mitogen-activated protein kinases, polo-kinase, and insulin receptor activation, depending on the concentration and cell type (24, 25). These actions might contribute to the hepatoprotective effects of corilagin following trauma-hemorrhagic shock. In addition, corilagin may function by different mechanisms/pathways. Corilagin is a weak carbonic anhydrase inhibitor (26). It remains unknown if the effect is related to p-Akt. Additional studies are needed to precisely elucidate the mechanism by which corilagin attenuates hepatic injury following trauma-hemorrhagic shock.
In summary, we determined the hepatoprotective mechanism of corilagin from P. urinaria extract in a trauma-hemorrhagic shock rodent model. Corilagin treatment effectively ameliorated neutrophil accumulation, production of pro-inflammatory mediators, and elevation of enzymes released following hepatic injury. When Akt activation was blocked, the hepatoprotective effects of corilagin were abolished. Our results suggest that the Akt-dependent pathway plays a critical role in corilagin-mediated hepatoprotection following trauma-hemorrhagic shock. These findings indicate that corilagin from P. urinaria extract is a potential therapeutic adjunct that can be used for organ protection following low flow conditions.
Footnotes
This work was supported in part by grants from the National Science Council (NSC102-2314-B-182A-051-MY3) and Chang Gung Memorial Hospital (CMRPG3E1551) to H-PY. Support was also provided by the National Science Council (NSC103-2314-B-182-046-MY2) and Chang Gung Memorial Hospital (CMRPG3E1542) to F-CL.
The authors report no conflicts of interest.
REFERENCES
- 1.Wu D, Zhou X, Ye L, Gan J, Zhang M. Emergency department crowding and the performance of damage control resuscitation in major trauma patients with hemorrhagic shock. Acad Emerg Med 2015; 22:915–921. [DOI] [PubMed] [Google Scholar]
- 2.Douzinas EE. Hemorrhagic shock resuscitation: a critical issue on the development of posttraumatic multiple organ failure. Crit Care Med 2012; 40:1348–1349. [DOI] [PubMed] [Google Scholar]
- 3.Karmaniolou II, Theodoraki KA, Orfanos NF, Kostopanagiotou GG, Smyrniotis VE, Mylonas AI, Arkadopoulos NF. Resuscitation after hemorrhagic shock: the effect on the liver—a review of experimental data. J Anesth 2013; 27:447–460. [DOI] [PubMed] [Google Scholar]
- 4.Liu FC, Yu HP, Hwang TL, Tsai YF. Protective effect of tropisetron on rodent hepatic injury after trauma-hemorrhagic shock through P38 MAPK-dependent hemeoxygenase-1 expression. PLoS One 2012; 7:e53203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu FC, Pang ST, Tsai YF, Chaudry IH, Yu HP. Hepatoprotective effect of casodex after trauma hemorrhage in a rodent model. Shock 2015; 43:470–474. [DOI] [PubMed] [Google Scholar]
- 6.Williams DL, Ozment-Skelton T, Li C. Modulation of the phosphoinositide 3-kinase signaling pathway alters host response to sepsis, inflammation, and ischemia/reperfusion injury. Shock 2006; 25:432–439. [DOI] [PubMed] [Google Scholar]
- 7.Kim M, Shin MS, Lee JM, Cho HS, Kim CJ, Kim YJ, Choi HR, Jeon JW. Inhibitory effects of isoquinoline alkaloid berberine on ischemia-induced apoptosis via activation of phosphoinositide 3-kinase/protein kinase B signaling pathway. Int Neurourol J 2014; 18:115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunawan-Puteri MD, Kato E, Kawabata J. Alpha-Amylase inhibitors from an Indonesian medicinal herb, Phyllanthus urinaria. J Sci Food Agric 2012; 92:606–609. [DOI] [PubMed] [Google Scholar]
- 9.Jin F, Cheng D, Tao JY, Zhang SL, Pang R, Guo YJ, Ye P, Dong JH, Zhao L. Anti-inflammatory and anti-oxidative effects of corilagin in a rat model of acute cholestasis. BMC Gastroenterol 2013; 13:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuandani, Ilangkovan M, Jantan I, Mohamad HF, Husain K, Abdul Razak AF. Inhibitory effects of standardized extracts of phyllanthus amarus and phyllanthus urinaria and their marker compounds on phagocytic activity of human neutrophils. Evid Based Complement Alternat Med 2013; 2013:603634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tong F, Zhang J, Liu L, Gao X, Cai Q, Wei C, Dong J, Hu Y, Wu G, Dong X. Corilagin attenuates radiation-induced brain injury in mice. Mol Neurobiol 2016; 53:6982–6996. [DOI] [PubMed] [Google Scholar]
- 12.Yeo SG, Song JH, Hong EH, Lee BR, Kwon YS, Chang SY, Kim SH, Lee SW, Park JH, Ko HJ. Antiviral effects of Phyllanthus urinaria containing corilagin against human enterovirus 71 and Coxsackievirus A16 in vitro. Arch Pharm Res 2015; 38:193–202. [DOI] [PubMed] [Google Scholar]
- 13.Moreira J, Klein-Junior LC, Cechinel Filho V, de Campos Buzzi F. Anti-hyperalgesic activity of corilagin, a tannin isolated from Phyllanthus niruri L (Euphorbiaceae). J Ethnopharmacol 2013; 146:318–323. [DOI] [PubMed] [Google Scholar]
- 14.Huang ST, Pang JH, Yang RC. Anti-cancer effects of phyllanthus urinaria and relevant mechanisms. Chang Gung Med J 2010; 33:477–487. [PubMed] [Google Scholar]
- 15.Shen ZQ, Dong ZJ, Peng H, Liu JK. Modulation of PAI-1 and tPA activity and thrombolytic effects of corilagin. Planta Med 2003; 69:1109–1112. [DOI] [PubMed] [Google Scholar]
- 16.Hau DK, Gambari R, Wong RS, Yuen MC, Cheng GY, Tong CS, Zhu GY, Leung AK, Lai PB, Lau FY, et al. Phyllanthus urinaria extract attenuates acetaminophen induced hepatotoxicity: involvement of cytochrome P450 CYP2E1. Phytomedicine 2009; 16:751–760. [DOI] [PubMed] [Google Scholar]
- 17.Yu HP, Shimizu T, Hsieh YC, Suzuki T, Choudhry MA, Schwacha MG, Chaudry IH. Tissue-specific expression of estrogen receptors and their role in the regulation of neutrophil infiltration in various organs following trauma-hemorrhage. J Leukoc Biol 2006; 79:963–970. [DOI] [PubMed] [Google Scholar]
- 18.Hannigan MO, Huang CK, Wu DQ. Roles of PI3K in neutrophil function. Curr Top Microbiol Immunol 2004; 282:165–175. [DOI] [PubMed] [Google Scholar]
- 19.Hsu JT, Kuo CJ, Chen TH, Wang F, Lin CJ, Yeh TS, Hwang TL, Jan YY. Melatonin prevents hemorrhagic shock-induced liver injury in rats through an Akt-dependent HO-1 pathway. J Pineal Res 2012; 53:410–416. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y, Lu Y, Li SY, Song YH, Hao Y, Wang Q. Extract from Phyllanthus urinaria L. inhibits hepatitis B virus replication and expression in hepatitis B virus transfection model in vitro. Chin J Integr Med 2015; 21:938–943. [DOI] [PubMed] [Google Scholar]
- 21.Hau DK, Zhu GY, Leung AK, Wong RS, Cheng GY, Lai PB, Tong SW, Lau FY, Chan KW, Wong WY, et al. In vivo anti-tumour activity of corilagin on Hep3B hepatocellular carcinoma. Phytomedicine 2010; 18:11–15. [DOI] [PubMed] [Google Scholar]
- 22.Huang YF, Zhang SL, Jin F, Cheng D, Zhou YP, Li HR, Tang ZM, Xue J, Cai W, Dong JH, et al. Activity of corilagin on post-parasiticide liver fibrosis in Schistosomiasis animal model. Int J Immunopathol Pharmacol 2013; 26:85–92. [DOI] [PubMed] [Google Scholar]
- 23.Kinoshita S, Inoue Y, Nakama S, Ichiba T, Aniya Y. Antioxidant and hepatoprotective actions of medicinal herb, Terminalia catappa L. from Okinawa Island and its tannin corilagin. Phytomedicine 2007; 14:755–762. [DOI] [PubMed] [Google Scholar]
- 24.Arcaro A, Guerreiro AS. The phosphoinositide 3-kinase pathway in human cancer: genetic alterations and therapeutic implications. Curr Genomics 2007; 8:271–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Codogno P, Meijer AJ. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ 2005; 12 suppl 2:1509–1518. [DOI] [PubMed] [Google Scholar]
- 26.Satomi H, Umemura K, Ueno A, Hatano T, Okuda T, Nora T. Carbonic anhydrase inhibitors from the pericarps of Punica granatum L. Biol Pharm Bull 1993; 16:787–790. [DOI] [PubMed] [Google Scholar]


