Abstract
The PI3K pathway is hyperactivated in most cancers, yet the capacity of PI3K inhibitors to induce tumor cell death is limited. The efficacy of PI3K inhibition can also derive from interference with the cancer cells’ ability to respond to stromal signals, as illustrated by the approved PI3Kδ inhibitor Idelalisib in B-cell malignancies. Inhibition of the leukocyte-enriched PI3Kδ or PI3Kγ may unleash more potent anti-tumor T-cell responses, by inhibiting regulatory T-cells and immune-suppressive myeloid cells. Moreover, tumor angiogenesis may be targeted by PI3K inhibitors to enhance cancer therapy. Future work should therefore focus on the effects of PI3K inhibitors on the stroma, in addition to their direct effects on tumors.
Significance
The PI3K pathway extends beyond the direct regulation of cancer cell proliferation and survival. In B-cell malignancies, targeting PI3K purges the tumor cells from their protective microenvironment. Moreover, we propose that PI3K isoform-selective inhibitors may be exploited in the context of cancer immunotherapy and by targeting angiogenesis to improve drug and immune cell delivery.
Introduction
Pathological activation of the PI3K pathway is among the most frequent signaling events associated with cellular transformation, cancer and metastasis (1–4). This is exemplified by the frequent activating mutations in PIK3CA and the loss of PTEN functionality in common cancers such as those of the breast, colon and ovaries. A significant pharmaceutical effort is therefore dedicated to inhibiting the PI3K pathway within cancer cells and this is starting to yield some positive results in combination trials.
However, in other cancer types, such as lung and pancreas, mutations that activate the PI3K pathway are less common. Mutational activation of the PI3K pathway is also rare in B-cell malignancies, such as chronic lymphocytic leukemia (CLL) and indolent non-Hodgkin’s lymphoma (iNHL), yet a PI3K pathway inhibitor (Idelalisib, an inhibitor of PI3Kδ) was recently approved as a therapy for this disease. A major component of the mechanism of action of PI3Kδ inhibition in these B-cell malignancies is to dampen the responsiveness of the tumor cells to supportive stimuli from the microenvironment (5).
The impact of PI3K inhibition on the tumor stroma (6) is under-investigated. The stroma can be defined as any cell that forms part of the tumor mass but which is not malignantly transformed. Typically, the stroma will include (a) the vasculature, (b) infiltrating immune cells, (c) fibroblasts and connective tissue. Emerging evidence indicates that PI3K activity has an important role in regulating each of these stromal elements, which could be exploited therapeutically.
In this review, we summarize the key observations made to date on PI3K intervention in cancer, and provide some examples of ongoing trials that combine PI3K inhibitors with other agents. We will concentrate on the emerging indications for the use of PI3K inhibitors to target the cancer stroma, with a special focus on immune modulation. For detailed overviews of the PI3K signaling pathway, PI3K inhibitors and ongoing clinical trials, we refer the reader to recent reviews (1–4, 7).
PI3k Pathway Inhibition In Cancer: Lessons Learned To Date
Preclinical and clinical experience with PI3K inhibitors in cancer has provided important insights, which can be summarized as follows:
First, despite PI3K signaling being commonly activated in tumor cells, PI3K inhibitors have shown only modest single-agent therapeutic efficacy in solid tumors. This could be due to various reasons, including insufficient target inhibition, intrinsic and acquired drug resistance and tolerability. Pan-class I PI3K inhibitors show serious adverse effects upon long-term continuous dosing, which limits the on-therapy time (reviewed in Ref. (8)). Emerging data indicate that isoform-selective PI3Kα inhibitors may have a more favorable safety profile than pan-class I PI3K inhibitors (9). Alternative dosing schedules are also being explored, as evidence from pre-clinical models suggests that transient, complete PI3K pathway interruption can increase the therapeutic index without compromising therapeutic efficacy (10, 11).
Second, cancer cells are very effective at resisting PI3K inhibition. This occurs through (1) non-genetic, intrinsic feedback regulation within the pathway upon short-term PI3K inactivation and (2) through genetic resistance that develops, or is selected for, upon long-term PI3K blockade [(12, 13), reviewed in Ref. (14)]. The presence of multiple mechanisms to counteract PI3K inhibition underlines the key importance of this pathway in cancer cells.
Third, inhibition of PI3K in cancer cells in vitro is rarely cytotoxic, but more commonly cytostatic, which most likely reflects the fundamental role of PI3K signaling as a growth factor/nutrient-sensor. Upon inhibition of the PI3K pathway, cells enter a dormant, nutrient-deprived state but do not necessarily die. This is akin to the key role of AGE-1, the single class I PI3K equivalent in C. elegans, in regulating the formation of dauer larva, a state of stasis that allows the survival of the organism under harsh conditions (15). It has also been demonstrated that cells can continue to proliferate with minimal residual class I PI3K activity (16). Long-term complete inhibition of all PI3K activity may not be achievable in the clinical setting due to unacceptable side effects. However, as mentioned above, it is possible that repetitive, short-term, more complete PI3K inhibition with a high dose of inhibitor may be effective as a ‘shock therapy’ that prevents the cancer cells from adapting to PI3K inhibition (10, 11).
Fourth, given that cancer cells can activate the PI3K pathway in many ways, the power of specific PI3K pathway mutations in predicting drug sensitivity is not absolute [reviewed in Refs. (17, 18)]. Indeed, while for example PIK3CA amplification/mutation in cancer cell lines has some predictive value in determining sensitivity to PI3K inhibitors, this correlation is not absolute and other genetic parameters also control this response (19). This complicates patient selection based on single-gene PI3K pathway mutation status.
Fifth, the relative merits of pan-class I versus isoform-selective class I PI3K inhibitors in the clinic remain unclear. While pan-class I PI3K inhibitors are less well tolerated, they are less likely than isoform-selective class I PI3K inhibitors to allow compensation by other PI3K isoforms, as was observed by activation of PI3Kβ upon selective blockade of PI3Kα (20) and vice versa (21).
Lastly, due to the central role of PI3Kα in regulating organismal glucose homeostasis, PI3K inhibition in patients often gives rise to hyperglycemia and/or hyperinsulinemia (22). High levels of circulating insulin could potentially be mitogenic and/or anti-apoptotic for cancer cells, and thus negate the anti-proliferative effects of PI3K inhibitors (23). However, it remains to be determined whether the observed changes in circulating insulin levels upon PI3K inhibition have a physiological impact on cancer cells. Insulin-stimulated PI3K activation in the cancer cells would be expected to be blocked by sufficient PI3K target inhibition; however, this would not be the case for other signaling pathways activated by insulin in the cancer cells, such as the MAPK pathway. In the setting of cancer with mutated PI3Kα, one way to overcome the problem of compensatory production of insulin and/or glucose upon systemic PI3Kα inhibition would be to develop inhibitors with enhanced selectivity for mutant PI3Kα over wild-type PI3Kα. This would create a window for drug dosing to inhibit PI3Kα in the cancer cells without affecting the wild-type PI3Kα in the host tissues that control systemic metabolism.
Examples Of Drug Combination Strategies Based On The Cancer Cell-Intrinsic Roles Of PI3K
The pro-survival role of PI3K signaling, and the notion that PI3K activation has also been found to be a major contributor to resistance against a diverse range of anti-cancer agents [reviewed in Ref. (14)], positions intervention with PI3K signaling as an important target for combination strategies. The challenge hereby is to identify the most rational combinations within an acceptable tolerability window. Indeed, despite the significant synergy between inhibitors of the PI3K and MAPK pathways in preclinical cancer models (24), moving this particular combination therapy forward in the clinic has been hampered by toxicity. Moreover, the exact molecular mechanism by which the PI3K pathway affects other anti-cancer therapy responses most often remains unknown.
A few examples of ongoing combination strategies in solid tumors are discussed below. For more extensive overviews, the reader is referred to Refs. (1, 4).
PI3K and endocrine therapy
In breast and prostate cancer, there is a clear, although mechanistically poorly understood, crosstalk between hormonal signaling and PI3K activity, with PI3K inhibition leading to increased estrogen and androgen pathway activity.
Breast cancer
In breast cancer, combination trials of PI3K inhibitors with endocrine therapy are in progress [reviewed in Ref. (1)]. These are based on evidence from preclinical models showing (1) that activation of PI3K/Akt can confer resistance to anti-estrogens, and (2) that there is reciprocal crosstalk between signaling by PI3K and the estrogen receptor (ER), whereby inhibition of each pathway enhances the function of the other (1, 25–27). One way in which PI3K inhibition could lead to sensitization to hormonal therapy is through the induction of estrogen-dependent transcriptional activity and increased ER expression, potentially mediated by increased FOXO3A-mediated transcription (25).
A recent update (28) of the BELLE-2 phase III trial (ClinicalTrials.gov NCT01610284) in ER-positive, HER2-negative metastatic breast cancer that had become resistant to aromatase inhibitors, showed that the combination of Buparlisib (BKM120; a pan-class I PI3K inhibitor) with fulvestrant (an ER antagonist) provides a significant improvement in progression-free survival (PFS) (6.9 versus 5 months). Interestingly, when considering only those patients with PIK3CA mutant cancers, the difference in PFS was more pronounced, namely 7 months for the combination versus 3.2 months for fulvestrant alone. In this trial, adverse events of Buparlisib led to frequent drug discontinuation, resulting in reduced treatment duration. The dosing of the pan-class I PI3K inhibitor pictilisib (Genentech, GDC0941) was also a limiting factor in another trial that combined PI3K inhibition with fulvestrant in patients with ER-positive, HER2-negative metastatic breast cancer, in which no significantly improved PFS was observed (29). The expectation (or hope) therefore is that the better-tolerated PI3Kα inhibitors might be more efficacious in this clinical setting.
It remains to be seen whether the observed extension in PFS in the BELLE-2 trial will translate in an overall survival benefit. Indeed, a 4.6 month prolongation in PFS by the combination of everolimus (a rapamycin analog that inhibits mTORC1) with emestane (an aromatase inhibitor that blocks estrogen production) in the same breast cancer setting did not result in an overall survival benefit (30).
Of interest in this context are recent studies in mouse models which indicate that the expression of mutant PIK3CA in early breast cancer induces cancer cell multipotency, possibly contributing to tumor heterogeneity (31, 32). This is of relevance, given that PIK3CA mutation can be an early lesion in breast cancer development (33). It is not clear whether PI3K inhibitor treatment of established, late-stage breast cancer will be able to reverse such early biological effects induced by PIK3CA mutation.
Prostate Cancer
PI3K and endocrine signaling also interact in prostate cancer, with PI3K pathway inhibition giving rise to an activation of the androgen receptor (AR) and, conversely, AR blockade leading to PI3K pathway activation (34). Combination trials of PI3K inhibitors with endocrine therapy in prostate cancer are in progress, also based on the notion that PTEN inactivation is a very common event in prostate cancer. PI3Kβ has been reported to be the main isoform mediating enhanced PI3K activity as a result of PTEN inactivation in some cancer contexts [reviewed in Ref. (17)]. Although this correlation is not absolute, it is of interest to note that PI3Kβ positively regulates AR transactivation in prostate cancer cell lines (35). In line with the dominant contribution of PI3Kβ and PI3Kδ over PI3Kα to enhanced PI3K activity upon PTEN loss in prostate cancer cell lines (36), a PI3Kβ/δ inhibitor was found to be very effective in a preclinical study of prostate cancer, particularly in combination with hormonal therapy (37). Another study (21) showed that combined inhibition of PI3Kα/β and AR signaling is effective at inhibiting PTEN mutant prostate cancer cells.
PI3K and PARP inhibitors
Another example of combination therapy is that of PI3K pathway inhibitors with PARP inhibitors (which block some forms of cellular DNA repair), which is currently being tested in trials in ovarian and breast cancer (ClinicalTrials.gov NCT01623349), using Buparlisib or Alpelisib (BYL719), a PI3Kα-selective inhibitor.
These trials are based on preclinical findings of a synergistic impact of Buparlisib and the PARP inhibitor olaparib (38, 39). The underlying mechanism by which Buparlisib sensitizes to PARP inhibitors is not entirely clear but has been linked to the capacity of Buparlisib on its own to increase markers of DNA damage and to reduce expression of the BRCA1/2 DNA repair enzymes (38, 39). It is important to mention that Buparlisib has been shown, in a PI3K-independent manner, and at higher doses, to inhibit microtubule dynamics (40), which can dampen DNA repair but also have anti-tumor effects in its own right. It therefore remains to be seen whether PI3K inhibitors without this off-target activity will be able to sensitize to PARP inhibition. PARP inhibitors are also being trialed in combination with inhibitors of Akt or mTOR (ClinicalTrials.gov NCT02338622 and NCT02576444).
Indirect Effects of PI3k Pathway Inhibition in Cancer
Despite the tremendous promise and rationale for oncogene-targeted therapies, most kinase inhibitors offer at best an extension of median survival, measured in months, with no improvement in overall survival in solid cancer. Complete responses are the exception rather than the rule. The last couple of years have seen a resurgence of cancer immunotherapy. New agents that target the CTLA4 or PD1 inhibitory receptors on T-cells, or PD1 ligands on tumor cells and the cancer stroma, are showing the potential for complete responses, with improved overall survival rates resulting in lifespan extension measured in years rather than months (41–43). However, only select patient populations benefit from immunotherapy so far.
Recent findings indicate that PI3K inhibitors could also be used to target the immune system, as well as the cancer stroma in a broader sense, in particular the vasculature. PI3K inhibitors could be used to target the stroma and enhance anti-tumor responses by four main mechanisms (Figure 1):
by improving circulation to the tumor by normalizing blood vessels to aid chemo- and immunotherapy, or by inhibiting angiogenesis-promoting tumor-associated myeloid cells to enhance VEGF-based anti-angiogenic therapy;
by interfering with desmoplasia, the generation of fibrous connective tissue surrounding tumors;
by interfering with the survival and homing signals provided by the stroma in B-cell malignancies;
by interfering with immunosuppressive mechanisms to enhance tumor killing by the immune system.
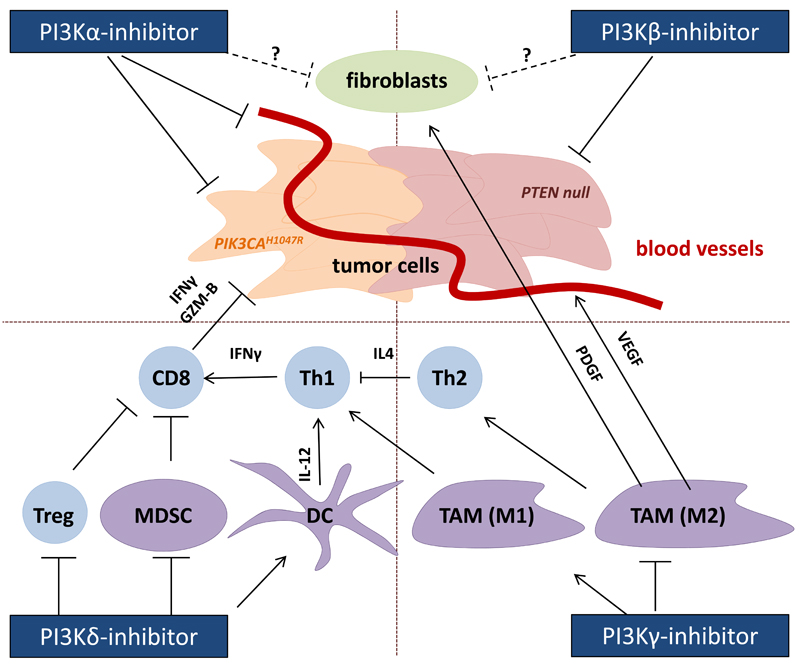
Figure 1. Direct and indirect anti-cancer effects of interference with PI3K isoform activity.
Different PI3K isoforms have the potential to interfere with cancer growth and survival, either by acting on the transformed cells directly, by interfering with the supportive stroma and nutrient supply, or by stimulating more potent immune responses against the transformed cells. PI3Kα inhibitors have shown promising results in cancers driven by activating PIK3CA mutations, such as p110αH1047R. PI3Kα inhibition may also affect nutrient supply by inhibiting angiogenesis, or enhance drug delivery by normalizing vessels, depending on the degree of inhibition and the particular tumor type. Evidence suggests that PTEN-deficient tumors are often (but not always) more sensitive to PI3Kβ inhibitors. Both PI3Kα and PI3Kβ inhibitors may be useful in targeting tumor-associated fibroblasts, although this is speculative at this stage. PI3Kγ inhibition has been shown to reduce the infiltration of tumor-suppressive macrophages, diverting them from an immune suppressive (wound healing) M2 to an immunostimulatory M1 phenotype and reducing the production of fibroblast-stimulating growth factors. PI3Kδ inhibitors can stimulate a more potent CD8+ T-cell-mediated cytotoxic anti-tumor response by activating DCs to produce more IL-12 and by inhibiting Treg and MDSCs, which antagonize cell-mediated immunity in tumors. Arrows indicate a stimulatory impact.
1. Effects of PI3K inhibitors on the tumor vasculature (Figure 2)
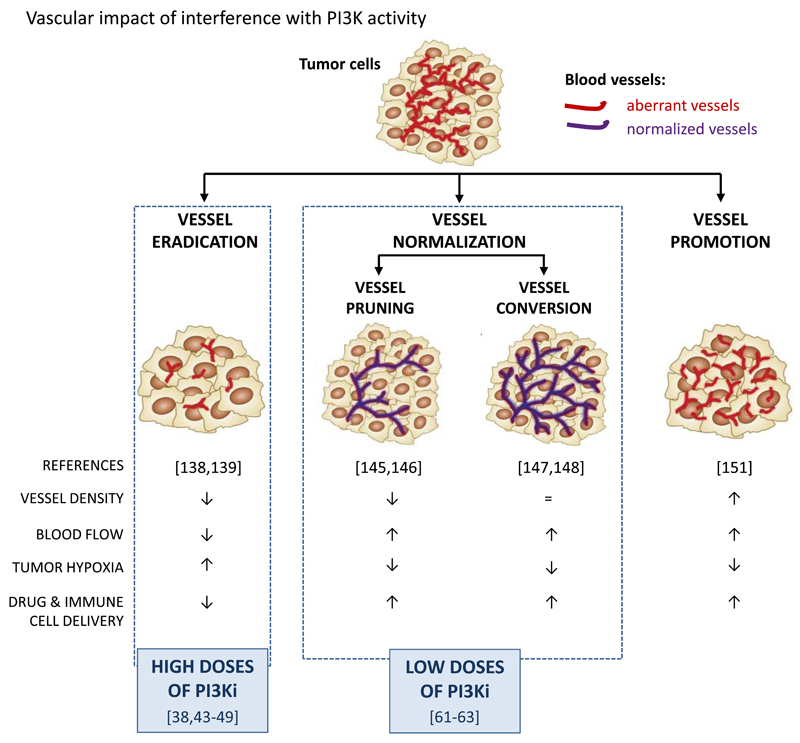
Figure 2. Vascular targeting strategies in cancer and possible role of PI3K isoforms therein.
PI3K inhibitors, at high doses, have been documented to induce a mild vessel eradication response, whereas at low doses, can lead to vessel normalization, associated with either reduced or no changes in vessel density. Key concepts to therapeutically exploit the dependence of tumors on the vasculature: (1) vessel eradication, aimed at destroying the tumor vasculature and ‘starving the tumor to death’ (146, 147). Problems with this strategy are both intrinsic and acquired resistance to vascular trimming (148, 149), reduced chemotherapy delivery to the tumor and induction of hypoxia, which can accelerate tumor progression (51, 150); (2) vessel normalization, aimed at improving vascular perfusion and oxygenation, allowing enhanced drug delivery and immunotherapy (151, 152). This normalization effect can be the consequence of vessel pruning, with only normal vessels left behind after therapy (153, 154) or of vessel conversion, a biological change in endothelial cells whereby the tubular structures are converted into more physiological, normal vessels (155, 156). The applicability of the normalization approach in the clinic has been limited by the transient window during which vessels are susceptible to normalization, difficulties to predict when and which agent will induce vessel normalization, and a highly context-dependent response, with every tumor relying to different extents on angiogenic cues to stimulate vessel growth (157, 158); (3) the vessel promotion strategy is based on stimulating vessel growth, response, with every tumor relying to different extents on angiogenic cues to stimulate vessel together with promotion of vasodilatation (159) and is aimed at enhancing delivery of chemotherapy and other anti-cancer agents to the tumor (160).
PI3K inhibition modulates the tumor vasculature, either directly (by inhibiting endothelial cells) or indirectly (by inhibiting angiogenesis-promoting tumor-associated myeloid cells and VEGF production by tumor cells). The direct effects appear PI3K inhibitor dose-dependent and range from mild vascular pruning to vessel normalization, which could be exploited to enhance drug delivery and possibly immune cell access to the tumor. At present, there are no clinical data on the impact of PI3K inhibitors on tumor angiogenesis, most likely because this parameter has thus far not been evaluated in any detail in trials.
Direct vascular impact of PI3K inhibitors
Anti-angiogenic effects of PI3K pathway inhibitors have been documented in multiple preclinical models of cancer (39, 44–50). Most of these studies have been performed with pan-class I PI3K inhibitors used at high doses, to mirror the maximum tolerated doses used in cancer. These studies have revealed that sustained PI3K inhibition results in (i) reduced total intra-tumor vessel area, which is most often accompanied by reduced vessel function (39, 44–50) and anti-tumor activity; (ii) a milder anti-angiogenic impact than VEGF-targeted therapies (51, 52), indicating that PI3K inhibitors are unlikely to be useful in pruning/destroying vessels, as discussed further below. The vascular responses to PI3K inhibitors can be attributed to effects on both the endothelial cells and the tumor cells, and the crosstalk between them, for example by dampening the production of VEGF by the tumor cells (47–49, 53, 54), with differences observed depending on the tumor model used (49, 52). Other than interfering with tumor angiogenesis, PI3K inhibitors may also dampen lymphangiogenesis in the lymph nodes (55), a possible way of limiting tumor cell dissemination via this tissue site.
PI3Kα in angiogenesis
Of the class I PI3K isoforms, PI3Kα has been shown to be the most important isoform in endothelial cells, both in blood vessels (49, 56, 57) and lymphatic vessels (58, 59). Gain-of-function mutations in PIK3CA have recently been reported to result in venous and lymphatic malformations, a specific type of congenital vascular anomaly (60–64), further highlighting the importance of PI3Kα activity in endothelial cells. PI3Kα-selective inhibitors are currently being tested in various cancers, with the main aim of reducing cancer cell-intrinsic PI3K activity. As part of the clinical evaluation of these compounds, it will be critical to also assess their impact on the tumor vasculature.
Roles of non-PI3Kα isoforms in angiogenesis
The generation of blood vessels, including in tumors, is a cooperative process between endothelial cells and numerous other cell types, including the tumor cells, mural cells, platelets and immune cells, which can secrete angiogenic factors (such as VEGF) and also contribute to vascular tube formation (57, 65). PI3K signaling plays a role in all of these cells, with specific isoforms displaying predominant roles in certain cell types (57), including PI3Kβ in platelets and PI3Kδ/PI3Kγ in leukocytes. Indeed, PI3Kγ inhibition has been shown to have anti-angiogenic effects in cancer, due to its ability to inhibit tumor-associated myeloid cells (66, 67). It is therefore likely that multiple PI3K isoforms contribute to tumor angiogenesis by different mechanisms.
Vascular impact of PI3K inhibitors can improve cancer treatment in multiple ways
Additional studies are required to assess the way in which PI3K inhibition compares to other vascular targeting therapies, but the data available to date indicate that high doses of PI3K inhibitors have a weaker impact on vessel eradication than VEGF-targeted therapies (51). Recent preclinical studies have highlighted alternative ways, beyond vascular pruning, that could be used to exploit PI3K inhibitors in the modulation of the tumor vasculature.
Notably, low doses of PI3K inhibitors were shown to improve vascular function (68–70), even upon short-term (3-day) administration (69), correlating with an enhanced capacity to deliver chemotherapy to the tumor (69). These findings point to a so-called ‘vessel normalization’ effect that is also observed upon administration of low (71) or single (69, 72) doses of anti-VEGF antibodies. These data suggest that the direct vascular effects of PI3K inhibitors on tumor angiogenesis could be exploited to improve vessel function, which could enhance drug delivery and potentially also the influx of anti-tumor immune cells to improve immunotherapy.
A prime example of an indirect anti-vascular effect of PI3K inhibition in cancer is the recent finding that resistance to VEGF-targeted therapy in tumors is partially mediated by pro-angiogenic tumor-associated myeloid cells, in a PI3Kγ-dependent manner (66). Accordingly, a PI3Kδ/PI3Kγ inhibitor was able to enhance tumor responsiveness to VEGF-based anti-angiogenic therapy (66). The contribution of PI3Kδ to this biological effect is unclear at present. Indeed, while PI3Kγ plays a clear role in tumor-associated myeloid cells (66), including macrophages (73), the exact role of PI3Kδ in these cells in cancer remains to be determined.
2. Effects of PI3K inhibitors on stromal fibroblasts
Desmoplasia, the growth of fibrous stromal tissue around tumors, is common in some cancers, such as pancreatic ductal adenocarcinoma, and has been thought to be a mechanical barrier for access of drugs and immune cells to the tumor, but could also dampen metastasis (74).
In a mouse model of pancreatic cancer, PI3Kγ inhibition was shown to dampen the secretion of PDGF-BB by tumor-associated macrophages (Figure 1), thereby reducing collagen secretion by tumor-associated fibroblasts, which most likely contributes to the marked reduction in fibrous tissue surrounding the tumors (73).
Functional inactivation of PTEN (which leads to PI3K activation) in stromal fibroblasts in breast cancer has been reported to contribute to cancer development and progression (75). A direct action of PI3K inhibitors (especially against PI3Kα/β) on such cancer-associated fibroblasts would be expected to inhibit their tumor-promoting activities (Figure 1), although this has not been formally tested, and will not be discussed further. However, it is likely that PI3K activity is an important regulator of this aspect of tumor-stroma interaction.
3. PI3Kδ inhibition disrupts stroma-cancer cell engagement in B-cell malignancies, rendering the tumor cells more susceptible to cell death (Figure 3)
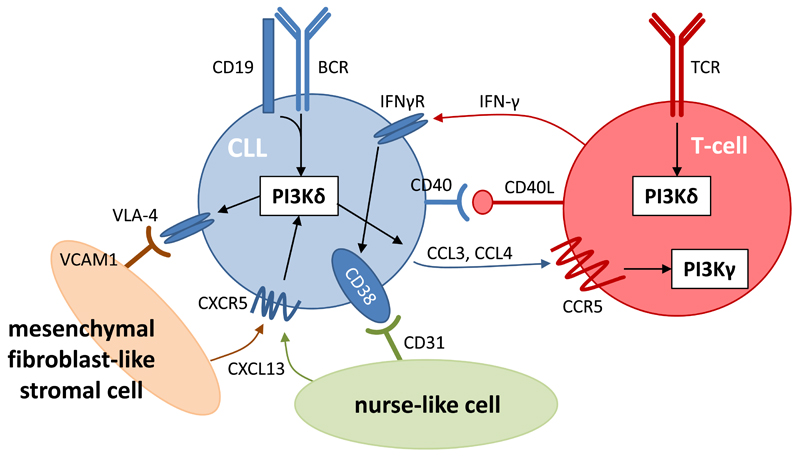
Figure 3. Roles of PI3Kδ in CLL cells and their interaction with their surrounding stroma.
CLL cells depend on signaling via the BCR to increase proliferation, adhesion to mesenchymal stromal cells (via VLA-4/V-CAM1), and secretion of the chemokines CCL3 and CCL4. The ligands (antigens) for the BCR may be expressed by the CLL cells themselves or by other cells in the stroma. CCL3 and CCL4 facilitate the recruitment of T-cells which provide CD40 ligand (CD40L), IFN-γ and other agonists that stimulate the CLL cells, as evidenced by increased expression of CD38 on the CLL cells. Other key cell types in the lymph node niche include: (1) mesenchymal stromal cells that secrete chemokines, such as CXCL12 and CXCL13, which bind to CXCR4 and CXCR5 on CLL cells and facilitate their recruitment to and retention in the lymph nodes; and (2) myeloid-derived nurse-like cells that also secrete CXCL13 and promote the survival of CLL cells. PI3Kδ inhibition interferes with many aspects of this intercellular communication, ultimately resulting in the ‘purging’ of CLL cells from their protective lymph node (or bone marrow) environment into circulation where they are more susceptible to undergoing apoptosis.
Early studies showed an important role for PI3Kδ in non-transformed B-cells, with an almost exclusive dependence of the B-cell antigen receptor signaling on PI3Kδ over other PI3K isoforms (76–78). In contrast to other B-cell malignancies, which show enhanced contribution of PI3Kα (79) and/or activating mutations in signaling pathways parallel to PI3K (such as in the NFκB pathway (80)), the biology of CLL and iNHL remains remarkably dependent on PI3Kδ activity, creating a unique vulnerability to PI3Kδ inhibition in these diseases. Interestingly, the susceptibility of CLL cells to PI3Kδ inhibition is independent of p53 status (81), indicating that activation of prominent oncogenic pathways does not inevitably reduce cellular susceptibility to PI3Kδ inhibitors.
The mechanism of action of the PI3Kδ inhibitor Idelalisib is best understood from clinical studies on CLL. Treatment of CLL patients with Idelalisib leads to ‘purging’ of the transformed B-cells from their protective lymph node and spleen niches into the blood circulation. This results in rapid shrinking of these tissues and correlates with a greatly increased number of lymphocytes in the blood, so-called lymphocytosis (81–83). This phenomenon is a consequence of Idelalisib interfering with the adhesion, survival and homing signals provided by stromal cells to the CLL cells. These critical signals are transduced in the CLL cells via receptors that critically depend on PI3Kδ, such as the tyrosine kinase-linked B-cell antigen receptor (BCR) and CD19 and G protein-coupled receptors such as the chemokine receptor CXCR5 (84).
Once in the circulation, CLL cells no longer receive survival signals from their stroma and as a result are thought to become more susceptible to spontaneous cell death. In addition, upon PI3Kδ inhibition, these cells no longer respond to homing and adhesion signals upon re-entry into their normal tumor niche. Cell death of circulating CLL cells can be accelerated by Rituximab (an antibody against the CD20 B-cell surface marker) and/or chemotherapy by bendamustine. The in vitro cytotoxic/cytostatic effect of PI3Kδ inhibitors on monocultures of CLL cells is modest (84). However, when CLL cells are co-cultured with stromal cells, PI3Kδ inhibition interferes with the stroma-dependent survival signals (5, 84, 85).
Idelalisib treatment also reduces the responsiveness of CLL cells to several chemokine agonists, including CXCL13, which is primarily released by fibroblast-like mesenchymal stromal cells and important for the recruitment and retention of CLL cells to their niche. In addition, Idelalisib prevents recruitment of other cells to the CLL niche, such as monocytes (which can give rise to nurse-like cells) and lymphocytes, through reducing the secretion of the chemokines CCL4 and CCL5 by CLL cells. Of the infiltrated lymphocytes in the CLL niche, Th1 T-cells might support the survival and differentiation of CLL cells by providing CD40 ligand (CD40L), IFN-γ and other agonists (86, 87), and it is possible that Idelalisib also inhibits the secretion of some of these factors by T-cells. Other recruited lymphocytes, especially CD8+ T-cells, have the potential to kill CLL cells. However, these CLL-associated CD8+T-cells are usually dysfunctional, probably as a consequence of the immune-suppressive environment (88). The function of the monocyte-derived nurse-like cells and mesenchymal stromal cells in the stroma is possibly also affected by Idelalisib (84–87, 89).
To summarise, in CLL, Idelalisib and other PI3Kδ inhibitors act to a large extent by disrupting the interactions between the malignant B-cells, their protective stromal cells and immune cells, without directly killing the cancer cells. This is facilitated by the unique dependency of CLL cells on PI3Kδ over other PI3K isoforms, but also potentially by the effect of PI3Kδ inhibition on other leukocytes in the stroma. As will be explained below, it is also possible that an adaptive immune response is being generated against the CLL cells, although this remains to be documented in CLL patients.
4. Immuno-modulatory effects of PI3K inhibition in cancer (Figure 1)
While PI3Kγ and PI3Kδ are present at low levels in most cell types, their expression is high in all leukocyte subtypes. Over the last few years, accumulating evidence has shown that dampening the activities of these PI3K isoforms has broad anti-cancer activity, which is not restricted to hematological malignancies. Indeed, while PI3Kδ inhibitors can dampen many immune cell functions, such as antibody production, their effect on regulatory immune cell subsets, such as Treg, results, unexpectedly, in a heightened immune response against cancer and certain infectious agents (90, 91). Similarly, whilst PI3Kγ inhibition reduces the immediate response to bacterial infections, it also decreases the immune suppressive effects of myeloid cells in cancer (67, 73).
Below, we provide a detailed description of the immunomodulatory effects of PI3Kγ and PI3Kδ inhibition in cancer, focusing on the stimulation of T-cell responses, either through acting on T-cell subpopulations (by PI3Kδ inhibition) or modulation of the myeloid cell compartment (by PI3Kδ and/or PI3Kγ inhibition). Before doing so, we first summarize the adverse effects observed in patients treated with Idelalisib, which we speculate could be related, at least in part, to a heightened immune response.
Adverse effects associated with PI3Kδ inhibition the clinic: the result of an overactive immune response against commensals and opportunistic infectious agents?
PI3Kδ kinase-dead mice are prone to the development of colitis (76, 92–94). Similarly, patients treated with Idelalisib often develop colitis (severe diarrhea) and, less frequently, pneumonitis and/or transaminitis (95–98). A few fatalities caused by infections with Pneumocystis jirovecii or cytomegalovirus, suggesting increased risk for opportunistic infections, have led to the discontinuation of several phase 3 trials of idelalisib (98).
Increasing evidence suggests that some of the more common adverse events associated with Idelalisib treatment are immune-mediated, as they are more frequently observed in first-line patients who are more immunocompetent, are associated with T-cell infiltrates (in the case of colitis and pneumonitis) and can often be alleviated by administration of steroids (83, 99, 100). Moreover, similar symptoms have been described in an individual with bi-allelic loss of PIK3CD (101), further suggesting that these are likely to be target-related adverse effects. Some Idelalisib-treated patients develop skin rash, which could be a result of inappropriate reactivity to commensal pathogens on the skin (99, 100). In fact, the spectrum of adverse effects associated with the use of PI3Kδ inhibitors is similar to that observed with the immune checkpoint inhibitors anti-PD1/PDL1 and anti-CTLA4 (102).
In mice, colitis is often correlated with defects in Treg function (103). In an experimental model of this disease, naïve CD4+ T-cells are injected into a lymphopenic host. A subset of these T-cells will be activated in a manner dependent on the gut microflora, including Helicobacter species. The disease can be effectively prevented by co-injection of functional Treg (103). Of relevance in this context, Treg with inactive PI3Kδ failed to suppress such experimentally-induced colitis in mice (92). In addition, colon-associated macrophages in PI3Kδ kinase-dead mice are more inflammatory and less bactericidal than in wild-type mice (93, 94). Colitis observed in patients on Idelalisib is characterized by infiltrating CD8+ T-cells, reminiscent of graft-versus-host disease, and is more prevalent in patients that have not been exposed to immune-suppressive therapies (83, 95–97, 99), indicative of a possible host T-cell immune response against the gut flora. There is also evidence that Treg are affected in patients on Idelalisib that develop colitis (100). It should be noted however, that different PI3Kδ inhibitor (TG-1202) shows similar efficacy as idelalisib in CLL and iNHL, but with fewer adverse effects (104). Although diarrhea was a common adverse effect with TG1202, it was less severe than with Idelalisib and rarely led to drug discontinuation. Transaminitis was also less common. It remains to be determined whether the less severe adverse effects of TG-1202 relative to other PI3Kδ inhibitors are due to a different formulation or dosing regimen of this agent, or whether these data present a challenge to the concept that colitis is a PI3Kδ target-related adverse effect in humans as it is in mice.
Emerging evidence suggests a possible connection of pneumonitis in Idelalisib-treated patients with infection with cytomegalovirus or the fungus Pneumocystis jirovecii (formerly known as Pneumonitis carinii). Cytomegalovirus pneumonitis may be related to a reduced function of Natural Killer cells upon PI3Kδ inactivation (105). So-called Pneumocystis jirovecii pneumonitis (PJP) is most often associated with lymphopenia caused by HIV/AIDS chemotherapy or other forms of immunodeficiencies. Injection of lymphopenic mice with naïve CD4+ T-cells can cause pneumonitis in a manner that correlates with levels of Pneumocystis jirovecii in the lungs (106). As with colitis, co-injection of Treg prevents this experimentally-induced PJP in mice (106); however, the specific role of PI3Kδ in Treg has not yet been assessed in this model. The immune etiology of PJP induced by Idelalisib as monotherapy has not been reported, but in a combination trial of Idelalisib with a Syk inhibitor, it was found to be accompanied by features of increased Th1-type T-cell responses (107). It is of interest to note that PJP has also been reported for mTOR inhibitors (rapamycin analogs), the mechanism of which is not understood to date but which may also involve an exacerbated Th1 T-cell–mediated autoimmune response (Ref. (107) and references therein). It will be important to assess whether PJP in Idelalisib-treated patients is due to an immune overreaction of the host to Pneumocystis jirovecii, rather than to an overall lack of immune response to this pathogen. The mechanism of transaminitis in patients on Idelalisib is also likely to be immune-related and is correlated with a reduction in Treg and infiltration of CD8+ T-cells (100).
Taken together, evidence suggests that adverse effects observed upon Idelalisib treatment may be target-related and caused in part by inhibition of Treg followed by unrestrained T-cell-mediated immune responses against otherwise innocuous non-infectious organisms. These findings highlight the concept that PI3Kδ inhibitors are not just immunosuppressive but can also act to heighten cellular immune responses against pathogens and, importantly, as will be described below, against tumors. Although PI3Kδ inhibition may also increase susceptibility to opportunistic infections, PI3Kδ kinase-dead mice display enhanced resistance to infection with certain pathogens, such as Leishmania, which is attributed to impaired expansion and effector functions of Treg, allowing weakened Th1 responses to contain parasites and prevent pathology (108). PI3Kδ kinase-dead mice are also more resistant to infection with the intracellular bacteria Listeria monocytogenes (109). Therefore, while initially assumed to primarily be a target for therapies aimed at reducing autoimmunity and inflammation (110), PI3Kδ may in fact also be targeted to increase certain innate and adaptive cell-mediated immune responses.
PI3Kδ inhibition can enhance T-cell-mediated anti-tumor responses
Diverse mechanisms are at play in tumors to promote the recruitment and expansion of Treg that dampen effector T-cell responses (111). PI3Kδ kinase-dead mice are resistant to diverse transplanted tumors and this resistance could not only be recapitulated but even improved by deleting PI3Kδ selectively from Treg (91). Moreover, PI3Kδ kinase-dead mice show enhanced resistance to secondary tumor administration, suggestive of the generation of tumor-specific immunological memory. Administration of a PI3Kδ-selective inhibitor in a syngeneic mouse model also led to reduced growth and metastasis of a breast tumor cell line that did not express functional levels of PI3Kδ (Ref. (91)).
These data indicate that PI3Kδ inhibitors, such as Idelalisib, have the potential to be used as cancer immunotherapeutic agents. However, we suspect that, in most cases, PI3Kδ inhibitors will not be sufficient as a monotherapy to eliminate cancer. There is increasing evidence for synergy between different immunotherapeutic agents (112) or for combining immunotherapy with chemotherapy (113), irradiation (114) or targeted therapies (115), including PI3K inhibitors (116). When used together with other immunotherapies or conventional therapies, PI3Kδ inhibitors may help in unleashing anti-tumor immune responses.
Several clinical trials are designed to test PI3Kδ inhibitors, either alone or in combination with other therapies, in non-hematological malignancies (Table 1). It will also be of interest to determine whether enhanced T-cell-mediated anti-tumor responses contribute to the impressive clinical responses to PI3Kδ inhibition in CLL and iNHL.
Table 1.
Ongoing clinical trials of testing inhibitors against the leukocyte-enriched PI3Kδ and PI3Kγ isoforms in solid tumors.
| Sponsor | Phase | Trial Identifier | Inhibitor | Cancer type(s) | Combination |
|---|---|---|---|---|---|
| PI3Kδ | |||||
| CRUK and AMGEN | II | NCT02540928 | AMG-319 | Head and Neck squamous cell carcinoma (non-viral) | None (no prior treatment) |
| Gilead Sciences | I | NCT02468557 | Idelalisib | Pancreatic Ductal Adenocarcinoma | Chemotherapy |
| TG Therapeutics | I | NCT02574663 | TGR-1202 | Various solid cancers | Chemotherapy |
| Incyte | I | NCT02646748 | INCB050465 | Various solid cancers | Pembrolizumab (anti-PD1) |
| Incyte | Ib | NCT02559492 | INCB050465 | Various solid cancers | INCB039110 (JAK1 inhibitor) |
| PI3Kγ | |||||
| Infinity | I/Ib | NCT02637531 | IPI-549 | Non-small cell lung cancer, melanoma | Pembrolizumab (anti-PD1) |
Below we describe the complex role of PI3K in the regulation of specific T-cell populations in cancer, mainly focusing on PI3Kδ for which most of the data in this context are available. An emerging theme is that, while specific cells and their responses can be inhibited in isolation or at early time points, the surprising and unanticipated end result is a net anti-tumor immune response in several instances.
PI3Kδ inhibition dampens Treg function (Figure 4)
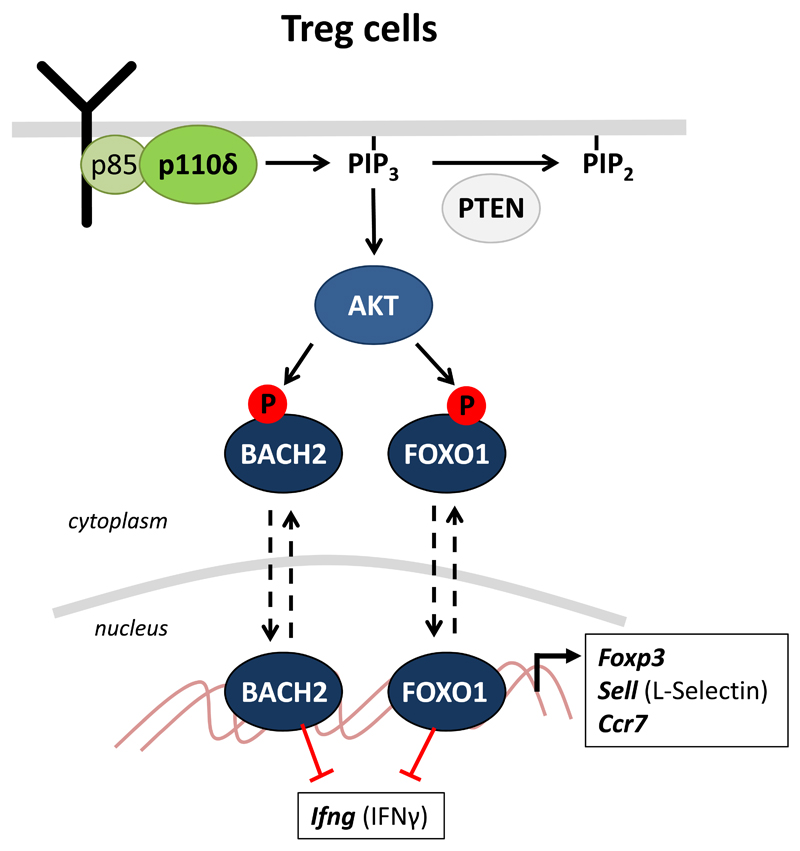
Figure 4. PI3K pathway components in Treg involved in the regulation of anti-cancer immunity.
The activation of Akt by PI3Kδ leads to the phosphorylation of the BACH2 and FOXO1 transcription factors and their retention in the cytoplasm. BACH2 and FOXO1 regulate expression of key genes in Treg differentiation and function, including genes encoding FOXP3, L-selectin, CCR7 and IFNγ. Failure to activate this pathway upon PI3Kδ inhibition may prevent Treg from undergoing a differentiation program and migrating effectively to tumors in order to suppress anti-tumor responses. By contrast, constitutive PI3Kδ activation upon loss of PTEN may destabilize the Treg lineage, loss of FOXP3 and CD25 expression, resulting in loss of suppressive function of the Treg and production of pro-inflammatory cytokines instead of the normal immune-suppressive factors produced by Treg.
The precise mechanism through which inhibition of PI3Kδ blocks Treg-mediated suppression of anti-tumor immune responses remains to be fully elucidated. PI3Kδ-deficient Treg make less IL-10 and express lower levels of CD38, a receptor involved in the metabolism of extracellular metabolites (117, 118).
A recent study showed that mice with Treg-specific expression of an Akt-insensitive mutant of the FOXO1 transcription factor, displayed resistance to tumors (119). The authors hypothesize that in wild-type mice, inactivation of FOXO1 by PI3K/Akt signaling is required for Treg to mature and suppress anti-cancer immune responses. These studies suggest that the loss of PI3K signaling in Treg leads to increased FOXO1 activity and a failure of these cells to suppress anti-tumor immune responses.
The transcription factor BACH2, which antagonizes AP1-dependent transcription in T-cells(120), has recently also been shown to be regulated by PI3K/Akt signaling, in an analogous manner to that of FOXO1 (120), and selective loss of BACH2 in Treg is sufficient to confer tumor resistance (121). This is consistent with a model in which elevated PI3K signaling leads to the loss of BACH2 function and could destabilize Treg. Indeed, increasing PI3K signaling by inhibiting PTEN in Treg has also been shown to stimulate potent anti-tumor immune responses by destabilizing the Treg lineage, leading to impaired Treg function and reduced immune suppression (122). Reduced tumor growth could also be achieved using a systemically-administered PTEN-inhibitor that appeared to act primarily on PTEN within Treg (122). A potential further advantage of administering a PTEN inhibitor is that tumor-reactive CD4+ T-effector cells are more potent in the absence of PTEN (123). However, it remains to be determined whether a PTEN inhibitor could be administered safely to humans, given that PTEN is one of the most commonly-inactivated tumor suppressor genes. The reduced stability of PTEN-deficient Treg could be due to reduced FOXO1 transcription with consequently reduced transcription of FOXP3, a transcription factor that is essential for Treg development (85, 86).
Taken together, these data establish a potential signaling pathway from PI3Kδ to Akt towards the FOXO1 and BACH2 transcription factors (Figure 4) in Treg, with both the loss or overactivation of FOXO1 or BACH2 transcriptional activities resulting in a dampening of the capacitiy of Treg to suppress anti-tumor immune responses. These results highlight how PI3K signaling needs to be balanced such that too high, too low or the inability to dynamically regulate PI3K signaling (the latter by constitutive genetic alterations in the PI3K pathway) can lead to similar effects on immune cell functions (90).
PI3Kδ inhibition can improve CD8+ T-cell activity in cancer
As in Treg, the exact role of the PI3Kδ pathway in CD8+ T-cells is enigmatic (109, 124–129). In this T-cell population, PI3K also acts via Akt to suppress the transcriptional activity of FOXO1 and BACH2 to maximize the expression of effector cytokines (such as IFN-γ) and granzymes (such as GzmB) (91, 120, 127, 130, 131). However, it appears that once CD8+ T-cells are fully differentiated, they become less dependent on PI3Kδ inhibition, but probably remain susceptible to suppression by Treg (91, 109, 126, 132–134).
Inhibition of downstream signaling components in the PI3K pathway has also been shown to lead to enhanced T-cell-mediated anti-tumor activity in vivo. CD8+ T-cells cultured in the presence of an Akt inhibitor showed enhanced efficacy in adaptive cancer immunotherapy (132–134). Similarly, the mTOR inhibitor rapamycin, which inhibits CD8+ T-cell effector functions, can enhance the generation of memory CD8+ T-cells (135). In PI3Kδ kinase-dead mice, the generation of effector T-cells in response to infection is impaired, but the generation of long-lived memory T-cells remains intact (109, 125), which may help to explain why these mice are able to reject tumors in a CD8+ T-cell-dependent manner (91). At least in the case of adoptive T-cell therapy, it has been shown that T-cells with a stem-like memory phenotype provide a more durable response than effector T-cells (136). Further, the differential effect of PI3Kδ inhibition on naïve T-cells versus memory T-cells helps alleviate the effect of graft versus host disease while maintaining effective graft versus leukemia in a bone marrow transplant setting (126).
Thus, although PI3Kδ inhibitors will initially reduce anti-tumor effector functions, they may also help to sustain the CD8+ T-cells, which may help persistent immune-mediated attacks on cancer, despite the blunted production of immediate effectors, such as IFN-γ and GzmB, by these cells.
Inhibition of PI3Kδ and/or PI3Kγ in myeloid cells can lead to enhanced T-cell anti-tumor responses
Tumor mass is often comprised of up to 50% of myeloid cells that can suppress immune responses and promote angiogenesis. Indeed, tumors have been described as non-healing wounds (137). In addition to directly affecting T-cells, there is significant potential for PI3Kγ and PI3Kδ inhibitors to stimulate anti-cancer immune responses through the modulation of myeloid cells. This can be either by inhibiting suppressive myeloid cells, by dampening immune-suppressive tumor-infiltrating macrophages (TAMs) or by stimulating macrophages and dendritic cells (DCs) to make cytokines that contribute to effective T-cell responses. Most studies assessing the impact of PI3K inhibition on cancer-associated myeloid cells have focused on PI3Kγ (66, 67, 73, 138). It is clear, however, that PI3Kδ can also modulate myeloid cells in cancer, which most likely contributes to the anti-tumor effects seen with PI3Kδ inhibitors (91, 139).
PI3Kγ-deficient mice are resistant to colon cancer that develops subsequent to oral administration of dextran sulfate sodium to induce chronic colitis (138). Moreover, inhibition of PI3Kγ reduced tumor progression in a range of mouse models and dampened the recruitment of myeloid cells to tumors, at least in part by interfering with Rap1-dependent integrin activation (67, 140). The majority of these myeloid cells were CD11b+, GR1low and considered to be TAMs (67). TAMs are a major source of IL-1β, IL-6 and VEGFα, all of which are tumor-promoting. In a mouse model of pancreatic ductal adenocarcinoma, PI3Kγ activity was shown to be important for the induction of an immunosuppressive transcriptional programme in TAMs that inhibits T-cell immune responses. By reprogramming TAMs from an immunosuppressive to an immunostimulatory phenotype, PI3Kγ inhibition was able to dampen tumor cell metastasis and desmoplasia, eventually allowing the development of CD8+ T-cell-mediated tumor suppression, without altering the presence of Treg (73).
In PI3Kδ kinase-dead mice, the expansion of MDSCs that express CD11b but, in contrast to TAMs, express high levels of GR1, and are distinguished from neutrophils by their lower expression of Ly6C, was reduced, which correlated with reduced suppressive activity and reduced tumor growth (91).
TAMs, and especially DCs, can be potent inducers of tumor immunity when exposed to IFN-γ and other pro-inflammatory cytokines, thus shifting the balance from a wound healing response to anti-tumor immunity (141, 142). There is evidence to suggest that this could be achieved by PI3Kδ inhibitors. Indeed, inhibition of PI3Kδ in LPS-stimulated DCs increases the production of pro-inflammatory cytokines (TNFα, IL-12, IL-1β) and reduces the levels of anti-inflammatory cytokines (IL-10, IFN-β) (143, 144). This is in line with observations from preclinical mouse models whereby administration of PI3K inhibitors reduces IL-10 and TGFβ production in favor of IL-12 production, and enhances DC-mediated anti-tumor therapy based on Toll-like receptor agonists (139). For example, combination of the TLR ligand flagellin with various class I PI3K inhibitors, either with or without a DC vaccine, delayed tumor growth and increased survival (139). Tumor growth suppression was associated with increased accumulation of polyfunctional T-cells that secreted multiple effector cytokines, including IFN-γ, IL-17, and IL-2 (139).
Future Directions
Over the last few years, it has become clear that harnessing the cancer cell-intrinsic anti-proliferative action of PI3K inhibition will be challenging as a monotherapy in cancer, largely due to intrinsic and acquired cancer cell resistance to PI3K inhibition. Drug tolerability is also a concern. Drug combination strategies and alternative dosing schemes are currently being explored. Identifying the most promising combination therapies will be challenging, with successes most likely to come from mechanism-based combination therapies that exploit specific vulnerabilities in the cancer cells that sensitize to PI3K therapy. An example is the clear, yet poorly understood, crosstalk between hormonal and PI3K signaling in breast and prostate cancer, with PI3K inhibition leading to increased signaling through the estrogen and androgen receptors, respectively. Identifying such specific vulnerabilities will take time, and illustrates that there is still a lot to be learned about basic mechanisms of PI3K signaling in cancer.
Recent evidence has highlighted the potential for PI3K inhibitors to target not only aberrant PI3K signaling in the tumor cells but also to interfere with the tumor-protective and immunosuppressive stroma. This is illustrated by the fact that the clinical efficacy of the FDA-approved PI3Kδ inhibitor Idelalisib in B-cell malignancies such as CLL derives mainly from its capacity to interfere with the interplay between the cancer cells and their surrounding stroma.
An exciting development is the potential use of PI3K inhibitors in cancer immunotherapy. To achieve therapeutic efficacy in cancer, these inhibitors will most likely have to be combined with other therapeutic modalities (such as irradiation or surgery) or other anti-cancer agents, an approach that is currently being tested in the clinic (Table 1). While these efforts will most likely focus on inhibitors of the leukocyte-enriched PI3Kδ and PI3Kγ, it cannot be excluded that other isoform-selectivities of existing PI3K inhibitors, such as dual PI3Kα/δ inhibitors might also be useful in this regard. A recent study (116) indicated that PI3K activation in cancer cells by PTEN loss renders cancer cells more resistant to immune-mediated killing, further highlighting the potential for immunotherapy on PI3K pathway mutant tumors but also leading to sensitization to PI3Kβ inhibitors. A key question is also whether immune activation is also part of the therapeutic mechanism of Idelalisib in B-cell malignancies.
The finding that agents with immune-suppressive effects on immune cells in vitro can have immune-stimulatory roles in cancer is an exciting recent development, and has also been observed for other signaling molecules, such as MEK inhibitors (145). However, this immunomodulation is not without risks. An important consideration will be the dose and scheduling regimen of immunomodulatory PI3K inhibitors. Indeed, it is not unlikely that the effective immunomodulatory dose and exposure time might differ from continuous drug administration at the maximum-tolerated dose level, which is currently the norm in cancer therapy. It will also be important to assess which patients are suitable for PI3K-based immunotherapies. Indeed, while Idelalisib is reasonably well-tolerated in therapy-resistant CLL, there are emerging data on toxicities, and even fatalities, in the upfront treatment setting of B-cell malignancies. The emerging evidence that toxicities of Idelalisib may have a strong immunological component may underlie the increased frequency of adverse effects in the first-line setting because a more intact cellular immune system in these non-pretreated patients (83, 99). It is anticipated that such challenges of toxicities can be overcome through a better understanding of the underlying mechanism of adverse effects, especially in the combination setting and in immune-compromised patients. For example, we speculate that several of the adverse immune effects associated with PI3Kδ inhibitors are in fact due to aberrant immune-stimulation against otherwise innocuous agents rather than a consequence of overall immune suppression. These on-target effects can be clinically managed and further support the rationale of using PI3Kδ inhibitors in cancer immunotherapy.
Another potentially exploitable indirect anti-cancer effect of PI3K inhibitors is their impact on the tumor vasculature. Data available to date suggest that PI3K inhibition does not result in acute vascular pruning but instead has more subtle vasculo-modulatory roles that could be exploited to normalize vessels in order to enhance delivery of chemo- and immunotherapy to the core of the tumors. Additional pre-clinical studies are required to validate this concept.
To be able to fully exploit the indirect role of PI3K in cancer, it will be critical, in ongoing and future clinical trials, to not only assess the impact on the cancer cells themselves, but also to better monitor the stromal environment, immunological characteristics and vascular status. We believe that PI3K-based therapy could be tailored to better exploit the indirect effects of PI3K in cancer, hopefully leading to more durable therapeutic responses than currently observed.
Acknowledgements
The authors thank Jordi Rodón for critical feedback and Maria Whitehead for help with all aspects of writing this review.
Grant Support
Work in the laboratory of K.O. is supported by the Wellcome Trust (095691/Z/11/Z) and the Biotechnology and Biological Sciences Research Council (BBS/E/B/000C0409, BBS/E/B/000C0407). Work in the laboratory of M.G. is supported by research grants SAF2014-59950-P from MINECO (Spain), 2014-SGR-725 from the Catalan Government, the People Programme (Marie Curie Actions; grant agreement 317250) of the European Union's Seventh Framework Programme FP7/2007-2013/, the Marie Skłodowska-Curie (grant agreement 675392) of the European Union's Horizon 2020 research and innovation programme, the Institute of Health Carlos III (ISC III) and the European Regional Development Fund (ERDF) under the integrated Project of Excellence no. PIE13/00022 (ONCOPROFILE). Work in the laboratory of B.V. is supported by Cancer Research UK [C23338/A15965] and the National Institute for Health Research (NIHR) University College London Hospitals Biomedical Research Centre.
Footnotes
Disclosure of Potential Conflicts of Interest
B.V. and K.O. are consultants for Karus Therapeutics, Oxford, UK. K.O. has received consultancy or speaker fees from Merck, GSK, Gilead and Incyte. No potential conflicts of interest were disclosed by M.G.
References
- 1.Mayer IA, Arteaga CL. The PI3K/AKT Pathway as a Target for Cancer Treatment. Annual review of medicine. 2016;67:11–28. doi: 10.1146/annurev-med-062913-051343. [DOI] [PubMed] [Google Scholar]
- 2.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nature reviews Drug discovery. 2014;13:140–56. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thorpe LM, Yuzugullu H, Zhao JJ. PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nature reviews Cancer. 2015;15:7–24. doi: 10.1038/nrc3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodon J, Dienstmann R, Serra V, Tabernero J. Development of PI3K inhibitors: lessons learned from early clinical trials. Nature reviews Clinical oncology. 2013;10:143–53. doi: 10.1038/nrclinonc.2013.10. [DOI] [PubMed] [Google Scholar]
- 5.Okkenhaug K, Burger JA. PI3K Signaling in Normal B Cells and Chronic Lymphocytic Leukemia (CLL) Current topics in microbiology and immunology. 2016;393:123–42. doi: 10.1007/82_2015_484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirsch E, Ciraolo E, Franco I, Ghigo A, Martini M. PI3K in cancer-stroma interactions: bad in seed and ugly in soil. Oncogene. 2014;33:3083–90. doi: 10.1038/onc.2013.265. [DOI] [PubMed] [Google Scholar]
- 7.Vanhaesebroeck B, Whitehead MA, Pineiro R. Molecules in medicine mini-review: isoforms of PI3K in biology and disease. J Mol Med (Berl) 2016;94:5–11. doi: 10.1007/s00109-015-1352-5. [DOI] [PubMed] [Google Scholar]
- 8.Toska E, Baselga J. Pharmacology in the Era of Targeted Therapies: The Case of PI3K Inhibitors. Clin Cancer Res. 2016;22:2099–101. doi: 10.1158/1078-0432.CCR-16-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayer IA, Abramson V, Formisano L, Balko JM, Estrada MV, Sanders M, et al. A Phase Ib Study of Alpelisib (BYL719), a PI3Kalpha-specific Inhibitor, with Letrozole in ER+/HER2-Negative Metastatic Breast Cancer. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-16-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang W, Hosford SR, Dillon LM, Shee K, Liu SC, Bean JR, et al. Strategically Timing Inhibition of Phosphatidylinositol 3-Kinase to Maximize Therapeutic Index in Estrogen Receptor Alpha-Positive, PIK3CA-Mutant Breast Cancer. Clin Cancer Res. 2016;22:2250–60. doi: 10.1158/1078-0432.CCR-15-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Will M, Qin AC, Toy W, Yao Z, Rodrik-Outmezguine V, Schneider C, et al. Rapid induction of apoptosis by PI3K inhibitors is dependent upon their transient inhibition of RAS-ERK signaling. Cancer discovery. 2014;4:334–47. doi: 10.1158/2159-8290.CD-13-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juric D, Castel P, Griffith M, Griffith OL, Won HH, Ellis H, et al. Convergent loss of PTEN leads to clinical resistance to a PI(3)Kalpha inhibitor. Nature. 2015;518:240–4. doi: 10.1038/nature13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei W, Shin YS, Xue M, Matsutani T, Masui K, Yang H, et al. Single-Cell Phosphoproteomics Resolves Adaptive Signaling Dynamics and Informs Targeted Combination Therapy in Glioblastoma. Cancer cell. 2016;29:563–73. doi: 10.1016/j.ccell.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown KK, Toker A. The phosphoinositide 3-kinase pathway and therapy resistance in cancer. F1000prime reports. 2015;7:13. doi: 10.12703/P7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris JZ, Tissenbaum HA, Ruvkun G. A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature. 1996;382:536–9. doi: 10.1038/382536a0. [DOI] [PubMed] [Google Scholar]
- 16.Foukas LC, Berenjeno IM, Gray A, Khwaja A, Vanhaesebroeck B. Activity of any class IA PI3K isoform can sustain cell proliferation and survival. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11381–6. doi: 10.1073/pnas.0906461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weigelt B, Downward J. Genomic Determinants of PI3K Pathway Inhibitor Response in Cancer. Frontiers in oncology. 2012;2:109. doi: 10.3389/fonc.2012.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Josephs DH, Sarker D. Pharmacodynamic Biomarker Development for PI3K Pathway Therapeutics. Transl Oncogenomics. 2015;7:33–49. doi: 10.4137/TOG.S30529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fritsch C, Huang A, Chatenay-Rivauday C, Schnell C, Reddy A, Liu M, et al. Characterization of the novel and specific PI3Kalpha inhibitor NVP-BYL719 and development of the patient stratification strategy for clinical trials. Molecular cancer therapeutics. 2014;13:1117–29. doi: 10.1158/1535-7163.MCT-13-0865. [DOI] [PubMed] [Google Scholar]
- 20.Costa C, Ebi H, Martini M, Beausoleil SA, Faber AC, Jakubik CT, et al. Measurement of PIP3 levels reveals an unexpected role for p110beta in early adaptive responses to p110alpha-specific inhibitors in luminal breast cancer. Cancer cell. 2015;27:97–108. doi: 10.1016/j.ccell.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz S, Wongvipat J, Trigwell CB, Hancox U, Carver BS, Rodrik-Outmezguine V, et al. Feedback suppression of PI3Kalpha signaling in PTEN-mutated tumors is relieved by selective inhibition of PI3Kbeta. Cancer cell. 2015;27:109–22. doi: 10.1016/j.ccell.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Busaidy NL, Farooki A, Dowlati A, Perentesis JP, Dancey JE, Doyle LA, et al. Management of metabolic effects associated with anticancer agents targeting the PI3K-Akt-mTOR pathway. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:2919–28. doi: 10.1200/JCO.2011.39.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blouin M-J, Birman E, Zhao Y, Zakikhani M, Pollak MN. Abstract 4615: The hyperinsulinemia caused by PI3K inhibitors attenuates their antineoplastic efficacy, but can be minimized by co-administration of metformin. Cancer Research. 2013;73:4615. [Google Scholar]
- 24.Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–6. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bosch A, Li Z, Bergamaschi A, Ellis H, Toska E, Prat A, et al. PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer. Science translational medicine. 2015;7:283ra51. doi: 10.1126/scitranslmed.aaa4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrison MM, Hutchinson K, Williams MM, Stanford JC, Balko JM, Young C, et al. ErbB3 downregulation enhances luminal breast tumor response to antiestrogens. The Journal of clinical investigation. 2013;123:4329–43. doi: 10.1172/JCI66764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu X, Creighton CJ, Biswal NC, Kumar V, Shea M, Herrera S, et al. Overcoming endocrine resistance due to reduced PTEN levels in estrogen receptor-positive breast cancer by co-targeting mammalian target of rapamycin, protein kinase B or mitogen-activated protein kinase kinase. Breast cancer research : BCR. 2014;16:430. doi: 10.1186/s13058-014-0430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.PI3K Inhibitor Improves PFS in BELLE-2 Trial. Cancer discovery. 2016;6:115–6. doi: 10.1158/2159-8290.CD-NB2015-176. [DOI] [PubMed] [Google Scholar]
- 29.Krop IE, Mayer IA, Ganju V, Dickler M, Johnston S, Morales S, et al. Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016;17:811–21. doi: 10.1016/S1470-2045(16)00106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piccart M, Hortobagyi GN, Campone M, Pritchard KI, Lebrun F, Ito Y, et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2dagger. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2014;25:2357–62. doi: 10.1093/annonc/mdu456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koren S, Reavie L, Couto JP, De Silva D, Stadler MB, Roloff T, et al. PIK3CA(H1047R) induces multipotency and multi-lineage mammary tumours. Nature. 2015;525:114–8. doi: 10.1038/nature14669. [DOI] [PubMed] [Google Scholar]
- 32.Van Keymeulen A, Lee MY, Ousset M, Brohee S, Rorive S, Giraddi RR, et al. Reactivation of multipotency by oncogenic PIK3CA induces breast tumour heterogeneity. Nature. 2015;525:119–23. doi: 10.1038/nature14665. [DOI] [PubMed] [Google Scholar]
- 33.Yates LR, Gerstung M, Knappskog S, Desmedt C, Gundem G, Van Loo P, et al. Subclonal diversification of primary breast cancer revealed by multiregion sequencing. Nat Med. 2015;21:751–9. doi: 10.1038/nm.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer cell. 2011;19:575–86. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu Q, Youn H, Tang J, Tawfik O, Dennis K, Terranova PF, et al. Phosphoinositide 3-OH kinase p85alpha and p110beta are essential for androgen receptor transactivation and tumor progression in prostate cancers. Oncogene. 2008;27:4569–79. doi: 10.1038/onc.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang X, Chen S, Asara JM, Balk SP. Phosphoinositide 3-kinase pathway activation in phosphate and tensin homolog (PTEN)-deficient prostate cancer cells is independent of receptor tyrosine kinases and mediated by the p110beta and p110delta catalytic subunits. The Journal of biological chemistry. 2010;285:14980–9. doi: 10.1074/jbc.M109.085696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marques RB, Aghai A, de Ridder CM, Stuurman D, Hoeben S, Boer A, et al. High Efficacy of Combination Therapy Using PI3K/AKT Inhibitors with Androgen Deprivation in Prostate Cancer Preclinical Models. European urology. 2015;67:1177–85. doi: 10.1016/j.eururo.2014.08.053. [DOI] [PubMed] [Google Scholar]
- 38.Ibrahim YH, Garcia-Garcia C, Serra V, He L, Torres-Lockhart K, Prat A, et al. PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer discovery. 2012;2:1036–47. doi: 10.1158/2159-8290.CD-11-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Juvekar A, Burga LN, Hu H, Lunsford EP, Ibrahim YH, Balmana J, et al. Combining a PI3K inhibitor with a PARP inhibitor provides an effective therapy for BRCA1-related breast cancer. Cancer discovery. 2012;2:1048–63. doi: 10.1158/2159-8290.CD-11-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brachmann SM, Kleylein-Sohn J, Gaulis S, Kauffmann A, Blommers MJ, Kazic-Legueux M, et al. Characterization of the mechanism of action of the pan class I PI3K inhibitor NVP-BKM120 across a broad range of concentrations. Molecular cancer therapeutics. 2012;11:1747–57. doi: 10.1158/1535-7163.MCT-11-1021. [DOI] [PubMed] [Google Scholar]
- 41.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 44.Kong D, Okamura M, Yoshimi H, Yamori T. Antiangiogenic effect of ZSTK474, a novel phosphatidylinositol 3-kinase inhibitor. Eur J Cancer. 2009;45:857–65. doi: 10.1016/j.ejca.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 45.Schnell CR, Stauffer F, Allegrini PR, O'Reilly T, McSheehy PM, Dartois C, et al. Effects of the dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 on the tumor vasculature: implications for clinical imaging. Cancer Res. 2008;68:6598–607. doi: 10.1158/0008-5472.CAN-08-1044. [DOI] [PubMed] [Google Scholar]
- 46.Murillo MM, Zelenay S, Nye E, Castellano E, Lassailly F, Stamp G, et al. RAS interaction with PI3K p110alpha is required for tumor-induced angiogenesis. The Journal of clinical investigation. 2014;124:3601–11. doi: 10.1172/JCI74134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu L, Hofmann J, Jaffe RB. Phosphatidylinositol 3-kinase mediates angiogenesis and vascular permeability associated with ovarian carcinoma. Clin Cancer Res. 2005;11:8208–12. doi: 10.1158/1078-0432.CCR-05-0206. [DOI] [PubMed] [Google Scholar]
- 48.Fang J, Ding M, Yang L, Liu LZ, Jiang BH. PI3K/PTEN/AKT signaling regulates prostate tumor angiogenesis. Cell Signal. 2007;19:2487–97. doi: 10.1016/j.cellsig.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soler A, Serra H, Pearce W, Angulo A, Guillermet-Guibert J, Friedman LS, et al. Inhibition of the p110alpha isoform of PI 3-1kinase stimulates nonfunctional tumor angiogenesis. The Journal of experimental medicine. 2013;210:1937–45. doi: 10.1084/jem.20121571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan TL, Choi HS, Matsui A, Benes C, Lifshits E, Luo J, et al. Class 1A PI3K regulates vessel integrity during development and tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9739–44. doi: 10.1073/pnas.0804123105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer cell. 2009;15:220–31. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soler A, Figueiredo AM, Castel P, Martin L, Monelli E, Angulo-Urarte A, et al. Therapeutic benefit of selective inhibition of p110alpha PI3-kinase in pancreatic neuroendocrine tumors. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xia C, Meng Q, Cao Z, Shi X, Jiang BH. Regulation of angiogenesis and tumor growth by p110 alpha and AKT1 via VEGF expression. J Cell Physiol. 2006;209:56–66. doi: 10.1002/jcp.20707. [DOI] [PubMed] [Google Scholar]
- 54.Jiang BH, Zheng JZ, Aoki M, Vogt PK. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:1749–53. doi: 10.1073/pnas.040560897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garmy-Susini B, Avraamides CJ, Desgrosellier JS, Schmid MC, Foubert P, Ellies LG, et al. PI3Kalpha activates integrin alpha4beta1 to establish a metastatic niche in lymph nodes. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9042–7. doi: 10.1073/pnas.1219603110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Graupera M, Guillermet-Guibert J, Foukas LC, Phng LK, Cain RJ, Salpekar A, et al. Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature. 2008;453:662–6. doi: 10.1038/nature06892. [DOI] [PubMed] [Google Scholar]
- 57.Soler A, Angulo-Urarte A, Graupera M. PI3K at the crossroads of tumour angiogenesis signaling pathways. Molecular and Cellular Oncology. 2015;2:e975624–1: 10. doi: 10.4161/23723556.2014.975624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stanczuk L, Martinez-Corral I, Ulvmar MH, Zhang Y, Lavina B, Fruttiger M, et al. cKit Lineage Hemogenic Endothelium-Derived Cells Contribute to Mesenteric Lymphatic Vessels. Cell Rep. 2015 doi: 10.1016/j.celrep.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 59.Gupta S, Ramjaun AR, Haiko P, Wang Y, Warne PH, Nicke B, et al. Binding of ras to phosphoinositide 3-kinase p110alpha is required for ras-driven tumorigenesis in mice. Cell. 2007;129:957–68. doi: 10.1016/j.cell.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 60.Castillo SD, Tzouanacou E, Zaw-Thin M, Berenjeno IM, Parker VE, Chivite I, et al. Somatic activating mutations in Pik3ca cause sporadic venous malformations in mice and humans. Sci Trans Med. 2016;8:332ra43. doi: 10.1126/scitranslmed.aad9982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Limaye N, Kangas J, Mendola A, Godfraind C, Schlogel MJ, Helaers R, et al. Somatic Activating PIK3CA Mutations Cause Venous Malformation. Am J Hum Genet. 2015;97:914–21. doi: 10.1016/j.ajhg.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Castel P, Carmona FJ, Grego-Bessa J, Berger MF, Viale A, Anderson KV, et al. Somatic PIK3CA mutations as a driver of sporadic venous malformations. Science translational medicine. 2016;8:332ra42. doi: 10.1126/scitranslmed.aaf1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Castillo SD, Tzouanacou E, Zaw-Thin M, Berenjeno IM, Parker VE, Chivite I, et al. Somatic activating mutations in Pik3ca cause sporadic venous malformations in mice and humans. Science translational medicine. 2016;8:332ra43. doi: 10.1126/scitranslmed.aad9982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boscolo E, Coma S, Luks VL, Greene AK, Klagsbrun M, Warman ML, et al. AKT hyper-phosphorylation associated with PI3K mutations in lymphatic endothelial cells from a patient with lymphatic malformation. Angiogenesis. 2015;18:151–62. doi: 10.1007/s10456-014-9453-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–87. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 66.Rivera LB, Meyronet D, Hervieu V, Frederick MJ, Bergsland E, Bergers G. Intratumoral myeloid cells regulate responsiveness and resistance to antiangiogenic therapy. Cell Rep. 2015;11:577–91. doi: 10.1016/j.celrep.2015.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmid MC, Avraamides CJ, Dippold HC, Franco I, Foubert P, Ellies LG, et al. Receptor tyrosine kinases and TLR/IL1Rs unexpectedly activate myeloid cell PI3kgamma, a single convergent point promoting tumor inflammation and progression. Cancer cell. 2011;19:715–27. doi: 10.1016/j.ccr.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qayum N, Muschel RJ, Im JH, Balathasan L, Koch CJ, Patel S, et al. Tumor vascular changes mediated by inhibition of oncogenic signaling. Cancer Res. 2009;69:6347–54. doi: 10.1158/0008-5472.CAN-09-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qayum N, Im J, Stratford MR, Bernhard EJ, McKenna WG, Muschel RJ. Modulation of the tumor microvasculature by phosphoinositide-3 kinase inhibition increases doxorubicin delivery in vivo. Clin Cancer Res. 2012;18:161–9. doi: 10.1158/1078-0432.CCR-11-1413. [DOI] [PubMed] [Google Scholar]
- 70.Fokas E, Im JH, Hill S, Yameen S, Stratford M, Beech J, et al. Dual inhibition of the PI3K/mTOR pathway increases tumor radiosensitivity by normalizing tumor vasculature. Cancer Res. 2012;72:239–48. doi: 10.1158/0008-5472.CAN-11-2263. [DOI] [PubMed] [Google Scholar]
- 71.Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 72.Tolaney SM, Boucher Y, Duda DG, Martin JD, Seano G, Ancukiewicz M, et al. Role of vascular density and normalization in response to neoadjuvant bevacizumab and chemotherapy in breast cancer patients. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:14325–30. doi: 10.1073/pnas.1518808112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaneda MM, Cappello P, Nguyen AV, Ralainirina N, Hardamon CR, Foubert P, et al. Macrophage PI3Kgamma Drives Pancreatic Ductal Adenocarcinoma Progression. Cancer discovery. 2016;6:870. doi: 10.1158/2159-8290.CD-15-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leake I. Pancreatic cancer: surprising role for fibrosis. Nat Rev Gastroenterol Hepatol. 2014;11:396. doi: 10.1038/nrgastro.2014.97. [DOI] [PubMed] [Google Scholar]
- 75.Trimboli AJ, Cantemir-Stone CZ, Li F, Wallace JA, Merchant A, Creasap N, et al. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature. 2009;461:1084–91. doi: 10.1038/nature08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E, et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297:1031–4. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 77.Jou ST, Carpino N, Takahashi Y, Piekorz R, Chao JR, Carpino N, et al. Essential, nonredundant role for the phosphoinositide 3-kinase p110delta in signaling by the B-cell receptor complex. Mol Cell Biol. 2002;22:8580–91. doi: 10.1128/MCB.22.24.8580-8591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clayton E, Bardi G, Bell SE, Chantry D, Downes CP, Gray A, et al. A crucial role for the p110delta subunit of phosphatidylinositol 3-kinase in B cell development and activation. The Journal of experimental medicine. 2002;196:753–63. doi: 10.1084/jem.20020805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iyengar S, Clear A, Bodor C, Maharaj L, Lee A, Calaminici M, et al. P110alpha-mediated constitutive PI3K signaling limits the efficacy of p110delta-selective inhibition in mantle cell lymphoma, particularly with multiple relapse. Blood. 2013;121:2274–84. doi: 10.1182/blood-2012-10-460832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Davis RE, Ngo VN, Lenz G, Tolar P, Young RM, Romesser PB, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gopal AK, Kahl BS, de Vos S, Wagner-Johnston ND, Schuster SJ, Jurczak WJ, et al. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370:1008–18. doi: 10.1056/NEJMoa1314583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.O'Brien SM, Lamanna N, Kipps TJ, Flinn I, Zelenetz AD, Burger JA, et al. A phase 2 study of idelalisib plus rituximab in treatment-naive older patients with chronic lymphocytic leukemia. Blood. 2015;126:2686–94. doi: 10.1182/blood-2015-03-630947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoellenriegel J, Meadows SA, Sivina M, Wierda WG, Kantarjian H, Keating MJ, et al. The phosphoinositide 3'-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118:3603–12. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ten Hacken E, Burger JA. Microenvironment interactions and B-cell receptor signaling in Chronic Lymphocytic Leukemia: Implications for disease pathogenesis and treatment. Biochim Biophys Acta. 2016;1863:401–13. doi: 10.1016/j.bbamcr.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Burgler S, Gimeno A, Parente-Ribes A, Wang D, Os A, Devereux S, et al. Chronic lymphocytic leukemia cells express CD38 in response to Th1 cell-derived IFN-gamma by a T-bet-dependent mechanism. J Immunol. 2015;194:827–35. doi: 10.4049/jimmunol.1401350. [DOI] [PubMed] [Google Scholar]
- 87.Os A, Burgler S, Ribes AP, Funderud A, Wang D, Thompson KM, et al. Chronic lymphocytic leukemia cells are activated and proliferate in response to specific T helper cells. Cell Rep. 2013;4:566–77. doi: 10.1016/j.celrep.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 88.Burger JA, Gribben JG. The microenvironment in chronic lymphocytic leukemia (CLL) and other B cell malignancies: insight into disease biology and new targeted therapies. Semin Cancer Biol. 2014;24:71–81. doi: 10.1016/j.semcancer.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 89.Zotes TM, Spada R, Mulens V, Perez-Yague S, Sorzano CO, Okkenhaug K, et al. PI3K p110delta is expressed by gp38(-)CD31(+) and gp38(+)CD31(+) spleen stromal cells and regulates their CCL19, CCL21, and LTbetaR mRNA levels. PloS one. 2013;8:e72960. doi: 10.1371/journal.pone.0072960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Okkenhaug K. Signaling by the phosphoinositide 3-kinase family in immune cells. Annu Rev Immunol. 2013;31:675–704. doi: 10.1146/annurev-immunol-032712-095946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ali K, Soond DR, Pineiro R, Hagemann T, Pearce W, Lim EL, et al. Inactivation of PI(3)K p110delta breaks regulatory T-cell-mediated immune tolerance to cancer. Nature. 2014;510:407–11. doi: 10.1038/nature13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Patton DT, Garden OA, Pearce WP, Clough LE, Monk CR, Leung E, et al. Cutting edge: the phosphoinositide 3-kinase p110 delta is critical for the function of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2006;177:6598–602. doi: 10.4049/jimmunol.177.10.6598. [DOI] [PubMed] [Google Scholar]
- 93.Steinbach EC, Kobayashi T, Russo SM, Sheikh SZ, Gipson GR, Kennedy ST, et al. Innate PI3K p110delta regulates Th1/Th17 development and microbiota-dependent colitis. J Immunol. 2014;192:3958–68. doi: 10.4049/jimmunol.1301533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Uno JK, Rao KN, Matsuoka K, Sheikh SZ, Kobayashi T, Li F, et al. Altered macrophage function contributes to colitis in mice defective in the phosphoinositide-3 kinase subunit p110delta. Gastroenterology. 2010;139:1642–53, 53 e1-6. doi: 10.1053/j.gastro.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Louie CY, DiMaio MA, Matsukuma KE, Coutre SE, Berry GJ, Longacre TA. Idelalisib-associated Enterocolitis: Clinicopathologic Features and Distinction From Other Enterocolitides. Am J Surg Pathol. 2015;39:1653–60. doi: 10.1097/PAS.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 96.Weidner AS, Panarelli NC, Geyer JT, Bhavsar EB, Furman RR, Leonard JP, et al. Idelalisib-associated Colitis: Histologic Findings in 14 Patients. Am J Surg Pathol. 2015;39:1661–7. doi: 10.1097/PAS.0000000000000522. [DOI] [PubMed] [Google Scholar]
- 97.Coutre SE, Barrientos JC, Brown JR, de Vos S, Furman RR, Keating MJ, et al. Management of adverse events associated with idelalisib treatment: expert panel opinion. Leuk Lymphoma. 2015;0:1–8. doi: 10.3109/10428194.2015.1022770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cheah CY, Fowler NH. Idelalisib in the management of lymphoma. Blood. 2016;128:331–6. doi: 10.1182/blood-2016-02-702761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thompson PA, Stingo F, Keating MJ, Ferrajoli A, Burger JA, Wierda WG, et al. Outcomes of patients with chronic lymphocytic leukemia treated with first-line idelalisib plus rituximab after cessation of treatment for toxicity. Cancer. 2016 doi: 10.1002/cncr.30069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lampson BL, Kasar SN, Matos TR, Morgan EA, Rassenti L, Davids MS, et al. Idelalisib given front-line for treatment of chronic lymphocytic leukemia causes frequent immune-mediated hepatotoxicity. Blood. 2016;128:195–203. doi: 10.1182/blood-2016-03-707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang KJ, Husami A, Marsh R, Jordan MB. Identification of a phosphoinositide 3-kinase (PI-3K) p110delta (PIK3CD) deficient individual. J Clin Immunol. 2013;33:673–4. [Google Scholar]
- 102.Callahan MK, Postow MA, Wolchok JD. Targeting T Cell Co-receptors for Cancer Therapy. Immunity. 2016;44:1069–78. doi: 10.1016/j.immuni.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 103.Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256–71. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 104.Burris HA, F I, Lunning MA, Vose J, Fowler NA, Nastoupil L, et al. Long-term follow-up of the PI3Kδ inhibitor TGR-1202 to demonstrate a differentiated safety profile and high response rates in CLL and NHL: Integrated-analysis of TGR-1202 monotherapy and combined with ublituximab. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2016;34:7512. [Google Scholar]
- 105.Kerr WG, Colucci F. Inositol phospholipid signaling and the biology of natural killer cells. J Innate Immun. 2011;3:249–57. doi: 10.1159/000323920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hori S, Carvalho TL, Demengeot J. CD25+CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur J Immunol. 2002;32:1282–91. doi: 10.1002/1521-4141(200205)32:5<1282::AID-IMMU1282>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 107.Barr PM, Saylors GB, Spurgeon SE, Cheson BD, Greenwald DR, O' Brien SM, et al. Phase 2 study of idelalisib and entospletinib: pneumonitis limits combination therapy in relapsed refractory CLL and NHL. Blood. 2016;127:2411–5. doi: 10.1182/blood-2015-12-683516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu D, Zhang T, Marshall AJ, Okkenhaug K, Vanhaesebroeck B, Uzonna JE. The p110delta isoform of phosphatidylinositol 3-kinase controls susceptibility to Leishmania major by regulating expansion and tissue homing of regulatory T cells. J Immunol. 2009;183:1921–33. doi: 10.4049/jimmunol.0901099. [DOI] [PubMed] [Google Scholar]
- 109.Pearce VQ, Bouabe H, MacQueen AR, Carbonaro V, Okkenhaug K. PI3Kdelta Regulates the Magnitude of CD8+ T Cell Responses after Challenge with Listeria monocytogenes. J Immunol. 2015;195:3206–17. doi: 10.4049/jimmunol.1501227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rommel C, Camps M, Ji H. PI3K delta and PI3K gamma: partners in crime in inflammation in rheumatoid arthritis and beyond? Nat Rev Immunol. 2007;7:191–201. doi: 10.1038/nri2036. [DOI] [PubMed] [Google Scholar]
- 111.Roychoudhuri R, Eil RL, Restifo NP. The interplay of effector and regulatory T cells in cancer. Curr Opin Immunol. 2015;33:101–11. doi: 10.1016/j.coi.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 112.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, et al. Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy. Immunity. 2016;44:343–54. doi: 10.1016/j.immuni.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–7. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic [bgr]-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–5. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 116.Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, et al. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer discovery. 2016;6:202–16. doi: 10.1158/2159-8290.CD-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Patton DT, Wilson MD, Rowan WC, Soond DR, Okkenhaug K. The PI3K p110delta regulates expression of CD38 on regulatory T cells. PloS one. 2011;6:e17359. doi: 10.1371/journal.pone.0017359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Okkenhaug K, Patton DT, Bilancio A, Garcon F, Rowan WC, Vanhaesebroeck B. The p110delta isoform of phosphoinositide 3-kinase controls clonal expansion and differentiation of Th cells. J Immunol. 2006;177:5122–8. doi: 10.4049/jimmunol.177.8.5122. [DOI] [PubMed] [Google Scholar]
- 119.Luo CT, Liao W, Dadi S, Toure A, Li MO. Graded Foxo1 activity in Treg cells differentiates tumour immunity from spontaneous autoimmunity. Nature. 2016;529:532–6. doi: 10.1038/nature16486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Roychoudhuri R, Clever D, Li P, Wakabayashi Y, Quinn KM, Klebanoff CA, et al. BACH2 regulates CD8(+) T cell differentiation by controlling access of AP-1 factors to enhancers. Nat Immunol. 2016;17:851–60. doi: 10.1038/ni.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Roychoudhuri R, Eil RL, Clever D, Klebanoff CA, Sukumar M, Grant FM, et al. The transcription factor BACH2 promotes tumor immunosuppression. The Journal of clinical investigation. 2016;126:599–604. doi: 10.1172/JCI82884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sharma MD, Shinde R, McGaha TL, Huang L, Holmgaard RB, Wolchok JD, et al. The PTEN pathway in Tregs is a critical driver of the suppressive tumor microenvironment. Sci Adv. 2015;1:e1500845. doi: 10.1126/sciadv.1500845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Soond DR, Garcon F, Patton DT, Rolf J, Turner M, Scudamore C, et al. Pten loss in CD4 T cells enhances their helper function but does not lead to autoimmunity or lymphoma. J Immunol. 2012;188:5935–43. doi: 10.4049/jimmunol.1102116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Abu-Eid R, Samara RN, Ozbun L, Abdalla MY, Berzofsky JA, Friedman KM, et al. Selective inhibition of regulatory T cells by targeting the PI3K-Akt pathway. Cancer Immunol Res. 2014;2:1080–9. doi: 10.1158/2326-6066.CIR-14-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gracias DT, Boesteanu AC, Fraietta JA, Hope JL, Carey AJ, Mueller YM, et al. Phosphatidylinositol 3-Kinase p110delta Isoform Regulates CD8+ T Cell Responses during Acute Viral and Intracellular Bacterial Infections. J Immunol. 2016;196:1186–98. doi: 10.4049/jimmunol.1501890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Doisne JM, Huber CM, Okkenhaug K, Colucci F. Immunomodulation of Selective Naive T Cell Functions by p110delta Inactivation Improves the Outcome of Mismatched Cell Transplantation. Cell Rep. 2015;10:702–10. doi: 10.1016/j.celrep.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Soond DR, Bjorgo E, Moltu K, Dale VQ, Patton DT, Torgersen KM, et al. PI3K p110delta regulates T-cell cytokine production during primary and secondary immune responses in mice and humans. Blood. 2010;115:2203–13. doi: 10.1182/blood-2009-07-232330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zebedin E, Simma O, Schuster C, Putz EM, Fajmann S, Warsch W, et al. Leukemic challenge unmasks a requirement for PI3Kdelta in NK cell-mediated tumor surveillance. Blood. 2008;112:4655–64. doi: 10.1182/blood-2008-02-139105. [DOI] [PubMed] [Google Scholar]
- 129.Putz EM, Prchal-Murphy M, Simma OA, Forster F, Koenig X, Stockinger H, et al. PI3Kdelta is essential for tumor clearance mediated by cytotoxic T lymphocytes. PloS one. 2012;7:e40852. doi: 10.1371/journal.pone.0040852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Macintyre AN, Finlay D, Preston G, Sinclair LV, Waugh CM, Tamas P, et al. Protein kinase B controls transcriptional programs that direct cytotoxic T cell fate but is dispensable for T cell metabolism. Immunity. 2011;34:224–36. doi: 10.1016/j.immuni.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Staron MM, Gray SM, Marshall HD, Parish IA, Chen JH, Perry CJ, et al. The transcription factor FoxO1 sustains expression of the inhibitory receptor PD-1 and survival of antiviral CD8(+) T cells during chronic infection. Immunity. 2014;41:802–14. doi: 10.1016/j.immuni.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Crompton JG, Sukumar M, Roychoudhuri R, Clever D, Gros A, Eil RL, et al. Akt inhibition enhances expansion of potent tumor-specific lymphocytes with memory cell characteristics. Cancer Res. 2015;75:296–305. doi: 10.1158/0008-5472.CAN-14-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.van der Waart AB, van de Weem NM, Maas F, Kramer CS, Kester MG, Falkenburg JH, et al. Inhibition of Akt signaling promotes the generation of superior tumor-reactive T cells for adoptive immunotherapy. Blood. 2014;124:3490–500. doi: 10.1182/blood-2014-05-578583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Abu Eid R, Friedman KM, Mkrtichyan M, Walens A, King W, Janik J, et al. Akt1 and -2 inhibition diminishes terminal differentiation and enhances central memory CD8 T-cell proliferation and survival. Oncoimmunology. 2015;4:e1005448. doi: 10.1080/2162402X.2015.1005448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–12. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–7. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–9. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 138.Gonzalez-Garcia A, Sanchez-Ruiz J, Flores JM, Carrera AC. Phosphatidylinositol 3-kinase gamma inhibition ameliorates inflammation and tumor growth in a model of colitis-associated cancer. Gastroenterology. 2010;138:1374–83. doi: 10.1053/j.gastro.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 139.Marshall NA, Galvin KC, Corcoran AM, Boon L, Higgs R, Mills KH. Immunotherapy with PI3K inhibitor and Toll-like receptor agonist induces IFN-gamma+IL-17+ polyfunctional T cells that mediate rejection of murine tumors. Cancer Res. 2012;72:581–91. doi: 10.1158/0008-5472.CAN-11-0307. [DOI] [PubMed] [Google Scholar]
- 140.Schmid MC, Franco I, Kang SW, Hirsch E, Quilliam LA, Varner JA. PI3-kinase gamma promotes Rap1a-mediated activation of myeloid cell integrin alpha4beta1, leading to tumor inflammation and growth. PloS one. 2013;8:e60226. doi: 10.1371/journal.pone.0060226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–96. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 142.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nature reviews Cancer. 2012;12:265–77. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Aksoy E, Taboubi S, Torres D, Delbauve S, Hachani A, Whitehead MA, et al. The p110delta isoform of the kinase PI(3)K controls the subcellular compartmentalization of TLR4 signaling and protects from endotoxic shock. Nat Immunol. 2012;13:1045–54. doi: 10.1038/ni.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fukao T, Tanabe M, Terauchi Y, Ota T, Matsuda S, Asano T, et al. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol. 2002;3:875–81. doi: 10.1038/ni825. [DOI] [PubMed] [Google Scholar]
- 145.Ebert PJ, Cheung J, Yang Y, McNamara E, Hong R, Moskalenko M, et al. MAP Kinase Inhibition Promotes T Cell and Anti-tumor Activity in Combination with PD-L1 Checkpoint Blockade. Immunity. 2016;44:609–21. doi: 10.1016/j.immuni.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 146.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 147.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nature reviews Drug discovery. 2007;6:273–86. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 148.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 149.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nature reviews Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer cell. 2009;15:232–9. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70:6171–80. doi: 10.1158/0008-5472.CAN-10-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nature reviews Drug discovery. 2011;10:417–27. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 153.Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer cell. 2004;6:553–63. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 154.Maes H, Kuchnio A, Peric A, Moens S, Nys K, De Bock K, et al. Tumor vessel normalization by chloroquine independent of autophagy. Cancer cell. 2014;26:190–206. doi: 10.1016/j.ccr.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 155.Mazzone M, Dettori D, Leite de Oliveira R, Loges S, Schmidt T, Jonckx B, et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell. 2009;136:839–51. doi: 10.1016/j.cell.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hamzah J, Jugold M, Kiessling F, Rigby P, Manzur M, Marti HH, et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature. 2008;453:410–4. doi: 10.1038/nature06868. [DOI] [PubMed] [Google Scholar]
- 157.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 159.Wong PP, Demircioglu F, Ghazaly E, Alrawashdeh W, Stratford MR, Scudamore CL, et al. Dual-action combination therapy enhances angiogenesis while reducing tumor growth and spread. Cancer cell. 2015;27:123–37. doi: 10.1016/j.ccell.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 160.Huang Y, Goel S, Duda DG, Fukumura D, Jain RK. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013;73:2943–8. doi: 10.1158/0008-5472.CAN-12-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]