Abstract
Background & Aims
Regulation of bile acid homeostasis in mammals is a complex process regulated via extensive cross-talk between liver, intestine and intestinal microbiota. Here we studied the effects of gut microbiota on bile acid homeostasis in mice.
Methods
Bile acid homeostasis was assessed in four mouse models. Germfree mice, conventionally-raised mice, Asbt-KO mice and intestinal-specific Gata4-iKO mice were treated with antibiotics (bacitracin, neomycin and vancomycin; 100 mg/kg) for five days and subsequently compared with untreated mice.
Results
Attenuation of the bacterial flora by antibiotics strongly reduced fecal excretion and synthesis of bile acids, but increased the expression of the bile acid synthesis enzyme CYP7A1. Similar effects were seen in germfree mice. Intestinal bile acid absorption was increased and accompanied by increases in plasma bile acid levels, biliary bile acid secretion and enterohepatic cycling of bile acids. In the absence of microbiota, the expression of the intestinal bile salt transporter Asbt was strongly increased in the ileum and was also expressed in more proximal parts of the small intestine. Most of the effects of antibiotic treatment on bile acid homeostasis could be prevented by genetic inactivation of either Asbt or the transcription factor Gata4.
Conclusions
Attenuation of gut microbiota alters Gata4-controlled expression of Asbt, increasing absorption and decreasing synthesis of bile acids. Our data support the concept that under physiological conditions microbiota stimulate Gata4, which suppresses Asbt expression, limiting the expression of this transporter to the terminal ileum. Our studies expand current knowledge on the bacterial control of bile acid homeostasis.
Keywords: Gut microbiota, Intestinal bacteria, Antibiotic treatment, Enterohepatic circulation, Germfree, Fgf15, Asbt, Gata4, Bile acid reabsorption, Bile acid synthesis, CYP7A1
Introduction
The dynamic community of bacteria colonizing the intestinal tract interacts symbiotically with the human host [1]. These bacteria, also known as gut microbiota, exert a variety of effects on the host, ranging from shaping the structure and functions of the gut and the immune system, to altering host energy metabolism [1]. In return, the bacteria benefit by inhabiting a protected, nutrient-rich environment.
Intestinal bacteria are also known to be involved in the metabolism of bile acids (BAs). BAs are produced in the liver from cholesterol through a complex multi-enzyme pathway. Intestinal bacteria can deconjugate BAs and metabolize primary BAs (i.e. those synthesized in the liver) through oxidation and dehydroxylation into more hydrophobic, so-called secondary BAs [2–4]. Under physiological conditions, BAs are efficiently reabsorbed by ileal enterocytes and transported back to the liver via the portal circulation, in a cycle known as the enterohepatic circulation. Normally, relatively small amounts of BAs reach the colon, where some are absorbed and the remainder is excreted in the feces. To maintain a constant circulating pool, a small amount of BAs is synthesized each cycle, which is equivalent to the loss in feces under steady state conditions.
Active BA absorption is mediated by the apical sodium-dependent BA transporter (Asbt) [5], which is almost exclusively expressed in the terminal part of the ileum. In Asbt knock-out (Asbt-KO) mice, fecal BA excretion is 10 to 20 times higher than in wildtype mice. Despite increased BA synthesis in these mice, the BA pool size is reduced by 80%, indicating that alternative (absorptive) mechanisms are unable to compensate for loss of Asbt function [6].
The expression of Asbt in the small intestine is under direct negative control of the transcription factor Gata4 [7]. In the adult mouse, Gata4 expression is repressed in the terminal ileum, thereby allowing expression of Asbt in the enterocytes of this part of the intestine. Consequently, intestinal-specific Gata4 knock-out (Gata4-iKO) induces the expression of Asbt in the duodenum and jejunum [8].
Previous studies in germfree animals have observed that these animals have less fecal BA excretion [9,10], while the total concentration of BAs in the small intestines is higher [9,11]. Accordingly, germfree animals have an increased half-life of 14C-labeled tauro-cholic acid [12]. Similar results have recently been reported in mice treated with antibiotics [13,14]. A possible explanation for these results is that microbial deconjugation of BAs is thought to enhance fecal BA excretion [15]. However, when germfree rats are colonized with a bacterial strain capable of deconjugating BAs, this has no effect on the excretion levels of either fecal BAs or of the tracer [12,16].
BAs are made from cholesterol and plasma cholesterol levels are a major risk factor for cardiovascular diseases. If we know how bacteria affect BA homeostasis, this could offer opportunities to modulate cholesterol levels in the future. In this study, we compared germfree and conventional mice and gave mice short-term antibiotic treatment. We aimed to elucidate the mechanism(s) by which absence of gut microbiota affects bile acid metabolism. We demonstrate that under normal conditions gut microbiota strongly controls enterohepatic recycling of bile acids by a GATA 4 dependent mechanism. Spatiotemporal regulation of Asbt expression plays a key role in the process.
Materials and methods
Animal experiments
All experiments were performed on male mice. C57BL/6 mice (Charles River, France), Asbt-KO mice (kindly provided by Prof. P.A. Dawson, Wake Forest University School of Medicine, Winston-Salem, NC, USA), Gata4-iKO mice [8] and their control littermates were housed individually in a temperature and light-controlled facility with a 12-h light-dark cycle. All mice were fed commercially available laboratory chow (RMH-B; Hope Farms, Woerden, the Netherlands) ad libitum, which was supplemented with antibiotics (bacitracin, neomycin and vancomycin; 100 mg/kg) as required. Germfree NMRI mice and their conventional counterparts were housed in groups of five and fed an irradiated diet (Ssniff® M-Z, Ssniff Spezialdiäten GmbH, Soest, Germany).
After five days of antibiotic or control treatment, gallbladder cannulation was performed to collect hepatic bile. After a five-min equilibration period, bile was collected for 20 min under Hypnorm (fentanyl/fluanisone; 1 ml/kg) and diazepam (10 mg/kg) anesthesia using a humidified incubator to maintain body temperature. Blood was obtained via heart puncture and, after sacrificing the mice, the liver and ileum were excised and snap-frozen in liquid nitrogen for gene expression and protein analysis. All experiments were approved by the Ethics Committee for Animal Experiments of the University of Groningen.
Measurements
Analysis of BAs, cholesterol and phospholipids, as well as cDNA measurements and western blotting were all performed as described previously. For detailed information please refer to Supplementary material and methods.
Results
Bile acid homeostasis in germfree mice
When we compared BA homeostasis between germfree and conventional mice, we found the BA concentration in the feces of germfree mice were to be less than one third of that in the feces of their conventional counterparts (Fig. 1A). Only primary BAs were present in the feces of germfree mice and virtually all fecal BAs were conjugated, mainly with taurine, while in the feces of conventional mice also secondary BAs were present and only 19% of BAs were conjugated (Supplementary Fig. 1A). The main BAs detected in plasma were tauro-cholic acid (TCA) and tauro-beta-muricholic acid (Tβ-MCA), levels that were much higher in germfree mice than in conventional controls (Supplementary Fig. 1B).
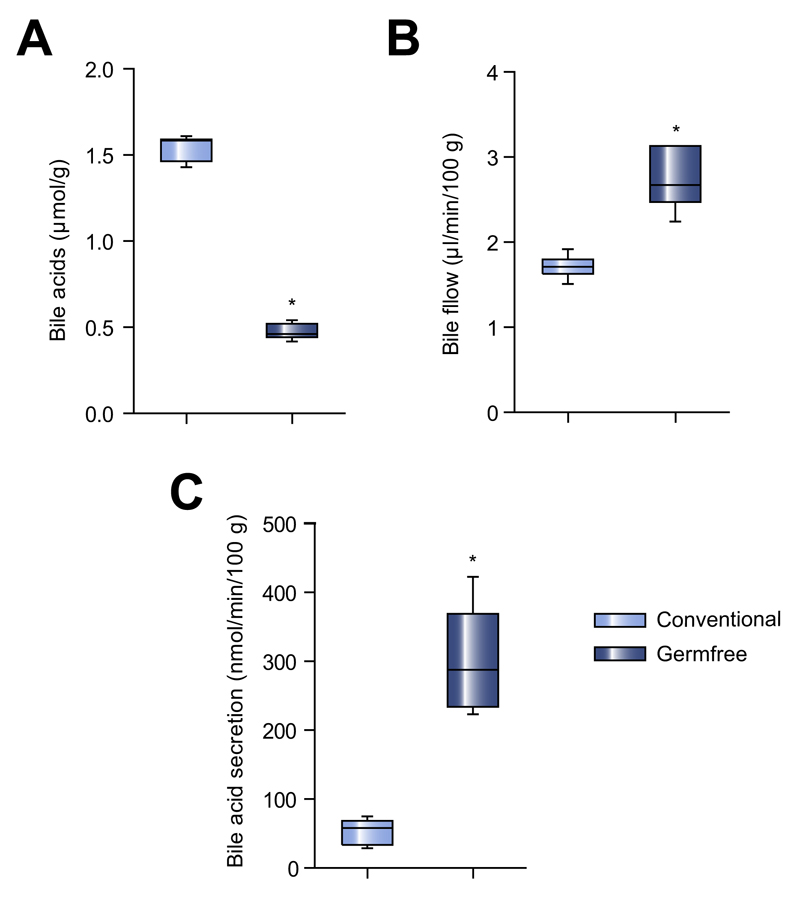
Fig. 1. Bile acid homeostasis in germfree mice.
(A) Total BA concentration in feces (μmol/g feces) of germfree and conventional mice. (B) Bile flow (μl/min/100 g bodyweight) measured by bile cannulation and (C) secretion of biliary BAs (nmol/min/100 g bodyweight) in germfree and conventional mice. Median ± range; n = 6–9/group; *p <0.05.
Bile flow was higher in germfree mice than in conventional mice (Fig. 1B). Secretion of biliary BAs was also significantly higher in these mice (Fig. 1C), and consisted mainly of CA and β-MCA (Supplementary Fig. 1C). When we compared the levels of cholesterol and phospholipids secreted in bile between the two groups of mice, we found germfree mice had 4.2-fold higher amounts of biliary cholesterol (3.3 ± 1.3 vs. 0.8 ± 0.2 nmol/min/100 g; p = 0.04) and 2.4-fold higher amounts of phospholipids (26.3 ± 7.2 vs. 10.8 ± 2.5 nmol/min/100 g; p = 0.04).
Bile acid homeostasis in conventional mice treated with antibiotics
BA homeostasis in germfree mice is clearly different to that in their conventional counterparts. However, since the differences in the colonization of the gut are present from birth, metabolic compensation may have occurred. Thus, to identify the short-term effects of a decrease in intestinal bacteria on BA metabolism, conventional mice were treated with cocktail of three non-absorbable antibiotics for five days, which significantly reduced fecal bacterial DNA concentrations (Supplementary Fig. 2A). Antibiotic treatment markedly reduced total fecal BA excretion (Fig. 2A), indicating an inhibition of BA synthesis. To validate this, we injected mice intravenously with 13C-cholate and subsequently determined the turnover and pool size of cholate. The cholate pool size was larger in antibiotic-treated mice than in control mice (34 ± 5 vs. 31 ± 8 μmol/100 g), as apparent from the lower 13C-cholate enrichment seen in the plasma of these mice (Supplementary Fig. 2B). In line with the lower fecal BA excretion, cholate turnover rate (Fig. 2B) and cholate synthesis were also lower in treated mice than in control mice (3.1 ± 2.5 vs. 6.7 ± 2.5 μmol/24 h/100 g).
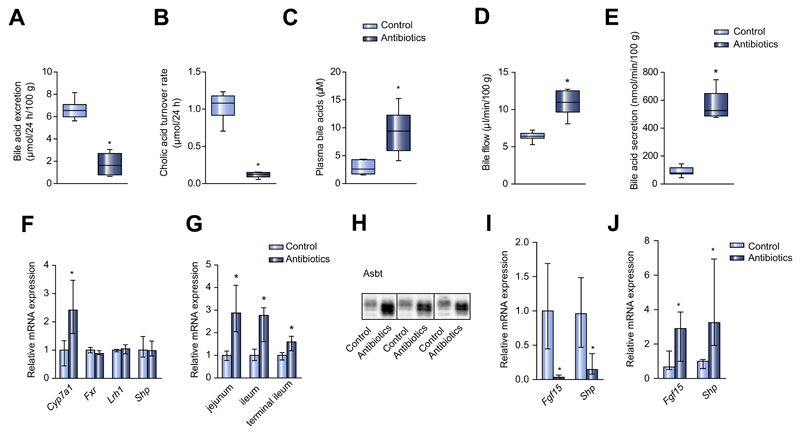
Fig. 2. Antibiotic treatment alters bile acid composition, recirculation and gene expression in the enterohepatic circulation.
(A) Fecal excretion of BAs in antibiotic-treated and control mice (μmol/24 h/100 g bodyweight). (B) Cholic acid turnover rate (μmol/24 h), (C) total plasma BA levels (μM), (D) bile flow (μl/min/100 g bodyweight) and (E) secretion of biliary BAs (nmol/min/100 g bodyweight) in antibiotic-treated and control mice. (F) Hepatic mRNA expression in antibiotic-treated and control mice. (G) Asbt mRNA expression in different segments of the small intestine. (H) Representative western blot of ileal Asbt from three control and three antibiotic-treated mice. (I) Ileal mRNA expression of BA-responsive genes. (J) mRNA expression of BA-responsive genes in the jejunum. Median ± range; n = 8/group; *p <0.05.
In mice given antibiotics, we also observed higher levels of plasma BAs, bile flow and biliary BA secretion (Fig. 2C–E), suggesting that antibiotic treatment enhanced intestinal BA absorption and enterohepatic cycling. Biliary secretion of CA and β-MCA was far higher in treated mice than in controls, and also the secretion of the secondary BAs HDCA and ω-MCA was higher indicating that treatment with antibiotics does not completely abrogate dihydroxylation of BA. (Supplementary Fig. 2C). As biliary BA secretion is the driving force behind the biliary secretion of cholesterol and phospholipids, it was not unexpected to see an increase in the biliary secretion of these lipids (Supplementary Fig. 2D and E). The fecal excretion of neutral sterols was lower in antibiotic-treated mice (Supplementary Fig. 2F). Treatment with a different antibacterial drug (ampicillin 100 mg/kg) showed similar results, and treatment of germfree mice with antibiotics had no effect on any of the parameters related to BA and cholesterol metabolism studied above (data not shown).
Effects of antibiotic therapy on gene expression and bile acid reabsorption
In order to study the mechanism by which gut microbiota influence BA reabsorption, we measured the gene expression of key enzymes and transporters within the enterohepatic circulation. While antibiotic treatment increased the hepatic expression of Cyp7a1, there were no significant differences between antibiotic-treated mice and controls with regard to the hepatic expression of the nuclear receptors Fxr and Lrh-1 or their target gene Shp, which are known to be involved in the regulation of Cyp7a1 expression (Fig. 2F).
Antibiotic treatment strongly increased Asbt expression in all segments of the small intestine (Fig. 2G), absolute amounts were highest in the terminal ileum. Western blot analysis showed that Asbt protein levels in the terminal ileum were higher than those in controls (Fig. 2H). Despite increased Asbt expression, ileal expression of the BA-responsive genes Shp and Fgf15 was much lower than in controls (Fig. 2I), suggesting a decreased BA activation of Fxr in this part of the small intestine.
Increased Asbt expression in the proximal small intestine may induce BA absorption in this part of the intestine. We analyzed luminal BA concentrations in several segments of the small intestine, luminal BA concentrations were lower in the jejunum and ileum of mice treated with antibiotics than in control mice (data not shown), indicating that increased absorption may have occurred in proximal parts. In line with proximal Asbt expression and BA absorption, antibiotic treatment induced the expression levels of the BA-responsive genes Shp and Fgf15 in the proximal small intestine (Fig. 2J). These findings are compatible with enhanced import of BAs into proximal enterocytes. These data suggest that when there is a reduction of bacterial flora, BAs are also absorbed in the proximal small intestine, which may result in a reduction of BA absorption and of subsequent Fxr activation in distal parts.
Effects of antibiotic therapy in Asbt-KO mice
The results described above suggested that the bacterial flora may influence Asbt expression and thereby BA absorption. To identify the role of Asbt in the relationship between gut microbiota and BA absorption, we treated Asbt-KO mice with antibiotics.
In untreated animals, fecal BA excretion was higher in Asbt-KO mice than in wildtype (WT) mice (Fig. 3A), which coincided with lower biliary BA secretion, despite no changes in either bile flow rate or plasma BA concentration (Fig. 3B–D). Secondary BAs made up 51% of the plasma BA pool in Asbt-KO mice, compared with only 12% in WT mice, suggesting that in Asbt-KO mice colonic absorption of secondary BAs compensates for enhanced fecal BA loss, as shown before [6]. Hepatic mRNA expression of Cyp7a1, Cyp8b1 and Hmgcr was higher in Asbt-KO mice than in WT mice (Fig. 3E), findings compatible with compensation for the increased fecal BA loss.
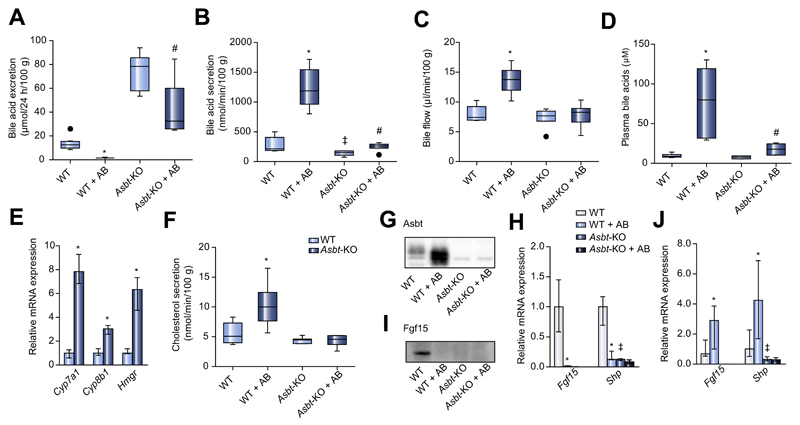
Fig. 3. Bile acid homeostasis in Asbt-KO mice treated with antibiotics.
(A) Fecal BA excretion (μmol/24 h/100 g bodyweight) in wildtype and Asbt-KO mice upon antibiotic treatment. (B) Secretion of biliary BAs (nmol/min/100 g bodyweight). (C) Bile flow (μl/min/100 g bodyweight). (D) Total plasma BAs (μM) in antibiotic-treated and control wildtype and Asbt-KO mice. (E) Hepatic mRNA expression of Cyp7a1, Cyp8b1 and Hmgcr. (F) Biliary cholesterol secretion (nmol/min/100 g bodyweight) in Asbt-KO and wildtype mice. (G) Ileal Asbt protein expression. (H) Ileal mRNA expression of BA-responsive genes. (I) Ileal FGF15 protein levels. (J) mRNA expression of Fgf15 and Shp in jejunum of wildtype and Asbt-KO mice. Median ± range; n = 8/group; *p <0.05 WT vs. WT + AB, ‡p <0.05 WT vs. Asbt-KO, #p <0.05 Asbt-KO vs. Asbt-KO + AB.
Antibiotic treatment reduced fecal BA excretion 9.1-fold in WT mice, but only 2.4-fold in Asbt-KO mice (Fig. 3A). While in antibiotic-treated WT mice we observed a dramatic 8.9-fold increase in plasma BAs, this increase was only 2.2-fold in antibiotic-treated Asbt-KO mice (Fig. 3D). Bile flow in Asbt-KO mice was not affected by antibiotic treatment (Fig. 3C). Biliary BA secretion did increase in Asbt-KO mice upon antibiotic treatment, although not as much as in WT mice (1.8-fold vs. 5.5-fold) (Fig. 3B). Biliary cholesterol secretion was induced in WT mice upon antibiotic treatment, but unchanged in treated Asbt-KO mice (Fig. 3F).
When we looked at the expression levels of BA-responsive genes in the intestine, we saw that antibiotic treatment induced ileal Asbt protein expression in WT mice, whereas it was absent in Asbt-KO mice (Fig. 3G). Ileal expression of Fgf15 was also virtually absent in Asbt-KO mice, as was the expression of Shp; neither changed upon treatment with antibiotics (Fig. 3H). Fgf15 protein levels were also undetectable in both Asbt-KO and WT mice treated with antibiotics (Fig. 3I). In the proximal intestine, antibiotic treatment increased the expression of Fgf15 and Shp in WT mice, whereas their expression remained unaffected in Asbt-KO mice (Fig. 3J). These results demonstrate that the bacterial flora mediate transcriptional changes in enterocyte Asbt, which in turn govern BA reabsorption.
Effects of antibiotic therapy in Gata4-iKO mice
It is not known how intestinal bacteria might influence the expression of Asbt. The major regulator of intestinal Asbt expression is the transcription factor Gata4, which is expressed throughout the small intestine apart from in the terminal ileum [18]. Gata4 inhibits the expression of Asbt, and therefore restricts Asbt expression to the terminal ileum [19,20].
Recently, it has been shown that intestinal bacteria influence the expression of genes regulated by Gata4 [21]. We therefore used intestinal-specific Gata4 knock-out (Gata4-iKO) mice to determine the role of Gata4 in BA reabsorption in response to changes in the intestinal microbiota. Compared with WT mice, untreated Gata4-iKO mice had higher expression levels of Asbt in proximal parts of the intestine (Fig. 4A), in line with previous studies [7,8,18,22]. These higher Asbt expression levels were accompanied by lower fecal BA excretion; absolute values were similar to the levels of fecal BA excretion seen in antibiotic-treated WT mice (Fig. 4B). Bile flow and biliary BA secretion were higher in Gata4-iKO mice than in WT mice (Fig. 4C/D), in accordance with the enhanced BA reabsorption observed in Gata4-iKO mice.
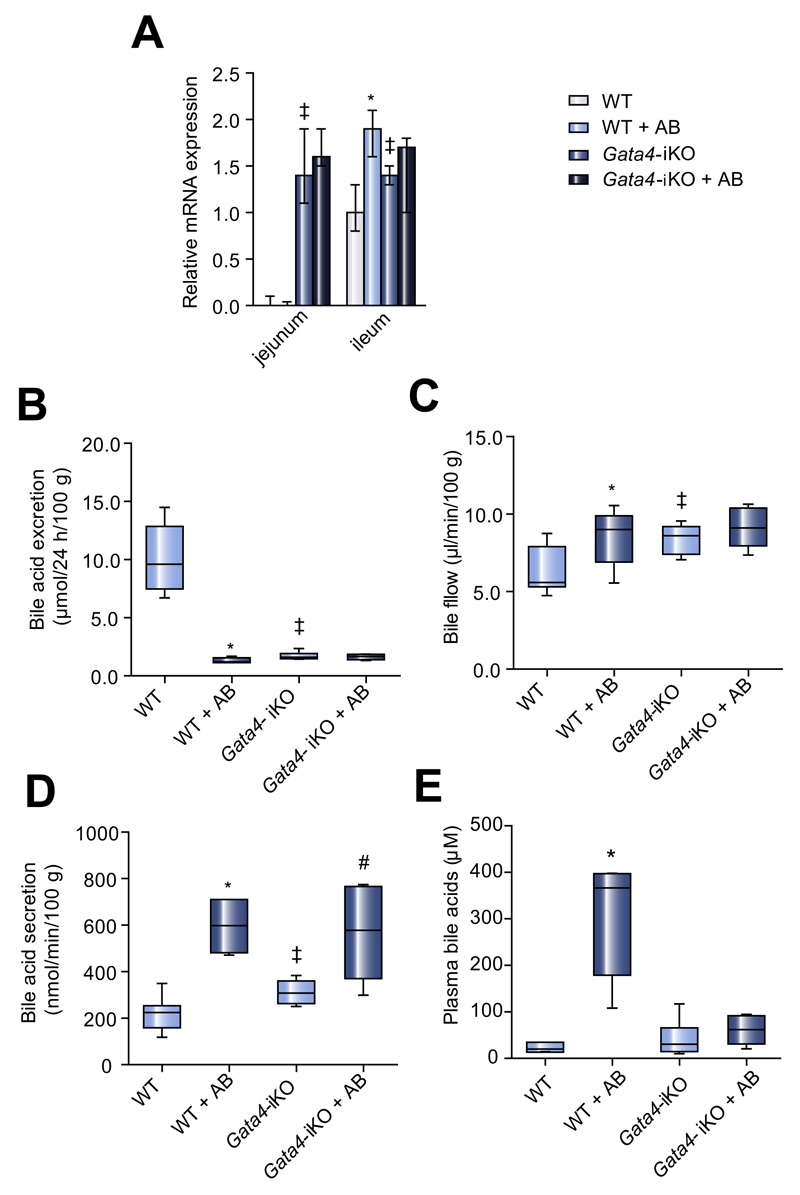
Fig. 4. Bile acid homeostasis in Gata4-iKO mice upon antibiotic treatment.
(A) Relative mRNA expression of Asbt in different segments of the small intestine from wildtype and Gata4-iKO mice. (B) Fecal excretion of BAs (μmol/24 h/100 g bodyweight). (C) Bile flow (μl/min/100 g bodyweight). (D) Biliary BA secretion (nmol/min/100 g bodyweight) in wildtype and Gata4-iKO mice. (E) Total plasma BA levels (μM). Median ± range; n = 6–7/group; *p <0.05 WT vs. WT + AB; ‡p <0.05 WT vs. Gata4-iKO; #p <0.05 Gata4-iKO vs. Gata4-iKO + AB.
Next, Gata4-iKO mice were treated with antibiotics. Antibiotic treatment influenced neither Asbt expression nor fecal BA excretion in Gata4-iKO mice (Fig. 4A/B). Bile flow and plasma BA levels were also unaffected by antibiotic treatment in Gata4-iKO mice (Fig. 4C/E). However, biliary BA secretion was induced by antibiotic treatment in Gata4-iKO mice (Fig. 4D). When we looked at individual BAs, we found that this increase was mainly due to increased biliary secretion of β-MCA (Supplementary Fig. 3). The induction of β-MCA secretion upon antibiotic treatment was 4.5-fold in WT mice and 1.6-fold in Gata4-iKO mice; some of the β-MCA detected in Gata4-iKO mice was present in the form of Δ22-β-MCA. Antibiotic treatment of WT mice greatly increased the biliary secretion of CA, which was unchanged in antibiotic-treated Gata4-iKO mice.
Biliary cholesterol and phospholipid secretion were higher in Gata4-iKO mice than in wildtype animals, but were not induced upon antibiotic treatment (data not shown). Taken together, most of the effects seen in WT mice treated with antibiotics were absent in Gata4-iKO mice.
Discussion
The regulation of BA homeostasis in mammals is a complex process regulated via extensive cross-talk between liver, intestine and intestinal microbiota. The role of bacterial metabolism was noted back in the early 1980s, when several studies reported that BA kinetics change considerably in germfree rats [9–12,16,23]. After a long standstill, interest in the underlying regulatory mechanisms has seen a revival, especially over the past three years.
In this study we have shown for the first time that under physiological conditions gut microbiota inhibit enterohepatic recycling of BAs. One hypothesis regarding the effect of gut microbiota on BA kinetics involves interference with the activation of the BA receptor Fxr. Sayin et al. recently argued that the shift in BA composition to tauro-β-muricholate antagonizes intestinal Fxr, leading to a decrease in ileal Fgf15 expression and an increase in BA synthesis [24]. Hu et al. have also recently postulated that α and β-muricholate antagonize Fxr [25]. However, when Miyata et al. treated Fxr knock-out mice with ampicillin, they reported increased Asbt expression, decreased fecal BA excretion, increased levels of portal BAs and decreased Fgf15 expression [13,14], supporting an Fxr-independent pathway for the changes in BA metabolism seen upon antibiotic treatment.
In the present study we investigated the effects of gut microbiota on BA physiology in more detail. Our data confirm that fecal BA excretion is decreased considerably in germfree animals and antibiotic-treated mice. We speculate that induction of Asbt expression throughout the ileum and expression in more proximal parts of the intestine, contribute to more efficient absorption of BAs from the intestinal lumen, resulting in elevated plasma BA levels, increased biliary BA secretion and decreased fecal BA excretion.
The fact that we observed increased hepatic expression of Cyp7a1 following antibiotic treatment suggested an increase in BA synthesis. However, the data presented in our study, as well as data presented by Miyata et al. and Sayin et al., show decreased fecal BA excretion in antibiotic-treated or germfree mice, indicating a decrease in BA synthesis. Both studies also speculated that intestinal BA reabsorption may be enhanced, contributing to the enhanced total BA pool size [14,24]. Other studies in germfree animals have also reported increases in the uptake of TCA in the ileal epithelium [26] and in the half-life of 14C-labeled tauro-cholic acid [12], indicating enhanced BA reabsorption and decreased turnover. The “classic view” on BA homeostasis implies that the BA pool size is maintained by BA synthesis, which, under steady state conditions, compensates for fecal BA loss. Therefore, despite increased hepatic Cyp7a1 expression, the strongly decreased fecal BA excretion rate in the face of increased intestinal Asbt expression, biliary BA secretion and circulating pool, command the conclusion that antibiotic treatment induces more effective intestinal BA conservation, instead of inducing BA synthesis (Supplementary Fig. 4). When we performed cholate kinetic studies in antibiotic-treated mice to validate the direct relationship between fecal excretion and BA synthesis once more, we saw a decrease in cholate synthesis, confirming that antibiotic treatment inhibited BA synthesis. One might question whether animals treated for five days with antibiotics are in a steady state, however germfree mice certainly are.
The homeostasis of cholesterol, the substrate for BA synthesis, has also been studied. In germfree animals hepatic cholesterol synthesis from labeled acetate is only 13% of that of conventional animals [27]. When germfree rats are injected with cholesterol-26-14C they expire 50% less 14C as 14CO2 than do conventional rats, with higher specific activities in plasma and liver [28]. Also, 7α-hydroxylation of [4-14C]-cholesterol and 5α-reduction and 12α-hydroxylation of the BA precursor 7α-hydroxy-4-cholesten-3-one are lower in germfree rats [29,30]. These findings are in accordance with germfree animals having slower cholesterol turnover than conventional animals. Although at the time no mechanism was known for the differences observed between germfree and conventional animals, such valuable data should not be forgotten.
Clearly, in the antibiotic-treated and germfree animals there is a discrepancy between CYP7A1 mRNA expression and actual in vivo BA synthesis. If synthesis was in fact increased in these mice, along with enhanced intestinal BA reabsorption and decreased fecal excretion, then the BA pool would also continuously expand because of a lack of balance between synthesis and disposal. Previous studies on BA synthesis under different conditions have shown that altered synthesis of BAs is not always correlated with changes in Cyp7a1 mRNA expression [31–33]. In a recent paper on Apobec-1, an enzyme involved in the production of apolipoprotein B48, Cyp7a1 mRNA expression was decreased by approximately 75% in Apobec-1−/− mice, while no difference in fecal BA excretion was found [34]. The authors suggested that post-transcriptional regulation of Cyp7a1 expression and alteration in Cyp7a1 mRNA stability accounted for the changes observed in Apobec-1−/− mice. These findings underscore the importance of physiological measurements, along with data on gene expression, to properly study the enterohepatic circulation of BAs in vivo.
Thus, increased intestinal BA reabsorption due to enhanced Asbt expression leads to a compensatory decrease in BA synthesis. The question then arises how the microbiota might regulate Asbt expression. Previously, Annaba et al. suggested a link between certain intestinal bacterial species and regulation of Asbt [17]. Post-transcriptional regulation of Asbt has also been suggested [35]. Our data confirm the importance of Asbt for the microbiota-induced alterations in BA homeostasis. The fact that a small effect remained in Asbt-KO mice treated with antibiotics could be related to the massive flow of BAs into the colon in the absence of Asbt, and hence increased uptake of these BAs across the colonocytes. This Asbt-independent BA absorption was also seen in untreated Asbt-KO mice, reflected in the higher amounts of circulating secondary BAs in these mice, DCA in particular. However, we cannot exclude the fact that DCA may also be taken up by the small intestine after coprophagic recycling of the excreted BAs. Taken together, Asbt is important in the interaction between the bacterial flora and BA reabsorption.
Asbt expression is thought to be primarily controlled by the transcription factor Gata4, which plays an important role in maintaining jejunal-ileal differences in absorptive enterocyte gene expression and restricts expression of Asbt to the terminal ileum [7,18,20,22]. Studies in mice have shown that intestinal Gata4 deletion induces Asbt expression and BA absorption in the proximal small intestine, leading to tauro-β-muricholate enrichment of the BA pool [7]. This phenotype shows a striking resemblance to that seen in our experiments with antibiotic treatment and it should also be noted that others have previously linked enterocyte Gata4 to the commensal flora [21]. We now show that antibiotic treatment in Gata4-iKO mice failed to elicit the changes on Asbt expression, fecal BA excretion, bile flow rate and plasma BA concentrations that were observed in WT mice. Therefore, we postulate that under physiological conditions gut microbiota stimulate Gata4-dependent Asbt repression throughout the intestine except in the terminal ileum, since Gata4 is not expressed here, thereby restricting BA reabsorption to this part of the small intestine. The exact mechanism by which intestinal bacteria regulate Gata4 remains to be elucidated.
Shulzhenko et al. recently proposed a molecular switch between Gata4-dependent metabolic and interferon-dependent immune processes within epithelial cells, influenced by gut microbiota [21]. Munakata et al. found that the absence of gut microbiota induces the expression of several interferon regulatory factors, as well as IFN-α-inducible genes and IFN receptors. The authors suggested that the regulation of this small subset of genes reflects an adaptive response of the immune system to prevent excessive inflammation during continuous exposure to commensal bacteria [36]. In line with these studies, the lack of bacteria-derived signals in germfree animals or following antibiotic treatment may change anti-inflammatory responses in these animals. This may shift the balance of enterocyte metabolism at the expense of Gata4-dependent metabolic functions, ultimately leading to diminished inhibition of Asbt expression and increased BA reabsorption.
We cannot exclude that the induction of Asbt in the ileum is at least in part Gata4-independent since Gata4 expression is low in the last part of the ileum. Increased ileal Asbt expression may be a compensatory effect to decreased import of bile acids and consequently decreased Fxr activation in this part of the intestine. However, it could also be caused by the increased concentration of the FXR antagonist tauro-β-muricholate [24]. Decreased Fxr activation results in decreased expression of Shp and Fgf15. Both are able to repress Asbt expression, their decrease may therefore lead to derepression of Asbt expression and hence increased Asbt expression in a situation where the distal intestine senses less BA than normal.
Since BA and cholesterol metabolism are tightly coupled, more efficient recirculation of BAs could result in slower rates of cholesterol catabolism for de novo BA synthesis. In fact, germ-free rats are known to accumulate more cholesterol than their conventional counterparts [10]. Long-term antibiotic treatment may therefore have negative effects on plasma cholesterol levels, which are a major risk factor for cardiovascular diseases. A further possible implication of our findings is that it may be possible to induce Asbt expression in the proximal small intestine by interventions that promote specific intestinal bacteria. Such interventions may be useful in the development of therapies for patients with BA malabsorption and associated maldigestion of fat due to ileal disease or resection.
Taken together, we demonstrate that antibiotic treatment induced a strong increase in the expression of Asbt in the ileum and a shift in expression in the direction of the jejunum. We saw an increase in enterohepatic cycling of BAs, resulting in decreased fecal BA excretion, higher levels of tauro-β-muricholate and taurocholate in the BA pool, and increased biliary BA secretion. As a consequence, hepatic BA synthesis is reduced. Regulation of Asbt expression by Gata4 is crucial in mediating the effects that intestinal microbiota exert on host BA reabsorption.
Supplementary Data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jhep.2015.04.030.
Acknowledgement
We thank Vincent Bloks for critically reading and editing the manuscript.
Financial support
Junior Scientific Masterclass Groningen and the Austrian Science Fund (P22832, DK-MCD W1226 and SFB F30).
Abbreviations
- BA
bile acid
- Asbt
apical sodium-dependent bile acid transporter
- KO
knock-out
- iKO
intestinal-specific knock-out
- WT
wildtype
- CA
cholic acid
- CDCA
chenodeoxycholic acid
- MCA
muricholic acid
- UDCA
urodeoxycholic acid
Footnotes
Conflict of interest
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Authors’ contributions
Study concept and design: CO, HJV, AKG.
Acquisition of data: CO, JVP, MB.
Analysis and interpretation of data: CO, JVP, TvD.
Drafting of the manuscript: CO, AKG.
Critical revision of the manuscript for important intellectual
content: all authors.
Statistical analysis: CO.
Obtained funding and study supervision: FK, HJV, AKG
Technical or material support: RH, HW, TB, SdB, RB, AS, AB.
References
- [1].Ley RE, Lozupone CA, Hamady M, et al. Worlds within worlds: evolution of the vertebrate gut microbiota. Nat Rev Microbiol. 2008;6:776–788. doi: 10.1038/nrmicro1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kitahara M, Takamine F, Imamura T, et al. Clostridium hiranonis sp. nov., a human intestinal bacterium with bile acid 7alpha-dehydroxylating activity. Int J Syst Evol Microbiol. 2001;51:39–44. doi: 10.1099/00207713-51-1-39. [DOI] [PubMed] [Google Scholar]
- [3].Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- [4].Batta AK, Salen G, Arora R, et al. Side chain conjugation prevents bacterial 7-dehydroxylation of bile acids. J Biol Chem. 1990;265:10925–10928. [PubMed] [Google Scholar]
- [5].Hagenbuch B, Dawson P. The sodium bile salt cotransport family SLC10. Pflugers Arch. 2004;447:566–570. doi: 10.1007/s00424-003-1130-z. [DOI] [PubMed] [Google Scholar]
- [6].Dawson PA, Haywood J, Craddock AL, et al. Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J Biol Chem. 2003;278:33920–33927. doi: 10.1074/jbc.M306370200. [DOI] [PubMed] [Google Scholar]
- [7].Beuling E, Kerkhof IM, Nicksa GA, et al. Conditional Gata4 deletion in mice induces bile acid absorption in the proximal small intestine. Gut. 2010;59:888–895. doi: 10.1136/gut.2009.204990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Patankar JV, Chandak PG, Obrowsky S, et al. Loss of intestinal GATA4 prevents diet-induced obesity and promotes insulin sensitivity in mice. Am J Physiol Endocrinol Metab. 2011;300:E478–E488. doi: 10.1152/ajpendo.00457.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Madsen D, Beaver M, Chang L, et al. Analysis of bile acids in conventional and germfree rats. J Lipid Res. 1976;17:107–111. [PubMed] [Google Scholar]
- [10].Kellogg TF, Wostmann BS. Fecal neutral steroids and bile acids from germfree rats. J Lipid Res. 1969;10:495–503. [PubMed] [Google Scholar]
- [11].Wostmann BS. The germfree animal in nutritional studies. Annu Rev Nutr. 1981;1:257–279. doi: 10.1146/annurev.nu.01.070181.001353. [DOI] [PubMed] [Google Scholar]
- [12].Gustafsson BE, Bergstrom S, Lindstedt S, et al. Turnover and nature of fecal bile acids in germfree and infected rats fed cholic acid-24-14C; bile acids and steroids 41. Proc Soc Exp Biol Med. 1957;94:467–471. doi: 10.3181/00379727-94-22981. [DOI] [PubMed] [Google Scholar]
- [13].Miyata M, Takamatsu Y, Kuribayashi H, et al. Administration of ampicillin elevates hepatic primary bile acid synthesis through suppression of ileal flbroblast growth factor 15 expression. J Pharmacol Exp Ther. 2009;331:1079–1085. doi: 10.1124/jpet.109.160093. [DOI] [PubMed] [Google Scholar]
- [14].Miyata M, Yamakawa H, Hamatsu M, et al. Enterobacteria modulate intestinal bile acid transport and homeostasis through apical sodium-dependent bile acid transporter (SLC10A2) expression. J Pharmacol Exp Ther. 2011;336:188–196. doi: 10.1124/jpet.110.171736. [DOI] [PubMed] [Google Scholar]
- [15].Chawla RK, Hersh T, Labme DW, Jr, et al. Effect of antibiotics on growth of the immature rat. J Nutr. 1976;106:1737–1746. doi: 10.1093/jn/106.12.1737. [DOI] [PubMed] [Google Scholar]
- [16].Kellogg TF, Knight PL, Wostmann BS. Effect of bile acid deconjugation on the fecal excretion of steroids. J Lipid Res. 1970;11:362–366. [PubMed] [Google Scholar]
- [17].Annaba F, Sarwar Z, Gill RK, et al. Enteropathogenic Escherichia coli inhibits ileal sodium-dependent bile acid transporter ASBT. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1216–G1222. doi: 10.1152/ajpgi.00017.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bosse T, Piaseckyj CM, Burghard E, et al. Gata4 is essential for the maintenance of jejunal-ileal identities in the adult mouse small intestine. Mol Cell Biol. 2006;26:9060–9070. doi: 10.1128/MCB.00124-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Beuling E, Bosse T, aan de Kerk DJ, et al. GATA4 mediates gene repression in the mature mouse small intestine through interactions with friend of GATA (FOG) cofactors. Dev Biol. 2008;322:179–189. doi: 10.1016/j.ydbio.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Beuling E, Baffour-Awuah NY, Stapleton KA, et al. GATA factors regulate proliferation, differentiation, and gene expression in small intestine of mature mice. Gastroenterology. 2011;140:1219–1229. e1–e2. doi: 10.1053/j.gastro.2011.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shulzhenko N, Morgun A, Hsiao W, et al. Crosstalk between B lymphocytes, microbiota and the intestinal epithelium governs immunity versus metabolism in the gut. Nat Med. 2011;17:1585–1593. doi: 10.1038/nm.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Battle MA, Bondow BJ, Iverson MA, et al. GATA4 is essential for jejunal function in mice. Gastroenterology. 2008;135:e1. doi: 10.1053/j.gastro.2008.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wostmann BS. Intestinal bile acids and cholesterol absorption in the germfree rat. J Nutr. 1973;103:982–990. doi: 10.1093/jn/103.7.982. [DOI] [PubMed] [Google Scholar]
- [24].Sayin SI, Wahlstrom A, Felin J, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- [25].Hu X, Bonde Y, Eggertsen G, et al. Muricholic bile acids are potent regulators of bile acid synthesis via a positive feedback mechanism. J Intern Med. 2014;275:27–38. doi: 10.1111/joim.12140. [DOI] [PubMed] [Google Scholar]
- [26].Riottot M, Sacquet E. Increase in the ileal absorption rate of sodium taurocholate in germ-free or conventional rats given an amylomaize-starch diet. Br J Nutr. 1985;53:307–310. doi: 10.1079/bjn19850038. [DOI] [PubMed] [Google Scholar]
- [27].Ukai M, Tomura A, Ito M. Cholesterol synthesis in germfree and conventional rats. J Nutr. 1976;106:1175–1183. doi: 10.1093/jn/106.8.1175. [DOI] [PubMed] [Google Scholar]
- [28].Wostmann BS, Wiech NL, Kung E. Catabolism and elimination of cholesterol in germfree rats. J Lipid Res. 1966;7:77–82. [PubMed] [Google Scholar]
- [29].Gustafsson BE, Einarsson K, Gustafsson J. Influence of cholesterol feeding on liver microsomal metabolism of steroids and bile acids in conventional and germ-free rats. J Biol Chem. 1975;250:8496–8502. [PubMed] [Google Scholar]
- [30].Einarsson K, Gustafsson JA, Gustafsson BE. Differences between germ-free and conventional rats in liver microsomal metabolism of steroids. J Biol Chem. 1973;248:3623–3630. [PubMed] [Google Scholar]
- [31].Hulzebos CV, Wolters H, Plosch T, et al. Cyclosporin a and enterohepatic circulation of bile salts in rats: decreased cholate synthesis but increased intestinal reabsorption. J Pharmacol Exp Ther. 2003;304:356–363. doi: 10.1124/jpet.102.041640. [DOI] [PubMed] [Google Scholar]
- [32].Liu Y, Havinga R, van der Leij FR, et al. Dexamethasone exposure of neonatal rats modulates biliary lipid secretion and hepatic expression of genes controlling bile acid metabolism in adulthood without interfering with primary bile acid kinetics. Pediatr Res. 2008;63:375–381. doi: 10.1203/PDR.0b013e318165b8af. [DOI] [PubMed] [Google Scholar]
- [33].Lukovac S, Los EL, Stellaard F, et al. Effects of essential fatty acid deficiency on enterohepatic circulation of bile salts in mice. Am J Physiol Gastrointest Liver Physiol. 2009;297:G520–G531. doi: 10.1152/ajpgi.00091.2009. [DOI] [PubMed] [Google Scholar]
- [34].Xie Y, Blanc V, Kerr TA, et al. Decreased expression of cholesterol 7alpha-hydroxylase and altered bile acid metabolism in Apobec-1-/- mice lead to increased gallstone susceptibility. J Biol Chem. 2009;284:16860–16871. doi: 10.1074/jbc.M109.010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Miyata M, Yamakawa H, Hayashi K, et al. Ileal apical sodium-dependent bile acid transporter protein levels are down-regulated through ubiquitin-dependent protein degradation induced by bile acids. Eur J Pharmacol. 2013;714:507–514. doi: 10.1016/j.ejphar.2013.06.036. [DOI] [PubMed] [Google Scholar]
- [36].Munakata K, Yamamoto M, Anjiki N, et al. Importance of the interferon-alpha system in murine large intestine indicated by microarray analysis of commensal bacteria-induced immunological changes. BMC Genomics. 2008;9:192. doi: 10.1186/1471-2164-9-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.