INTRODUCTION
Eosinophilia represents an increased number of eosinophils in the tissues and/or blood. Although enumeration of tissue eosinophil numbers would require examination of biopsied tissues, blood eosinophil numbers are more readily and routinely measured. Hence, eosinophilia is often recognized based on an elevation of eosinophils in the blood. Absolute eosinophil counts exceeding 450 to 550 cells/µL, depending on laboratory standards, are reported as elevated. Percentages generally above 5% of the differential are regarded as elevated in most institutions, although the absolute count should be calculated before a determination of eosinophilia is made. This is done by multiplying the total white cell count by the percentage of eosinophils.
Eosinophils are bone marrow–derived cells of the granulocyte lineage. They have an approximate half-life of 8 to 18 hours in the bloodstream, and mostly reside in tissues1 where they can persist for at least several weeks. Their functional roles are multifaceted and include antigen presentation; the release of lipid-derived, peptide, and cytokine mediators for acute and chronic inflammation; responses to helminth and parasite clearance through degranulation; and ongoing homeostatic immune responses. They can be part of the overall cellular milieu in malignant neoplasms and autoimmune conditions, and connective tissue disorders, and are also found in less well characterized entities as described elsewhere in this paper.
The approach to eosinophilia is largely based on clinical history. Often, a few aspects of a case alert the clinician as to the likely underlying cause of abnormally elevated eosinophils. However, at times, more significant investigations need to occur to more clearly define the cause of their presence and possible role in disease presentation.
Eosinophilia → 450 to 550 cells/µL in the blood stream
Allergic Sensitization
Mild eosinophilia is present often in patients with allergic disease (<1500 cells/µL will be used for the definition of mild, whereas hypereosinophilic syndromes, defined elsewhere in the article, are generally considered with sustained eosinophilia > 1500 cells/µL2). Allergic rhinitis and asthma often produce a mild eosinophilia. Atopic dermatitis may produce a more significant eosinophilia if affecting a large part of the body and if associated with significant atopy. Eosinophilic esophagitis as well as other eosinophilic gastrointestinal diseases can cause a mild peripheral eosinophilia.
Chronic sinusitis, especially of the polypoid variety seen in aspirin-exacerbated respiratory disease, produces a more robust eosinophilic response that can be in the mild to moderate range. Often these patients start with nasal allergies and asthma, but then develop abnormal arachidonic acid metabolizing cascades and hence have a more dramatic presentation both of their disease entity and of the eosinophilia.3,4
Allergic bronchopulmonary aspergillosis, related both to a fungus (Aspergillus) and to sensitization in an allergic/asthmatic host, can also produce varied and sometimes significant degrees of eosinophilia and also elevated total immunoglobulin (Ig)E.5
Chronic eosinophilic pneumonia often starts in a sensitized, asthmatic host. Although these patients may have milder peripheral eosinophilia at disease onset, they often have more moderate range eosinophilia later in the course. They also have bronchoalveolar lavage fluid that contains at least 40% eosinophils in up to 80% of cases.6 This form of eosinophilic pneumonia can be premonitory to the later development of the eosinophilic vasculitis, eosinophilic granulomatosis with polyangiitis (EGPA), previously known as Churg-Strauss vasculitis.
Drug allergy can cause anywhere from mild to severe eosinophilia and often waxes quickly and wanes in a slower fashion; it can take months for eosinophilia from drug allergy to clear. There is usually, although not always, an associated drug rash of the diffuse/maculopapular variety. Patients can also present with asymptomatic eosinophilia owing to drugs, especially penicillins, cephalosporins, or quinolones.7 Pulmonary infiltrates and peripheral eosinophilia have been associated with varied medications, including nonsteroidal antiinflammatory drugs, sulfa drugs, and nitrofurantoin.7 Drug-induced diseases of other organs can also elicit tissue and blood eosinophilia (eg, drug-induced interstitial nephritis). Box 1 summarizes causes of allergen-induced eosinophilia.
Box 1: Causes of allergy associated eosinophilia.
Mild degree of eosinophilia
Allergic rhinitis
Asthma
Atopic dermatitis
Eosinophilic esophagitis
Drug allergy
Moderate to severe degree of eosinophilia
Chronic sinusitis (especially polypoid and aspirin-exacerbated respiratory disease)
Allergic bronchopulmonary aspergillosis
Chronic eosinophilic pneumonia
Drug allergy (drug rash with eosinophilia and systemic symptoms [DRESS] syndrome)
The drug rash with eosinophilia and systemic symptoms (DRESS) syndrome often produces significant eosinophil elevations in addition to liver function abnormalities, temperature dysregulation and lymphadenopathy. In reviews of 2 large, hospital-based cohorts of monitored drug allergies in Brazil and Malaysia, the DRESS syndrome was caused mainly by antibiotics, antiepileptics, antigout regimens, antiretrovirals, and nonsteroidal antiinflammatory drugs.8,9 Stevens-Johnson syndrome and toxic epidermal necrolysis, severe and life-threating forms of drug allergy, do not usually produce eosinophilia, but rather neutrophilia and lymphocytopenia. Box 2 lists pharmaceuticals commonly implicated in DRESS syndrome.
Box 2: Commonly implicated pharmaceuticals in drug rash with eosinophilia and systemic symptoms (DRESS) syndrome.
Antibiotics: Penicillins, cephalosporins, dapsone, sulfa-based antibiotics
Xanthine oxidase inhibitor: Allopurinol
Antiepileptics: Carbamazepine, phenytoin, lamotrigine, valproic acid
Antiretrovirals: Nevirapine, efavirenz
Nonsteroidal antiinflammatory drugs: Ibuprofen
Parasite- and Infection-Related Eosinophilia
Tissue-dwelling helminths (“worms”) are parasitic infections that often produce mild to moderate eosinophilia. Strongyloides infection is a common cause, whereas Giardia, a luminal parasite, does not cause eosinophilia. See Box 3 for parasites that cause eosinophilia.
Box 3: Select parasitic infections that cause eosinophilia.
Helminthic infections
Nematodes
Angiostrongyliasis costaricensis
Ascariasis
Hookworm infection
Strongyloidiasis
Trichinellosis
Visceral larva migrans
Gnathostomiasis
Cysticercosis
Echinococcosis
Filariases
Tropical pulmonary eosinophilia
Loiasis
Onchocerciasis
Flukes
Schistosomiasis
Fascioliasis
Clonorchiasis
Paragonimiasis
Fasciolopsiasis
Protozoan infections
Isospora belli
Dientamoeba fragilis
Sarcocystis
In the United States, parasites that can be contracted without any travel to foreign countries include strongyloides, trichinella, ascaris, hookworm, and visceral larva migrans (Toxocara from dogs/cats). In evaluating a patient for a possible helminthic etiology of the eosinophilia, testing for Strongyloides must be done because, unique among helminths, it can persist even decades after initial infection. Strongyloides can be asymptomatic or cause fleeting hives, dermatographism, angioedema, and/or abdominal pain. If a patient with undiagnosed Strongyloides infection receives high-dose systemic corticosteroids, disseminated strongyloidiasis can ensue. This is sometimes associated with enteric bacterial sepsis and can be fatal.10 The enzymelinked immunosorbent antibody assay is a sensitive test for Strongyloides. Stool examinations for larvae are insensitive. Serologic or other evidence of ongoing strongyloidiasis merits ivermectin treatment, especially in an eosinophilic subject who may eventually be treated with corticosteroids.
Some fungi such as coccidioidomycosis (both acute and chronic), disseminated histoplasmosis (less commonly eosinophilic), and cryptococcosis (especially with central nervous system infections) also have been associated with eosinophilia. Coccidioides is found in the Southwestern United States as well as Mexico, and Central and South Americas. Histoplasma contaminates bird and bat droppings, so cave exploring and living in areas with heavy pigeon populations can lead to significant exposures. Cryptococcus is found in soil throughout the world. These infections are often more significant and disseminated in immunocompromised hosts.
The retrovirus, human T-cell lymphocytic virus-1, is one of the few viruses associated with eosinophilia. It is prevalent in Japan, Africa, the Caribbean Islands, and South America, and newly found in Eastern Europe and the Middle East.11 Human immunodeficiency virus-1–infected patients may have eosinophilia not directly related to the infection but from secondary phenomena (drug allergy, eosinophilic folliculitis12).
In contrast with the “rule” that only helminthic parasites may elicit eosinophilia, there are 3 protozoa that can cause peripheral eosinophilia. Isospora belli and Dientamoeba fragilis,13 both intestinal protozoa, and Sarcocystitis, a rare tissue-dwelling protozoa, are found only in those with exposures in areas of Southeast Asia.
Autoimmune Disease
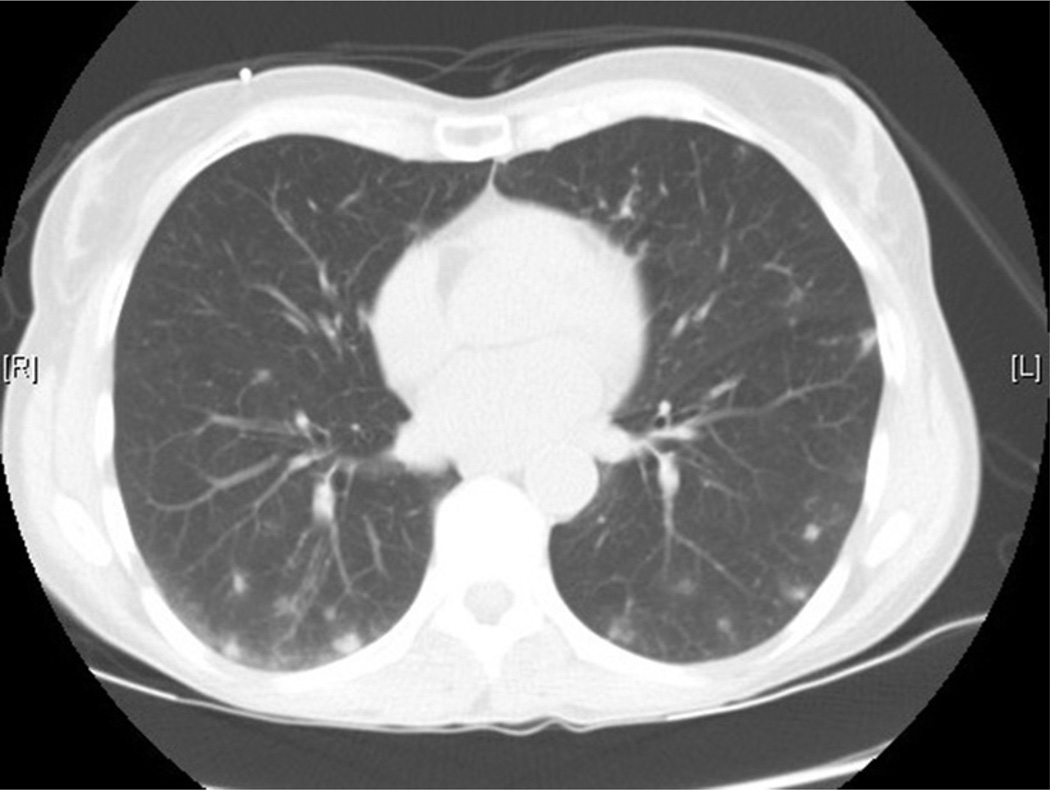
EGPA, previously known as Churg-Strauss syndrome, arises usually in an atopic individual and produces varying degrees of sinus disease, lung disease, and kidney disease and can cause mononeuritis multiplex and vascular disease as well. EGPA is associated with significant eosinophilia, increased inflammatory markers, and tissue eosinophilia in affected areas. It carries in its differential diagnosis and shares many aspects with hypereosinophilic syndromes (discussed elsewhere in this article). The vasculitis is often identified late in the course of the disease, especially if the kidney is not involved. EGPA with eosinophilia and predominantly nonhemorrhagic lung manifestations has a significantly lower prevalence of antineutrophil cytoplasmic antibody positivity (perinuclear antineutrophil cytoplasmic antibody) than EGPA with renal involvement, so antineutrophil cytoplasmic antibody tests do not exclude EGPA disease.14 EGPA produces both eosinophilia and symptoms out of proportion to usual allergic complications, with sinus, lung, and other organ involvement. In the initial evaluation of patients with possible EGPA, the latest consensus recommendations from 2015 suggest serologic testing for toxocariasis and human immunodeficiency virus, specific IgE and IgG for Aspergillus spp., search for Aspergillus spp. in sputum and/or bronchoalveolar lavage fluid (to evaluate for allergic bronchopulmonary aspergillosis), tryptase, and vitamin B12 levels (to evaluate for myeloproliferative hypereosinophilic syndrome), peripheral blood smear (looking for dysplastic eosinophils or blasts suggestive of primary eosinophilic bone marrow process), and chest computed tomography scan (to evaluate for lung involvement). Additional workup should be guided by presentation. Referral for more specific evaluation by a qualified rheumatologist or immunologist at a center that treats patients with vasculitis is recommended given the elusive nature of EGPA as well as clinical implications if it is undertreated.15 Fig. 1 is a characteristic computed tomography scan of the chest done to evaluate a patient with EGPA treated at our institution.
Fig. 1.
Computed tomography (CT) scan of the chest of a patient with eosinophilic granulomatosis with polyangiitis. Areas of diffuse ground glass opacities and consolidations in both right and left lungs. This finding is characteristic of how small-vessel vasculitides affect the lungs. (Patient of Anna Kovalszki, MD; CT scan taken at Beth Israel Deaconess Medical Center, Boston, MA.)
Connective tissue/autoimmune diseases to a variable extent can be associated with peripheral eosinophilia. Eosinophils are present as part of the overall inflammatory milieu, even if they are not the cell types implicated in disease pathogenesis necessarily. The degree of eosinophilia is not well-established in these cases. For example, in a retrospective analysis, mild degrees of eosinophilia were felt to be related to certain rheumatic disorders, but the authors felt that treatment with nonsteroidal antiinflammatory drugs and corticosteroids confounded the frequency of eosinophilia found.16 In a case report of severe rheumatoid arthritis, up to 24,000 eosinophils/mm3 were seen.17
In inflammatory bowel diseases, blood eosinophil levels can be elevated, yet the roles of eosinophils in gastrointestinal diseases are still being elucidated. For instance, levels of the eosinophil-recruiting chemokine, eotaxin-1, in the tissues were correlated with tissue eosinophilia of ulcerative colitis patients in the active disease state.18 In sarcoidosis, mild peripheral eosinophilia was common, even without other atopic disease present in a retrospective cohort of sarcoid patients.19 In IgG4-related disease, a relatively newly recognized entity known to produce tissue infiltration and tumorlike destruction of certain glands, organs, and lymph nodes, blood eosinophilia can be present in about 25% and both IgE elevations and mild to moderate blood eosinophilia occurred in an atopy-independent fashion.20
Often with these disorders (listed in Box 4), an exhaustive search is usually done if the eosinophilia is persistent and marked. Even though these disorders can be associated with eosinophilia to some degree, often patients are on many medications and more common reasons for eosinophilia such as drug allergy need to be ruled out.
Box 4: Eosinophilia associated with connective tissue, rheumatologic, and autoimmune disease.
Eosinophilic fasciitis
Eosinophilic granulomatosis with polyangiitis (Churg-Strauss vasculitis)
Dermatomyositis
Severe rheumatoid arthritis
Progressive systemic sclerosis
Sjögren syndrome
Thromboangiitis obliterans with eosinophilia of the temporal arteries
Granulomatosis with polyangiitis (Wegener syndrome)
Systemic lupus erythematosus
Behçet syndrome
IgG4-related disease
Inflammatory bowel disease
Sarcoidosis
Bullous pemphigoid
Dermatitis herpetiformis (celiac disease)
Primary Eosinophilia
Hypereosinophilic syndromes (idiopathic, myeloproliferative variant, and lymphocytic variant) are a group of disorders in which eosinophilia is always present and can be moderate to severe. The underlying cause can have a defined etiology or be completely idiopathic. Affected organs can include lungs, skin, heart, blood vessels, sinuses, kidneys, and brain. Patients with idiopathic disease can present asymptomatically or with ongoing fatigue, myalgias, weakness, and general malaise. Workup can be unrevealing except for the eosinophilia, which may be recalcitrant even to corticosteroid treatment.
Often, patients with a lymphocytic variant have diffuse skin manifestations (pruritus, ongoing erythema, rash). They often have a CD3− CD4+ population of T cells and/or abnormal T-cell receptor clonality. They are at risk for developing T-cell lymphomas. They can be especially difficult to manage with ongoing monitoring for neoplasm development and ongoing need for oral corticosteroids (with response) and other disease-modifying drugs.21
Patients with a myeloproliferative variant often have heart disease or thrombotic complications (endomyocardial inflammation and stroke), splenomegaly, elevated tryptase level, elevated lactate dehydrogenase, and increased vitamin B12 level. They can have FIP1L1-PDGFRA and other related chromosomal mutations, and are responsive to imatinib. Imatinib can be useful in some who lack the FIP1L1-PDGFRA mutation if they have myeloproliferative features.22
Episodic angioedema associated with eosinophilia (Gleich syndrome23) is associated with marked episodic eosinophilia, angioedema, urticaria, pruritus, fever, weight gain, and often an elevated IgM level. These patients have an underlying immune dyscrasia driving their presentation, with an elevation in eosinophils associated with antecedent increases in cytokine interleukin-5 levels. Many also have an aberrant CD3− CD4+ T-cell population and T-cell receptor clonality as well.24
In 25% of patients with mastocytosis, there is an associated eosinophilia, usually in the mild to moderate range. There is thought to be significant cross-talk between mast cells and eosinophils.25 Most patients have a mutation (D816V) in the c-KIT gene. Systemic mastocytosis patients with D816VKIT mutations have concurrently presented with FIP1L1-PDGFRA mutations and chronic eosinophilic leukemia, although this is a rare event. Bone marrow biopsy in mastocytosis and chronic eosinophilic leukemia exhibit some common pathologic features, such as CD25 expression on mast cells and spindle shaped mast cell morphology.26
Box 5 summarizes clinical entities associated with primary eosinophil burden and likely disease-related pathogenesis.
Box 5: Primary eosinophilias.
Idiopathic hypereosinophilic syndrome: sustained peripheral eosinophilia at greater than 1500 cells/µL with associated end-organ damage.
Lymphoproliferative hypereosinophilic syndrome: sustained peripheral eosinophilia at greater than 1500 cell/µL, often associated with rash, aberrant T-cell immunophenotypic profile, often steroid responsive.
Myeloproliferative hypereosinophilic syndrome: sustained peripheral eosinophilia at greater than 1500 cell/µL, often features of splenomegaly, heart related complications, and thrombosis. Can have associated FIP1L1-PDGFRA and other mutations and are often steroid resistant. Patients can be considered to have a diagnosis of chronic eosinophilic leukemia.
Episodic eosinophilia associated with angioedema (G syndrome): cyclical fevers, swelling, hives, pruritus, marked eosinophilia, and IgM elevation. Aberrant T-cell phenotypes often associated.
Malignancy-Related Eosinophilia
There are eosinophil-derived malignancies (acute and chronic eosinophilic leukemia) and malignancies in which eosinophils are increased as part of the overall cellular milieu. Box 6 lists malignancies associated with eosinophilia.
Box 6: Malignancy-associated eosinophilia.
Blood-related neoplasms
Acute or chronic eosinophilic leukemia
Lymphoma (T cell and Hodgkin)
Chronic myelomonocytic leukemia
Solid organ–associated neoplasms
Adenocarcinomas of the gastrointestinal tract (gastric, colorectal)
Lung cancer
Squamous epithelium related cancers (cervix, vagina, penis, skin, nasopharynx, bladder)
Thyroid cancer
Immunodeficiency-Related Eosinophilia
Autosomal-dominant hyper-IgE syndrome (Job syndrome), with recurrent abscesses, lung infections, and severe eczema, is associated with eosinophilia. Wiskott-Aldrich syndrome, with eczema, thrombocytopenia, and recurrent infections in an X-linked fashion, also causes peripheral and tissue eosinophilia. Adenosine deaminase severe combined immunodeficiency also has eosinophilic associations. Atopy is a common feature in this entity in addition to severe immunodeficiency with recurrent infections starting in infancy. Omenn syndrome causes profound peripheral eosinophilia, IgE elevation, and abnormally elevated T cells as well as autoimmunity and an erythrodermic rash. Patients are often discovered in newborn screening and undergo transplantation.27
Miscellaneous Entities Associated with Eosinophilia
Rejection of transplanted solid organs including liver, pancreas, kidney, and heart have been associated with peripheral and organ-specific eosinophilia. The eosinophilia can be moderate to severe.28
Chronic graft-versus-host disease after hematopoietic stem cell transplantation has also caused peripheral eosinophilia. Level of skin involvement and severity of graft-versus-host disease could not be reliably predicted based on the presence of eosinophilia in 1 cohort of patients.29
Kimura disease, a disease of mostly Asian males, is defined as masslike lymph node or subcutaneous tissue swelling mainly in the head and neck, peripheral eosinophilia, IgE elevation, and eosinophilic pathologic infiltrates with follicular hyperplasia and proliferation of postcapillary venules in biopsies. Surgical excision and steroid therapy is often used.30
Epithelioid hemangioma, also known as angiolymphoid hyperplasia with eosinophilia, also most often affects the head and neck, especially on and around the auricles. It is seen in all races and both sexes, affects the dermis or epidermis, and is thought of as a benign vascular proliferative disease. Excision and laser therapy seem to be the most often used treatment modalities, often undertaken for cosmetic reasons.31 Peripheral eosinophilia is variable and IgE elevation is not common.
Historical syndromes caused by toxic ingestions included the eosinophilia–myalgia syndrome and the toxic oil syndrome. Contaminated (impure) l-tryptophan caused the eosinophilia–myalgia syndrome in 1989 in the United States, characterized by myalgias, skin induration, and eosinophilia. This occurred from a single source of l-tryptophan, which has since been banned by the US Food and Drug Administration. Analine-denatured cooking grade oils produced by a specific refinery in Seville, Spain, in 1981 caused the toxic oil syndrome. Symptoms were myalgias, eosinophilia, and pulmonary infiltrates. Both of these episodes caused significant morbidity and mortality. These are historical events, but could in theory occur again with other manufactured ingestible items.32 The specific mechanisms and subtypes of contaminants responsible for the disorders were never fully elucidated.
Eosinophils are sensitive even to endogenous corticosteroid production. Therefore, absence of corticosteroids can induce a peripheral eosinophilia. Such hypoadrenalism may happen in Addison disease or adrenal hemorrhage.
Irritation of serosal surfaces can cause peripheral eosinophilia. Peritoneal dialysis catheters can produce peritoneal eosinophilia, which can in turn be detected peripherally.
Cholesterol embolization, owing to instrumentation or other trauma to the aorta, can cause purplish discoloration of toes, livedo reticularis, renal disease, elevated inflammatory markers, and hypocomplementemia, along with mild to moderate transient eosinophilia, which can provide a diagnostic clue.33 Box 7 lists miscellaneous entities that can be associated with eosinophilia.
Box 7: Entities that can be associated with eosinophilia.
Rejection of a transplanted solid organ
Graft-versus-host disease after hematopoietic stem cell transplantation
Kimura disease and epithelioid hemangioma
Eosinophilia-myalgia syndrome/toxic oil syndrome
Adrenal insufficiency
Irritation of serosal surfaces
Cholesterol embolus
SUMMARY
Eosinophilia, greater than 450 to 500 eosinophils/µL in peripheral blood, is a hallmark of or a related finding in many allergic, infectious, autoimmune, idiopathic, malignant, and miscellaneous clinical scenarios. The clinical history is often the most important clue in discovering a pathway by which the patient is possibly affected by eosinophilia. Tailored evaluation based on scenario, including allergy testing, laboratory testing, imaging, and pathologic biopsy of affected areas can be useful in confirming a diagnosis. A broad differential diagnosis is often narrowed based on careful observation and clinical evaluation. Should findings point to specific areas, referral to appropriate specialists may be warranted. This referral spectrum in the case of eosinophilia is broad and often related to which organ system is involved.
KEY POINTS.
Eosinophilia is an elevation in the total number of bloodstream eosinophils, can be transient or sustained, and can exist in milder versus more significant levels.
Sustained and significant eosinophilia in the 1500 cells/µL or above range, without clear cause, should prompt evaluation.
Processes known to cause modest eosinophilia include allergic disease, parasitic disease, drug allergy, and mastocytosis.
More significant eosinophilia is often caused by drug allergy, aspirin exacerbated respiratory disease, sustained and significant atopic dermatitis, and some parasitic disorders.
If no apparent cause of the eosinophilia is known and levels above 1500 cells/µL exist for greater than 1 month, an exhaustive search guided by clinical presentation should ensue.
Footnotes
Disclosure Statement: Neither author has relevant items to disclose for this article.
REFERENCES
- 1.Kovalszki A, Sheikh J, Weller PF. Eosinophils and Eosinophilia. In: Rich RR, editor. Clinical immunology principles and practice. 4th. London: Elsevier Saunders; 2013. pp. 298–309. [Google Scholar]
- 2.Ogbogu PU, Bochner BS, Butterfield JH, et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol. 2009;124(6):1319–1325.e3. doi: 10.1016/j.jaci.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevens WW, Ocampo CJ, Berdnikovs S, et al. Cytokines in chronic rhinosinusitis. role in eosinophilia and aspirin-exacerbated respiratory disease. Am J Respir Crit Care Med. 2015;192(6):682–694. doi: 10.1164/rccm.201412-2278OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laidlaw TM, Boyce JA. Aspirin-exacerbated respiratory disease–new prime suspects. N Engl J Med. 2016;374(5):484–488. doi: 10.1056/NEJMcibr1514013. [DOI] [PubMed] [Google Scholar]
- 5.Saxena S, Madan T, Shah A, et al. Association of polymorphisms in the collagen region of SP-A2 with increased levels of total IgE antibodies and eosinophilia in patients with allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol. 2003;111(5):1001–1007. doi: 10.1067/mai.2003.1395. [DOI] [PubMed] [Google Scholar]
- 6.Akuthota P, Weller PF. Eosinophilic pneumonias. Clin Microbiol Rev. 2012;25(4):649–660. doi: 10.1128/CMR.00025-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nutman TB. Evaluation and differential diagnosis of marked, persistent eosinophilia. Immunol Allergy Clin North Am. 2007;27(3):529–549. doi: 10.1016/j.iac.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grando LR, Schmitt TA, Bakos RM. Severe cutaneous reactions to drugs in the setting of a general hospital. An Bras Dermatol. 2014;89(5):758–762. doi: 10.1590/abd1806-4841.20142997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choon SE, Lai NM. An epidemiological and clinical analysis of cutaneous adverse drug reactions seen in a tertiary hospital in Johor, Malaysia. Indian J Dermatol Venereol Leprol. 2012;78(6):734–739. doi: 10.4103/0378-6323.102367. [DOI] [PubMed] [Google Scholar]
- 10.Newberry AM, Williams DN, Stauffer WM, et al. Strongyloides hyperinfection presenting as acute respiratory failure and gram-negative sepsis. Chest. 2005;128(5):3681–3684. doi: 10.1378/chest.128.5.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stienlauf S, Yahalom V, Schwartz E, et al. Epidemiology of human T-cell lymphotropic virus type 1 infection in blood donors, Israel. Emerg Infect Dis. 2009;15(7):1116–1118. doi: 10.3201/eid1507.080796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rane SR, Agrawal PB, Kadgi NV, et al. Histopathological study of cutaneous manifestations in HIV and AIDS patients. Int J Dermatol. 2014;53(6):746–751. doi: 10.1111/ijd.12298. [DOI] [PubMed] [Google Scholar]
- 13.Gray TJ, Kwan YL, Phan T, et al. Dientamoeba fragilis: a family cluster of disease associated with marked peripheral eosinophilia. Clin Infect Dis. 2013;57(6):845–848. doi: 10.1093/cid/cit389. [DOI] [PubMed] [Google Scholar]
- 14.Sinico RA, Di Toma L, Maggiore U, et al. Prevalence and clinical significance of antineutrophil cytoplasmic antibodies in Churg-Strauss syndrome. Arthritis Rheum. 2005;52(9):2926–2935. doi: 10.1002/art.21250. [DOI] [PubMed] [Google Scholar]
- 15.Groh M, Pagnoux C, Baldini C, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss) (EGPA) Consensus Task Force recommendations for evaluation and management. Eur J Intern Med. 2015;26(7):545–553. doi: 10.1016/j.ejim.2015.04.022. [DOI] [PubMed] [Google Scholar]
- 16.Kargili A, Bavbek N, Kaya A, et al. Eosinophilia in rheumatologic diseases: a prospective study of 1000 cases. Rheumatol Int. 2004;24(6):321–324. doi: 10.1007/s00296-004-0469-6. [DOI] [PubMed] [Google Scholar]
- 17.Rosenstein RK, Panush RS, Kramer N, et al. Hypereosinophilia and seroconversion of rheumatoid arthritis. Clin Rheumatol. 2014;33(11):1685–1688. doi: 10.1007/s10067-014-2566-6. [DOI] [PubMed] [Google Scholar]
- 18.Adar T, Shteingart S, Ben-Ya’acov A, et al. The importance of intestinal eotaxin-1 in inflammatory bowel disease: New insights and possible therapeutic implications. Dig Dis Sci. 2016;61(7):1915–1924. doi: 10.1007/s10620-016-4047-z. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi A, Konno S, Hatanaka K, et al. A case of sarcoidosis with eosinophilia in peripheral blood and bronchoalveolar lavage fluid. Respir Med Case Rep. 2013;8:43–46. doi: 10.1016/j.rmcr.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Della Torre E, Mattoo H, Mahajan VS, et al. Prevalence of atopy, eosinophilia, and IgE elevation in IgG4-related disease. Allergy. 2014;69(2):269–272. doi: 10.1111/all.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lefevre G, Copin MC, Staumont-Sallé D, et al. The lymphoid variant of hypereosinophilic syndrome: study of 21 patients with CD3-CD41+ aberrant T-cell phenotype. Medicine (Baltimore) 2014;93(17):255–266. doi: 10.1097/MD.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khoury P, Desmond R, Pabon A, et al. Clinical features predict responsiveness to imatinib in platelet-derived growth factor receptor-alpha-negative hypereosinophilic syndrome. Allergy. 2016;71(6):803–810. doi: 10.1111/all.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gleich GJ, Schroeter AL, Marcoux JP, et al. Episodic angioedema associated with eosinophilia. N Engl J Med. 1984;310(25):1621–1626. doi: 10.1056/NEJM198406213102501. [DOI] [PubMed] [Google Scholar]
- 24.Khoury P, Herold J, Alpaugh A, et al. Episodic angioedema with eosinophilia (Gleich syndrome) is a multilineage cell cycling disorder. Haematologica. 2015;100(3):300–307. doi: 10.3324/haematol.2013.091264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovalszki A, Weller PF. Eosinophilia in mast cell disease. Immunol Allergy Clin North Am. 2014;34(2):357–364. doi: 10.1016/j.iac.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gotlib J, Akin C. Mast cells and eosinophils in mastocytosis, chronic eosinophilic leukemia, and non-clonal disorders. Semin Hematol. 2012;49(2):128–137. doi: 10.1053/j.seminhematol.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Williams KW, Milner JD, Freeman AF. Eosinophilia associated with disorders of immune deficiency or immune dysregulation. Immunol Allergy Clin North Am. 2015;35(3):523–544. doi: 10.1016/j.iac.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weir MR, Bartlett ST, Drachenberg CB. Eosinophilia as an early indicator of pancreatic allograft rejection. Clin Transplant. 2012;26(2):238–241. doi: 10.1111/j.1399-0012.2011.01440.x. [DOI] [PubMed] [Google Scholar]
- 29.Mortensen KB, Gerds TA, Bjerrum OW, et al. The prevalence and prognostic value of concomitant eosinophilia in chronic graft-versus-host disease after allogeneic stem cell transplantation. Leuk Res. 2014;38(3):334–339. doi: 10.1016/j.leukres.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Kar IB, Sethi AK. Kimura’s disease: report of a case & review of literature. J Maxillofac Oral Surg. 2013;12(1):109–112. doi: 10.1007/s12663-012-0388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adler BL, Krausz AE, Minuti A, et al. Epidemiology and treatment of angiolymphoid hyperplasia with eosinophilia (ALHE): A systematic review. J Am Acad Dermatol. 2016;74(3):506–512.e11. doi: 10.1016/j.jaad.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 32.Gelpi E, de la Paz MP, Terracini B, et al. The Spanish toxic oil syndrome 20 years after its onset: a multidisciplinary review of scientific knowledge. Environ Health Perspect. 2002;110(5):457–464. doi: 10.1289/ehp.110-1240833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kasinath BS, Lewis EJ. Eosinophilia as a clue to the diagnosis of atheroembolic renal disease. Arch Intern Med. 1987;147(8):1384–1385. [PubMed] [Google Scholar]