Abstract
Achieving and maintaining safe and reliable lineage specific differentiation of stem cells is important for clinical translation of tissue engineering strategies. In an effort to circumvent the multitude of problems arising from the usage of growth factors and growth factor delivery systems, we have explored the use of exosomes as biomimetic tools to induce stem cell differentiation. Working on the hypothesis that cell-type specific exosomes can trigger lineage-specific differentiation of stem cells, we have evaluated the potential of exosomes derived from dental pulp cells cultured on under growth and odontogenic differentiation conditions to induce odontogenic differentiation of naïve human dental pulp stem cells (DPSCs) and human bone marrow derived stromal cells (HMSCs) in vitro and in vivo. Results indicate that the exosomes can bind to matrix proteins such as type I collagen and fibronectin enabling them to be tethered to biomaterials. The exosomes are endocytosed by both DPSCs and HMSCs in a dose-dependent and saturable manner via the caveolar endocytic mechanism and trigger the P38 mitogen activated protein kinase (MAPK) pathway. In addition, the exosomes also trigger the increased expression of genes required for odontogenic differentiation. When tested in vivo in a tooth root slice model with DPSCs, the exosomes triggered regeneration of dental pulp-like tissue. However, our results indicate that exosomes isolated under odontogenic conditions are better inducers of stem cell differentiation and tissue regeneration. Overall, our results highlight the potential exosomes as biomimetic tools to induce lineage specific differentiation of stem cells. Our results also show the importance of considering the source and state of exosome donor cells before a choice is made for therapeutic applications.
Keywords: Biomimetics, exosomes, dental pulp stem cells, dental pulp regeneration, regenerative endodontics
Introduction
Predictable and reliable induction of lineage specific differentiation of stem cells is one of the key requirements for tissue engineering applications. In many cases, the biomaterial properties and choice of biomaterials are also dependent on this factor. Traditionally, growth factor delivery systems are used for induction of lineage specific differentiation of stem cells. The FDA has approved the use of growth factors such as bone morphogenetic factor 2 (BMP2) for clinical use. However, current clinical applications of growth factors have caused several adverse side effects and ectopic interactions. Many complications have been reported recently from BMP2 usage causing serious safety concerns among clinicians [1, 2]. In an effort to develop biomimetic approaches that can circumvent growth factor usage and complex controlled release mechanisms, we explored the possibility of utilizing cell-type specific exosomes.
We have tested this approach for dental pulp tissue regeneration using exosomes and an existing clinical material (collagen tape) in a translationally relevant small animal model. Clinically relevant dental pulp tissue regeneration can serve as a regenerative endodontic treatment to replace the existing root canal therapy used to treat necrotic permanent teeth arising from dental caries.
Dental caries is one of the most prevalent infectious disease in the world second only to the common cold [3]. A report from the world health organization (WHO) states that 90% of the world’s population has experienced dental caries. Poor oral hygiene is the primary cause for dental caries. However, treatments such as chemotherapy, radiation therapy and ingestion of medicines that cause excessive dryness of the oral cavity and affect salivation are also responsible for caries. Dental caries causes irreversible damage to the mineralized tissues of the teeth. Exacerbation leads to infection of the soft pulp tissue and causes acute to chronic pain, distress and ultimately loss in quality of life. Advanced dental caries is characterized by bacterial infection of the dental pulp leading to inflammation and necrosis of the vital dental pulp tissue.
The dental pulp is a highly vascularized and innervated tissue that performs several functions ranging from response to bacterial insult and injury, providing neuronal sensitivity to transmission of mechanical stimuli for repair and regeneration. Therefore, loss of this tissue results in loss of tooth vitality. Root canal therapy is the current clinical treatment of choice for treating necrotic permanent teeth. More than 20 million root canal treatments are performed in the United States alone every year and several million around the world. The clinical filling material used for this procedure is an inorganic compound that is biologically inert. Therefore, this procedure leads to permanent loss of tooth vitality and sensitivity (Dead tooth). Due to lack of immune response from an insensitive tooth, subsequent infections can go unnoticed increasing the likelihood for secondary infections. In the immature permanent teeth of young adolescents, root canal therapy prevents tooth maturation and root development leading to weak tooth structures prone to cervical root fractures [4]. Therefore, maintaining the functionality of the dental pulp is necessary for tooth integrity and longevity. Tissue engineering approaches aimed at regenerating the dental pulp can readily address these issues. Our group and several other groups have attempted dental pulp tissue regeneration using a variety of growth factors [5–7], biomaterials and mesenchymal stem cells [8–13]. However, the dosage, delivery and safety concerns regarding the growth factors limit the translatory potential of these approaches. On the other hand, exosomes have never been explored as biomimetic tools for dental pulp tissue regeneration.
Exosomes are micro vesicles that are secreted by cells to facilitate inter cellular communication[14]. They contain RNA material (both mRNA and micro RNA (miRNA)), cytosolic proteins as well as trans membrane proteins [15]. Originally, exosomes were believed to be mediators of cellular homeostasis by secreting cellular waste [16]. Recent studies on exosomes have mainly focused on immunology and cancer biology[16, 17]. However, after discovery of their role in transference of mRNA and miRNA [18], there has been a renewed focus on their applications in regenerative medicine. Recently, exosomes have been shown to increase the proliferative ability of mesenchymal and epithelial cells via the mitogen activated protein kinase (MAPK) pathway [19, 20]. Exosomes from endothelial cells[21], endothelial progenitor cells [22–24] and mesenchymal stem cells (MSCs) [25, 26] all possess pro-angiogenic properties that have been attributed to the miRNAs localized within them [21]. Finally, exosomes have been identified as the driving force behind the immunomodulatory effects of MSCs by enabling secretion of anti-inflammatory cytokines and facilitating the formation of M2 macrophages [27, 28].
Although these studies show the potential of exosomes for use in regenerative medicine, their ability to induce lineage specific differentiation of stem cells has not been studied rigorously. Additionally, the effects of using exosomes from one cell type on the overall biology of the recipient cell needs to be studied. We hypothesized that exosomes isolated from differentiated cells could be used to induce lineage specific differentiation of naïve MSCs. In this study, we explore the possibility of using exosomes isolated from odontogenic human dental pulp cells as biomimetic tools to effect lineage specific differentiation of primary human dental pulp stem cells (DPSCs) and primary human marrow stromal cells (HMSCs) in vitro and in vivo.
Materials and Methods
Cell culture
The primary human DPSCs used in this study were a gift from Dr. Songtao Shi (University of Pennsylvania, School of Dental Medicine). The primary human HMSCs used in this study were purchased from ATCC. Both cell types were cultured in minimum essential medium- alpha (αMEM) containing 20% fetal bovine serum (FBS, Gibco), 1% antibiotic-antimycotic solution (anti-anti, Gibco) and 1% L-glutamine (Gibco). The cells used in the all the experiments presented in this manuscript were passage 4 or under. For induction of odontogenic differentiation, containing growth media supplemented with 100μg/ml ascorbic acid, 10mM β-glycerophosphate and 10mM dexamethasone was used.
Isolation of exosomes
DPSCs were seeded to confluence in 100mm cell culture dishes. Exosomes were isolated from the culture medium of cells cultured in the presence of either growth (DPSC-Exo) or odontogenic differentiation media (DPSC-OD-Exo) for a period of 4 weeks. Exosomes were isolated as per previously published protocols [29]. Briefly, one day prior to isolation, the cell cultures were washed in serum free media and cultured for 24 hours in serum free media. When odontogenic media was used, the serum free media was supplemented with the odontogenic media cocktail of ascorbic acid, β-glycerophosphate and dexamethasone. The exosomes from the culture medium was isolated using the ExoQuick-TC (System Biosciences) exosome isolation reagent as per the manufacturer’s protocol.
The isolated exosomes from cells cultured under growth and differentiation were suspended in PBS. Exosome suspensions were normalized to cell number from the tissue culture plate they were isolated from and diluted to ensure that 100μl of suspension contained exosomes isolated from 1 million cells. Cross-verification was performed by measuring RNA and total protein isolated from the exosome suspensions to ensure that RNA/protein concentration from the same volume of exosomes remained consistent. Immunoblotting was performed with exosome markers CD63 (Abcam, 1/1000) and CD9 (Abcam 1/1000) antibodies as positive exosomal markers using protein extracted from 50μl of exosomes (equivalent to exosomes from 500,000 cells) and also with tubulin as negative control for intracellular proteins (Sigma 1/10,000) as per previously published protocols [29]. For comparison, the expression of the same proteins in DPSC lysates corresponding to 50,000 cells is provided.
Transmission electron microscopy (TEM)
TEM was used to verify the presence of exosomes in the purified samples and also to evaluate binding to type I collagen. 10μl of a 1 in 10 dilution of exosome suspension (equivalent to exosomes isolated from 10,000 cells) was placed on to fomvar/carbon coated nickel TEM grids and incubated for 30 minutes. For immunogold labeling, the exosomes bound to the grids were permeablized in PBS containing 0.5% Triton X-100 followed by blocking with PBS containing 5% BSA. The exosomes were then incubated for 2 hours at room temperature in mouse monoclonal anti CD63 antibody (Abcam, 1/100 dilution). Followed by washing (3 times) and 1 hour incubation at room temperature in 10nm gold-labeled secondary antibody. The grids were then washed, dried and imaged using a JOEL JEM-3010 TEM.
For collagen binding experiments, fomvar/carbon coated nickel grids were incubated for 15 minutes with 10μl of collagen solution containing 1μg of type I collagen (BD Biosciences). The grids were then washed in double deionized water and incubated with protein free blocking buffer (Thermo scientific) for blocking non-specific binding for 30 minutes. The grids were then incubated with 10μl of diluted exosome solution as described previously for 1hr at room temperature and then washed extensively in double deionized water and immunolabeled for CD63 as described above. The grids were imaged using a JOEL JEM 3010 TEM.
Fluorescent labeling of exosomes
The exosomes were labeled using the Exo-Glow-Green labeling kit (System Biosciences) as per the manufacturer’s recommended protocol. During each labeling reaction, a control reaction was performed using PBS not containing exosomes. This mixture was used in subsequent experiments as control to ensure against non-specific staining from the labeling procedure.
Endocytosis experiments
100,000 DPSCs or HMSCs were seeded on to glass coverslips placed inside 6 well tissue culture plates. 24 hours post seeding, 50μl of fluorescently labeled exosomes (corresponding to exosomes isolated from 500,000 cells) or control mixture was added to the culture medium and incubated for 1 hour at 37°C. Integrins mediated endocytosis was blocked by treating the cells with 2mM RGD peptide (Abcam) for 1 hour at 37°C followed by treatment with exosomes. Energy dependency was verified by performing the endocytosis experiment for 1 hour at 4°C. The coverslips were then washed in PBS 3 times, fixed in 4% neutral buffered formalin solution and immunostained as per previously published protocols [29]. The slides were then imaged using a Zeiss LSM 710 confocal microscope.
Quantitation of endocytosis and dose dependence experiments were performed in 96 well Elisa plates specifically used for fluorescence quantitation. Briefly, 20,000 DPSCs were seeded onto each well of a 96 well plate. 24 hours post seeding, increasing amounts of exosome suspensions were added to the wells and incubated for 1 hour at 37°C. When blocking experiments were performed, the exosome amount was fixed at 10μl per 20,000 cells. The cells were pre-treated with increasing concentrations of methyl beta cyclodextrin (MBCD, 0, 2.5, 5 and 10mM) prior to endocytosis or treated with heparin (0, 5 and 10μM) at the start of the experiment. The experiments were conducted in quadruplicates. The wells were washed 3 times in PBS, fixed using 4% neutral buffered formalin solution and the fluorescence from the wells was measured using a BioTek 96 well plate reader equipped with the appropriate band pass filter sets. The fluorescence was normalized to background signal from wells that did not contain exosomes and plotted using Matlab software for endocytosis dose dependence and using Excel for blocking experiments. The wells were also imaged for representative fluorescent images using a BioRad Zoe fluorescent microscope.
Binding experiments
100,000 DPSCs were seeded onto glass coverslips placed in 6 well plates. 48 hours post seeding, the coverslips were decellularized using previously published standardized procedures to leave behind the cell secreted ECM [30–35]. The ECM coated coverslips were then incubated with 50μl of fluorescently labeled exosome suspension for 1 hour at 37°C, washed extensively using PBS, fixed using 4% neutral buffered formalin and immunostained for fibronectin (red fluorescent secondary antibody). Blocking of integrin mediated binding was analyzed by pre-incubating the exosomes with 2mM RGD peptide prior to performing the ECM binding experiment.
Dose dependence and saturation in the binding of DPSC exosomes to the native ECM of DPSCs was analyzed using an Elisa based assay. For analyzing binding to native ECM, 50,000 DPSCs were seeded on to 96 well plates suitable for fluorescence. 48 hours post seeding, the plates were decellularized leaving behind the cell secreted ECM as per previously published protocols [36, 37]. The ECM was incubated with increasing amounts of exosome suspension for 1 hour at room temperature, washed 3 times in PBS and fixed. Experiments were performed in quadruplicates. To analyze the role of integrins in mediating exosome binding to the ECM, exosomes were also preincubated for 1 hour with 2mM RGD peptide (Abcam) after which they were incubated on the ECM coated plates. The bound exosomes were quantitated by measuring the fluorescence using a BioTek 96 well plate reader.
Dose dependence and saturation in the binding of DPSC exosomes to type I collagen was analyzed using Elisa as per previously published protocol [38]. Briefly, type I collagen was coated on to 96 well plates (5μg of type I collagen/well). The coated wells were blocked for non-specific binding using PBS containing 5% BSA and then incubated with increasing amounts of exosome suspensions. The wells were washed extensively, fixed in 4% neutral buffered formalin, permeablized using PBS containing 0.5% triton-x-100. The bound exosomes were quantitated by colorimetric immunostaining for the exosome marker protein CD63 (1/1000 dilution, Abcam) as per published protocols[29, 38] and measuring the absorbance using a BioTek microtiter plate reader. The experiments were performed in quadruplicates and the results were plotted using Matlab.
Exosome mediated stem cell differentiation (In vitro)
100,000 DPSCs or HMSCs cultured in 6 well plates or within clinical grade collagen sponges (Zimmer CollaCote1cm×1cm) were incubated for 8 or 48 hours with exosomes isolated from 500,000 cells or an equivalent volume of the similarly diluted isolation reagent (control solution). Exosomes isolated from cells cultured for 4 weeks using growth (DPSC-Exo) as well as odontogenic differentiation media (DPSC-OD-Exo) were used. P38 signaling was blocked using the inhibitor SB203580 (10μM). For these experiments, the cells were embedded in collagen sponges and the time point was fixed at 8hours. The control group contained an equivalent amount of DMSO to the group containing the SB inhibitor. Experiments were performed in triplicate. After specified time points, RNA from the cells was isolated followed by cDNA synthesis. Quantitative real time RTPCR (qRT PCR) was performed to analyze the expression levels of 16 genes representative of odontogenic differentiation of MSCs. Table 1 lists the gene-specific primers used in this study. Only the genes that showed a statistically significant change are listed in the results section. Data is presented as mean fold change with respect to control samples (ones that did not contain exosomes but were treated similarly in every other way). Statistical significance is represented as P value calculated using student’s t-test.
Table 1.
List of primers used in this study for qRT PCR.
| GENE | FORWARD | REVERSE |
|---|---|---|
|
| ||
| BMP2 | 5′ – ACT ACC AGA AAC GAG TGG GAA – 3′ | 5′ – GCA TCT GTT CTC GGA AAA CCT – 3′ |
| BMP6 | 5′ - TGT TGG ACA CCC GTG TAG TAT - 3′ | 5′ – AAC CCA CAG ATT GCT AGT GGC – 3′ |
| TGFB1 | 5′ – CAA TTC CTG GCG ATA CCT CAG – 3′ | 5′ – GCA CAA CTC CGG TGA CAT CAA – 3′ |
| VEGFA | 5′ – AGG GCA GAA TCA TCA CGA AGT – 3′ | 5′ – AGG GTC TCG ATT GGA TGG CA – 3′ |
| FGF2 | 5′-AGA AGA GCG ACC CTC ACA TCA – 3′ | 5′ – CGG TTA GCA CAC ACT CCT TTG – 3′ |
| GDF10 | 5′ – AGA TCG TTC GTC CAT CCA ACC - 3′ | 5′ – GGG AGT TCA TCT TAT CGG GAA CA– 3′ |
| RUNX2 | 5′ – TGG TTA CTG TCA TGG CGG GTA -3′ | 5′ – TCT CAG ATC GTT GAA CCT TGC TA -3′ |
| OSX | 5′ – CCT CTG CGG GAC TCA ACA AC – 3′ | 5′ – AGC CCA TTA GTG CTT GTA AAG G – 3′ |
| OCN | 5′ – AGC CCA TTA GTG CTT GTA AAG G – 3′ | 5′ – CCC TCC TGC TTG GAC ACA AAG – 3′ |
| ALPL | 5′ – ACT GGT ACT CAG ACA ACG AGA T – 3′ | 5′ – ACG TCA ATG TCC CTG ATG TTA TG – 3′ |
| OPN | 5′ – GAA GTT TCG CAG ACC TGA CAT – 3′ | 5′ – GTA TGC ACC ATT CAA CTC CTC G – 3′ |
| COL1 | 5′ – GAG GGC CAA GAC GAA GAC ATC – 3′ | 5′ – CAG ATC ACG TCA TCG CAC AAC -3′ |
| PHEX | 5′ – GAG GCA CTC GAA TTG CCC T – 3′ | 5′ – ACT CCT GTT TAG CTT GGA GAC TT – 3′ |
| DSPP | 5′ – TTT GGG CAG TAG CAT GGG C – 3′ | 5′ – CCA TCT TGG GTA TTC TCT TGC CT – 3′ |
| GAPDH | 5′ – CAG GGC TGC TTT TAA CTC TGG - 3′ | 5′ – TGG GTG GAA TCA TAT TGG AAC A -3′ |
| B2M | 5′ – GAG GCT ATC CAG CGT ACT CCA – 3′ | 5′ – CGG CAG GCA TAC TCA TCT TTT – 3′ |
In vivo experiments in root slice model
All animal experiments were performed in accordance with protocols approved by the UIC animal care committee (A3460-01). Exosomes isolated from 1.25 million cells under growth and differentiation conditions were added to 1cm × 1cm clinical grade type I collagen membranes (Zimmer CollaCote). 250,000 DPSCs were then seeded on to the membranes homogeneously. Note that the cell to exosome ratio was maintained constant for the in vitro and in vivo experiments. The collagen membranes treated similarly, but not containing exosomes served as control. The membranes were then used to fill the root canal spaces of human tooth root slices measuring 3–4mm in thickness. The root slices were obtained from extracted and discarded human molars from the clinics after proper disinfection. The root canal spaces in the root slices were filled with the control and experimental materials and implanted subcutaneously in the back of athymic nude mice for a period of 2 weeks. The slices were implanted immediately after cell seeding and were not cultured in vitro prior to implantation.
The animals were sacrificed and the extracted samples were fixed in 4% neutral buffered formalin and demineralized in 10% EDTA solution for 2 weeks with the EDTA solution changed once in 2 days. The demineralized samples were then paraffin embedded and sectioned in to 5μm thick sections. The sections were subjected to H&E staining and immunostaining using a mouse monoclonal anti DMP1 antibody (a kind gift from Dr. Anne George, UIC, 1/2000 dilution), rabbit polyclonal anti DPP antibody (a kind gift from Dr. Anne George UIC 1/100 dilution) and a mouse monoclonal antibody to von Willebrand factor (vWF, santacruz 1/100 dilution) followed by biotinylated secondary antibody (1/2500, Vector Labs) followed by colorimetric development using the DAB kit (Vector Labs). In addition, fluorescent immunohistochemistry (IHC) was performed using mouse monoclonal anti BMP2 antibody (1/100 dilution, Abcam), rabbit polyclonal anti TGFβ1 antibody (1/100 dilution Abcam), mouse monoclonal anti Runx2 antibody (1/100, Abcam) and finally rabbit polyclonal anti PDGF (platelet derived growth factor) antibody (1/100 dilution, Abcam). The immunostained sections were imaged using a Zeiss LSM 710 confocal microscope.
Results
Endocytosis of exosomes by DPSCs
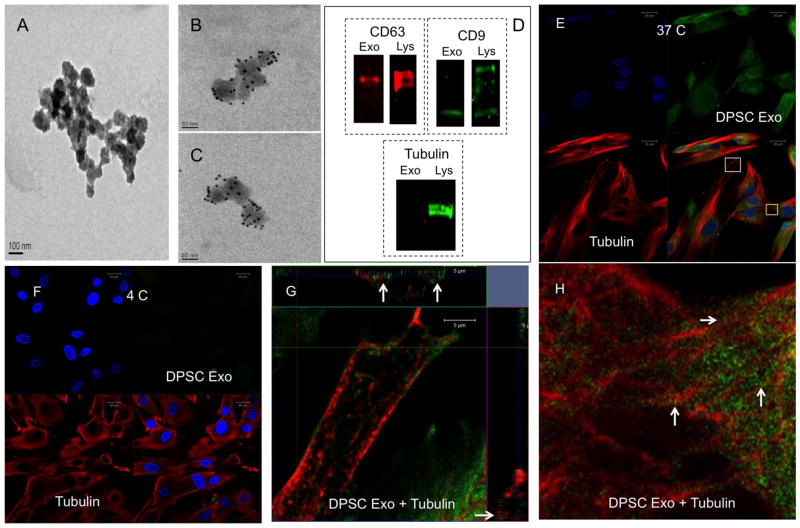
Presence of exosomes in the isolates was verified by TEM. Figure 1A, B and C are electron micrographs showing the presence of exosomes. Figures 1B and 1C were immunogold labeled for the exosomal marker protein CD63. Figure 1A is a secondary antibody control showing the absence of non-specific labeling of the exosomes. Proteins from the exosomes and DPSC lysates were subjected to SDS PAGE and immunoblotting was performed for exosomal marker proteins CD63 and CD9. Representative immunoblots presented in figure 1D show the presence of both marker proteins. Immunoblotting for the intracellular protein tubulin was negative indicating the absence of proteins from lysed cells (figure 1D). The isolated exosomes were labeled fluorescently (green) and DPSC cultures were incubated with the labeled exosomes at 37°C and 4°C. A control labeling reaction was performed without exosomes and was subjected to the same procedure as the experimental sample. This preparation was used in the endocytosis experiments as control. The cells treated with this control preparation did not show any positive green fluorescent signal. The cells treated with the labeled exosomes were immunostained with anti tubulin antibody to observe the microtubules and also to verify if the endocytosed exosomes are transported intracellularly via the microtubules. Result presented in Figure 1 indicates that the DPSC generated exosomes are endocytosed by undifferentiated primary DPSCs at 37°C (figure 1E) but not at 4°C (Figure 1F). Figure 1G shows an orthogonal representation of a z-stack of confocal images obtained from the area represented by a white box in Figure 1E. The arrows in figure 1G point to endocytosed exosomes co-localizing with the microtubules in the x-z and y-z planes. Figure 1H is an enlarged view of the area indicated by the yellow box in Figure 1E. Figure 1H demonstrates the presence of endocytosed exosomes (green) on the microtubules (red). White arrows point to representative regions. Overall, the results presented in Figure 1 indicate that primary human DPSCs can endocytose pulp cell derived exosomes in an energy dependent manner and that the endocytosed exosomes are transported via the microtubules.
Figure 1. Endocytosis of exosomes by DPSCs.
(A) Representative TEM image of exosomes on nickel grids coated with carbon/fomvar film. This image also serves as secondary antibody negative control for the immune-gold labeled images in (B) and (C) that represent exosomes labeled for CD63 antigen with 10nm gold particles. (D) Immunoblots of protein extracts from exosome isolates and DPSCs showing the presence of marker proteins CD63 and CD9 and negative control tubulin (exo represents exosome and Lys represents cell lysate). (E) Representative confocal image of fluorescently labeled exosomes (green) endocytosed by DPSCs at 37°C counter stained with tubulin (red). (F) Representative confocal image of fluorescently labeled exosomes (green) endocytosed by DPSCs at 4°C counter stained with tubulin (red) (G) Orthogonal representation of z-stack confocal images of the area represented by white box in (E). Arrows point to exosomal presence on the microtubules in the x-z and y-z planes. (H) Higher magnification image of the area represented by the yellow box in (E) showing the presence of exosomes on the microtubules (white arrows).
Figure 2A shows that the process of exosome endocytosis by DPSCs is dose dependent and saturable. This result coupled with the result presented in figure 1 showing that endocytosis is energy dependent, indicated that the endocytic process was not a random uptake of exosomes, but rather a controlled mechanism possibly receptor mediated. Published studies have shown that exosome endocytosis by dendritic cells is mediated by integrins [39, 40]. We therefore, blocked integrin-mediated endocytosis by pre-treating the DPSCs with 2mM of RGD peptide. However, this did not affect the endocytosis of the exosomes by DPSCs indicating the absence of integrin-mediated endocytosis (Figure 2B, 2C).
Figure 2. Characterization of exosomal endocytic pathway.
(A) Graph showing the dose dependent and saturable endocytosis of exosomes by DPSCs. Error bars represent standard deviation. The red line indicates a rectangular hyperbola fit to the data indicating that endocytosis follows a saturable binding curve suggesting a controlled mechanism. (B), (C) Representative confocal micrographs of untreated DPSCs (B) or DPSCs pre-treated with 2mM RGD peptide (C) and subjected to exosome endocytosis using fluorescently labeled exosomes (green) and counter stained with tubulin antibody (red). Note that the endocytosis was not blocked by the treatment. (D) Representative confocal micrograph of DPSCs containing endocytosed exosomes (green) stained for clathrin (red). No colocalization was observed between the two. (E) Representative confocal micrograph of DPSCs showing colocalization of endocytosed exosomes (green) with caveolin 1(red). (F) Orthogonal representation of z-stack confocal images showing three-dimensional colocalization of endocytosed exosomes with caveolin 1. Arrows point to regions of colocalization in the x-z and y-z planes. (G) Graph showing inhibition of exosomal endocytosis by MBCD at various concentrations. (H) Graph showing inhibition of exosomal endocytosis by DPSCs in the presence of heparin. For the graphs (G) and (H), (*) represents statistical significance with respect to the control group (P<0.05, student’s t-test)) and (#) represents statistical significance between the experimental groups (P<0.05, student’s t-test).
Depending on the target cell type, exosomes are endocytosed by either clathrin or caveolin mediated endocytosis and in some cases via macropinocytois and also via phagocytosis [39]. We performed immunolocalization experiments using clathrin and caveolin antibodies to ascertain the mode of endocytosis of exosomes by DPSCs. Representative confocal images presented in Figures 2D and 2E indicate that DPSCs endocytosed the exosomes via the lipid raft/caveolae mediated pathway (arrows in Figure 2E) and not by clathrin mediated endocytosis. No colocalization of the endocytosed exosomes was observed with clathrin. Caveolin 1 (red) co-localized with the endocytosed exosomes (green) near the plasma membrane (white arrows in Figure 2E, 2F). Figure 2F is an orthogonal representation of z-stack confocal images that shows co-localization of caveolin 1 with the endocytosed exosomes in then x-z and y-z planes. In addition to these qualitative evaluations quantitative analysis was performed to analyze the role of lipid rafts/caveolae in exosome endocytosis by DPSCs. Lipid raft/caveolae mediated endocytosis was blocked using the inhibitor MBCD. MBCD removes the cholesterol from the plasma membrane and is an effective inhibitor of lipid raft/caveolae mediated endocytosis [41]. Results presented in in figure 2G indicate that MBCD blocking inhibits the endocytosis of exosomes in DPSCs even at 2.5mM concentration.
Recent research suggests the involvement of heparan sulfate proteoglycans (HSPGs) in the endocytosis of glioblastoma cell derived exosomes [42]. HSPGs act as both receptors and co-receptors on the plasma membrane and are actively involved in endocytosis of several viruses [43, 44]. Sulfated heparin mimics the extracellular heparan sulfate domains of the HSPGs and can competitively block endocytosis via HSPGs by actively binding to the exosomes [42]. We therefore investigated if HSPGs are involved in the endocytosis of exosomes by DPSCs. Results presented in figure 2H indicate that heparin can dose dependently inhibit the endocytosis of exosomes by DPSCs. In Figures 2G and 2H, * represents statistical significance with respect to the control group and # represents statistical significance between the experimental groups with greater than 95% confidence. Qualitative fluorescence images for both MBCD and heparin blocking experiments are presented as supplementary data (please refer to supplementary Figures 1 and 2).
Overall our experiments indicted that exosome endocytosis by DPSCs is saturable and occurs via HSPGs and is lipid raft/caveolae mediated.
Endocytosis of exosomes by DPSCs triggers the P38 MAPK pathway and odontogenic differentiation in vitro
Receptor mediated endocytosis is usually accompanied by a corresponding signaling mechanism. MSC derived exosomes have been shown to promote vascularization via the P38 mitogen activated protein kinase (MAPK) pathway [45]. Additionally, published studies have shown that this pathway is triggered during exosome mediated cell proliferation albeit in cancer cells [46]. We therefore evaluated if the pulp cell derived exosomes triggered the MAPK pathway in DPSCs upon endocytosis. Figure 3 shows representative confocal micrographs of control and exosome treated DPSCs 4 hours post treatment immunostained for phosphorylated P38 (pP38). Compared to untreated controls (Figure 3A), the DPSC-Exo and DPSC-OD-Exo treatment triggered nuclear translocation of pP38 (white arrows in Figure 3B, 3C) indicating the activation of this pathway. Western blot analyses indicated an increase in the amount of pP38 upon exosome treatment. The increased presence of pP38 was observed at 8 hours. At 24 hours post treatment only the group treated with odontogenic exosomes showed significant increase. The phosphorylated protein levels returned to basal at 72 hours. Figure 3D shows representative western blots and Figure 3E is a quantitation of data obtained from triplicate experiments. The total P38 levels were normalized to tubulin. The normalized P38 levels were used to obtain relative levels of pP38 and the resulting data are represented as fold change with respect to control (no exosomes) for each of the time points. Statistical significance of the experimental groups with respect to the control group (*) and among themselves (# representing exosome treatment vs odontogenic exosome treatment) was obtained using student’s t-test (95% confidence interval).
Figure 3. Endocytosis of exosomes triggers the activation of P38 MAPK pathway.
(A) Representative confocal micrograph of DPSCs treated with control sample and immunostained with pP38 antibody (red). (B) Representative confocal micrograph of DPSCs treated with fluorescently labeled DPSC-Exo (green) immunostained with pP38 antibody (red). Note the enhanced nuclear translocation of pP38 (white arrows). (C) Representative confocal micrograph of DPSCs treated with fluorescently labeled DPSC-OD-Exo (green) immunostained with pP38 antibody (red). White arrows point to nuclear translocation of pP38. (D) Representative western blots showing the time dependent increase in pP38 levels. (E) Quantitation of western blot data for triplicate experiments showing normalized mean fold change in pP38 intensity. Total P38 expression was normalized to tubulin expression and the normalized P38 was used to obtain relative levels of pP38. Fold change was calculated for the exosome treatments with respect to the control. Data shows mean +/− SD. (*) Represents statistical significance (P<0.05, student’s t-test) with respect to control. (#) Represents statistical significance of DPSC-OD-Exo group with respect to DPSC-Exo group (P<0.05, student’s t-test). (F and G) Graph representing the expression levels of BMP2 and BMP9 respectively after 8 hours of treatment with DPSC-Exo in the absence and presence of P38 inhibitor SB203580 (SB). Note the reduction in the effect of exosome-mediated change in the presence of the inhibitor. Data shows mean +/− SD. (*) Represents statistical significance (P<0.05, student’s t-test) with respect to control. (#) Represents statistical significance between the exosome group and the exosome group with SB inhibitor (P<0.05, student’s t-test).
To identify the role of P38 in exosome endocytosis mediated signaling, DPSCs seeded on collagen sponges were treated with exosomes (DPSC-Exo) in the presence and absence of the P38 pharmacological inhibitor SB 203580. The control group contained an equivalent amount of DMSO to the SB 203580 group to rule out any interference caused by the DMSO solvent. The expression of 16 odontogenic marker genes (table 1) was evaluated 8 hours post treatment. This early time point was chosen to ensure that the observed effects would be due to endocytosis and P38 signaling and not influenced by exosomal miRNAs. Results presented in Figures 3F and 3G indicate that at 8 hours, only BMP2 and BMP9 genes were significantly regulated as a result of exosome treatment. At this time point P38 blocking significantly blocked the up regulation of these two genes. In the figures 3F and 3G, * represents statistical significance with greater than 95% confidence with respect to control and # represents statistical significance between the experimental groups.
Next, we evaluated if the endocytosis of exosomes triggered differentiation of DPSCs by affecting the expression of regulatory genes (listed in table 1) over a longer period of time. When DPSCs plated in 6 well plates were treated with either DPSC-Exo or DPSC-OD-Exo for 48 hours, we were able to observe a statistically significant increase in the expression of genes that regulate odontogenic differentiation including the dentin sialophosphoprotein (DSPP) gene. Table 2 lists the genes and the mean fold change in expression with respect to control. Statistical significance with respect to control was measured using student’s t-test. Results presented in Table 2 indicate that DPSC-Exo and DPSC-OD-Exo can induce odontogenic differentiation of DPSCs in vitro.
Table 2.
Exosome mediated change in DPSC gene expression in 2D cultures.
| GENES | DPSC EXO (T-test) | DPSC OD EXO (T-test) |
|---|---|---|
|
| ||
| GROWTH FACTORS | ||
| BMP2 | 3.29 (0.0305) | 2.53 (0.0232) |
| BMP6 | 1.62 (0.1456) | 1.50 (0.0185) |
| TGFB1 | 2.05 (0.0001) | 4.70 (0.0216) |
| VEGFA | 0.98 (0.0188) | 1.62 (0.0376) |
| FGF2 | 2.70 (0.2517) | 1.53 (0.0010) |
| TRANSCRIPTION FACTORS | ||
| RUNX2 | 2.63 (0.0092) | 2.90 (0.0125) |
| ECM PROTEINS | ||
| COL1 | 3.02 (0.0068) | 2.63 (0.0006) |
| OPN | 2.33 (0.0987) | 2.40 (0.0152) |
| DSPP | 2.42 (0.0739) | 3.49 (0.0879) |
Data represents mean fold change in the expression of genes involved in odontogenic differentiation after DPSCs cultured on 2D tissue culture surfaces were treated for 48 hours with DPSC-Exo and DPSC-OD-Exo. Statistical significance with respect to control was measured using student’s t-test.
Exosome binding to fibronectin and type I collagen
Binding of exosomes to ECM proteins is important to understand from a regenerative medicine perspective and to evaluate if they can be tethered to biomaterials for delivery. We analyzed if pulp cell derived exosomes have the ability to bind to structural proteins in the ECM. When fluorescently labeled exosomes were incubated with the native ECM of DPSCs and counter stained with fibronectin, we were able to observe binding of the exosomes to fibrillar fibronectin in the ECM. Representative confocal micrographs and z-stack orthogonal representations presented in Figures 4B and 4D respectively, elucidate this interaction.
Figure 4. Binding of exosomes to ECM proteins.
(A) Representative confocal micrograph of native DPSC generated ECM treated with control sample (no exosomes) and immunostained with fibronectin antibody (red). (B) Representative confocal micrograph of DPSC ECM treated with fluorescently labeled exosomes (green) immunostained with fibronectin antibody (red). Note the colocalization of the exosomes with fibronectin (white arrows). (C) Representative confocal micrograph of DPSC ECM treated with fluorescently labeled exosomes (green) that were pre-treated with 2mM RGD peptide to block integrins on the exosomal plasma membrane and immunostained with fibronectin antibody (red). Note the absence of green fluorescence indicating blocking of integrin mediated binding of exosomes to fibronectin. (D) Orthogonal representation of z-stack confocal images showing the co-localization of exosomes with fibronectin. Arrows point to colocalization in the x-z and y-z planes. (E) Graph representing the quantitation of exosomal binding to DPSC ECM and the effect of RGD peptide mediated integrin blocking on the binding. Note the significant decrease (*, P<0.05, student’s t-test) in the amount of bound exosome with RGD blocking, but not complete abrogation of binding. Data points represent mean of quadruplicate experiments +/− SD. (F) Graph representing the dose dependent and saturable binding of exosomes to type I collagen. Data points represent mean of quadruplicate experiments +/− SD. The red line represents a rectangular hyperbola fit to the data indicating saturable binding. (G, H, I) Representative transmission electron micrographs of exosomes alone (G), type I collagen alone (H) and exosome bound to type I collagen (I).
Exosomal membranes are made up of plasma membrane similar to that of the originating cell. We therefore tested if exosomal binding to the ECM is mediated by integrins present on the exosomal membranes. When these exosomal integrins were blocked using RGD peptides, exosomal binding to fibronectin was completely abrogated indicating the role of integrins in mediating the binding of exosomes to fibronectin (Figure 4C).
Exosome binding to the ECM was then analyzed quantitatively. Results presented in Figure 4E indicate a dose dependent and saturable binding to the ECM of DPSCs. When exosomal integrins were blocked using an RGD peptide, the binding to the ECM was significantly reduced, but not completely abrogated indicating that the exosomes may also bind to other proteins in the ECM (figure 4E). We therefore evaluated if exosomes could bind to type I collagen, as it is one of the most abundant proteins in the mesenchymal ECM. Quantitative binding assays indicated a dose dependent and saturable binding to type I collagen (Figure 4F). Binding to type I collagen was also analyzed qualitatively using TEM. Figure 4I is a representative micrograph that shows the presence of a CD63 immunolabeled exosome on a type I collagen fibril (white arrow). Figures 4G and 4H show the presence of exosomes and collagen fibrils individually respectively. Overall, results demonstrated that the pulp cell derived exosomes bind to fibronectin via an integrin-mediated process and also bind to type I collagen.
Exosome mediated differentiation of DPSCs in 3D cultures
Having observed exosomal binding to type I collagen, we investigated if type I collagen bound exosomes could induce differentiation of DPSCs cultured in a 3D environment within type I collagen hydrogels. Results presented in supplementary figure 3 show that DPSCs can endocytose exosomes loaded on to clinical grade type I collagen sponges. Results in Table 3 indicate that DPSC-Exo and DPSC-OD-Exo induce increased expression of odontogenic marker genes. However, compared to the 2D results, the change in gene expression was more robust with respect to DPSC-OD-Exo than the change presented in Table 2 indicating that the odontogenic exosome (DPSC-OD-Exo) mediated differentiation is more efficient when the cells are present in 3D culture resembling their in vivo environment. Another observation from this experiment was that the DPSC-OD-Exo triggered a more robust increase in the expression of odontogenic marker genes compared to DPSC-Exo indicating that exosomes from cells subjected to odontogenic differentiation were more potent in inducing differentiation of naïve DPSCs.
Table 3.
Exosome mediated change in DPSC gene expression in 3D cultures.
| GENES | DPSC EXO (T-test) | DPSC OD EXO (T-test) |
|---|---|---|
|
| ||
| GROWTH FACTORS | ||
| GDF10 | 1.87 (0.1447) | 14.28 (0.0320) |
| BMP9 | 0.58 (0.2303) | 12.27 (0.0015) |
| FGF2 | 0.870551 | 3.62 (0.0415) |
| TRANSCRIPTION FACTORS | ||
| RUNX2 | 0.93 (0.2386) | 2.28 (0.0592) |
| OSX | 0.69 (0.0006) | 1.82 (0.0321) |
| ECM PROTEINS | ||
| ALPL | 1.05 (0.4412) | 2.42 (0.0184) |
| OPN | 1.03 (0.3890) | 1.74 (0.0118) |
| COL1 | 0.91 (0.2399) | 3.66 (4.75E-05) |
| DSPP | 2.57 (0.0075) | 5.38 (0.0714) |
Data represents mean fold change in the expression of genes involved in odontogenic differentiation after DPSCs were cultured within clinical grade collagen membranes incorporated with DPSC-Exo and DPSC-OD-Exo for 48 hours. Statistical significance with respect to control was measured using student’s t-test.
Exosome mediated dental pulp-like tissue regeneration in a tooth root slice model
With in vitro experiments highlighting the potential of DPSC-Exo and DPSC-OD-Exo to induce differentiation of DPSCs, we investigated if exosomes could trigger the regeneration of pulp-like tissue in a translationally relevant model. For this purpose, we chose a collagen membrane that is used clinically for endodontic treatment (Zimmer CollaCote) in a tooth root-slice regeneration model. Published studies have shown the efficiency of this model for evaluating scaffolds and tools developed for dental pulp tissue regeneration [47, 48]. The root canal spaces of tooth root slices were filled primary human DPSCs embedded within either control or exosome incorporated collagen membranes and implanted subcutaneously on the back of nude mice. The explant sections were then subjected histology and IHC. Figure 5 shows representative micrographs of explant sections stained with odontogenic differentiation marker proteins DMP1 (Figure 5A, A1 and A2) and DPP (Figure 5B, B1, B2). Figure 5 also shows explant sections stained for the endothelial cell marker protein von Willebrand factor (vWF, Figures 5C, C1 and C2) and fluorescent images that show the presence of vasculature by looking at red blood corpuscle (RBC) autofluorescence (Figures 5D, D1 and D2). Figures 5E, E1 and E2 are H&E stained sections that show the overall morphology. No secondary antibody non-specific staining was observed (Figures 5F, 5G). The data presented in Figure 5 shows that DPSC-Exo and DPSC-OD-Exo triggered increased expression of DMP1 and DPP. An increased concentration in the expression of DMP1 and DPP was observed at the interface between the dentin and soft tissue (black arrows in Figure 5). We could also observe that the DPSC-OD-Exo triggered a more robust expression of these proteins especially at the interface. However, only DPSC-OD-Exo group stained for the endothelial cell marker von Willebrand Factor (vWF). Additionally, we could observe active blood vessels (indicated by the presence of RBCs marked by white arrows in Figure 5 D2) only in the DPSC-OD-Exo incorporated explant sections indicating that DPSC-OD-Exo improved the vascularization of the implants.
Figure 5. Histology and IHC of explant sections from the in vivo tooth root slice model.
(A, A1, A2) Representative micrographs of explant sections from control (A), DPSC-Exo treated (A1) and DPSC-OD-Exo treated (C) immunostained with DMP1 antibody. Note the increased expression at the interphase between the soft tissue and the dentin (arrows) in exosome treated samples. Scale bar represents 50μm. (B, B1 and B2) Representative micrographs presented in the same order showing the localization of DPP. Only DPSC-OD-Exo treated samples showed a concentrated expression at the interface. Scale bar represents 50μm. (C, C1 and C2) Representative micrographs presented in the same order showing the localization of vWF. Only DPSC-OD-Exo treated samples showed increased expression. Scale bar represents 100μm. (D, D1, D2) Representative fluorescent micrographs showing autofluorescence in the red channel. Only DPSC-OD-Exo treated samples showed the presence of RBCs (white arrows) indicating the presence of capillaries within. Scale bar represents 10μm. (E, E1 and E2) Representative micrographs of H&E stained sections showing the overall morphology of the explants. Scale bar represents 20μm. (F, G) Representative micrographs showing absence of rabbit and mouse secondary antibody non-specific staining.
In addition to IHC and histology, fluorescence IHC was also performed to observe the expression levels of growth factors BMP2, TGFβ and the pro-vasculogenic factor PDGF (platelet derived growth factor) and the transcription factor Runx2 in the developing pulp-like tissue. Results presented in Figure 6 indicate that both DPSC-Exo and DPSC-OD-Exo triggered an increase in the expression levels these proteins. However, DPSC-OD-Exo treated samples showed an increased level of expression compared to sample treated with DPSC-Exo indicating that the improved efficiency of exosomes isolated from odontogenic pulp cells.
Figure 6. Fluorescence IHC of explant sections from the in vivo tooth root slice model.
(A, A1, A2) Representative confocal micrographs of explant sections from control (A), DPSC-Exo treated (A1) and DPSC-OD-Exo treated (C) immunostained with BMP2 antibody. (B, B1, B2) Similarly presented representative confocal images showing the expression of TGFβ1. (C, C1, C2) Confocal images showing the expression of the transcription factor Runx2. (D, D1, D2) Confocal images showing the expression of the pro-angiogenic factor PDGF. Note the increased expression of all the proteins in the sections of samples treated with exosomes. Also note the increased expression in the DPSC-OD-Exo treated samples with respect to DPSC-Exo treated samples and the control. Scale bar represents 20μm in all images.
Exosome mediated odontogenic differentiation of HMSCs
To evaluate if pulp cell derived exosomes can induce odontogenic differentiation of other somatic MSCs, we investigated if pulp cell derived exosomes can induce odontogenic differentiation of primary HMSCs. Results presented in Figure 7 indicate that DPSC exosomes are endocytosed by HMSCs. Gene expression analysis of HMSCs after 48 hours of treatment with DPSC-Exo and DPSC-OD-Exo indicated that the exosomes triggered the increase in the expression levels of several growth factors and ECM proteins along with the transcription factor Runx2 (Table 4). However, the most important result from this experiment was the one that relates to DSPP. The average GAPDH and B2M Ct values used for normalization of data were between in the 19 to 21-cycle range for the samples. For this expression level of control genes, we could not obtain any amplification of the DSPP gene in the control sample. However, in the samples containing the DPSC-Exo and DPSC-OD-Exo, DSPP was expressed with an average Ct value of 35.93 and 32.43 respectively. Therefore, DSPP expression is indicated as “turned on” in Table 4. After normalization to respective housekeeping genes, the fold change between DPSC-Exo treated HMSC group and DPSC-OD-Exo treated HMSC group is 60.55 fold (n=3, P= 0.0039 by student’s t-test). This increase in DSPP expression in conjunction with the expression levels of other genes indicated that exosomes isolated from the cells subjected to odontogenic differentiation were more effective in triggering odontogenic differentiation of naïve HMSCs.
Figure 7. Endocytosis of DPSC exosomes by HMSCs.
(A) Representative confocal micrograph of HMSCs treated with control sample and stained with phalloidin TRITC. (B) 3D representation of z-stack confocal images of HMSCs treated with fluorescently labeled DPSC exosomes (green) immunostained with tubulin antibody (red).
Table 4.
Exosome mediated change in HMSC gene expression in 3D cultures.
| GENES | DPSC EXO (T-test) | DPSC OD EXO (T-test) |
|---|---|---|
|
| ||
| GROWTH FACTORS | ||
| BMP2 | 7.03 (9.05E-5) | 2.04(0.033) |
| TGFB1 | 5.92 (7.5E-6) | 1.41 (0.008) |
| VEGFA | 2.23 (7.13E-6) | 1.84 (0.0005) |
| GDF10 | 1.72 (0.030) | 0.858 (0.258) |
| BMP9 | 1.035 (0.465) | 4.337 (0.034) |
| TGFβ1 | 2.31 (0.040) | 3.09 (0.0068) |
| VEGFA | 2.01 (0.084) | 3.24 (0.0074) |
| TRANSCRIPTION FACTORS | ||
| RUNX2 | 2.38 (0.00015) | 2.11 (0.0152) |
| ECM PROTEINS | ||
| ALPL | 1.48 (0.075) | 5.701 (0.002) |
| COL1 | 2.72 (5.29E-5) | 1.00 (0.476) |
| DSPP | Turned on | Turned on |
Data represents mean fold change in the expression of genes involved in odontogenic differentiation after HMSCs were cultured within collagen membranes incorporated with DPSC-Exo and DPSC-OD-Exo for 48 hours. Statistical significance with respect to control was measured using student’s t-test.
Discussion
The use of exosomes as tools in regenerative medicine has only recently gained prominence. However, most of the research efforts have focused on the immunomodulatory roles of exosomes and also on their use as vehicles to deliver specific genetic materials [20, 28, 49]. There have been no prior studies on the uptake of exosomes by DPSCs or on the effects of pulp-specific exosomes on the odontogenic differentiation of DPSCs and HMSCs. DPSCs are mesenchymal stem cells derived from the neural crest and possess multi-lineage differentiation potential [50–53]. On the other hand HMSCs are marrow derived stromal cells also capable of multi-lineage differentiation. The primary source of autologous DPSCs for stem cell therapy is the third molar. However, in several cases, the third molar may not be available for stem cell extraction or the number cells that can be isolated may not be sufficient for therapeutic use. Under these circumstances, HMSCs can serve as a potential stem cell source as the marrow serves as a renewable stem cell source in healthy individuals.
Therefore, in this study, we have evaluated the potential of exosomes isolated from differentiating DPSC cultures grown in the presence of growth as well as odontogenic differentiation media to induce odontogenic differentiation of naïve DPSCs and HMSCs.
Our results indicate that DPSCs can endocytose exosomes via the caveolar endocytic pathway. The endocytosed exosomes are transported via the microtubules. The endocytic process is dose dependent, energy dependent and saturable and is possibly mediated by HSPGs, but is not integrin mediated. In addition, the endocytic process triggers the activation of P38 MAPK pathway resulting in increased phosphorylation of P38 and nuclear translocation of the phorphorylated protein.
We hypothesize that exosome mediated control of cell fate is a -step process. The first step involves endocytosis mediated signaling and the second step involves exosomal ‘cargo’ (miRNA, protein and mRNA) mediated control of gene expression and cell fate. Our results indicate that the endocytic process mediated by P38 MAPK pathway may regulate BMP2 and BMP9 gene expression. However, at this point it is unclear if other BMPs are also regulated. Further studies are required to completely characterize this process and delineate the differences between endocytosis-mediated effects from exosomal miRNA or protein-mediated effects. On the other hand, gene expression studies showed that the exosomes trigger odontogenic differentiation of the DPSCs in both 2D and 3D culture models by significantly regulating several odontogenic marker genes including DSPP over a longer period of time validating the two-step process hypothesis. The exosomes derived from cells cultured in the presence of odontogenic differentiation media were more potent in inducing lineage specific differentiation. Specifically, a very robust and significant positive regulation of DSPP was observed when DPSCs were cultured in the presence of DPSC-OD-Exo in 3D culture. In addition to the odontogenic marker DSPP, DPSC-OD-Exos induced significant up regulation of several growth factors (GDF 10, BMP9), transcription factors such as Runx2 and osterix as well as several key ECM proteins such as alkaline phosphatase (ALPL) and type I collagen required for odontogenic differentiation and reparative dentin formation.
In order to use exosomes as therapeutic agents locally during endodontic procedures, they should be compatible for use with existing clinical materials and any other materials that may be developed in the future for regenerative medicine. One way of estimating this ability is to evaluate the binding of exosomes to structural ECM proteins. Exosomal membranes are derived from the cellular plasma membrane. We therefore hypothesized that exosomes should be able to bind to ECM proteins such as fibronectin and collagen. Results presented in this study indicate that exosomes bind to both type I collagen and fibronectin in a dose dependent and saturable manner and that their binding to fibronectin is integrin mediated and can be abrogated by blocking the integrins with an RGD peptide.
The abrogation of exosomal binding to fibronectin by RGD peptide suggests that exosomes can be tethered to biomaterials functionalized with an RGD peptide. This prospect opens up several avenues of research using exosomes and multiple combinations of biomaterials not only for dental pulp tissue regeneration, but for regeneration of other tissues as well. In this study however, we investigated the use of exosomes with a collagen membrane used in endodontic treatments clinically. Results from our animal model experiments indicated that both DPSC-Exo and DPSC-OD-Exo could trigger the regeneration of dental pulp-like tissue in a tooth root slice model. However, the DPSC-OD-Exo triggered a more robust expression of the odontogenic marker proteins as wells as growth factors and transcription factors required for odontogenic differentiation of DPSCs. We were particularly encouraged to note the increased expression of DPP and DMP1 along the interface of the soft tissue and dentin. DPP and DMP1 are key regulators of matrix mineralization and odontogenic differentiation [54]. Therefore, their expression at the interface of the pulp dentin complex is indicative of enhanced odontogenic differentiation and reparative dentin formation. The formation of reparative dentin is key to functional dental pulp tissue regeneration and the fact that DPSC-OD-Exo triggers this process is explicitly indicative of its potential in pulp regenerative therapy.
In order to test the hypothesis that cell type-specific exosomes can induce linage specific differentiation of stem cells, we evaluated the effect of pulp cell derived exosomes on marrow MSCs. Our results indicated that HMSCs can endocytose pulp cell derived exosomes and that these exosomes can direct the differentiation of the HMSCs towards an odontogenic lineage. Specifically, the fact that the exosomes can trigger the transcriptional activation of the DSPP gene in HMSCs is indicative of their potential in future regenerative applications.
Overall, our results highlight the potential of exosomes as tools in dental pulp tissue regeneration using both DPSCs and HMSCs. We hypothesize that the exosome-mediated changes in gene and protein expression observed in our study are a result of both endocytic and miRNA mediated control of cellular events. We also speculate that the difference in the potential of DPSC-Exo and DPSC-OD-Exo is a result of altered genetic and protein exosomal cargo. Therefore, in order to use exosomes as therapeutic agents, careful consideration should be given to the source of exosome and the state of the cells from which exosomes are isolated.
This study only explores the role of exosomes in inducing lineage specific differentiation of DPSCs. Further studies are required to characterize the mechanism by which exosomes control stem cell fate and the exosomal miRNAs and proteins that contribute towards this process. Furthermore, the ability of exosomes to induce odontogenic differentiation of bone marrow derived MSCs requires further investigation and characterization using in vitro and in vivo techniques.
Conclusion
We believe that the data presented in this manuscript can serve as a starting point for us and other groups to further investigate the possibility of using cell type-specific exosomes from cells cultured under defined conditions to elicit specific responses in target stem cells. Form a translational medicine perspective; we envision that purified exosomes can be produced in large quantities, lyophilized and distributed commercially for use with autologous stem cells and existing clinical materials such as collagen sponges in regenerative endodontics. Further studies are required to characterize the differences between early (endocytosis mediated) and later (miRNA mediated) signaling mechanisms triggered by exosomes to understand the mechanisms behind the specificity of action. We hypothesize that along with cell-derived ECM incorporated biomaterials, exosomes can be used to recreate a complete extracellular environment that can enable safe and reliable differentiation of stem cells.
Supplementary Material
Supplementary figure 1: MBCD blocking of exosome endocytosis
(A) Representative light and fluorescent microscopic images of fluorescently labeled exosomes endocytosed by DPSCs. (B) Representative light and fluorescent microscopic images of fluorescently labeled exosomes endocytosed by DPSCs pre-treated with 10mM MBCD.
Supplementary figure 2: Heparin blocking of exosome endocytosis
(A) Representative light and fluorescent microscopic images of fluorescently labeled exosomes endocytosed by DPSCs. (B) Representative light and fluorescent microscopic images of fluorescently labeled exosomes endocytosed by DPSCs pre-treated with 10μg of heparin.
Supplementary figure 3: Endocytosis of exosomes bound to collagen sponge by DPSCs
A representative 3D reconstruction of z-stack confocal images showing the endocytosis of fluorescently labeled exosomes (green) bound to CollaCote collagen sponge endocytosed by DPSCs (red actin stain). Apart from the cellular localization (white arrows), exosome presence is also visible in fibrillar patterns indicating collagen binding (yellow arrows).
Acknowledgments
This work was supported by NIH grant R56 DE023806 and UIC Chancellor’s Discovery fund award to Dr. Sriram Ravindran. We would also like to thank the UIC electron microscopy core facility and the confocal microscopy core facility for their assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zara JN, Siu RK, Zhang X, Shen J, Ngo R, Lee M, et al. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue engineering Part A. 2011;17:1389–99. doi: 10.1089/ten.tea.2010.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tannoury CA, An HS. Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. The spine journal: official journal of the North American Spine Society. 2014;14:552–9. doi: 10.1016/j.spinee.2013.08.060. [DOI] [PubMed] [Google Scholar]
- 3.Islam B, Khan SN, Khan AU. Dental caries: from infection to prevention. Medical science monitor: international medical journal of experimental and clinical research. 2007;13:RA196–203. [PubMed] [Google Scholar]
- 4.Cvek M. Prognosis of luxated non-vital maxillary incisors treated with calcium hydroxide and filled with gutta-percha. A retrospective clinical study. Endod Dent Traumatol. 1992;8:45–55. doi: 10.1111/j.1600-9657.1992.tb00228.x. [DOI] [PubMed] [Google Scholar]
- 5.Morito A, Kida Y, Suzuki K, Inoue K, Kuroda N, Gomi K, et al. Effects of basic fibroblast growth factor on the development of the stem cell properties of human dental pulp cells. Archives of histology and cytology. 2009;72:51–64. doi: 10.1679/aohc.72.51. [DOI] [PubMed] [Google Scholar]
- 6.Nakashima M. Induction of dentine in amputated pulp of dogs by recombinant human bone morphogenetic proteins-2 and -4 with collagen matrix. Archives of oral biology. 1994;39:1085–9. doi: 10.1016/0003-9969(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 7.Nakashima M, Reddi AH. The application of bone morphogenetic proteins to dental tissue engineering. Nature biotechnology. 2003;21:1025–32. doi: 10.1038/nbt864. [DOI] [PubMed] [Google Scholar]
- 8.Demarco FF, Casagrande L, Zhang Z, Dong Z, Tarquinio SB, Zeitlin BD, et al. Effects of morphogen and scaffold porogen on the differentiation of dental pulp stem cells. Journal of endodontics. 2010;36:1805–11. doi: 10.1016/j.joen.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 9.Karaoz E, Demircan PC, Saglam O, Aksoy A, Kaymaz F, Duruksu G. Human dental pulp stem cells demonstrate better neural and epithelial stem cell properties than bone marrow-derived mesenchymal stem cells. Histochemistry and cell biology. 2011;136:455–73. doi: 10.1007/s00418-011-0858-3. [DOI] [PubMed] [Google Scholar]
- 10.Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. Journal of dental research. 2002;81:531–5. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 11.Rosa V, Zhang Z, Grande RH, Nor JE. Dental pulp tissue engineering in full-length human root canals. Journal of dental research. 2013;92:970–5. doi: 10.1177/0022034513505772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, et al. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod. 2008;34:962–9. doi: 10.1016/j.joen.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Huang GT, Yamaza T, Shea LD, Djouad F, Kuhn NZ, Tuan RS, et al. Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue engineering Part A. 2010;16:605–15. doi: 10.1089/ten.tea.2009.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. International journal of molecular sciences. 2014;15:4142–57. doi: 10.3390/ijms15034142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nature reviews Immunology. 2002;2:569–79. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 16.Johnstone RM, Mathew A, Mason AB, Teng K. Exosome formation during maturation of mammalian and avian reticulocytes: evidence that exosome release is a major route for externalization of obsolete membrane proteins. Journal of cellular physiology. 1991;147:27–36. doi: 10.1002/jcp.1041470105. [DOI] [PubMed] [Google Scholar]
- 17.Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer metastasis reviews. 2013;32:623–42. doi: 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature cell biology. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y, et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem cell research & therapy. 2013;4:34. doi: 10.1186/scrt194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, et al. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PloS one. 2012;7:e33115. doi: 10.1371/journal.pone.0033115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Balkom BW, de Jong OG, Smits M, Brummelman J, den Ouden K, de Bree PM, et al. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood. 2013;121:3997–4006. S1–15. doi: 10.1182/blood-2013-02-478925. [DOI] [PubMed] [Google Scholar]
- 22.Cantaluppi V, Biancone L, Figliolini F, Beltramo S, Medica D, Deregibus MC, et al. Microvesicles derived from endothelial progenitor cells enhance neoangiogenesis of human pancreatic islets. Cell transplantation. 2012;21:1305–20. doi: 10.3727/096368911X627534. [DOI] [PubMed] [Google Scholar]
- 23.Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, et al. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circulation research. 2011;109:724–8. doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ranghino A, Cantaluppi V, Grange C, Vitillo L, Fop F, Biancone L, et al. Endothelial progenitor cell-derived microvesicles improve neovascularization in a murine model of hindlimb ischemia. International journal of immunopathology and pharmacology. 2012;25:75–85. doi: 10.1177/039463201202500110. [DOI] [PubMed] [Google Scholar]
- 25.Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. Journal of molecular medicine. 2014;92:387–97. doi: 10.1007/s00109-013-1110-5. [DOI] [PubMed] [Google Scholar]
- 26.Zhang HC, Liu XB, Huang S, Bi XY, Wang HX, Xie LX, et al. Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem cells and development. 2012;21:3289–97. doi: 10.1089/scd.2012.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mokarizadeh A, Delirezh N, Morshedi A, Mosayebi G, Farshid AA, Mardani K. Microvesicles derived from mesenchymal stem cells: potent organelles for induction of tolerogenic signaling. Immunology letters. 2012;147:47–54. doi: 10.1016/j.imlet.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Zhang B, Yin Y, Lai RC, Tan SS, Choo AB, Lim SK. Mesenchymal stem cells secrete immunologically active exosomes. Stem cells and development. 2014;23:1233–44. doi: 10.1089/scd.2013.0479. [DOI] [PubMed] [Google Scholar]
- 29.Narayanan R, Huang CC, Ravindran S. Hijacking the Cellular Mail: Exosome Mediated Differentiation of Mesenchymal Stem Cells. Stem cells international. 2016;2016:3808674. doi: 10.1155/2016/3808674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravindran S, Gao Q, Kotecha M, Magin RL, Karol S, Bedran-Russo A, et al. Biomimetic extracellular matrix-incorporated scaffold induces osteogenic gene expression in human marrow stromal cells. Tissue engineering Part A. 2012;18:295–309. doi: 10.1089/ten.tea.2011.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ravindran S, George A. Biomimetic extracellular matrix mediated somatic stem cell differentiation: applications in dental pulp tissue regeneration. Frontiers in physiology. 2015;6:118. doi: 10.3389/fphys.2015.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ravindran S, Huang C-C, Gajendrareddy P, Narayanan R. Biomimetically Enhanced Demineralized Bone Matrix for Bone Regenerative Applications. Frontiers in physiology. 2015:6. doi: 10.3389/fphys.2015.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ravindran S, Huang CC, George A. Extracellular matrix of dental pulp stem cells: applications in pulp tissue engineering using somatic MSCs. Frontiers in physiology. 2014;4:395. doi: 10.3389/fphys.2013.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ravindran S, Kotecha M, Huang CC, Ye A, Pothirajan P, Yin Z, et al. Biological and MRI characterization of biomimetic ECM scaffolds for cartilage tissue regeneration. Biomaterials. 2015;71:58–70. doi: 10.1016/j.biomaterials.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravindran S, Zhang Y, Huang CC, George A. Odontogenic induction of dental stem cells by extracellular matrix-inspired three-dimensional scaffold. Tissue engineering Part A. 2014;20:92–102. doi: 10.1089/ten.tea.2013.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ravindran S, Grys TE, Welch RA, Schapira M, Patston PA. Inhibition of plasma kallikrein by C1-inhibitor: role of endothelial cells and the amino-terminal domain of C1-inhibitor. Thrombosis and haemostasis. 2004;92:1277–83. doi: 10.1160/TH04-01-0008. [DOI] [PubMed] [Google Scholar]
- 37.Ravindran S, Huang CC, Gajendrareddy P, Narayanan R. Biomimetically enhanced demineralized bone matrix for bone regenerative applications. Frontiers in physiology. 2015;6:292. doi: 10.3389/fphys.2015.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ravindran S, Gao Q, Ramachandran A, Blond S, Predescu SA, George A. Stress chaperone GRP-78 functions in mineralized matrix formation. The Journal of biological chemistry. 2011;286:8729–39. doi: 10.1074/jbc.M110.179341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. Journal of extracellular vesicles. 2014:3. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD, et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104:3257–66. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- 41.Nabi IR, Le PU. Caveolae/raft-dependent endocytosis. The Journal of cell biology. 2003;161:673–7. doi: 10.1083/jcb.200302028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Christianson HC, Svensson KJ, van Kuppevelt TH, Li JP, Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:17380–5. doi: 10.1073/pnas.1304266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shukla D, Liu J, Blaiklock P, Shworak NW, Bai X, Esko JD, et al. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell. 1999;99:13–22. doi: 10.1016/s0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]
- 44.Belting M. Heparan sulfate proteoglycan as a plasma membrane carrier. Trends in biochemical sciences. 2003;28:145–51. doi: 10.1016/S0968-0004(03)00031-8. [DOI] [PubMed] [Google Scholar]
- 45.Alcayaga-Miranda F, Varas-Godoy M, Khoury M. Harnessing the Angiogenic Potential of Stem Cell-Derived Exosomes for Vascular Regeneration. Stem cells international. 2016;2016:11. doi: 10.1155/2016/3409169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li C, Liu DR, Li GG, Wang HH, Li XW, Zhang W, et al. CD97 promotes gastric cancer cell proliferation and invasion through exosome-mediated MAPK signaling pathway. World journal of gastroenterology. 2015;21:6215–28. doi: 10.3748/wjg.v21.i20.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakai VT, Cordeiro MM, Dong Z, Zhang Z, Zeitlin BD, Nor JE. Tooth slice/scaffold model of dental pulp tissue engineering. Advances in dental research. 2011;23:325–32. doi: 10.1177/0022034511405325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Albuquerque MT, Valera MC, Nakashima M, Nor JE, Bottino MC. Tissue-engineering-based strategies for regenerative endodontics. Journal of dental research. 2014;93:1222–31. doi: 10.1177/0022034514549809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X, Li JJ, Yang JY, Wang DS, Zhao W, Song WJ, et al. Tolerance induction by exosomes from immature dendritic cells and rapamycin in a mouse cardiac allograft model. PloS one. 2012;7:e44045. doi: 10.1371/journal.pone.0044045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grottkau BE, Purudappa PP, Lin YF. Multilineage differentiation of dental pulp stem cells from green fluorescent protein transgenic mice. International journal of oral science. 2010;2:21–7. doi: 10.4248/IJOS10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okolicsanyi RK, Camilleri ET, Oikari LE, Yu C, Cool SM, van Wijnen AJ, et al. Human Mesenchymal Stem Cells Retain Multilineage Differentiation Capacity Including Neural Marker Expression after Extended In Vitro Expansion. PloS one. 2015;10:e0137255. doi: 10.1371/journal.pone.0137255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 53.Liu H, Gronthos S, Shi S. Dental pulp stem cells. Methods in enzymology. 2006;419:99–113. doi: 10.1016/S0076-6879(06)19005-9. [DOI] [PubMed] [Google Scholar]
- 54.Ravindran S, George A. Multifunctional ECM proteins in bone and teeth. Experimental cell research. 2014;325:148–54. doi: 10.1016/j.yexcr.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1: MBCD blocking of exosome endocytosis
(A) Representative light and fluorescent microscopic images of fluorescently labeled exosomes endocytosed by DPSCs. (B) Representative light and fluorescent microscopic images of fluorescently labeled exosomes endocytosed by DPSCs pre-treated with 10mM MBCD.
Supplementary figure 2: Heparin blocking of exosome endocytosis
(A) Representative light and fluorescent microscopic images of fluorescently labeled exosomes endocytosed by DPSCs. (B) Representative light and fluorescent microscopic images of fluorescently labeled exosomes endocytosed by DPSCs pre-treated with 10μg of heparin.
Supplementary figure 3: Endocytosis of exosomes bound to collagen sponge by DPSCs
A representative 3D reconstruction of z-stack confocal images showing the endocytosis of fluorescently labeled exosomes (green) bound to CollaCote collagen sponge endocytosed by DPSCs (red actin stain). Apart from the cellular localization (white arrows), exosome presence is also visible in fibrillar patterns indicating collagen binding (yellow arrows).