Abstract
Background:
Historically, complication rates after pressure ulcer reconstruction utilizing flap coverage have been high. Patients undergoing operations for pressure ulcer coverage typically have multiple risk factors for postoperative complications. The purpose of this study was to examine a large patient series in the pressure ulcer population to uncover objective evidence of the linkage between risk factors and outcomes after flap coverage.
Methods:
This study was a retrospective chart review of patients who underwent flap reconstruction for a pressure ulcer between 1997 and 2015. The characteristics of patients were analyzed to determine those who had complications such as pressure ulcer recurrence, wound dehiscence, and wound infection.
Results:
All patients (N = 276) underwent flap coverage of their pressure ulcers. The overall complication rate was 58.7% (162 patients). Wound dehiscence was the most common complication (31.2%), and the pressure ulcer recurrence rate was 28.6%. Multivariate regression for pressure ulcer recurrence revealed that body mass index <18.5 [relative risk (RR) 3.13], active smoking (RR 2.33), and ischial pressure ulcers (RR 3.46) were independent risk factors for pressure ulcer recurrence. Ischial pressure ulcers (RR 2.27) and preoperative osteomyelitis (RR 2.78) were independent risk factors for wound dehiscence. Diabetes was an independent risk factor for wound infection (RR 4.34).
Conclusions:
Our retrospective analysis revealed numerous factors that are associated with high rates of major postoperative complications. Risk factors must be taken into account when offering flap coverage, and risk-reducing strategies must be implemented in patients before pressure ulcer reconstruction.
INTRODUCTION
Pressure ulcers are common in hospitalized and long-term institutional care patients. Approximately 2.5 million pressure ulcers are treated in the United States yearly,1, 2 and related hospitalizations are becoming common.3 Their cost is estimated to be $9.1–11.6 billion per year in the United States.4–7 One susceptible patient population is are those with spinal cord injury. It is estimated that approximately 30% of spinal cord injury patients have pressure ulcers 20 years post injury.8 Pressure ulcers are especially morbid after spinal cord injury, leading to high rates of hospitalization and longer hospital stays.9, 10
The utilization of risk assessment tools can reduce incidence rates; nevertheless, high-risk patients will inevitably develop stage 3 and 4 ulcers that require surgical intervention.1, 2, 7 Operative approaches using primary closure and skin grafts have been abandoned as flaps for wound coverage are becoming popular.11 However, complication and recurrence rates for flap coverage remain alarmingly high.11–14 Outcomes analyses to date are limited to small retrospective reviews and have reported conflicting conclusions on risk factors.12–17
Spinal cord injury patients make up the majority of patients undergoing surgical reconstruction of pressure ulcers.13, 15–17 Given their relatively long life expectancies,18 flap coverage of pressure ulcers can prevent hospitalization and decrease morbidity. However, the risks and benefits of operative management of pressure ulcers must be balanced given the potential complications.
Ulcer recurrence and wound dehiscence are the most common surgical complications with recurrence rates as high as 80% in some series.12, 13, 16, 17, 19–21 The wide variation in reported outcomes suggests that patient-specific risk factors should be investigated further to determine the optimal operative settings. We hypothesized that there are patient-specific and potentially modifiable risk factors associated with complications after flap reconstruction for pressure ulcers. Our retrospective study design was utilized to query a large patient series in the pressure ulcer population to uncover objective evidence of the linkage between risk factors and outcomes after flap coverage.
METHODS
Study Design
This retrospective study was approved by the Institutional Review Board of Vanderbilt University Medical Center. Inclusion criteria were adult patients of any age and sex with sacral, ischial, and trochanteric pressure ulcers that required flap reconstruction between 1997 and 2016. Patients with pressure ulcers managed conservatively or that required wound debridement and/or primary closure without flap coverage were excluded. Further exclusion criteria were not used to maximize our study population.
Data Collection
Patient data collected included age, sex, body mass index (BMI), race, medical comorbidities, surgical history, medications, American Society of Anesthesiologists (ASA) physical status classification system, and tobacco use. Data from preoperative labs were also examined, including prealbumin, albumin, C-reactive protein (CRP), hematocrit, white blood cell (WBC) count, international normalized ratio, creatinine, and perioperative blood transfusion data. Psychiatric factors were not collected given the inconsistent collection of such data. Pressure ulcer characteristics including wound location, size, and presence of osteomyelitis (acute or chronic) were also recorded. Operative details recorded included donor site, flap composition, and procedure length. Postoperative management and outcomes collected included length of follow-up and complications including recurrence, wound dehiscence, and infection.
The primary outcomes for our review were major complications, which included ulcer recurrence, wound dehiscence, postoperative infection, flap necrosis, and minor complications such as seroma and hematoma. Patient characteristics were compared between those who did and did not have the aforementioned complications.
Statistical Analysis
Study data were collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at Vanderbilt University Medical Center.22 REDCap is a secure, web-based application designed to support data capture for research studies, providing (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for importing data from external sources.
Quantitative analyses of continuous and categorical data were completed using Fisher’s exact test, chi-square analysis, Mann-Whitney U tests, and unpaired t tests when appropriate. Statistical significance was achieved at two-tailed P values below 0.05. All analyses were performed within SPSS Statistics version 19 (IBM Corporation, Chicago, IL).
RESULTS
From January 1997 to December 2015, 276 patients underwent flap coverage of their pressure ulcers. The average patient age was 42.9 (range 4–85), and an analysis of sex revealed that 73.2% in our study series were males. Table 1 illustrates patient demographic and wound characteristics. Whites comprised 82.6% of patients during this study period. The average BMI was 25.1 and 24.6% of patients were active smokers. Paralysis was noted in 82.6% of patients, with 11.2% having quadriplegia and 55.8% having paraplegia. Of note, 62.3% of patients had ischial pressure ulcers. The most commonly performed flap utilized the gluteal musculature (62.3%). The majority of reconstructive flaps were myocutaneously based (80.8%) (Table 1).
Table 1.
Epidemiology of All Patients Undergoing Pressure Ulcer Reconstruction

Complications including major ones (ulcer recurrence, wound dehiscence, postoperative infection, flap necrosis) and minor ones (seroma and hematoma) occurred in 162 (58.7%) patients in this series (Fig. 1, Table 2). The average prealbumin of all patients was low (15.7 ± 6.9), but the average prealbumin of those who had a complication was significantly lower than those without complication (14.8 vs 17.0, P = 0.037). Additionally, the average albumin was significantly lower in those with complications (2.97 vs 3.40, P < 0.01). Interestingly, 71.1% of patients who received a perioperative blood transfusion experienced postoperative complications compared with a 50.3% complication rate in those not receiving a blood transfusion (P < 0.01). Longer operative times were associated with complications (190.6 vs 160.4 minutes, P < 0.01). Age, sex, race, BMI, diabetes, smoking status, preoperative osteomyelitis, and flap choice were not risk factors for complications (Table 2).
Fig. 1.
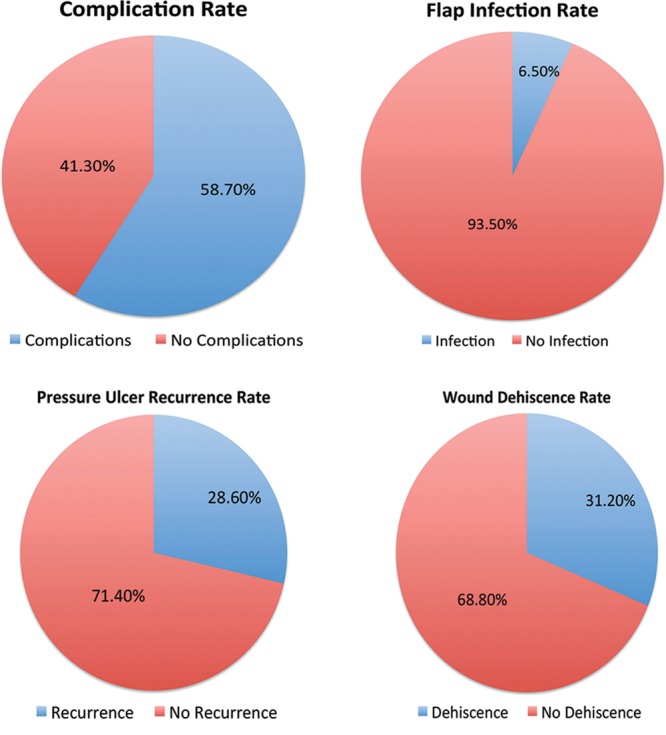
Complication rates.
Table 2.
Comparison of Patients With and Without Complications
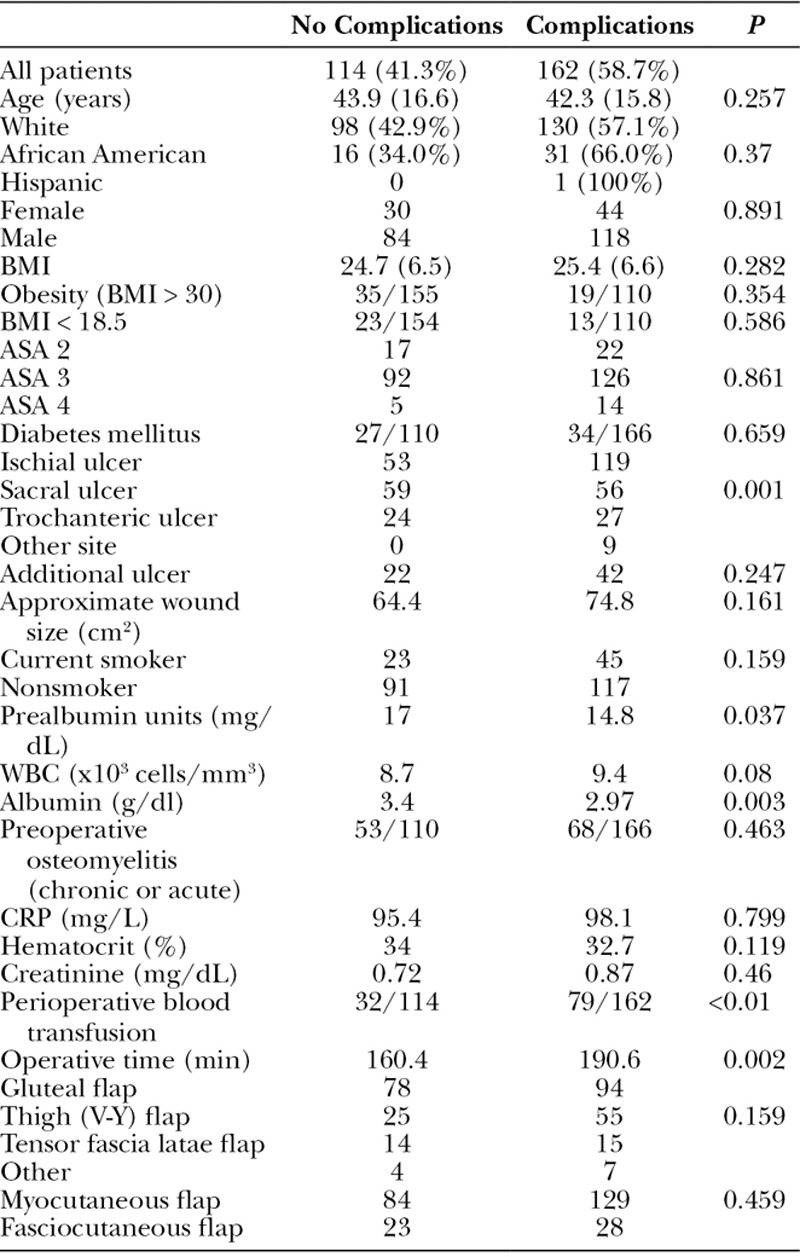
Postoperative flap infection rates were low (6.5%; Table 3). Advanced ASA class was associated with higher rates of infection. Pressure ulcer wound size was significantly larger in those who had a postoperative infection (109.8 cm2 vs 67.7 cm2, P = 0.047). The perioperative blood transfusion rate was significantly higher in those with a postoperative infection compared with those without (77.8% vs 37.6%, P < 0.01), and longer operative times were associated with postoperative infections (206.5 vs 176.1 minutes, P = 0.013). Interestingly, age sex, race, BMI, diabetes, smoking status, preoperative osteomyelitis, and flap choice were not risk factors for postoperative flap infections in our series (Table 3).
Table 3.
Comparison of Characteristics for Patients With and Without Postoperative Infections
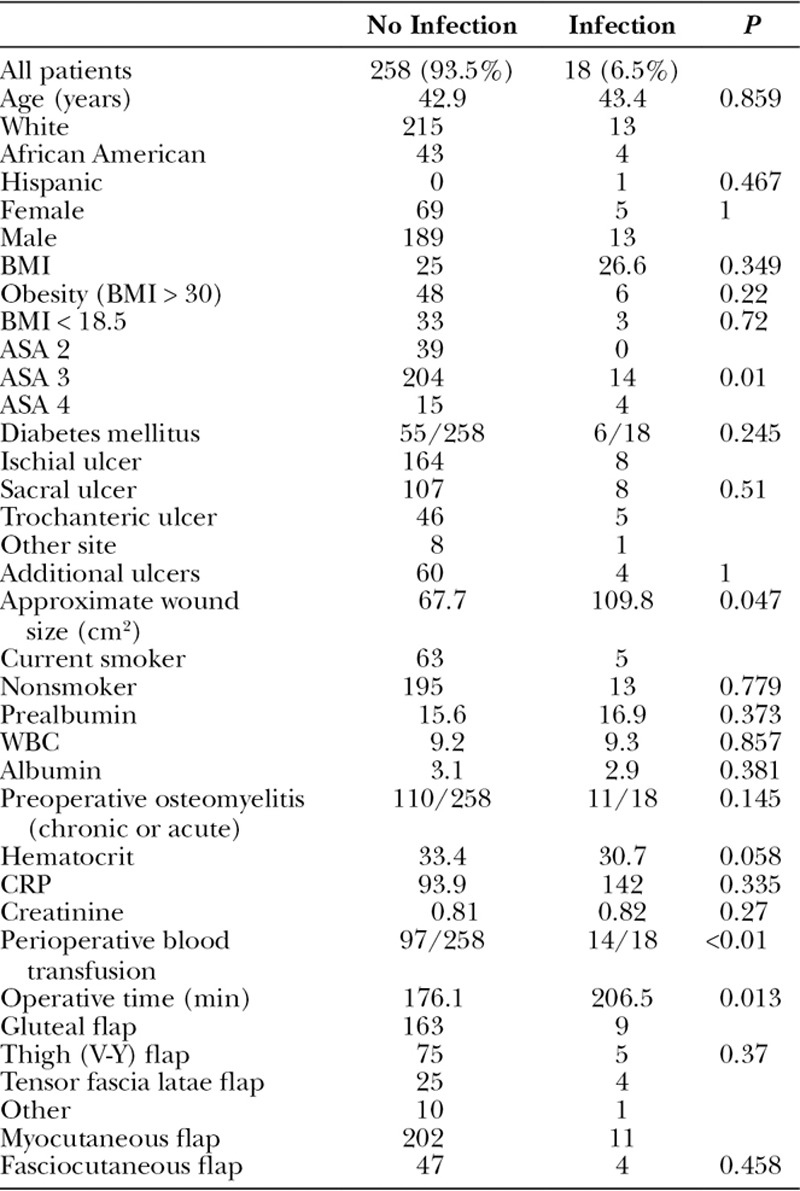
The same site pressure ulcer recurrence rate was 28.6% (Table 4), and the average time to recurrence was 357.9 days. The average age for those having an ulcer recurrence was younger than those not suffering a recurrence (39.4 vs 44.4 years old, P = 0.018). African Americans had higher rates of recurrence compared with whites (74.1% vs 25.9%, P = 0.02). Underweight patients (BMI < 18.5) had higher rates of recurrences (74.7% vs 25.3%, P = 0.027). Curiously, obesity was not a risk factor for recurrence in this series. Patients with pressure ulcers involving the ischium or in multiple sites were more likely to have ulcer recurrences compared with other sites. Active smokers had higher rates of recurrence (42.6% vs 24%, P < 0.01), and those with recurrences had high rates of perioperative blood transfusions (51.9% vs 35.5%, P = 0.015). Interestingly, thigh (V-Y) flaps had increased rates of pressure ulcer recurrences compared with other flap choices (Table 4). Of note, sex, diabetes, and preoperative osteomyelitis were not risk factors for pressure ulcer recurrence.
Table 4.
Patient Characteristics of Those Who Had a Recurrence
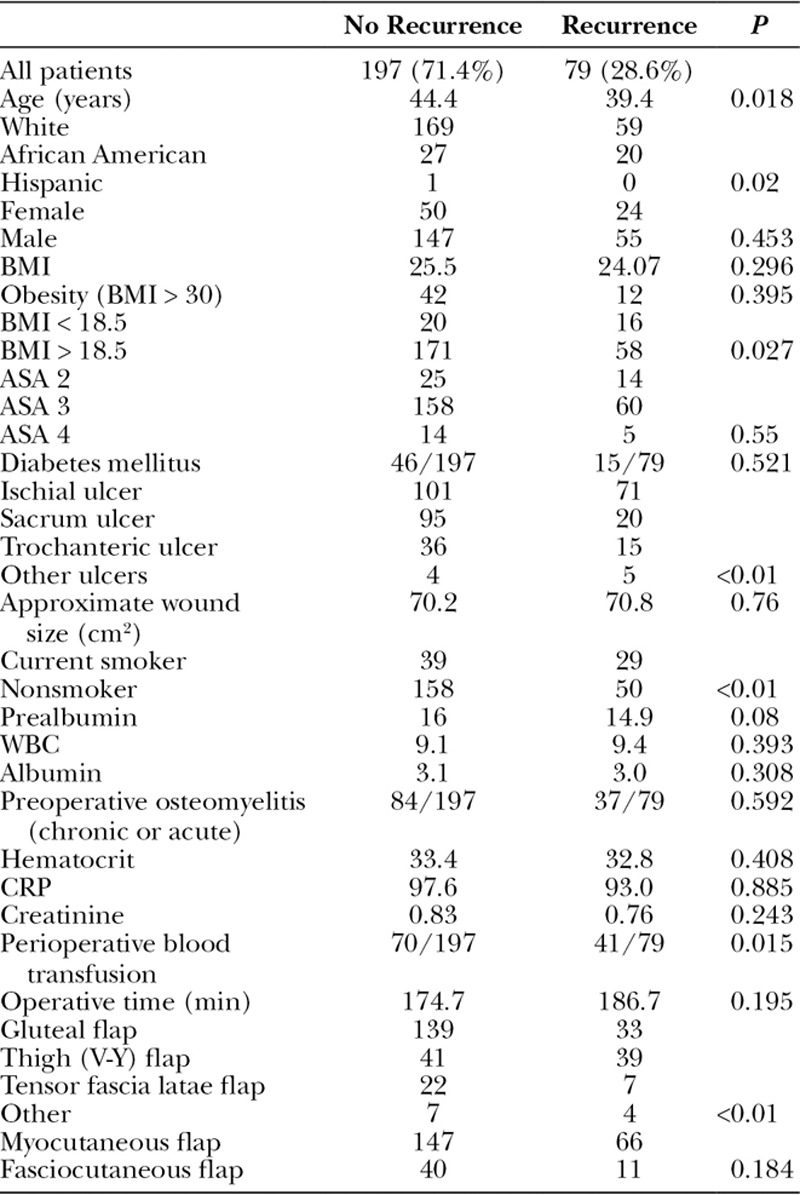
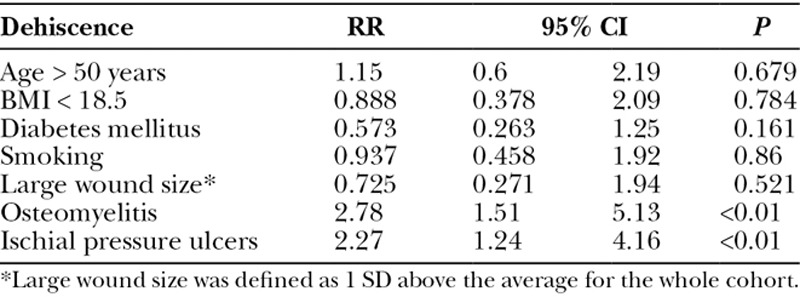
Wound dehiscence was the most common complication (31.2%), and the average time to wound dehiscence was 28.1 days (Table 5). Patients with pressure ulcers involving the ischium were more likely to have wound dehiscence compared with other sites. Patients with postoperative wound dehiscence had lower albumin levels (2.8 vs 3.3, P < 0.01), but prealbumin levels were not significantly lower in those with wound dehiscence (14.3 vs 16.2, P = 0.125). An unexpected finding was patients with preoperative osteomyelitis had a lower prevalence of wound dehiscence (24% vs 37%, P = 0.026). The perioperative blood transfusion rate was significantly higher in those with a postoperative wound dehiscence compared with those without (38.7% vs 26.1%, P < 0.034), and longer operative times were associated with postoperative wound dehiscence (194.9 vs 170.5 minutes, P = 0.031). Of note, age, sex, race, BMI, smoking status, and flap choice were not risk factors for wound dehiscence.
Table 5.
Patient Characteristics of Those With and Without a Wound Dehiscence
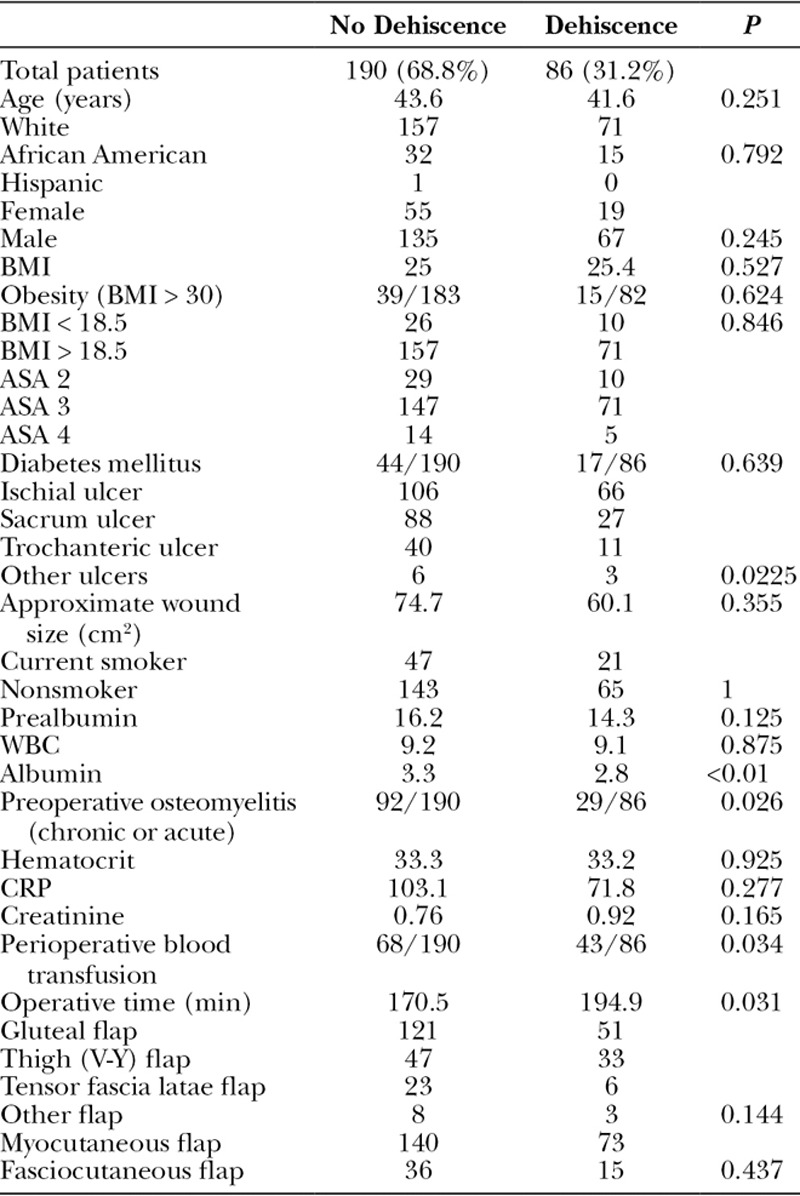
When a multivariate regression analysis was applied, ischial wounds were shown to be an independent risk factor for all complications [relative risk (RR) 2.63, 95% confidence interval (CI) 1.52–4.54, P < 0.01]. Age, BMI, diabetes, active smoking, wound size, and preoperative osteomyelitis were not independent risk factors for complications (Table 6). Not surprisingly, multivariate regression analysis revealed that diabetes was an independent risk factor for wound infection (RR 4.34, 95% CI 1.15–16.43, P = 0.031). Age, BMI, active smoking, wound size, preoperative osteomyelitis, and ischial wounds were not independent risk factors for complications in our patient population (Table 7). The multivariate regression for pressure ulcer recurrence revealed that BMI <18.5 (RR 3.13, 95% CI 1.34–7.27, P < 0.01), active smoking (RR 2.33, 95% CI 1.16–4.7, P = 0.018), and ischial pressure ulcers (RR 3.46, 95% CI 1.76–6.81, P < 0.01) were independent risk factors for pressure ulcer recurrence. Age, diabetes, wound size, and preoperative osteomyelitis were not independent risk factors for pressure ulcer recurrence (Table 8). Ischial pressure ulcers (RR 2.27, 95% CI 1.24–4.16, P < 0.01) and preoperative osteomyelitis (RR 2.78, 95% CI 1.51–5.13, P < 0.01) were independent risk factors for wound dehiscence. Age, BMI < 18.5, diabetes, active smoking, and wound size were not independent risk factors for wound dehiscence (Table 9).
Table 6.
Multivariate Regression for Any Complication
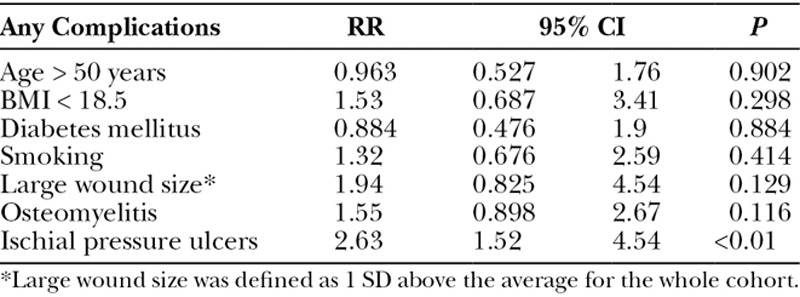
Table 7.
Multivariate Regression for Postoperative Infection
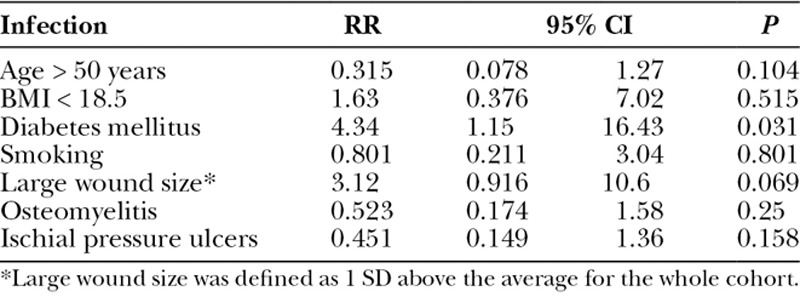
Table 8.
Multivariate Regression for Pressure Ulcer Recurrence
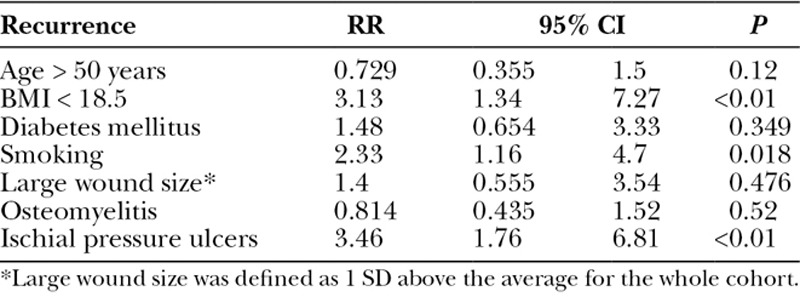
Table 9.
Multivariate Regression for Wound Dehiscence
DISCUSSION
Our retrospective analysis of two hundred seventy-six pressure ulcer patients who underwent reconstructive flap coverage has revealed numerous factors that are associated with high rates of major postoperative complications. Although prior studies have suggested that complication rates including recurrence and wound dehiscence were high, clinical evidence of the risk factors for complications was sparce.23 The challenge of pressure ulcer management is that pressure sores develop due to the interplay of many factors such as ischemic regions over bony prominences, poor nutrition, and infection.24 Flap coverage does not address the root causes, and therefore, careful patient selection may be the best way to improve operative outcomes.
A younger age at the time of onset has previously been cited as a risk factor for the development of pressure ulcers.25 Keys et al found that a chronologic age <45 years was a risk factor for recurrence and reoperation.13 We also noted that the average age of patients who had recurrence was significantly younger than those who did not have a recurrence. A variety of reasons may explain why a younger age is associated with elevated risk of recurrence. One potential explanation is that younger patients have a longer time span for a recurrence after flap surgery. However, importantly, patients who are younger may be offered more aggressive flap coverage for pressure ulcers compared with older counterparts. This ultimately may lead to higher rates of recurrence. Our retrospective analysis did not address this hypothesis.
We believe our study is the first large series to report a racial disparity in the postoperative outcomes for patients undergoing flap surgery for pressure ulcers. Our analysis revealed that African Americans had higher rates of recurrence. Previous reports have noted that African Americans have higher rates of pressure ulcer development26–28 and display a noted increase in pressure ulcer recurrence after initial healing.29 Poorer clinical and surgical outcomes in African Americans is a well-documented occurrence across different surgical specialties,30, 31 but the relationship remains poorly understood and is likely due to the interplay of multiple factors. Race was not an independent risk factor for pressure ulcer recurrence in our review, but the association with increased recurrence is an interesting finding in light of current literature.
Ischial pressure ulcers were an independent risk factor for pressure ulcer recurrence and wound dehiscence. This finding is not surprising given that ischial tuberosities have increased pressure over other bony prominences in the sitting position.32 Previous reviews have also found ischial pressure ulcers are at risk for recurrence.13, 33 Unfortunately, ischial pressure ulcers are the most common pressure ulcer, and avoidance of flap coverage for ischial pressure ulcers is not an ethical option. Rather, reducing other patient risk factors for flap complications may be important in those with ischial pressure ulcers.
Flap choice for pressure ulcer reconstruction continues to be an unresolved topic. Our study found that thigh (V-Y) flaps had higher rates of recurrence. Thigh (V-Y) flaps are considered reliable flaps; however, a disadvantage is that their closure is often under tension. This may increase the risk for recurrence, explaining our study’s results. In our review, gluteal- and tensor fascia latae-based flaps had lower recurrence rates. Additionally, our study did not find any difference between musculocutaneous- or fasciocutaneous-based flaps, which are in concordance with the findings of a recent meta-analysis.11 Despite these findings, an overarching rule for pressure ulcer reconstruction is not possible. Surgeons must use flaps that are individually tailored to each patient with the consideration that thigh-based flaps may be at higher risk for recurrence.
Smoking was an independent risk factor for pressure ulcer recurrence in our retrospective series. Smoking has not been previously linked to pressure ulcer flap complications, so this appears to be a novel finding. Smoking is a risk factor in wound complications in the general plastic surgery population and especially those undergoing free flaps.34, 35 Because smoking is a modifiable risk factor, motivational coaching directed toward smoking cessation may be an important step in preventing recurrence. Our data indicate that surgeons should be cautious about offering pressure ulcer flap coverage in smokers, and patients should be counseled on the risks associated with smoking.
Diabetes was found to be an independent risk factor for flap infection in our study. A recent review of the national CosmetAssure database found that diabetes was an independent risk factor in infections in all patients receiving elective, esthetic surgery.36 In our review, diabetes was not a risk factor for recurrence or wound dehiscence, but given the elevated risk of infection, a patient with a diagnosis of diabetes should be considered a risk, especially when other risk factors are present.
ASA class 3 also had an association with flap infection in this series. This finding was expected given that ASA class has been noted previously to be a risk factor for surgical site infections.37–39 A similar retrospective analysis of wound complications after abdominal contouring operations noted that ASA classification is also a significant independent risk factor for wound complications including infections.40 An interesting finding was that ASA class did not predict recurrence or wound dehiscence in our student population. Given these findings, those with higher ASA class should be considered at higher risk for infection, and proper precautions for infection prevention should be taken.
Nutritional factors are considered to play a role in the development and recovery from a pressure ulcer. Specifically, low prealbumin and albumin levels have been implicated in the development of pressure ulcers.41–43 Our review revealed that low albumin and prealbumin were associated with complications. Low albumin was specifically associated with wound dehiscence. Previous studies have had mixed findings on albumin and prealbumin and their predictive power for pressure ulcer reconstruction.13, 44 Albumin and prealbumin have traditionally been used to evaluate nutritional status, but their accuracy and reliability of nutritional status have recently been questioned.45, 46 However, albumin and prealbumin have inversely correlated with morbidity and mortality across surgical disciplines.47–49 Because these are imperfect markers, serum levels of albumin and prealbumin should be considered in light of other clinical findings when making the decision to offer elective flap coverage for a pressure ulcer,
Perioperative blood transfusions were associated with postoperative complications, including infection, pressure ulcer recurrence, and wound dehiscence. The deleterious effects of blood transfusion in the perioperative setting are highly debated,50, 51 and the surgical arena is experiencing a paradigm shift recently moving toward a conservative approach to transfusion.52 Optimal oxygen delivery is an integral part of wound healing and plays an important role in a successful outcome when flap coverage is selected in the setting of a pressure ulcer. Regardless of being a marker of high risk or an immunosuppressant, the data from our retrospective series suggest that blood transfusion in the perioperative setting should be used only when necessary to minimize risk in this high-risk patient population.
Longer operative times and large wounds had higher rates of infection, and longer operative times were associated with higher rates of wound dehiscence. These findings are difficult to interpret because pressure ulcers requiring longer operative times may be inherently more complicated and at a higher risk for infection and wound dehiscence. However, longer operative times have been associated with negative postoperative outcomes including wound complications.53, 54 Thus, our current finding of higher infection rates in larger pressure ulcers is not unexpected. No previous review of pressure ulcer reconstruction has found wound size as a risk factor.13 Large wounds are intrinsically at higher risk for infection and wound dehiscence, and hence, wound size is an important factor when selecting operative candidates.
Underlying osteomyelitis can be challenging to diagnose in patients with pressure ulcers and is likely underdiagnosed.55 In our study, acute osteomyelitis was an independent risk factor for wound dehiscence. Curiously, those with chronic osteomyelitis were not at increased risk for wound complications nor was there an increased risk of infection or ulcer recurrence noted in those patients with active osteomyelitis. Given these findings, it is appropriate to delay pressure ulcer reconstruction in those with active infections to avoid wound complications.
Although our study is limited by its retrospective nature, it is one of the largest reviews of pressure ulcer reconstruction to date. Another limitation is that rehabilitation data of our patients were not uniformly available, and given rehabilitation status may play a role in postoperative complications, future studies should address this.
Successful outcomes after pressure ulcer reconstruction remain a formidable challenge. Our data should provide the impetus for surgeons to renew their risk reduction efforts aimed at improving outcomes. Our report identifies numerous risk factors that should be considered when offering flap reconstruction. However, each patient must be evaluated independently and appropriately stratified by risk. Prospective clinical trials are needed in pressure ulcer reconstruction to validate risk factors and develop proper patient selection criteria that will reduce risk and improve outcomes.
ACKNOWLEDGMENTS
The authors would like to thank the attending plastic surgeons (R. Bruce Shack, MD; Kevin F. Hagan, MD; and J. Blair Summitt, MD) who performed the flap procedures for reconstruction of pressure ulcers.
Footnotes
Disclosure: None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript. R. Bamba and W.P. Thayer were supported in part by W81XWH-12-PRORP-TRPA from the Department of Defense. L.B. Nanney was supported in part by 1R01 AR056138-01A2, 1R01EB019409, and 1R01 GM118300-01 from the NIH. The Article Processing Charge was paid for by the Department of Plastic Surgery, Vanderbilt University Medical Center.
REFERENCES
- 1.Lyder CH. Pressure ulcer prevention and management. JAMA. 2003;289:223–226. doi: 10.1001/jama.289.2.223. [DOI] [PubMed] [Google Scholar]
- 2.Reddy M, Gill SS, Rochon PA. Preventing pressure ulcers: a systematic review. JAMA. 2006;296:974–984. doi: 10.1001/jama.296.8.974. [DOI] [PubMed] [Google Scholar]
- 3.Russo CA, Steiner C, Spector W. Hospitalizations Related to Pressure Ulcers, 2006. Rockville, MD:: Agency for Healthcare Research and Quality; 2008. Dec, HCUP Statistical Brief #64. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb64.pdf. [PubMed] [Google Scholar]
- 4.Preventing Pressure Ulcers in Hospitals. Rockville, MD:: Agency for Healthcare Research and Quality; September 2012 (content last reviewed October 2014). Available at: http://www.ahrq.gov/professionals/systems/hospital/pressureulcertoolkit/index.html. Accessed June 1, 2016. [Google Scholar]
- 5.Filius A, Damen TH, Schuijer-Maaskant KP, et al. Cost analysis of surgically treated pressure sores stage III and IV. J Plast Reconstr Aesthet Surg. 2013;66:1580–1586. doi: 10.1016/j.bjps.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Hirshberg J, Rees RS, Marchant B, et al. Osteomyelitis related to pressure ulcers: the cost of neglect. Adv Skin Wound Care. 2000;13:25–29. [PubMed] [Google Scholar]
- 7.Levi B, Rees R. Diagnosis and management of pressure ulcers. Clin Plast Surg. 2007;34:735–748. doi: 10.1016/j.cps.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 8.McKinley WO, Jackson AB, Cardenas DD, et al. Long-term medical complications after traumatic spinal cord injury: a regional model systems analysis. Arch Phys Med Rehabil. 1999;80:1402–1410. doi: 10.1016/s0003-9993(99)90251-4. [DOI] [PubMed] [Google Scholar]
- 9.Middleton JW, Lim K, Taylor L, et al. Patterns of morbidity and rehospitalisation following spinal cord injury. Spinal Cord. 2004;42:359–367. doi: 10.1038/sj.sc.3101601. [DOI] [PubMed] [Google Scholar]
- 10.Rish BL, Dilustro JF, Salazar AM, et al. Spinal cord injury: a 25-year morbidity and mortality study. Mil Med. 1997;162:141–148. [PubMed] [Google Scholar]
- 11.Sameem M, Au M, Wood T, et al. A systematic review of complication and recurrence rates of musculocutaneous, fasciocutaneous, and perforator-based flaps for treatment of pressure sores. Plast Reconstr Surg. 2012;130:67e–77e. doi: 10.1097/PRS.0b013e318254b19f. [DOI] [PubMed] [Google Scholar]
- 12.Berry RB. The late results of surgical treatment of pressure sores in paraplegics. Br J Surg. 1980;67:473–474. doi: 10.1002/bjs.1800670707. [DOI] [PubMed] [Google Scholar]
- 13.Keys KA, Daniali LN, Warner KJ, et al. Multivariate predictors of failure after flap coverage of pressure ulcers. Plast Reconstr Surg. 2010;125:1725–1734. doi: 10.1097/PRS.0b013e3181d51227. [DOI] [PubMed] [Google Scholar]
- 14.Relander M, Palmer B. Recurrence of surgically treated pressure sores. Scand J Plast Reconstr Surg Hand Surg. 1988;22:89–92. doi: 10.3109/02844318809097940. [DOI] [PubMed] [Google Scholar]
- 15.Goodman CM, Cohen V, Armenta A, et al. Evaluation of results and treatment variables for pressure ulcers in 48 veteran spinal cord-injured patients. Ann Plast Surg. 1999;42:665–672. doi: 10.1097/00000637-199906000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Kenneweg KA, Welch MC, Welch PJ. A 9-year retrospective evaluation of 102 pressure ulcer reconstructions. J Wound Care. 2015;24(Suppl 4a):S12–S21. doi: 10.12968/jowc.2015.24.Sup4a.S12. [DOI] [PubMed] [Google Scholar]
- 17.Biglari B, Büchler A, Reitzel T, et al. A retrospective study on flap complications after pressure ulcer surgery in spinal cord-injured patients. Spinal Cord. 2014;52:80–83. doi: 10.1038/sc.2013.130. [DOI] [PubMed] [Google Scholar]
- 18.National Spinal Cord Injury Statistical Center, Facts and Figures at a Glance. Birmingham, AL: University of Alabama at Birmingham; 2016. Accessed June 17, 2016. https://www.nscisc.uab.edu/Public/Facts%202016.pdf. [Google Scholar]
- 19.Disa JJ, Carlton JM, Goldberg NH. Efficacy of operative cure in pressure sore patients. Plast Reconstr Surg. 1992;89:272–278. doi: 10.1097/00006534-199202000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Kierney PC, Engrav LH, Isik FF, et al. Results of 268 pressure sores in 158 patients managed jointly by plastic surgery and rehabilitation medicine. Plast Reconstr Surg. 1998;102:765–772. doi: 10.1097/00006534-199809030-00022. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto Y, Tsutsumida A, Murazumi M, et al. Long-term outcome of pressure sores treated with flap coverage. Plast Reconstr Surg. 1997;100:1212–1217. doi: 10.1097/00006534-199710000-00021. [DOI] [PubMed] [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cushing CA, Phillips LG. Evidence-based medicine: pressure sores. Plast Reconstr Surg. 2013;132:1720–1732. doi: 10.1097/PRS.0b013e3182a808ba. [DOI] [PubMed] [Google Scholar]
- 24.Bauer J, Phillips LG. MOC-PSSM CME article: pressure sores. Plast Reconstr Surg. 2008;121(1 Suppl):1–10. doi: 10.1097/01.prs.0000294671.05159.27. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi PY, Chandra A, Cha SS. Risk factors for pressure ulceration in an older community-dwelling population. Adv Skin Wound Care. 2011;24:72–77. doi: 10.1097/01.ASW.0000394030.49530.b4. [DOI] [PubMed] [Google Scholar]
- 26.Bliss DZ, Gurvich O, Savik K, et al. Are there racial-ethnic disparities in time to pressure ulcer development and pressure ulcer treatment in older adults after nursing home admission? J Aging Health. 2015;27:571–593. doi: 10.1177/0898264314553895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fogerty M, Guy J, Barbul A, et al. African Americans show increased risk for pressure ulcers: a retrospective analysis of acute care hospitals in America. Wound Repair Regen. 2009;17:678–684. doi: 10.1111/j.1524-475X.2009.00522.x. [DOI] [PubMed] [Google Scholar]
- 28.Fogerty MD, Abumrad NN, Nanney L, et al. Risk factors for pressure ulcers in acute care hospitals. Wound Repair Regen. 2008;16:11–18. doi: 10.1111/j.1524-475X.2007.00327.x. [DOI] [PubMed] [Google Scholar]
- 29.Guihan M, Garber SL, Bombardier CH, et al. Predictors of pressure ulcer recurrence in veterans with spinal cord injury. J Spinal Cord Med. 2008;31:551–559. doi: 10.1080/10790268.2008.11754570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smedley BD, Stith AY, Nelson AR, editors. Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- 31.Haider AH, Scott VK, Rehman KA, et al. Racial disparities in surgical care and outcomes in the United States: a comprehensive review of patient, provider, and systemic factors. J Am Coll Surg. 2013;216:482–492.e12. doi: 10.1016/j.jamcollsurg.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindan O, Greenway RM, Piazza JM. Pressure distribution on the surface of the human body. I. Evaluation in lying and sitting positions using a “bed of springs and nails”. Arch Phys Med Rehabil. 1965;46:378–385. [PubMed] [Google Scholar]
- 33.Foster RD, Anthony JP, Mathes SJ, et al. Flap selection as a determinant of success in pressure sore coverage. Arch Surg. 1997;132:868–873. doi: 10.1001/archsurg.1997.01430320070011. [DOI] [PubMed] [Google Scholar]
- 34.Chang DW, Reece GP, Wang B, et al. Effect of smoking on complications in patients undergoing free TRAM flap breast reconstruction. Plast Reconstr Surg. 2000;105:2374–2380. doi: 10.1097/00006534-200006000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Coon D, Tuffaha S, Christensen J, et al. Plastic surgery and smoking: a prospective analysis of incidence, compliance, and complications. Plast Reconstr Surg. 2013;131:385–391. doi: 10.1097/PRS.0b013e318277886a. [DOI] [PubMed] [Google Scholar]
- 36.Bamba R, Gupta V, Shack RB, et al. Evaluation of diabetes mellitus as a risk factor for major complications in patients undergoing aesthetic surgery. Aesthet Surg J. 2016;36:598–608. doi: 10.1093/asj/sjv241. [DOI] [PubMed] [Google Scholar]
- 37.Berger RL, Li LT, Hicks SC, et al. Development and validation of a risk-stratification score for surgical site occurrence and surgical site infection after open ventral hernia repair. J Am Coll Surg. 2013;217:974–982. doi: 10.1016/j.jamcollsurg.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Kaoutzanis C, Leichtle SW, Mouawad NJ, et al. Risk factors for postoperative wound infections and prolonged hospitalization after ventral/incisional hernia repair. Hernia. 2015;19:113–123. doi: 10.1007/s10029-013-1155-y. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen TJ, Costa MA, Vidar EN, et al. Effect of immediate reconstruction on postmastectomy surgical site infection. Ann Surg. 2012;256:326–333. doi: 10.1097/SLA.0b013e3182602bb7. [DOI] [PubMed] [Google Scholar]
- 40.Greco JA, 3rd, Castaldo ET, Nanney LB, et al. The effect of weight loss surgery and body mass index on wound complications after abdominal contouring operations. Ann Plast Surg. 2008;61:235–242. doi: 10.1097/SAP.0b013e318166d351. [DOI] [PubMed] [Google Scholar]
- 41.Byrne DW, Salzberg CA. Major risk factors for pressure ulcers in the spinal cord disabled: a literature review. Spinal Cord. 1996;34:255–263. doi: 10.1038/sc.1996.46. [DOI] [PubMed] [Google Scholar]
- 42.Guenter P, Malyszek R, Bliss DZ, et al. Survey of nutritional status in newly hospitalized patients with stage III or stage IV pressure ulcers. Adv Skin Wound Care. 2000;13(4 Pt 1):164–168. [PubMed] [Google Scholar]
- 43.Reed RL, Hepburn K, Adelson R, et al. Low serum albumin levels, confusion, and fecal incontinence: are these risk factors for pressure ulcers in mobility-impaired hospitalized adults? Gerontology. 2003;49:255–259. doi: 10.1159/000070407. [DOI] [PubMed] [Google Scholar]
- 44.Larson DL, Hudak KA, Waring WP, et al. Protocol management of late-stage pressure ulcers: a 5-year retrospective study of 101 consecutive patients with 179 ulcers. Plast Reconstr Surg. 2012;129:897–904. doi: 10.1097/PRS.0b013e3182442197. [DOI] [PubMed] [Google Scholar]
- 45.Baker JP, Detsky AS, Wesson DE, et al. Nutritional assessment: a comparison of clinical judgement and objective measurements. N Engl J Med. 1982;306:969–972. doi: 10.1056/NEJM198204223061606. [DOI] [PubMed] [Google Scholar]
- 46.Forse RA, Shizgal HM. Serum albumin and nutritional status. JPEN J Parenter Enteral Nutr. 1980;4:450–454. doi: 10.1177/014860718000400503. [DOI] [PubMed] [Google Scholar]
- 47.Fuhrman MP, Charney P, Mueller CM. Hepatic proteins and nutrition assessment. J Am Diet Assoc. 2004;104:1258–1264. doi: 10.1016/j.jada.2004.05.213. [DOI] [PubMed] [Google Scholar]
- 48.Gibbs J, Cull W, Henderson W, et al. Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study. Arch Surg. 1999;134:36–42. doi: 10.1001/archsurg.134.1.36. [DOI] [PubMed] [Google Scholar]
- 49.Seres DS. Surrogate nutrition markers, malnutrition, and adequacy of nutrition support. Nutr Clin Pract. 2005;20:308–313. doi: 10.1177/0115426505020003308. [DOI] [PubMed] [Google Scholar]
- 50.Talbot TR, D’Agata EM, Brinsko V, et al. Perioperative blood transfusion is predictive of poststernotomy surgical site infection: marker for morbidity or true immunosuppressant? Clin Infect Dis. 2004;38:1378–1382. doi: 10.1086/386334. [DOI] [PubMed] [Google Scholar]
- 51.Vamvakas EC, Blajchman MA. Deleterious clinical effects of transfusion-associated immunomodulation: fact or fiction? Blood. 2001;97:1180–1195. doi: 10.1182/blood.v97.5.1180. [DOI] [PubMed] [Google Scholar]
- 52.Carson JL, Grossman BJ, Kleinman S, et al. Clinical Transfusion Medicine Committee of the AABB. Red blood cell transfusion: a clinical practice guideline from the AABB*. Ann Intern Med. 2012;157:49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 53.Daley BJ, Cecil W, Clarke PC, et al. How slow is too slow? Correlation of operative time to complications: an analysis from the Tennessee Surgical Quality Collaborative. J Am Coll Surg. 2015;220:550–558. doi: 10.1016/j.jamcollsurg.2014.12.040. [DOI] [PubMed] [Google Scholar]
- 54.Fogarty BJ, Khan K, Ashall G, et al. Complications of long operations: a prospective study of morbidity associated with prolonged operative time (> 6 h). Br J Plast Surg. 1999;52:33–36. doi: 10.1054/bjps.1998.3019. [DOI] [PubMed] [Google Scholar]
- 55.Larson DL, Gilstrap J, Simonelic K, et al. Is there a simple, definitive, and cost-effective way to diagnose osteomyelitis in the pressure ulcer patient? Plast Reconstr Surg. 2011;127:670–676. doi: 10.1097/PRS.0b013e3181fed66e. [DOI] [PubMed] [Google Scholar]


