Abstract
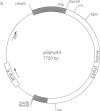
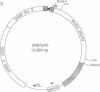
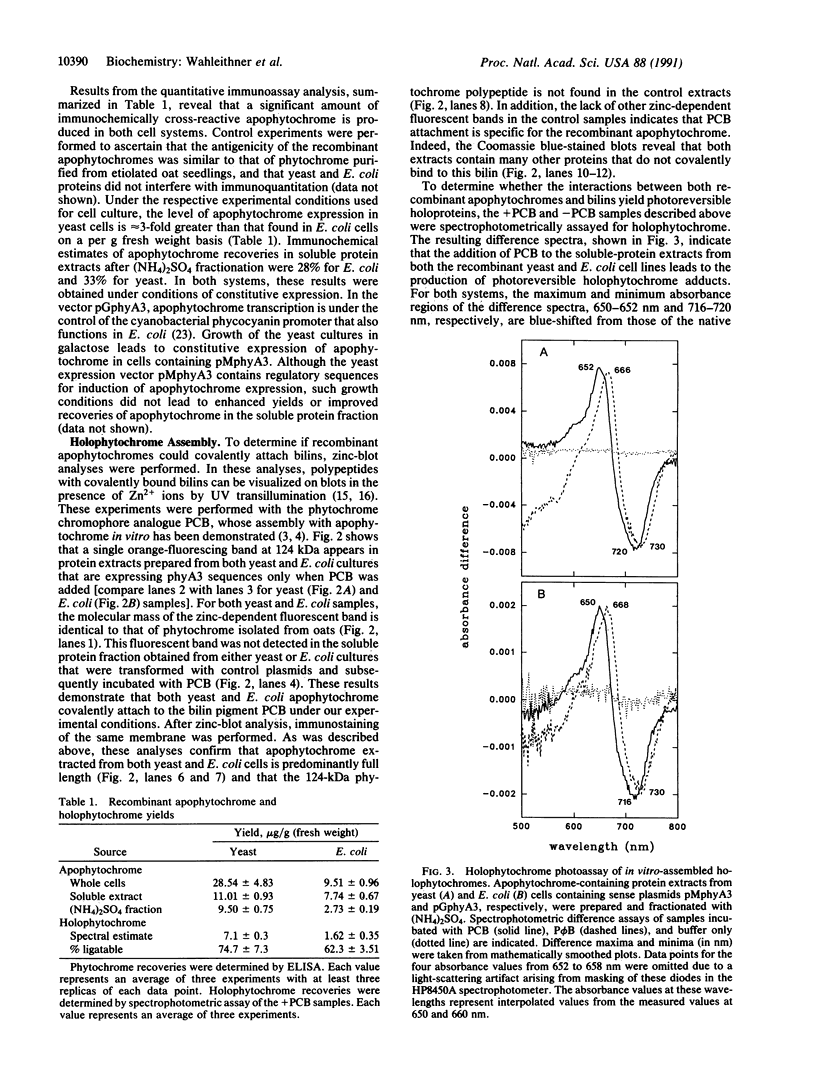
To develop an in vitro phytochrome assembly system, we have expressed an oat phytochrome cDNA in both the yeast Saccharomyces cerevisiae and the bacterium Escherichia coli. Analysis of soluble protein extracts showed that the recombinant apophytochromes were full-length and capable of covalently attaching the phytochrome chromophore analogue phycocyanobilin. Difference spectra indicated that in vitro-assembled holophytochrome species were photoreversible; however, maxima and minima difference absorption values were blue-shifted relative to those of the native photoreceptor. Extracts containing the recombinant apophytochromes were also incubated with phytochromobilin, the natural chromophore synthesized from biliverdin by cucumber etioplast preparations. In these experiments, the difference spectrum obtained was identical to that of native oat holophytochrome. These results suggest that the recombinant apophytochromes adopt a structure similar to that of the apoprotein biosynthesized in vivo. ELISAs were used to quantitate phytochrome expression levels in both yeast and E. coli extracts. These measurements show that 62-75% of the phytochrome apoprotein in the soluble protein extract was competent to assemble with bilins to form spectrally active holophytochrome.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkelman T. R., Lagarias J. C. Visualization of bilin-linked peptides and proteins in polyacrylamide gels. Anal Biochem. 1986 Jul;156(1):194–201. doi: 10.1016/0003-2697(86)90173-9. [DOI] [PubMed] [Google Scholar]
- Birkett C. R., Foster K. E., Johnson L., Gull K. Use of monoclonal antibodies to analyse the expression of a multi-tubulin family. FEBS Lett. 1985 Aug 5;187(2):211–218. doi: 10.1016/0014-5793(85)81244-8. [DOI] [PubMed] [Google Scholar]
- Elich T. D., Lagarias J. C. Formation of a photoreversible phycocyanobilin-apophytochrome adduct in vitro. J Biol Chem. 1989 Aug 5;264(22):12902–12908. [PubMed] [Google Scholar]
- Elich T. D., Lagarias J. C. Phytochrome Chromophore Biosynthesis : Both 5-Aminolevulinic Acid and Biliverdin Overcome Inhibition by Gabaculine in Etiolated Avena sativa L. Seedlings. Plant Physiol. 1987 Jun;84(2):304–310. doi: 10.1104/pp.84.2.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elich T. D., McDonagh A. F., Palma L. A., Lagarias J. C. Phytochrome chromophore biosynthesis. Treatment of tetrapyrrole-deficient Avena explants with natural and non-natural bilatrienes leads to formation of spectrally active holoproteins. J Biol Chem. 1989 Jan 5;264(1):183–189. [PubMed] [Google Scholar]
- Hershey H. P., Barker R. F., Idler K. B., Murray M. G., Quail P. H. Nucleotide sequence and characterization of a gene encoding the phytochrome polypeptide from Avena. Gene. 1987;61(3):339–348. doi: 10.1016/0378-1119(87)90197-1. [DOI] [PubMed] [Google Scholar]
- Innis M. A., Holland M. J., McCabe P. C., Cole G. E., Wittman V. P., Tal R., Watt K. W., Gelfand D. H., Holland J. P., Meade J. H. Expression, Glycosylation, and Secretion of an Aspergillus Glucoamylase by Saccharomyces cerevisiae. Science. 1985 Apr 5;228(4695):21–26. doi: 10.1126/science.228.4695.21. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lagarias J. C., Lagarias D. M. Self-assembly of synthetic phytochrome holoprotein in vitro. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5778–5780. doi: 10.1073/pnas.86.15.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litts J. C., Kelly J. M., Lagarias J. C. Structure-function studies on phytochrome. Preliminary characterization of highly purified phytochrome from Avena sativa enriched in the 124-kilodalton species. J Biol Chem. 1983 Sep 25;258(18):11025–11031. [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tomizawa K., Nayatani A., Furuya M. Phytochrome genes: studies using the tools of molecular biology and photomorphogenetic mutants. Photochem Photobiol. 1990 Jul;52(1):265–275. doi: 10.1111/j.1751-1097.1990.tb01784.x. [DOI] [PubMed] [Google Scholar]