ABSTRACT
Male microchimerism, the presence of a small number of male cells, in women has been attributed to prior pregnancies. However, male microchimerism has also been reported in women with only daughters, in nulliparous women and prepubertal girls suggesting that other sources of male microchimerism must exist. The aim of the present study was to examine the presence of male microchimerism in a cohort of healthy nulliparous Danish girls aged 10–15 y using DNA extracted from cells from whole blood (buffy coats) and report the association with potential sources of male cells. A total of 154 girls were studied of which 21 (13.6%) tested positive for male microchimerism. There was a tendency that girls were more likely to test positive for male microchimerism if their mothers previously had received transfusion, had given birth to a son or had had a spontaneous abortion. Furthermore, the oldest girls were more likely to test positive for male microchimerism. However, less than half of microchimerism positivity was attributable to these factors. In conclusion, data suggest that male microchimerism in young girls may originate from an older brother either full born or from a discontinued pregnancy or from transfusion during pregnancy. We speculate that sexual intercourse may be important but other sources of male cells likely exist in young girls.
KEYWORDS: Denmark, girls, male microchimerism, microchimerism
Introduction
Microchimerism is defined as the presence of a small amount of genetically distinct cells in an individual. During pregnancy there is bidirectional exchange of cells between the pregnant woman and her fetus through the placenta, potentially leading to the establishment of microchimerism in both the woman and the fetus. This phenomenon is referred to as fetal microchimerism (FMc) and maternal microchimerism (MMc), respectively. FMc acquired during pregnancies can persist in the mothers for decades after giving birth.1 Furthermore, FMc has been detected in several different tissues and shown to express specific cell surface marker indicating that the cells are tissue specific. FMc has also been found in T cells, B cells, macrophages and NK cells.2 Studies have suggested that FMc may be involved in autoimmune diseases, pregnancy complications and malignancy,1,3-6 but the exact role of FMc is still uncertain.
In several studies, the level of male microchimerism has been tested using the presence of Y chromosome as a marker in women who have given birth to a son. In healthy women, the determined prevalence of male microchimerism varies from 6% to 70%.3,6-8 This heterogeneity likely reflects the choice of assay and group of women studied. Surprisingly male microchimerism has also been reported in women with only daughters, in nulliparous women and in prepubertal girls.9-12 Besides pregnancy other established sources of Y chromosome include transfusion and transplantation.13,14 Although rare, male microchimerism may also stem from a vanished twin or an older brother.15 In addition, sexual intercourse has been suggested as a possible source.
To gain insight into male microchimerism in young girls, we examined Y chromosome presence in a cohort of nulliparous Danish girls aged 10 to 15 y. To our knowledge this is the first study systematically investigating presence of male microchimerism in a cohort of young girls. The study was approved by the Danish Health Research Ethics Committee (reference H-4-2013-129).
Results
A total of 154 girls aged 10 to 15 y at time of phlebotomy were bled and tested for the presence of male microchimerism using DNA extracted from cells from whole blood (buffy coats). Overall 21 out of 154 girls (13.6%) tested microchimerism positive. Mean (SD) level of male microchimerism was 0.035 (0.143), and among male microchimerism positive girls the level ranged from 0.087 to 1.360 male genome equivalents (GE) per 100,000 female GE. The level of male microchimerism in the 154 studied girls is illustrated in Figure 1.
Figure 1.
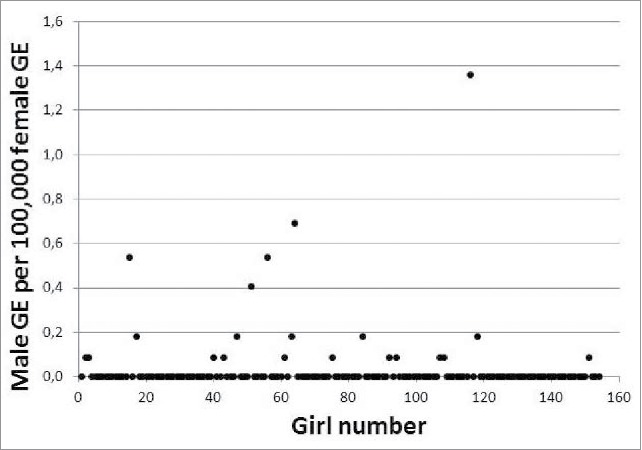
Male microchimerism level in girls aged 10–15 years, expressed as male genome equivalents (GE) per 100,000 female G.
Table 1 shows the distribution and association between exposures during index pregnancy, the mother's prior pregnancy history, the girls' age at phlebotomy and maternal male microchimerism status, respectively, and presence of male microchimerism in the girl. Several tendencies, although statistically insignificant, were observed. Among girls whose mother received one or more transfusions during the index pregnancy 28.6% tested male microchimerism positive, whereas only 12.4% of girls whose mother did not receive transfusion tested positive. This translates to an OR of 2.83 (95% CI 0.50–15.89) of testing male microchimerism positive among girls born to mothers who received transfusion compared with girls whose mother did not. Also, girls whose mother had one or more spontaneous abortions before the index pregnancy, girls with an older brother, and girls aged 13 y or older at time of phlebotomy were more likely to test positive for male microchimerism. We observed no obvious pattern according to whether girls were born to mothers with an index pregnancy complicated by HIP/preeclampsia, induced abortion, the spacing to the nearest older brother or the mother's male microchimerism status. We found no evidence of male contamination during sample handling (see Fig. 3), and also we found perfect reproducibility in both runs of the assay. Finally, we calculated that 40% of male microchimerism positivity in the young girls could be attributed to maternal transfusion during the index pregnancy, prior maternal spontaneous abortion, having an older brother, and being 13 y or older at time of phlebotomy (data not shown).
Table 1.
Distribution and crude OR (95% CI) of male microchimerism presence in girls aged 10–15 years according to exposures during index pregnancy, the mother's pregnancy history, the girl's age at phlebotomy and maternal male microchimerism status.
| Male microchimerism negative (n = 133) | Male microchimerism positive (n = 21) | Crude OR (95% CI) | |
|---|---|---|---|
| HIP/preeclampsia1 | |||
| – Yes | 16 (88.9) | 2 (11.1) | 0.79 (0.17–3.80) |
| – No | 95 (86.4) | 15 (13.6) | 1 (ref.) |
| Transfusion1 | |||
| – Yes | 5 (71.4) | 2 (28.6) | 2.83 (0.50–15.89) |
| – No | 106 (87.6) | 15 (12.4) | 1 (ref.) |
| Spontaneous abortion2 | |||
| – Yes | 12 (80.0) | 3 (20.0) | 1.75 (0.44–6.88) |
| – No | 112 (87.5) | 16 (12.5) | 1 (ref.) |
| – Trend per extra spontaneous abortion | 1.25 (0.40–3.84) | ||
| Induced abortion2 | |||
| – Yes | 36 (85.7) | 6 (14.3) | 1.13 (0.40–3.20) |
| – No | 88 (87.1) | 13 (12.9) | 1 (ref.) |
| – Trend per extra induced abortion | 0.93 (0.38–2.26) | ||
| Discontinued pregnancy2,3 | |||
| – Yes | 44 (83.0) | 9 (17.0) | 1.64 (0.62–4.33) |
| – No | 80 (88.9) | 10 (11.1) | 1 (ref.) |
| Trend per extra discontinued pregnancy | 0.98 (0.50–1.92) | ||
| Older brother2 | |||
| – Yes | 31 (81.6) | 7 (18.4) | 1.75 (0.63–4.84) |
| – No | 93 (88.6) | 12 (11.4) | 1 (ref.) |
| – Trend per extra older brother | 1.59 (0.76–3.34) | ||
| Spacing4 | |||
| – < 4 years | 15 (83.3) | 3 (16.7) | 0.80 (0.15–4.18) |
| – ≥ 4 years | 16 (80.0) | 4 (20.0) | 1 (ref.) |
| – Trend per extra year | 1.00 (0.76–1.31) | ||
| Age at phlebotomy2 | |||
| – < 13 years | 62 (91.2) | 6 (8.2) | 1 (ref.) |
| – ≥13 years | 62 (82.7) | 13 (17.3) | 2.17 (0.77–6.07) |
| – Trend per extra year | 1.31 (0.91–1.89) | ||
| Maternal male microchimerism status5 | |||
| – Negative | 83 (86.5) | 13 (13.5) | 1 (ref.) |
| – Positive | 14 (93.3) | 1 (6.7) | 0.53 (0.06–4.51) |
| Any male microchimerism source1,6 | |||
| – Yes | 58 (82.9) | 12 (17.1) | 2.19 (0.72–6.64) |
| – No | 53 (91.4) | 5 (8.6) | 1 (ref.) |
Relevant data was available for 128 girls.
Relevant data was available for 143 girls.
Prior spontaneous abortion, induced abortion, extrauterine pregnancy or hydatidiform mole.
Data on spacing to nearest older brother was available only for the 38 girls with an older brother.
Data on maternal male microchimerism status was available for 111 girls.
Any male microchimerism source includes having an older brother, having a mother with a discontinued pregnancy, or having a mother who received transfusion during pregnancy.
Figure 3.

Contamination during sample handling was tested by repeating the microchimerism analysis on 2–3 different samples from 10 anonymous women.
Discussion
In the present study, we show that 13.6 % of nulliparous young girls test positive for male microchimerism. This prevalence is very similar to what was reported in prepubertal healthy girls used as controls in a study of systemic lupus erythematosus (14.3%).12 Also, it does not differ much from the prevalence in healthy women without sons.3,8,9 Although comparing the prevalence of male microchimerism positivity across studies is hampered by the application of different techniques, similar prevalence may suggest that 1 in 7 girls and women testing positive represents a naturally occurring background level acquired during the fetal state i.e. it is not related to a history of pregnancies. Alternatively, it may reflect that girls in the current study may have had their sexual debut and that sexual intercourse is a source of male microchimerism. In a recent report on young adolescent behavior in Denmark, 16% of girls aged 14 y and 36% of girls aged 15 y report to have had sexual intercourse.16 Of these approximately 80% stated that they used condom, which prevents the transfer of male cells.16 Thus, at most 7.2% of the studied girls would expectedly test Y chromosome positive if sexual intercourse was the source of male microchimerism. We report that 13.6 % test positive indicating that even though sexual intercourse may be involved other sources likely exist which causes male microchimerism in young girls. In accordance with this, other groups have documented male microchimerism in liver and blood from young girls and female fetuses17,18 as well as in cord blood from female newborns.19 This raises questions regarding the origin of the male microchimerism. Some groups have suggested that male microchimerism may be due to trans-maternal flow of cells of non-maternal or non-fetal origin during pregnancy leading to the transfer of male cells from older male siblings or vanished twins to the female fetus.9,15,20 In our study, however, we do not observe a positive association between male microchimerism positivity in girls and their mothers. However, this discrepancy may be due to homing of the male positive cells into tissues leading to a misrepresent level of male microchimerism in the blood. We do find a tendency of having an older brother raises the odds of being male microchimerism positive and that the odds increases when having several older brothers. Similarly, our study suggests that a prior discontinued pregnancy affects whether girls test positive or not for male microchimerism. Yan et al.9 showed that women without sons were more likely to test male microchimerism positive if they had experienced a discontinued pregnancy. This suggest that women are likely to attain male microchimerism during discontinued pregnancies and our data suggest that these cells originating from older male siblings either full born or from discontinued pregnancies can further be passed on to the next sibling. Gammill et al.3 previously showed, that women with a pregnancy complicated by preeclampsia were more likely to test male microchimerism positive and with a higher concentration than women with uncomplicated pregnancies. Therefore, we expected that the girls were more likely to test positive for male microchimerism if the index pregnancy was complicated by preeclampsia. However, if anything we observed a tendency toward decreased odds of testing positive for male microchimerism if the index pregnancy was complicated by preeclampsia. Finally, our data show that the odds of being male microchimerism positive increases if the mother has received blood transfusion during pregnancy. Previously, data has shown that blood transfusion is associated with microchimerism.21 However, young women mainly receive blood transfusion during obstetric complications and therefore male microchimerism could also arise from reasons stated above. Also, we were surprised by the fact that less than half of the observed male microchimerism positivity in young girls can be attributed to either maternal transfusion during the index pregnancy, prior maternal spontaneous abortion, having an older brother, or being 13 y or older at time of phlebotomy. This underlines the need for a better understanding of the sources of microchimerism in this population and likely other populations as well. In conclusion, we show that approximately 1 in 7 nulliparous young girls test positive for male microchimerism, a level similar to what has been reported in healthy young girls and women without sons. We speculate that male microchimerism in these girls may originate from an older brother either full born or from a discontinued pregnancy, from transfusion during pregnancy, or possibly from sexual intercourse. However, other common sources of male microchimerism likely exist because less than half of male microchimerism positivity is attributable to these factors.
Patients and methods
Subjects
We recruited 154 girls born to women participating in the Danish National Birth Cohort (DNBC), which is a birth cohort of approximately 100,000 Danish women and their offspring.22 For the purpose of the present study girls and their mothers were bled and gave informed consent. Data regarding the mother's pregnancy history were obtained from the Danish Medical Birth Registry,23 and exposures during the index pregnancy were obtained from self-administered DNBC questionnaires filled in by the mother at pregnancy weeks 12 and 30 and 6 months after birth. Exposures during pregnancy (hypertension in pregnancy (HIP)/preeclampsia and transfusion), the mothers' prior pregnancy history (spontaneous abortion, induced abortion, extrauterine pregnancy, mola) and age at phlebotomy could be established for 143 (92.8%) girls. Blood samples were obtained from only 111 (72.1%) of the 154 girls' mothers, why male microchimerism status could be established for these girls only.
Preparation of samples
Blood samples were obtained by a female technician wearing gloves into 10 mL BD Vacutainer EDTA tubes. Samples were centrifuged at 3000 rpm for 10 minutes at 4°C. Approximately 1 mL of the buffy coat layer was carefully collected for each subject and stored at −80°C until further processing. DNA was extracted from the buffy coat samples using Maxwell Buffy coat DNA extraction kit on a Maxwell robot (Promega) according to the manufactures instructions. The DNA concentration was determined by use of the PicoGreen (Invitrogen) assay, in accordance to the instructions provided by the manufacturer. The DNA concentration ranged from 70–200 ng/µL.
DYS14 real-time PCR analysis
The real-time PCR analysis was performed by use of the Lightcycler 480 II instrument (Roche), and the DYS14 sequence was selected as the Y-chromosome marker. A PCR mastermix for the DYS14 assay was prepared on cooling blocks in a safety hood and contained 1x Lightcycler 480 probes master mix (Roche), 300 nM forward primer, 900 nM reverse primer and 250 nM probe (see Table 2). A PCR mastermix for the reference gene was prepared on cooling blocks in a safety hood and contained 1x Lightcycler 480 probes master mix (Roche), 300 nM of each primer and 250 nM probe. The reaction volume was 25 µL. Primer and probes sequences were as listed in Table 2. The probes were labeled with VIC (DYS14) and Cy5 (CCR5). For each sample, the amount of DNA added to each well were approximately 100,000 genome equivalents (GE) (6.6 pg DNA equals 1 GE [7]). The possible inhibition of the PCR by the amount of used DNA was tested by measuring the presence of 3 different concentrations of male DNA in the background of different levels of female DNA (see Fig. 2). There was no inhibition of the PCR for DYS14 in the background of 100.000 GE XX DNA. Furthermore, the limit of detection was set to 1 GE XY in the background of 100.000 GE XX DNA. Comparing the signal from wells with 100.000 GE female DNA with wells containing 0.5 GE male DNA in the background of 100.000 GE female DNA, the cut off value for positive XY signal was set to a Ct value of 40 (data not shown). Each sample was analyzed in a total of 12 wells, corresponding to a total of 1.2 million GE. The assay was run with the following cycling conditions 10 min at 95°C for pre-incubation, followed by 50 cycles of 10 s at 95°C, 30 s at 60°C and 1 s at 72°C. A calibration curve using 0.5, 1 and 10 GE male DNA in the background of 100.000 GE female DNA as well as a negative control (only female DNA) was included on each plate and run in triplicate. The negative control had to turn out negative in all 3 tests or the analysis was rerun. Two of 3 positive signals from 0.5 and 1GE male DNA was accepted. To study possible risk of contamination during sample handling 100 anonymous women were tested for male microchimerism. Five samples tested positive. For these samples and an equal number of negative samples, analysis was repeated on a second and if possibly a third blood sample taken previously andstored at −80°C (see Fig. 3). The sensitivity of the second and third test was increased by increasing the number of wells tested per sample (from 8 to 24) and results were interpreted by use of the Poisson distribution. The level of microchimerism in the positive samples were within the range 0,067–1,92 GE per 100.000 female GE; the same level detected in the women and girls tested. The level did not increase upon retest. Five samples were negative and remained negative when retested except for 1, which was detected with a low level of 0,021GE per 100.000 female GE. This is likely due to the increase in sensitivity and the method of interpretation where all wells with a CT value below 40 from a 24-plicates run have been taken into account using Poisson distribution.
Table 2.
Primer and the probe sequences used in DYS 14-analyses.
| Primer | Sequence 5′–3′ |
|---|---|
| DYS14_1582F, forward | CCAATTGAGTGGTATCCGGATT |
| DYS14_1649R, reverse | TTAAGGCTGCTGTTGTGGTGTCT |
| CCR5_F, forward | TACCTGCTCAACCTGGCCAT |
| CCR5_R, reverse | TTCCAAAGTCCCACTGGGC |
| DYS14_P | VIC_TGA AGT GGA GGC CTA TC |
| CCR5_P | Cy5-TTT CCT TCT TAC TGT CCC CTT CTG GGC TC |
Figure 2.

The sensitivity of the assay was determined by measuring 3 different concentration of male DNA in the background of varying concentration of female DNA.
Statistics
The association between exposures during the index pregnancy, maternal pregnancy history, the girls' age at phlebotomy and maternal male microchimerism status, respectively, and presence of male microchimerism in the girl was evaluated by crude odds ratio (OR) estimates with 95% confidence intervals (95% CI), obtained in logistic regression models. We chose a priori not to adjust OR estimates. To evaluate the fraction of male microchimerism positivity which could be attributed to ‘risk’ factors we calculated the population attributable fraction among the young girls using the method suggested by Bruzzi et al.24 Data management and analysis was conducted using SAS software version 9.4.25
Abbreviations
- CI
confidence interval
- DNBC
Danish National Birth Cohort
- FMc
fetal microchimerism
- GE
genome equivalents
- HIP
hypertension in pregnancy
- MMc
maternal microchimerism
- OR
odds ratio
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Line Hjort for blood sample assistance, Anne-Marie Nybo Andersen for sharing DNBC experience, and Susanne Hansen and Marin Ström for data delivery and approval help.
Funding
The study was supported by Department of Public Health, University of Copenhagen. The Danish National Research Foundation has established the Danish Epidemiology Science Center that initiated and created the Danish National Birth Cohort. The cohort is furthermore a result of a major grant from this Foundation. Additional support for the Danish National Birth Cohort is obtained from the Pharmacy Foundation, the Egmont Foundation, the March of Dimes Birth Defects Foundation, the Augustinus Foundation, and the Health Foundation.
References
- [1].Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci U S A 1996. January 23; 93(2):705-8; PMID:8570620; http://dx.doi.org/ 10.1073/pnas.93.2.705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gammill HS, Nelson JL. Naturally acquired microchimerism. Int J Dev Biol 2010; 54(2–3):531-43; PMID:19924635; http://dx.doi.org/ 10.1387/ijdb.082767hg [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gammill HS, Aydelotte TM, Guthrie KA, Nkwopara EC, Nelson JL. Cellular fetal microchimerism in preeclampsia. Hypertension 2013. December; 62(6):1062-7; http://dx.doi.org/ 10.1161/HYPERTENSIONAHA.113.01486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gammill HS, Stephenson MD, Aydelotte TM, Nelson JL. Microchimerism in recurrent miscarriage. Cell Mol Immunol 2014. November; 11(6):589-94; http://dx.doi.org/ 10.1038/cmi.2014.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kamper-Jorgensen M, Biggar RJ, Tjonneland A, Hjalgrim H, Kroman N, Rostgaard K, Stamper CL, Olsen A, Andersen AM, Gadi VK. Opposite effects of microchimerism on breast and colon cancer. Eur J Cancer 2012. September; 48(14):2227-35; http://dx.doi.org/ 10.1016/j.ejca.2012.02.006 [DOI] [PubMed] [Google Scholar]
- [6].Lambert NC, Lo YM, Erickson TD, Tylee TS, Guthrie KA, Furst DE, Nelson JL. Male microchimerism in healthy women and women with scleroderma: cells or circulating DNA? A quantitative answer. Blood 2002 Oct 15; 100(8):2845-51; http://dx.doi.org/ 10.1182/blood-2002-01-0295 [DOI] [PubMed] [Google Scholar]
- [7].Kamper-Jorgensen M, Mortensen LH, Andersen AM, Hjalgrim H, Gadi VK, Tjonneland A. Predictors of male microchimerism. Chimerism 2012. July; 3(3):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bloch EM, Reed WF, Lee TH, Montalvo L, Shiboski S, Custer B, Barcellos LF. Male microchimerism in peripheral blood leukocytes from women with multiple sclerosis. Chimerism 2011. January; 2(1):6-10; http://dx.doi.org/ 10.4161/chim.15151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yan Z, Lambert NC, Guthrie KA, Porter AJ, Loubiere LS, Madeleine MM, Stevens AM, Hermes HM, Nelson JL. Male microchimerism in women without sons: quantitative assessment and correlation with pregnancy history. Am J Med 2005. August; 118(8):899-906; http://dx.doi.org/ 10.1016/j.amjmed.2005.03.037 [DOI] [PubMed] [Google Scholar]
- [10].Lambert NC, Pang JM, Yan Z, Erickson TD, Stevens AM, Furst DE, Nelson JL. Male microchimerism in women with systemic sclerosis and healthy women who have never given birth to a son. Ann Rheum Dis 2005. June; 64(6):845-8; http://dx.doi.org/ 10.1136/ard.2004.029314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Klintschar M, Schwaiger P, Regauer S, Mannweiler S, Kleiber M. Persisting fetal microchimerism does not interfere with forensic Y-chromosome typing. Forensic Sci Int 2004 Jan 28; 139(2–3):151-4; http://dx.doi.org/ 10.1016/j.forsciint.2003.10.011 [DOI] [PubMed] [Google Scholar]
- [12].Stevens AM, Howsmon R, Gadi VK, Gammill HS, Lambert NC, Sunku CC, Hermes HM, Porter AJ, Lu C, Nelson JL. Male microchimerism in girls with systemic lupus erythematosus (SLE). Chimerism 2013; 4(2):51. [Google Scholar]
- [13].Imanishi D, Miyazaki Y, Yamasaki R, Sawayama Y, Taguchi J, Tsushima H, Fukushima T, Yoshida S, Sasaki H, Hata T, et al.. Donor-derived DNA in fingernails among recipients of allogeneic hematopoietic stem cell transplantation. Blood 2007; 110(7):2231-4. [DOI] [PubMed] [Google Scholar]
- [14].Utter GH, Reed WF, Lee TH, Busch MP. Transfusion-associated microchimerism. Vox Sang 2007. October; 93(3):188-95; http://dx.doi.org/ 10.1111/j.1423-0410.2007.00954.x [DOI] [PubMed] [Google Scholar]
- [15].de Bellefon LM, Heiman P, Kanaan SB, Azzouz DF, Rak JM, Martin M, Roudier J, Roufosse F, Lambert N. Cells from a vanished twin as a source of microchimerism 40 years later. Chimerism 2011; 1:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].National Board of Health National report on lifestyle and health on adolescence age 11–15 years. 2015. Report. Available at: https://sundhedsstyrelsen.dk/da/sundhed-og-livsstil/boern-og-unge/unges-livsstil-og-dagligdag/11-15-aariges-livsstil-og-sundhedsvaner
- [17].Guettier C, Sebagh M, Buard J, Feneux D, Ortin-Serrano M, Gigou M, Tricottet V, Reynes M, Samuel D, Feray C. Male cell microchimerism in normal and diseased female livers from fetal life to adulthood. Hepatology 2005. July; 42(1):35-43; http://dx.doi.org/ 10.1002/hep.20761 [DOI] [PubMed] [Google Scholar]
- [18].Sanchez R, Lee TH, Wen L, Montalvo L, Schechterly C, Colvin C, Alter HJ, Luban NL, Busch MP. Absence of transfusion-associated microchimerism in pediatric and adult recipients of leukoreduced and gamma-irradiated blood components. Transfusion 2012. May; 52(5):936-45; http://dx.doi.org/ 10.1111/j.1537-2995.2011.03366.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dierselhuis MP, Blokland EC, Pool J, Schrama E, Scherjon SA, Goulmy E. Transmaternal cell flow leads to antigen-experienced cord blood. Blood 2012. July 19; 120(3):505-10; http://dx.doi.org/ 10.1182/blood-2012-02-410571 [DOI] [PubMed] [Google Scholar]
- [20].Dierselhuis MP, Jankowska-Gan E, Blokland E, Pool J, Burlingham WJ, van Halteren AG, Goulmy E. HY immune tolerance is common in women without male offspring. PLoS One 2014; 9(3):e91274; PMID:24646895; http://dx.doi.org/ 10.1371/journal.pone.0091274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Reed W, Lee TH, Norris PJ, Utter GH, Busch MP. Transfusion-associated microchimerism: a new complication of blood transfusions in severely injured patients. Semin Hematol 2007. January; 44(1):24-31; http://dx.doi.org/ 10.1053/j.seminhematol.2006.09.012 [DOI] [PubMed] [Google Scholar]
- [22].Olsen J, Melbye M, Olsen SF, Sorensen TI, Aaby P, Andersen AM, Taxbol D, Hansen KD, Juhl M, Schow TB, et al.. The Danish National Birth Cohort–its background, structure and aim. Scand J Public Health 2001. December; 29(4):300-7; http://dx.doi.org/ 10.1177/14034948010290040201 [DOI] [PubMed] [Google Scholar]
- [23].Knudsen LB, Olsen J. The danish medical birth registry. Dan Med Bull 1998. June; 45(3):320-3. [PubMed] [Google Scholar]
- [24].Bruzzi P, Green SB, Byar DP, Brinton LA, Schairer C. Estimating the population attributable risk for multiple risk factors using case-control data. Am J Epidemiol 1985. November; 122(5):904-14. [DOI] [PubMed] [Google Scholar]
- [25].SAS Institute Inc SAS/STAT software release 9.4 Cary Cary, NC: SAS Institute Inc; 2008. Computer program. [Google Scholar]


