ABSTRACT
Development of Schwann cells is tightly regulated by concerted action of activating and inhibiting factors. Most of the regulatory feedback loops identified to date are transcriptional activators promoting induction of genes coding for integral myelin proteins and lipids. The mechanisms by which inhibitory factors are silenced during Schwann cell maturation are less well understood. We could recently show a pivotal function for the transcription factor zinc finger E-box binding homeobox 2 (Zeb2) during Schwann cell development and myelination as a transcriptional repressor of maturation inhibitors. Zeb2 belongs to a family of highly conserved 2-handed zinc-finger proteins and represses gene transcription by binding to E-box sequences in the regulatory region of target genes. The protein is known to repress E-cadherin during epithelial to mesenchymal transition (EMT) in tumor malignancy and mediates its functions by interacting with multiple co-factors. During nervous system development, Zeb2 is expressed in neural crest cells, the precursors of Schwann cells, the myelinating glial cells of peripheral nerves. Schwann cells lacking Zeb2 fail to fully differentiate and are unable to sort and myelinate peripheral nerve axons. The maturation inhibitors Sox2, Ednrb and Hey2 emerge as targets for Zeb2-mediated transcriptional repression and show persistent aberrant expression in Zeb2-deficient Schwann cells. While dispensible for adult Schwann cells, re-activation of Zeb2 is essential after nerve injury to allow remyelination and functional recovery. In summary, Zeb2 emerges as an “inhibitor of inhibitors,” a novel concept in Schwann cell development and nerve repair.
keywords: PNS, Schwann cell, Schwann cell development, transcription factor, regeneration
Zeb2 in Schwann cell development
Schwann cells (SC) stem from the neural crest, myelinate and support peripheral nervous system (PNS) axons and are key players in regeneration after nerve injury.1 The Schwann cell lineage is well defined and divided into 3 main stages: Schwann cell precursors (SCP), immature Schwann cells and fully differentiated myelinating or non-myelinating Schwann cells. Each stage of Schwann cell development is characterized by the expression of a set of specific transcription and growth factors, some of them promoting maturation, others acting as differentiation inhibitors.2 Most of the regulatory input during Schwann cell development and myelination known to date occurs via feedforward cascades of transcription factors. The pluripotency gene Sox10 has emerged as one important transcriptional co-activator promoting maturation and is crucial at each stage of Schwann cell development. Conventional Sox10 knockout mice do not develop any peripheral glia and die prenatally while inactivation specifically in Schwann cells leads to their developmental arrest at the immature stage.3,4 Furthermore, Sox10 drives myelination by binding to Krox20 (Egr2).5,6 In mice deficient of Krox20, Schwann cells manage to radially sort axons, but do not myelinate them.7 In addition, Krox20 is important for myelin maintenance8 and missense mutations can be found in patients with congenital hypomyelinating neuropathy and Charcot Marie Tooth disease Type 1D.9 Other positive regulators of Schwann cell myelination include neuregulin 1 (NRG1),10 laminin–integrin signaling11,12 and G-protein-coupled receptors (GPCRs) such as Gpr126.13 In addition to these factors promoting Schwann cell maturation, negative modulators of the myelination program need to be silenced to ensure timely lineage progression.14 It is still unclear, how these inhibitory proteins are regulated in development and pathological conditions, such as neuropathies and acute nerve injury. We have identified the transcriptional repressor Zeb2 as one of the crucial players in the silencing of Schwann cell maturation inhibitors.15 Proteins of the Zeb family are involved in regulation of cell type specification and differentiation and highly conserved in vertebrates.16 They bind to the E-box like sequence 5′-CACCT(G) in regulatory regions of their target genes and their ability to activate or repress gene transcription stems from interaction with co-effectors, such as CtBP,17 NurD18 and Smad transcription factors.19 Smads are downstream effectors of the transforming growth factor β (TGFβ) superfamily of proteins, which have been shown to regulate Schwann cell proliferation and death in vivo.20 Smad signaling plays a major role in organogenesis21 and Zeb2 is especially important during nervous system development22 as evident from the study of genetically altered mouse mutants.23,24 Zeb2 knockout mice die early embryonically from failed neural tube closure and severe neural plate defects.25,26 In humans, mutations of the Zeb2 gene cause a severe neurodevelopmental disorder, Mowat-Wilson syndrome, characterized by mental retardation, Hirschsprung's disease and craniofacial abnormalities.27 Notably, Hirschsprung's disease also occurs in patients having Waardenburg syndrome type 4C, caused by mutations in the Sox10 gene.28 Both, Sox10 and Zeb2, show a remarkably similar expression pattern in neural crest cells, a highly motile pluripotent cell population, which forms in neurodevelopment as part of the neuroectoderm, and have even been proposed to interact in these cells.29 We hypothesized, that Zeb2 could represent a second inhibitory axis in Schwann cell development, acting in concert with transcriptional activators such as Sox10 to ensure timely lineage progression. In proof of principle experiments we could indeed demonstrate, that Zeb2 regulates 2 signaling systems described previously as crucial to Schwann cell development. One of these is Notch signaling, which plays multiple roles in Schwann cells. Notch governs the transition from SCPs to immature Schwann cells, regulates Schwann cell proliferation, and is downregulated by Krox20 at the initiation of myelination. Furthermore, enforced re-expression of the Notch intracellular domain in mature Schwann cells leads to demyelination.30 We found that Zeb2 binds in the promoter of Hairy/enhancer-of-split related with YRPW motif protein 2 (Hey2), a downstream effector of Notch signaling.15 Hey2 has not been shown to be expressed by Schwann cells before, however, it is a marker for boundary cap cells, a specialized cell population situated at the border of CNS and PNS known to be a source for Schwann cells in the ventral and dorsal roots.31 In Zeb2-deficient Schwann cells, we found highly elevated Hey2 mRNA levels compared with controls and additional inactivation of Hey2 led to a partial improvement of the severe sorting defect observed in conditional Zeb2 knockout mice. It has to be mentioned, that steady-state levels of Hey2 mRNA in sciatic nerves of 25 d old control mice were very low. Hence, aberrant Hey2 expression at that time point seems to be a feature characteristic of Zeb2-deficient cells.15 In a second study published in the same issue as our own work, it has recently been shown that there is an early increase in Hey2 expression at postnatal day 5 followed by a rapid downregulation of Hey2 mRNA levels in sciatic nerve of wild type mice.32
The second Zeb2-regulated pathway is the endothelin- endothelin receptor signaling system, consisting of the 3 small peptides endothelin (ET) 1, 2 and 3 and 2 G-protein coupled receptors, endothelin receptors A and B (EDNRA and B). Endothelin signaling is crucial for neural crest derivate development. While loss of ET-1 or EDNRA leads to craniofacial abnormalities,33,34 loss of ET3 or EDNRB results in a melanocyte defect and Hirschsprung's disease.35 During Schwann cell development, activation of EDNRB delays the generation of immature Schwann cells from precursors in vitro and in vivo, thereby acting as a brake in Schwann cell maturation.36 We identified Ednrb as a target gene for Zeb2-mediated repression. Similar to our results for Hey2, levels of Ednrb mRNA remained high in Zeb2-deficient Schwann cells and additional deletion of Ednrb in Zeb2-deficient cells led to a partial amelioration of the sorting defect observed in Zeb2 conditional mutants.
In addition to Hey2 and Ednrb, we identified a third Zeb2 target gene, the transcription factor Sox2.15 Sox2 is a known inhibitor of myelination,37,38 which could be recently demonstrated by transgenic mice with enforced expression in Schwann cells. This led to persistent Schwann cell proliferation and repressed peripheral nerve myelination (D. B. Parkinson personal communication). In early Schwann cell development, Sox2 and Sox10 act in concert to promote RNA polymerase transcriptional elongation by recruiting P-TEFb to induce myelin gene expression,39 however, Sox2 expression is absent from mature Schwann cells. We found, that Zeb2-deficiency led to persistent Sox2 expression in Schwann cells and identified functional Zeb2 binding sites in the Sox2 promoter.15
Recently, epigenetics and alterations of chromatin structure came into the focus of Schwann cell biology since loss of histone deacetylases 1 and 2 during development impairs radial sorting, SC development and myelination.40,41 It has been shown that the histone deacetylases 1 and 2 (HDAC 1 and 2) induce the expression of Pax3, which itself is required for expression of Sox10 during SC development, and induce important myelin genes like FABP7 and MPZ.42 Furthermore, inactivation of Cdh4, the core unit of the NuRD chromatin-remodeling complex leads to mild defects of peripheral myelination.43 On the other hand loss of Brg1, the ATPase of the BAF complex, leads to a severe differentiation and myelination defect.44 In a companion paper to ours, Wu et al. demonstrated that Zeb2 mediates gene repression in Schwann cell differentiation by recruiting HDAC1/2-NuRD complexes which then suppress the maturation inhibitors Sox2 and Hey2 during Schwann cell development.32
In summary, Zeb2 functions as an inhibitor of inhibition in Schwann cell development, repressing the expression of Hey2, Ednrb, Sox2 and possibly other factors stalling maturation. Transcriptional repression by Zeb2 emerges as a novel concept in Schwann cell maturation acting in concert with transcriptional activation by Sox10, Oct6 and Krox20 (Fig. 1a).
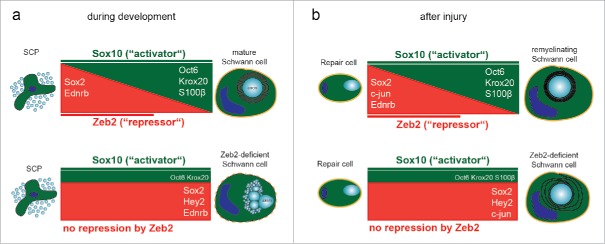
Figure 1.
Hypothetical model of Zeb2 function in Schwann cells. During the development of Schwann cell precursors (SCP) to mature Schwann cells Krox20 is activated by binding of positive regulators ((a), top panel, depicted in green) such as Oct6 and the co-activator Sox10, leading to the upregulation of genes coding for myelin proteins and enzymes of the lipid biosynthesis pathway. In addition, the cytoplasmic protein S100β is upregulated at the transition from Schwann cell precursors to immature Schwann cells. At the same time, Sox2 and Ednrb, which act as developmental brakes (depicted in red), are repressed by Zeb2. Loss of Zeb2 in Schwann cells leads to continuous expression of maturation inhibitors, resulting in a failure to sort axons and myelinate ((a), bottom panel). (b) After nerve injury Sox2, c-Jun and Ednrb expression is induced and a specialized repair cell is generated, leading to the formation of regenerative tracks and allowing axon regrowth. These genes need to be silenced again at later stages to enable successful Schwann cell re-differentiation and remyelination (top panel). Zeb2-deficient Schwann cells are capable of de-differentiation after nerve injury, however, a subpopulation of mutant cells maintains expression of negative regulatory factors and cannot re-differentiate and remyelinate (bottom panel).
Zeb2 in Schwann cell re-differentiation after injury
Zeb2 has been described in cancer metastasis as a key promoter of epithelial-to-mesenchymal transition (EMT), a process in which differentiated stationary cells convert to a more immature, motile, proliferative phenotype.45 This conversion, which does not only occur in pathological states, but is also crucial for embryonic organ development and wound healing, can be compared with the massive phenotypical changes undergone by Schwann cells after peripheral nerve injury. The “reprogrammed” cell type generated after nerve injury shares some features of immature Schwann cells, however, the generation of regenerative tracts (bands of Bungner) and support of axonal regeneration are unique to these repair cells.46 Notably, maturation inhibitors and markers of immature Schwann cells such as c-Jun, Sox2, and Notch are reactivated after nerve injury.37,38 Furthermore, re-expression of c-Jun and Sox2 has been shown to be absolutely essential for successful peripheral nerve regeneration. C-Jun-deficient Schwann cells are unable to form functional repair cells47 and Sox2 mediates fibroblast-dependent Schwann cell sorting during nerve regeneration.48 Importantly, just as in development, these factors necessary to form the repair cell need to be inactivated again to allow remyelination.46 When we deleted Zeb2 in Schwann cells of adult mice by tamoxifen-inducible Cre-recombination and induced a sciatic nerve compression injury, we found a severe delay in regeneration and functional recovery in conditional mutants. Even as late as 8 weeks after injury, remyelination by Zeb2-deficient Schwann cells had not been completed. These cells showed the same upregulation of c-Jun and Sox2 after nerve transection as the corresponding controls. In contrast, re-expression of Krox20 as a differentiation marker was reduced 56 d after nerve crush and expression of Sox2 remained high. We therefore conclude, that loss of Zeb2 leads to a failure of Schwann cells to re-differentiate to a mature remyelinating cell after acute injury, whereas the generation of the repair cell proceeds normally. Interestingly, if myelin repair did initiate in conditional Zeb2 mutants, remyelination proceeded normally.15 This is similar to c-Jun-deficient Schwann cells.47 Impaired functional recovery is common to both conditional mutants, however, substantial loss of sensory fibers was observed when Schwann cells lacked c-Jun, while axons seemed to be preserved in conditional Zeb2 mutant mice after nerve crush.47,15 It has been hypothesized before, that the specialized repair cell, in addition to gaining support functions, may also lose some characteristics of immature Schwann cells in development and thereby share similarities with Schwann cell precursors.47,46 One study showed the ability of Schwann cells after nerve transection to re-differentiate into melanocytes, a feature characteristic of precursors.49 In wild type nerves, we found a highly adaptable Zeb2 re-expression after nerve crush with the highest number of cells positive for the protein at 7 d after compression injury. This may imply, that not all repair cells simultaneously become dependent on Zeb2 re-expression after acute nerve injury. Accordingly, in Zeb2-deficient repair cells continued expression of maturation inhibitors such as Sox2, c-Jun and ectopic expression of Hey2 may, in a subpopulation of cells, prevent normal execution of the maturation and myelination program after nerve injury (Fig. 1b).
We describe for the first time an essential role for a gene involved in cellular reprogramming in cancer metastasis during Schwann cell lineage progression and re-differentiation of the repair cell after sciatic nerve crush. Characterization of genes involved in the molecular and morphological changes common to these 3 processes may provide novel therapeutic strategies for the treatment of human neuropathies and nerve injuries.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Monk KR, Feltri ML, Taveggia C. New insights on Schwann cell development. Glia 2015; 63:1376-93; PMID:25921593; http://dx.doi.org/ 10.1002/glia.22852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jessen KR, Mirsky R, Lloyd AC. Schwann Cells: Development and Role in Nerve Repair. Cold Spring Harb Perspect Biol 2015; 7:a020487; PMID:25957303; http://dx.doi.org/ 10.1101/cshperspect.a020487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Britsch S, Goerich DE, Riethmacher D, Peirano RI, Rossner M, Nave KA, Birchmeier C, Wegner M. . The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev 2001; 15:66-78; PMID:11156606; http://dx.doi.org/ 10.1101/gad.186601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Finzsch M, Schreiner S, Kichko T, Reeh P, Tamm ER, Bösl MR, Meijer D, Wegner M. Sox10 is required for Schwann cell identity and progression beyond the immature Schwann cell stage. J Cell Biol 2010; 189:701-12; PMID:20457761; http://dx.doi.org/ 10.1083/jcb.200912142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ghislain J, Charnay P. Control of myelination in Schwann cells: a Krox20 cis-regulatory element integrates Oct6, Brn2 and Sox10 activities. EMBO Rep. 2006; 7:52-8; PMID:16311519; http://dx.doi.org/ 10.1038/sj.embor.7400573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Stolt CC, Wegner M. Schwann cells and their transcriptional network: Evolution of key regulatorsof peripheral myelination. Brain Res 2016; 1641:101-10; http://dx.doi.org/ 10.1016/j.brainres.2015.09.025 [DOI] [PubMed] [Google Scholar]
- [7].Topilko P, Schneider-Maunoury S, Levi G, Baron-Van Evercooren A, Chennoufi AB, Seitanidou T, Babinet C, Charnay P. Krox-20 controls myelination in the peripheral nervous system. Nature 1994; 371:796-9; PMID:7935840; http://dx.doi.org/ 10.1038/371796a0 [DOI] [PubMed] [Google Scholar]
- [8].Decker L, Desmarquet-Trin-Dinh C, Taillebourg E, Ghislain J, Vallat JM, Charnay P. . Peripheral myelin maintenance is a dynamic process requiring constant Krox20 expression. J Neurosci 2006; 26:9771-9; PMID:16988048; http://dx.doi.org/ 10.1523/JNEUROSCI.0716-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Warner LE, Mancias P, Butler IJ, McDonald CM, Keppen L, Koob KG, Lupski JR. Mutations in the early growth response 2 (EGR2) gene are associated with hereditary myelinopathies. Nat Genet 1998; 18:382-4; PMID:9537424; http://dx.doi.org/ 10.1038/ng0498-382 [DOI] [PubMed] [Google Scholar]
- [10].Newbern J, Birchmeier C. Nrg1/ErbB signaling networks in Schwann cell development and myelination. Semin Cell Dev Biol 2010; 21:922-8; PMID:20832498; http://dx.doi.org/ 10.1016/j.semcdb.2010.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nodari A, Zambroni D, Quattrini A, Court FA, D'Urso A, Recchia A, Tybulewicz VLJ, Wrabetz L, Feltri ML. β1 integrin activates Rac1 in Schwann cells to generate radial lamellae during axonal sorting and myelination. J Cell Biol 2007; 177:1063-75; PMID:17576799; http://dx.doi.org/ 10.1083/jcb.200610014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Court FA, Hewitt JE, Davies K, Patton BL, Uncini A, Wrabetz L, Feltri ML. A laminin-2, dystroglycan, utrophin axis is required for compartmentalization and elongation of myelin segments. J Neurosci 2009; 29:3908-19; PMID:19321787; http://dx.doi.org/ 10.1523/JNEUROSCI.5672-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Monk KR, Oshima K, Jörs S, Heller S, Talbot WS. Gpr126 is essential for peripheral nerve development and myelination in mammals. Development 2011; 138:2673-80; PMID:21613327; http://dx.doi.org/ 10.1242/dev.062224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jessen KR, Mirsky R. Negative regulation of myelination: Relevance for development, injury, and demyelinating disease. Glia 2008; 56:1552-65; PMID:18803323; http://dx.doi.org/ 10.1002/glia.20761 [DOI] [PubMed] [Google Scholar]
- [15].Quintes S, Brinkmann BG, Ebert M, Fröb F, Kungl T, Arlt FA, Tarabykin V, Huylebroeck D, Meijer D, Suter U, et al.. Zeb2 is essential for Schwann cell differentiation, myelination and nerve repair. Nat Neurosci 2016; 19:1050-9; PMID:27294512; http://dx.doi.org/ 10.1038/nn.4321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hegarty SV, Sullivan AM, O'Keeffe GW. Zeb2: A multifunctional regulator of nervous system development. Prog Neurobiol 2015:1-15; http://dx.doi.org/ 10.1016/j.pneurobio.2015.07.001 [DOI] [PubMed] [Google Scholar]
- [17].van Grunsven LA, Michiels C, Van de Putte T, Nelles L, Wuytens G, Verschueren K, Huylebroeck D. Interaction between Smad-interacting protein-1 and the corepressor C-terminal binding protein is dispensable for transcriptional repression of E-cadherin. J Biol Chem 2003; 278:26135-45; PMID:12714599; http://dx.doi.org/ 10.1074/jbc.M300597200 [DOI] [PubMed] [Google Scholar]
- [18].Verstappen G, van Grunsven LA, Michiels C, Van de Putte T, Souopgui J, Van Damme J, Bellefroid E, Vandekerckhove J, Huylebroeck D. Atypical Mowat-Wilson patient confirms the importance of the novel association between ZFHX1B/SIP1 and NuRD corepressor complex. Hum Mol Genet 2008; 17:1175-83; PMID:18182442; http://dx.doi.org/ 10.1093/hmg/ddn007 [DOI] [PubMed] [Google Scholar]
- [19].Verschueren K, Remacle JE, Collart C, Kraft H, Baker BS, Tylzanowski P, Nelles L, Wuytens G, Su MT, Bodmer R, et al.. SIP1, a novel zinc finger/homeodomain repressor, interacts with smad proteins and binds to 5′-CACCT sequences in candidate target genes. J Bioll Chem 1999; 274:20489-98; PMID:10400677; http://dx.doi.org/ 10.1074/jbc.274.29.20489 [DOI] [PubMed] [Google Scholar]
- [20].D'Antonio M, Droggiti A, Feltri ML, Roes J, Wrabetz L, Mirsky R, Jessen KR. TGF Type II Receptor Signaling Controls Schwann Cell Death and Proliferation in Developing Nerves. J Neurosci 2006; 26:8417-27; PMID:16914667; http://dx.doi.org/ 10.1523/JNEUROSCI.1578-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Conidi A, Cazzola S, Beets K, Coddens K, Collart C, Cornelis F, Cox L, Joke D, Dobreva MP, Dries R, et al.. Few Smad proteins and many Smad-interacting proteins yield multiple functions and action modes in TGF Cytokine & growth factor reviews. Cytokine and Growth Factor Reviews 2011; 22:287-300; PMID:22119658; http://dx.doi.org/ 10.1016/j.cytogfr.2011.11.006 [DOI] [PubMed] [Google Scholar]
- [22].Hegarty SV, O'Keeffe GW, Sullivan AM. BMP-Smad 1/5/8 signalling in the development of the nervous system. Prog Neurobiol 2013; 109:28-41; PMID:23891815; http://dx.doi.org/ 10.1016/j.pneurobio.2013.07.002 [DOI] [PubMed] [Google Scholar]
- [23].Seuntjens E, Nityanandam A, Miquelajauregui A, Debruyn J, Stryjewska A, Goebbels S, Nave KA, Huylebroeck D, Tarabykin V. Sip1 regulates sequential fate decisions by feedback signaling from postmitotic neurons to progenitors. Nat Neurosci 2009; 12:1373-80; PMID:19838179; http://dx.doi.org/ 10.1038/nn.2409 [DOI] [PubMed] [Google Scholar]
- [24].Parthasarathy S, Srivatsa S, Nityanandam A, Tarabykin V. Ntf3 acts downstream of Sip1 in cortical postmitotic neurons to control progenitor cell fate through feedback signaling. Development 2014; 141:3324-30; PMID:25085976; http://dx.doi.org/ 10.1242/dev.114173 [DOI] [PubMed] [Google Scholar]
- [25].Higashi Y, Maruhashi M, Nelles L, Van de Putte T, Verschueren K, Miyoshi T, Yoshimoto A, Kondoh H, Huylebroeck D. Generation of the floxed allele of the SIP1 (Smad-interacting protein 1) gene for Cre-mediated conditional knockout in the mouse. Genesis 2002; 32:82-4; PMID:11857784; http://dx.doi.org/ 10.1002/gene.10048 [DOI] [PubMed] [Google Scholar]
- [26].Van de Putte T, Maruhashi M, Francis A, Nelles L, Kondoh H, Huylebroeck D, Higashi Y. Mice lacking ZFHX1B, the gene that codes for Smad-interacting protein-1, reveal a role for multiple neural crest cell defects in the etiology of Hirschsprung disease-mental retardation syndrome. Am. J. Hum. Genet. 2003; 72:465-70; PMID:12522767; http://dx.doi.org/ 10.1086/346092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mowat DR, Croaker GD, Cass DT, Kerr BA, Chaitow J, Adès LC, Chia NL, Wilson MJ. Hirschsprung disease, microcephaly, mental retardation, and characteristic facial features: delineation of a new syndrome and identification of a locus at chromosome 2q22-q23. J. Med. Genet. 1998; 35:617-23; PMID:9719364; http://dx.doi.org/ 10.1136/jmg.35.8.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bondurand N, Sham MH. The role of SOX10 during enteric nervous system development. Dev Biol 2013; 382:330-43; PMID:23644063; http://dx.doi.org/ 10.1016/j.ydbio.2013.04.024 [DOI] [PubMed] [Google Scholar]
- [29].Stanchina L, Van de Putte T, Goossens M, Huylebroeck D, Bondurand N. Genetic interaction between Sox10 and Zfhx1b during enteric nervous system development. Dev Biol 2010; 341:416-28; PMID:20206619; http://dx.doi.org/ 10.1016/j.ydbio.2010.02.036 [DOI] [PubMed] [Google Scholar]
- [30].Woodhoo A, Alonso MB, Droggiti A, Turmaine M, D'Antonio M, Parkinson DB, Wilton DK, Al-Shawi R, Simons P, Shen J, et al.. Notch controls embryonic Schwann cell differentiation, postnatal myelination and adult plasticity. Nat Neurosci 2009; 12:839-47; PMID:19525946; http://dx.doi.org/ 10.1038/nn.2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Coulpier F, Le Crom S, Maro GS, Manent J, Giovannini M, Maciorowski Z, Fischer A, Gessler M, Charnay P, Topilko P. Novel features of boundary cap cells revealed by the analysis of newly identified molecular markers. Glia 2009; 57:1450-7; PMID:19243017; http://dx.doi.org/ 10.1002/glia.20862 [DOI] [PubMed] [Google Scholar]
- [32].Wu LMN, Wang J, Conidi A, Zhao C, Wang H, Ford Z, Zhang L, Zweier C, Ayee BG, Maurel P, et al.. Zeb2 recruits HDAC-NuRD to inhibit Notch and controls Schwann cell differentiation and remyelination. Nat Neurosci 2016; 19:1060-72; PMID:27294509; http://dx.doi.org/ 10.1038/nn.4322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kurihara Y, Kurihara H, Suzuki H, Kodama T, Maemura K, Nagai R, Oda H, Kuwaki T, Cao WH, Kamada N, et al.. Elevated blood pressure and craniofacial abnormalities in mice deficient in endothelin-1. Nature 1994; 368:703-10; PMID:8152482; http://dx.doi.org/ 10.1038/368703a0 [DOI] [PubMed] [Google Scholar]
- [34].Clouthier DE, Hosoda K, Richardson JA, Williams SC, Yanagisawa H, Kuwaki T, Kumada M, Hammer RE, Yanagisawa M. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development 1998; 125:813-24; PMID:9449664 [DOI] [PubMed] [Google Scholar]
- [35].AG Baynash, Hosoda K, Giaid A, Richardson JA, Emoto N, Hammer RE, Yanagisawa M. Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell 1994; 79:1277-85; PMID:8001160; http://dx.doi.org/ 10.1016/0092-8674(94)90018-3 [DOI] [PubMed] [Google Scholar]
- [36].Brennan A, Dean CH, Zhang AL, Cass DT, Mirsky R, Jessen KR. Endothelins control the timing of Schwann cell generation in vitro and in vivo. Dev Biol 2000; 227:545-57; PMID:11071773; http://dx.doi.org/ 10.1006/dbio.2000.9887 [DOI] [PubMed] [Google Scholar]
- [37].Le N, Nagarajan R, Wang JY, Araki T, Schmidt RE, Milbrandt J. Analysis of congenital hypomyelinating Egr2Lo/Lo nerves identifies Sox2 as an inhibitor of Schwann cell differentiation and myelination. Proc Natl Acad Sci U S A 2005; 102:2596-601; PMID:15695336; http://dx.doi.org/ 10.1073/pnas.0407836102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Parkinson DB, Bhaskaran A, Arthur-Farraj P, Noon LA, Woodhoo A, Lloyd AC, Feltri ML, Wrabetz L, Behrens A, Mirsky R, et al.. c-Jun is a negative regulator of myelination. J Cell Biol 2008; 181:625-37; PMID:18490512; http://dx.doi.org/ 10.1083/jcb.200803013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Arter J, Wegner M. Transcription factors Sox10 and Sox2 functionally interact with positive transcription elongation factor b in Schwann cells. J Neurochem 2015; 132:384-93; PMID:25524031; http://dx.doi.org/ 10.1111/jnc.13013 [DOI] [PubMed] [Google Scholar]
- [40].Chen Y, Wang H, Yoon SO, Xu X, Hottiger MO, Svaren J, Nave KA, Kim HA, Olson EN, Lu QR. HDAC-mediated deacetylation of NF-B is critical for Schwann cell myelination. Nat Neurosci 2011; 14:437-41; PMID:21423191; http://dx.doi.org/ 10.1038/nn.2780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jacob C, Christen CN, Pereira JA, Somandin C, Baggiolini A, Lötscher P, Özcelik M, Tricaud N, Meijer D. Yamaguchi T et al. HDAC1 and 2 control the transcriptional program of myelination and the survival of schwann cells. Nat Neurosci 2011; 14:429-36; PMID:21423190; http://dx.doi.org/24760871 10.1038/nn.2762 [DOI] [PubMed] [Google Scholar]
- [42].Jacob C, Lötscher P, Engler S, Baggiolini A, Varum Tavares S, Brügger V, John N, Büchmann-Møller S, Snider PL, Conway SJ, et al.. HDAC1 and HDAC2 control the specification of neural crest cells into peripheral glia. J Neurosci 2014; 34:6112-22; PMID:24760871; http://dx.doi.org/ 10.1523/JNEUROSCI.5212-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hung H, Kohnken R, Svaren J. The Nucleosome Remodeling and Deacetylase Chromatin Remodeling (NuRD) complex is required for peripheral nerve myelination. J Neurosci 2012; 32:1517-27; PMID:22302795; http://dx.doi.org/ 10.1523/JNEUROSCI.2895-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Weider M, Küspert M, Bischof M, Vogl MR, Hornig J, Loy K, Kosian T, Müller J, Hillgärtner S, Tamm ER et al. Chromatin-remodeling factor Brg1 is required for schwann cell differentiation and myelination. Dev Cell 2012; 23:193-201; PMID:22814607; http://dx.doi.org/18343170 10.1016/j.devcel.2012.05.017 [DOI] [PubMed] [Google Scholar]
- [45].Baum B, Settleman J, Quinlan MP. Transitions between epithelial and mesenchymal states in development and disease. Semin Cell Dev Biol 2008; 19:294-308; PMID:18343170; http://dx.doi.org/ 10.1016/j.semcdb.2008.02.001 [DOI] [PubMed] [Google Scholar]
- [46].Jessen KR, Mirsky R, Arthur-Farraj P. The role of cell plasticity in tissue repair: Adaptive cellular reprogramming. Dev Cell 2015; 34:613-20; PMID:26418293; http://dx.doi.org/ 10.1016/j.devcel.2015.09.005 [DOI] [PubMed] [Google Scholar]
- [47].PJ Arthur-Farraj, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman M, Turmaine M, et al.. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron 2012; 75:633-47; PMID:22920255; http://dx.doi.org/ 10.1016/j.neuron.2012.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Parrinello S, Napoli I, Ribeiro S, Wingfield Digby P, Fedorova M, Parkinson DB, Doddrell RD, Nakayama M, Adams RH, Lloyd AC. EphB Signaling Directs Peripheral Nerve Regeneration through Sox2-Dependent Schwann Cell Sorting. Cell 2010; 143:145-55; PMID:20869108; http://dx.doi.org/ 10.1016/j.cell.2010.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Adameyko I, Lallemend F, Aquino JB, Pereira JA, Topilko P, Müller T, Fritz N, Beljajeva A, Mochii M, Liste I, et al.. Schwann Cell Precursors from Nerve Innervation Are a Cellular Originof Melanocytes in Skin. Cell 2009; 139:366-79; PMID:19837037; http://dx.doi.org/ 10.1016/j.cell.2009.07.049 [DOI] [PubMed] [Google Scholar]