Abstract
Background and objectives
Mean corpuscular volume is the measure of the average size of the circulatory erythrocyte, and it is principally used as an index for the differential diagnosis of anemia. Recently, mean corpuscular volume has been associated with mortality in many clinical settings. However, the association of mean corpuscular volume with mortality in patients with CKD has not been fully addressed.
Design, setting, participants, & measurements
We conducted a retrospective observational cohort study of 1439 patients with stages 3–5 CKD and baseline mean corpuscular volume values from 2004 to 2012 in a medical center. The study cohort was divided into the high–mean corpuscular volume group and the low–mean corpuscular volume group by the median value (90.8 fl) of mean corpuscular volume. The baseline patient information included demographic data, laboratory parameters, medications, and comorbid conditions. The independent association of mean corpuscular volume with mortality was examined using multivariate Cox regression analysis.
Results
Of the 1439 participants, 234 patients (16.2%) died during a median follow-up of 1.9 years (interquartile range, 1.1–3.8 years). The crude overall mortality rate was significantly higher in the high–mean corpuscular volume group (high–mean corpuscular volume group, 22.7%; low–mean corpuscular volume group, 9.7%; P<0.001). In the fully adjusted models, the high–mean corpuscular volume group was associated with higher risks of all-cause mortality (hazard ratio, 2.19; 95% confidence interval, 1.62 to 2.96; P<0.001), cardiovascular mortality (hazard ratio, 3.57; 95% confidence interval, 1.80 to 7.06; P<0.001), and infection-related mortality (hazard ratio, 2.22; 95% confidence interval, 1.41 to 3.49; P=0.001) compared with the low–mean corpuscular volume group.
Conclusions
In patients with stages 3–5 CKD, mean corpuscular volume was associated with all-cause mortality, cardiovascular disease mortality, and infection-associated mortality, independent of other factors. The underlying pathophysiologic mechanisms warrant additional investigation.
Keywords: cardiovascular disease; chronic kidney disease; mean corpuscular volume (MCV); mortality; anemia; Biomarkers; Cohort Studies; Confidence Intervals; Demography; Diagnosis, Differential; Erythrocyte Indices; Erythrocytes; Follow-Up Studies; Humans; Regression Analysis; Renal Insufficiency, Chronic; Retrospective Studies; Risk
Introduction
Anemia is a common comorbid condition in patients with CKD, especially for those with a moderate to severe stage (1). The underlying etiologies of anemia are multifactorial, and impaired endogenous erythropoietin production by the dysfunctional kidneys is the predominant cause (2). Other reasons that have been identified include shortened erythrocyte lifespan, iron deficiency, folate and vitamin B12 deficiency, and bone marrow suppression by uremic toxins (3–6). Recently, an increasing body of evidence has unveiled the effect of inflammation on renal anemia (7).
Although the typical renal–related anemia is normocytic and normochromic (8), a fair proportion of patients with ESRD have macrocytosis (9). Several pathophysiologic mechanisms have been suggested to explain the observed phenomenon in patients on dialysis, including intravenous iron supplementation, megaloblastic anemia due to folate or vitamin B12 deficiency, and dialysis-related changes in erythrocytes (5,10,11). In addition, because reticulocytosis has been correlated with high mean corpuscular volume (MCV), the use of erythropoiesis-stimulating agents (ESAs) may be associated with macrocytosis, which is supported by the study by Tennankore et al. (12) that concluded that a higher ESA dosage was linked with macrocytosis in patients on chronic hemodialysis.
MCV is a measure of the mean size of erythrocytes and has long been a useful index for approaching the differential diagnosis of anemia, and it is also a possible biomarker of bone marrow dysfunction. Recently, however, MCV has emerged as an independent risk factor for mortality in several diseases. For example, Zheng et al. (13) reported that preoperative MCV was associated with mortality in patients with resectable esophageal cell carcinoma. Unfortunately, little information on the associations of MCV with clinical outcomes among patients with CKD is currently available in the literature. For this reason, we conducted this study to examine the association between MCV and mortality among patients with stages 3–5 CKD.
Materials and Methods
Participants and Measurements
We conducted a single–center retrospective study from the medical records and electronic data in Taiwan. Between January 1, 2004 and December 31, 2011, patients who joined the integrated CKD care program at the outpatient clinic were screened for eligibility. The diagnosis of CKD was defined according to National Kidney Foundation Kidney Disease Outcomes Quality Initiative criteria. Renal function status was determined by eGFR using the simplified four–variable Modification of Diet in Renal Disease (MDRD) Study equation as follows: eGFR in milliliters per minute per 1.73 m2 =186× serum creatinine−1.154× age−0.203×0.742 (if the patient is a woman) ×1.212 (if black patient). After excluding those with stages 1 and 2 CKD, younger than 20 years of age, or older than 80 years of age, a final cohort of 1439 patients was left for final analysis. All of the study participants were followed until death or the end of study on December 31, 2012. The study was approved by the institutional review board of Changhua Christian Hospital, and all of the clinical investigation was conducted according to the principles of the Declaration of Helsinki.
For each patient, the collected baseline data at enrollment included demographic features (sex and age), smoking, alcohol status, comorbid conditions, the cause of CKD, educational status, body mass index (BMI), medications, and laboratory parameters. The comorbidities encompassed diabetes mellitus (DM), hypertension, coronary artery disease, congestive heart failure, cerebrovascular disease and peripheral artery disease, cancer, dementia, autoimmune disease, chronic lung disease, and liver cirrhosis. The medication history included angiotensin–converting enzyme inhibitors, angiotensin II receptor blockers, iron, folic acid, vitamin B12, lipid-lowering agents (statin and fibrate), and ESAs. The laboratory measurements included blood levels of BUN, creatinine, albumin, white blood cell counts, hemoglobin, red cell distribution width, MCV, platelets, cholesterol, triglycerides, glutamic-pyruvic transaminase, uric acid, calcium, phosphate, and 24-hour proteinuria. The median value of MCV for our study population was 90.8 fl. The study cohort was stratified into two groups according to this median value: the high-MCV group (≥90.8 fl) and the low-MCV group (<90.8 fl). Regarding the causes of death, the two most common reasons were infection and cardiovascular disease (CVD). CVD-related mortality was defined as death that is attributed to cardiovascular events, such as sudden cardiac death, congestive heart failure, acute myocardial infarction, aortic aneurysm, stroke, or peripheral atherosclerotic vascular disease. The primary aim of the investigation was to assess whether MCV was associated with all-cause mortality as well as CVD mortality and infection-associated mortality independent of other risk factors.
Statistical Analyses
The study cohort was stratified into two groups by the median value of MCV. Continuous variables with approximately normal distribution were expressed as mean±SD, and those with non-normal distribution were expressed as median and interquartile range. Comparisons of distribution between the two groups were assessed using the paired t test for parametric data and the Mann–Whitney U test for nonparametric data as appropriate. Categorical variables are shown as number (n) and percentage, and the difference of the two groups was compared using chi-squared test or Fisher exact test as appropriate. The comparison of survival status between the two groups was done using the Kaplan–Meier curve with log rank test to determine significance levels. Cox proportional hazards model were used for the analyses of predictors for mortality. We implemented five models for the adjustments of the covariates: model 1, adjusted for sex, age categories, educational level, marital status, and BMI; model 2, adjusted for all variables in model 1 plus alcohol and smoking; model 3, adjusted for all variables in model 2 plus laboratory parameters; model 4, adjusted for all variables in model 3 plus medications; and model 5, adjusted for all variables in model 4 plus comorbid conditions.
One sensitivity analysis was done with the hazard ratio (HR) of MCV adjusted for quintiles of the propensity score in addition to all of the covariates in model 5 (model S1). The propensity scores were estimated by using the logistic regression model to control for the differences between the high-MCV (≥90.8 fl) and low-MCV (<90.8 fl) groups; <10% of our patients had serum levels of folate and vitamin B12. We performed another sensitivity test with adjustment for iron profile in 417 patients who had blood levels of iron profile (model S2). All statistics were two-sided tests, and the results were considered significant if the P value was <0.05. All statistical analyses were carried out using the statistical package for Windows, SAS 9.2 (SAS Institute Inc., Cary, NC), and SPSS 16.0 (SPSS, Chicago, IL).
Results
Baseline Characteristics of the Study Cohort
The median MCV level among the 1439 participants was 90.8 fl, with a range of 58.4–114.5 fl. The mean age was 64.1±12.2 years old, 795 patients (55.3%) were men, and the median follow-up was 1.9 years (interquartile range, 1.1–3.8 years). The three most common causes of CKD included DM, hypertension, and chronic GN. Primary school was the most common educational level, and 20.4% of the participants were current smokers. Additional information was collected according to the median (90.8 fl) of the MCV values (dividing the cohort into low- and high-MCV groups) and is presented in Table 1. The high-MCV group was older and had more men than the low-MCV group. The high-MCV group had higher levels of albumin, eGFR, hemoglobin, and calcium than the low-MCV group. Comorbid conditions of DM and hypertension were more common in the low-MCV group. The prevalence of cirrhosis and cancer was higher in the high-MCV group. The high-MCV group had a lower proportion of prescriptions for angiotensin–converting enzyme inhibitors, angiotensin II receptor blockers, iron supplements, fibrates, and statins than the low-MCV group.
Table 1.
Baseline characteristics of the study population by the median of mean corpuscular volume
| Variables | Total, n=1439 | MCV<90.8, n=717 | MCV≥90.8, n=722 | P Value |
|---|---|---|---|---|
| Age, yr | 64.1±12.2 | 60.9±13.1 | 67.3±10.3 | <0.001 |
| Age category, yr | <0.001 | |||
| <50 | 124 (17.3%) | 51 (7.1%) | 175 (12.2%) | |
| 50–64 | 258 (36%) | 179 (24.8%) | 437 (30.3%) | |
| ≥65 | 335 (46.7%) | 492 (68.1%) | 827 (57.5%) | |
| Sex, % men | 795 (55.3%) | 362 (50.5%) | 433 (59.9%) | <0.001 |
| Educational level | 0.11 | |||
| Illiterate | 309 (21.5%) | 150 (20.9%) | 159 (22%) | |
| Primary school | 691 (48%) | 327 (45.6%) | 364 (50.4%) | |
| Junior high school | 153 (10.6%) | 79 (11.0%) | 74 (10.3%) | |
| Senior high school | 171 (11.9%) | 98 (13.7%) | 73 (10.1%) | |
| Junior college | 48 (3.3%) | 30 (4.2%) | 18 (2.5%) | |
| University and graduate school | 67 (4.7%) | 33 (4.6%) | 34 (4.7%) | |
| Marital status | 0.02 | |||
| Single | 61 (4.2%) | 42 (5.9%) | 19 (2.6%) | |
| Married | 1149 (79.9%) | 566 (78.9%) | 583 (80.8%) | |
| Widowed | 209 (14.5%) | 97 (13.5%) | 112 (15.5%) | |
| Separated | 8 (0.6%) | 4 (0.6%) | 4 (0.6%) | |
| Divorced | 12 (0.8%) | 8 (1.1%) | 4(0.6%) | |
| Causes of CKD | <0.001 | |||
| DM | 536 (37.2%) | 316 (44.1%) | 220 (30.5%) | |
| Hypertension | 356 (24.7%) | 157 (21.9%) | 199 (27.6%) | |
| Chronic GN | 160 (11.1%) | 74 (10.3%) | 86 (11.9%) | |
| BMI, kg/m2 | 24.9±4.0 | 25.3±4.1 | 24.5±4.0 | <0.001 |
| Smoker, % | 0.47 | |||
| Current smoker | 294 (20.4%) | 141 (19.7%) | 153 (21.2%) | |
| Alcohol, % | 0.56 | |||
| Never | 1257 (87.4%) | 633 (88.3%) | 624 (86.4%) | |
| Current | 73 (5.1%) | 33 (4.6%) | 40 (5.5%) | |
| Former | 109 (7.6%) | 51 (7.1%) | 58 (8.0%) | |
| Medication prescription | ||||
| ACE inhibitor/ARB | 910 (63.2%) | 486 (67.8%) | 424 (58.7%) | <0.001 |
| Vitamin B12 (cyanocobalamin) | 295 (20.5) | 132 (18.4) | 163 (22.6) | 0.05 |
| Iron preparations | 212 (14.7) | 128 (17.9) | 84 (11.6) | 0.001 |
| Folic acid | 358 (24.9) | 155 (21.6) | 203 (28.1) | 0.004 |
| Erythropoiesis-stimulating agents | 324 (22.5) | 176 (24.6) | 148 (20.5) | 0.06 |
| Fibrate | 75 (5.2) | 48 (6.7) | 27 (3.7) | 0.01 |
| Statin | 536 (37.3) | 298 (41.6) | 238 (33.0) | 0.001 |
| Comorbidity | ||||
| Cancer | 140 (9.7%) | 49 (6.8%) | 91 (12.6%) | <0.001 |
| Cerebrovascular disease | 202 (14.0%) | 112 (15.6%) | 90 (12.5%) | 0.09 |
| Chronic lung disease | 216 (15.0%) | 102 (14.2%) | 114 (15.8%) | 0.42 |
| Congestive heart failure | 147 (10.2%) | 82 (11.4%) | 65 (9%) | 0.14 |
| Coronary artery disease | 361 (25.1%) | 174 (24.3%) | 187 (25.9%) | 0.50 |
| Dementia | 34 (2.4%) | 11 (1.5%) | 23 (3.2%) | 0.05 |
| DM | 672 (46.7%) | 389 (54.3%) | 283 (39.2%) | <0.001 |
| Hypertension | 1004 (69.8%) | 530 (73.9%) | 474 (65.7%) | 0.001 |
| Liver cirrhosis | 24 (1.7%) | 5 (0.7%) | 19 (2.6%) | <0.01 |
| Peripheral arterial disease | 19 (1.3%) | 8 (1.1%) | 11 (1.5%) | 0.65 |
| Autoimmune disease | 75 (5.2%) | 36 (5.0%) | 39 (5.4%) | 0.81 |
| Laboratory data | ||||
| WBC count ×103/L | 7.1 (5.7–8.8) | 7.4 (6.0–9.1) | 6.8 (5.6–8.4) | <0.001 |
| Hemoglobin, g/dl | 10.4 (9–12) | 10.1 (8.8–11.7) | 10.6 (9.2–12.3) | 0.001 |
| Platelet count ×103/L | 218 (177–267) | 230 (190–279) | 203 (166–254) | <0.001 |
| Albumin, g/dl | 3.8 (3.4–4.2) | 3.7 (3.3–4.2) | 3.9 (3.6–4.3) | <0.001 |
| MCV, fl | 90.8 (87.2–95.6) | 87.17 (84.16–89.1) | 95.6 (93.83–98) | <0.001 |
| RDW, % | 14.3 (13.5–15.4) | 14.5 (13.6–15.6) | 14.2 (13.5–15.1) | <0.001 |
| BUN, mg/dl | 42.1 (29–63.3) | 44.2 (30–65.9) | 39.6 (28.3–60.8) | 0.001 |
| Creatinine, mg/dl | 2.9 (2.0–5.0) | 3.0 (2.0–5.2) | 2.7 (2.0–4.7) | 0.04 |
| eGFR, ml/min per 1.73 m2 | 19.7 (10.6–30.9) | 18.4 (10.1–30.3) | 20.6 (11.0–31.9) | 0.03 |
| Ca, mg/dl | 8.8 (8.4–9.2) | 8.8 (8.3–9.1) | 8.9 (8.4–9.2) | <0.01 |
| Phosphate, mg/dl | 4 (3.4–4.7) | 4.2 (3.5–5) | 3.9 (3.3–4.5) | <0.001 |
| Cholesterol, mg/dl | 180 (153–210) | 184 (155–217) | 177 (151–204) | <0.01 |
| Triglyceride, mg/dl | 130 (92–184) | 141 (103–203) | 119 (85–171) | <0.001 |
| GPT, U/L | 19 (13–31) | 18 (13–30) | 19 (13–31) | 0.28 |
| Uric acid, mg/dl | 7.9 (6.7–9) | 8.1 (6.9–9.3) | 7.7 (6.5–8.8) | <0.001 |
| 24-h Proteinuria, mg | 684.4 (169.5–2143.7) | 884.6 (215.4–2855.1) | 556.8 (117.1–1679.8) | <0.001 |
Values are expressed as means±SD, medians (interquartile range), or numbers (percentage). MCV, mean corpuscular volume; BMI, body mass index, ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; DM, diabetes mellitus; WBC, white blood cell; RDW, red cell distribution width; Ca, calcium; GPT, glutamic-pyruvic transaminase.
MCV and All-Cause Mortality
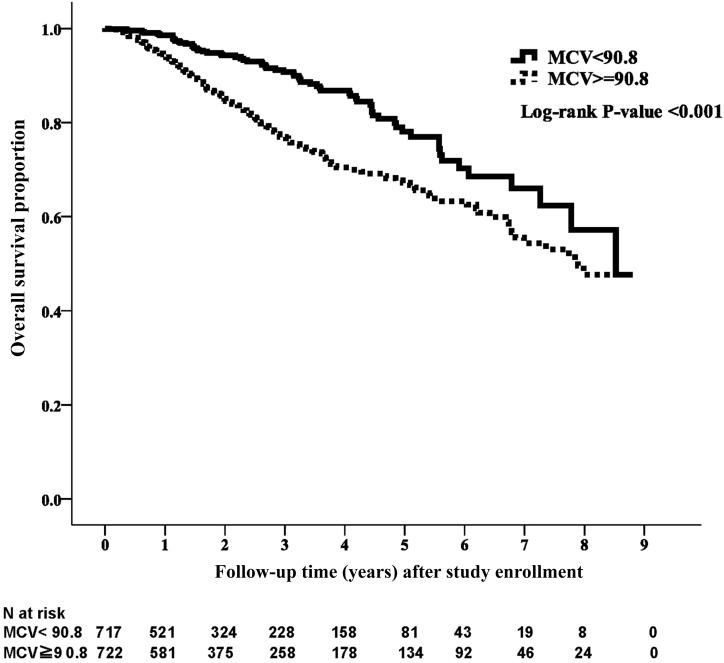
During the median follow-up period of 1.9 years, 234 patients (16.2%) died. The crude mortality rate was 22.7% (n=164) for the high-MCV group and 9.8% (n=70) for the low-MCV group (P<0.001). The Kaplan–Meier analysis revealed that the patient survival was significantly better for the low-MCV group than the high-MCV group (Figure 1) (P<0.001). The unadjusted and adjusted HRs are presented in Table 2. Compared with the low-MCV group, the HR for the high-MCV group was 1.94 (95% confidence interval [95% CI], 1.46 to 2.57; P<0.001) for all-cause mortality in the unadjusted model. In the fully adjusted model (model 5), the risk of mortality in the high-MCV group was 119% higher (HR, 2.19; 95% CI, 1.62 to 2.96; P<0.001).
Figure 1.
Kaplan–Meier curve of overall patient survival according to mean corpuscular volume (MCV) category. Patient survival was significantly better for the low MCV group than for the high MCV group (log rank test, P<0.001).
Table 2.
Cox proportional hazard models of all-cause and cause-specific mortality by mean corpuscular volume category
| All-Cause Mortality | CVD Mortality | Infection Mortality | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Unadjusted model | 1.94 (1.46 to 2.57) | <0.001 | 2.78 (1.45 to 5.31) | 0.002 | 1.94 (1.28 to 2.94) | 0.002 |
| Model 1 | 1.70 (1.28 to 2.26) | <0.001 | 2.29 (1.20 to 4.39) | 0.01 | 1.66 (1.09 to 2.53) | 0.01 |
| Model 2 | 1.69 (1.27 to 2.25) | <0.001 | 2.29 (1.20 to 4.39) | 0.01 | 1.67 (1.11 to 2.55) | 0.01 |
| Model 3 | 2.13 (1.59 to 2.86) | <0.001 | 3.64 (1.83 to 7.23) | <0.001 | 2.02 (1.31 to 3.11) | 0.002 |
| Model 4 | 2.12 (1.58 to 2.85) | <0.001 | 3.67 (1.84 to 7.33) | <0.001 | 2.02 (1.31 to 3.11) | 0.002 |
| Model 5 | 2.19 (1.62 to 2.96) | <0.001 | 3.57 (1.80 to 7.06) | <0.001 | 2.22 (1.41 to 3.49) | 0.001 |
The referent group for all models is mean corpuscular volume below the median of 90.8. The variables for adjustments in models 1–5 are described. Model 1: mean corpuscular volume, age categories, sex, body mass index, educational level, marital status, and causes of CKD. Model 2: model 1 plus alcohol and smoking. Model 3: model 2 plus albumin, BUN, eGFR, glutamic-pyruvic transaminase, white blood cell, hemoglobin, platelet, red cell distribution width, calcium, phosphate, cholesterol, triglyceride, uric acid, and proteinuria. Model 4: model 3 plus angiotensin–converting enzyme inhibitor/angiotensin II receptor blocker, vitamin B12, iron preparation, folic acid, erythropoiesis-stimulating agent, fibrate, and statin. Model 5: model 4 plus cancer, cerebrovascular disease, chronic lung disease, congestive heart failure, coronary artery disease, dementia, diabetes mellitus, hypertension, liver cirrhosis, peripheral arterial disease, and autoimmune disease. CVD, cardiovascular disease; 95% CI, 95% confidence interval.
MCV and CVD Mortality
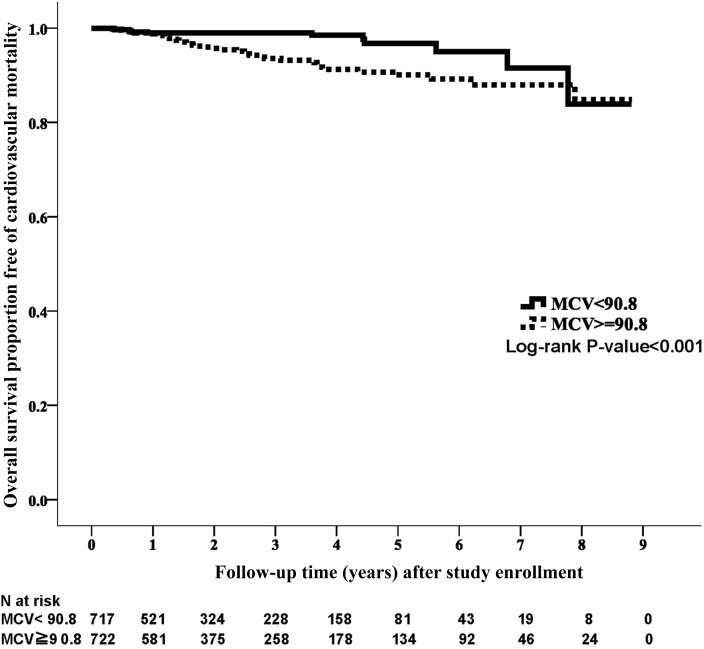
Fifty-two patients (3.6%) died of CVD during the study period. There was a difference in the crude CVD mortality rate between the two groups (high-MCV group: n=40, 5.5%; low-MCV group: n=12, 1.7%; P<0.001). A significant survival difference was observed between the two groups in the Kaplan–Meier analysis with respect to CVD death (Figure 2) (P<0.001). The high-MCV group had a higher CVD death rate than the low-MCV group in the univariate model (HR, 2.78; 95% CI, 1.45 to 5.31; P=0.002) and the fully adjusted model (HR, 3.57; 95% CI, 1.80 to 7.06; P<0.001) (Table 2).
Figure 2.
Kaplan–Meier curve of cardiovascular disease survival according to mean corpuscular volume (MCV) category. Cardiovascular disease survival was significantly better for the low MCV group than for the high MCV group (log rank test, P<0.001).
MCV and Infection-Associated Mortality
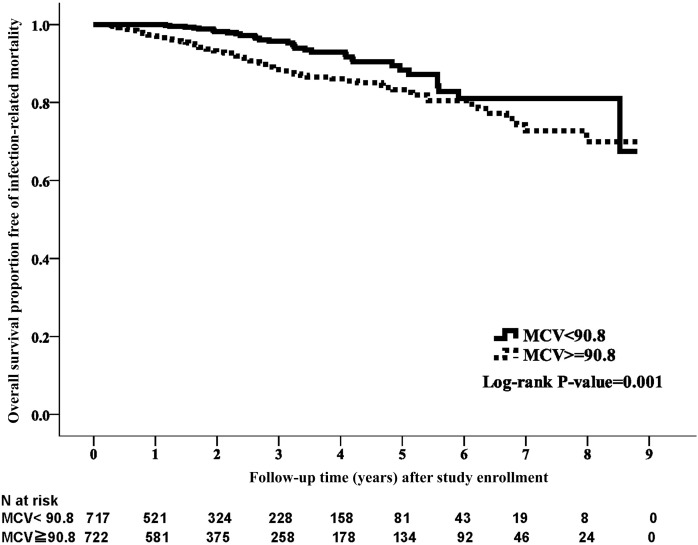
During the study period, 108 patients (7.51%) died of infection. The crude rate of infection-associated mortality was significantly higher in the high-MCV group (n=76, 10.5%) than in the low-MCV group (n=32, 4.5%; P=0.001). A distinct survival difference was observed in the Kaplan–Meier analysis (Figure 3) (P=0.001). In the fully adjusted model, the high-MCV group had higher infection–associated mortality compared with the low-MCV group (HR, 2.22; 95% CI, 1.41 to 3.49; P=0.001) (Table 2).
Figure 3.
Kaplan–Meier curve of infection-associated survival according to mean corpuscular volume (MCV) category. The low MCV group was associated with better survival with respect to infection-related mortality than the high MCV group (log rank test, P=0.001).
Sensitivity Analyses
In the sensitivity analysis adjusted for propensity scores (Table 3, model S1), the high-MCV group had a worse survival compared with the low-MCV group for all-cause mortality (HR, 1.84; 95% CI, 1.35 to 2.51; P<0.001), CVD mortality (HR, 2.06; 95% CI, 1.02 to 4.12; P=0.04), and infection-associated mortality (HR, 1.78; 95% CI, 1.14 to 2.79; P=0.01). Repeated analysis was carried out in a subgroup of patients (n=417) who had iron profile measurements. After adjusting for serum levels of ferritin and transferrin saturation, MCV remained independently associated with mortality (Table 3, model S2).
Table 3.
Sensitivity analysis of Cox proportional hazard models of all-cause and cause-specific mortality by mean corpuscular volume category
| All-Cause Mortality | CVD Mortality | Infection Mortality | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Model S1, n=1439 | ||||||
| MCV | 1.84 (1.35 to 2.51) | <0.001 | 2.06 (1.02 to 4.12) | 0.04 | 1.78 (1.14 to 2.79) | 0.01 |
| Model S2, n=417 | ||||||
| MCV | 3.35 (1.76 to 6.37) | <0.001 | 2.92 (1.57 to 5.42) | 0.001 | 3.44 (1.35 to 8.74) | <0.01 |
| Ferritin | 1.00 (0.99 to 1.00) | 0.85 | 1.00 (0.99 to 1.00) | 0.96 | 0.99 (0.99 to 1.00) | 0.19 |
| Transferrin saturation | 0.16 (0.01 to 1.59) | 0.12 | 0.37 (0.03 to 3.94) | 0.42 | 0.68 (0.01 to 29.10) | 0.85 |
The referent group for all models is MCV below the median of 90.8. Model S1: adjusted for strata by quintiles on the basis of propensity scores in addition to all of the covariates in model 5 in Table 2. Model S2: adjusted for ferritin and transferrin saturation in addition to all of the variables in model 5 in Table 2. CVD, cardiovascular disease; 95% CI, 95% confidence interval; MCV, mean corpuscular volume.
Discussion
In this study of patients with stages 3–5 CKD, we identified that a high MCV was independently associated with all-cause mortality, infection-related mortality, and cardiovascular mortality. To the best of our knowledge, this is the largest study to evaluate the association of MCV with mortality among patients with CKD.
MCV has been reported as being a part of complete blood cell counts, and it is used principally in the diagnostic approach of anemia in clinical practice. From the available evidence, few studies have assessed the role of MCV in the clinical settings, and most of these were for patients with malignancy. Wenzel et al. (14) reported the relation of an increase in MCV with clinical response in patients with advanced solid malignancies after capecitabine. Development of macrocytosis after capecitabine, defined as an increase in MCV of >10 fl, was also significantly associated with better overall survival and response rate in patients with advanced gastric cancer (15). A recent investigation of Chinese patients with esophageal squamous cell carcinoma found the preoperative MCV, determined by receiver operating characteristic curve analysis, to be associated with mortality (13). As for other clinical settings, a higher MCV was associated with all-cause and CVD death in patients with acute decompensated heart failure (16). MCV was also related to adverse clinical outcomes for patients subjected to percutaneous coronary intervention (17). In addition, macrocytosis, both as a continuous variable and using a cutoff point of 102 fl, was also noted to be associated with mortality in stable patients on chronic hemodialysis (12).
Although we found in this study that MCV was associated with mortality in patients with CKD, the underlying mechanisms are still not fully known. One possible explanation linking MCV to the prognosis is that macrocytosis is merely a biomarker of malnutrition. Crystal osmotic pressure has been reported to be an important regulator of erythrocyte volume (18), and a reduced crystal osmotic pressure as a result of malnutrition may cause erythrocyte swelling and higher MCV. In this study, nutritional markers, such as cholesterol, triglycerides, uric acid, and BMI, were significantly lower in the high-MCV group than in the low-MCV group, but albumin was higher in the high-MCV group. However, even after adjusting for these nutrition markers, the association of MCV with mortality remained unchanged; thus, malnutrition alone cannot explain the relationship.
Alternatively, a higher MCV may be a manifestation of bone marrow malfunction with altered hematopoiesis. A study of 49 elderly patients with unexplained macrocytosis who underwent bone marrow biopsy showed that 25 patients had dysplastic features or pathologic findings consistent with myelodysplastic syndrome (19). A recent investigation of patients with heart failure concluded that erythrocyte, white blood cell, and platelet counts were significantly lower in the macrocytic group than in the nonmacrocytic group, which prompted the authors to suggest that the development of all cell lineages in bone marrow is impaired (16). However, we did not observe such a phenomenon in our patients.
Hyperhomocysteinemia is a common finding in CKD and frequently put forward as being a risk factor for CVD and endothelial dysfunction. Another consideration is that a higher MCV may be a surrogate marker of folic acid or vitamin B12 deficiency. The consequent hyperhomocysteinemia reportedly improves the CVD risk classification on the basis of a Framingham risk score (20). However, a prospective study of predialysis adult patients with CKD showed that lowering hyperhomocysteinemia by high–dose folic acid failed to achieve an improvement of endothelial dysfunction (21). Another meta-analysis failed to show the beneficial effect of homocysteine-lowering treatment on CVD events, stroke, and all-cause mortality in both predialysis patients with CKD and patients with CKD on dialysis (22). Therefore, although we did not have data on the homocysteine level, this hypothesis cannot explain the association between the high-MCV level and mortality.
Oxidative stress as a result of increased reactive oxygen species production has been shown to have a negative effect on clinical outcomes (23). Metabolism and homeostasis of the erythrocytes strongly affect the antioxidant capacities of the whole body, because their membranes are exposed to oxidative stress and involved in oxygen and carbon dioxide transport as well as have high intracellular antioxidant enzyme activities (24,25). Macrocytosis is considered a morphologic and functional abnormality of the erythrocyte. From this study, a higher MCV level, even in the normal range, was a negative predictor of mortality, and therefore, we speculate that a modest increase in MCV may lead to an increased reactive oxygen species with a resultant higher mortality risk in the long term. Higher MCV value and reduced antioxidant capacity may be an appealing issue that warrants additional research.
Another explanation may be the direct relation of MCV with endothelial function. Flow-mediated dilation was introduced as a noninvasive examination to assess vascular function. Endothelial dysfunction has been considered as one of the key mechanisms linking high cardiovascular burden and CKD (26). A recent study of 309 patients with stages 1–5 CKD by Solak et al. (27) found an inverse association of MCV with flow-mediated dilation independent of insulin resistance and inflammation. The mechanistic hypothesis was unclear but proposed to be impaired antioxidant capacity of macrocytic erythrocytes.
As its name indicates, MCV is a measure of average size of erythrocytes. The most common causes of macrocytosis are alcoholism, vitamin B12 or folate deficiency, hypothyroidism, and certain medications. However, clinicians have to be aware that macrocytosis may be masked by other coexisting conditions, such as thalassemia, iron deficiency, and chronic illness. The term megaloblastic anemia without macrocytosis indicates the presence of folate or vitamin B12 deficiency without apparent macrocytosis. Therefore, the findings of our study could also be explained by the above phenomenon in that, even within the normal range, patients with higher MCV level may have a higher proportion of macrocytes than those with a lower MCV level.
Some limitations of this study deserve to be mentioned. First, information on blood levels of thyroid function, folate, vitamin B12, and iron profiles was lacking. Instead, the collected medications included supplementation with folic acid, vitamin B12, and iron, which were adjusted for in the analysis. In addition, reticulocytosis caused by acute bleeding, hemolysis, or decreased red cell survival also can contribute to a higher MCV, but we did not have related data, such as reticulocyte count and lactate dehydrogenase. Second, the single-center investigation and retrospective nature also limit the generalizability of our findings. Despite the extensive adjustment, residual confounding is still possible. Third, instead of measuring GFR by the clearance of exogenous filtration markers, eGFR was used. Nevertheless, a recent study has shown that acceptable longitudinal performance was achieved by the MDRD equation for GFR estimation (28). The Chronic Kidney Disease Epidemiology Collaboration equation is superior to the MDRD equation in estimating GFR when GFR is normal or mildly reduced (29). However, only 34 patients (2.4%) had eGFR of >50 ml/min per 1.73 m2 in our cohort. Fourth, the most common cause of mortality was infection in this cohort, but racial differences or distinct patient characteristics may contribute to the inconsistency between the studies in the literature. Nevertheless, MCV was still independently associated with mortality, irrespective of the etiology of death.
In conclusion, this study found that MCV was an independent risk factor for mortality among patients with stages 3–5 CKD. Identifying high-risk patients for mortality by MCV could provide benefits for risk classification in patient care. A large–scale, multicenter study with a prospective design is needed to confirm these findings, and the underlying pathophysiologic mechanisms also deserve to be clarified.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Voormolen N, Grootendorst DC, Urlings TA, Boeschoten EW, Sijpkens YW, Huisman RM, Krediet RT, Dekker FW: Prevalence of anemia and its impact on mortality and hospitalization rate in predialysis patients. Nephron Clin Pract 115: c133–c141, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Eschbach JW, Adamson JW: Recombinant human erythropoietin: Implications for nephrology. Am J Kidney Dis 11: 203–209, 1988 [DOI] [PubMed] [Google Scholar]
- 3.Shaw AB: Haemolysis in chronic renal failure. BMJ 2: 213–216, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eschbach JW, Cook JD, Scribner BH, Finch CA: Iron balance in hemodialysis patients. Ann Intern Med 87: 710–713, 1977 [DOI] [PubMed] [Google Scholar]
- 5.Hampers CL, Streiff R, Nathan DG, Snyder D, Merrill JP: Megaloblastic hematopoiesis in uremia and in patients on long-term hemodialysis. N Engl J Med 276: 551–554, 1967 [DOI] [PubMed] [Google Scholar]
- 6.Toto RD: Anemia of chronic disease: Past, present, and future. Kidney Int Suppl 64: S20–S23, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Yilmaz MI, Solak Y, Covic A, Goldsmith D, Kanbay M: Renal anemia of inflammation: The name is self-explanatory. Blood Purif 32: 220–225, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Zachée P, Vermylen J, Boogaerts MA: Hematologic aspects of end-stage renal failure. Ann Hematol 69: 33–40, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Afshar R, Sanavi S, Salimi J, Ahmadzadeh M: Hematological profile of chronic kidney disease (CKD) patients in Iran, in pre-dialysis stages and after initiation of hemodialysis. Saudi J Kidney Dis Transpl 21: 368–371, 2010 [PubMed] [Google Scholar]
- 10.Pollak VE, Lorch JA, Means RT Jr.: Unanticipated favorable effects of correcting iron deficiency in chronic hemodialysis patients. J Investig Med 49: 173–183, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Bartels PC, Helleman PW, Soons JB: Investigations on red cell size distribution histograms in subjects treated by maintenance haemodialysis. J Clin Chem Clin Biochem 28: 113–118, 1990 [DOI] [PubMed] [Google Scholar]
- 12.Tennankore KK, Soroka SD, West KA, Kiberd BA: Macrocytosis may be associated with mortality in chronic hemodialysis patients: A prospective study. BMC Nephrol 12: 19, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng YZ, Dai SQ, Li W, Cao X, Li Y, Zhang LJ, Fu JH, Wang JY: Prognostic value of preoperative mean corpuscular volume in esophageal squamous cell carcinoma. World J Gastroenterol 19: 2811–2817, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wenzel C, Mader RM, Steger GG, Pluschnig U, Kornek GV, Scheithauer W, Locker GJ: Capecitabine treatment results in increased mean corpuscular volume of red blood cells in patients with advanced solid malignancies. Anticancer Drugs 14: 119–123, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Jung HA, Kim HJ, Maeng CH, Park SH, Lee J, Park JO, Park YS, Lim HY, Kang WK: Changes in the mean corpuscular volume after capecitabine treatment are associated with clinical response and survival in patients with advanced gastric cancer. Cancer Res Treat 47: 72–77, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ueda T, Kawakami R, Horii M, Sugawara Y, Matsumoto T, Okada S, Nishida T, Soeda T, Okayama S, Somekawa S, Takeda Y, Watanabe M, Kawata H, Uemura S, Saito Y: High mean corpuscular volume is a new indicator of prognosis in acute decompensated heart failure. Circ J 77: 2766–2771, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Myojo M, Iwata H, Kohro T, Sato H, Kiyosue A, Ando J, Sawaki D, Takahashi M, Fujita H, Hirata Y, Nagai R: Prognostic implication of macrocytosis on adverse outcomes after coronary intervention. Atherosclerosis 221: 148–153, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Porath-Furedi A: The mutual effect of hydrogen ion concentration and osmotic pressure on the shape of the human erythrocyte as determined by light scattering and by electronic cell volume measurement. Cytometry 4: 263–267, 1983 [DOI] [PubMed] [Google Scholar]
- 19.Mahmoud MY, Lugon M, Anderson CC: Unexplained macrocytosis in elderly patients. Age Ageing 25: 310–312, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Veeranna V, Zalawadiya SK, Niraj A, Pradhan J, Ference B, Burack RC, Jacob S, Afonso L: Homocysteine and reclassification of cardiovascular disease risk. J Am Coll Cardiol 58: 1025–1033, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Thambyrajah J, Landray MJ, McGlynn FJ, Jones HJ, Wheeler DC, Townend JN: Does folic acid decrease plasma homocysteine and improve endothelial function in patients with predialysis renal failure? Circulation 102: 871–875, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Pan Y, Guo LL, Cai LL, Zhu XJ, Shu JL, Liu XL, Jin HM: Homocysteine-lowering therapy does not lead to reduction in cardiovascular outcomes in chronic kidney disease patients: A meta-analysis of randomised, controlled trials. Br J Nutr 108: 400–407, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Madamanchi NR, Vendrov A, Runge MS: Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol 25: 29–38, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Tsantes AE, Bonovas S, Travlou A, Sitaras NM: Redox imbalance, macrocytosis, and RBC homeostasis. Antioxid Redox Signal 8: 1205–1216, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Lucantoni G, Pietraforte D, Matarrese P, Gambardella L, Metere A, Paone G, Bianchi EL, Straface E: The red blood cell as a biosensor for monitoring oxidative imbalance in chronic obstructive pulmonary disease: An ex vivo and in vitro study. Antioxid Redox Signal 8: 1171–1182, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Stam F, van Guldener C, Becker A, Dekker JM, Heine RJ, Bouter LM, Stehouwer CD: Endothelial dysfunction contributes to renal function-associated cardiovascular mortality in a population with mild renal insufficiency: The hoorn study. J Am Soc Nephrol 17: 537–545, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Solak Y, Yilmaz MI, Saglam M, Demirbas S, Verim S, Unal HU, Gaipov A, Oguz Y, Kayrak M, Caglar K, Vural A, Turk S, Covic A, Kanbay M: Mean corpuscular volume is associated with endothelial dysfunction and predicts composite cardiovascular events in patients with chronic kidney disease. Nephrology (Carlton) 18: 728–735, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Lee D, Levin A, Roger SD, McMahon LP: Longitudinal analysis of performance of estimated glomerular filtration rate as renal function declines in chronic kidney disease. Nephrol Dial Transplant 24: 109–116, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]