Abstract
Background and objectives
Patients receiving hemodialysis are at risk of cardiovascular events. A novel blood test (T50 test) determines the individual calcification propensity of blood.
Design, setting, participants, & measurements
T50 was determined in 2785 baseline serum samples of patients receiving hemodialysis enrolled in the Evaluation of Cinacalcet Therapy to Lower Cardiovascular Events (EVOLVE) trial and the T50 results were related to patient outcomes.
Results
Serum albumin, bicarbonate, HDL cholesterol, and creatinine were the main factors positively/directly and phosphate was the main factor negatively/inversely associated with T50. The primary composite end point (all-cause mortality, myocardial infarction [MI], hospitalization for unstable angina, heart failure, or peripheral vascular event [PVE]) was reached in 1350 patients after a median follow-up time of 619 days. After adjustments for confounding, a lower T50 was independently associated with a higher risk of the primary composite end point as a continuous measure (hazard ratio [HR] per 1 SD lower T50, 1.15; 95% confidence interval [95% CI], 1.08 to 1.22; P<0.001). Furthermore, lower T50 was associated with a higher risk in all-cause mortality (HR per 1 SD lower T50, 1.10; 95% CI, 1.02 to 1.17; P=0.001), MI (HR per 1 SD lower T50, 1.38; 95% CI, 1.19 to 1.60; P<0.001), and PVE (HR per 1 SD lower T50, 1.22; 95% CI, 1.05 to 1.42; P=0.01). T50 improved risk prediction (integrated discrimination improvement and net reclassification improvement, P<0.001 and P=0.001) of the primary composite end point.
Conclusions
Blood calcification propensity was independently associated with the primary composite end point, all-cause mortality, MI, and PVE in the EVOLVE study and improved risk prediction. Prospective trials should clarify whether T50-guided therapies improve outcomes.
Keywords: calcification propensity; T50; calciprotein particles; CPP; hemodialysis; EVOLVE; angina, unstable; bicarbonates; cholesterol, HDL; cinacalcet hydrochloride; creatinine; follow-up studies; heart failure; hematologic tests; hospitalization; humans; myocardial infarction; phosphates; prospective studies; renal dialysis; serum albumin
Introduction
Patients receiving hemodialysis suffer from a dramatically increased cardiovascular (CV) morbidity and mortality compared with age-matched persons with normal or near normal kidney function (1,2). A large portion of this excess risk is attributable to CV causes, which can only partially be explained by the traditional CV risk factors BP, cholesterol, smoking, body mass index (BMI), and diabetes (3,4). Rather, so-called nontraditional CV risk factors (5,6) reflecting disturbances in bone and mineral metabolism appear to play an important role (7,8). Current therapeutic concepts are accordingly aimed at lowering elevated serum concentrations of phosphate and parathyroid hormone (PTH), which are associated with an increased mortality in patients receiving hemodialysis (9,10,11) and with dystrophic calcification within vascular walls and cardiac valves (12,13). Such ectopic calcifications are often present even at young age (14,15) and may progress rapidly (16). The number of sites with calcified vessels (17) and the degree of calcifications at specific sites have been associated with mortality in this patient population (18–20).
Recently, a novel functional in vitro test (T50 test) for the determination of calcification propensity in blood was developed (21). This test quantifies the calcification inhibition inherent in blood by challenging the patient’s serum with supersaturated calcium and phosphate solutions. This leads to the instantaneous formation of primary calciprotein particles (CPP). The timing of the spontaneous transformation of these particles into secondary CPP depends on the individual composition of serum, and more specifically on the concentrations and interplay of well established calcification-inhibiting factors, including the proteins fetuin-A and albumin, and the small molecules calcium, phosphate, magnesium, pyrophosphate, and others (21,22). A shorter transformation time indicates a more rapid precipitation of calcium and phosphate in the presence of serum and lower T50 values have been associated with higher risk of worse outcome in cohort studies. Specifically, the T50 test was recently shown to predict all-cause mortality and to outperform the predictive value of its individual components in patients with stages 3 and 4 CKD (22) and in kidney transplant recipients (23). Furthermore, the T50 value was closely associated with progressive stiffening of the aorta (22).
The Evaluation of Cinacalcet Therapy to Lower Cardiovascular Events (EVOLVE) trial (24) is the largest (in size) and longest (in duration) prospective, randomized, placebo-controlled event-driven CV outcome trial performed in patients with secondary hyperparathyroidism receiving hemodialysis. This trial was conducted over 64 months and assessed the benefits and risks of cinacalcet (Mimpara/Sensipar) compared with placebo on all-cause mortality and major CV events (24). As the T50 test is a composite of established nontraditional CV risk factors related to bone and mineral metabolism, we hypothesized that the T50 result would be associated with the primary composite end point (all-cause mortality, myocardial infarction [MI], hospitalization for unstable angina, heart failure, or peripheral vascular event [PVE]), as well as its individual components.
Materials and Methods
Study Population and Design
The EVOLVE trial was an interventional trial which assessed the benefits and risks of cinacalcet (Mimpara/Sensipar) compared with placebo. The dose of cinacalcet was titrated according to PTH and calcium levels from 30 mg/d to a maximum dose of 180 mg/d. A tabulated overview of the baseline treatments relevant to mineral metabolism is given at the end of Supplemental Table 1. Blood was drawn before the first dialysis session of the week. Additional serum samples were collected in nonrandomly preselected 394 out of 467 sites from patients who had agreed to contribute additional serum samples for additional studies beyond the original EVOLVE study. Details on the patient characteristics at baseline (25), the study design (26), and the primary trial results (24) have been reported previously. The EVOLVE trial was sponsored by Amgen Inc. and was led by an academic executive committee. Ethics committee approval was obtained from all sites and all patients gave informed consent. The trial was registered under ClinicalTrials.gov number, NCT00345839.
Determination of Serum Calcification Propensity (T50)
For this post hoc analysis, serum calcification propensity was measured in a blinded manner at a single site (21) in 2785 patients with stored baseline (i.e., week 0) serum samples (stored at −70°C throughout without thawing). In short, serum samples were challenged with highly concentrated calcium and phosphate solutions to induce the formation of CPP. Pipetting was performed with a high precision manual pipetting device using a 96-channel pipetting head (Liquidator; Mettler Toledo, Greifensee, Switzerland). The ripening and spontaneous transformation from primary to secondary CPP was then monitored in a time-resolved manner using a standard nephelometer (Nephelostar; BMG Labtech, Ortenberg, Germany). The results of these measurements were used to calculate the one-half maximal transition time (T50). Upon analysis of the T50 values with reference to storage duration, no significant association between storage duration and T50 values was found: Period 1, September of 2006 to March of 2007: T50 median (10th percentile, 90th percentile): 215 (110, 326); Period 2, March of 2007 to August of 2007: 207 (106, 327); Period 3, August of 2007 to January of 2008: 214 (111, 334). The intra-assay coefficients of variation of standards precipitating at 130, 170, and 400 minutes were 3.3%, 3.2%, and 6.0%, respectively. The inter-assay coefficients of variation of standards precipitating at 120, 260, and 390 minutes were 7.8%, 5.1%, and 5.9%, respectively.
Clinical Study End Points
The EVOLVE primary composite end point was the time to death or the first nonfatal CV event (myocardial infarction, hospitalization for unstable angina, heart failure, or a PVE). PVE was defined as lower limb amputation for peripheral vascular disease, revascularization procedure for peripheral vascular disease, or hospitalization for ischemic rest pain with documented gangrene/tissue necrosis. Secondary end points included the time to the individual components of the primary composite end point, death from CV causes, and a tertiary CV composite end point (CV mortality, MI, heart failure, and hospitalization for unstable angina). All end points were adjudicated by an independent clinical events classification group.
Statistical Analyses
All randomized patients with baseline serum T50 were included in these analyses. To assess the associations of baseline variables and T50, we performed a general multivariable linear regression analysis using a backward elimination procedure at significance level of 0.10. Demographics, patient characteristics, prior CV history, and laboratory measures at baseline were assessed as factors potentially associated with baseline T50.
To examine the association of T50 and clinical events, hazard ratios (HRs) (per 1 SD decrease) and 95% confidence intervals (95% CIs) were calculated using Cox proportional hazards regression models. We performed multivariable analysis which adjusted for baseline covariates using a backward selection procedure at a significance level of 0.10. Potential baseline covariates assessed include patient characteristics, demographics, concomitant medication use, CV disease history, and laboratory measures (Supplemental Table 2).
Harrell C statistic was calculated to assess the capacity of the estimated risk score of the fitted survival model. Improvement in risk prediction was assessed using integrated discrimination improvement (IDI) and net reclassification improvement (NRI) methods. Three-year risk IDI and NRI were calculated accounting for time-to-event data using SAS macro and PROC PHREG (27).
Kaplan–Meier event-free survival times were computed and compared T50 groups using a two-sided log-rank test, stratified by country and diabetes status. Baseline quintile cutpoints were used to define the T50 groups within each randomized group.
As previously reported (24), the primary analysis of the EVOLVE trial did not reach statistical significance (unadjusted log-rank test using the intention-to-treat approach). The analyses presented herewith were not adjusted for multiplicity and we considered two-tailed P values <0.05 nominally statistically significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Study Population
Of the 3883 randomized patients, calcification propensity (T50) was determined in 2785 (72%) patients (“T50 cohort”), who had additional serum samples collected at baseline. Median (10th–90th percentiles) T50 was 212 (109–328) minutes in the T50 cohort. Median (10th –90th percentiles) age was 54 (34–73) years with 41% women and 57% white participants. Median Quetelet index (BMI) was 26 (20–36) kg/m2 and dialysis vintage was 47 (10–148) months. One third of patients had a history of diabetes mellitus, and 26% were current smokers. The T50 cohort was representative of the overall population of patients randomized in the EVOLVE trial (Supplemental Table 3, Table 1).
Table 1.
Baseline demographics of all EVOLVE patients and the T50 cohort
| Demographics | All EVOLVE Patients (n=3883) | T50 Cohort | ||
|---|---|---|---|---|
| All Patients (n=2785) | Placebo Group (n=1366) | Cinacalcet Group (n=1419) | ||
| Age, yr | 55 (35–73) | 54 (34–73) | 54 (34–72) | 55 (34–74) |
| Women, % | 41 | 41 | 40 | 41 |
| BMI, kg/m2 | 26 (21–37) | 26 (20–36) | 26 (20–36) | 26 (21–37) |
| Race or ethnic group, % | ||||
| White | 57.7 | 57.4 | 57.0 | 57.8 |
| Black | 21.6 | 19.8 | 19.8 | 19.9 |
| Other | 20.8 | 22.8 | 23.2 | 22.3 |
| Dialysis vintage, mo | 45 (9–146) | 47 (10–148) | 48 (11–152) | 46 (9–145) |
| Current tobacco use, % | 27.4 | 26.2 | 26.5 | 26.0 |
| Hypertension, % | 92.1 | 91.7 | 91.9 | 91.6 |
| Diabetes (types 1 and 2), % | 33.6 | 31.4 | 31.7 | 30.9 |
| Coronary artery disease, % | 24.5 | 23.5 | 22.5 | 24.5 |
| Dyslipidemia, % | 39.6 | 37.4 | 36.9 | 37.9 |
| Calcification propensity (T50), min | 212 (109–328) | 216 (111–333) | 209 (108–323) | |
| iPTH, pg/ml | 693 (363–1694) | 705 (371–1734) | 698 (372–1717) | 707 (371–1755) |
| Serum calcium, mg/dl | 9.8 (9.0–10.7) | 9.8 (9.0–10.7) | 9.8 (9.0–10.7) | 9.8 (9.0–10.7) |
| Serum phosphorus, mg/dl | 6.2 (4.9–8.4) | 6.3 (4.9–8.4) | 6.2 (4.9–8.4) | 6.3 (4.9–8.3) |
| Serum albumin, g/dl | 3.7 (3.2–4.1) | 3.7 (3.2–4.1) | 3.7 (3.2–4.1) | 3.7 (3.2–4.1) |
Data are given as median (p10–p90) unless stated otherwise. Mean (SD) T50 was 215 (84) minutes in the T50 cohort. The “other” race or ethnic group includes Hispanic ethnicity. EVOLVE, Evaluation of Cinacalcet Therapy to Lower Cardiovascular Events; BMI, body mass index; iPTH, intact parathyroid hormone.
Baseline characteristics of the T50 cohort were comparable among patients randomized to placebo (n=1366) and cinacalcet (n=1419) (Supplemental Table 4, Table 1). Median (10th–90th percentiles) baseline serum T50 was 216 (111–333) minutes and 209 (108–323) minutes in the placebo and cinacalcet groups, respectively (P=0.16).
Factors Associated with T50
Baseline serum T50 levels were nearly normally distributed (Supplemental Figure 1). Supplemental Table 1 gives an overview of the distribution of factors associated with demographics, race and region, medical history, laboratory parameters, and medications by quintile of T50. Interestingly, increasing T50 quintiles were associated with decreasing quintiles of factors associated with bone resorption (intact PTH, N-telopeptide, phosphorus) and increasing quintiles of factors associated with bone formation (alkaline phosphatase, bone-specific alkaline phosphatase, 1,25-dihydroxy vitamin D). In the multivariate general linear regression model, lower T50 values (i.e., lower calcium phosphate crystallization resistance) were associated with older age, men, nonwhite race, lower serum albumin, bicarbonate, creatinine, HDL cholesterol, triglyceride, and 1,25-dihydroxy vitamin D concentrations and higher serum urea nitrogen and phosphate concentrations (Table 2). Notably, variables not associated with serum T50 included the traditional CV risk factors: smoking, hypertension, LDL cholesterol, diabetes mellitus, and BMI. Furthermore, the nontraditional risk factors PTH, alkaline phosphatase, and corrected serum calcium had dropped out of the model during the backward selection procedure.
Table 2.
Factors associated with T50, identified by multivariate linear regression
| Parameter | Estimate (min) | 95% CI | P Value |
|---|---|---|---|
| Intercept | 249 | (236 to 261) | <0.001 |
| Epidemiologic variables | |||
| Age, yr | −0.46 | (−0.67 to −0.25) | <0.001 |
| Women (versus men) | 9.95 | (3.91 to 15.99) | 0.001 |
| Ethnicity (versus white) | |||
| Black | −29.18 | (−36.76 to −21.60) | <0.001 |
| Other | −29.54 | (−36.30 to −22.77) | <0.001 |
| Blood values per 1 SD increase | |||
| Serum phosphorus, mg/dl | −45.23 | (−48.13 to −42.33) | <0.001 |
| Albumin, g/dl | 9.08 | (6.26 to 11.90) | <0.001 |
| Bicarbonate, mEq/L | 6.45 | (3.36 to 9.55) | <0.001 |
| 1,25(OH)D, pg/ml | 3.86 | (1.09 to 6.62) | 0.006 |
| HDL, mg/dl | 6.71 | (3.63 to 9.78) | <0.001 |
| Triglycerides, mg/dl | 7.97 | (4.96 to 10.98) | <0.001 |
| BUN, mg/dl | −6.66 | (−10.07 to −3.26) | <0.001 |
| Creatinine, mg/dl | 9.41 | (5.62 to 13.19) | <0.001 |
The “estimate” column quantifies the difference in T50 value related to an increment of 1 SD in the corresponding parameter value (continuous parameters) or comparing the two groups of a binary parameter. A positive estimate indicates a positive correlation between the parameter and T50 value, and a negative estimate indicates a negative correlation between the parameter and T50 value. Only variables that were found significant by the backward selection processes were included in the table. Variables assessed: age (years), sex, race group, cause of renal disease, history of diabetes, history of smoking, dialysis vintage (years), weight (kg), BMI (kg/m2), systolic BP (mmHg), diastolic BP (mm/Hg), pulse pressure (systolic–diastolic BP), parathyroid hormone (pg/ml), corrected serum calcium (mg/dl), serum phosphorus (mg/dl), calcium phosphate product (mg2/dl2), bone alkaline phosphatase (ng/ml), alkaline phosphatase (U/L), BUN (mg/dl), creatinine (mg/dl), albumin (g/dl), total protein (g/dl), sodium (mEq/L), potassium (mEq/L), chloride (mEq/L), bicarbonate (mEq/L), N-telopeptide (nmol/L), 25 hydroxy vitamin D (ng/ml), 1,25(OH)D (pg/ml), hemoglobin (g/dl), glucose (mg/dl), triglycerides (mg/dl), total cholesterol (mg/dl), HDL (mg/dl), LDL (mg/dl), uric acid (mg/dl), fibroblast growth factor 23 (pg/ml), baseline vitamin D use, baseline calcium phosphate binder use, β-adrenergic antagonists use, Angiotensin-converting enzyme-inhibitors/angiotensin receptor blocker use, antiplatelet use, statin use, erythropoietin use, iron use. Magnitude of SD: albumin 0.35 g/dl, bicarbonate 3.85 mEq/L, creatinine 18.29 mg/dl, HDL 2.85 mg/dl, phosphorus 1.49 mg/dl, triglycerides 1.41 mg/dl, 1,25(OH)2D 10.24 pg/dl. 95% CI, 95% confidence interval; 1,25(OH)D, 1,25 dihydroxy vitamin D.
T50 and Clinical Outcomes
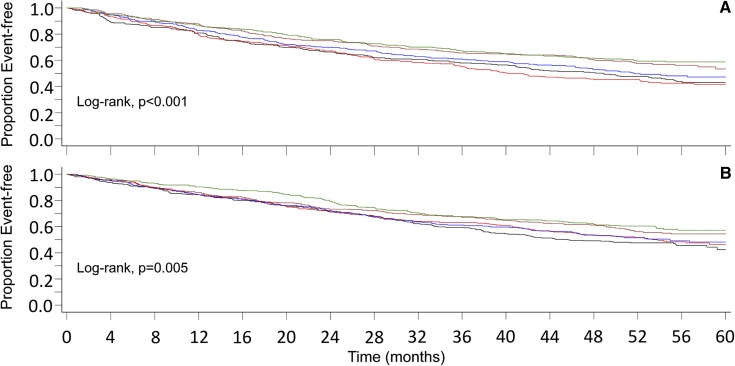
The primary composite end point (all-cause mortality, MI, hospitalization for unstable angina, heart failure, or PVE) was reached in 671 of 1366 patients (49%) randomized to placebo, and by 679 of 1419 randomized to cinacalcet (48%). Figure 1A shows the Kaplan–Meier curves for the primary composite end point of the placebo group, and Figure 1B for the cinacalcet group, according to quintiles of T50. In both groups, the groups/quintiles with higher T50 values had a significantly better outcome than those with a lower T50 value. This was also the case when the single components of the composite end point, i.e., all-cause mortality, MI, and PVE, as well as the tertiary CV composite end point (CV mortality, MI, heart failure, and hospitalization for unstable angina) were considered separately in the placebo group. In the cinacalcet group, statistical significance was reached for all-cause mortality and MI, and was close for all-cause mortality (log rank P=0.05) and PVE (log rank P=0.06, Supplemental Figure 2).
Figure 1.
Kaplan–Meier curves of the primary composite end point demonstrate that quintiles of T50 are significantly associated with outcome. (A) Placebo and (B) cinacalcet treatment group. Colors of lines representing T50 quintiles: lower: black, lower mid: red, middle: blue, upper mid: brown, upper: green.
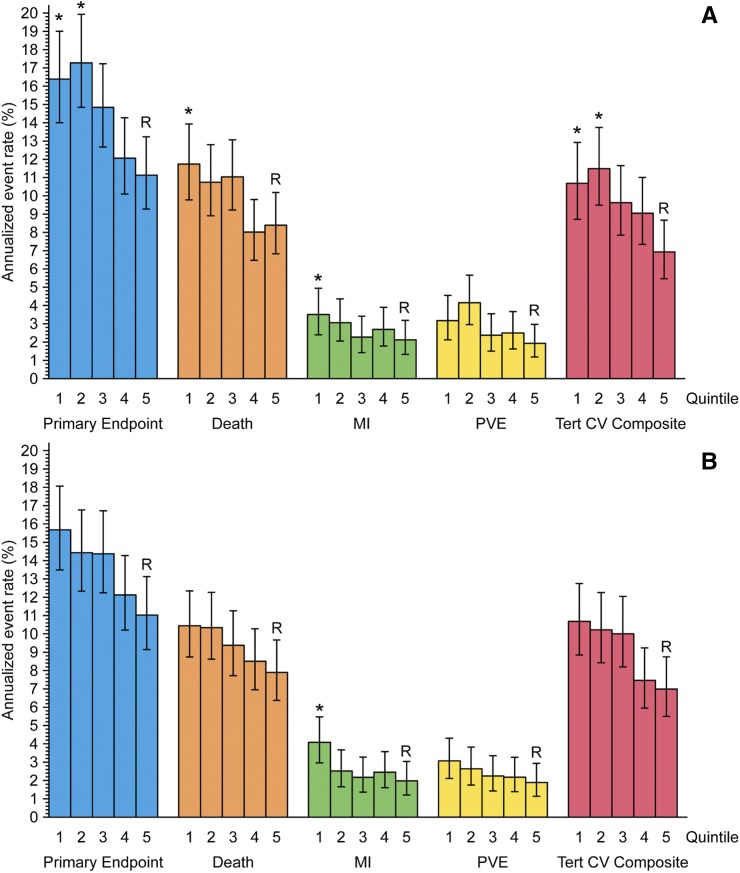
Figure 2 shows graphical representations of the annualized event rates by end point and quintiles of T50 in the placebo (Figure 2A) and the cinacalcet group (Figure 2B) where T50 was put into the adjusted Cox regression analysis as a categoric variable. With reference to quintile 5, significant differences were found in the placebo group for the primary composite end point (first and second quintile), death (first quintile), MI (first quintile), and the tertiary CV composite (first and second quintile) and in the cinacalcet group for MI (first quintile). Supplemental Table 2 provides general information about the variables and Supplemental Table 5 detailed information about the significant variables in the final Cox regression model for the primary composite end point.
Figure 2.
T50 is associated with annualized event rates according to quintiles of T50. (A) Placebo and (B) cinacalcet group. | represent 95% confidence intervals. Asterisks indicate P values <0.05 from a multivariable adjusted Cox regression analysis in which T50 was included as a categoric variable (quintiles, Supplemental Table 6). Quintile 5 was the reference group. “R” means the reference group. “*” indicates that the subjects in this T50 quintile have significantly different annualized event rate compared with subjects in the 5th quintile. The T50 quintile ranges for the placebo group were, q1: 49–138 minutes; q2: 139–189 minutes; q3: 190–236 minutes; q4: 237–289 minutes; q5: 290–521 minutes. The T50 quintile ranges for the cinacalcet group were, q1: 42–134 minutes; q2: 135–184 minutes; q3: 185–234 minutes; q4: 235–285 minutes; q5: 286–550 minutes. MI, myocardial infarctions; PVE, peripheral vascular events; Tert CV Composite, tertiary composite end point of the EVOLVE study.
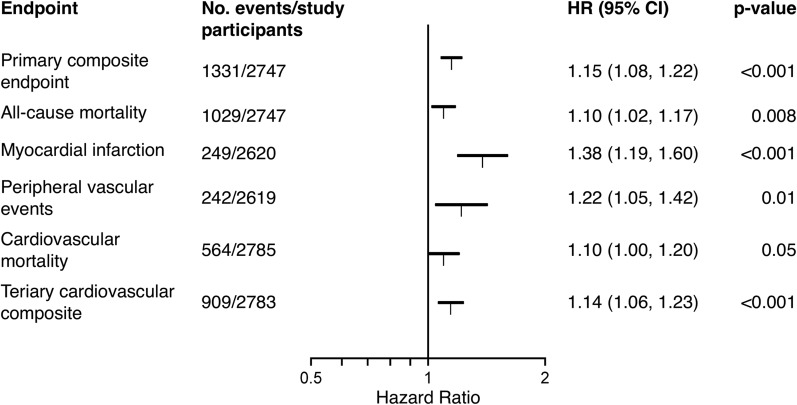
When T50 was considered a continuous variable in the pooled data, which had of note been adjusted for planned cinacalcet treatment, T50 was associated with the primary composite end point independent of other important baseline factors (HR per 1 SD decrease, 1.15; 95% CI, 1.08 to 1.22). The association was also observed for all-cause mortality (HR per 1 SD decrease, 1.10; 95% CI, 1.02 to 1.17), myocardial infarction (HR per 1 SD decrease, 1.38; 95% CI, 1.19 to 1.60), and PVE (HR per 1 SD decrease, 1.22; 95% CI, 1.05 to 1.42) (Figure 3). The Harrell C statistics calculated from the Cox regression model were 0.65 (95% CI, 0.60 to 0.70) both adjusting and not adjusting for baseline T50. Harrell C statistic is limited in its ability to quantify improvement in model performance after addition of a new marker (28). The IDI at 3-year risk was 0.004 (95% CI, 0.003 to 0.008; P<0.001) and NRI at 3-year risk was 0.02 (95% CI, 0.01 to 0.04; P=0.001).
Figure 3.
T50 is associated with clinical outcomes. Forest plot showing the magnitude of the association between lower T50 values and worse outcome per SD of T50, (84 minutes). T50 was not significantly associated with unstable angina or heart failure, therefore these end points are not shown here. 95% CI, 95% confidence interval; HR, hazard ratio.
Discussion
The T50 test is a novel in vitro blood test, which enumerates the propensity of individual sera to resist mineralization by measuring the timing of the transformation from primary to secondary CPP (21). Supplemental Figures 3 and 4 show the factors associated with the transformation from primary to secondary CPP found in this study and a schematic illustration of the T50 test principle, respectively. Here we observed an association between the results of the T50 test and the primary composite end point of the EVOLVE study and several of its individual components. Primarily nontraditional CV risk factors related to disorders of mineral metabolism appear to play an important role in patients receiving hemodialysis (29–32). Previous reports have linked these factors individually to vascular calcifications (12,33,34) and to survival (10,34,35) in cohorts with CKD. It has been speculated that several of these factors may serve as surrogate markers (e.g., albumin for inflammation [36]), or may be directly involved in active cellular processes (e.g., phosphate as a trigger of the osteogenic transformation of vascular smooth muscle cells [37]), or the mechanism has remained elusive (e.g., bicarbonate has not been attributed a specific role other than serving as a buffer involved in acid-base disturbances). Interestingly, BUN and creatinine had opposing effects in the multivariate regression analysis. Although the reason for these opposing effects is not known, we speculate that it might rather reflect a negative association with protein catabolism (BUN) and a positive association with muscle mass (creatinine) than with the urea and creatinine molecules themselves.
The individual associations of factors like phosphate, bicarbonate, and albumin with vascular calcifications and survival have been thoroughly studied, but the possibility of a direct functional interplay of these factors has not been tested because of the lack of a suitable in vitro test system. Such a direct interplay is however apparent in the calcification cascade, which proceeds in aqueous solutions from the formation of amorphous calcium phosphate to the transient formation of octacalcium phosphate, i.e., a hydroxyapatite precursor, and the final product hydroxyapatite (38,39), the main constituent of physiologic (bone and teeth) and pathologic (soft tissue and vasculature) calcifications. This sequence of events also occurs in body fluids like serum, where it is however a vastly delayed and regulated process integrated into a system of colloidal chemistry (Supplemental Figure 3) (40,41). Besides phosphate (destabilizing), also magnesium, pyrophosphate, and bicarbonate (all stabilizing) and likely other yet unidentified factors influence this process. Also pointing toward a functional interplay, a recent study reported that the serum magnesium concentration modifies the CV mortality risk associated with hyperphosphatemia (42). Lending plausibility to the major factors we found to be associated with T50 in our study, these are largely congruent with factors identified in previous in vitro experiments (i.e., phosphate, albumin [21]) and/or have been identified in previous clinical studies (phosphate, albumin, bicarbonate [22,23,43]).
The T50 test is a functional test, which can conceptually best be compared with functional clotting tests. While these tests give an estimate of the efficiency of an individual’s clotting system to form fibrin cross-links, the T50 test provides an estimate of the efficiency of an individual’s anticalcification system to inhibit the formation of calcium phosphate nanocrystals. Both tests are not representative of any single “independent” factor but instead depend as composite functional tests on the presence and interaction of multiple factors. Therefore, adjusting for factors already known to affect T50 will weaken the association between the T50 test result and the clinical outcome. For this reason, we did not include bone and mineral-related factors associated with T50 in our regression analyses.
Patients receiving hemodialysis suffer from an excess morbidity and mortality largely related to CV events (44). Unfortunately, controlled clinical outcome trials investigating the effects of intensified dialysis (45), lipid-lowering (46), or phosphate-binder choice (47) in patients receiving dialysis did not enhance survival or reduce the risk of CV events. The recognition, description, and characterization of the functional humoral mineralization system appears relevant, as it may help to better understand the pathophysiology and mechanisms involved in CV events in patients with CKD (22,23). Of note, the T50 test improved prediction despite the inclusion of clinical variables like “history of myocardial infarction” and “history of PVEs” which are strongly associated with future events and could therefore have considerably weakened the predictive value of the T50 test, as CV events may be in its “causal pathway.” It is tempting to speculate that going forward, the T50 test may guide multimodal and personalized interventions with the aim to e.g., co-ordinate meaningful phosphate-lowering and magnesium- and bicarbonate-increasing interventions.
Although the precise mechanism(s) which links T50 with outcome has not been elucidated in our study, the nature of the test indicates a role of the propensity of serum to form calcium phosphate nanocrystals.
Limitations of our analysis include that the EVOLVE trial only studied patients with moderate-to-severe secondary hyperparathyroidism receiving hemodialysis; hence, the findings reported here may not extend to other clinical settings, including patients new to dialysis, patients with mild-to-moderate CKD, or patients with only mild disturbances of mineral metabolism. This post hoc analysis also precludes conclusions concerning the causality of our findings. A further shortcoming is that vascular calcification itself was not evaluated in EVOLVE. Although the NRI and IDI were statistically significant, their magnitude as well as the magnitude of the c-statistic was modest. Finally, we only evaluated the predictive value of T50 testing on baseline samples. Longitudinal T50 testing could improve diagnostic accuracy. Major strengths of our study include the large sample size, diverse by age, sex, race/ethnicity, and geographic origin; detailed clinical characterization; and adjudication of CV end points.
In summary, we observed associations between lower T50 and higher risk of death, MI, and PVE in patients of the EVOLVE trial. The nature of the T50 test indicates that pathologic disturbances of serum-based calcification resistance provide a mechanism which links T50 to the pronounced morbidity and mortality in patients receiving hemodialysis. Prospective interventional studies are needed to determine whether these associations can be causally linked.
Disclosures
A.P. is an employee and stock holder of Calciscon. W.J.-D. is a stock holder of Calciscon; G.M.C. reports to receive grant support from Amgen; E.R.S. reports to hold stock in Calciscon and to receive grant support from Amgen and Baxter. J.F. reports consulting fees from Chugai, Bayer, Fresenius, Vifor, and lecture fees from Amgen, Vifor, Fresenius, Shire. P.P. reports lecture fees from Amgen. G.A.B. reports consulting fees from Amgen and to own stock in Ardelyx; X.M. is an employee of Amgen. M.B. is a part-time employee of Calciscon. S.A. has nothing to disclose. Rheinisch-Westfälische-Technische Hochschule Aachen, University of Aachen, Germany has submitted a patent regarding the T50 test, licenced to Calciscon. This study was supported by a grant from Amgen for the measurement of T50, which was carried out in the research lab of A.P. Ethical approval: None required for this ancillary post hoc analysis.
Supplementary Material
Acknowledgments
We gratefully acknowledge the help of Beatrix Blanchard, lab technician at the University Hospital Bern, Switzerland, for her valuable technical support. A.P. contributed to the conception, design, data analysis and interpretation, and drafting/revision of the manuscript. G.A.B., M.B., E.R.S., W.J.-D., S.A., G.M.C., P.P., and J.F. contributed to the design, data analysis and interpretation, and drafting/revision of the manuscript. X.M. performed the statistical analyses and contributed to data analysis and interpretation and to drafting/revision of the manuscript. X.M. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors critically revised the manuscript for important intellectual content and approved the final version of the manuscript. X.M. is the study guarantor.
Amgen Inc. provided financial support for the measurement of the T50 values in the research lab of A.P.
Parts of the data presented here have been presented as a free communication at the American Society of Nephrology Renal Week November 15, 2014 in Philadelphia (SA-OR065).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.04720416/-/DCSupplemental.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32[Suppl 3]: S112–S119, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Shah DS, Polkinghorne KR, Pellicano R, Kerr PG: Are traditional risk factors valid for assessing cardiovascular risk in end-stage renal failure patients? Nephrology (Carlton) 13: 667–671, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Huang JC, Chen SC, Su HM, Chang JM, Hwang SJ, Chen HC: Performance of the Framingham risk score in patients receiving hemodialysis. Nephrology (Carlton) 18: 510–515, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Floege J, Gillespie IA, Kronenberg F, Anker SD, Gioni I, Richards S, Pisoni RL, Robinson BM, Marcelli D, Froissart M, Eckardt KU: Development and validation of a predictive mortality risk score from a European hemodialysis cohort. Kidney Int 87: 996–1008, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adragao T, Herberth J, Monier-Faugere MC, Branscum AJ, Ferreira A, Frazao JM, Dias Curto J, Malluche HH: Low bone volume--a risk factor for coronary calcifications in hemodialysis patients. Clin J Am Soc Nephrol 4: 450–455, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group : KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl (113): S1–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK: Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol 12: 2131–2138, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Block GA, Hulbert-Shearon TE, Levin NW, Port FK: Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis 31: 607–617, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL: Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 16: 520–528, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Adeney KL, Siscovick DS, Ix JH, Seliger SL, Shlipak MG, Jenny NS, Kestenbaum BR: Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol 20: 381–387, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ribeiro S, Ramos A, Brandão A, Rebelo JR, Guerra A, Resina C, Vila-Lobos A, Carvalho F, Remédio F, Ribeiro F: Cardiac valve calcification in haemodialysis patients: Role of calcium-phosphate metabolism. Nephrol Dial Transplant 13: 2037–2040, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB: Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 342: 1478–1483, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Civilibal M, Caliskan S, Kurugoglu S, Candan C, Canpolat N, Sever L, Kasapcopur O, Arisoy N: Progression of coronary calcification in pediatric chronic kidney disease stage 5. Pediatr Nephrol 24: 555–563, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Ohtake T, Ishioka K, Honda K, Oka M, Maesato K, Mano T, Ikee R, Moriya H, Hidaka S, Kobayashi S: Impact of coronary artery calcification in hemodialysis patients: Risk factors and associations with prognosis. Hemodial Int 14: 218–225, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM: Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 38: 938–942, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Abdelmalek JA, Stark P, Walther CP, Ix JH, Rifkin DE: Associations between coronary calcification on chest radiographs and mortality in hemodialysis patients. Am J Kidney Dis 60: 990–997, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu S, Zhang DL, Guo W, Cui WY, Liu WH: Left ventricular mass index and aortic arch calcification score are independent mortality predictors of maintenance hemodialysis patients. Hemodial Int 16: 504–511, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Górriz JL, Molina P, Cerverón MJ, Vila R, Bover J, Nieto J, Barril G, Martínez-Castelao A, Fernández E, Escudero V, Piñera C, Adragao T, Navarro-Gonzalez JF, Molinero LM, Castro-Alonso C, Pallardó LM, Jamal SA: Vascular calcification in patients with nondialysis CKD over 3 years. Clin J Am Soc Nephrol 10: 654–666, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasch A, Farese S, Gräber S, Wald J, Richtering W, Floege J, Jahnen-Dechent W: Nanoparticle-based test measures overall propensity for calcification in serum. J Am Soc Nephrol 23: 1744–1752, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith ER, Ford ML, Tomlinson LA, Bodenham E, McMahon LP, Farese S, Rajkumar C, Holt SG, Pasch A: Serum calcification propensity predicts all-cause mortality in predialysis CKD. J Am Soc Nephrol 25: 339–348, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keyzer CA, de Borst MH, van den Berg E, Jahnen-Dechent W, Arampatzis S, Farese S, Bergmann IP, Floege J, Navis G, Bakker SJ, van Goor H, Eisenberger U, Pasch A: Calcification propensity and survival among renal transplant recipients. J Am Soc Nephrol 27: 239–248, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chertow GM, Block GA, Correa-Rotter R, Drüeke TB, Floege J, Goodman WG, Herzog CA, Kubo Y, London GM, Mahaffey KW, Mix TC, Moe SM, Trotman ML, Wheeler DC, Parfrey PS; EVOLVE Trial Investigators : Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med 367: 2482–2494, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Chertow GM, Correa-Rotter R, Block GA, Drueke TB, Floege J, Goodman WG, Herzog CA, Kubo Y, London GM, Mahaffey KW, Mix TC, Moe SM, Wheeler DC, Parfrey PS: Baseline characteristics of subjects enrolled in the Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) trial. Nephrol Dial Transplant 27: 2872–2879, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Chertow GM, Pupim LB, Block GA, Correa-Rotter R, Drueke TB, Floege J, Goodman WG, London GM, Mahaffey KW, Moe SM, Wheeler DC, Albizem M, Olson K, Klassen P, Parfrey P: Evaluation of Cinacalcet Therapy to Lower Cardiovascular Events (EVOLVE): rationale and design overview. Clin J Am Soc Nephrol 2: 898–905, 2007 [DOI] [PubMed] [Google Scholar]
- 27.D’Agostino RB, Lee ML, Belanger AJ, Cupples LA, Anderson K, Kannel WB: Relation of pooled logistic regression to time dependent Cox regression analysis: The Framingham Heart Study. Stat Med 9: 1501–1515, 1990 [DOI] [PubMed] [Google Scholar]
- 28.Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS: Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med 27: 157–172, discussion 207–212, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Becker BN, Himmelfarb J, Henrich WL, Hakim RM: Reassessing the cardiac risk profile in chronic hemodialysis patients: A hypothesis on the role of oxidant stress and other non-traditional cardiac risk factors. J Am Soc Nephrol 8: 475–486, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Spiegel DM, Raggi P, Smits G, Block GA: Factors associated with mortality in patients new to haemodialysis. Nephrol Dial Transplant 22: 3568–3572, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Noordzij M, Cranenburg EM, Engelsman LF, Hermans MM, Boeschoten EW, Brandenburg VM, Bos WJ, Kooman JP, Dekker FW, Ketteler M, Schurgers LJ, Krediet RT, Korevaar JC; NECOSAD Study Group : Progression of aortic calcification is associated with disorders of mineral metabolism and mortality in chronic dialysis patients. Nephrol Dial Transplant 26: 1662–1669, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Chertow GM, Raggi P, Chasan-Taber S, Bommer J, Holzer H, Burke SK: Determinants of progressive vascular calcification in haemodialysis patients. Nephrol Dial Transplant 19: 1489–1496, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Oka M, Ohtake T, Mochida Y, Ishioka K, Maesato K, Moriya H, Hidaka S, Kobayashi S: Correlation of coronary artery calcification with pre-hemodialysis bicarbonate levels in patients on hemodialysis. Ther Apher Dial 16: 267–271, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Bommer J, Locatelli F, Satayathum S, Keen ML, Goodkin DA, Saito A, Akiba T, Port FK, Young EW: Association of predialysis serum bicarbonate levels with risk of mortality and hospitalization in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 44: 661–671, 2004 [PubMed] [Google Scholar]
- 35.Sigrist MK, Taal MW, Bungay P, McIntyre CW: Progressive vascular calcification over 2 years is associated with arterial stiffening and increased mortality in patients with stages 4 and 5 chronic kidney disease. Clin J Am Soc Nephrol 2: 1241–1248, 2007 [DOI] [PubMed] [Google Scholar]
- 36.de Mutsert R, Grootendorst DC, Indemans F, Boeschoten EW, Krediet RT, Dekker FW; Netherlands Cooperative Study on the Adequacy of Dialysis-II Study Group : Association between serum albumin and mortality in dialysis patients is partly explained by inflammation, and not by malnutrition. J Ren Nutr 19: 127–135, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Shroff RC, McNair R, Figg N, Skepper JN, Schurgers L, Gupta A, Hiorns M, Donald AE, Deanfield J, Rees L, Shanahan CM: Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation 118: 1748–1757, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Boskey AL, Posner AS: Conversion of amorphous calcium phosphate to microcrystalline hydroxyapatite. A pH-dependent, solution-mediated, solid-solid conversion. J Phys Chem 77: 2313–2317, 1973 [Google Scholar]
- 39.Meyer JL, Eanes ED: A thermodynamic analysis of the amorphous to crystalline calcium phosphate transformation. Calcif Tissue Res 25: 59–68, 1978 [DOI] [PubMed] [Google Scholar]
- 40.Heiss A, DuChesne A, Denecke B, Grötzinger J, Yamamoto K, Renné T, Jahnen-Dechent W: Structural basis of calcification inhibition by alpha 2-HS glycoprotein/fetuin-A. Formation of colloidal calciprotein particles. J Biol Chem 278: 13333–13341, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Heiss A, Eckert T, Aretz A, Richtering W, van Dorp W, Schäfer C, Jahnen-Dechent W: Hierarchical role of fetuin-A and acidic serum proteins in the formation and stabilization of calcium phosphate particles. J Biol Chem 283: 14815–14825, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Sakaguchi Y, Fujii N, Shoji T, Hayashi T, Rakugi H, Iseki K, Tsubakihara Y, Isaka Y; Committee of Renal Data Registry of the Japanese Society for Dialysis Therapy : Magnesium modifies the cardiovascular mortality risk associated with hyperphosphatemia in patients undergoing hemodialysis: A cohort study. PLoS One 9: e116273, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dahle DO, Åsberg A, Hartmann A, Holdaas H, Bachtler M, Jenssen TG, Dionisi M, Pasch A: Serum calcification propensity is a strong and independent determinant of cardiac and all-cause mortality in kidney transplant recipients. Am J Transplant 16: 204–212, 2016 [DOI] [PubMed] [Google Scholar]
- 44.Thompson S, James M, Wiebe N, Hemmelgarn B, Manns B, Klarenbach S, Tonelli M; Alberta Kidney Disease Network : Cause of death in patients with reduced kidney function. J Am Soc Nephrol 26: 2504–2511, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, Allon M, Bailey J, Delmez JA, Depner TA, Dwyer JT, Levey AS, Levin NW, Milford E, Ornt DB, Rocco MV, Schulman G, Schwab SJ, Teehan BP, Toto R; Hemodialysis (HEMO) Study Group : Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 347: 2010–2019, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Wanner C, Krane V, März W, Olschewski M, Mann JF, Ruf G, Ritz E; German Diabetes and Dialysis Study Investigators : Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 353: 238–248, 2005 [DOI] [PubMed] [Google Scholar]
- 47.St Peter WL, Liu J, Weinhandl E, Fan Q: A comparison of sevelamer and calcium-based phosphate binders on mortality, hospitalization, and morbidity in hemodialysis: A secondary analysis of the Dialysis Clinical Outcomes Revisited (DCOR) randomized trial using claims data. Am J Kidney Dis 51: 445–454, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.