Abstract
Given the high comorbidity in patients on hemodialysis and the complexity of the dialysis treatment, it is remarkable how rarely a life-threatening complication occurs during dialysis. The low rate of dialysis emergencies can be attributed to numerous safety features in modern dialysis machines; meticulous treatment and testing of the dialysate solution to prevent exposure to trace elements, toxins, and pathogens; adherence to detailed treatment protocols; and extensive training of dialysis staff to handle medical emergencies. Most hemodialysis emergencies can be attributed to human error. A smaller number are due to rare idiosyncratic reactions. In this review, we highlight major emergencies that may occur during hemodialysis treatments, describe their pathogenesis, offer measures to minimize them, and provide specific interventions to prevent catastrophic consequences on the rare occasions when such emergencies arise. These emergencies include dialysis disequilibrium syndrome, venous air embolism, hemolysis, venous needle dislodgement, vascular access hemorrhage, major allergic reactions to the dialyzer or treatment medications, and disruption or contamination of the dialysis water system. Finally, we describe root cause analysis after a dialysis emergency has occurred to prevent a future recurrence.
Keywords: dialysis disequilibrium, air embolism, hemolysis, Clinical Protocols, Dialysis Solutions, Embolism, Air, Emergencies, Hemolysis, Humans, Hypersensitivity, Kidneys, Artificial, Needles, Pharmaceutical Solutions, Recurrence, renal dialysis, Root Cause Analysis, Trace Elements, Water
Introduction
There are currently approximately 400,000 patients with ESRD on maintenance hemodialysis (HD) in the United States (1). Each one receives dialysis at least thrice weekly (156 times per year) for a total of over 62 million dialysis sessions annually. Given the high comorbidity in patients on HD and the complexity of the dialysis treatment, it is remarkable how rarely a life-threatening complication occurs during dialysis. For example, a cardiac arrest occurs only seven times per 100,000 HD sessions (2). The low rate of major complications can be attributed to numerous safety features in modern dialysis machines; meticulous treatment and testing of the dialysate solution to prevent exposure to trace elements, toxins, and pathogens; adherence to detailed treatment protocols; and extensive training of dialysis staff to handle medical emergencies. Most HD emergencies can be attributed to human error. A smaller number are due to rare idiosyncratic reactions. Ongoing dialysis staff training is essential to both prevent human error as well as ensure prompt and effective interventions when complications happen.
This review highlights major emergencies that may occur during HD treatments (Table 1), measures to minimize them, and specific interventions to prevent catastrophic consequences on the rare occasions when such complications arise. We have provided case reports to illustrate these emergencies in Supplemental Appendix. Complications related to more frequent HD are not addressed in this review. Intradialytic hypotension, a relatively common complication during dialysis, is also beyond the scope of this review but has been the subject of some recent comprehensive papers (3–6).
Table 1.
Major dialysis emergencies
| Type of Emergency | Estimated Frequency, per Million HD Sessions | Refs. |
| Dialysis disequilibrium | ||
| Air embolism | 8.5–33 | Tennankore et al. (31), Wong et al. (32) |
| Hemolysis | ||
| Vascular access hemorrhage | ||
| Venous needle dislodgement | 14–91 | Tennankore et al. (31), Wong et al. (32), VA study (65), Pennsylvania patient safety study (64) |
| Allergic reaction | 21–170 | Villarroel and Ciarkowski (71), Daugirdas et al. (72), Simon et al. (129) |
| Cardiac arrest | 70 | Karnik et al. (2) |
| Errors in following the HD prescription |
HD, hemodialysis.
Dialysis Disequilibrium Syndrome
Dialysis disequilibrium syndrome (DDS) is a rare syndrome occurring in patients with severe azotemia undergoing their initial HD session. It is characterized by nausea, vomiting, headache, encephalopathy, and seizures (7,8). DDS is attributed to the faster decline of urea concentration in the blood than in the brain during the dialysis session. This lag (reverse urea effect) creates an osmotic gradient that promotes net water shift from the blood into the brain, leading to cerebral edema and its associated manifestations (9,10). Rosen et al. (11) studied 10 patients with AKI (baseline BUN concentrations of 210–460 mg/dl) undergoing their initial HD session. They obtained concurrent plasma and cerebrospinal fluid (CSF) samples before dialysis, immediately after dialysis, and 24 hours after dialysis. The CSF-to-plasma BUN ratio was 0.91 predialysis, 1.99 immediately after dialysis, and back to baseline 24 hours later.
In animal models of uremia, the alteration in brain urea and other electrolytes concentrations does not completely account for the increase in brain osmolality during rapid HD (12). Arieff et al. (12) suggested that generation of new solutes (“idiogenic osmoles”) in brain tissue accounted for brain edema during rapid HD. In contrast, Silver et al. (13) reported that retained urea in brain was sufficient to cause a change in brain water content in rapidly dialyzing animals. Moreover, the content of brain organic osmolytes (myoinosotol, glutamine, and taurine, etc.) did not increase in rapidly dialyzing animals (9,13,14). Idiogenic osmoles may be generated in brain during acute azotemia as an adaptive response to increased plasma BUN to prevent brain cell shrinkage (15) but are probably not generated during rapid HD (9). Finally, in a CKD animal model, there was a decrease in brain urea transporters and an increase in aquaporins (AQPs; AQP4 and AQP9), providing a potential molecular mechanism for the reverse urea effect (16).
The spectrum of DDS ranges from headache and restlessness in mild forms to nausea, vomiting, and hypertension in patients with moderate cases to seizures and coma in patients with severe cases (7,17,18). There is no set BUN value above which patients predictably develop DDS. Both a high BUN level (>175 mg/dl) and its rapid decline are risk factors for DDS (7,18,19). Additional risk factors include preexisting neurologic conditions, the first session of HD, hyponatremia, and liver disease (7,18,20). It is unknown whether the risk of DDS is similar in patients with advanced CKD and those with AKI. Computed tomography or magnetic resonance may show cerebral edema in patients with DDS.
Several strategies may prevent DDS in patients with very high serum BUN undergoing their first HD session (Table 2) (7,12,17). The most important measure is to slow the urea removal rate. In a dog model of uremia, Arieff et al. (12) compared rapid and slow HD that achieved a similar reduction in BUN. Rapid HD (100 minutes with a blood flow of 12 ml/kg per minute and a dialysate flow rate of 500 ml/min) produced an elevated CSF pressure and seizures. In contrast, slower HD (200 minutes at 5 ml/kg per minute blood flow rate and a dialysate flow rate of 500 ml/min) elevated the CSF pressure without causing seizures (12). Thus, a short HD session (2 hours) with a low blood flow (200 ml/min) and a urea reduction ratio goal of 0.4 is recommended as the initial prescription for patients at risk for DDS (7,17). Continuous RRT (CRRT) may be considered in patients at high risk for DDS, including those with intracranial mass or brain injury (20). Kidney Disease Improving Global Outcomes recommends CRRT over intermittent dialysis for AKI in patients with brain injury/edema or elevated intracranial pressure (21).
Table 2.
Potential strategies to prevent dialysis disequilibrium syndrome in high-risk patients
| (1) Limit the first HD session to 2–2.5 h |
| (2) Limit the blood flow to 200–250 ml/min |
| (3) Sodium modeling or a high-sodium dialysate |
| (4) Consider intravenous mannitol (1 g/kg) |
| (5) Consider CRRT in patients at high risk for DDS (traumatic brain injury, intracerebral hemorrhage, intracranial mass) |
HD, hemodialysis; CRRT, continuous RRT; DDS, dialysis disequilibrium syndrome.
Port et al. (22) reported that a higher dialysate sodium concentration (144–154 mmol/L) prevented DDS symptoms. Each 1-mmol/L increase in serum sodium offset the osmotic effect of 12 mg/dl BUN. Thus, sodium modeling may be useful in preventing DDS during the initial HD sessions in patients at risk (22). Adding glucose or glycerol to the dialysate may also prevent DDS (23). Rodrigo et al. (24) studied patients on HD at risk for DDS by adding glucose to the dialysate or administering intravenous mannitol. Increasing the dialysate glucose concentration to 450 mg/dl contributed 2–3 mosM/kg H2O, whereas intravenous mannitol (1 g/kg) added 8.5–10 mosM/kg H2O (24).
Air Embolism
Venous air embolism (VAE) during HD is thought to be rare, but because signs and symptoms of air embolism may mimic other more common complications, careful vigilance and high suspicion are required for the diagnosis. Air bubbles trapped in the systemic (pulmonary or cerebral) microcirculation may cause local ischemia, circulatory arrest, activation of complement and coagulation system, localized inflammation, and vascular endothelial cell damage (25–27).
Owing to safeguards in the modern HD machine, symptomatic air embolism is exceedingly rare during HD. Air may enter the extracorporeal HD circuit either as a result of residual air trapped in the tubing or dialyzer due to incomplete priming or because of a broken or loose luer connection prepump (where the arterial pressure is negative) (25,28–30) (Figure 1). Air entering the extracorporeal circuit presents to the venous air trap placed distal to the dialyzer, immediately decreasing the blood level in the chamber. The change in fluid level in this chamber is recognized by a sensor that triggers an alarm and stops the blood pump. As a consequence of these technologic safeguards, air embolism in the modern era results from human error. In a retrospective cohort study of 202 patients on home HD, only six patients had suspected air embolism that occurred during 183,603 dialysis sessions for an overall incidence less than one episode per 30,000 dialysis sessions. These episodes were related to inadequate priming or lack of clamping of the catheter or tubing (31). Another study reported a single case of air embolism during follow-up of 190 patients on home HD (approximately 117,000 HD sessions) for an incidence of less than one episode per 100,000 HD sessions. This case resulted from not clamping the arterial line during disconnection, thereby allowing air to enter the tubing (32).
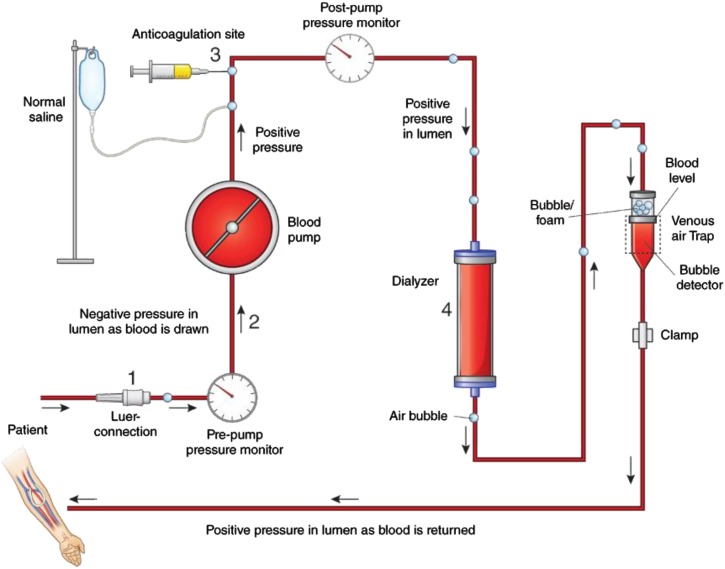
Figure 1.
Venous air embolism may arise from 4 possible areas of air entry into the dialysis circuit. Schematic diagram of a hemodialysis circuit depicting four possible areas of air entry. (1) A broken or loose luer connection between the arterial needle and the tubing can result in air entry, because this segment has negative intraluminal pressure. (2) A hole in the arterial tubing can suck air into the arterial line. (3) Air entry can occur during administration of anticoagulation or saline. (4) Inadequate priming can result in air entry from the dialyzer or dialysis tubing. A crack in the venous bloodline will not cause air entry due to the positive intraluminal pressure. Air entering the circuit presents to the venous air trap and forms foam/bubble at the top of blood level. As soon as the blood level in the venous air trap chamber falls below the air detector level, it immediately triggers an alarm and stops blood flow. As a consequence, venous air embolism occurs due to human error.
Most microbubbles <50 μm in diameter and many microbubbles between 50 and 200 μm pass through the venous bubble catcher without triggering an alarm (33). The rate of microbubble formation is dependent on the blood flow rate and negative arterial pressure. The total volume of microbubbles during an HD session is a few milliliters, a volume insufficient to cause acute symptoms (34). The venous air trap and air detector prevent infusion of larger amounts of air. If the air detector triggered an alarm for every microbubble, HD would be regularly interrupted (28). Therefore, a safe limit of air infusion (0.1 ml/kg body wt for bolus infusion and 0.03 ml/kg per minute for continuous infusion) has been suggested (25,28).
Measures to minimize the risk of air embolism include avoidance of extremely high dialysis blood flow, keeping the arterial luer lock tightened, adequately priming the dialyzer and tubing system before initiation of an HD session, and maintenance of a high blood level in the venous air catcher (29,35).
Massive VAE manifests with chest pain, dyspnea, and syncope. Cerebral air embolism may cause blurry vision, altered mental status, seizures, or ischemic stroke. Patients may develop hypotension and tachycardia due to right ventricular overload with involvement of the pulmonary capillary bed (26,27,36). The degree of end organ damage depends on the rate of air entry, volume of air, the patient’s position, and underlying cardiac status. In dogs, rapid injection of 7.5 ml/kg air is lethal. In humans, a volume of 100–300 ml air is considered fatal (37,38). A high clinical suspicion is required to diagnose VAE. Precordial Doppler can detect 0.05 ml/kg air, whereas transesophageal echocardiogram can detect 0.02 ml/kg air (39). Computed tomography may detect air in suspected cases of cerebral embolism.
After VAE is suspected, the patient should be provided with 100% oxygen (27). Aspiration of air may be attempted if the catheter is still in place (26). Early studies suggested that the left lateral recumbent (LLR) position could prevent right ventricular failure by preventing outflow tract obstruction during air embolism by moving the air more superiorly in the right ventricle (40–42). Geissler et al. (43) and Mehlhorn et al. (44) studied the effect of injecting 2.5 ml/kg air at a rate of 5 ml/s in dogs and concluded that the LLR position did not provide hemodynamic advantage over the supine position. Although traditional teaching has been to maintain an LLR with head down position for suspected VAE, the supine position has been recommended more recently (26). The supine position also provides additional advantage of appropriate delivery of oxygen and hemodynamic support, a critical part of the treatment (26). An LLR position has been recommended by Muth and Shank (26) if aspiration of air is to be attempted through an existing central venous catheter (CVC) for VAE (26,43).
Air embolism may also occur during placement of an HD CVC, accidental disconnection during its use, or at its removal. Vesely (45) reported 15 cases of air embolism occurring during placement of 11,583 tunneled and nontunneled CVCs (0.13%). All 15 cases occurred during insertion of tunneled CVCs; most patients had mild to moderate symptoms, except one who died. Rinsing the catheter, placing the patient in a supine position, and inserting the needle during expiration may prevent air embolism during CVC placement (46). A break in the catheter or accidental disconnection may also lead to fatal air embolism (47). During CVC removal, the patient should be supine, with catheter removal performed during exhalation or a Valsalva maneuver to increase intrathoracic pressure (46). An air-occlusive dressing should be in place for 24 hours to prevent delayed air entry through the subcutaneous track (48).
Hemolysis
Red blood cells (RBCs) undergo shear stress when they circulate through the HD circuit, and are, therefore, at risk for fragmentation. Additionally, blood osmotic changes, dialysate contaminants, or hyperthermia may each enhance hemolysis (Table 3) (49–52). Because blood flow is higher at the center of a laminar flow than at the edges (wall) of the extracorporeal circuit, the RBC membrane is exposed to a differential force on two different sides, thereby causing a shear stress (51). The low degree of hemolysis that typically occurs during HD is insufficient to produce a measurable drop in hematocrit. More significant hemolysis may occur in cases of dialysate contamination with trace metals (copper or zinc), disinfectant added to city water (chloramine), or nitrate (49,53). The normal starting dialysis blood flow with a new arteriovenous (AV) fistula is 250 ml/min with a 17-gauge needle. In comparison, a dialysis blood flow of 500 ml/min can be delivered using a 14-gauge needle without inducing hemolysis (54). The positive and negative pressures (approximately 1500 and −500 mmHg, respectively) sustained by RBCs in the extracorporeal circuit usually do not cause any hemolysis (51). However, attempting a high dialysis blood flow with a small gauge needle may induce hemolysis.
Table 3.
Causes of hemolysis during hemodialysis
| Dialysate related |
| Contamination with copper, zinc, nitrate, nitrite, and chloramine |
| Hypo-osmolar dialysate |
| Hyperthermia |
| Extracorporeal circuit related |
| Blood pump malocclusion |
| Single–needle high–flow dialysis |
| Partial occlusion of HD catheter |
| Kinked tubing, faulty blood tubing |
| Patient related |
| Sickle cell anemia |
| Hereditary spherocytosis |
| Autoimmune hemolysis |
HD, hemodialysis.
Overheated dialysate may cause thermal injury and hemolysis. A 1948 study by Ham et al. (55) showed changes in RBC morphology, osmotic fragility, and hemolysis at temperatures >47°C. In 1975, Berkes et al. (56) reported on a patient with delayed hemolysis 48 hours after exposure to overheated dialysate of 50°C. Because modern HD machines trigger an alarm at dialysate temperature >39.5°C, hyperthermic hemolysis is rare (55,57). If the HD machine triggers an alarm for overheated dialysate, an appropriate stepwise protocol should be followed: responding to the alarm, immediate cessation of dialysis, and informing concerned authorities and supervisor to find the source of heated water (57). Jepson and Alonso (57) reported an issue with a valve system that led to heated dialysate of 39.8°C; no harm was caused to the patient due to the attentiveness of the nurse who followed an appropriate protocol (57).
Hemolysis due to osmotic changes and dialysate impurities is also rare. Several investigators have reported mechanical injury to RBCs caused by kinked dialysis tubing (52,58). Sudden bends, turns, and the entry point into the dialyzer are additional vulnerable areas for kinking. A simultaneous decrease of >25 mmHg in both the arterial (prepump) and venous (postpump) pressures suggests a severe postpump kinked tubing that may result in hemolysis; this drop in pressure results from decrease blood flow due to severe obstruction. Therefore, it essential to use the length of tubing recommended by the manufacturer, monitor the arterial and venous dialysis pressures, and avoid sudden bends in the tubing (59). A multistate outbreak of hemolysis during HD led to the finding of faulty blood tubing causing hemolysis. Narrowing of the blood tubing before its entry to the dialyzer led to exposure of RBCs to increased pressure and consequent hemolysis (60).
Patients with severe intradialytic hemolysis complain of nausea, shortness of breath, abdominal/back pain, and chills and initially develop acute hypertension (61). Confirmatory laboratory data include low serum haptoglobin, elevated lactate dehydrogenase, reduction in hematocrit, and pink serum. When hemolysis is suspected, the HD session should be stopped immediately. Blood should not be returned from the extracorporeal circulation to the bloodstream due to the risk of precipitating severe hyperkalemia by infusing potassium released from hemolyzed erythrocytes. If several patients develop hemolysis in a single dialysis unit, contamination of the dialysate, faulty tubing, and altered dialysate osmolality should be suspected. A systematic assessment of the potential causes is imperative to avoid recurrent hemolysis during a subsequent HD session (Figure 2). A root cause analysis (RCA) should be performed in each case, and every aspect of the HD session should be well examined; blood tubing and the needle system should be saved for future investigation.
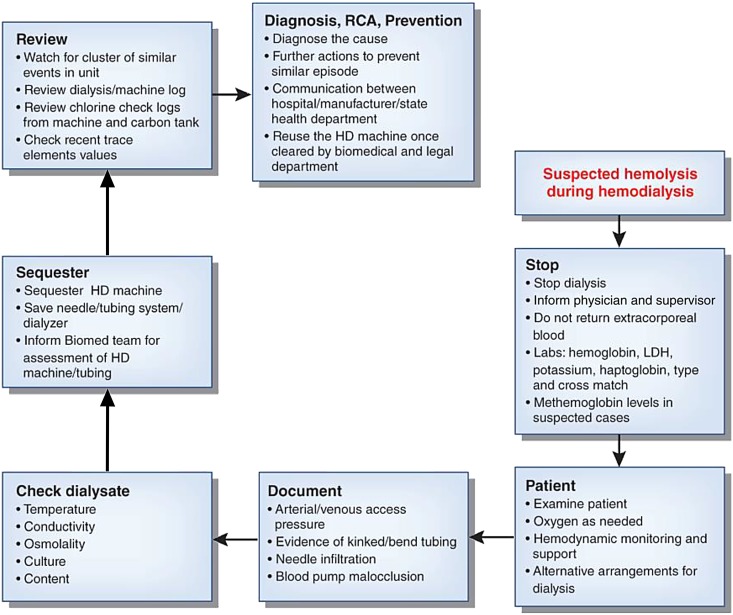
Figure 2.
Schematic diagram illustrating an approach to evaluation of a suspected case of hemolysis during hemodialysis (HD) and its root cause analysis to prevent future episodes. LDH, lactate dehydrogenase.
Venous Needle Dislodgement
Venous needle dislodgement (VND) is a rare but life-threatening complication of HD. With a typical dialysis blood flow of 300–500 ml/min, hemorrhagic shock ensues within minutes (after loss of 30%–40% of total blood volume) (62). The Veterans Administration National Center for Patient Safety reported 40 major hemorrhages due to VND or disconnection at the dialysis catheter site during 2.5 million dialysis sessions or approximately one episode per 60,000 HD sessions (63). Two large Canadian series of patients on home HD reported VND in one in 11,000 (31) and one in 20,000 HD sessions (32). Data from Pennsylvania public safety reported 32 patients with VND during 2.26 million HD session, for a frequency of about one in 70,000 HD sessions (64).
The major factors leading to needle dislodgement are related to access care (improper taping of access tubing to the skin, loose luer lock tubing connection, bloodlines not being looped loosely, or access site not being visible) and patient factors (a confused patient pulling the needle out of the access) (65). An acute decrease in dialysis venous pressure should theoretically ensue rapidly after dislodgement of the venous needle from the access and trigger a pressure alarm to alert the dialysis nurse. However, the venous alarm monitor on HD machines is affected by not only the intra-access pressure but also, the dialysis blood flow, blood viscosity, flow resistance of the extracorporeal tubing, and the height difference between the access and venous drip chamber (66). In addition, the intra-access pressure is higher for grafts than fistulas (by 27/15 mmHg) (67). Ideally, to ensure early detection of blood loss, the venous pressure alarm would be set 10 mmHg below the baseline dialysis venous pressure. However, the venous pressure varies by 30–40 mmHg during a typical dialysis session due to patient position (reclining versus sitting) and movement of the access extremity (66,68). To prevent triggering multiple false pressure alarms during each dialysis session, the pressure monitor is usually set below that threshold. As a consequence, the dialysis staff may have a false sense of security when, in fact, a substantial blood leak may occur before the venous pressure drops by 40 mmHg and triggers the pressure alarm.
Various sensors can detect blood leaks during VND. Some were initially developed to detect moisture related to enuresis but later, used off label for detection of VND by some dialysis units (65,66). Redsense, a device developed for detection of blood leakage, has been Food and Drug Administration cleared for in-center and home HD. It consists of an alarm unit connected by an optical fiber to a sensor patch; the sensor patch has an absorbent patch in the center. The patch is placed over the venous needle, with the absorbent area placed directly over the needle entry point. In the event of VND or significant blood leakage, the blood comes in contact with the optical sensor that is embedded within the patch and generates a continuous alarm (69). Ahlmén et al. (70) reported that Redsense correctly alarmed in 92.5% of cases of blood leakage; when the patch was modified and placed closer to the venous puncture site, the device functioned in 97.2% of all tests (70). Although blood sensors add substantially to the cost of HD (Redsense costs about $550), they can be considered for additional safety in high-risk patients and patients on home HD. However, sensors should never replace an appropriate stepwise protocol to prevent VND. This includes proper taping of access needle, adequate tightening of luer lock at all connections, and ensuring that all bloodlines are loosely looped to prevent accidental dislodgement. The access site should always be examined whenever the venous pressure monitor suggests a drop in pressure, even if the blood leakage detector does not generate an alarm. The two most important measures are maintaining the access site visible at all times and keeping high-risk patients close to the nurse’s station (65). A detailed 12-step protocol, including taping techniques, assessment of risks for VND, and its prevention, is available at https://www.annanurse.org/download/reference/journal/vndArticle.pdf (65).
Allergic Reaction during HD
An allergic or allergic-like reaction during HD must be closely investigated, because re-exposure to the allergen may result in worse signs and symptoms and a poor outcome. A 1982–1983 survey documented 3.3 allergic reactions per 1000 patient-years of dialysis with a hollow fiber dialyzer (71). Daugirdas et al. (72) reported 21 severe allergic reactions to dialyzers in 260,000 dialysis sessions for a frequency of approximately one episode in 12,000 HD sessions. These allergic reactions included four respiratory arrests and one death. A patient may get an allergic reaction to the dialyzer itself or more commonly, the sterilizer (kills all micro-organisms, including bacterial spores), disinfectant (kills micro-organisms on the surface but not bacterial spores), heparin, or other medications infused during dialysis (antibiotics, blood, or iron) (73,74).
Allergic reactions are classified as type A and type B (75,76). Type A allergic reactions occur within 5–20 minutes of HD initiation and present with pruritus, urticaria, bronchospasm, laryngeal edema, or anaphylactic shock. Type B reactions occur later in the dialysis session and are associated with less intense symptoms, such as chest and back pain. Type A reactions are mediated through IgE, whereas type B reactions are complement mediated (77).
Earlier dialyzers were composed of cellulose (a cotton derivative). Owing to their organic component, they activated the alternate complement cascade and were considered bioincompatible (77,78). Subsequent modifications that added acetate side chains to the cellulose (cellulose acetate, diacetate, and triacetate) reduced complement activation, making the dialyzers more biocompatible. At present, most United States dialysis centers use synthetic dialyzers, which are considered highly biocompatible, because they only minimally activate complement (78). Synthetic dialyzers are composed of polymethylmethacrylate, polyether sulfone, polysulfone, or polyacrylonitrile (PAN) (78). Dialyzers are sterilized with chemicals (ethylene oxide), steam (heat), or radiation (γ or β). Ethylene oxide has fallen out of favor due to its propensity to attach to the potting compound of the dialyzer and cause type A allergic reactions (79–82).
Various mechanisms (Figure 3) have been proposed for allergic and allergic-like reactions during HD depending on the allergen (77,82–88). Ethylene oxide mediates anaphylaxis through IgE-mediated hypersensitivity, whereas dialyzer components typically activate complement or bradykinin. Tielemans et al. (89) reported five cases of anaphylactoid reactions to acrylonitrile 69 (AN69) dialyzers occurring within minutes of HD initiation in patients on an angiotensin–converting enzyme inhibitor (ACEi). This reaction is mediated by bradykinin rather than IgE, histamine, or complement (89). The negatively charged moiety of AN69 during contact phase with blood may activate Hageman factor (factor 12), leading to bradykinin formation. ACEi also inhibits kininase, an enzyme that inactivates bradykinin, thereby further increasing plasma bradykinin levels and producing hypotension and angioedema in the absence of urticaria (90,91). PAN membranes pretreated with positively charged polyethyleneimine, are associated with markedly reduced bradykinin activation (92). Patients on ACEi can be safely dialyzed with AN69 dialyzers that are surface treated with polyethyleneimine (93).
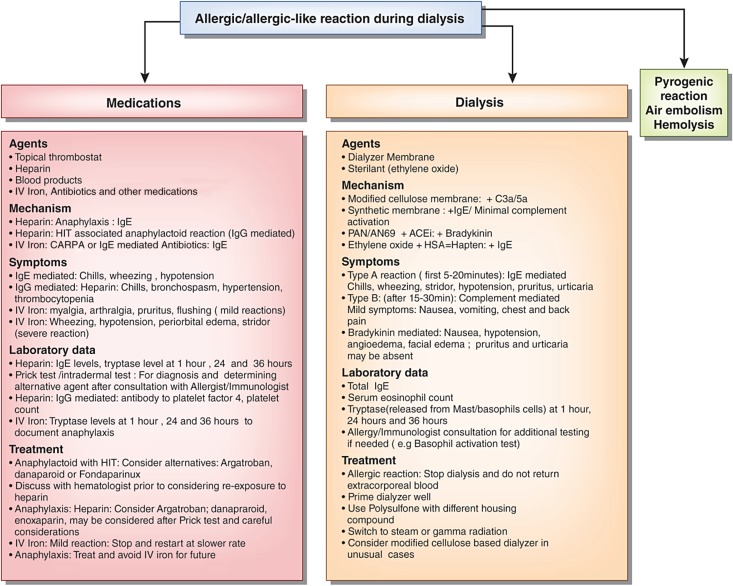
Figure 3.
Flow chart of possible causes of allergic or allergic-like reactions during hemodialysis. Similar symptoms could also be caused by other etiologies, like endotoxin back filtration causing pyrogenic reaction, hemolysis, and rarely, air embolism. Heparin can cause anaphylaxis or anaphylactoid associated with positive heparin–induced thrombocytopenia (HIT) antibodies. Blood products, antibiotics, and other medications used with dialysis may also cause allergic reaction. Intravenous iron may cause a reaction due to IgE-mediated or complement activation–related pseudoallergy (CARPA); at-risk patients have history of atopy, faster infusion, and possible iron dextran exposure than iron sucrose. Ethylene oxide may bind to HSA and act as a hapten to induce an allergic reaction. Although an allergic reaction to synthetic biocompatible dialyzers is rare, it has been reported. A dialyzer with different housing compound or modified cellulose dialyzer may be considered if other causes are ruled out. Occasionally, measuring tryptase and IgE levels may be helpful; additional immunoassays and prick testing may be undertaken after consultation with an allergist. ACEi, angiotensin–converting enzyme inhibitor; AN69, acrylonitrile; HSA, human serum albumin; IV, intravenous; PAN, polyacrylonitrile.
Disinfectants, such as hypochlorite (bleach) and formaldehyde, which are frequently used to reprocess dialyzers, may also cause an allergic reaction if they are not adequately rinsed out before the dialysis session (76).
Heparin may result in heparin-induced thrombocytopenia (HIT) (94). Five percent of patients with HIT develop allergic-like reactions characterized by nausea, cough, fever, and chills (83). Additional clinical features include hypertension initially, transient global amnesia, and profuse diarrhea. HIT–associated allergic–like reaction is mediated by IgG rather than IgE. In 2007–2008, an epidemic of anaphylaxis to heparin was caused by oversulfated chondroitin sulfate contaminants introduced during the manufacturing process (83).
Several measures can minimize the risk or severity of an allergic reaction during a dialysis session (Table 4). First, the dialyzer should be primed with sufficient saline to wash out the sterilant. Second, switching from ethylene oxide sterilization to γ-radiation or steam sterilization is helpful (81,86). Third, if a suspected allergic reaction occurs, it is critical to not return the blood into the extracorporeal circuit so as to avoid aggravating the hypersensitivity reaction (86). When a suspected allergic reaction occurs, one should also rule out other complications that can mimic this condition, such as air embolism, hemolysis, or a pyrogenic reaction. Severe hypersensitivity reactions are treated with antihistamines, corticosteroids, and epinephrine (77,86).
Table 4.
Measures to minimize an allergic reaction to a dialyzer
| (1) Prime the dialyzer well |
| (2) Switch from ethylene oxide sterilization to γ- or steam-sterilized dialyzer |
| (3) In the event of a hypersensitivity reaction, do not return the blood, because this may aggravate the allergic reaction |
| (4) Treat with antihistamines, corticosteroids, and epinephrine for a hypersensitivity reaction |
| (5) Rule out other conditions that may simulate an allergic reaction (air embolism, hemolysis, pyrogenic reaction) |
Adverse Reactions with Intravenous Iron
Intravenous iron, which is more efficacious than oral iron in raising hemoglobin in the HD population, is administered to approximately 70% of patients on HD each month (95). Adverse drug events to intravenous iron were estimated at 94 per million intravenous doses in 1998–2000 and decreased to 38 per million by 2001–2003 after a switch to safer formulations (96,97). The rate of fatal adverse drug events was highest for higher molecular weight dextran (11.3 per million), intermediate for lower molecular weight dextran (3.3 per million), and lowest for sodium ferric gluconate (0.9 per million) and iron sucrose (0.6 per million). Wysowski et al. (98) reported that the mortality rate between 2002 and 2006 was exceedingly low (0.06–0.32 deaths per million doses of iron purchased).
Minor adverse reactions can occur with any intravenous iron preparation, but severe life–threatening reactions are rare. Minor symptoms, such as pruritus, flushing, mild chest discomfort, arthralgia, myalgia, and nausea, usually abate with cessation of the infusion; it can be restarted at a lower rate after symptoms resolve (73,87). If patients develop urticaria, then infusion should be stopped, and the patient should be observed. Restarting the infusion after treatment with steroids may be considered after symptoms resolve (99). A more severe reaction may present with severe chest pain, persistent hypotension, and cough, and it may warrant stopping the infusion and treating with steroids and epinephrine. Premedication with steroids should be considered in patients at high risk of developing a reaction: history of inflammatory arthritis, multiple drug allergies, or severe asthma (100,101). Diphenhydramine should be avoided as a premedication, because it may cause symptoms similar to minor reactions and be falsely interpreted as an adverse effect (102,103). A life-threatening reaction (stridor, wheezing, periorbital edema, or symptomatic hypotension) to intravenous iron infusion precludes future iron infusion (99). Intravenous iron is contraindicated in the first trimester of pregnancy and should be used with caution during the second and third trimesters (87).
Emergencies Related to Dialysis Water System
This is a very brief review of the HD emergencies that can arise due to water system issues. Several excellent review articles provide greater detail (104–106). Acute loss of water in a dialysis unit is an emergency. It may be localized due to breakage in water pipes in the dialysis unit only or represent a hospital-wide problem. If it is localized to dialysis units, immediate notification to concerned authorities, nursing supervisor, and medical director should be made. If water from other areas of the hospital can be used, then a portable reverse osmosis system can be used. In case of more widespread loss of water, stepwise protocol should be followed, including saving water, alerting higher authorities, and communication with the city water body. Patients needing acute dialysis may be switched to alternative RRT, like CRRT, or transferred to a different medical facility (107).
Patients on in-center HD are exposed to close to 200 L water during an average dialysis session. With the dialysis membrane being the only barrier between the dialysate and blood, it is critical that municipal water undergo rigorous purification before its use during dialysis. Each dialysis unit has its own water system for purification of municipal water, protocol for sampling and monitoring, and management to adhere to the guidelines for water quality set by the American National Standards Institute/Association for the Advancement of Medical Instrumentation (104).
Pyrogenic reaction during a dialysis session may be due to multiple causes, including infection from various sources; water/dialysate bacterial contamination should be considered if there is cluster of similar events. Chloramine/chlorine is added to city water for decontamination; this level of protection is abolished downstream of carbon tanks in dialysis water systems, and thus, the reverse osmosis system, storage tank, and pipes are subjected to contamination (105,108,109). Dialysis units follow strict protocols to deliver purified water for dialysis, including running water system, maintenance, disinfection process, and periodic checks and evaluating with water cultures and endotoxin assays. Nonadherence to the protocol may result in water contamination.
Chloramine and chlorine are removed by primary and secondary carbon tanks of the dialysis water system. Chloramine may cause hemolysis and methemoglobinemia in patients on dialysis due to exhaustion of carbon tanks or excess load of chloramine in city water exceeding the capacity of carbon tanks (49,108–111). Thus, total chlorine, which is the sum of free chlorine and bound chlorine (chloramine), is measured from both carbon tanks every 4 hours. The total chlorine level should be <0.1 parts per million (1 part per million =1 mg/L) (104,112).
Methemoglobinemia usually manifests as cyanosis with chocolate brown color blood in the tubing and saturation gap (113). In such cases, the oxygen saturation calculated from arterial blood gas (PaO2≥70 mmHg) is higher than that measured by pulse oximetry (SaO2≤90%) (114,115). The diagnosis is confirmed by direct methemoglobin measurement.
Hydrogen peroxide is commonly used for disinfection of water storage tanks in hospitals. It is usually removed by carbon filters but not by reverse osmosis. There have been reports of hemolysis and methemoglobinemia from exposure of patients’ blood to hydrogen peroxide. This may happen if the potable water system does not have a carbon filter or if the carbon tanks gets exhausted when larger quantities of chlorine/chloramine and hydrogen peroxide have to be processed (113,116,117).
An outbreak of fluoride toxicity leading to severe pruritus, headache, and cardiac arrest has been reported (118). It was found that an exhausted resin of deionizer was releasing fluoride into the water. Fluoride (an anion) binds to calcium and magnesium and lowers their serum level. Additionally, it may cause hyperkalemia by its action on the sodium-potassium ATPase pump and indirectly cause efflux of potassium from cells (119).
To prevent such complications, appropriate protocol should always be followed. A periodic communication with the city water body and hospital maintenance may prevent additional complications. A cluster of adverse symptoms or events should prompt a thorough investigation.
Vascular Access Hemorrhage
Hemorrhage from an AV access is an uncommon but potentially fatal complication if it is not recognized promptly and acted on with an appropriate intervention. Most fatal vascular access hemorrhages occur outside of the dialysis facility, but occasionally, they rupture at the dialysis unit (120). Patients and their families should be educated about the recognition and emergent management of a bleeding AV access. Pseudoaneurysm (PSA) is a false aneurysm, because it does not have all of the layers of a vein, but it is rather composed of hematoma and fibrous tissue. It results from trauma and repeated cannulation during HD. Aneurysms usually form at the outflow vein/graft of an AV access and result from increasing dilation due to high blood flow and vascular damage (121,122). Physical examination of an aneurysm is the most important tool to determine the need for an intervention (Figure 4). Any rapidly enlarging PSA, evidence of outflow stenosis (arm elevation test—failure to collapse, high-pitch bruit), thinning or ulceration of skin over the PSA, pulsatility, or evidence of infection should prompt urgent intervention (121,123). Proper cannulation techniques may prevent PSA formation. Rope ladder technique prevents aneurysm formation, whereas repeated cannulation of the same area may promote aneurysm formation (123).
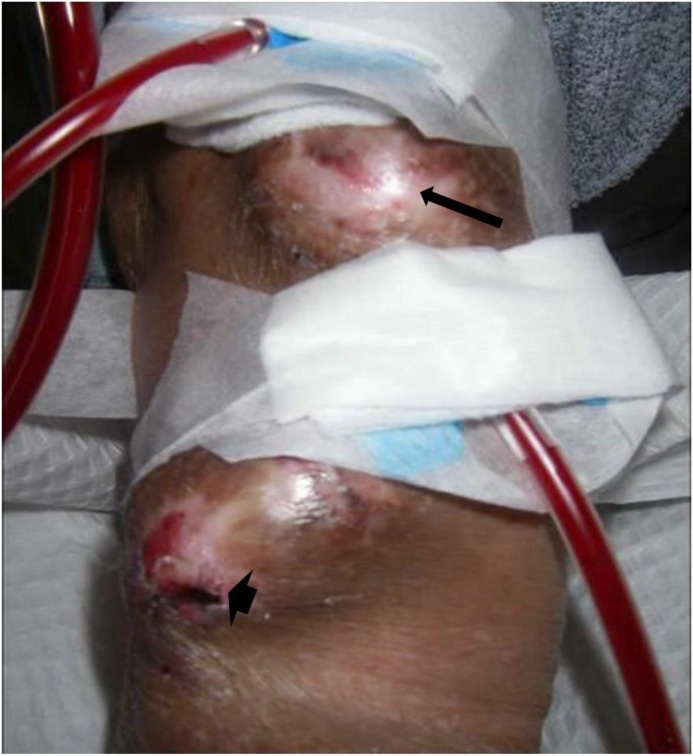
Figure 4.
An arteriovenous graft with evidence of thin shiny surface (arrow) and superficial ulceration (arrow head) over a pseudoaneurysm.
If the aneurysm is stable without evidence of imminent rupture (ulceration, thinning or shiny skin, or infection), then referral should be made for a fistulogram to evaluate for potential underlying outflow stenosis. Angioplasty to decrease the intra-access pressure may prevent aneurysm formation or slow its growth.
In the event of bleeding from vascular access site, direct continuous pressure with a finger for 15–20 minutes is the most effective method of controlling the bleeding. In the event of rupture of a PSA or aneurysm away from dialysis unit or hospital, direct pressure with a finger at the site of bleeding is the best method of controlling bleeding. Patients should be advised to continue holding direct pressure until emergency medical help arrives and avoid applying a tourniquet, towel, or BP cuff to the extremity (124).
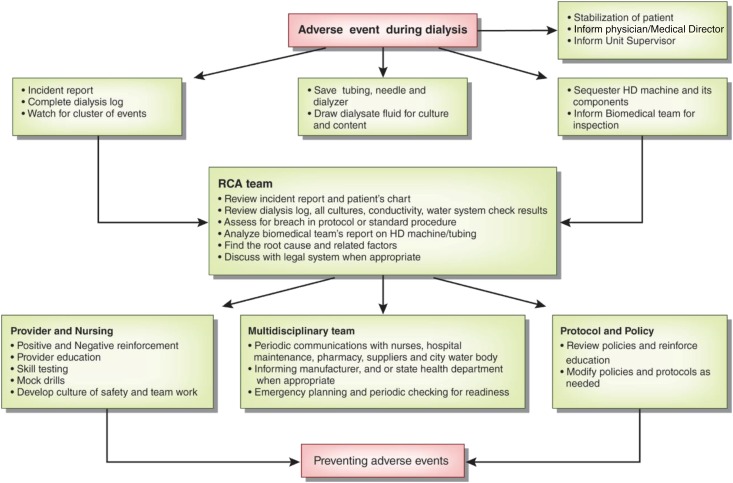
RCA
Any adverse event, whether it has occurred, has the potential to occur, or was averted by appropriate intervention, requires a thorough investigation to identify its root cause (125). All dialysis units must have protocols for reporting such events and taking appropriate measures toward RCA. The culture of safety in the health care field comes from education, following protocols, and a balance between staffing and work burden (126,127). There is often one main root cause leading to an adverse event and multiple other related factors; all of these factors should be evaluated. There are various guidelines regarding performing an RCA, including one by the Centers for Medicare and Medicaid Services (128).
During an adverse event, patient safety and management of the adverse event are paramount. The nursing staff and other care providers should work as a team to achieve that goal rather than blaming each other or attempting to find a cause. After the patient has been stabilized, additional stepwise measures should be undertaken toward an RCA. This may start with an incident report completed by nursing staff. The nurses are encouraged to elaborate the sequence of events, including those related to the patient, surrounding environment, and the dialysis machine. The dialysis log, including patient and machine vital measurements, should be completed. The HD machine, dialyzer, and tubing should be sequestered pending completion of the investigation. In an isolated event, like hemolysis during HD, a stepwise protocol toward that event may be considered (Figure 2). In other cases, a broader view may be more appropriate (Figure 5).
Figure 5.
A basic scheme for root cause analysis (RCA) after an adverse event related to hemodialysis (HD).
The appropriate authorities, including the dialysis supervisor, legal system, and hospital authorities, should be informed of the complication. An RCA should be exhaustive and confidential. After a cause is found, appropriate measures should be implemented to prevent a similar occurrence. These measures may include a change in policy or protocol, provider education, communication among different staff members and hospital personnel, multidisciplinary approach, and periodic mock drills and skills testing.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported, in part, by National Institutes of Health grant T32DK007545 (to M.S.).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.05260516/-/DCSupplemental.
References
- 1.USRDS: US Renal Data System USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Vol. 2, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2013 [Google Scholar]
- 2.Karnik JA, Young BS, Lew NL, Herget M, Dubinsky C, Lazarus JM, Chertow GM: Cardiac arrest and sudden death in dialysis units. Kidney Int 60: 350–357, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Agarwal R: How can we prevent intradialytic hypotension? Curr Opin Nephrol Hypertens 21: 593–599, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Dheenan S, Henrich WL: Preventing dialysis hypotension: A comparison of usual protective maneuvers. Kidney Int 59: 1175–1181, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Perazella MA: Pharmacologic options available to treat symptomatic intradialytic hypotension. Am J Kidney Dis 38[Suppl 4]: S26–S36, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Reilly RF: Attending rounds: A patient with intradialytic hypotension. Clin J Am Soc Nephrol 9: 798–803, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel N, Dalal P, Panesar M: Dialysis disequilibrium syndrome: A narrative review. Semin Dial 21: 493–498, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Arieff AI: Dialysis disequilibrium syndrome: Current concepts on pathogenesis and prevention. Kidney Int 45: 629–635, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Silver SM, Sterns RH, Halperin ML: Brain swelling after dialysis: Old urea or new osmoles? Am J Kidney Dis 28: 1–13, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Kennedy AC, Linton AL, Eaton JC: Urea levels in cerebrospinal fluid after haemodialysis. Lancet 1: 410–411, 1962 [DOI] [PubMed] [Google Scholar]
- 11.Rosen SM, O’Connor K, Shaldon S: Haemodialysis disequilibrium. BMJ 2: 672–675, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arieff AI, Massry SG, Barrientos A, Kleeman CR: Brain water and electrolyte metabolism in uremia: Effects of slow and rapid hemodialysis. Kidney Int 4: 177–187, 1973 [DOI] [PubMed] [Google Scholar]
- 13.Silver SM, DeSimone JA Jr., Smith DA, Sterns RH: Dialysis disequilibrium syndrome (DDS) in the rat: Role of the “reverse urea effect.” Kidney Int 42: 161–166, 1992 [DOI] [PubMed] [Google Scholar]
- 14.Silver SM: Cerebral edema after rapid dialysis is not caused by an increase in brain organic osmolytes. J Am Soc Nephrol 6: 1600–1606, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Trachtman H, Futterweit S, Tonidandel W, Gullans SR: The role of organic osmolytes in the cerebral cell volume regulatory response to acute and chronic renal failure. J Am Soc Nephrol 3: 1913–1919, 1993 [DOI] [PubMed] [Google Scholar]
- 16.Trinh-Trang-Tan MM, Cartron JP, Bankir L: Molecular basis for the dialysis disequilibrium syndrome: Altered aquaporin and urea transporter expression in the brain. Nephrol Dial Transplant 20: 1984–1988, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Zepeda-Orozco D, Quigley R: Dialysis disequilibrium syndrome. Pediatr Nephrol 27: 2205–2211, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bagshaw SM, Peets AD, Hameed M, Boiteau PJ, Laupland KB, Doig CJ: Dialysis disequilibrium syndrome: Brain death following hemodialysis for metabolic acidosis and acute renal failure--a case report. BMC Nephrol 5: 9, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez-Almaraz E, Correa-Rotter R: Dialysis disequilibrium syndrome and other treatment complications of extreme uremia: A rare occurrence yet not vanished. Hemodial Int 12: 301–306, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Esnault P, Lacroix G, Cungi PJ, D’Aranda E, Cotte J, Goutorbe P: Dialysis disequilibrium syndrome in neurointensive care unit: The benefit of intracranial pressure monitoring. Crit Care 16: 472, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kidney Disease Improving Global Outcome: Clinical Practice Guideline for Acute Kidney Injury, 2012. Available at: http://www.kdigo.org/clinical_practice_guidelines/pdf/KDIGO%20AKI%20Guideline.pdf. Accessed July 1, 2016
- 22.Port FK, Johnson WJ, Klass DW: Prevention of dialysis disequilibrium syndrome by use of high sodium concentration in the dialysate. Kidney Int 3: 327–333, 1973 [DOI] [PubMed] [Google Scholar]
- 23.Arieff AI, Lazarowitz VC, Guisado R: Experimental dialysis disequilibrium syndrome: Prevention with glycerol. Kidney Int 14: 270–278, 1978 [DOI] [PubMed] [Google Scholar]
- 24.Rodrigo F, Shideman J, McHugh R, Buselmeier T, Kjellstrand C: Osmolality changes during hemodialysis. Natural history, clinical correlations, and influence of dialysate glucose and intravenous mannitol. Ann Intern Med 86: 554–561, 1977 [DOI] [PubMed] [Google Scholar]
- 25.Barak M, Nakhoul F, Katz Y: Pathophysiology and clinical implications of microbubbles during hemodialysis. Semin Dial 21: 232–238, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Muth CM, Shank ES: Gas embolism. N Engl J Med 342: 476–482, 2000 [DOI] [PubMed] [Google Scholar]
- 27.van Hulst RA, Klein J, Lachmann B: Gas embolism: Pathophysiology and treatment. Clin Physiol Funct Imaging 23: 237–246, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Polaschegg HD: Hemodialysis machine air detectors need not detect microbubbles. Artif Organs 31: 911–912, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Forsberg U, Jonsson P, Stegmayr C, Jonsson F, Nilsson B, Nilsson Ekdahl K, Stegmayr B: A high blood level in the venous chamber and a wet-stored dialyzer help to reduce exposure for microemboli during hemodialysis. Hemodial Int 17: 612–617, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Riddick L, Brogdon BG: Fatal air embolism during renal dialysis. Am J Forensic Med Pathol 33: 110–112, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Tennankore KK, d’Gama C, Faratro R, Fung S, Wong E, Chan CT: Adverse technical events in home hemodialysis. Am J Kidney Dis 65: 116–121, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Wong B, Zimmerman D, Reintjes F, Courtney M, Klarenbach S, Dowling G, Pauly RP: Procedure-related serious adverse events among home hemodialysis patients: A quality assurance perspective. Am J Kidney Dis 63: 251–258, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Keshavarzi G, Barber TJ, Yeoh G, Simmons A, Reizes JA: Two-dimensional computational analysis of microbubbles in hemodialysis. Artif Organs 37: E139–E144, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Stegmayr B, Forsberg U, Jonsson P, Stegmayr C: The sensor in the venous chamber does not prevent passage of air bubbles during hemodialysis. Artif Organs 31: 162–166, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Wagner S, Rode C, Wojke R, Canaud B: Observation of microbubbles during standard dialysis treatments. Clin Kidney J 8: 400–404, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King MB, Harmon KR: Unusual forms of pulmonary embolism. Clin Chest Med 15: 561–580, 1994 [PubMed] [Google Scholar]
- 37.Palmon SC, Moore LE, Lundberg J, Toung T: Venous air embolism: A review. J Clin Anesth 9: 251–257, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Toung TJ, Rossberg MI, Hutchins GM: Volume of air in a lethal venous air embolism. Anesthesiology 94: 360–361, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Rossi UG, Torcia P, Rigamonti P, Colombo F, Giordano A, Gallieni M, Cariati M: Tunneled central venous catheter exchange: Techniques to improve prevention of air embolism. J Vasc Access 17: 200–203, 2016 [DOI] [PubMed]
- 40.Durant TM, Long J, Oppenheimer MJ: Pulmonary (venous) air embolism. Am Heart J 33: 269–281, 1947 [DOI] [PubMed] [Google Scholar]
- 41.Oppenheimer MJ, Durant TM, Lynch P: Body position in relation to venous air embolism and the associated cardiovascular-respiratory changes. Am J Med Sci 225: 362–373, 1953 [DOI] [PubMed] [Google Scholar]
- 42.Durant TM, Oppenheimer MJ, Lynch PR, Ascanio G, Webber D: Body position in relation to venous air embolism: A roentgenologic study. Am J Med Sci 227: 509–520, 1954 [PubMed] [Google Scholar]
- 43.Geissler HJ, Allen SJ, Mehlhorn U, Davis KL, Morris WP, Butler BD: Effect of body repositioning after venous air embolism. An echocardiographic study. Anesthesiology 86: 710–717, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Mehlhorn U, Burke EJ, Butler BD, Davis KL, Katz J, Melamed E, Morris WP, Allen SJ: Body position does not affect the hemodynamic response to venous air embolism in dogs. Anesth Analg 79: 734–739, 1994 [DOI] [PubMed] [Google Scholar]
- 45.Vesely TM: Air embolism during insertion of central venous catheters. J Vasc Interv Radiol 12: 1291–1295, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Feil M: Preventing central line air embolism. Am J Nurs 115: 64–69, 2015 [DOI] [PubMed] [Google Scholar]
- 47.Halliday P, Anderson DN, Davidson AI, Page JG: Management of cerebral air embolism secondary to a disconnected central venous catheter. Br J Surg 81: 71, 1994 [DOI] [PubMed] [Google Scholar]
- 48.Turnage WS, Harper JV: Venous air embolism occurring after removal of a central venous catheter. Anesth Analg 72: 559–560, 1991 [DOI] [PubMed] [Google Scholar]
- 49.Junglee NA, Rahman SU, Wild M, Wilms A, Hirst S, Jibani M, Seale JR: When pure is not so pure: Chloramine-related hemolytic anemia in home hemodialysis patients. Hemodial Int 14: 327–332, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Yoon J, Thapa S, Chow RD, Jaar BG: Hemolysis as a rare but potentially life-threatening complication of hemodialysis: A case report. BMC Res Notes 7: 475, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Polaschegg HD: Red blood cell damage from extracorporeal circulation in hemodialysis. Semin Dial 22: 524–531, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Sweet SJ, McCarthy S, Steingart R, Callahan T: Hemolytic reactions mechanically induced by kinked hemodialysis lines. Am J Kidney Dis 27: 262–266, 1996 [DOI] [PubMed] [Google Scholar]
- 53.Manzler AD, Schreiner AW: Copper-induced acute hemolytic anemia. A new complication of hemodialysis. Ann Intern Med 73: 409–412, 1970 [DOI] [PubMed] [Google Scholar]
- 54.Techert F, Techert S, Woo L, Beck W, Lebsanft H, Wizemann V: High blood flow rates with adjustment of needle diameter do not increase hemolysis during hemodialysis treatment. J Vasc Access 8: 252–257, 2007 [PubMed] [Google Scholar]
- 55.Ham TH, Shen SC, Fleming EM, Castle WB: Studies on the destruction of red blood cells; thermal injury; action of heat in causing increased spheroidicity, osmotic and mechanical fragilities and hemolysis of erythrocytes; observations on the mechanisms of destruction of such erythrocytes in dogs and in a patient with a fatal thermal burn. Blood 3: 373–403, 1948 [PubMed] [Google Scholar]
- 56.Berkes SL, Kahn SI, Chazan JA, Garella S: Prolonged hemolysis from overheated dialysate. Ann Intern Med 83: 363–364, 1975 [DOI] [PubMed] [Google Scholar]
- 57.Jepson R, Alonso E: Overheated dialysate: A case study and review. Nephrol Nurs J 36: 551–553, 2009 [PubMed] [Google Scholar]
- 58.Gault MH, Duffett S, Purchase L, Murphy J: Hemodialysis intravascular hemolysis and kinked blood lines. Nephron 62: 267–271, 1992 [DOI] [PubMed] [Google Scholar]
- 59.Malinauskas RA: Decreased hemodialysis circuit pressures indicating postpump tubing kinks: A retrospective investigation of hemolysis in five patients. Hemodial Int 12: 383–393, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Duffy R, Tomashek K, Spangenberg M, Spry L, Dwyer D, Safranek TJ, Ying C, Portesi D, Divan H, Kobrenski J, Arduino M, Tokars J, Jarvis W: Multistate outbreak of hemolysis in hemodialysis patients traced to faulty blood tubing sets. Kidney Int 57: 1668–1674, 2000 [DOI] [PubMed] [Google Scholar]
- 61.Murcutt G: Guarding against hidden haemolysis during dialysis: An overview. Summary of the EDTNA/ERCA Journal Club discussion Spring 2007. J Ren Care 33: 191–195, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Cherkas D: Traumatic hemorrhagic shock: Advances in fluid management. Emerg Med Pract 13: 1–19, 2011 [PubMed] [Google Scholar]
- 63.Bleeding Episodes during dialysis. Veterans Health Administration Warning System. Available at: http://www.patientsafety.va.gov/docs/alerts/BleedingEpisodesDuringDialysisAD09-02.pdf. Published 2008. Accessed July 1, 2016
- 64.Pennsylvania Patient Safety Authority: Hemodialysis Administration: Strategies to Ensure Safe Patient Care, 2010. Available at: http://patientsafetyauthority.org/ADVISORIES/AdvisoryLibrary/2010/Sep7(3)/Pages/87.aspx. Accessed March 16, 2016
- 65.Axley B, Speranza-Reid J, Williams H: Venous needle dislodgement in patients on hemodialysis. Nephrol Nurs J 39: 435–445, 2012 [PubMed] [Google Scholar]
- 66.Polaschegg HD: Venous needle dislodgement: The pitfalls of venous pressure measurement and possible alternatives, a review. J Ren Care 36: 41–48, 2010 [DOI] [PubMed] [Google Scholar]
- 67.Besarab A, Frinak S: The prevention of access failure: Pressure monitoring. ASAIO J 44: 35–37, 1998 [DOI] [PubMed] [Google Scholar]
- 68.Ribitsch W, Schilcher G, Hafner-Giessauf H, Krisper P, Horina JH, Rosenkranz AR, Schneditz D: Prevalence of detectable venous pressure drops expected with venous needle dislodgement. Semin Dial 27: 507–511, 2014 [DOI] [PubMed] [Google Scholar]
- 69.Redsense Medical: How it works. Available at: http://www.redsensemedical.com/index.php/product/how-it-works. Accessed September 1, 2016
- 70.Ahlmén J, Gydell KH, Hadimeri H, Hernandez I, Rogland B, Strömbom U: A new safety device for hemodialysis. Hemodial Int 12: 264–267, 2008 [DOI] [PubMed] [Google Scholar]
- 71.Villarroel F, Ciarkowski AA: A survey on hypersensitivity reactions in hemodialysis. Artif Organs 9: 231–238, 1985 [DOI] [PubMed] [Google Scholar]
- 72.Daugirdas JT, Ing TS, Roxe DM, Ivanovich PT, Krumlovsky F, Popli S, McLaughlin MM: Severe anaphylactoid reactions to cuprammonium cellulose hemodialyzers. Arch Intern Med 145: 489–494, 1985 [PubMed] [Google Scholar]
- 73.Fishbane S: Safety in iron management. Am J Kidney Dis 41[Suppl]: 18–26, 2003 [DOI] [PubMed] [Google Scholar]
- 74.Centers for Disease Control and Prevention: Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. Available at: http://www.cdc.gov/hicpac/Disinfection_Sterilization/19_00glossary.html. Accessed May 1, 2016
- 75.Daugirdas JT, Ing TS: First-use reactions during hemodialysis: A definition of subtypes. Kidney Int Suppl 24: S37–S43, 1988 [PubMed] [Google Scholar]
- 76.Salem M, Ivanovich PT, Ing TS, Daugirdas JT: Adverse effects of dialyzers manifesting during the dialysis session. Nephrol Dial Transplant 9[Suppl 2]: 127–137, 1994 [PubMed] [Google Scholar]
- 77.Sayeed K, Murdakes C, Spec A, Gashti C: Anaphylactic shock at the beginning of hemodialysis. Semin Dial 29: 81–84, 2016 [DOI] [PubMed] [Google Scholar]
- 78.Bouré T, Vanholder R: Which dialyser membrane to choose? Nephrol Dial Transplant 19: 293–296, 2004 [DOI] [PubMed] [Google Scholar]
- 79.Heegard KD, Tilley MA, Stewart IJ, Edgecombe HP, Lundy JB, Renz EM, Chung KK: Anaphylactoid reaction during first hemofiltration with a PUREMA polysulfone membrane. Int J Artif Organs 36: 363–366, 2013 [DOI] [PubMed] [Google Scholar]
- 80.Ansorge W, Pelger M, Dietrich W, Baurmeister U: Ethylene oxide in dialyzer rinsing fluid: Effect of rinsing technique, dialyzer storage time, and potting compound. Artif Organs 11: 118–122, 1987 [DOI] [PubMed] [Google Scholar]
- 81.Müller TF, Seitz M, Eckle I, Lange H, Kolb G: Biocompatibility differences with respect to the dialyzer sterilization method. Nephron 78: 139–142, 1998 [DOI] [PubMed] [Google Scholar]
- 82.Dolovich J, Bell B: Allergy to a product(s) of ethylene oxide gas: Demonstration of IgE and IgG antibodies and hapten specificity. J Allergy Clin Immunol 62: 30–32, 1978 [DOI] [PubMed] [Google Scholar]
- 83.Warkentin TE, Greinacher A: Heparin-induced anaphylactic and anaphylactoid reactions: Two distinct but overlapping syndromes. Expert Opin Drug Saf 8: 129–144, 2009 [DOI] [PubMed] [Google Scholar]
- 84.Shu KH, Kao TW, Chiang WC, Wu VC: A case of anaphylactic shock induced by FX60 polysulfone hemodialyzer but not F6-HPS polysulfone hemodialyzer. Hemodial Int 18: 841–845, 2014 [DOI] [PubMed] [Google Scholar]
- 85.Santosa A, Tan SH, Cheng YK: Recurrent intradialytic heparin induced anaphylaxis: Workup and management. Asia Pac Allergy 3: 285–288, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ebo DG, Bosmans JL, Couttenye MM, Stevens WJ: Haemodialysis-associated anaphylactic and anaphylactoid reactions. Allergy 61: 211–220, 2006 [DOI] [PubMed] [Google Scholar]
- 87.Rampton D, Folkersen J, Fishbane S, Hedenus M, Howaldt S, Locatelli F, Patni S, Szebeni J, Weiss G: Hypersensitivity reactions to intravenous iron: Guidance for risk minimization and management. Haematologica 99: 1671–1676, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ward RA: Ultrapure dialysate. Semin Dial 17: 489–497, 2004 [DOI] [PubMed] [Google Scholar]
- 89.Tielemans C, Madhoun P, Lenaers M, Schandene L, Goldman M, Vanherweghem JL: Anaphylactoid reactions during hemodialysis on AN69 membranes in patients receiving ACE inhibitors. Kidney Int 38: 982–984, 1990 [DOI] [PubMed] [Google Scholar]
- 90.Verresen L, Fink E, Lemke HD, Vanrenterghem Y: Bradykinin is a mediator of anaphylactoid reactions during hemodialysis with AN69 membranes. Kidney Int 45: 1497–1503, 1994 [DOI] [PubMed] [Google Scholar]
- 91.Schulman G, Hakim R, Arias R, Silverberg M, Kaplan AP, Arbeit L: Bradykinin generation by dialysis membranes: Possible role in anaphylactic reaction. J Am Soc Nephrol 3: 1563–1569, 1993 [DOI] [PubMed] [Google Scholar]
- 92.Thomas M, Valette P, Mausset AL, Déjardin P: High molecular weight kininogen adsorption on hemodialysis membranes: Influence of pH and relationship with contact phase activation of blood plasma. Influence of pre-treatment with poly(ethyleneimine). Int J Artif Organs 23: 20–26, 2000 [PubMed] [Google Scholar]
- 93.Maheut H, Lacour F: Using AN69 ST membrane: A dialysis centre experience. Nephrol Dial Transplant 16: 1519–1520, 2001 [DOI] [PubMed] [Google Scholar]
- 94.Greinacher A: CLINICAL PRACTICE. Heparin-induced thrombocytopenia. N Engl J Med 373: 252–261, 2015 [DOI] [PubMed] [Google Scholar]
- 95.TDOaPP Study: DOPPS Practice Monitor: Most Recent Trends in US Hemodialysis Practice. Available at: http://www.dopps.org/dpm/DPMSlideBrowser.aspx. Accessed July 31, 2016
- 96.Chertow GM, Mason PD, Vaage-Nilsen O, Ahlmén J: On the relative safety of parenteral iron formulations. Nephrol Dial Transplant 19: 1571–1575, 2004 [DOI] [PubMed] [Google Scholar]
- 97.Chertow GM, Mason PD, Vaage-Nilsen O, Ahlmén J: Update on adverse drug events associated with parenteral iron. Nephrol Dial Transplant 21: 378–382, 2006 [DOI] [PubMed] [Google Scholar]
- 98.Wysowski DK, Swartz L, Borders-Hemphill BV, Goulding MR, Dormitzer C: Use of parenteral iron products and serious anaphylactic-type reactions. Am J Hematol 85: 650–654, 2010 [DOI] [PubMed] [Google Scholar]
- 99.Macdougall IC, Bircher AJ, Eckardt KU, Obrador GT, Pollock CA, Stenvinkel P, Swinkels DW, Wanner C, Weiss G, Chertow GM; Conference Participants: Iron management in chronic kidney disease: Conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) controversies conference. Kidney Int 89: 28–39, 2016 [DOI] [PubMed] [Google Scholar]
- 100.Auerbach M, Chaudhry M, Goldman H, Ballard H: Value of methylprednisolone in prevention of the arthralgia-myalgia syndrome associated with the total dose infusion of iron dextran: A double blind randomized trial. J Lab Clin Med 131: 257–260, 1998 [DOI] [PubMed] [Google Scholar]
- 101.Cançado RD, Muñoz M: Intravenous iron therapy: How far have we come? Rev Bras Hematol Hemoter 33: 461–469, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Auerbach M, Macdougall IC: Safety of intravenous iron formulations: Facts and folklore. Blood Transfus 12: 296–300, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barton JC, Barton EH, Bertoli LF, Gothard CH, Sherrer JS: Intravenous iron dextran therapy in patients with iron deficiency and normal renal function who failed to respond to or did not tolerate oral iron supplementation. Am J Med 109: 27–32, 2000 [DOI] [PubMed] [Google Scholar]
- 104.Kasparek T, Rodriguez OE: What medical directors need to know about dialysis facility water management. Clin J Am Soc Nephrol 10: 1061–1071, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ward RA: Water processing for hemodialysis. Part I: A historical perspective. Semin Dial 10: 26–31, 1997 [DOI] [PubMed] [Google Scholar]
- 106.Layman-Amato R, Curtis J, Payne GM: Water treatment for hemodialysis: An update. Nephrol Nurs J 40: 383–404, 2013 [PubMed] [Google Scholar]
- 107.Centers for Medicare and Medicaid Services: Emergency preparedness for dialysis facilities. Available at: https://www.cms.gov/Medicare/End-Stage-Renal-Disease/ESRDNetworkOrganizations/downloads/emergencypreparednessforfacilities2.pdf. Accessed July 31, 2016
- 108.Fluck S, McKane W, Cairns T, Fairchild V, Lawrence A, Lee J, Murray D, Polpitiye M, Palmer A, Taube D: Chloramine-induced haemolysis presenting as erythropoietin resistance. Nephrol Dial Transplant 14: 1687–1691, 1999 [DOI] [PubMed] [Google Scholar]
- 109.Eaton JW, Kolpin CF, Swofford HS, Kjellstrand CM, Jacob HS: Chlorinated urban water: A cause of dialysis-induced hemolytic anemia. Science 181: 463–464, 1973 [DOI] [PubMed] [Google Scholar]
- 110.Davidovits M, Barak A, Cleper R, Krause I, Gamzo Z, Eisenstein B: Methaemoglobinaemia and haemolysis associated with hydrogen peroxide in a paediatric haemodialysis centre: A warning note. Nephrol Dial Transplant 18: 2354–2358, 2003 [DOI] [PubMed] [Google Scholar]
- 111.de Torres JP, Strom JA, Jaber BL, Hendra KP: Hemodialysis-associated methemoglobinemia in acute renal failure. Am J Kidney Dis 39: 1307–1309, 2002 [DOI] [PubMed] [Google Scholar]
- 112.de Oliveira RM, de los Santos CA, Antonello I, d’Avila D: Warning: An anemia outbreak due to chloramine exposure in a clean hemodialysis unit--an issue to be revisited. Ren Fail 31: 81–83, 2009 [DOI] [PubMed] [Google Scholar]
- 113.Newbigging N, Peel W, Bell E, Isles C: Unexpected cyanosis in a haemodialysis patient-did someone add hydrogen peroxide to the dialysis water? NDT Plus 2: 158–160, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Barker SJ, Tremper KK, Hyatt J: Effects of methemoglobinemia on pulse oximetry and mixed venous oximetry. Anesthesiology 70: 112–117, 1989 [DOI] [PubMed] [Google Scholar]
- 115.Guay J: Methemoglobinemia related to local anesthetics: A summary of 242 episodes. Anesth Analg 108: 837–845, 2009 [DOI] [PubMed] [Google Scholar]
- 116.Hoenich NA: Disinfection of the hospital water supply: A hidden risk to dialysis patients. Crit Care 13: 1007, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bek MJ, Laule S, Reichert-Jünger C, Holtkamp R, Wiesner M, Keyl C: Methemoglobinemia in critically ill patients during extended hemodialysis and simultaneous disinfection of the hospital water supply. Crit Care 13: R162, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Arnow PM, Bland LA, Garcia-Houchins S, Fridkin S, Fellner SK: An outbreak of fatal fluoride intoxication in a long-term hemodialysis unit. Ann Intern Med 121: 339–344, 1994 [DOI] [PubMed] [Google Scholar]
- 119.Mohapatra M, Anand S, Mishra BK, Giles DE, Singh P: Review of fluoride removal from drinking water. J Environ Manage 91: 67–77, 2009 [DOI] [PubMed] [Google Scholar]
- 120.Gill JR, Storck K, Kelly S: Fatal exsanguination from hemodialysis vascular access sites. Forensic Sci Med Pathol 8: 259–262, 2012 [DOI] [PubMed] [Google Scholar]
- 121.Georgiadis GS, Lazarides MK, Panagoutsos SA, Kantartzi KM, Lambidis CD, Staramos DN, Vargemezis VA: Surgical revision of complicated false and true vascular access-related aneurysms. J Vasc Surg 47: 1284–1291, 2008 [DOI] [PubMed] [Google Scholar]
- 122.Pasklinsky G, Meisner RJ, Labropoulos N, Leon L, Gasparis AP, Landau D, Tassiopoulos AK, Pappas PJ: Management of true aneurysms of hemodialysis access fistulas. J Vasc Surg 53: 1291–1297, 2011 [DOI] [PubMed] [Google Scholar]
- 123.Saeed F, Kousar N, Sinnakirouchenan R, Ramalingam VS, Johnson PB, Holley JL: Blood loss through AV fistula: A case report and literature review. Int J Nephrol 2011: 350870, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ball LK: Fatal vascular access hemorrhage: Reducing the odds. Nephrol Nurs J 40: 297–303, 2013 [PubMed] [Google Scholar]
- 125.Kliger AS: Patient safety in the dialysis facility. Blood Purif 24: 19–21, 2006 [DOI] [PubMed] [Google Scholar]
- 126.Thomas-Hawkins C, Flynn L, Clarke SP: Relationships between registered nurse staffing, processes of nursing care, and nurse-reported patient outcomes in chronic hemodialysis units. Nephrol Nurs J 35: 123–130, 2008 [PMC free article] [PubMed] [Google Scholar]
- 127.Davis KK, Harris KG, Mahishi V, Bartholomew EG, Kenward K: Perceptions of culture of safety in hemodialysis centers. Nephrol Nurs J 43: 119–126, 2016 [PubMed] [Google Scholar]
- 128.Centers for Medicare and Medicaid Services: Guidance for Performing Root Cause Analysis with Performance Improvement projects, 2010. Available at: https://www.cms.gov/medicare/provider-enrollment-and-certification/qapi/downloads/guidanceforrca.pdf. Accessed July 31, 2016
- 129.Simon P, Potier J, Thebaud HE: Risk factors for acute hypersensitivity reactions in hemodialysis. Nephrologie 17: 163–170, 1996 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.