Abstract
Background and objectives
Hyperkalemia is associated with adverse outcomes in patients with CKD and in hospitalized patients with acute medical conditions. Little is known regarding hyperkalemia, cardiovascular disease (CVD), and mortality in community-living populations. In a pooled analysis of two large observational cohorts, we investigated associations between serum potassium concentrations and CVD events and mortality, and whether potassium-altering medications and eGFR<60 ml/min per 1.73 m2 modified these associations.
Design, setting, participants, & measurements
Among 9651 individuals from the Multi-Ethnic Study of Atherosclerosis (MESA) and the Cardiovascular Health Study (CHS), who were free of CVD at baseline (2000–2002 in the MESA and 1989–1993 in the CHS), we investigated associations between serum potassium categories (<3.5, 3.5–3.9, 4.0–4.4, 4.5–4.9, and ≥5.0 mEq/L) and CVD events, mortality, and mortality subtypes (CVD versus non-CVD) using Cox proportional hazards models, adjusting for demographics, time-varying eGFR, traditional CVD risk factors, and use of potassium-altering medications.
Results
Compared with serum potassium concentrations between 4.0 and 4.4 mEq/L, those with concentrations ≥5.0 mEq/L were at higher risk for all-cause mortality (hazard ratio, 1.41; 95% confidence interval, 1.12 to 1.76), CVD death (hazard ratio, 1.50; 95% confidence interval, 1.00 to 2.26), and non-CVD death (hazard ratio, 1.40; 95% confidence interval, 1.07 to 1.83) in fully adjusted models. Associations of serum potassium with these end points differed among diuretic users (Pinteraction<0.02 for all), such that participants who had serum potassium ≥5.0 mEq/L and were concurrently using diuretics were at higher risk of each end point compared with those not using diuretics.
Conclusions
Serum potassium concentration ≥5.0 mEq/L was associated with all-cause mortality, CVD death, and non-CVD death in community-living individuals; associations were stronger in diuretic users. Whether maintenance of potassium within the normal range may improve clinical outcomes requires future study.
Keywords: Epidemiology and outcomes; mortality risk; serum potassium; Atherosclerosis; Cardiovascular Diseases; Demography; diuretics; Ethnic Groups; glomerular filtration rate; Humans; Hyperkalemia; Potassium; Proportional Hazards Models; Reference Values; Renal Insufficiency, Chronic; risk factors
Introduction
Severe hyperkalemia is well recognized as causing fatal arrhythmias, including ventricular fibrillation, asystole, and cardiac arrest (1–4). Although most individuals with hyperkalemic events concurrently have chronic or acute kidney disease, recent studies have suggested that patients admitted to the hospital with milder hyperkalemia and normal kidney function may also have a higher mortality risk (5). As a result of modest reductions in kidney function and widespread use of nonsteroidal anti-inflammatory drugs (NSAIDs) and medications that block the renin-angiotensin system, hyperkalemia is common in clinical practice. It is often tolerated clinically, especially when observed in the setting of the renin-angiotensin blockade, because it is commonly believed that the benefits of these medications may outweigh the risks associated with low-grade hyperkalemia.
Research regarding hyperkalemia has focused almost exclusively on patients admitted to the hospital with acute medical conditions (6). Thus, little is known regarding the implications of hyperkalemia in community-living populations. Moreover, it is unknown whether various comorbid conditions or medications may modify the associations between hyperkalemia and either cardiovascular disease (CVD) events or mortality. To address these questions, we evaluated two large observational studies in community-living individuals with long-term follow-up and adjudication of CVD events in the Multi-Ethnic Study of Atherosclerosis (MESA) and the Cardiovascular Health Study (CHS). The MESA recruited younger individuals without CVD at baseline, whereas the CHS recruited older individuals, some of whom had prevalent CVD. Therefore, we pooled these two representative samples of community-living individuals, and excluded participants with prevalent CVD in the CHS in order to investigate associations between serum potassium, incident CVD, and mortality in a large and geographically diverse cohort free of CVD at baseline. We also examined whether the associations differed depending on whether individuals had eGFR<60 ml/min per 1.73 m2 or were using potassium-altering medications.
Materials and Methods
Study Populations
The MESA enrolled 6814 participants between 2000 and 2002 who were free of CVD (defined as physician diagnosed myocardial infarction (MI), angina, nitroglycerine use, stroke or transient ischemic attack, heart failure, current atrial fibrillation, or having undergone cardiovascular procedures [coronary artery bypass grafting, angioplasty, valve replacement, pacemaker or defibrillator implantation, any surgery on the heart or arteries]). The population was recruited to be multiethnic (black, Hispanic, Asian, and non-Hispanic white Americans) and was drawn from six cities across the United States (7). From this group, we excluded 346 who were missing serum potassium measurements or covariate data, resulting in an analytic sample of 6468 participants within the MESA.
Participants in the CHS were all aged ≥65 years at baseline and there was no exclusion for prevalent CVD (7,8). The CHS enrolled 5201 participants between 1989 and 1990 from four regions across the United States. An additional 687 black participants were enrolled between 1992 and 1993. From these 5888 participants, 711 lacked eGFR measurements, 80 lacked serum potassium measurements, 25 lacked covariate measurements, and 1926 had prevalent CVD (defined as MI, stroke, coronary heart disease [CHD], angina, coronary bypass, transient ischemic attack, or heart failure). As these groups were not mutually exclusive, these exclusions resulted in a sample of 3206 CHS participants. Twenty-three individuals had participated in both the MESA and CHS, so after excluding them from the MESA (and retaining them in the CHS), we ultimately derived an analytic dataset of 9651 participants for pooled analysis.
The institutional review boards for both MESA and CHS sites, and the University of California, San Diego approved this study, and all participants provided written informed consent.
Serum Potassium
In the MESA, serum samples were collected at baseline, between 2000 and 2002, and frozen at −80°C. They were thawed in 2011 for measurement at the Kidney Research Institute at University of Washington utilizing indirect potentiometry with an ion selective electrode on an automated clinical chemistry analyzer (UniCel DxC 800; Beckman Coulter, Inc., Brea, CA). Coefficients of variation (CVs) for this assay were 1.3%–2.0%.
In the CHS, serum potassium was measured in 5132 samples from the 1989–1990 and 1992–1993 exams at the University of Vermont Laboratory for Clinical Biochemistry Research following standard procedures for collection and storage, and using the Kodak Ektachem 700 Analyzer (Eastman Kodak, Rochester, NY), a colorimetric method (9). CVs were approximately 2.0% (10).
Cardiovascular Events
In both the MESA and CHS, a CVD event was defined as a composite of MI, resuscitated cardiac arrest, definite angina, probable angina (if followed by revascularization), stroke, stroke death, CHD death, other atherosclerotic death, or other CVD death. Cardiovascular events were adjudicated by a centralized events committee, which entailed detailed review of medical records by a panel of physicians, compared with prespecified criteria, which came to consensus on each event (7,11).
Total and CVD Mortality
Total and CVD mortality were adjudicated by centralized events committees in both cohorts (11,12). In both the MESA and CHS, CVD death was defined as death from atherosclerotic CVD, stroke, atherosclerotic disease other than coronary disease or stroke (e.g., aortic aneurysm), or other CVD death (e.g., valvular heart disease).
In the MESA, non-CVD death was defined as a death that was not related to CVD. In the CHS, non-CVD death was first classified into 19 disease and organ system categories. We then collapsed non-CVD death into four categories representing the most common causes: pulmonary (e.g., chronic obstructive pulmonary disease, pneumonia), cancer, neurologic (e.g., Parkinson disease, dementia, amyotrophic lateral sclerosis), or other, consistent with prior CHS manuscripts (13).
Sudden cardiac death (SCD) was defined according to the National Heart, Lung, and Blood Institute working group on SCD, where SCD is a pulseless condition of cardiac origin in a previously stable person, occurring out of the hospital or in the emergency department (14). Investigation of SCD in the CHS was performed ancillary to the collection of main events, and was followed until 2006.
Covariates
Covariates were measured at baseline concurrent with serum potassium measurement. Participants reported age, sex, race/ethnicity, highest attained education, smoking status, pack-years of smoking, and ever having been diagnosed with cancer. Medication use was obtained through a validated medication inventory, as described previously (7,15). Medications of particular interest for this study included diuretics, NSAIDS (both prescribed and over-the-counter), potassium supplements, angiotensin-converting enzyme (ACE) inhibitors/angiotensin II receptor blockers (ARB), β agonists, β blockers, and any other antihypertensive medications (15). ARBs were not available at the CHS baseline visit (1989–1990), and were therefore not included in the CHS. Diabetes mellitus was defined as fasting blood glucose ≥126 mg/dl or the use of hypoglycemic medications. eGFR<60 ml/min per 1.73 m2 was calculated using serum concentrations of both creatinine and cystatin C (16). Trained study personnel measured systolic and diastolic BP, height, and weight. Standard clinical analyzers were used to measure fasting blood glucose, total cholesterol level, triglycerides, and urine albumin and creatinine, the latter two of which were combined to create the urine albumin-to-creatinine ratio in analysis in the MESA.
Statistical Methods
Before analysis, we elected to model serum potassium by categories: <3.5, 3.5–3.9, 4.0–4.4, 4.5–4.9, and ≥5.0 mEq/L. We examined the distribution of covariates by categories of serum potassium using ANOVA for continuous variables and chi-squared tests for categorical variables.
In the MESA, participants provided risk time from their baseline visit (2000–2002) until April 13, 2011. In the CHS, participants were considered at risk from their baseline visit (1989–1990 or 1992–1993, depending upon date of each participant’s enrollment) until December of 2011, except for sudden death, which was available up to 2006.
We calculated the unadjusted incident rates of CVD events, and total and cause-specific mortality for each category of serum potassium concentration separately. We then used Cox proportional hazards models to examine associations of serum potassium concentrations with each outcome, setting the 4.0–4.4 mEq/L category as the referent category. With regard to cause-specific mortality, we utilized competing risks models as described by Lunn and McNeil (17). Analyses were performed in sequential models. Model 1 adjusted for age, sex, and race/ethnicity. Model 2 additionally added time-varying eGFR. Model 3 further added diabetes mellitus and systolic BP, and model 4 added current smoking, pack-years of smoking, ever having cancer, study cohort (MESA versus CHS), ACE/ARB, diuretics (potassium sparing and all other diuretics), NSAIDs, potassium supplements, β agonists, β blockers, and use of any other antihypertensive medication.
To examine the functional form of fully adjusted associations between unit change in serum potassium concentration with each outcome, we used penalized smoothing splines with evenly spaced knots among the inner 95% distribution (18).
In order to address whether associations differed by eGFR<60 ml/min per 1.73 m2 versus eGFR≥60 ml/min per 1.73 m2, ACE/ARB use, NSAIDs use, or diuretics use, we evaluated multiplicative interaction terms, and conducted stratified analyses when statistically significant interactions were observed. Additionally, we evaluated interactions by sex and race/ethnicity for the main outcomes only: all-cause mortality, CVD death, non-CVD death, and CVD events.
All statistical tests were two-sided, and P<0.05 was considered statistically significant for all analyses, including interaction terms. No adjustments were made for multiple comparisons, as our a priori hypothesis focused on these specific outcomes (19). Analyses were conducted using SAS, version 9.4 (SAS Institute, Inc., Cary, NC) except for spline analysis, which was conducted in R software (2013; http://www.R-project.org/).
Results
Among 9651 individuals in pooled analysis, the mean age was 66 ± 10 years, 58% were women, 56% were non-Hispanic white, 40% were from the CHS cohort, and mean eGFR was 80 ± 18 ml/min per 1.73 m2. The mean serum potassium was 4.2 ± 0.4 mEq/L and 269 participants (3%) had serum potassium ≥5.0 mEq/L. Compared with persons with lower serum potassium concentrations, those with concentrations ≥5.0 mEq/L were more frequently men, had lower eGFR, were more likely to be former smokers and have more pack-years of smoking, were more likely to have diabetes, to have a cancer history, and to be current users of ACE/ARB medications (Table 1).
Table 1.
Baseline characteristics by serum potassium categories in a pooled cohort of MESA and CHS participants (N=9651)
| Baseline Characteristics | Potassium Concentration, mEq/L | ||||
|---|---|---|---|---|---|
| <3.5 | 3.5–3.9 | 4.0–4.4 | 4.5–4.9 | ≥5.0 | |
| Total, n | 323 | 1582 | 5134 | 2343 | 269 |
| Clinical measures | |||||
| Age, yr | 69.8 (8) | 67.1 (9) | 65.8 (10) | 65.5 (11) | 66.1 (11) |
| Women, % | 70.0 | 67.9 | 58.9 | 49.7 | 41.6 |
| BMI, kg/m2 | 28.3 (5) | 27.7 (6) | 27.5 (5) | 27.6 (5) | 27.3 (5) |
| Systolic BP, mmHg | 139.4 (21) | 135.7 (23) | 129.2 (22) | 127.2 (21) | 129.4 (21) |
| Diastolic BP, mmHg | 73.7 (11) | 73.2 (11) | 71.4 (11) | 70.6 (10) | 71.2 (10) |
| Fasting blood glucose, mg/dl | 117.4 (50) | 106.2 (30) | 105.6 (32) | 105.5 (32) | 115.6 (49) |
| Total cholesterol, mg/dl | 204.5 (42) | 200.1 (37) | 202.4 (38) | 200.5 (38) | 200.2 (34) |
| HDL-C, mg/dl | 55.7 (16) | 54.6 (16) | 53.0 (15) | 52.2 (15) | 51.2 (15) |
| LDL-C, mg/dl | 121.9 (37) | 119.7 (33) | 123.0 (34) | 122.0 (34) | 123.6 (30) |
| Triglycerides, mg/dl median (25%, 75%) | 120 (86, 166) | 113 (84, 158) | 114 (84, 162) | 116 (83, 161) | 108 (78, 160) |
| eGFR, ml/min per 1.73 m2 | 75.1 (18) | 80.4 (18) | 81.8 (17) | 78.6 (18) | 73.9 (24) |
| Demographics, % | |||||
| Non-Hispanic white | 60.7 | 52.0 | 55.8 | 59.5 | 50.9 |
| Education greater than high school | 45.2 | 53.6 | 56.3 | 57.3 | 55.8 |
| Cigarette smoking status | |||||
| Never | 52.3 | 53.9 | 50.8 | 44.6 | 43.9 |
| Former | 37.2 | 34.5 | 37.1 | 39.7 | 43.5 |
| Current | 10.5 | 11.6 | 12.1 | 15.7 | 12.6 |
| Pack-years cigarette smoking, median, (25%, 75%) | 0 (0, 18) | 0 (0, 15) | 0 (0, 17.5) | 1.9 (0, 24) | 2.0 (0, 22) |
| Comorbidities, % | |||||
| Diabetes | 20.7 | 13.2 | 13.3 | 13.2 | 23.4 |
| eGFR<60 ml/min per 1.73 m2 | 22.0 | 13.7 | 10.4 | 16.2 | 26.8 |
| Hypertension | 85.8 | 61.1 | 37.8 | 35.9 | 42.0 |
| Cancer diagnosis, ever | 9.6 | 11.6 | 9.8 | 9.6 | 12.3 |
| Medications, % | |||||
| Any diuretic | 74.5 | 39.1 | 12.8 | 9.2 | 10.0 |
| Thiazide diuretics | 63.8 | 33.1 | 9.4 | 6.5 | 7.1 |
| Loop diuretics | 2.5 | 3.2 | 2.3 | 2.2 | 2.6 |
| Potassium-sparing agents | 1.9 | 0.8 | 0.5 | 0.5 | 0.7 |
| Nonsteroidal anti-inflammatories | 17.7 | 15.6 | 14.6 | 16.9 | 17.1 |
| Potassium supplements | 20.5 | 6.0 | 2.2 | 1.0 | 0.7 |
| β Agonists (sympathomimetics, oral and inhaled) | 3.4 | 3.6 | 2.5 | 2.6 | 1.5 |
| β Blockers | 14.3 | 10.7 | 8.4 | 8.9 | 11.2 |
| ACE inhibitor and/or ARB use | 13.0 | 14.2 | 11.3 | 14.7 | 22.7 |
MESA, Multi-Ethnic Study of Atherosclerosis; CHS, Cardiovascular Health Study; BMI, body mass index; ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blockers.
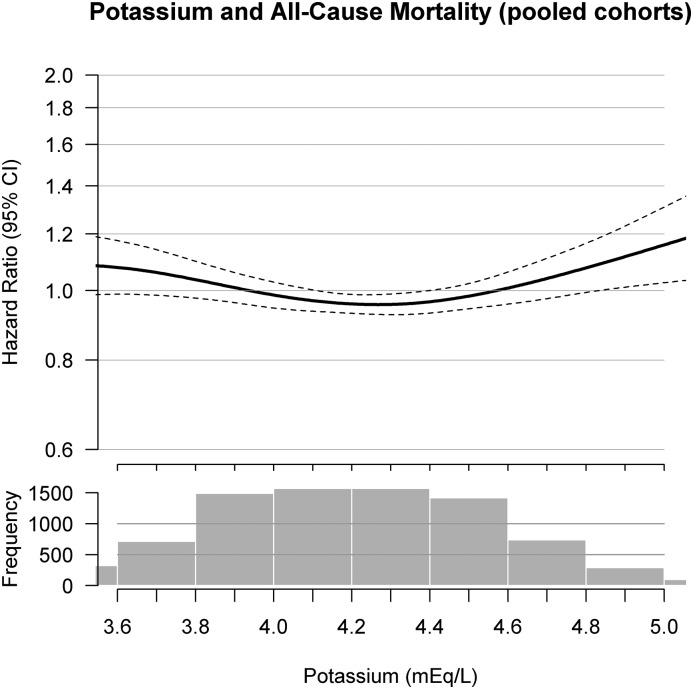
During a median follow-up of 10.5 (interquartile range, 9.7–12.3) years, there were 3529 deaths; 1087 because of CVD and 2442 because of non-CVD causes. Combining CVD deaths and nonfatal CVD events, there were 2376 CVD events during follow-up. The nature of the relationship of serum potassium concentrations with all-cause mortality was J-shaped (Figure 1). Compared with those with serum potassium of 4.0–4.4 mEq/L, those with serum potassium <3.5 mEq/L had a nonsignificant 13% (95% confidence interval [95% CI], 0.96 to 1.33) higher mortality risk, and those with serum potassium ≥5.0 mEq/L had 41% (95% CI, 1.12 to 1.76) higher mortality risk; an association that was statistically significantly independent of kidney function and other CVD risk factors (Table 2). The association between serum potassium, CVD death, and non-CVD death showed similar relationships. Compared with serum potassium of 4.0–4.4 mEq/L, those with serum potassium ≥5.0 mEq/L had a 50% (95% CI, 1.00 to 2.26) higher risk for CVD death and a 40% (95% CI, 1.07 to 1.83) higher risk for non-CVD death in fully adjusted analyses.
Figure 1.
Functional form of serum potassium as a continuous variable with all-cause mortality in a pooled cohort of MESA and CHS participants. The upper figure presents the hazard ratios and 95% confidence intervals (95% CIs) for associations between milliequivalent per liter of serum potassium and all-cause mortality, adjusted for age, sex, race, time-varying eGFR, diabetes mellitus, systolic BP, study cohort (MESA versus CHS), current smoking, pack-years smoking, ever having cancer, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, diuretics (potassium sparing and all others), nonsteroidal anti-inflammatory drugs, potassium supplements, β blockers, β agonists, and other antihypertensive medications. The lower histogram presents the distribution of serum potassium concentrations in milliequivalent per liter for 9651 combined individuals from the MESA and CHS. CHS, Cardiovascular Health Study; MESA, Multi-Ethnic Study of Atherosclerosis.
Table 2.
Associations between categories of serum potassium and all-cause mortality, CVD death, non-CVD death, and CVD events in MESA and CHS (pooled, n=9651)
| Outcomes | Potassium Concentration, mEq/L | ||||
|---|---|---|---|---|---|
| <3.5 | 3.5–3.9 | 4.0–4.4 | 4.5–4.9 | ≥5.0 | |
| All-cause mortality | |||||
| Events/P-Y | 199/4086 | 723/18,835 | 1777/61,187 | 739/25,691 | 91/ 2758 |
| Event rate (per 1000 P-Y) | 48.70 | 38.39 | 29.04 | 28.76 | 32.99 |
| Age, sex, race | 1.33 (1.15 to 1.54) | 1.28 (1.17 to 1.40) | 1 (ref) | 1.07 (0.98 to 1.16) | 1.37 (1.11 to 1.69) |
| Fully adjusteda | 1.13 (0.96 to 1.33) | 1.18 (1.08 to 1.30) | 1 (ref) | 1.06 (0.97 to 1.16) | 1.41 (1.12 to 1.76) |
| CVD deathb | |||||
| Events/P-Y | 65/5858 | 233/24,635 | 549/75,102 | 212/31,245 | 28/3308 |
| Event rate (per 1000 P-Y) | 11.10 | 9.46 | 7.31 | 6.79 | 8.46 |
| Age, sex, race | 0.96 (0.74 to 1.24) | 0.99 (0.85 to 1.16) | 1 (ref) | 1.26 (1.08 to 1.48) | 1.60 (1.09 to 2.34) |
| Fully adjusteda | 0.84 (0.63 to 1.12) | 0.98 (0.79 to 1.09) | 1 (ref) | 1.21 (1.02 to 1.42) | 1.50 (1.00 to 2.26) |
| Non-CVD deathb | |||||
| Events/P-Y | 134/5858 | 490/24,635 | 1228/75,102 | 527/31,245 | 63/3308 |
| Event rate (per 1000 P-Y) | 22.87 | 19.89 | 16.35 | 16.87 | 19.04 |
| Age, sex, race | 0.89 (0.74 to 1.06) | 0.91 (0.82 to 1.01) | 1 (ref) | 1.45 (1.31 to 1.61) | 1.56 (1.21 to 2.02) |
| Fully adjusteda | 0.99 (0.81 to 1.21) | 1.02 (0.85 to 1.06) | 1 (ref) | 1.22 (1.10 to 1.36) | 1.40 (1.07 to 1.83) |
| CVD events | |||||
| Events/P-Y | 137/3300 | 472/16,590 | 1224/53,951 | 484/23,084 | 59/2417 |
| Event rate (per 1000 P-Y) | 41.52 | 28.45 | 22.69 | 20.97 | 23.17 |
| Age, sex, race | 1.58 (1.33 to 1.89) | 1.24 (1.11 to 1.38) | 1 (ref) | 0.95 (0.85 to 1.05) | 1.14 (0.88 to 1.49) |
| Fully adjusteda | 1.13 (0.93 to 1.37) | 1.08 (0.96 to 1.20) | 1 (ref) | 1.02 (0.91 to 1.13) | 1.20 (0.90 to 1.56) |
Data are displayed as Hazard Ratio (95% confidence intervals) for age, sex, race, and fully adjusted models. CVD, cardiovascular disease; MESA, Multi-Ethnic Study of Atherosclerosis; CHS, Cardiovascular Health Study; P-Y, person-years; ref, reference.
Adjusted for age, sex, race, study cohort, time-varying eGFR, diabetes mellitus, systolic BP, current smoking, pack-years smoking, ever having cancer, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, potassium-sparing diuretics, other diuretics, nonsteroidal anti-inflammatory drugs, potassium supplements, other antihypertensive medications, β blockers, and β agonists.
Analyses and events per P-Y accounted for participants up to December of 2011.
The nature of the associations of serum potassium categories with CVD events also appeared J-shaped, but none of the serum potassium categories were significantly associated with CVD events in fully adjusted models (Table 2).
The CHS provided an opportunity to examine cause-specific death. Compared with the 4.0–4.4 mEq/L category, those with serum potassium between 4.5 and 4.9 mEq/L were at a significantly higher risk of cancer (hazard ratio [HR], 1.23; 95% CI, 1.00 to 1.52) and other deaths (HR, 1.46; 95% CI, 1.13 to 1.89) in fully adjusted models. Trends were similar for those with serum potassium ≥5.0 mEq/L but there were few events within strata of cause-specific death, and associations were not significant. None of the categories of serum potassium concentration were significantly associated with either SCD, pulmonary death, or neurologic deaths (Supplemental Table 1).
Across the different end points, we found consistent effect modification by diuretic use. Table 3 shows associations of serum potassium concentrations with all-cause mortality, CVD death, and non-CVD death stratified by diuretic use (Pinteractions=0.02, 0.01, and 0.002, respectively). Among participants who used diuretics, those with serum potassium ≥5.0 mEq/L were at a 2.15-fold (95% CI, 1.17 to 3.95) higher risk of all-cause mortality compared with a 37% (95% CI, 1.08 to 1.75) higher risk in participants who did not use diuretics. Similarly, individuals with serum potassium ≥5.0 mEq/L were at a 4.24-fold (95% CI, 1.66 to 10.86) higher risk of CVD death, whereas no association was observed in those not using diuretics. We also detected a statistically significant interaction by diuretic use for the association of higher serum potassium with non-CVD death, (Pinteraction=0.002) with stronger associations among those using diuretics, although associations were not significant in either stratum.
Table 3.
Stratified analysis of serum potassium categories with all-cause mortality, CVD death, and non-CVD death by diuretics
| Outcomes | Potassium Concentration, mEq/L | ||||
|---|---|---|---|---|---|
| <3.5 | 3.5–3.9 | 4.0–4.4 | 4.5–4.9 | ≥5.0 | |
| All-cause mortality | |||||
| No diuretics | |||||
| Events/P-Y | 41/986 | 389/11,271 | 1438/53,496 | 639/23,486 | 78/2543 |
| Event rate (per 1000 P-Y) | 41.58 | 34.51 | 26.88 | 27.21 | 30.67 |
| Age, sex, race | 1.44 (1.06 to 1.97) | 1.35 (1.21 to 1.51) | 1 (ref) | 1.09 (0.99 to 1.20) | 1.37 (1.09 to 1.72) |
| Fully adjusteda | 1.27 (0.92 to 1.77) | 1.31 (1.17 to 1.47) | 1 (ref) | 1.07 (0.97 to 1.18) | 1.37 (1.08 to 1.75) |
| Diuretics | |||||
| Events/P-Y | 157/3095 | 332/7489 | 334/7591 | 100/2206 | 13/215 |
| Event rate (per 1000 P-Y) | 50.73 | 44.33 | 44.00 | 45.33 | 60.47 |
| Age, sex, race | 0.99 (0.82 to 1.20) | 0.98 (0.84 to 1.14) | 1 (ref) | 1.03 (0.82 to 1.29) | 1.74 (0.99 to 3.05) |
| Fully adjusteda | 1.01 (0.83 to 1.23) | 0.98 (0.84 to 1.14) | 1 (ref) | 1.07 (0.85 to 1.35) | 2.15 (1.17 to 3.95) |
| P for interaction=0.02 | |||||
| CVD death | |||||
| No diuretics | |||||
| Events/P-Y | 10/1304 | 119/14,346 | 426/64,313 | 174/28,249 | 21/3040 |
| Event rate (per 1000 P-Y) | 7.67 | 8.29 | 6.62 | 6.16 | 6.91 |
| Age, sex, race | 1.17 (0.62 to 2.19) | 0.88 (0.72 to 1.08) | 1 (ref) | 1.23 (1.03 to 1.48) | 1.27 (0.82 to 1.98) |
| Fully adjusteda | 1.03 (0.54 to 1.95) | 0.90 (0.73 to 1.11) | 1 (ref) | 1.15 (0.96 to 1.38) | 0.98 (0.61 to 1.58) |
| Diuretics | |||||
| Events/P-Y | 54/4532 | 114/10,199 | 121/10,652 | 38/2996 | 7/268 |
| Event rate (per 1000 P-Y) | 11.92 | 11.18 | 11.36 | 12.68 | 26.12 |
| Age, sex, race | 0.80 (0.58 to 1.10) | 1.05 (0.81 to 1.34) | 1 (ref) | 1.71 (1.18 to 2.48) | 9.25 (4.21 to 20.34) |
| Fully adjusteda | 0.87 (0.62 to 1.22) | 1.02 (0.78 to 1.33) | 1 (ref) | 1.63 (1.11 to 2.38) | 4.24 (1.66 to 10.86) |
| P for interaction=0.01 | |||||
| Non-CVD death | |||||
| No diuretics | |||||
| Events/P-Y | 31/1304 | 270/14,346 | 1012/64,313 | 465/28,249 | 57/3040 |
| Event rate (per 1000 P-Y) | 23.77 | 18.82 | 15.74 | 16.46 | 18.75 |
| Age, sex, race | 1.44 (1.01 to 2.06) | 0.83 (0.72 to 0.95) | 1 (ref) | 1.43 (1.28 to 1.60) | 1.47 (1.12 to 1.94) |
| Fully adjusteda | 1.19 (0.81 to 1.75) | 0.92 (0.80 to 1.06) | 1 (ref) | 1.24 (1.11 to 1.39) | 1.06 (0.79 to 1.43) |
| Diuretics | |||||
| Events/P-Y | 103/4532 | 218/10,199 | 213/10,652 | 62/2996 | 6/268 |
| Event rate (per 1000 P-Y) | 22.73 | 21.37 | 19.20 | 20.69 | 22.39 |
| Age, sex, race | 0.83 (0.65 to 1.05) | 1.11 (0.91 to 1.34) | 1 (ref) | 1.60 (1.20 to 2.14) | 3.30 (1.45 to 7.53) |
| Fully adjusteda | 0.90 (0.70 to 1.15) | 1.01 (0.83 to 1.23) | 1 (ref) | 1.23 (0.92 to 1.66) | 1.28 (0.55 to 3.01) |
| P for interaction=0.002 | |||||
Data are displayed as Hazard Ratio (95% confidence intervals) for age, sex, race, and fully adjusted models. CVD, cardiovascular disease; P-Y, person years; ref, reference.
Diuretics: fully adjusted for age, sex, race, time-varying eGFR, diabetes mellitus, systolic BP, current smoking, pack-years smoking, ever having cancer, nonsteroidal anti-inflammatory drugs, any angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, potassium supplements, β blockers, β agonists, and other antihypertensive medications.
We observed several other interactions which were not consistent across end points. Associations were similar by race/ethnicity for all end points except non-CVD death (Pinteraction=0.001), where serum potassium ≥5.0 mEq/L was most strongly associated with this end point in non-Hispanic white individuals (HR, 1.82; 95% CI, 1.30 to 2.53) compared with other races/ethnicities. The association of serum potassium and non-CVD death was modified by eGFR<60 ml/min per 1.73 m2 (Pinteraction=0.02); those with serum potassium ≥5.0 mEq/L and lower eGFR had a 1.76-fold (95% CI, 1.14 to 2.71) higher risk of non-CVD death compared with the reference group, whereas associations were weaker and nonsignificant for non-CVD death among those with higher eGFR. There was no evidence of effect modification by eGFR category for all-cause mortality, CVD death, and CVD events (Pinteractions>0.20 for all). Associations of serum potassium with all end points were similar by sex, use of NSAIDs, and use of ACE inhibitors or ARBs.
Discussion
In 9651 individuals from two large cohorts of community-living individuals free from CVD at baseline, we found that high serum potassium concentrations were associated with a 41% higher risk of all-cause mortality, independent of kidney function or other CVD risk factors. Associations were similar for CVD and non-CVD death overall. These associations were consistently of greater magnitude in individuals using diuretics.
To our knowledge, only two prior studies have evaluated the association of serum potassium with mortality and/or CVD in community-living populations. These studies provided inconsistent results. One used data from the National Health and Nutrition Examination Survey (NHANES) I study (1971–1975), and showed that individuals with serum potassium of 4.5–5.4 mEq/L, compared with a reference range of 3.8–4.4 mEq/L, were at higher risk of all-cause mortality (20). We confirmed this finding in our study. Interestingly, the NHANES I study also found that associations of higher potassium concentrations with CVD mortality were limited to those using diuretics (20), which we also confirmed here. In the Framingham Offspring Study, Walsh and colleagues (21) found no associations between serum potassium concentration and incident CVD events, CVD death, or all-cause mortality among 3151 participants followed for 16 years. Thus, our study advances this area of investigation in several important ways. Our study sample was much larger than either previous study, which allowed us to test interactions and evaluate associations within strata to determine whether associations were similar in users and nonusers of common medications that influence serum potassium, and in persons with lower eGFR. The older age and long-term follow-up in the CHS also allowed us to evaluate different causes of death, including adjudicated SCD events, for the first time. Our findings generally confirm and extend those from the NHANES I study. The much larger number of events may have led to the discrepant findings in the Framingham study, relative to our study.
We observed consistent associations between higher serum potassium concentrations and all-cause mortality, CVD death, and non-CVD death. Although estimates trended in similar directions, associations with CVD events were not significant despite the substantial statistical power available in our study. Clinical observations in patients with severe hyperkalemia in hospitalized patients demonstrate a predisposition to cardiac arrhythmias attributed to hyperkalemia. Therefore, we hypothesized that high serum potassium concentrations would be associated with SCD. A unique strength of the CHS is that the sudden death outcome has been adjudicated and the long-term follow-up provided reasonable numbers of sudden death events (n=74). Surprisingly, no category of serum potassium concentration was significantly associated with SCD. Whether this reflects limited power or if alternative mechanisms are responsible for associations with the other end points requires future study.
We found that higher serum potassium was significantly associated with cancer death and other death in the CHS. The “other” death category in the CHS consisted of deaths from trauma, bladder, infection, sepsis, liver disease, gastrointestinal disease, renal failure, metabolic conditions, amyloid, fractures, failure to thrive, myelodysplastic syndrome, and other musculoskeletal disease. The number of deaths for each subcategory was small, precluding precise evaluation of associations with any specific subtype of death.
The finding for stronger associations of serum potassium concentrations ≥5.0 mEq/L with all-cause, CVD, and non-CVD death among users of diuretics was consistent across the three end points, and this is the second observational study to demonstrate stronger associations of higher potassium with CVD death among diuretic users. These findings may have clinical implications. Although our data are observational, it may be reasonable to address other factors that influence serum potassium if found to be elevated in diuretic users (22).
This study has important limitations. Serum potassium was measured at one point in time. Intraindividual variation over time is to be expected, which may have biased results toward the null hypothesis. We did not have concurrent measurements of serum magnesium concentrations, which are known to influence serum potassium concentrations (23), and have previously been linked with mortality risk (24,25). We also were not able to adjust for covariates that were not measured in both cohorts, such as urine albumin-to-creatinine ratio, bicarbonate, and serum albumin and hemoglobin; these factors should be evaluated as potential confounders in future studies as there may be some residual confounding.
In summary, in community-living individuals, serum potassium concentrations ≥5.0 mEq/L were associated with all-cause mortality, CVD death, and non-CVD death independent of kidney function and CVD risk factors. These associations were consistently stronger in persons using diuretics. We did not observe an independent association of higher serum potassium with risk of SCD. Future clinical trials are required to determine if intervening to lower potassium may be warranted in community-living individuals, and to determine mechanisms by which diuretic use influences relationships linking higher potassium with mortality.
Disclosures
J.M.H.-A. received salary support during this project from ZS Pharma, Inc (San Mateo, CA). B.M.P. serves on the Data Safety and Monitoring Board for a clinical trial funded by the manufacturer (Zoll LifeCor, Chelmsford, MA) and on the Steering Committee of the Yale Open Data Access Project, funded by Johnson & Johnson. The other authors report no conflicts.
Supplementary Material
Acknowledgments
The authors would like to thank the participants in the Multi-Ethnic Study of Atherosclerosis and the Cardiovascular Health Study, as well as the clinic staff who received and examined these participants, and the respective coordinating centers for each study. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org.
This research was supported by grants R01HL096875, R01HL102214, and R01HL080295, contracts N01HC95159–N01HC95169, and contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant U01HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. J.M.H.-A. was supported by a grant from ZS Pharma, Inc. J.H.I. was supported by an American Heart Association Established Investigator Award (14EIA18560026), and by the National Institutes of Diabetes, Digestive, and Kidney Diseases (R01DK098234). Additional support was provided by grant R01AG023629 from the National Institute on Aging.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Serum Potassium and Cardiovascular Outcomes: The Highs and the Lows,” on pages 220–221.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06290616/-/DCSupplemental.
References
- 1.Ettinger PO, Regan TJ, Oldewurtel HA: Hyperkalemia, cardiac conduction, and the electrocardiogram: A review. Am Heart J 88: 360–371, 1974 [DOI] [PubMed] [Google Scholar]
- 2.Mattu A, Brady WJ, Robinson DA: Electrocardiographic manifestations of hyperkalemia. Am J Emerg Med 18: 721–729, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Ohmae M, Rabkin SW: Hyperkalemia-induced bundle branch block and complete heart block. Clin Cardiol 4: 43–46, 1981 [DOI] [PubMed] [Google Scholar]
- 4.Spodick DH: Effects of severe hyperkalemia. Am Heart Hosp J 6: 68, 2008 [DOI] [PubMed] [Google Scholar]
- 5.An JN, Lee JP, Jeon HJ, Kim DH, Oh YK, Kim YS, Lim CS: Severe hyperkalemia requiring hospitalization: Predictors of mortality. Crit Care 16: R225, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phillips BM, Milner S, Zouwail S, Roberts G, Cowan M, Riley SG, Phillips AO: Severe hyperkalaemia: Demographics and outcome. Clin Kidney J 7: 127–133, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP: Multi-Ethnic Study of Atherosclerosis: Objectives and design. Am J Epidemiol 156: 871–881, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O’Leary D, Psaty BM, Rautaharju P, Tracy RP, Weiler PG: The Cardiovascular Health Study: Design and rationale. Ann Epidemiol 1: 263–276, 1991 [DOI] [PubMed] [Google Scholar]
- 9.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP: Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem 41: 264–270, 1995 [PubMed] [Google Scholar]
- 10.Meigs JB, Muller DC, Nathan DM, Blake DR, Andres R; Baltimore Longitudinal Study of Aging : The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes 52: 1475–1484, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S: Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol 5: 278–285, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR: The relationship of left ventricular mass and geometry to incident cardiovascular events: The MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol 52: 2148–2155, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agarwal I, Glazer NL, Barasch E, Biggs ML, Djoussé L, Fitzpatrick AL, Gottdiener JS, Ix JH, Kizer JR, Rimm EB, Siscovick DS, Tracy RP, Zieman SJ, Mukamal KJ: Fibrosis-related biomarkers and risk of total and cause-specific mortality: The Cardiovascular Health Study. Am J Epidemiol 179: 1331–1339, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deo R, Katz R, de Boer IH, Sotoodehnia N, Kestenbaum B, Mukamal KJ, Chonchol M, Sarnak MJ, Siscovick D, Shlipak MG, Ix JH: Fibroblast growth factor 23 and sudden versus non-sudden cardiac death: The Cardiovascular Health Study. Am J Kidney Dis 66: 40–46, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Psaty BM, Lee M, Savage PJ, Rutan GH, German PS, Lyles M; The Cardiovascular Health Study Collaborative Research Group : Assessing the use of medications in the elderly: Methods and initial experience in the Cardiovascular Health Study. J Clin Epidemiol 45: 683–692, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lunn M, McNeil D: Applying Cox regression to competing risks. Biometrics 51: 524–532, 1995 [PubMed] [Google Scholar]
- 18.Eilers PH, Marx BD: Flexible smoothing with B-splines and penalties. Stat Sci 11: 89–102, 1996 [Google Scholar]
- 19.Rothman KJ: No adjustments are needed for multiple comparisons. Epidemiology 1: 43–46, 1990 [PubMed] [Google Scholar]
- 20.Fang J, Madhavan S, Cohen H, Alderman MH: Serum potassium and cardiovascular mortality. J Gen Intern Med 15: 885–890, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walsh CR, Larson MG, Leip EP, Vasan RS, Levy D: Serum potassium and risk of cardiovascular disease: The Framingham Heart Study. Arch Intern Med 162: 1007–1012, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Sica DA, Struthers AD, Cushman WC, Wood M, Banas JS Jr, Epstein M: Importance of potassium in cardiovascular disease. J Clin Hypertens (Greenwich) 4: 198–206, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alfonzo AV, Isles C, Geddes C, Deighan C: Potassium disorders--clinical spectrum and emergency management. Resuscitation 70: 10–25, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Liao F, Folsom AR, Brancati FL: Is low magnesium concentration a risk factor for coronary heart disease? The Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 136: 480–490, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Qu X, Jin F, Hao Y, Li H, Tang T, Wang H, Yan W, Dai K: Magnesium and the risk of cardiovascular events: A meta-analysis of prospective cohort studies. PLoS One 8: e57720, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.