Abstract
Background and objectives
AKI is an increasingly common and devastating complication in hospitalized patients. Severe AKI requiring RRT is associated with in–hospital mortality rates exceeding 40%. Clinical decision making related to RRT initiation for patients with AKI in the medical intensive care unit is not standardized.
Design, setting, participants, & measurements
We conducted a 13-month (November of 2013 to December of 2014) prospective cohort study in an academic medical intensive care unit involving the implementation of an AKI Standardized Clinical Assessment and Management Plan, a decision-making algorithm to assist front-line clinicians caring for patients with AKI. The Standardized Clinical Assessment and Management Plan algorithms provided recommendations about optimal indications for initiating and discontinuing RRT on the basis of various clinical parameters; 176 patients managed by nine nephrologists were included in the study. We captured reasons for deviation from the recommended algorithm as well as mortality data.
Results
Patients whose clinicians adhered to the Standardized Clinical Assessment and Management Plan recommendation to start RRT had lower in-hospital mortality (42% versus 63%; P<0.01) and 60-day mortality (46% and 68%; P<0.01), findings that were confirmed after multivariable adjustment for age, albumin, and disease severity. There was a differential effect of Standardized Clinical Assessment and Management Plan adherence in low (<50% mortality risk) versus high (≥50% mortality risk) disease severity on in-hospital mortality (interaction term P=0.02). In patients with low disease severity, Standardized Clinical Assessment and Management Plan adherence was associated with lower in–hospital mortality (odds ratio, 0.21; 95% confidence interval, 0.08 to 0.54; P=0.001), but no significant association was evident in patients with high disease severity.
Conclusions
Physician adherence to an algorithm providing recommendations on RRT initiation was associated with lower in–hospital mortality.
Keywords: acute renal failure, clinical nephrology, hemodialysis, Acute Kidney Injury, Albumins, Algorithms, Clinical Decision-Making, Hospital Mortality, Humans, Intensive Care Units, Prospective Studies, Renal Replacement Therapy, Risk
Introduction
Each year, >0.5 million United States hospitalizations are complicated by AKI at a cost of approximately $10 billion (1,2). Severe AKI requiring RRT is one of the most ominous of clinical complications, associated with in-hospital mortality rates exceeding 40% (3). Several absolute indications for the initiation of RRT in AKI are widely accepted: refractory hyperkalemia or metabolic acidosis, volume overload with pulmonary edema, and uremic pericarditis. Delays in initiating RRT can result in serious preventable complications and even death (4). However, early initiation carries the risk of starting RRT (an invasive procedure with non-negligible complications, including infection and hypotension) in a patient who may recover renal function without needing RRT.
Studies that have examined timing of RRT initiation in AKI have used varied biochemical and clinical parameters to define early versus late initiation and shown mixed results (4–9). Four meta-analyses concluded that earlier institution of RRT may be associated with a survival benefit (10–13). However, the studies were heterogeneous in design. Two recent randomized, controlled trials (RCTs) (14,15) showed disparate results when randomizing patients to early versus late initiation; Zarbock et al. (15) showed a benefit to early initiation, whereas Gaudry et al. (14) showed no benefit. Notably, the studies used different criteria for RRT initiation. The Kidney Disease Improve Global Outcomes (KDIGO) AKI practice guidelines provide the following recommendation: “Initiate RRT emergently, when life-threatening changes in fluid, electrolyte, and acid-base balance exist. Consider the broader clinical context, the presence of conditions that can be modified with RRT and the trends of laboratory tests—rather than single BUN and creatinine thresholds alone—when making the decision to start RRT” (16).
Data regarding RRT discontinuation are even more limited. A post hoc analysis of a prospective, multicenter, observational study of 529 patients who survived RRT showed that urine output at the time of cessation of RRT was the most important predictor of successful discontinuation (17). The KDIGO AKI practice guidelines provide the following recommendation: “Discontinue RRT when it is no longer required, either because intrinsic kidney function has recovered to the point that it is adequate to meet patient needs, or because RRT is no longer consistent with the goals of care” (16).
To address uncertainties in the care of patients with AKI requiring RRT, we implemented a structured decision–making algorithm for clinicians managing patients with AKI in the medical intensive care unit (MICU). The algorithm prompted clinicians to document criteria relevant for decisions on RRT initiation and discontinuation. The algorithm provided management recommendations, which the clinicians could follow or ignore. We measured whether clinicians followed or deviated from the recommendations provided, the reasons for deviation, and whether outcomes differed among patients whose clinicians adhered versus did not adhere to the algorithm.
Materials and Methods
Description of the Standardized Clinical Assessment and Management Plan
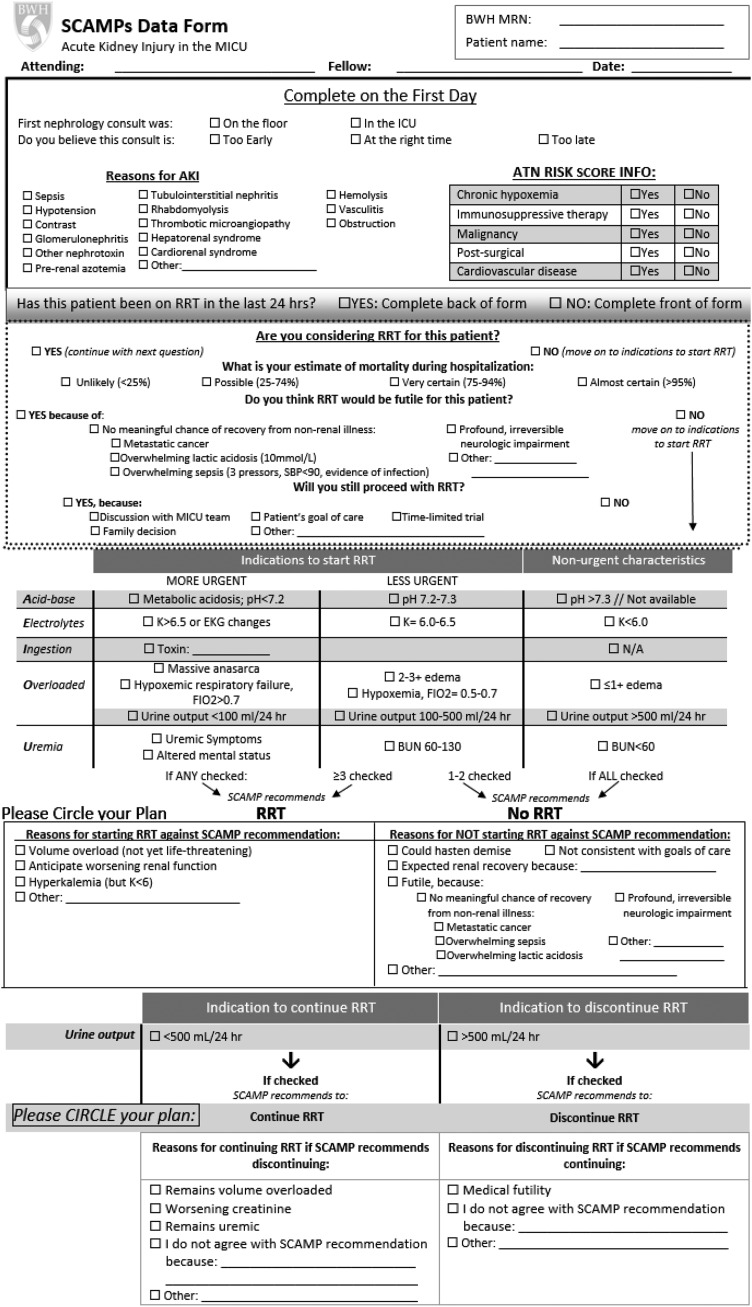
Standardized Clinical Assessment and Management Plans (SCAMPs) are a quality improvement approach that aims to provide insight into undefined areas of care and optimize outcomes through implementation of a standardized care pathway. Oversight of SCAMPs’ production is provided by the Institute for Relevant Clinical Data Analytics, a nonprofit, tax-exempt organization that provides the education and resources for the development, implementation, and analysis of SCAMPs at its member institutions. Through an unstructured, iterative process involving four intensive care unit (ICU) nephrology attending physicians, we identified several areas of uncertainty in the care of patients with severe AKI, including criteria for RRT initiation and discontinuation. Specific criteria were agreed on by nephrologists on the basis of clinical experience and review of existing literature. Structured forms were developed for clinicians to document decision making. The SCAMP included a phase 1 enrollment period from October 31, 2012 to November 7, 2013, during which preliminary data were collected on 142 patients; these data are not included in this study, because they were incomplete. Operational issues related to enrollment and data collection were identified that informed the design of phase 2. In phase 2, the SCAMP focused specifically on RRT initiation/discontinuation. Figure 1 illustrates the SCAMP form used by clinicians and the criteria included.
Figure 1.
Standardized Clinical Assessment and Management Plan (SCAMP) form completed by nephrologists regarding RRT initiation or discontinuation. ATN, acute tubular necrosis; BWH MRN, Brigham and Women's Medical Record Number; EKG, electrocardiogram; FiO2, fraction of inspired oxygen; ICU, intensive care unit; MICU, medical intensive care unit; N/A, not applicable.
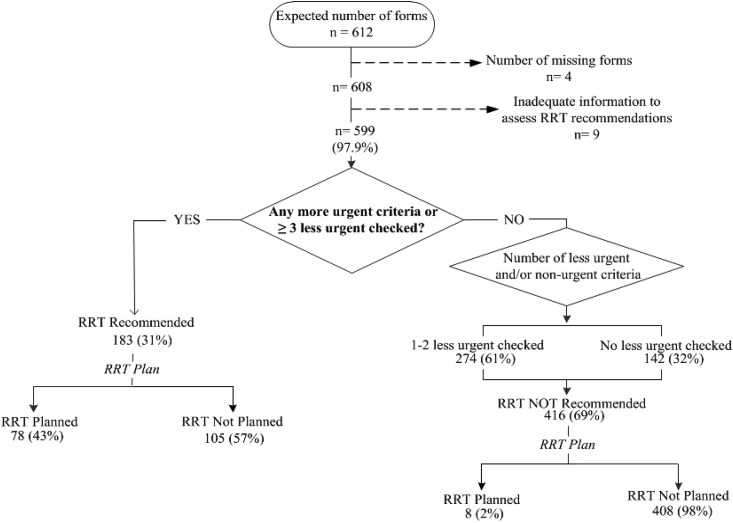
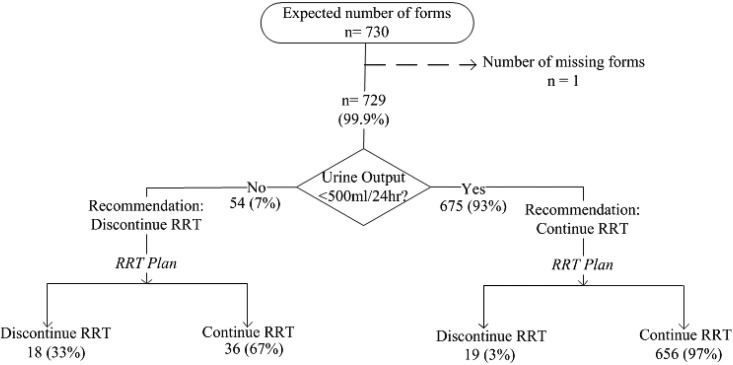
Figure 2 shows the SCAMP workflow for RRT initiation. For patients not on RRT, each day, clinicians entered clinical data relevant to AKI initiation (4–9) (pH, potassium, suspicion of toxic ingestion, volume overload, fraction of inspired oxygen, urine output, BUN, and uremic symptoms). On the basis of the presence or absence of prespecified criteria, the SCAMP recommended whether to initiate RRT. Clinicians were free to follow or ignore the recommendation but asked to provide reasons for deviation if recommendations were ignored. Figure 3 shows the SCAMP workflow for RRT discontinuation; the SCAMP recommended RRT discontinuation if urine output exceeded 500 ml/24 h. This criterion was adapted from the work by Uchino et al. (17). If clinicians ignored the recommendation for RRT discontinuation, they were prompted to record the reason. Renal physicians (attending physicians or fellows) completed one data form per patient each day of enrollment. Adherence to the SCAMP recommendations at the patient level was defined as adhering to all SCAMP recommendations regarding RRT initiation or discontinuation (separately) on every day that the patient was enrolled in the SCAMP. Nonadherence was defined as at least one instance of not adhering to the SCAMP recommendation: either discontinuation or initiation of RRT (separately) on any day that the patient was enrolled in the SCAMP. Patients were considered to have completed the SCAMP on death, discharge, transfer from MICU, or renal team signing off.
Figure 2.
Standardized Clinical Assessment and Management Plan (SCAMP) indications to start or not start RRT. N (percentage) refers to the number of SCAMP forms (each patient received one SCAMP form per day that he/she was in the medical intensive care unit and met criteria for AKI).
Figure 3.
Standardized Clinical Assessment and Management Plan (SCAMP) indications to continue or discontinue RRT. N (percentage) refers to the number of SCAMP forms (each patient received one SCAMP form per day that he/she was in the medical intensive care unit and met criteria for AKI).
Study Design and Patient Population
The SCAMP was implemented as a quality improvement initiative at the 20-bed MICU of Brigham and Women’s Hospital (BWH), a tertiary care academic medical center in Boston, Massachusetts. The Institutional Review Board approved data collection and analysis of the SCAMP study as a quality improvement project and waived the need for informed consent. Phase 2 of the SCAMP described in this report includes patients enrolled between November 8, 2013 and December 5, 2014. Eligible patients were identified by a study coordinator who identified patients in the MICU documented to have AKI by the nephrology consult service. Patients with previous MICU admissions during hospitalization were excluded. Patients were enrolled on the date that the initial nephrology service consultation was completed. Additional details regarding study design are provided in Supplemental Material.
Data Sources and Collection
The BWH electronic medical record was used to abstract data from nephrology consult notes as well as demographics, comorbidities, and laboratory results. Data related to vital signs, urine output, fluid balance, and RRT were obtained from the patients’ daily flow sheets. Data about clinician adherence or deviation, reasons for deviation, and causes of AKI were obtained from the SCAMP forms. Compliance with completion of the SCAMP forms was monitored by a data coordinator, who followed clinicians daily to ensure form completion; 99% of SCAMPs forms were completed in their entirety in phase 2.
Statistical Analyses
Patient demographic and clinical characteristics are reported as counts and percentages or medians and interquartile ranges (IQRs) as appropriate. Chi-squared and Fisher exact tests were used to evaluate differences in hospital mortality for those whose physicians adhered to start RRT recommendations compared with those who did not. Univariable logistic regression was used to identify patient characteristics associated with in-hospital mortality. Multivariable models were used to estimate the association between adherence to the recommendation to start RRT and death adjusting for age, albumin, and AKI–specific disease severity (18) at enrollment. Disease severity was estimated using the mortality risk equation by Demirjian et al. (18) derived in an RCT of patients with AKI RRT. The risk score by Demirjian et al. (18) has been shown to have improved performance for mortality prediction in patients with AKI compared with Sequential Organ Failure Assessment and Acute Physiology and Chronic Health Evaluation II scores. Laboratory data for the disease severity score were missing on enrollment in 36 of 177 patients (most commonly arterial Po2). In 15 of 36 with missing laboratory data, values were available from the preceding 48 hours before enrollment and used. In 21 of 36 with missing data for laboratory values, we imputed normal values given the likelihood that missing data on variables, such as pH, indicated low clinical suspicion for abnormal values. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
Clinical Characteristics
Table 1 shows characteristics of the 176 patients included in the study; one patient was admitted more than once to the hospital, resulting in 177 enrollments. The median age was 61 (IQR, 51–70) years old, and 58% were men. Most patients were white and non-Hispanic (84%). Hypotension (58%), sepsis (51%), and prerenal azotemia (30%) were the most common recorded etiologies of AKI. The median hospital and MICU lengths of stay were 16.3 (IQR, 7–29.1) days and 5.9 (IQR, 2.5–13.7) days, respectively.
Table 1.
Patient demographics and clinical characteristics
| Patient Characteristics, n=177 enrollments | N (%) |
|---|---|
| Men | 103 (58) |
| Age, yr, median (IQR) | 61 (51–70) |
| Race | |
| White | 146 (84) |
| Black | 16 (9) |
| Hispanic | 9 (5) |
| Other | 6 (3) |
| Reasons for AKIa | |
| Hypotension | 102 (58) |
| Sepsis | 89 (51) |
| Prerenal azotemia | 52 (30) |
| Other nephrotoxin | 16 (9) |
| Obstruction | 7 (4) |
| Contrast | 16 (9) |
| Hepatorenal syndrome | 7 (4) |
| Thrombotic microangiopathy | 6 (3) |
| Cardiorenal syndrome | 7 (4) |
| Rhabdomyolysis | 6 (3) |
| GN | 4 (2) |
| Hemolysis | 5 (3) |
| Vasculitis | 4 (2) |
| Tubulointerstitial nephritis | 4 (2) |
| Other | 26 (15) |
| Chronic health condition | |
| Chronic hypoxemia | 26 (15) |
| Malignancy | 69 (39) |
| Immunosuppresive therapy | 57 (32) |
| Cardiovascular disease | 47 (27) |
| Postsurgery | 15 (8) |
| Vitals at enrollment | |
| Mean arterial pressure, mean+SD | 76.8+13.8 |
| Serum albumin, mean+SD | 2.5+0.6 |
| FiO2>0.6 | 23 (13) |
| Mechanical ventilation | 91 (51) |
| Type (s) of RRT during enrollment | |
| None | 91 (51) |
| CVVH | 44 (25) |
| HD | 16 (9) |
| CVVH and HD | 26 (15) |
| Length of stay, median (IQR) | |
| MICU, d | 5.9 (2.5–13.7) |
| Hospital, d | 16.3 (7–29.1) |
| Probability of 60-d mortality,b median (IQR) | 0.48 (0.04–0.96) |
Values represent N (%) unless otherwise stated. IQR, interquartile range; FiO2, fraction of inspired oxygen; CVVH, continuous venovenous hemofiltration; HD, hemodialysis; MICU, medical intensive care unit.
Clinical diagnosis of AKI specified by clinicians caring for the patient.
Risk equation by Demirjian et al. (18).
Indications to Start RRT and Reasons for Deviation
Figure 2 depicts clinical decision making regarding initiation of RRT. RRT initiation was recommended in 31% of SCAMP forms in 176 enrolled patients. In 57% of forms where RRT was recommended by the SCAMP, clinicians deviated from the suggestions and did not initiate RRT. Table 2 shows the reasons for these deviations. The most common reason for not initiating RRT when recommended was expected renal recovery (48%). Of these patients, 33% ultimately required RRT, and 50% died. In 69% of the SCAMP forms, RRT was not recommended; virtually all of these recommendations (98%) were followed by the clinicians. The most common reasons for initiating RRT when not recommended were anticipation of worsening renal function and volume overload.
Table 2.
Reasons for Standardized Clinical Assessment and Management Plan deviation related to starting RRT
| Reasons for Deviation | N (%) |
|---|---|
| Reasons for not starting RRT when SCAMP recommended, n=105a | |
| Expected renal recovery | 50 (48) |
| Futile | 21 (20) |
| Could hasten demise | 8 (8) |
| Not consistent with goals of care | 7 (7) |
| Not recorded | 5 (5) |
| Comfort measures only | 4 (4) |
| Unclear goals of care | 3 (3) |
| Otherb | 25 (24) |
| Reasons for starting RRT when not SCAMP recommended, n=8 | |
| Anticipate worsening renal function | 6 (75) |
| Volume overload | 3 (38) |
| Hyperkalemia | 1 (13) |
| Anticipation of tumor lysis syndrome and volume overload | 1 (13) |
| Metabolic alkalosis | 1 (13) |
Values represent N (%) unless otherwise stated. SCAMP, Standardized Clinical Assessment and Management Plan.
Multiple selections were possible; therefore, the total number of deviations was greater than n=105.
Examples include end stage liver disease, with intravenous sodium bicarbonate, bacteremia, line holiday, medical intensive care unit team did not perceive benefit, responding to small fluid boluses, and large intracranial bleed.
Indications to Discontinue RRT and Reasons for Deviation
Figure 3 depicts a flow diagram illustrating clinical decision making regarding discontinuation of RRT. In 7% of the SCAMP forms, RRT discontinuation was recommended. In 67% of forms where RRT discontinuation was recommended, clinicians deviated from the suggestion and continued RRT. The most common reasons for deviation were volume overload (69%) and worsening renal function or uremia (42%). In 93% of the SCAMP forms, RRT was recommended to continue, and virtually all of these recommendations (97%) were followed. The most common reasons for discontinuing RRT against recommendations to continue were medical futility (12 of 19) and catheter malfunction (two of 12).
Adherence to and Deviation from the SCAMP Algorithm and Outcomes
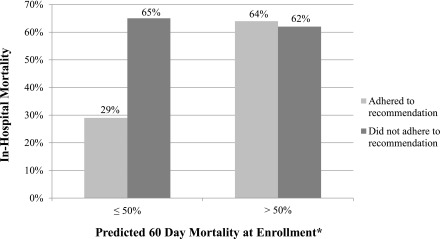
The association between adherence to the SCAMP recommendation to start RRT and in-hospital mortality is presented in Table 3; 162 patients had SCAMP information detailed about recommending RRT (of 176 total patients; 14 did not). We observed a significantly lower risk of in-hospital mortality in patients whose clinicians adhered to all SCAMP recommendations to start RRT compared with those whose clinicians did not adhere to the SCAMP on at least one occasion (42% versus 63%, respectively; P<0.01); the overall mortality of the entire cohort was 50%. There was a higher prevalence of cardiovascular disease, mechanical ventilation, and predicted 60-day mortality in the nonadherence cohort at baseline (Supplemental Tables 1–3). We performed prespecified subgroup analyses using multivariable analyses (adjusted for age, albumin, and disease severity at enrollment) in those with higher (≥50% predicted mortality) versus lower disease severity (≤50% predicted mortality) to account for observed baseline differences. In multivariable analyses in the lower–disease severity group, adherence to the SCAMP recommendation was associated with a 79% lower odds of death (95% confidence interval [95% CI], 46% to 92%; P=0.001). The results were nearly identical after excluding 32 patients in whom futility or goals of care were documented as reasons for deviation (odds ratio, 0.21; 95% CI, 0.11 to 0.85; P=0.02). In the higher–disease severity group, we found no difference in odds of death according to SCAMP adherence (odds ratio, 1.06; 95% CI, 0.38 to 2.95; P=0.91) (Table 4). In-hospital mortality according to disease severity and SCAMP adherence are shown in Figure 4. Results were similar with adjustment for propensity scores for adherence versus nonadherence (data not shown). The mean serum creatinine concentration at enrollment was similar among patients whose physicians adhered versus did not adhere to the SCAMP recommendation on RRT initiation (3.6±2.7 and 3.1±1.5 mg/dl, respectively; P=0.14). Of note, the overall in–hospital mortality rate of a separate historical control cohort from October of 2010 to July of 2012 captured using SCAMP entry criteria and matched by acute tubular necrosis risk score severity was not statistically significantly different (48% in n=183 historical controls versus 50% in this study; P=0.56, [data not shown]).
Table 3.
Relationship between adherence to standardized clinical assessment and management plan recommendation to start RRT and in-hospital mortality
| Followed Recommendation to Start RRT?a | N | Hospital Mortality, N (%) | P Value |
|---|---|---|---|
| Adhered | 102 | 43 (42) | <0.01b |
| Did not start RRT when not recommended | 55 | 15 (27) | <0.01b |
| Started RRT when recommended | 47 | 28 (60) | <0.01b |
| Did not adhere | 60 | 38 (63) | <0.01b |
| Did not start RRT when recommended | 53 | 35 (66) | <0.01b |
| Started RRT when not recommended | 7 | 2 (29) | <0.01b |
Refers to whether the nephrologist ever deviated from the recommendation to start RRT, and n=14 enrollments did not include standardized clinical assessment and management plan information related to RRT recommendation.
P value refers to adhered (42%) versus did not adhere (63%).
Table 4.
Univariate- and multivariable-adjusted logistic regression models for in-hospital mortality
| Covariate | Unadjusted OR (95% CI) | P Value | Multivariable-Adjusted OR (95% CI) | P Value |
|---|---|---|---|---|
| Adherence versus nonadherence | ||||
| Higher disease severitya | 1.09 (0.40 to 2.95) | 0.86 | 1.06 (0.38 to 2.95)b | 0.91 |
| Lower disease severitya | 0.22 (0.09 to 0.55) | 0.001 | 0.21 (0.08 to 0.54)b | 0.001 |
| Age at enrollment | 1.02 (1.00 to 1.04) | 0.12 | 1.02 (1.00 to 1.04) | 0.10 |
| Albumin at enrollment | 0.49 (0.29 to 0.82) | <0.01 | 0.49 (0.27 to 0.89) | 0.02 |
Adherence versus nonadherence refers to whether physicians implemented the recommendation regarding initiation of RRT; prespecified subgroups were higher– versus lower–risk disease severity. OR, odds ratio; 95% CI, 95% confidence interval.
Higher disease severity indicates >50% predicted risk of mortality. Lower disease severity indicates ≤50% predicted risk of mortality.
Model incorporates an interaction term between risk group and adherence; interaction term of disease severity and adherence, P=0.02.
Figure 4.
Lower mortality with adherence vs. non-adherence to the SCAMP recommendation was observed only in those with lower disease severity. *Risk equation by Demirjian et al. (18).
There were no differences in outcomes among patients whose nephrologists adhered or did not adhere to recommendations for discontinuing RRT. Our analyses were limited by sample size, because there were few patients whose nephrologists deviated from RRT discontinuation recommendations.
Discussion
The primary finding of this study on the implementation of an AKI RRT decision–making algorithm (SCAMP) was that mortality was lower among patients whose clinicians adhered to SCAMP recommendations on RRT initiation compared with those whose clinicians did not adhere to the recommendations; this difference was observed in prespecified analyses of those with lower severity of illness. Our study is unique in that it examines the issue of RRT timing on the basis of clinical decision making and sheds light on actual clinical practice.
Few small observational studies have examined the timing of RRT for AKI on the basis of varying clinical parameters. Some have found that earlier initiation results in improved outcomes. The Finnish Acute Kidney Injury Study found that patients meeting at least one indication of five ([1] potassium >6 meq/L, [2] pH<7.15, [3] urea >100.8 mg/dl, [4] urine output <0.3 ml/kg per hour for 24 hours, and [5] pulmonary edema) had a higher adjusted 90-day mortality than those not meeting criteria (9). A large multicenter, observational study including 1238 patients in the ICU developing AKI RRT in 23 countries stratified patients into early (median urea <24.2 mmol/L [67.8 mg/dl], creatinine <309 μmol/L [3.48 mg/dl], and <2 days from ICU admission) and late (>24.2 mmol/L [67.8 mg/dl], creatinine >309 μmol/L [3.48 mg/dl], and >5 days from ICU admission) RRT initiation. Late RRT initiation on the basis of creatinine was associated with lower adjusted mortality, whereas late RRT initiation on the basis of days from ICU admission was associated with higher adjusted mortality (4). Conversely, Crescenzi et al. (6) conducted a prospective single–center trial of early (urine output <0.5 ml/kg per hour for 6 hours) versus late initiation (urine output <0.5/mg per kilogram for 12 hours) of RRT in patients with AKI after cardiac surgery. There was no significant difference in survival between the two groups. A nested observational cohort study of 469 patients from the Randomized Evaluation of Normal Versus Augmented Level Replacement Therapy Study found that earlier commencement of continuous RRT relative to RIFLE AKI (<7.1 versus >46.0 hours) was not associated with improved survival (7). These studies highlight the fact that additional study is needed to delineate clear indications for RRT initiation and understand drivers of clinician decision making. Recently, a multicenter, randomized trial of early versus delayed RRT initiation failed to show any difference in 60-day mortality between the two RRT initiation strategies in both groups (14). Another recently published trial, however, found that early initiation of RRT led to lower 90-day mortality (15). There were key differences in the designs of the two studies in terms of criteria for initiating RRT. The discordance in the findings of these two well-designed RCTs highlights the need for a standardized approach to clinical decision support regarding dialysis initiation.
In our study, we implemented a decision-making algorithm on the basis of aggregated findings in the established literature (4–9). On the basis of the limitations of previous studies and the absence of clear guidelines about RRT initiation and discontinuation, we recognized the importance of allowing nephrologists to deviate from the SCAMP recommendations and sought to capture these reasons for deviation. Deviations from the algorithm shed light on the practical decisions that face nephrologists caring for critically ill patients with AKI. Notably, in the majority of patients (57%), the clinicians did not follow the recommendation to start RRT when recommended. The most common reason for not starting RRT when clinical parameters indicate need for initiation was a clinician’s sense of anticipated renal recovery, which suggests that clinical judgement should be balanced with recommended guidelines. Conversely, the most common reason for continuing RRT when the SCAMP algorithm suggested discontinuation was volume overload, implying that our discontinuation criterion was ineffective and could be more comprehensive by incorporating other factors, including volume status and creatinine clearance.
In addition to shedding light on clinical decision making, SCAMPs may lead to improved clinical care by providing clinicians with specific recommendations for patient-level care at the locus of patient contact and reducing variability in clinical practice. Real-time prompts through the SCAMP for implementation of evidence-based practice may guide clinicians to make the best evidence–based decisions. Our finding of improved survival among patients whose clinicians adhered to the SCAMP recommendation for RRT initiation is a promising piece of evidence in support of the ability of SCAMPs to improve patient care.
There are a number of limitations that should temper enthusiasm about the finding of improved outcomes. First, the most important limitation is residual confounding by disease severity (measured or unmeasured) or factors related to futility of care: it is possible that lower survival among those whose nephrologists declined to initiate RRT when recommended reflects higher severity of illness and/or perceived futility of care rather than a beneficial effect of initiating RRT. A cluster–stepped wedge RCT of SCAMP implementation versus usual care would shed light on causality. Future iterations of the algorithm incorporating, for example, a clear definition of futility are needed. Notably, outcomes appeared to be improved only among those with lower predicted risk of mortality. This suggests that high severity of illness may negate benefit of a structured algorithm. Second, the study involved a single academic medical center with a relatively small sample size, and single-center trials, such as this, often find exaggerated effect sizes. Clinical shared decision making in AKI can vary on the basis of site of practice and often involves non-nephrology intensivists, which further limits the generalizability of this study, particularly to institutions in regions where RRT is prescribed by non-nephrologists. On the basis of these factors, we believe an expansion of our study to multiple, diverse clinical sites is warranted before general adoption. Third, a potential confounding factor is that nephrologists who chose to adhere to the SCAMP recommendation may have been inherently better clinicians and therefore, had better patient outcomes compared with those that did not adhere to the SCAMP. Fourth, the possibility of the Hawthorne effect, in which knowledge of study participation leads to behavior changes affecting the outcome of interest, should not be ignored. Fifth, implementation of the SCAMP required tracking of patients and reminders to clinicians to use the decision-making algorithm, calling into question the feasibility of implementation outside of a quality improvement study. Implementation of the SCAMP algorithm into an electronic medical record template may facilitate clinical use.
In conclusion, our study provides preliminary evidence that an RRT decision–making algorithm may lead to improved outcomes in severe AKI. Additional study is needed to elucidate the benefit that clinical decision support for RRT initiation could provide in improving clinical outcomes.
Disclosures
None.
Supplementary Material
Acknowledgments
M.L.M. and S.S.W. take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Guiding Physician Decisions for Initiating Dialysis for AKI: Is Progress on the Horizon?,” on pages 217–219.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.07170716/-/DCSupplemental.
References
- 1.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM: Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol 17: 1143–1150, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Waikar SS, Liu KD, Chertow GM: Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol 3: 844–861, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Bagshaw SM, Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Oudemans-van Straaten HM, Ronco C, Kellum JA; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators : Timing of renal replacement therapy and clinical outcomes in critically ill patients with severe acute kidney injury. J Crit Care 24: 129–140, 2009. 19272549 [Google Scholar]
- 5.Chou YH, Huang TM, Wu VC, Wang CY, Shiao CC, Lai CF, Tsai HB, Chao CT, Young GH, Wang WJ, Kao TW, Lin SL, Han YY, Chou A, Lin TH, Yang YW, Chen YM, Tsai PR, Lin YF, Huang JW, Chiang WC, Chou NK, Ko WJ, Wu KD, Tsai TJ; NSARF Study Group : Impact of timing of renal replacement therapy initiation on outcome of septic acute kidney injury. Crit Care 15: R134, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crescenzi G, Torracca L, Pierri MD, Rosica C, Munch C, Capestro F: ‘Early’ and ‘late’ timing for renal replacement therapy in acute kidney injury after cardiac surgery: A prospective, interventional, controlled, single-centre trial. Interact Cardiovasc Thorac Surg 20: 616–621, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Jun M, Bellomo R, Cass A, Gallagher M, Lo S, Lee J; Randomized Evaluation of Normal Versus Augmented Level of Replacement Therapy (RENAL) Study Investigators : Timing of renal replacement therapy and patient outcomes in the randomized evaluation of normal versus augmented level of replacement therapy study. Crit Care Med 42: 1756–1765, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Lee J, Cho JH, Chung BH, Park JT, Lee JP, Chang JH, Kim DK, Kim S: Classical indications are useful for initiating continuous renal replacement therapy in critically ill patients. Tohoku J Exp Med 233: 233–241, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Vaara ST, Reinikainen M, Wald R, Bagshaw SM, Pettilä V; FINNAKI Study Group : Timing of RRT based on the presence of conventional indications. Clin J Am Soc Nephrol 9: 1577–1585, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Jie Yuan W: Timing of initiation of renal replacement therapy in acute kidney injury: A systematic review and meta-analysis. Ren Fail 34: 396–402, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Pannu N, Klarenbach S, Wiebe N, Manns B, Tonelli M; Alberta Kidney Disease Network : Renal replacement therapy in patients with acute renal failure: A systematic review. JAMA 299: 793–805, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Seabra VF, Balk EM, Liangos O, Sosa MA, Cendoroglo M, Jaber BL: Timing of renal replacement therapy initiation in acute renal failure: A meta-analysis. Am J Kidney Dis 52: 272–284, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Karvellas CJ, Farhat MR, Sajjad I, Mogensen SS, Leung AA, Wald R, Bagshaw SM: A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: A systematic review and meta-analysis. Crit Care 15: R72, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaudry S, Hajage D, Schortgen F, Martin-Lefevre L, Pons B, Boulet E, Boyer A, Chevrel G, Lerolle N, Carpentier D, de Prost N, Lautrette A, Bretagnol A, Mayaux J, Nseir S, Megarbane B, Thirion M, Forel JM, Maizel J, Yonis H, Markowicz P, Thiery G, Tubach F, Ricard JD, Dreyfuss D,; AKIKI Study Group : Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med 375: 122–133, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Zarbock A, Kellum JA, Schmidt C, Van Aken H, Wempe C, Pavenstädt H, Boanta A, Gerß J, Meersch M: Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: The ELAIN randomized clinical trial. JAMA 315: 2190–2199, 2016 [DOI] [PubMed] [Google Scholar]
- 16.KDIGO: KDIGO Clinical Practice Guideline for Acute Kidney Injury, 2015. Available at: http://www.kdigo.org/clinical_practice_guidelines/pdf/KDIGO%20AKI%20Guideline.pdf. Accessed July 2, 2015
- 17.Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Straaten HO, Ronco C, Kellum JA: Discontinuation of continuous renal replacement therapy: A post hoc analysis of a prospective multicenter observational study. Crit Care Med 37: 2576–2582, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Demirjian S, Chertow GM, Zhang JH, O’Connor TZ, Vitale J, Paganini EP, Palevsky PM; VA/NIH Acute Renal Failure Trial Network : Model to predict mortality in critically ill adults with acute kidney injury. Clin J Am Soc Nephrol 6: 2114–2120, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.