Abstract
Following the observation that dopaminergic components are present in normal and malignant B cells, we now provide evidence that they additionally express the functionally related trace amine-associated receptor-1 (TAAR1). Immunodetectable TAAR1 was found in lines derived from a broad range of B-cell malignancy; and in tonsillar B cells, particularly when activated. L3055 Burkitt’s lymphoma cells were shown to respond to prototypical TAAR1 agonists in cytotoxicity assays with features of apoptotic death evident; normal B cells were somewhat less sensitive to the agonists. These data raise the possibility that TAAR1 may have therapeutic relevance to leukemia, lymphoma, and wider B-cell pathologies.
Keywords: Apoptosis, B cells, Dopamine, Lymphoma, MDMA, Trace amine
1. Introduction
B lymphocytes, whether normal or malignant express multiple components more commonly associated with the central nervous system; including constituent proteins of the serotonergic and dopaminergic pathways [1]. Correspondingly, ligands that target associated receptors and transporters for the monoamines impact normal and malignant B-cell behaviour [1,2]. Based on their affinities for the serotonin transporter (SERT) and dopamine transporter (DAT), 3,4-methylenedioxymethamphetamine (MDMA/’ecstasy’) and chemically redesigned analogues thereof are being explored for anti-lymphoma potential; engaging as they do proliferation arrest and cytotoxicity in a broad range of B-cell malignancies [3,4]. MDMA has also been mooted as a ligand for the trace amine-associated receptor-1 (TAAR1) [5]: one of a family of G-protein coupled receptors thought to be activated physiologically by endogenous trace amines such as tyramine, octopamine and tryptamine as well as thyroid hormone derivatives like 3-iodothyronamine (T1AM). TAAR1 is functionally linked to the dopaminergic system by regulating the dopamine transporter via the dopamine D2R receptor [6]: both these proteins are expressed by human B cells [1]. To date a single report has addressed the presence of trace amine receptors in immune cells, showing the presence of TAAR1 transcripts within murine B cells and NK cells and within mitogen-activated human peripheral blood mononuclear cells [7]. Here we report immunodetectable TAAR1 protein in both normal and malignant human B cells and cytotoxic activity from TAAR1 agonists, indicating a potentially novel therapeutic avenue for the treatment of lymphoid neoplasia, and B-cell pathologies broadly.
2. Materials and methods
2.1. Trace amines
3-Iodothyronamine (T1AM) and o-phenyl-3-iodotyramine (o-PIT) were provided by co-authors T.S. and M.J.M., respectively, p-tyramine was purchased from Sigma–Aldrich (Poole, Dorset, UK); o-PIT differs from T1AM by substitution of the hydroxyl group for hydrogen in the para-position on the aryl group distal to the amine. A depiction of the trace amine structures (in relation to dopamine and MDMA) are shown in Fig. 1.
Fig. 1.
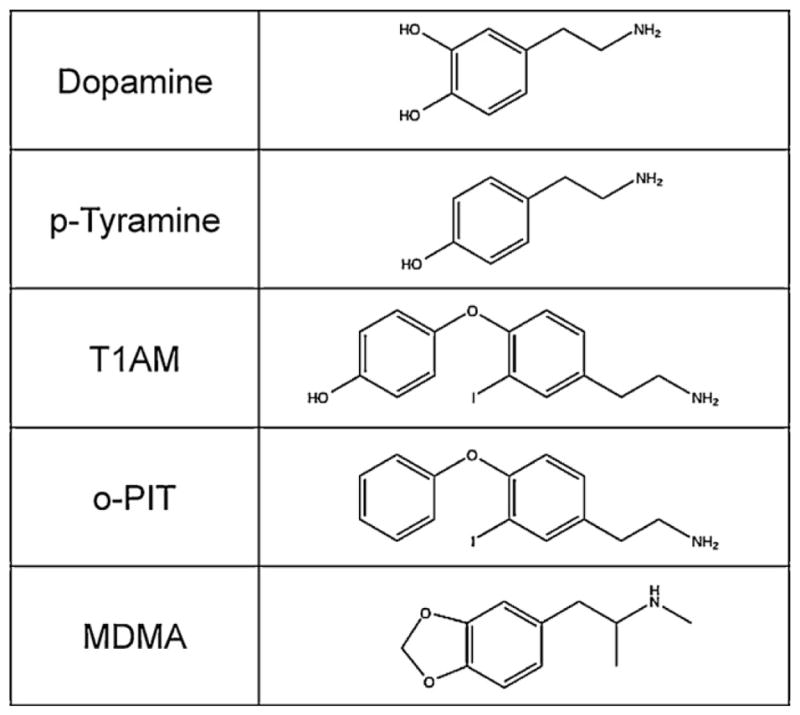
Chemical structures of the trace amines used in this study in relation to those of dopamine and MDMA.
2.2. Cells
Cell lines deriving from different B-cell malignancies, variants of the L3055 Burkitt’s lymphoma (BL) cell line, and HEK 293 cells, and primary B cells from human tonsils were obtained and cultured as described elsewhere [3]. EBV-transformed lymphoblastoid cell lines (LCL) were from the School of Cancer Sciences, University of Birmingham, UK.
2.3. Western blotting for TAAR1
Using the verified rabbit polyclonal anti-TAAR1 antibody from Chemicon International (Temecula, USA) [6], TAAR1 protein content on a per cell equivalent basis was determined by Western blot protocol described elsewhere [3].
2.4. Cytotoxicity/apoptosis studies
Cellular cytotoxicity/viability was assessed by staining treated cells with propidium iodide (PI; Sigma–Aldrich, Dorset, UK) prior to flow cytometric analysis of PI+ve versus PI−ve cells. Apoptosis was assessed by dual staining of cells with PI and PhiPhiLux (Oncoimmunin, Gaithersburg, MD, USA) an indicator of active caspase-3 followed by analysis on FACS as described previously [3].
3. Results
3.1. Western blotting of TAAR1 protein in derived B-cell lines and tonsillar B cells
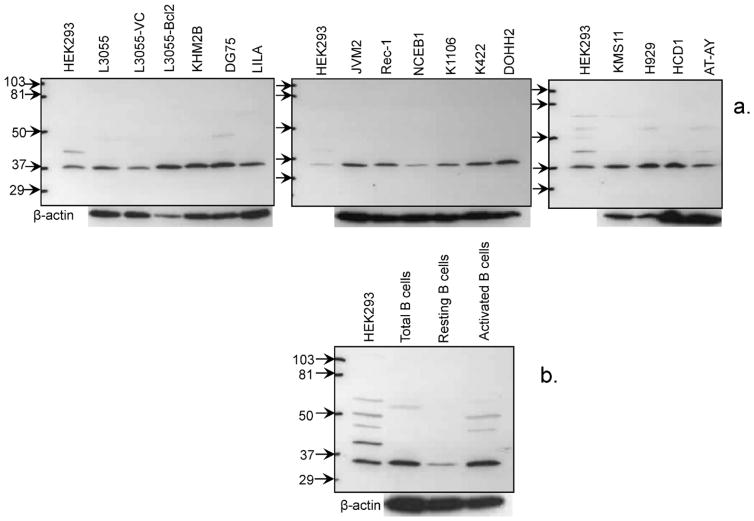
The constituent populations of B-cell lines generated from a diverse range of malignancies were assessed for TAAR1 expression by Western blotting. The origin of cells included those from patients diagnosed with Burkitt’s lymphoma (L3055 series; KHM2B): precursor acute lymphoblastic leukemia (LILA), prolymphocytic leukemia (JVM2), mantle cell lymphoma (Rec-1; NCEB-1), primary mediastinal B-cell lymphoma (K1106), diffuse large B-cell lymphoma (K422; DoHH2), multiple myeloma (KMS11; H929). Immortalized B-cell lines generated from the peripheral blood of three donors by transformation with Epstein–Barr virus (EBV) were also included (HCD1; AT-AY; ViWo). HEK293 cells shown to express TAAR1 endogenously served as positive controls [6]. From Fig. 2a it is evident that all lines examined irrespective of diagnosis/origin contained immuno-detectable TAAR1 with an apparent mol. wt. of approximately 37 kDa, which is close to the predicted protein size of 39 kDa (ExpasyProteomicsServer); and as reported previously using the same antibody as here [6].
Fig. 2.
Western blot analysis of TAAR1 protein expression in B cells from patient-derived cell lines and normal tonsil populations. Immunoblot for TAAR1 contained in cell lysates from: (a) derived cell lines as detailed in Section 3.1; (b) purified (total) tonsillar B cells, the CD38−ve (resting) fraction, CD38−ve B cells cultured (activated) for 4 days with 2 ng/ml PMA + 1 μg/ml ionomycin; mol. wt. markers (kDa) and β-actin loading controls as shown.
Next we examined TAAR1 expression in normal B cells isolated from tonsils comparing total B cells with resting B cells, the latter pre- and post-stimulation with phorbol myristate acetate (PMA) + ionomycin. Once more the major TAAR1-related band detected was at ~37 kDa similar to cell lines. A more intense immunoreactive band tended to develop from freshly isolated total B cells compared to the specifically isolated resting B-cell subset contained therein with activation of the latter producing a stronger immunoreactive band akin to that observed in the unseparated B-cell population (Fig. 2b).
3.2. Cytotoxicity of TAAR1 agonists towards L3055 and tonsillar B cells
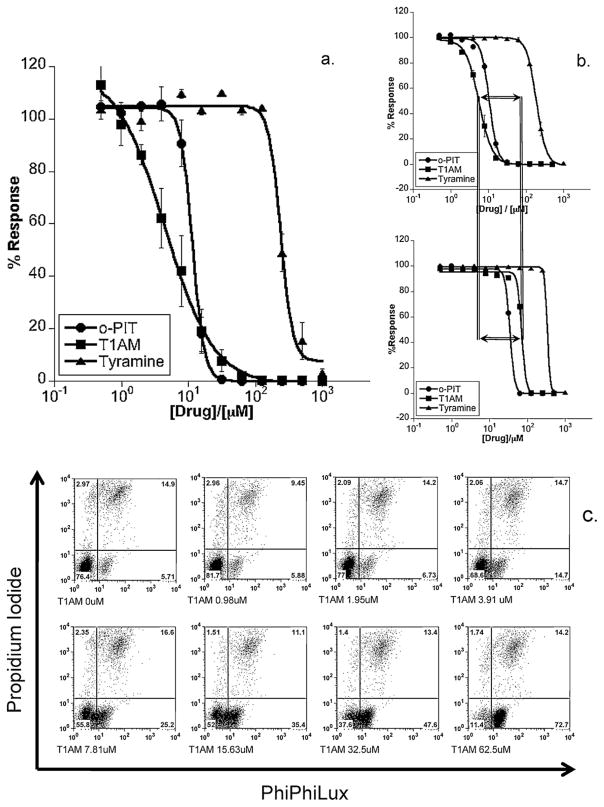
Wild-type L3055 BL cells (which undergo apoptosis in response to MDMA [1,3,4]) were used here to assess potential cytotoxicity from TAAR1 agonists. Fig. 3a shows the concentration response curves of killing. Of the three agonists, T1AM was the most potent followed closely by o-PIT, with tyramine the least potent; this being consistent with the differing affinities for the ligands at the receptor [8] which should be noted are lower against human TAAR1 than its more commonly studied rodent equivalents. L3055 cells carrying a BCL2 transgene that results in overexpression of the Bcl-2 protein were somewhat less susceptible to the TAAR1 agonists than their corresponding vector-only controls with relative resistance being most notable against the most potent agonist T1AM (Fig. 3b). This indication of apoptosis being involved was confirmed when assessing caspase-3 activation in L3055 wild-type cells in response to T1AM. Thus Fig. 3c illustrates the progressive early (4.5 h) appearance of active caspase-3 with increasing concentrations of T1AM; the vast majority of cells being positive for active caspase-3 at the highest concentration of agonist tested.
Fig. 3.
Cytotoxicity of TAAR1 agonists towards L3055 Burkitt’s lymphoma cells. (a) Concentration–response curves showing cytotoxic performance of trace amines against the L3055 BL cell line. Cells were cultured at 105 ml for 24 h prior to measuring cytotoxicity by PI uptake using flow cytometry. Results are represented as the mean of three independent experiments ± SEM in terms of the percentage of cells remaining viable with respect to vehicle (no drug) control; (b) as in (a) but here trace amines versus L3055 BCL2- (lower panel) or vehicle (control)-transfectants (upper panel); (c) L3055 cells cultured for 4.5 h with indicated concentrations of T1AM before dual staining with PhiPhiLux and PI revealing four subpopulations of cells: PIlo/PPLlo = viable (bottom left quadrant), PIlo/PPLhi = early apoptotic (bottom right), PIhi/PPLhi = late apoptotic (top right), and PIhi/PPLhi = necrotic (top left).
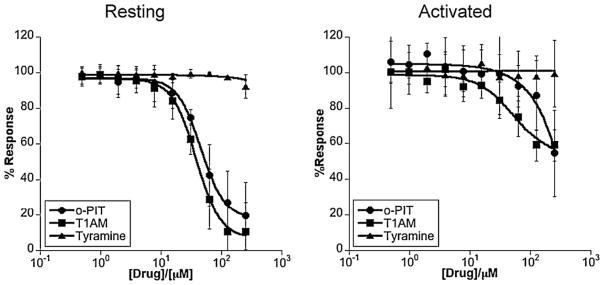
Resting tonsillar B cells displayed the same rank order of susceptibility to the TAAR1 agonists as L3055 wild-type cells with an absolute potency (from T1AM and o-PIT at least) close to that observed against L3055-Bcl-2 transfectants (Fig. 4a). Once activated by PMA/ionomycin, tonsillar B cells acquired relative resistance to TAAR1 agonists (Fig. 4b) despite an apparent increase in the amount of TAAR1 within the cells compared to resting controls (Fig. 3 above). Such resistance may reflect the appearance of anti-apoptotic proteins additional to Bcl-2 on activation: e.g. Bcl-xL and Mcl-1; or the impact of other changes downstream of the receptor.
Fig. 4.
Cytotoxicity of TAAR1 agonists towards resting and activated normal B cells. As for Fig. 3a but here versus tonsil B cells either resting or activated as in Fig. 1b.
4. Conclusions
Herein we have offered evidence for human B cells of normal and malignant derivation expressing TAAR1 and, furthermore, that TAAR1 agonists kill the expressing cells. The mode of death, at least in a Burkitt’s lymphoma line, bears hallmarks of apoptosis. Further work is needed to address precisely how trace amines exert their actions; whether they act directly and/or solely via a TAAR1-mediated mechanism. RNA knockdown and selective human TAAR1 antagonists when generally available will help resolve this. Whatever the answer, the observation that malignant B cells express immunodetectable TAAR1 protein raises interesting questions around its relevance to both the pathogenesis and, potentially, the treatment of the spectrum of neoplasia studied here. Moreover, the presence of TAAR1 in normal B cells, especially when activated, suggests a significance that may extend beyond malignancy; for example, to B-cell associated autoimmune diseases.
Acknowledgments
This work was supported in part by funding from Leukaemia and Lymphoma Research (U.K.) and National Institutes of Health grant (DK-52798). J.G. wrote the paper while in receipt of a Raine Visiting Professorship at the University of Western Australia. We thank Clotilde Mannoury la Cour for technical advice and help.
Footnotes
Conflict of interest
None.
Contributions. A.W. performed all experiments and contributed to their design. M.J.M. and T.S. provided key reagents and advice with experimental design and contributed to the preparation of the manuscript. J.G. and N.M.B. contributed equally to the concept of the study and writing the manuscript. All authors approved the final version of the manuscript.
References
- 1.Barnes NM, Gordon J. Harnessing serotonergic and dopaminergic pathways for lymphoma therapy: evidence and aspirations. Semin Cancer Biol. 2008;18:218–25. doi: 10.1016/j.semcancer.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Chamba A, Holder MJ, Jarrett RF, et al. SLC6A4 expression and anti-proliferative responses to serotonin transporter ligands chlomipramine and fluoxetine in B-cell malignancies. Leuk Res. 2010;36:1103–6. doi: 10.1016/j.leukres.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Meredith EJ, Holder MJ, Chamba A, et al. The serotonin transporter (SLC6A4) is present in B-cell clones of diverse malignant origin: probing a potential anti-tumor target for psychotropics. FASEB J. 2005;19:1187–9. doi: 10.1096/fj.04-3477fje. [DOI] [PubMed] [Google Scholar]
- 4.Gandy M, McIldowie M, Lewis K, et al. Redesigning the designer drug ecstasy: non-psychoactive MDMA analogues exhibiting Burkitt’s lymphoma cytotoxicity. Med Chem Commun. 2010;1:287–93. [Google Scholar]
- 5.Sotnikova TD, Caron MG, Gainetdinov RR. Trace amine-associated receptors as emerging therapeutic targets. Mol Pharmacol. 2009;76:229–35. doi: 10.1124/mol.109.055970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie Z, Westmoreland SV, Bahn ME, Chen G-L, Yang H, Vallender EJ, et al. Rhesus monkey trace amine-associated receptor 1 signaling: enhancement by monoamine transporters and attenuation by the D2 autoreceptor in vitro. JPET. 2007;116:164–76. doi: 10.1124/jpet.106.116863. [DOI] [PubMed] [Google Scholar]
- 7.Nelson DA, Tolbert MD, Singh SJ, Bost KL. Expression of neuronal trace amine-associated receptor (Taar) mRNAs in leukocytes. J Neuroimmunol. 2007;192:21–30. doi: 10.1016/j.jneuroim.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grandy DK. Trace amine-associated receptor 1-family archetype or iconoclast? Pharmacol Ther. 2007;116:355–90. doi: 10.1016/j.pharmthera.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]