Abstract
Ca2+ mobilization in response to crosslinking of IgE bound to its high affinity receptor, FcεRI, on mast cells is central to immune allergic responses. Stimulated tyrosine phosphorylation caused by this crosslinking activates store-operated Ca2+ entry that results in sustained Ca2+ oscillations dependent on Rho family GTPases and phosphoinositide synthesis. Coupling of the endoplasmic reticulum (ER) Ca2+ sensor, STIM1, to the Ca2+-selective channel, Orai1, is regulated by these elements and depends on membrane organization, both at the plasma membrane and at the ER. Mitochondria also contribute to the regulation of Ca2+ mobilization, and we describe recent evidence that the ER membrane protein VAP plays a significant role in the coupling between ER and mitochondria in this process. In addition to granule exocytosis, Ca2+ mobilization in these cells also contributes to stimulated outward trafficking of recycling endosomes and to antigen-stimulated chemotaxis, and it is pathologically regulated by protozoan parasitic invasion.
Keywords: calcium mobilization, mast cells, IgE receptors, VAP proteins, mitochondrial-endoplasmic reticulum coupling
Introduction
Granule exocytosis by mast cells in response to antigen-mediated crosslinking of immunoglobulin E (IgE) receptors, FcεRI, on the plasma membrane is among the most studied Ca2+-dependent processes in non-excitable cells [1, 2]. Other processes, including cytokine biogenesis and secretion, are also mediated by activation of these receptors and depend on Ca2+ mobilization in these cells [3]. In 2012 we reviewed the roles for Ca2+ mobilization in mast cell function [4]. The present article provides a summary of more recent progress on this topic from our laboratory and others.
Key to the role of Ca2+ mobilization in these processes is antigen-stimulated store-operated Ca2+ entry (SOCE), which involves coupling of the endoplasmic reticulum (ER) Ca2+ sensor, STIM1, with the Ca2+ channel protein, Orai1, in the plasma membrane (Figure 1). We previously identified a sequence containing six acidic amino acids in the C-terminus of Orai1 that that are important for functional coupling [5]. More recently, we identified a corresponding basic amino acid sequence in the C-terminus of STIM1 that is essential for SOCE [6]. Supporting our conclusions, a recent NMR study provided direct evidence for a complex between this C-terminal segment from Orai1 and two anti-parallel helical segments that contain this basic sequence in STIM1 [7]. We have also characterized the roles for two different pools of PIP2 in the regulation of stimulated STIM1-Orai1 coupling [8]. One of these pools fractionates with detergent-resistant membranes, and this pool exhibits a positive role in STIM1-Orai1 coupling. More recent data provides evidence for preferential association of Orai1 with detergent-resistant, liquid order-preferring lipids (D. Holowka, unpublished results; [9]).
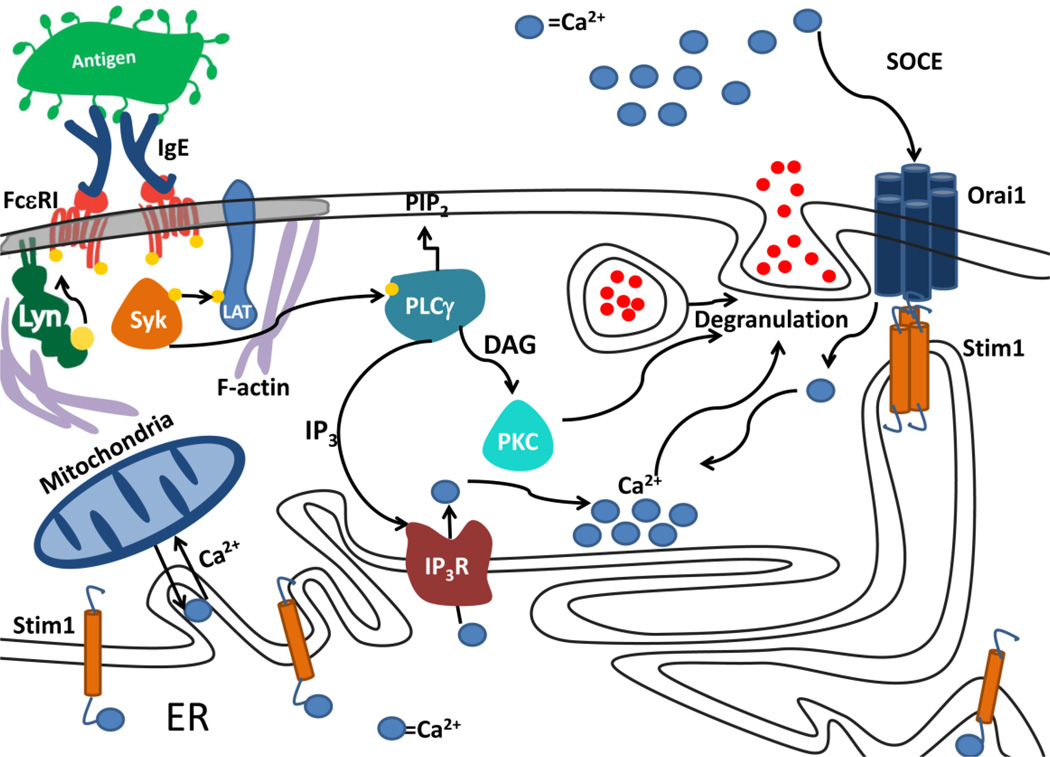
Figure 1.
Schematic of intracellular signaling after antigen-mediated crosslinking of IgE-FcεRI on the surface of mast cells. Binding of antigen-specific IgE to FcεRI sensitizes these receptors to antigen-stimulated signaling that is initiated by a tyrosine phosphorylation cascade. IP3 produced by hydrolysis of PIP2 activates SOCE that is important for granule exocytosis. Activation of protein kinase C (PKC) contributes to the exocytotic response. As described in this review, mitochondria contribute to buffering of cytoplasmic Ca2+ levels. Adapted from [9].
TRPC channel proteins also appear to participate in Ca2+ mobilization in mast cells. Ma and Beaven [10] provided evidence that TRPC5 contributes to SOCE in RBL mast cells, and Cohen et al. [11] described a role for TRPC1 in the initiation of antigen-stimulated Ca2+ waves that emanate from the tips of cell protrusions to contribute to spatially regulated granule exocytosis. Suzuki et al. [12] also provided evidence for a role for TRPC1 in mouse bone marrow-derived mast cell degranulation. The mechanism of activation of these TRPC channels is not fully understood, but the Ambudkar laboratory has described evidence for STIM1-mediated activation that involves association of TRPC proteins with Orai proteins in other cell types [13].
Another recent study pointed to additional regulatory roles for FcεRI in signaling events with the identification an alternate-spliced form of the β subunit of FcεRI that regulates functional responses in mast cells [14]. This variant does not contain exon 3, which includes the first two transmembrane sequences of the tetraspan FcεRI β protein. In addition to contributing to inhibition of degranulation responses in both mouse and human mast cells, knockdown of this variant, together with knockdown of full-length FcεRI β, causes greater reduction in Ca2+ responses stimulated by either antigen or the ER Ca2+ ATPase inhibitor thapsigargin than knockdown of full length FcεRI β alone. Earlier signaling events in antigen-mediated FcεRI responses are not detectably affected by knockdown of this altered splice form of FcεRI, suggesting a more downstream role for this protein in mast cell stimulation. The mechanism by which this alternatively spliced form of FcεRI β participates in these cellular responses is not yet clear, but some evidence points to regulation of microtubule assembly as a possible explanation [14].
Ca2+ oscillations and phosphoinositides
We previously established that Rho family GTPases Cdc42 and/or Rac1 participate in FcεRI-mediated Ca2+ mobilization and degranulation [15, 16], but the mechanistic basis was not determined. These studies took advantage of the RBL mutant cell line B6A4C1, which is defective in FcεRI-mediated, Rho family-dependent signaling. Expression of a constitutively GTP-bound mutant, Cdc42 G12V, reconstituted normal antigen-stimulated Ca2+ mobilization and granule exocytosis in these cells, providing evidence that their principal defect is activation of Cdc42, which normally results from receptor stimulation. More recently, we found that these mutant B6A4C1 cells do not sustain normal antigen-stimulated Ca2+ oscillations, and that these oscillations can be restored by a Dock family Rho GEF or by constitutively active Cdc42 G12V, but not by Cdc42 G12V/QQ with two arginine residues in its C-terminal sequence mutated to glutamines [17]. Furthermore, we showed that these mutant cells are defective in a Ca2+-independent process, antigen-stimulated FcεRI endocytosis, and this can be reconstituted by Cdc42 G12V expression, similar to reconstitution of Ca2+ oscillations. These and other results led us to hypothesize that activated Cdc42 participates in both of these activities by facilitating PIP2 synthesis, which is necessary for both sustained Ca2+ oscillations and for FcεRI endocytosis. Monitoring PIP2 at the plasma membrane with a fluorescent protein-tagged PIP2-specific PH domain, PLCδ-PH-EGFP, we found that oscillations in PIP2 levels stimulated by antigen in the parent RBL 2H3 cells are absent in the mutant B6A4C1 cells, but they are restored by expression of Cdc42 G12V. These results strongly support the hypothesis that stimulated PIP2 synthesis is important to maintain sustained Ca2+ oscillations that are necessary for granule exocytosis.
Other studies on phosphoinositides in IgE/FcεRI signaling yielded evidence that two different inhibitors of antigen-stimulated Ca2+ mobilization and degranulation, phenylarsine oxide and quercetin, are effective because they inhibit PI4-kinase and PIP5-kinase, respectively. These small molecule inhibitors of PI kinases also prevent antigen-stimulated morphological changes and FcεRI endocytosis in RBL mast cells [18], further implicating phosphoinositide synthesis in these multiple functional outcomes of IgE/FcεRI crosslinking and activation. These results also establish phenylarsine oxide and quercetin as useful pharmacologic tools for investigating roles for phosphoinositides in cellular processes.
STIM1-Orai1 coupling
We recently characterized the mechanism by which polyunsaturated fatty acids (PUFAs), including linoleic acid (C18:2 (n-6)), cause rapid inhibition of STIM1-Orai1 coupling. In this study, we developed an effective fluorimetry-based fluorescence resonance energy transfer (FRET) method for monitoring stimulated association of fluorescently tagged STIM1 and Orai1 in transfected COS7 and RBL cells, and we found that micromolar concentrations of linoleic acid disrupt the physical coupling between these proteins in parallel with inhibition of SOCE. Saturated fatty acids of the same acyl chain length do not inhibit STIM1/Orai1 coupling, and the mechanism for this inhibition is downstream of FcεRI-mediated tyrosine phosphorylation [19]. Our current evidence suggests that PUFAs inhibit STIM1/Orai1 coupling by disrupting ER membrane organization, possibly by electrostatic interference with oligomerization of STIM1 dimers or their conformational transition that is normally activated by depletion of Ca2+ from ER stores.
We and others continue to characterize changes in the structural interactions occurring between STIM1 proteins and between STIM1 and Orai1 that result from Ca2+ depletion from ER stores and lead to STIM1-Orai1 coupling in SOCE. We observed that store depletion increases FRET between C-terminally labeled STIM1-AcGFP and STIM1-mApple, consistent with expectations of oligomerization from previous studies with N-terminally labeled STIM1 donors and acceptors [20]. However, if the calcium activation domain (CAD) of STIM1 is deleted from the cytoplasmic segment of acceptor STIM1-mApple, we observe a time-dependent decrease in FRET between wt STIM1-AcGFP and this STIM1-mApple ΔCAD upon stimulation of store depletion, even though these proteins oligomerize and co-localize in stimulation-dependent puncta (Korzeniowski et al., submitted for publication). A minimal model to account for these results is that full-length STIM1-AcGFP undergoes a conformational change in its C-terminal segment during activation, causing the AcGFP donor probe to move farther away from the acceptor probe, mApple, on STIM1ΔCAD, which does not undergo a conformational change. Our further experiments identified a 14 amino acid sequence just C-terminal to CAD that is necessary for the conformational transition in the donor protein: The stimulated decrease in FRET observed requires that the donor construct STIM1-AcGFP contains both the CAD sequence and the subsequent 14 amino acids (STIM1 1-462), but no additional amino acids in the distal C-terminal sequence are needed (Korzeniowski et al., submitted for publication). These results provide the first evidence in live cells for a stimulated conformational change in the C-terminal segment of STIM1, and they complement in vitro studies showing a more extended conformation of the C-terminal segment of STIM1 due to activating mutations [21, 22].
Mitochondria
It has become increasingly apparent that mitochondria participate in buffering cytoplasmic Ca2+ levels, even under conditions of modest elevations in these levels due to receptor-mediated Ca2+ mobilization. A recent study has implicated mitochondrial buffering of cytoplasmic Ca2+ as a contributor to sustained Ca2+ oscillations stimulated by the leukotriene LTC4 [23]. Molecular mechanisms for this role for mitochondria are just beginning to be elucidated, and the VAP family of ER-localized Type I transmembrane proteins are receiving increasing attention, in part because of a familial genetic mutation in VAP B (P58S) that is a dominant marker for the fatal neuromuscular disease known as amyotrophic lateral sclerosis (ALS; [24]). Because mast cells exhibit robust Ca2+ responses to antigens via IgE/FcεRI, we embarked on an effort to investigate the roles for VAP proteins and the effects of this point mutation on these responses.
Initial examination by confocal microscopy of an EGFP-VAP B construct expressed in RBL cells confirmed its distribution throughout the ER, including the nuclear envelope. Expression of mutant EGFP-VAP B P56S showed frequent localization in large ER aggregates throughout the cytoplasm, as expected from similar imaging in other cell types [24, 25]. Upon store depletion of Ca2+ by thapsigargin, however, VAP B exhibits a tendency to cluster together with STIM1-mRFP in puncta, both for wt VAP B and the P56S mutant, suggesting that activated STIM1 exhibits some preferential association with VAP B (D. Holowka, unpublished results). In initial experiments, we employed siRNA knockdown of VAP A and VAP B to evaluate their contributions to antigen or thapsigargin-stimulated Ca2+ responses. We observed modest inhibition of antigen-stimulated SOCE by VAP A + VAP B siRNA cocktails: 13 +/- 2% (SE, n=4), and similar inhibition by either VAP A or VAP B siRNA alone. In subsequent experiments, we evaluated the effect of the mutant VAP B P56S on responses to either antigen or thapsigargin. We consistently observed an elevation in basal Ca2+ levels in the VAP B P56S-expressing cells. In three experiments, these basal levels were 10.1 ± 0.2% greater than in control, vector only-transfected cells. We also observed that expression of the mutant VAP B protein (P56S) causes about 20% inhibition of antigen-stimulated Ca2+ release from ER stores (determined in the absence of Ca2+ influx), as well as ~20% inhibition of SOCE by antigen and somewhat less inhibition for SOCE stimulated by thapsigargin, compared to control, vector only, cells (D. Holowka, unpublished results). Over-expression of wt VAP B under these conditions causes statistically insignificant inhibition of stimulated responses. These results suggest that the VAP proteins participate in the regulation of Ca2+ mobilization stimulated by antigen in these cells.
To further investigate the mechanism by which VAP contributes to this process, we monitored the level of intramitochondrial Ca2+ under stimulating conditions using the FRET-based reporter, mitocameleon [26]. Antigen stimulation by an optimal dose of DNP-BSA resulted in a rapid increase in mitochondrial Ca2+and knockdown of VAP A + VAP B by siRNA cocktails resulted in substantial inhibition of this response. Stimulation by either antigen or thapsigargin was inhibited by these siRNAs by ~50–60% in multiple experiments (D. Holowka, unpublished results). These results indicate a significant role for VAP A and B in antigen-stimulated Ca2+ filling of mitochondria and in the communication between ER and mitochondrial stores.
Other roles for Ca2+ responses
Recycling endosomes (REs) are now appreciated as a complex organelle that contributes to multiple trafficking processes in many cell types and, in particular, has been shown to play a role in cellular events that require extensive membrane remodeling, such as migration [27] and cell division [28]. In RBL mast cells, we previously showed that antigen engagement of IgE/FcεRI causes stimulated outward trafficking of these RE membranes to the plasma membrane in a Ca2+-dependent manner [29]. To study the dynamics of this process, we constructed several different pHluorin-tagged proteins, including transferrin receptors, VAMP3, and VAMP8, which all co-localize with cholera toxin B bound to the ganglioside GM1 in perinuclear recycling endosomes. We also constructed and characterized β-hexosaminidase-pHluorin (β-hex-pHluorin), which co-localizes with secretory granule markers. All of these undergo antigen-stimulated outward trafficking that can be monitored as an increase in fluorescence by fluorimetry or by TIRF microscopy. This trafficking, including that of β-hex-pHluorin, is inhibited by dominant negative Rab11, indicating its broad impact on the endo-lysosomal system (Wilson, J.D., Holowka, D., Baird, B., submitted for publication). A recent study described evidence that Rab11-dependent trafficking of recycling endosomes to the plasma membrane necessarily precedes cytotoxic granule exocytosis in T cells [30], providing a precedent for our finding of a role for Rab11 in secretory granule exocytosis in mast cells. These results offer new insights to recent reports of oscillatory PIP2 and actin in conjunction with secretory events in mast cells [31], and they provide new tools for elucidating functional roles for recycling endosomal exocytosis in hematopoietic cell biology.
Mast cell chemotaxis toward IgE-specific antigen was first described for RBL mast cells almost 30 years ago, and we recently demonstrated that Ca2+ influx via Orai1 plays an important role in regulating both spontaneous motility and directional migration of mast cells toward stimulating antigen [32]. Furthermore, RBL cells expressing the Ca2+ sensor, GCaMP3, exhibit spontaneous Ca2+ transients that depend on Ca2+ influx, and the appearance of these transients correlates with spontaneous cell motility [26].
We discovered that infection of RBL mast cells by the obligate intracellular parasite, Toxoplasma gondii, inhibits antigen-stimulated Ca2+ mobilization and granule exocytosis, and phosphorylation of phospholipase Cγ by the tyrosine kinase Syk appears to be the earliest biochemical event that is disrupted in this process. Interestingly, inhibition of IgE/FcεRI signaling is retained when tachyzoite invasion is arrested at the attachment stage by cytochalasin D treatment, suggesting that inhibition of Syk is mediated by a parasite-derived factor that is secreted during the invasion process [33]. This study provides the first direct evidence that initiation of immune subversion by T. gondii occurs concurrently with invasion. Infection by T. gondii often suppresses immune responses in host cells, and we hypothesize that this suppression results from inhibition of Ca2+-dependent transcriptional activation of cytokine genes via NFAT and/or NFκB.
In summary, Ca2+ mobilization is a ubiquitous mediator of mast cells functions in adaptive immune responses, and the mechanisms by which this central process is regulated by phosphoinositides and by mitochondrial Ca2+ buffering are being defined from a variety of experimental approaches. Questions for the future include the molecular mechanism by which the Rho family protein, Cdc42, regulates PIP2 and Ca2+ oscillations in these cells. The mechanism by which PUFAs regulate STIM1-Orai1 coupling remains to be determined, as does the apparent role of ordered membrane structure in the activation of SOCE. Recent evidence for participation of VAP B in ER-mitochondrial Ca2+ coupling summarized here warrants further investigation. Potential contributions of these particular proteins (Cdc42, VAP A and B) and the processes they regulate (Ca2+ mobilization, RE exocytosis) to the mechanism of mast cell chemotaxis are also intriguing and compel pursuit of more detailed information. Lastly, the generality of our findings regarding regulation of stimulated mast cell Ca2+ mobilization and granule exocytosis by T. gondii infection for its suppression of immunity in other hematopoietic cells and their responses is another question that will drive future efforts in understanding the ubiquitous roles for Ca2+-mediated signaling in immune responses.
Acknowledgments
Our work was supported by grants R01 AI018306 and R01 AI022499 from the National Institutes of Health (NIAID).
References
- 1.Beaven MA. Our perception of the mast cell from Paul Ehrlich to now. European Journal of Immunology. 2009;39:11–25. doi: 10.1002/eji.200838899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kraft S, Kinet JP. New developments in FcepsilonRI regulation, function and inhibition. Nat Rev Immunol. 2007;7:365–378. doi: 10.1038/nri2072. [DOI] [PubMed] [Google Scholar]
- 3.Ma HT, Beaven MA. Regulation of Ca2+ signaling with particular focus on mast cells. Critical Reviews in Immunology. 2009;29:155–186. doi: 10.1615/critrevimmunol.v29.i2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holowka D, Calloway N, Cohen R, Gadi D, Lee J, Smith NL, Baird B. Roles for Ca(2+) mobilization and its regulation in mast cell functions. Frontiers in Immunology. 2012;3:104. doi: 10.3389/fimmu.2012.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calloway N, Vig M, Kinet JP, Holowka D, Baird B. Molecular clustering of STIM1 with Orai1/CRACM1 at the plasma membrane depends dynamically on depletion of Ca2+ stores and on electrostatic interactions. Mol Biol Cell. 2009;20:389–399. doi: 10.1091/mbc.E07-11-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calloway N, Holowka D, Baird B. A basic sequence in STIM1 promotes Ca2+ influx by interacting with the C-terminal acidic coiled coil of Orai1. Biochemistry. 2010;49:1067–1071. doi: 10.1021/bi901936q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stathopulos PB, Schindl R, Fahrner M, Zheng L, Gasmi-Seabrook GM, Muik M, Romanin C, Ikura M. STIM1/Orai1 coiled-coil interplay in the regulation of store-operated calcium entry. Nature Communications. 2013;4:2963. doi: 10.1038/ncomms3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calloway N, Owens T, Corwith K, Rodgers W, Holowka D, Baird B. Stimulated association of STIM1 and Orai1 is regulated by the balance of PtdIns(4,5)P(2) between distinct membrane pools. Journal of Cell Science. 2011;124:2602–2610. doi: 10.1242/jcs.084178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holowka D, Baird B. Nanodomains in early and later phases of FcepsilonRI signalling. Essays Biochem. 2015;57:147–163. doi: 10.1042/bse0570147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma H-T, Peng Z, Hirgun T, Iwaki S, Gilfillan AM, Beaven MA. TRPC5/Orai1/STIM1-dependent store-operated entry of Ca2+ is linked to degranulation in a rat mast cell line. Journal of Immunology. 2008;180:2233–2239. doi: 10.4049/jimmunol.180.4.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen R, Corwith K, Holowka D, Baird B. Spatiotemporal resolution of mast cell granule exocytosis reveals correlation with Ca2+ wave initiation. J Cell Sci. 2012;125:2986–2994. doi: 10.1242/jcs.102632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki R, Liu X, Olivera A, Aguiniga L, Yamashita Y, Blank U, Ambudkar I, Rivera J. Loss of TRPC1-mediated Ca2+ influx contributes to impaired degranulation in Fyn-deficient mouse bone marrow-derived mast cells. J Leukoc Biol. 2010;88:863–875. doi: 10.1189/jlb.0510253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung TK, Ong HL, Li X, Ambudkar IS. Contribution and regulation of TRPC channels in store-operated Ca2+ entry. Current Topics in Membranes. 2013;71:1–24. doi: 10.1016/B978-0-12-407870-3.00007-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cruse G, Beaven MA, Ashmole I, Bradding P, Gilfillan AM, Metcalfe DD. A truncated splice variant of the FcεRI β receptor subunit is critical for microtubule formation and degranulaton in mast cells. Immunity. 2013;38:906–917. doi: 10.1016/j.immuni.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Field KA, Apgar JR, Hong-Geller E, Siraganian RP, Baird B, Holowka D. Mutant RBL mast cells defective in Fc epsilon RI signaling and lipid raft biosynthesis are reconstituted by activated Rho-family GTPases. Mol Biol Cell. 2000;11:3661–3673. doi: 10.1091/mbc.11.10.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong-Geller E, Holowka D, Siraganian RP, Baird B, Cerione RA. Activated Cdc42/Rac reconstitutes Fc epsilon RI-mediated Ca2+ mobilization and degranulation in mutant RBL mast cells. Proceedings of the National Academy of Sciences USA. 2001;98:1154–1159. doi: 10.1073/pnas.98.3.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilkes MM, Wilson JD, Baird B, Holowka D. Activation of Cdc42 is necessary for sustained oscillations of Ca2+ and PIP2 stimulated by antigen in RBL mast cells. Biology Open. 2014;3:700–710. doi: 10.1242/bio.20148862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santos M deSousa, Naal RM, Baird B, Holowka D. Inhibitors of PI(4,5)P2 synthesis reveal dynamic regulation of IgE receptor signaling by phosphoinositides in RBL mast cells. Mol Pharmacol. 2013;83:793–804. doi: 10.1124/mol.112.082834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holowka D, Korzeniowski MK, Bryant KL, Baird B. Polyunsaturated fatty acids inhibit stimulated coupling between the ER Ca(2+) sensor STIM1 and the Ca(2+) channel protein Orai1 in a process that correlates with inhibition of stimulated STIM1 oligomerization. Biochimica et Biophysica Acta. 2014;1841:1210–1216. doi: 10.1016/j.bbalip.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proceedings of the National Academy of Sciences USA. 2007;104:9301–9306. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muik M, Fahrner M, Schindl R, Stathopulos P, Frischauf I, Derler I, Plenk P, Lackner B, Groschner K, Ikura M, Romanin C. STIM1 couples to ORAI1 via an intramolecular transition into an extended conformation. The EMBO journal. 2011;30:1678–1689. doi: 10.1038/emboj.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y, Srinivasan P, Razavi S, Seymour S, Meraner P, Gudlur A, Stathopulos PB, Ikura M, Rao A, Hogan PG. Initial activation of STIM1, the regulator of store-operated calcium entry. Nature Structural & Molecular Biology. 2013;20:973–981. doi: 10.1038/nsmb.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samanta K, Douglas S, Parekh AB. Mitochondrial calcium uniporter MCU supports cytoplasmic Ca2+ oscillations, store-operated Ca2+ Entry and Ca2+-dependent gene expression in response to receptor stimulation. PLoS ONE. 2014;9(7):e101188. doi: 10.1371/journal.pone.0101188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishimura AL, Mitne-Neto M, Silva HC, Richieri-Costa A, Middleton S, Cascio D, Kok F, Oliveira JR, Gillingwater T, Webb J, Skehel P, Zatz M. A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. American Journal of Human Genetics. 2004;75:822–831. doi: 10.1086/425287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teuling E, Ahmed S, Haasdijk E, Demmers J, Steinmetz MO, Akhmanova A, Jaarsma D, Hoogenraad CC. Motor neuron disease-associated mutant vesicle-associated membrane protein-associated protein (VAP) B recruits wild-type VAPs into endoplasmic reticulum-derived tubular aggregates. The Journal of Neuroscience. 2007;27:9801–9815. doi: 10.1523/JNEUROSCI.2661-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmer AE, Giacomello M, Kortemme T, Hires SA, Lev-Ram V, Baker D, Tsien RY. Ca2+ indicators based on computationally redesigned calmodulin-peptide pairs. Chemistry & Biology. 2006;13:521–530. doi: 10.1016/j.chembiol.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Balasubramanian N, Scott DW, Castle JD, Casanova JE, Schwartz MA. Arf6 and microtubules in adhesion-dependent trafficking of lipid rafts. Nat Cell Biol. 2007;9:1381–1391. doi: 10.1038/ncb1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schweitzer JK, Sedgwick AE, D'Souza-Schorey C. ARF6-mediated endocytic recycling impacts cell movement, cell division and lipid homeostasis. Semin Cell Dev Biol. 2011;22:39–47. doi: 10.1016/j.semcdb.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naal RM, Holowka EP, Baird B, Holowka D. Antigen-stimulated trafficking from the recycling compartment to the plasma membrane in RBL mast cells. Traffic. 2003;4:190–200. doi: 10.1034/j.1600-0854.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- 30.Marshall MR, Pattu V, Halimani M, Maier-Peuschel M, Muller ML, Becherer U, Hong W, Hoth M, Tschernig T, Bryceson YT, Rettig J. VAMP8-dependent fusion of recycling endosomes with the plasma membrane facilitates T lymphocyte cytotoxicity. The Journal of Cell Biology. 2015;210:135–151. doi: 10.1083/jcb.201411093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wollman R, Meyer T. Coordinated oscillations in cortical actin and Ca2+ correlate with cycles of vesicle secretion. Nat Cell Biol. 2012;14:1261–1269. doi: 10.1038/ncb2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee J, Veatch SL, Baird B, Holowka D. Molecular mechanisms of spontaneous and directed mast cell motility. J Leukoc. Biol. 2012;92:1029–1041. doi: 10.1189/jlb.0212091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith NL, Abi Abdallah DS, Butcher BA, Denkers EY, Baird B, Holowka D. Toxoplasma gondii inhibits mast cell degranulation by suppressing phospholipase Cγ-mediated Ca(2+) mobilization. Front Microbiol. 2013;4:179. doi: 10.3389/fmicb.2013.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]