Abstract
Promoter mutations in telomerase reverse transcriptase (TERT) and telomere length have been studied in various tumors. In the present study, the frequency and clinical characteristics of TERT promoter mutation and telomere length were studied in hepatocellular carcinoma (HCC). TERT promoter mutation and telomere length were analyzed in 162 tumor samples of the patients with HCC by sequencing and real-time PCR, respectively. The TERT promoter mutation rate was 28.8% (46/160) in HCC and was associated with males (P = 0.027). The telomere length was not significantly different in the presence of a TERT promoter mutation but was shorter in high-grade tumor stages (P = 0.048). Survival analyses showed that poor overall survival was associated with longer telomere length (P = 0.013). However, the TERT promoter mutation did not have a prognostic value for HCC. Multivariate survival analyses demonstrated that the telomere length was an independent prognostic marker for poor overall survival (hazard ratio = 1.75, 95% confidence interval: 1.046–2.913, P = 0.033). These data demonstrated that TERT promoter mutation is a frequent event in HCC; however, telomere length, but not the presence of a TERT promoter mutation, might have potential value as a prognostic indicator of HCC.
Keywords: HCC, prognosis, telomere length, TERT promoter mutation
1. Introduction
Telomerase is uniquely composed of both protein and RNA components. The human telomerase reverse transcriptase (TERT) gene, located on chromosome 5p15.33, is a catalytic subunit of the telomerase complex, and functions in conjunction with the telomerase RNA component (TERC). In progenitor cells, the telomerase complex plays a central role in repairing chromosome ends, known as telomeres, during cell division. TERT provides the reverse transcriptase activity to the complex and uses TERC as a template.[1] The core promoter of TERT includes 330 bp upstream of the translation start site, as well as 37 bp in exon 2 of the TERT gene.[2] The TERT gene has recently garnered interest because of its involvement in the oncogenic process. Cytosine to thymine transitions at C228 and C250 in the TERT promoter locus were identified as 2 cancer-specific “hotspot” mutations.[3,4] The mutations are related to functional increases in TERT protein, telomerase activity, and telomere length. This promoter mutation and dysregulated expression of TERT was demonstrated in diverse human malignancies such as glioblastoma,[5] malignant melanoma,[3,4] and mesothelioma,[6] and also in epithelial malignancies including genitourinary cancers.[7] An association between decreased survival of cancer patients and TERT mutations has been reported.[8] Therefore, studies on telomeres and the related gene, TERT, are considered important in the fight against cancer. The present study investigated TERT gene mutation and telomerase length in malignant tumor, especially hepatocellular carcinoma (HCC).
HCC comprises the majority of primary liver cancers in adults and is a leading cause of cancer-related deaths worldwide.[9] Moreover, the incidence of HCC continues to increase. The 5-year survival rate for patients with HCC was recently reported to be 2% to 29%, thus indicating poor prognosis.[10] To date, there have been many studies on diagnostic genes, prognostic factors, and targeted genes for HCC therapy. The majority of HCC cases occur in a background of chronic liver disease due to viral hepatitis (B or C), alcohol-related cirrhosis, or possibly related steatohepatitis. For all stages, the relative 5-year survival rate of HCC is in the range of 15% to 55%.[11] However, even with hepatectomy, which is expected to yield the best results, the 5-year survival rate and tumor recurrence rate are 30% to 50% and 70% to 85%, respectively.[12] The only chemotherapeutic agent that is currently used and is effective against HCC is sorafenib, a multikinase inhibitor.[13] Therefore, more effective and diverse approaches for the treatment of HCC are urgently needed. However, only a few experimental studies have been performed on the TERT gene in tumor tissues of HCC. The objective of this study was to recognize the clinicopathological significance of telomere length and TERT mutation status in HCC and identify their potential roles as prognostic factors.
2. Methods
2.1. Patients and tissue samples
A total of 162 cases were included in the study, by retrieval of the pathology reports of patients who underwent conventional surgery for HCC at the Kyungpook National University Hospital from January 2005 to December 2010. The clinicopathological parameters of patients were re-evaluated by a review of the patients’ medical records and slides. Patients receiving preoperative therapies such as transarterial chemoembolization, radiofrequency ablation, and systemic chemotherapy with sorafenib (Nexavar; Bayer Corp., Pittsburgh, PA) were excluded from this study. Patients with other malignancies or incomplete survival data were also excluded. Tumor specimens and corresponding nonmalignant liver tissue specimens were formalin-fixed and paraffin-embedded. Paraffin blocks containing representative tumor lesions were selected after review of the corresponding hematoxylin and eosin-stained specimens. The representative lesion from each case was marked on the paraffin blocks and manually cored with a cylindrical device 3 mm in diameter. The study was approved by the institutional review board of the Kyungpook National University Hospital (KNUH-2014-04-056-001).
2.2. TERT promoter mutation
Genomic DNA was extracted using a QIAamp DNA Mini Kit (QIAGEN). Polymerase chain reaction (PCR) amplification of the TERT promoter region was performed as described previously, with 1 minor modification.[14] PCR was performed using AmpliTaq Gold (Applied Biosystems). The PCR products were electrophoresed on a 1.5% agarose gel and stained with ethidium bromide to confirm the size of the bands. Subsequently, direct DNA sequencing was performed using the ABI 3730 DNA sequencer by Bionics Inc, Korea.
2.3. Telomere length analysis
Telomere length was examined by real-time PCR assay. For quantitative determination of content relative to DNA, primers for the specific amplification of telomeres and the β-globin gene were selected, according to previous studies.[14] Real-time PCR was then performed in the LightCycler 480 II system (Roche Diagnostics, Germany), with a total reaction volume of 20 μL. Each measurement was repeated in triplicate and 5 serially diluted control samples were included in each experiment.
2.4. Statistical analysis
The Chi-square, Fischer's exact, Mann–Whitney U, and Simple correlation tests were performed to analyze the relationship between variables. Survival curves, estimated using the Kaplan–Meier method (univariate analysis), were compared by a log-rank test. Overall survival (OS) was defined as the time between diagnosis and either death from disease or death from other causes. Disease-free survival (DFS) was defined as the time between diagnosis and either disease recurrence or development of distant metastasis. Hazard ratios (HR) and 95% confidence intervals (CI) were estimated using the multivariate Cox proportional hazard model, with adjustment for age (≤ 65 vs > 65 years), gender, aspartate transaminase (AST), and alanine transaminase (ALT). All statistical analyses were carried out using the SPSS version 19.0 software package (SPSS-IBM Inc, Chicago, IL), and P-values < 0.05 were considered statistically significant.
3. Results
3.1. Frequency and clinicopathological characteristics of TERT promoter mutation and telomere length in HCC
The presence of the TERT promoter mutation could not be performed on 2 HCC samples. And, we successfully sequenced the TERT promoter region in 160 patients with HCC and found the mutation rate to be 28.8% (46/160). Thirty-two cases with TERT promoter mutations harbored the C228T mutation and 14 carried the C250T mutation (Fig. 1). Clinicopathological characteristics of TERT promoter mutations in HCC are presented in Table 1. A TERT promoter mutation was statistically associated with males (P = 0.027). The frequency of this mutation was higher in patients with HCC and infected with hepatitis C virus (HCV; 60.0%) compared to those infected with hepatitis B virus (HBV; 32.8%) or having other underlying disease. However, this was not statistically significant (P = 0.285). Other clinical parameters did not have any relationships with TERT promoter mutations.
Figure 1.
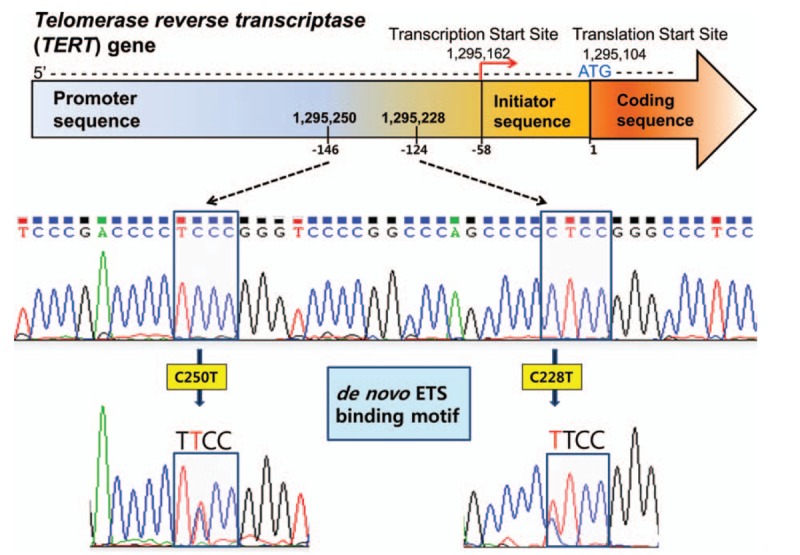
Identification of TERT promoter mutation in hepatocellular carcinomas. Two hot spot mutations, C250T and C228T, are identified by sequencing chromatographs of TERT promoter locus. Both mutations generate a de novo ETS (E26 transformation-specific) binding motif (5′-TTCC-3′), recruiting some transcription factors from ETS family. TERT = telomerase reverse transcriptase.
Table 1.
Clinicopathological characteristics of TERT promoter mutation and telomere length.

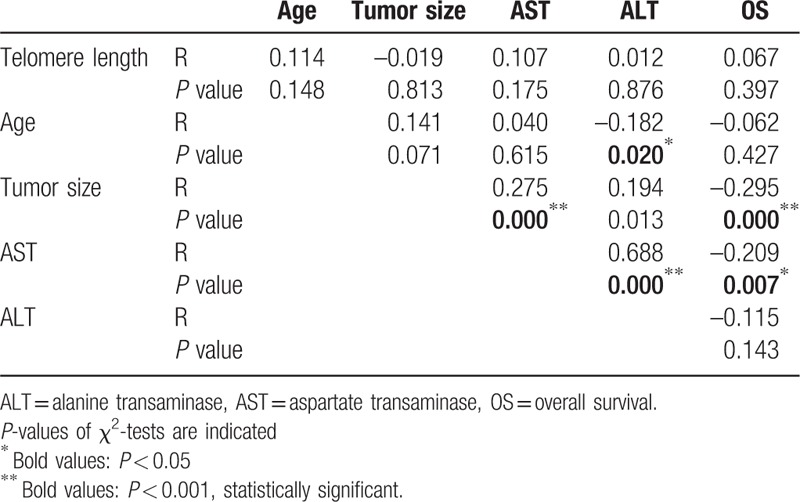
Telomere length was analyzed in 162 patients with HCC. The average telomere length in HCC was 3.97 ± 1.38, when comparing the ratio of telomere length in tumors to that of paired normal tissues. The mean telomere lengths in patients with HCC with and without TERT promoter mutations were 4.01 ± 0.79 and 3.92 ± 1.74, respectively, and showed no statistical difference (P = 0.963). To further explore the correlation between telomere length and clinicopathological parameters of HCC, patients were categorized into 2 subgroups, according to the average telomere length ratio (tumor/normal) of 3.97. The clinicopathological characteristics of telomere length are summarized in Table 1. Shortening of telomere length was more frequent in older (51.4%) patients compared to younger ones (66.3%). However, this did not achieve statistical significance (P = 0.056). This parameter had a deep relationship with T stage (P = 0.048). Other variables showed no association with telomere length.
Quantitative correlation analysis of telomere length with clinical parameters was also performed. The associations between telomere length, age, tumor size, AST, ALT, and overall survival (OS) are presented in Table 2. Telomere length had no correlation with any clinical parameters, and age was associated only with ALT (r = –0.182, P = 0.020). However, tumor size was associated with ALT (r = 0.194, P = 0.013), AST (r = 0.275, P < 0.001), and OS (r = –0.295, P < 0.001). AST was also related to other parameters including ALT (r = 0.688, P < 0.001) and OS (r = –0.209, P = 0.007). Other parameters had no quantitative correlation with each other.
Table 2.
Correlation with telomere length and clinical variables.
3.2. Prognostic value of TERT promoter mutation and telomere length in HCC
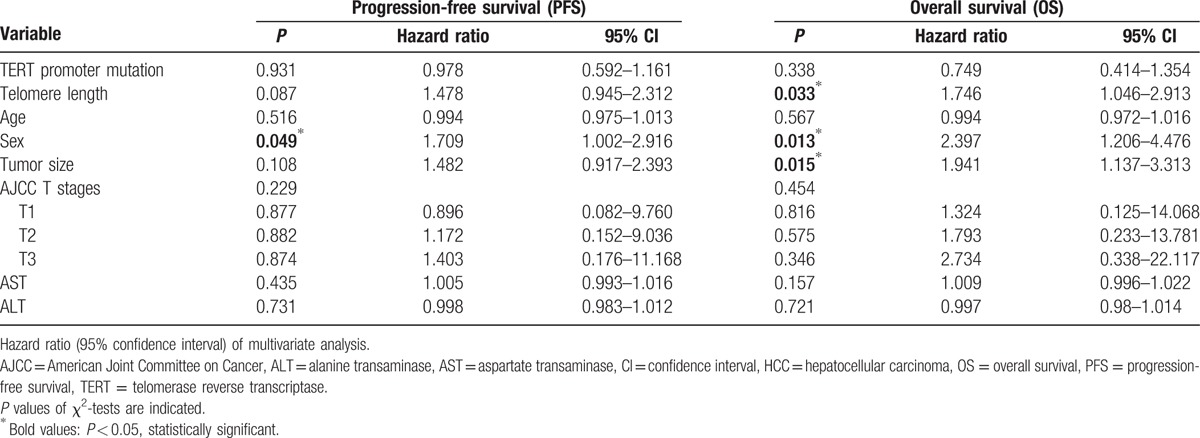
We then performed survival analysis to clarify the prognostic significance of TERT mutations and telomere length in HCC. The median follow-up of patients for survival analysis was 75.1 months (3–101). Univariate survival analysis was performed using a Kaplan–Meier curve; this showed a shorter overall survival in patients with HCC and longer telomere lengths (63.07 vs 75.70 months, χ2 = 6.24, P = 0.013) (Fig. 2). However, the presence of a TERT mutation was not associated with HCC prognosis. Tumor size also had a prognostic value for both OS and DFS. To evaluate if telomere length is an independent prognostic predictor in HCC, we further analyzed the data, using the Cox proportional hazards regression model after adjusting for possible confounders of survival (Table 3). Multivariate analysis showed that telomere length (HR for OS = 1.746, 95% CI: 1.05–2.91, P = 0.033; HR of DFS = 1.478, 95% CI: 0.95–2.31, P = 0.087) was a potential independent prognostic factor. However, the presence of a TERT mutation did not show any statistical significance as an independent prognostic marker (HR for OS = 0.749, 95% CI: 0.41–1.35, P = 0.338; HR of DFS = 0.978, 95% CI: 0.59–1.62, P = 0.931). In addition, sex and tumor size have a statistical significance for HCC prognosis.
Figure 2.
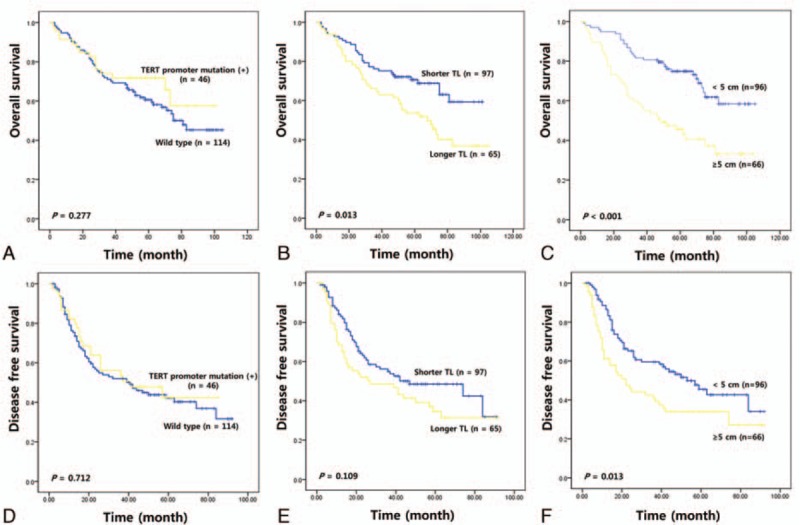
Kaplan–Meier plots showing overall survival (upper) and disease-free survival (lower) according to TERT promoter mutation (A and D), telomere length (B and E), and tumor size (C and F). TERT = telomerase reverse transcriptase.
Table 3.
Multivariated analysis of the prognostic values of various factors in HCC.
4. Discussion
HCC, the most common type of liver cancer, occurs secondary to HBV, HCV, alcohol consumption, and rare metabolic disorders. Its pathogenesis and progression have been studied extensively; the genetic landscape of HCC includes recurrent somatic mutations in CTNNB1, AXIN1, TP53, RB1, CDKN2A, ARID1A, ARID2, PIK3CA, and VEGFA, among others.[15] Since the discovery of TERT promoter mutations in malignant melanoma, this mutation was subsequently observed in a number of other malignancies including liver cancer, bladder cancer, glioblastoma, and thyroid cancer, and is established as a genetic mechanism of human tumorigenesis.[3–5,16,17]
To define molecular subtypes of HCC for personalized therapy, it is necessary to understand TERT promoter mutations and their molecular mechanisms, which are still largely unknown. Therefore, we evaluated the clinicopathological characteristics and prognostic effect of TERT promoter mutations and telomere length in patients with HCC. Here, we identified TERT promoter mutations in 28.8% (46/160) of patients with HCC. Previous studies showed that the frequency of TERT promoter mutations in HCC is approximately 50% in Western countries and 30% in Eastern countries.[15,17–19,20] Our result was in agreement with that in these previous reports. However, a relatively higher frequency of the C250T mutation was an interesting finding because most TERT promoter mutations in HCC have previously been reported to occur at C228T (95%).
Previous studies have demonstrated that TERT promoter mutations are positively associated with age and hepatitis C infection, but negatively associated with HBV infection.[17,20] Our results also showed a positive association of TERT promoter mutations with HCV, but not with HBV, with a higher frequency in males. Taken together, TERT promoter mutations are the most frequent genetic alterations observed in HCC.[21] Considering the association of TERT overexpression with TERT promoter mutations, questions have been raised about the change in telomere length with respect to TERT promoter mutations in HCC. However, TERT promoter mutation did not have any effect on the regulation of telomere length.
Recent studies reported that the mechanisms of telomere length and TERT regulation were not simple, and the association with other genetic alternations could be required.[22–24] Therefore, the regulatory mechanism of telomere length may be through various pathways, including the TERT promoter mutations in hepatic carcinogenesis. In our study, telomere length was associated with age, T stage, and infection status. However, its quantitative analysis showed no correlation with clinical parameters. A previous study demonstrated that leukocyte telomere length might serve as an independent prognostic marker for HCC. Some studies have reported that short telomere length is associated with a higher risk of HCC development and postoperative recurrence.[25,26] Notably, we demonstrated, for the first time, poor prognosis with longer telomere length in HCC tissue. In early stage of hepatocellular carcinogenesis, telomere shortening occurs, while telomerase is reactivated during its progression to more advanced HCCs, thus maintaining the telomere length for immortalization. Therefore, HCCs with longer telomere were shown to be associated with aggressive clinicopathological characteristics inducing poor prognosis.
Many reports have shown no prognostic value of TERT promoter mutations, similar to our results.[17] However, Kawai–Kitahata et al[27] showed that TERT promoter mutation was significantly associated with shorter disease-free survival and poor overall survival. A recent study also suggested that combination of the rs2853669 and a TERT promoter mutation indicates poor prognosis in HCC.[28]
Interestingly, we also demonstrated poorer prognosis of HCC in male than female, which is in agreement with previous study with HCC in Korea population.[29,30] This result may be originated from high prevalence of HCC in Korean male by specific epidemiologic factors like hepatitis infection and alcohol. Considering the strong association between TERT and telomere length, additional factors regulating the telomere system and HCC progression should be considered.
In conclusion, we studied both TERT mutations and telomere length in HCC by survival analysis. TERT promoter mutation was frequent, but it had no effect on telomere length and tumor progression. Instead, our study indicates that telomere length is an independent prognostic marker for poor overall survival. The combination of telomere length and clinical parameters has the potential to become prognostic markers for HCC and may further aid clinicians in therapeutic decisions.
Footnotes
Abbreviations: ALT = alanine transaminase, AST = aspartate transaminase, CI = confidence intervals, DFS = disease-free survival, HCC = hepatocellular carcinoma, HR = hazard ratios, OS = overall survival, PCR = polymerase chain reaction, TERC = telomerase RNA component, TERT = telomerase reverse transcriptase.
Funding: This study was supported by grants of the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2014R1A6A3A04058057) and by the Korean Government (MSIP) (No. 2014R1A5A2010008).
The authors have no conflicts of interest to disclose.
References
- [1].Millar SE. Cell biology: the not-so-odd couple. Nature 2009;460:44–5. [DOI] [PubMed] [Google Scholar]
- [2].Cong YS, Wen J, Bacchetti S. The human telomerase catalytic subunit hTERT: organization of the gene and characterization of the promoter. Hum Mol Genet 1999;8:137–42. [DOI] [PubMed] [Google Scholar]
- [3].Horn S, Figl A, Rachakonda PS, et al. TERT promoter mutations in familial and sporadic melanoma. Science 2013;339:959–61. [DOI] [PubMed] [Google Scholar]
- [4].Huang FW, Hodis E, Xu MJ, et al. Highly recurrent TERT promoter mutations in human melanoma. Science 2013;339:957–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A 2013;110:6021–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tallet A, Nault JC, Renier A, et al. Overexpression and promoter mutation of the TERT gene in malignant pleural mesothelioma. Oncogene 2014;33:3748–52. [DOI] [PubMed] [Google Scholar]
- [7].Kinde I, Munari E, Faraj SF, et al. TERT promoter mutations occur early in urothelial neoplasia and are biomarkers of early disease and disease recurrence in urine. Cancer Res 2013;73:7162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Melo M, da Rocha AG, Vinagre J, et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J Clin Endocrinol Metab 2014;99:E754–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [10].Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29. [DOI] [PubMed] [Google Scholar]
- [11].Vauthey JN, Lauwers GY, Esnaola NF, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol 2002;20:1527–36. [DOI] [PubMed] [Google Scholar]
- [12].Lu WP, Dong JH. Hepatectomy for hepatocellular carcinoma in the era of liver transplantation. World J Gastroenterol 2014;20:9237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–90. [DOI] [PubMed] [Google Scholar]
- [14].Liu T, Wang N, Cao J, et al. The age- and shorter telomere-dependent TERT promoter mutation in follicular thyroid cell-derived carcinomas. Oncogene 2014;33:4978–84. [DOI] [PubMed] [Google Scholar]
- [15].Nault JC, Zucman-Rossi J. TERT promoter mutations in primary liver tumors. Clin Res Hepatol Gastroenterol 2016;40:9–14. [DOI] [PubMed] [Google Scholar]
- [16].Nonoguchi N, Ohta T, Oh JE, et al. TERT promoter mutations in primary and secondary glioblastomas. Acta Neuropathol 2013;126:931–7. [DOI] [PubMed] [Google Scholar]
- [17].Chen YL, Jeng YM, Chang CN, et al. TERT promoter mutation in resectable hepatocellular carcinomas: a strong association with hepatitis C infection and absence of hepatitis B infection. Int J Surg 2014;12:659–65. [DOI] [PubMed] [Google Scholar]
- [18].Pinyol R, Tovar V, Llovet JM. TERT promoter mutations: gatekeeper and driver of hepatocellular carcinoma. J Hepatol 2014;61:685–7. [DOI] [PubMed] [Google Scholar]
- [19].Cevik D, Yildiz G, Ozturk M. Common telomerase reverse transcriptase promoter mutations in hepatocellular carcinomas from different geographical locations. World J Gastroenterol 2015;21:311–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tornesello ML, Buonaguro L, Izzo F, et al. Molecular alterations in hepatocellular carcinoma associated with hepatitis B and hepatitis C infections. Oncotarget 2016;7:25087–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nault JC, Mallet M, Pilati C, et al. High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat Commun 2013;4:2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Labussiere M, Boisselier B, Mokhtari K, et al. Combined analysis of TERT, EGFR, and IDH status defines distinct prognostic glioblastoma classes. Neurology 2014;83:1200–6. [DOI] [PubMed] [Google Scholar]
- [23].Chiba K, Johnson JZ, Vogan JM, et al. Cancer-associated TERT promoter mutations abrogate telomerase silencing. Elife 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zucman-Rossi J, Villanueva A, Nault JC, et al. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology 2015;149: 1226-1239 e1224. [DOI] [PubMed] [Google Scholar]
- [25].Yokota T, Suda T, Igarashi M, et al. Telomere length variation and maintenance in hepatocarcinogenesis. Cancer 2003;98:110–8. [DOI] [PubMed] [Google Scholar]
- [26].Plentz RR, Caselitz M, Bleck JS, et al. Hepatocellular telomere shortening correlates with chromosomal instability and the development of human hepatoma. Hepatology 2004;40:80–6. [DOI] [PubMed] [Google Scholar]
- [27].Kawai-Kitahata F, Asahina Y, Tanaka S, et al. Comprehensive analyses of mutations and hepatitis B virus integration in hepatocellular carcinoma with clinicopathological features. J Gastroenterol 2016;51:473–86. [DOI] [PubMed] [Google Scholar]
- [28].Ko E, Seo HW, Jung ES, et al. The TERT promoter SNP rs2853669 decreases E2F1 transcription factor binding and increases mortality and recurrence risks in liver cancer. Oncotarget 2016;7:684–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Park KW, Park JW, Kim TH, et al. Five-year survival analysis of a cohort of hepatocellular carcinoma patients who treated at the National Cancer Center, Korea. Korean J Hepatol 2007;13:530–42. [DOI] [PubMed] [Google Scholar]
- [30].Lee SH, Song IH, Noh R, et al. Clinical outcomes of patients with advanced hepatocellular carcinoma treated with sorafenib: a retrospective study of routine clinical practice in multi-institutions. BMC Cancer 2015;15:236. [DOI] [PMC free article] [PubMed] [Google Scholar]


