Abstract
Background:
This meta-analysis aimed to provide a pooled analysis of prospective controlled trials comparing the diagnostic accuracy of 22-G and 25-G needles on endoscopic ultrasonography (EUS-FNA) of the solid pancreatic mass.
Methods:
We established a rigorous study protocol according to Cochrane Collaboration recommendations. We systematically searched the PubMed and Embase databases to identify articles to include in the meta-analysis. Sensitivity, specificity, and corresponding 95% confidence intervals were calculated for 22-G and 25-G needles of individual studies from the contingency tables.
Results:
Eleven prospective controlled trials included a total of 837 patients (412 with 22-G vs 425 with 25-G). Our outcomes revealed that 25-G needles (92% [95% CI, 89%–95%]) have higher sensitivity than 22-G needles (88% [95% CI, 84%–91%]) on solid pancreatic mass EUS-FNA (P = 0.046). However, there were no significant differences between the 2 groups in overall diagnostic specificity (P = 0.842). The pooled positive and negative likelihood ratio of the 22-G needle were 12.61 (95% CI, 5.65–28.14) and 0.16 (95% CI, 0.12–0.21), respectively. The pooled positive likelihood ratio was 12.61 (95% CI, 5.65–28.14), and the negative likelihood ratio was 0.16 (95% CI, 0.12–0.21) for the 22-G needle. The pooled positive likelihood ratio was 8.44 (95% CI, 3.87–18.42), and the negative likelihood ratio was 0.13 (95% CI, 0.09–0.18) for the 25-G needle. The area under the summary receiver operating characteristic curve was 0.97 for the 22-G needle and 0.96 for the 25-G needle.
Conclusion:
Compared to the study of 22-G EUS-FNA needles, our study showed that 25-G needles have superior sensitivity in the evaluation of solid pancreatic lesions by EUS–FNA.
Keywords: 22-G, 25-G, EUS, FNA, Pancreas
1. Introduction
Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is a widely used, safe, and accurate technique for sampling the tissues of pancreatic masses.[1,2] EUS-FNA provides a tissue diagnosis for pancreatic masses and has high sensitivity and specificity (75%–92% and 82%–100%, respectively) with an accuracy >85%.[3,4] Bleeding, infection, perforation, and pancreatitis are among the most common complications during the targeting of pancreatic masses, but their incidences are as low as 1%.[5]
Several factors can affect EUS-FNA results, such as endosonographer experience, endoscope position, needle diameter, number of passes, sedation, FNA technique, and the presence of an onsite cytopathologist.[6–8] EUS-FNA needles of various diameters (19-, 22-, and 25-gauge (G]) are commercially available. Therefore, the decision to use a specific needle size involves tradeoffs between possible risks and benefits. In fact, 19-G needles, the largest of the 3, carry an increased risk of bleeding and have lower accuracy in diagnosing pancreatic lesions than conventional 22-G FNA needles.[9,10]
The 22-G FNA needle has been the most commonly used for EUS-FNA of pancreatic masses.[7,8] Recently, several studies demonstrate that the 25-G is more safe and effective than the 22-G needle for EUS-FNA of solid pancreatic masses.[11–13] The 25-G needle is considered the best choice because of its easy maneuverability in every endoscope position and its penetrating thin tip that is easy to advance, even into deep lesions. However, only a few comparative studies with small numbers of patients have examined whether a 25-G needle is comparable to a standard 22-G needle in terms of specimen cellularity, quality, and diagnostic accuracy. The results of several studies comparing diagnostic accuracy of 22-G and 25-G needles remain inconclusive. Considering the results of a recent meta-analysis that shows a potential advantage of the 25-G needle over the 22-G needle for the diagnosis of pancreatic malignancy.[14] Although this study compared the effects of the 2 methods, there are no evidence-based consensus regarding the optimal needle for use in EUS-FNA of solid pancreatic masses. The aim of this meta-analysis was to compare the diagnostic accuracy of 22- and 25-G needles on the solid pancreatic mass.
2. Materials and methods
The rigorous study protocol was established according to Cochrane Collaboration recommendations. And Ethical approval was not necessary for this meta-analysis. Abstracts of the citations identified by the search were then scrutinized by 2 observers to determine eligibility for inclusion in the meta-analysis. We conducted a systematic review and meta-analysis to compare the diagnostic accuracy of 22-G and 25-G needles on EUS-FNA of solid pancreatic masses. A systematic electronic search of the English literature was independently performed by 2 investigators using Cochrane, EMBASE, and MEDLINE; the databases were searched for studies published between December 2015 and February 2016. The search terms were “EUS-FNA,” “22-G,” “25-G,” and “pancreatic” and MeSH headings “EUS-FNA” (MeSH), “22-G” (MeSH), “25-G” (MeSH), and “pancreatic” (MeSH) were used in combination with the Boolean operators AND or OR. We also checked reference lists of relevant articles and review articles. No study design restrictions or time limits were applied to the initial search.
Studies were included if they met both criteria: prospective controlled trial; and separation into groups based on the use of 22-G and 25-G needles for EUS-FNA of the solid pancreatic mass. Exclusion criteria: retrospective study; literature with no original data; or case report or case series. We included studies that evaluated the association between the needle size and the diagnostic accuracy for EUS-FNA of solid pancreatic masses.
2.1. Statistical analysis
Synchronized extraction results were pooled statistically as effect estimates in the meta-analyses. Sensitivity, specificity, and corresponding 95% CI were calculated for 22-G and 25-G needles of individual studies from the contingency tables. Pooled results were constructed using both the fixed-effect model and the random-effects model based on whether significant heterogeneity was present or absent. We used the Cochran Q statistic and I2 value to assess heterogeneity among the studies. Values of I2 > 50% indicated significant heterogeneity. The fixed-effect model was used first to calculate pooled HR; if the assumption of homogeneity had to be rejected, a random-effects model would be used. All of the statistical analyses were performed using Meta-DiSc and SPSS 13.0.
2.2. Results Study identifications and selection
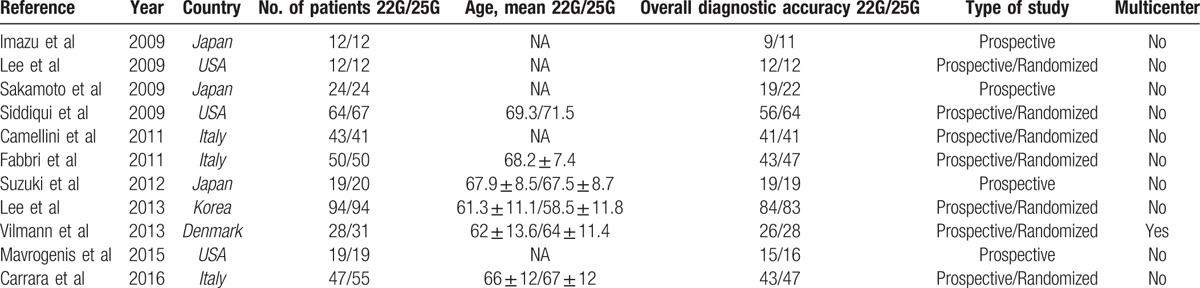
The search query resulted in 2811 records. Of them, 2613 were excluded by 2 independent reviewers after preliminary review of the titles and abstracts, leaving 198 articles for full-text review. After duplicate filtering, a total of 11 studies [12,13,15–23] including a total of 837 patients (412 22-G vs 425 25-G) were examined. All were prospective cohort studies and compared 22-G with 25-G needles. A PRISMA flowchart (Fig. 1) describes the details of the literature search for this systematic review. The main characteristics of the included studies and patients are summarized in Table 1.
Figure 1.
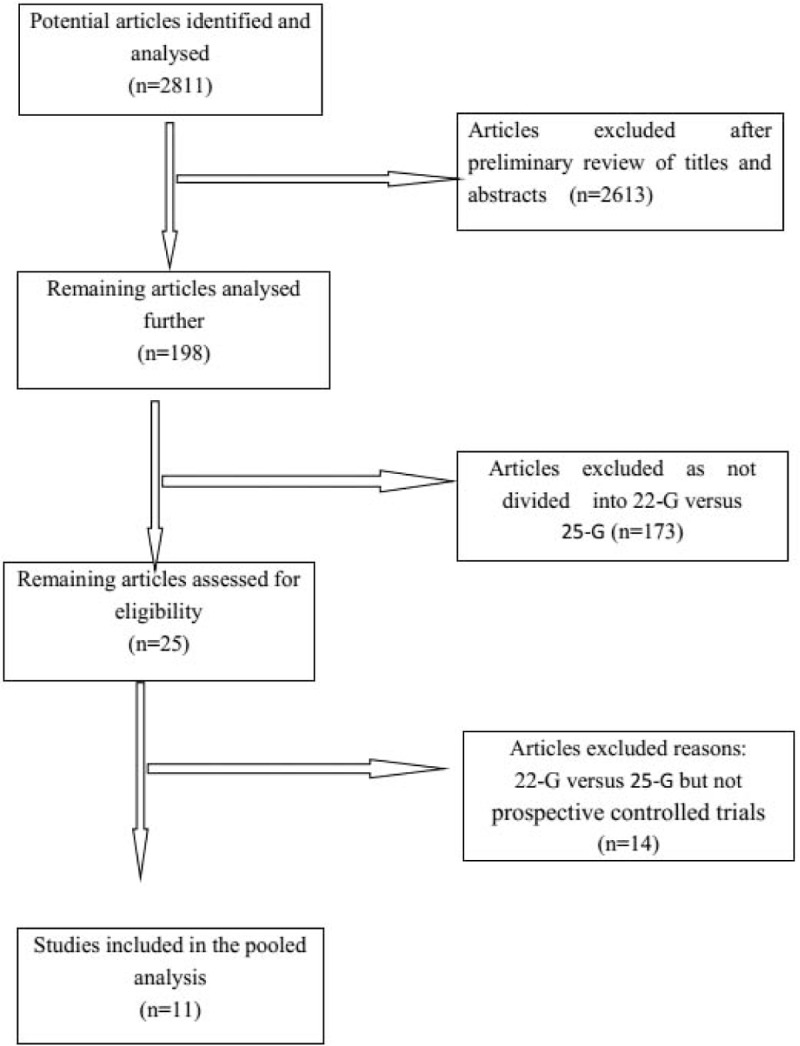
Flowchart of the study selection process for eligible studies in the systematic review.
Table 1.
Characteristics of the selected studies.
2.3. Diagnostic accuracy
In this meta-analysis, we separately calculated the diagnostic accuracy of EUSFNA with 22-G and 25-G needles. Eleven studies including 412 patients were eligible for the 22-G needle analysis. The pooled sensitivity was 88% (95% CI, 84%–91%), and the pooled specificity was 100% (95% CI, 95%–100%) for the 22-G needle. (Fig. 2). Statistical analyses of heterogeneity revealed I2 values of 22.2% and 0.0%, respectively. Eleven studies including 425 patients were eligible for the 25-G needle analysis. The pooled sensitivity and specificity of 25-G needles were 92% (95% CI, 89%–95%) and 100% (95% CI, 94%–100%), respectively (Fig. 3). Statistical analyses of heterogeneity revealed I2 values of 0.0% and 0.0%, respectively.
Figure 2.
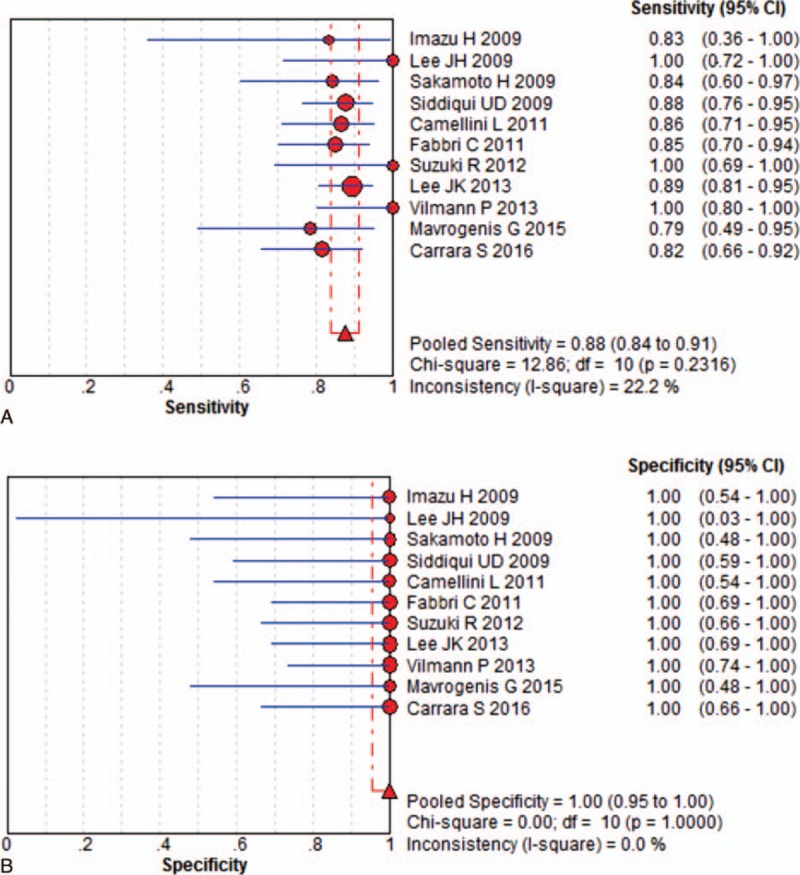
Results for the 22-gauge needle in individual studies and from pooled data shown as forest plots for: (A) sensitivity and (B) specificity.
Figure 3.
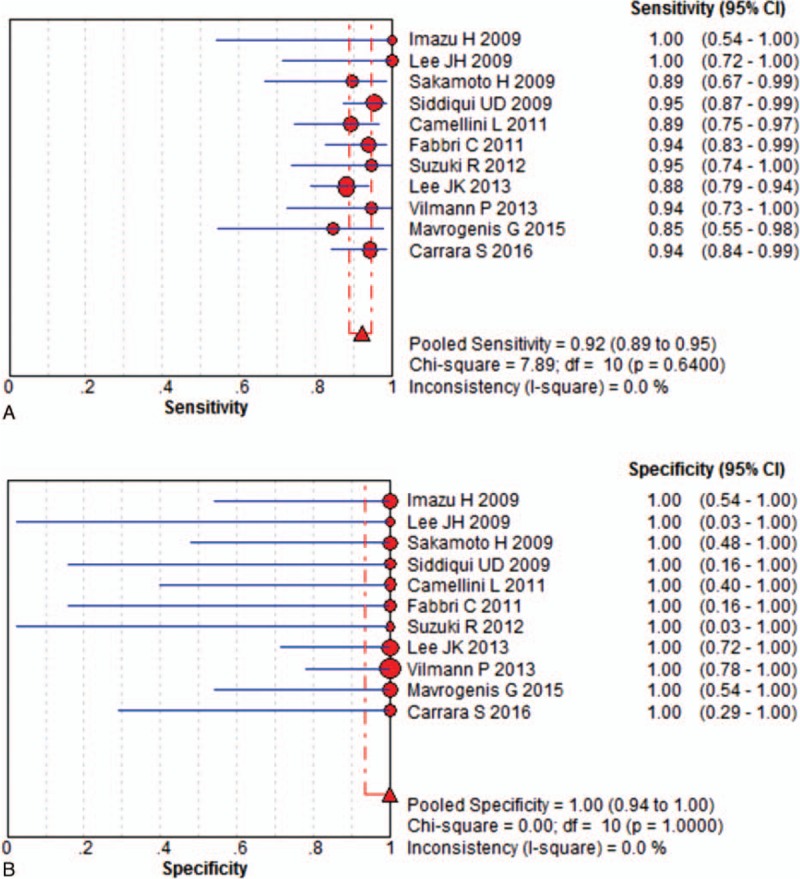
Results for the 25-gauge needle in individual studies and from pooled data shown as forest plots for: (A) sensitivity and (B) specificity.
The bivariate generalized linear random-effects model indicated that 25-G needles have better pooled sensitivity than 22-G needles for solid pancreatic mass EUS-FNA (P = 0.046). However, there was no significant difference in specificity between the 2 groups (P = 0.842).
For solid pancreatic mass EUS-FNA, the pooled positive and negative likelihood ratios (PLR and NLR, respectively) of the 22-G needle were 12.61 (95% CI, 5.65–28.14) and 0.16 (95% CI, 0.12–0.21), respectively (Fig. 4A). The pooled PLR was 8.44 (95% CI, 3.87–18.42), and the pooled NLR was 0.13 (95% CI, 0.09–0.18) for the 25-G needle. (Fig. 4B). The area under the summary receiver operating characteristic curve (SROC) was 0.97 for the 22-G needle and 0.96 for the 25-G needle, which indicates high accuracy (Fig. 5).
Figure 4.
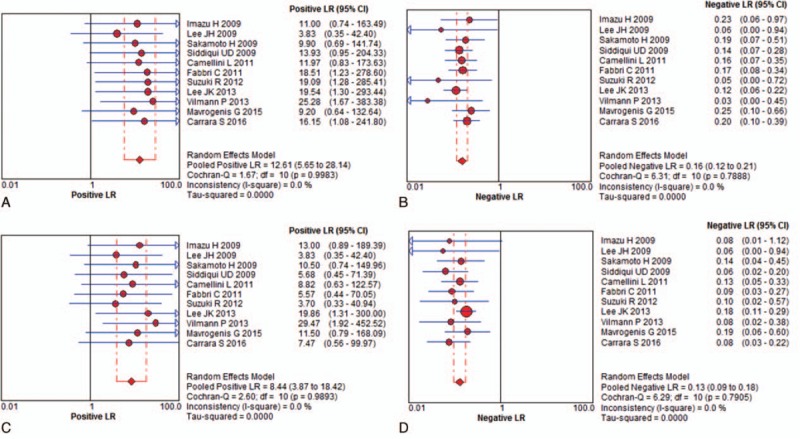
The pooled positive and negative likelihood ratios for solid pancreatic mass fine-needle aspiration guided by endoscopic ultrasonography for: (A) PLR for 22-gauge needle; (B) NLR for 22-gauge; (C) PLR for 25-gauge needles; (D) NLR for 25-gauge. NLR = negative likelihood ratios, PLR = positive likelihood ratios.
Figure 5.
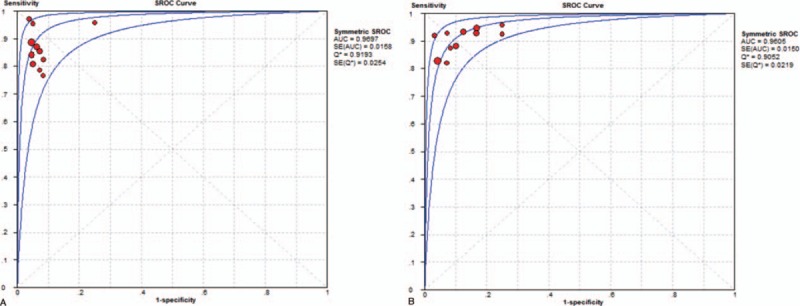
Summary receiver operating characteristic curves summarize the overall diagnostic accuracy for solid pancreatic mass fine-needle aspiration guided by endoscopic ultrasonography: (A) 22-gauge needles and (B) 25-gauge needles.
3. Discussion
Recently, many comparative studies have aimed to clarify which needle size is more suitable for diagnosing solid pancreatic masses, especially in the comparison of 22-G and 25-G needles. Published data on the sensitivity of EUS-FNA for detecting pancreatic masses have varied. Some authors concluded that a larger diameter needle has the advantage of acquiring more tissue than a smaller-diameter needle.[24–26] Indeed, this advantage may have a beneficial diagnostic effect in the use of diagnosis by 22-G needles for histology and immunohistochemistry examinations. However, the score of ease of puncture by the 25-G needle was significantly higher than that of the 22-G needle. A limitation of these studies was the relatively small number of patients with pancreatic lesions that lack the statistical power to make a clear statement regarding the utility of needle size.
A meta-analysis, such as that performed in this study, is a potentially useful tool in this situation because pooling data can create very powerful results compared to the data obtained from smaller individual studies. To our knowledge, our study is the first meta-analysis to include a pooled analysis of prospective controlled trials comparing the 22-G and 25-G needles with regard to operating characteristics and diagnostic yield in EUS-guided sampling of pancreatic masses. Our review provides a comprehensive summation of the current literature describing the sensitivity and specificity of 22-G and 25-G needles in EUS-FNA for the diagnosis of solid pancreatic masses. For this study, attempts were made wherever possible to closely follow the Cochrane Collaboration recommendations. We prespecified a rigorous study protocol and searched several electronic databases, identified international conference abstracts, and searched the study reference lists for relevant trials without language restrictions.
The search yielded 2811 original citations, of which 11 studies with 837 patients were eligible for the review. The 22-G needle size was used for a total of 412 patients and resulted in a pooled sensitivity of 88% (range, 84–91%) and specificity of 100% (95–100%). The 25-G needle size was used for a total of 425 patients and resulted in pooled sensitivity and specificity of 92% (range, 89–95) and 100% (range, 94–100%), respectively.
We also used both PLR and NLR as our measures of diagnostic accuracy since they can be more easily interpreted and applied to clinical practice. The PLR is a measure of how well the diagnosis identified the malignant pancreatic mass, whereas the NLR assesses how well the same diagnosis excludes the disease. Likelihood ratios >10 and <0.1 provide strong evidence to include or exclude a diagnosis, respectively. According to our analysis, the PLR and NLR were 12.61 (5.65–28.14) and 0.16 (0.1–0.21) for the 22-G needle and 8.44 (3.87–18.42), and 0.13 (0.09–0.18) for the 25-G needle, respectively. SROC curves were drawn to see any heterogeneity between studies and an AUC of 1 for any test indicates that the test is excellent. The AUC value was 0.97 for the 22-G and 25-G needles. Compared to the study of 22-G EUS-FNA needles, our study showed that 25-G needles have superior sensitivity for solid pancreatic mass EUS-FNA. The pooled results from this analysis are consistent with the trend from previous studies, which lack the statistical power to make a clear statement regarding selection of the most adequate needles for EUS-FNA. The reason for the observed superior diagnostic accuracy of the 25-G needle in this analysis is uncertain. One possible reason is that the smaller 25-G needle might provide less bloody and contaminated specimens and more easily penetrate a calcified or hard mass. Needle size selection is, in practice, a complex process. For instance, whether the lesion is solid or cystic may influence the endosonographer's decision to use a particular needle size.
The present study has several limitations. First, it had a small sample size. However, we contacted each author to supplement the missing data. Second, only English-language studies were included from this analysis. If the search had been extended to include literature published in other languages, it is possible that additional relevant trials may have been identified. Additionally, any other factor would influence the result of the meta-analysis such as the baseline characteristics of the patients (including age, sex, pathologic stage, lesion location).
4. Conclusion
In conclusion, the present meta-analysis showed that EUS-guided FNA using the 25-G needle system reliably provides overall diagnostic accuracy in patients with solid pancreatic masses. However, it should be interpreted in combination with clinical data and other conventional tests because the negative predictive value of this test was insufficient.
Footnotes
Abbreviations: 22-G = 22-gauge, 25-G = 25-gauge, CI = confidence interval, EUS-FNA= Endoscopic ultrasound-guided fine needle aspiration, HR =hazard ratio, NLR = negative likelihood ratios, PLR = positive likelihood ratios, SROC = summary receiver operating characteristic curve.
Authorship: YZ contributed substantially to conception and design and gave final approval of the version to be published. S-SL and H-YJ contributed to the analysis and interpretation of all data and drafted the article. M-MX and L-LY revised the article critically for important intellectual content.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Cahn M, Chang K, Nguyen P, et al. Impact of endoscopic ultrasound with fine-needle aspiration on the surgical management of pancreatic cancer. Am J Surg 1996;172:470–2. [DOI] [PubMed] [Google Scholar]
- [2].Erickson RA, Garza AA. Impact of endoscopic ultrasound on the management and outcome of pancreatic carcinoma. Am J Gastroenterol 2000;95:2248–54. [DOI] [PubMed] [Google Scholar]
- [3].O’Toole D, Palazzo L, Arotcarena R, et al. Assessment of complications of EUS-guided fine-needle aspiration. Gastrointest Endosc 2001;53:470–4. [DOI] [PubMed] [Google Scholar]
- [4].Kida M. Pancreatic masses. Gastrointest Endosc 2009;69(2 suppl):S102–9. [DOI] [PubMed] [Google Scholar]
- [5].Itoi T, Tsuchiya T, Itokawa F, et al. Histological diagnosis by EUS-guided fine-needle aspiration biopsy in pancreatic solid masses without on-site cytopathologist: a single-center experience. Dig Endosc 2011;23(suppl 1):34–8. [DOI] [PubMed] [Google Scholar]
- [6].Klapman JB, Logrono R, Dye CE, et al. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol 2003;98:1289–94. [DOI] [PubMed] [Google Scholar]
- [7].Yusuf TE, Ho S, Pavey DA, et al. Retrospective analysis of the utility of endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) in pancreatic masses, using a 22-gauge or 25-gauge needle system: a multicenter experience. Endoscopy 2009;41:445–8. [DOI] [PubMed] [Google Scholar]
- [8].Song TJ, Kim JH, Lee SS, et al. The prospective randomized, controlled trial of endoscopic ultrasound-guided fine-needle aspiration using 22G and 19G aspiration needles for solid pancreatic or peripancreatic masses. Am J Gastroenterol 2010;105:1739–45. [DOI] [PubMed] [Google Scholar]
- [9].Iglesias-Garcia J, Poley JW, Larghi A, et al. Feasibility and yield of a new EUS histology needle: results from a multicenter, pooled cohort study. Gastrointest Endosc 2011;73:1189–96. [DOI] [PubMed] [Google Scholar]
- [10].Hucl T, Wee E, Anuradha S, et al. Feasibility and efficiency of a new 22G core needle: a prospective comparison study. Endoscopy 2013;45:792–8. [DOI] [PubMed] [Google Scholar]
- [11].Gerke H. EUS-guided FNA: better samples with smaller needles? Gastrointest Endosc 2009;70:1098–100. [DOI] [PubMed] [Google Scholar]
- [12].Sakamoto H, Kitano M, Komaki T, et al. Prospective comparative study of the EUS guided 25-gauge FNA needle with the 19-gauge Trucut needle and 22-gauge FNA needle in patients with solid pancreatic masses. J Gastroenterol Hepatol 2009;24:384–90. [DOI] [PubMed] [Google Scholar]
- [13].Lee JK, Lee KT, Choi ER, et al. A prospective, randomized trial comparing 25-gauge and 22-gauge needles for endoscopic ultrasound-guided fine needle aspiration of pancreatic masses. Scand J Gastroenterol 2013;48:752–7. [DOI] [PubMed] [Google Scholar]
- [14].Madhoun MF, Wani SB, Rastogi A, et al. The diagnostic accuracy of 22-gauge and 25-gauge needles in endoscopic ultrasound-guided fine needle aspiration of solid pancreatic lesions: a meta-analysis. Endoscopy 2013;45:86–92. [DOI] [PubMed] [Google Scholar]
- [15].Suzuki R, Irisawa A, Bhutani MS, et al. Prospective evaluation of the optimal number of 25-gauge needle passes for endoscopic ultrasound-guided fine-needle aspiration biopsy of solid pancreatic lesions in the absence of an onsite cytopathologist. Dig Endosc 2012;24:452–6. [DOI] [PubMed] [Google Scholar]
- [16].Lee JH, Stewart J, Ross WA, et al. Blinded prospective comparison of the performance of 22-gauge and 25-gauge needles in endoscopic ultrasound-guided fine needle aspiration of the pancreas and peri-pancreatic lesions. Dig Dis Sci 2009;54:2274–81. [DOI] [PubMed] [Google Scholar]
- [17].Camellini L, Carlinfante G, Azzolini F, et al. A randomized clinical trial comparing 22G and 25G needles in endoscopic ultrasound-guided fine-needle aspiration of solid lesions. Endoscopy 2011;43:709–15. [DOI] [PubMed] [Google Scholar]
- [18].Imazu H, Uchiyama Y, Kakutani H, et al. A prospective comparison of EUS-guided FNA using 25-gauge and 22-gauge needles. Gastroenterol Res Pract 2009;2009:546390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vilmann P, Saftoiu A, Hollerbach S, et al. Multicenter randomized controlled trial comparing the performance of 22 gauge versus 25 gauge EUS-FNA needles in solid masses. Scand J Gastroenterol 2013;48:877–83. [DOI] [PubMed] [Google Scholar]
- [20].Siddiqui UD, Rossi F, Rosenthal LS, et al. EUS-guided FNA of solid pancreatic masses: a prospective, randomized trial comparing 22-gauge and 25-gauge needles. Gastrointest Endosc 2009;70:1093–7. [DOI] [PubMed] [Google Scholar]
- [21].Carrara S, Anderloni A, Jovani M, et al. A prospective randomized study comparing 25-G and 22-G needles of a new platform for endoscopic ultrasound-guided fine needle aspiration of solid masses. Dig Liver Dis 2016;48:49–54. [DOI] [PubMed] [Google Scholar]
- [22].Mavrogenis G, Weynand B, Sibille A, et al. 25-gauge histology needle versus 22-gauge cytology needle in endoscopic ultrasonography-guided sampling of pancreatic lesions and lymphadenopathy. Endosc Int Open 2015;3:E63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fabbri C, Polifemo AM, Luigiano C, et al. Endoscopic ultrasound-guided fine needle aspiration with 22- and 25-gauge needles in solid pancreatic masses: a prospective comparative study with randomisation of needle sequence. Dig Liver Dis 2011;43:647–52. [DOI] [PubMed] [Google Scholar]
- [24].Rong L, Kida M, Yamauchi H, et al. Factors affecting the diagnostic accuracy of endoscopic ultrasonography-guided fine-needle aspiration (EUS-FNA) for upper gastrointestinal submucosal or extraluminal solid mass lesions. Dig Endosc 2012;24:358–63. [DOI] [PubMed] [Google Scholar]
- [25].Kida M, Araki M, Miyazawa S, et al. Comparison of diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration with 22- and 25-gauge needles in the same patients. J Interv Gastroenterol 2011;1:102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Berzosa M, Villa N, El-Serag HB, et al. Comparison of endoscopic ultrasound guided 22-gauge core needle with standard 25-gauge fine-needle aspiration for diagnosing solid pancreatic lesions. Endosc Ultrasound 2015;4:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]


