Abstract
We have taken advantage of an enhancer trap event in a line of transgenic mice to identify a unique developmentally regulated endothelial cell locus (Del1). The protein encoded in this locus contains three EGF-like repeats homologous to those in Notch and related proteins, including an EGF-like repeat that contains an RGD motif, and two discoidin I-like domains. Del1 is shown to be a matrix protein and to promote adhesion of endothelial cells through interaction with the αvβ3 integrin receptor. Embryonic endothelial-like yolk sac cells expressing recombinant Del1 protein, or grown on an extracellular matrix containing Del1 protein, are inhibited from forming vascular-like structures. Expression of Del1 protein in the chick chorioallantoic membrane leads to loss of vascular integrity and promotes vessel remodeling. Del1 is thus a new ligand for the αvβ3 integrin receptor and may function to regulate vascular morphogenesis or remodeling in embryonic development.
Keywords: Endothelial, integrin, cloning, angiogenesis, embryogenesis, vasculature
Formation of the vasculature is an essential and fundamental process in mammalian development. The origin and differentiation of endothelial cells is closely linked to hematopoietic development in the yolk sac, and to development of the heart and outflow tract in the embryo proper (Gilbert 1994). The yolk sac vasculature and the embryonic vasculature must develop and function as a cardiovascular system by embryonic day 9 for the embryo to survive. Understanding of this process requires elucidation of the mechanisms that regulate the origin and differentiation of the endothelial cell lineage, the morphogenetic processes by which the vascular network is established, and the structured remodeling of the primitive vasculature to create the definitive vascular pattern.
Classical embryological studies in avians complemented by recent genetic studies in mice have provided significant insights into the mechanisms of embryonic blood vessel formation. Chick-quail chimera experiments have revealed that some blood vessel growth depends on angiogenesis, that is, budding and sprouting from existing vessels (Peault et al. 1983; Noden 1989; Pardanaud et al. 1989; Coffin and Poole 1991). A second process, vasculogenesis, depends on the incorporation of migratory individual endothelial precursor cells (angioblasts) into the developing blood vessel (Peault et al. 1983; Noden 1989; Pardanaud et al. 1989; Coffin and Poole 1991). Both of these processes are regulated at the molecular level by signaling through two receptor tyrosine kinase pathways. One pathway includes the angiopoietin ligands and the tie1 and tek/tie2 receptors, whereas the other includes the various forms of vascular endothelial growth factor and their receptors (Matthews et al. 1991; Dumont et al. 1992; Partanen et al. 1992; Millauer et al. 1993; Sato et al. 1993; Davis et al. 1996; Maisonpierre et al. 1997). Gene targeting experiments have indicated that these receptor tyrosine kinases have nonoverlapping essential roles in various aspects of vascular development (Dumont et al. 1994; Fong et al. 1995; Sato et al. 1995; Shalaby et al. 1995).
The signals received by endothelial cells from soluble ligands must be supported by appropriate signals from integrin receptors. The integrins are a family of transmembrane adhesion molecules that bind primarily to extracellular matrix proteins and have important roles in cell adhesion and migration (Hynes 1992). It is clear that integrins expressed by endothelial cells play critical roles in vascular formation in the embryo, and wound healing and tumorigenesis in the adult (Brooks et al. 1994a,b; Drake et al. 1992, 1995). Arg–Gly–Asp (RGD) is a common integrin recognition sequence found in extracellular matrix proteins like fibronectin and vitronectin, and recombinant peptides containing RGD have been shown to influence endothelial cell behavior (Hynes 1992; Brooks et al. 1994a,b).
While receiving less attention, the mechanisms by which the embryo limits vascular formation and remodels early vascular networks are of great importance. Vascular formation is an invasive and widespread process in the embryo and thus must be limited by specific molecular pathways. Although vascular inhibitory signals clearly have been linked to certain cell types during development, the molecular nature of these signals has not been elucidated fully. For instance, hypertrophic chondrocytes inhibit vascularization in regions of endochondral bone formation (Hallmann et al. 1987). Recently, a proteolytic cleavage product of plasminogen has been identified as an inhibitor of tumor-induced angiogenesis, providing experimental support for such mechanisms (O’Reilly et al. 1996). The ability of the embryo to remodel early vascular patterns also implies an intricate control of vascular development. The vasculature of many organs is formed initially as a mesh-like structure, with the final pattern resulting from attrition of some vessels and growth of others (Noden 1989; Coffin and Poole 1991).
To search for new molecular pathways of vascular development we have investigated Del1 (developmentally regulated endothelial cell locus), which was identified through an enhancer trap event in a transgenic mouse. A gene encoded in this locus has been cloned and studied by RNA blot, in situ hybridization, protein blot, and immunohistochemistry experiments. These data confirm reporter transgene analysis indicating that Del1 is an early endothelial cell marker and suggest that Del1 is deposited in the extracellular matrix. Del1 has an RGD motif and can mediate attachment of endothelial cells through integrin binding. In vivo and in vitro functional studies suggest a role for this novel factor in the complex process of vascular remodeling.
Results
Transgenic mice containing the SPARC–lacZ transgene were initially evaluated for cell-specific and developmental-specific expression of the transgene by X-gal staining of embryos at 9 days postcoitum (dpc). One line of mice exhibited an expression pattern distinct from that of the native SPARC gene and also different from that seen with the other transgenic lines (Holland et al. 1987). This line of mice, which expressed the reporter transgene in an endothelial cell-restricted manner, was employed in these studies.
Cell-specific and developmental-specific expression of the locus
Expression of the reporter transgene was first detected at 7.5 dpc in cells of the extraembryonic mesoderm that give rise to the endothelial and hematopoietic elements of the yolk sac (Fig. 1A). By 8.5 dpc, with formation of the blood islands, expression is not seen in the mature endothelial cells that line these structures but, rather, in a small number of round hematopoietic-appearing cells that occur in clusters within the blood island (Fig. 1B). Expression within the embryo at 8.5 dpc is found in the endothelial cells of the paired dorsal aortae and endocardial precursors migrating into the heart-forming region above the anterior intestinal portal (Fig. 1C). At this stage, all endothelial cells and their immediate precursors appear to express the transgene. By 9.0 dpc, expression of the reporter transgene is seen in endothelial cells associated with all large vasculature (Fig. 1D). High-level expression is seen in endothelial cells in the outflow prior and subsequent to epithelial–mesenchymal transformation (Fig 1E).
Figure 1.
Cell- and developmental-specific expression of murine Del1 as assessed by transcription of the β-galactosidase reporter transgene. (A) X-gal staining of whole mount murine embryo at 7.5 dpc, (original magnification, 70×). Short arrows indicate X-gal staining in the extraembryonic mesoderm. (B) Section of 8.5-dpc yolk sac stained with X-gal as a whole mount, embedded, sectioned, and counterstained with nuclear fast red (bright-field photograph at 400×). Endothelial cells lining the blood islands do not stain (short arrows), whereas rare clusters of cells within the blood island do stain. (C) Whole mount X-gal-stained embryo at 8.5 dpc, (70×). X-gal staining is present in endothelial cells associated with all blood vessels and the endocardium. (D) Section of 9.5-dpc embryo; X-gal staining is seen in endothelial cells forming endocardium, proximal aorta, and other developing vessels. (E) Section of outflow tract of X-gal-stained embryo at 9.5 dpc, (400×). Expression of the transgene persists after epithelial mesenchymal transformation. (F) Whole mount 13.5-dpc embryo, with prominent staining of lung (lu) and regions of endochondral bone formation (bn). Expression in the endocardium of the ventricle (v) and atrium (a) and the endothelium of the aorta (ao) is diminished but visible. Some organs, such as the liver (li), show no staining. (G) Section of outflow tract valve from X-gal-stained embryo, 13.5 dpc (200×). (H) Limb bud of whole mount embryo at 13.5 dpc, showing staining of endothelial progenitors forming marginal vessels (short arrows). Also, staining is seen in hypertrophic chondrocytes of limb bones and in endothelial cells of an umbilical vessel (ribbon-like structure). (I) Section of vertebral bodies from X-gal-stained embryo, 13.5 dpc (200×). Staining is visualized in hypertrophic chondrocytes. (J) Section of lung from X-gal-stained embryo, 13.5 dpc (200×). (K) Section of eye from X-gal-stained embryo, 15.5 dpc (200×).
Del1 transcription in large vessels and the endocardium progressively declines after 9.5 dpc and becomes prominent in the microvasculature of the lung, gut, neural tube, and kidney (Fig. 1F,J; and data not shown). Expression continues to be prominent in cells of the outflow tract and the endocardial cushions. At 13.5 dpc in the outflow tract, Del1 expression in mesenchymal cells that originated from the endothelium continues, even after the valves have been primarily formed (Fig. 1G). Also, by 13.5 dpc, expression is apparent in a restricted group of nonendothelial cells. These include hypertrophic chondrocytes, retinal neurons, and other cell types synthesizing the secondary vitreous in the developing posterior chamber of the eye (Fig. 1I,K; data not shown). After ∼15.5 days of development, transcription of the reporter transgene diminishes in these sites and is completely gone by the time of birth (data not shown).
Genomic and cDNA cloning
A genomic library was constructed in phage λ and used to clone both regions of sequence flanking the integrated transgene complex. This DNA was subsequently employed to clone ∼50 kb of the native murine locus from a wild-type 129/SvJ phage λ library. Mapping these phage λ clones indicated that ∼8 kb of genomic sequence had been deleted at the time of transgene integration. Subsequently, genomic fragments were employed in exon trapping, and a single exon identified ∼10 kb from the integration site. This exon was employed for cDNA cloning from murine embryonic and human embryonic lung libraries.
The transcript represented in most cDNA clones—the “major” transcript—encodes a 480-amino-acid protein in mouse and human (Fig. 2A). The amino acid sequence is highly conserved between mouse and human, with ∼95% identity of the primary sequence. The major transcript encodes a protein that contains a signal peptide, three epidermal growth factor- (EGF)-like repeats, and two discoidin I-like domains (Fig. 2A). A less frequently represented “minor” transcript is composed of a signal peptide, three EGF repeats, and a portion of the amino-terminal discoidin I-like domain. Additional complexity is added by the variable inclusion or exclusion of 10 amino acids in the spacer region between EGF repeat 1 and EGF repeat 2 (Fig. 2A).
Figure 2.

Deduced amino acid sequence of Del1 and expression pattern of the Del1 gene. (A) The major form is composed of a signal peptide, three EGF-like repeats, and two discoidin I-like domains. The signal peptide cleavage site, as predicted by the work of von Heijne (1985) is shown by the vertical arrow at amino acid 23. The minor Del1 transcript encodes a signal peptide, three EGF repeats, the amino portion of the first discoidin I-like domain, and the unique four amino acids VTVG at the carboxyl terminus. The amino acid sequence of this form is indicated by the arrow showing the region of identity with the major form. The 10-amino-acid boxed sequence between EGF repeats 1 and 2 was not present in some cDNA clones. An RGD recognition sequence in EGF repeat 2 is boxed. (B) Homology of Del1 EGF repeats to those found in other proteins. Notch-1 is the murine sequence for this homeotic receptor protein; Crumbs and Delta are the Drosophila sequences for two ligands of Notch; Fibrop(ellin) is a developmental sea urchin protein; Pref-1 is an adipocyte differentiation factor; and Tie(-1) is an orphan tyrosine kinase receptor expressed by endothelial cells (Hursh et al. 1987; Tepass et al. 1990; Rebay et al. 1991; Partanen et al. 1992; del Amo et al. 1993; Sato et al. 1993; Smas and Sul 1993). (C) RNA blot analysis of Del1 expression in organs derived from a 15.5-dpc mouse embryo or an adult mouse. (D) RNA blot analysis of Del1 expression in tumor lines and cultured cells. (E15.5), Whole embryo at 15.5 dpc; (P19 and F9) embryonal cell carcinoma lines; (NIH-3T3 and Ltk) embryonic fibroblast lines; (YS cells) adapted into culture from the embryonic yolk sac at 8.0 dpc; (HUVEC) human umbilical vein endothelial cell; (BAEC) bovine aortic endothelial cell; (EOMA) mouse endothelioma line; (MEL) mouse erythroleukemia line; (Molt4) human T-cell malignancy; (K562) human erythroleukemia; (HeLa) human epithelial cell tumor.
The EGF repeats of Del1 are homologous to molecules such as Notch and its ligands Crumbs and Delta (Tepass et al. 1990; Rebay et al. 1991; del Amo et al. 1993; Artavanis-Tsakonas et al. 1995). An extended region of homology in the carboxyl terminus of the second EGF repeat, the intervening spacer, and the third EGF repeat is shown in Figure 2B. There is also considerable homology in this region to the developmental sea urchin protein fibropellin, a factor shown to function in lineage commitment of the adipocyte (Pref-1), and an endothelial cell-specific receptor tyrosine kinase known to be essential for embryonic blood vessel development (Tie) (Hursh et al. 1987; Partanen et al. 1992; Sato et al. 1993; Smas and Sul 1993).
In its discoidin I-like domains, Del1 is homologous to the mammary epithelial cell marker milk fat globule membrane protein, coagulation factors V and VIII, the extracellular domain of a group of tumor-associated orphan receptor tyrosine kinases, and the archetypal domain of discoidin I (Poole et al. 1981; Toole et al. 1984; Jenny et al. 1987; Stubbs et al. 1990; Alves et al. 1995).
RNA and protein analysis of Del1 gene expression
To verify that the cloned gene is expressed in the unique cell-specific and developmental-specific pattern indicated by the reporter transgene, mRNA blot and in situ hybridization experiments were conducted. An EGF repeat probe detected an appropriate-length 6.5 kb band with RNA samples from highly vascular organs of a 15.5-dpc embryo, whereas no hybridization was detected to RNA samples from an adult animal (Fig. 2C). Del1 expression was detected with RNAs derived from human umbilical vein endothelial cells (HUVECs) and a mouse endothelioma cell line (EOMA) known to express markers consistent with an early developmental phenotype (Fig. 2D) (Obeso et al. 1990). No signal was seen in the cultured endothelial cells derived from adult bovine aortic endothelial cells (BAECs). Interestingly, a murine erythroleukemia cell line, MEL, and an embryonal carcinoma cell line, P19, appeared to have multiple transcripts. Whether these bands represent transcripts from homologous genes or the expression of alternative Del1 transcripts is unclear. NIH-3T3 embryonic fibroblasts also showed low level expression of Del1.
In situ hybridization documented Del1 expression in the endocardium of the developing heart at 9.5 dpc (Fig. 3A,B), the transformed mesenchymal-like endothelial cells forming the valves of the outflow tract at 13.5 dpc (Fig. 3C,D), in endothelial cells of a renal artery (Fig. 3E,F), and in hypertrophic chondrocytes at 13.5 dpc (Fig. 3G,H). The only potential disparity between X-gal staining and in situ hybridization is in the ventral neural tube, where a strong in situ signal is observed in later development (data not shown). Whether this represents expression by neural tissue or the forming vasculature has not been investigated.
Figure 3.

In situ hybridization analysis of Del1 expression. Bright-field (A,C,E,G) and dark-field (B,D,F,H) images reveal histology and cell-specific pattern of expression of the Del1 gene. (A,B) Hybridization to endocardial cells of the folding heart tube at 9.5 dpc. Arrows indicate the endocardial cell layer, which is separated from the myocardial cells by cardiac jelly. Background staining in the myocardial cell layer represents endothelial cells in forming trabeculae (original magnification, 200×). (C,D) Del1 expression in transformed mesenchymal-like endothelial cells forming the valves of the outflow tract at 13.5 dpc. Asterisks indicate forming valve leaflets (bar, 100 μm). (E,F) Hybridization to endothelial cells of a renal artery in a 15.5-dpc embryo. Asterisk indicates vessel lumen (400×). (G,H) Hybridization to hypertrophic chondrocytes at 13.5 dpc (200×).
To provide for biochemical and in vitro functional studies and to evaluate the specificity of polyclonal antisera, stably transfected cells were produced. An expression vector encoding the major form of Del1 or the empty expression vector was transfected into the YS-B embryonic yolk sac cell line. This cell line was chosen for these studies because it has characteristics of embryonic endothelial cells, does not express Del1, is clonal, and is long lived in culture (Figs. 2D and 4B; data not shown) (Corn et al. 1991; Wei et al. 1995). Populations of transfected cells were employed for Western blotting experiments (Fig. 4A), and individual colonies expressing varying levels of Del1 protein were identified and expanded for in vitro functional studies (Fig. 4B). Clonal cell lines transfected with the empty expression plasmid were selected to serve as negative controls.
Figure 4.
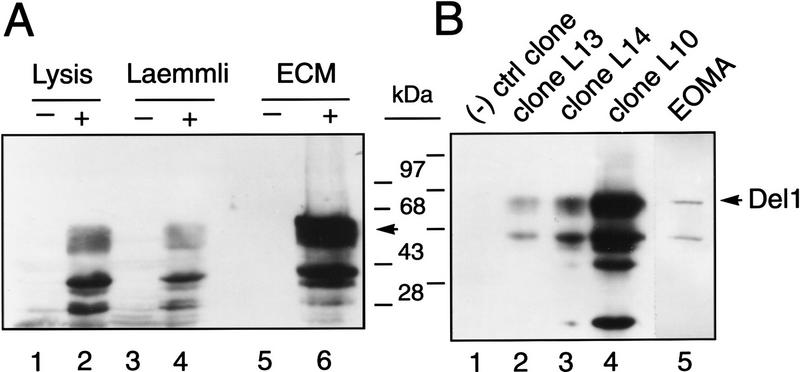
Immunoblotting and immunohistochemistry employing polyclonal Del1 antisera. (A) Yolk sac YS-B cells stably transfected with a eukaryotic expression vector encoding the murine major form of Del1 (+) or an empty expression vector (−) were selected and evaluated as pools for expression of Del1 protein. Protein was isolated from cells lysed in cell lysis buffer (Lysis), standard Laemmli gel loading buffer, or the extracellular matrix remaining after transfected cells were removed from the culture dish (ECM). The dominant band corresponds to a molecular mass of 52 kD, the predicted size for the major form of Del1. Lower molecular mass bands most likely represent protein degradation, although the use of alternative translation initiation sites is also possible. (B) YS-B cells were stably transfected with the Del1 expression construct or the empty expression plasmid and selected as individual clones. Clone L10 shows the highest level of Del1 protein, clones L13 and L14 have an intermediate amount of protein, and a negative control clone does not express Del1. Extracellular matrix deposited by a mouse endothelioma cell line (EOMA) also contains Del1 protein of the appropriate size. (C) Staining in and around endothelial cells of an artery in a 15.5-dpc embryo. Naphthol red was employed as an indicator, with alkaline phosphatase labeled secondary antibody, and specific antibody staining is thus red (original magnification, 630×). (D) Staining in the vascular wall of the aorta, and around cardiomyocytes of the atrium. Staining is seen beneath some of the endothelial cells, between the endothelial layer and the smooth muscle cells. Arrows indicate the luminal surface of the endothelial cells. Staining in association with the atrial cardiomyocytes could be detecting protein in the extracellular matrix, on the cell surface, or both. (630×). (E) The heart at 14.5 dpc, with staining appearing in a subendocardial pattern (40×). (F) High power view of the same section as in E. Most intense staining is seen in the endocardium and subendocardium, with a gradient extending from the endocardium to the epicardium (400×). (G) Staining in the extracellular matrix of cartilage in the developing vertebral bodies (100×). (H) Regions of endochondral bone formation at different stages of development. Staining is present around condensing mesodermal cells in early cartilage formation and persists in the matrix throughout the lifespan of the hypertrophic chondrocyte (400×). (I) Staining in the lung at 13.5 dpc (200×). (J) Negative control experiment, employing pre-immune serum. This slide was not counterstained.
Western blotting was performed with cell lysates, cell culture supernatant, and extracellular matrix. With Del1-transfected cells, a 52-kD protein was detected in cell lysates obtained by harvesting the cells in a lysis buffer (Lysis) or standard gel loading (Laemmli) buffer, and in extracellular matrix (ECM) (Fig. 4A). This is the predicted molecular mass for Del1, based on the deduced amino acid sequence, and corresponds to a signal at the identical molecular mass with protein samples from the EOMA line (Fig. 4B). Methodology for harvesting matrix proteins was verified by using a commercially available antibody to detect fibronectin in these samples (data not shown). No signal was obtained with culture supernatant harvested from transfected cells or EOMA cells, even when concentrated 100-fold (data not shown). These data suggest that Del1 is secreted across the abluminal surface of the endothelial cell and deposited in the extracellular matrix.
Immunohistochemistry experiments were conducted on sections of 13.5-dpc embryos with the polyclonal antisera. Some vessels at this stage show intense staining of the entire endothelial cell layer (Fig. 4C). In other vessels, such as the proximal aorta, there is patchy staining with only some of the endothelial cells labeling (Fig. 4D). This patchy pattern parallels the results obtained with X-gal staining (Fig. 1F). In these regions, there is no staining over the cell, but it appears that Del1 protein is localized beneath the endothelium, in a pattern consistent with abluminal secretion. In the atrium, which is only a few cell layers thick at this stage, Del1 is detected on the cardiac cells or in the matrix surrounding these cells. In the ventricle, in addition to the endocardium, Del1 is localized in the subendocardium, either in association with the cardiac cells or the matrix of the subendocardium (Fig. 4E,F). In regions of endochondral bone formation, Del1 expression is detected with early cellular condensation and continues to be expressed throughout the life of the hypertrophic chondrocyte in the surrounding matrix (Fig. 4G,H). Staining in the lung is not in a vascular pattern but is found diffusely throughout the mesenchyme (Fig. 4I). The cellular expression pattern in these tissues is identical to that observed with X-gal staining and in situ hybridization, although the staining is highly suggestive that Del1 is secreted into the extracellular matrix. Specificity of immunohistochemistry was verified by competition experiments with recombinant protein and staining with preimmune serum (Fig. 4J).
Endothelial cell-binding studies
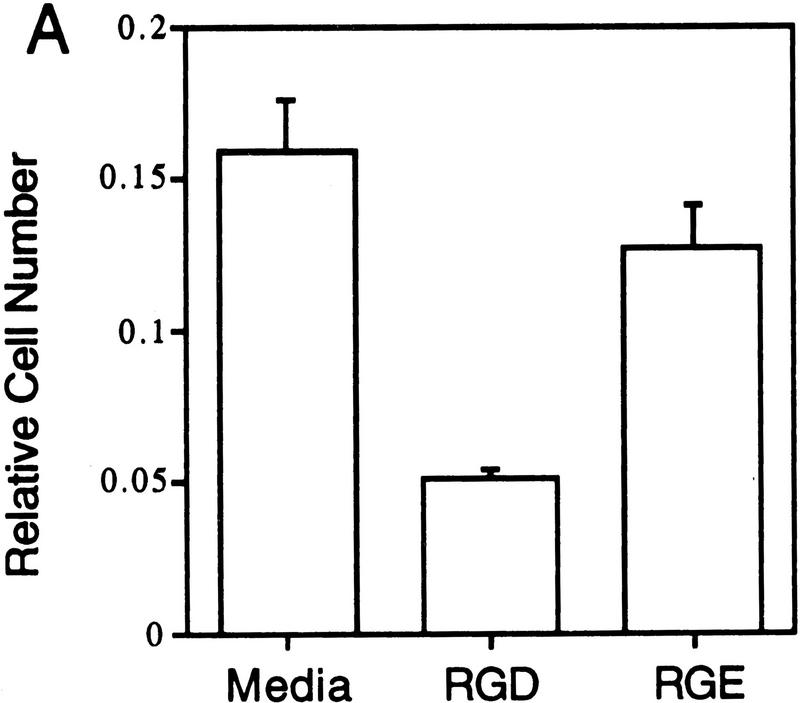
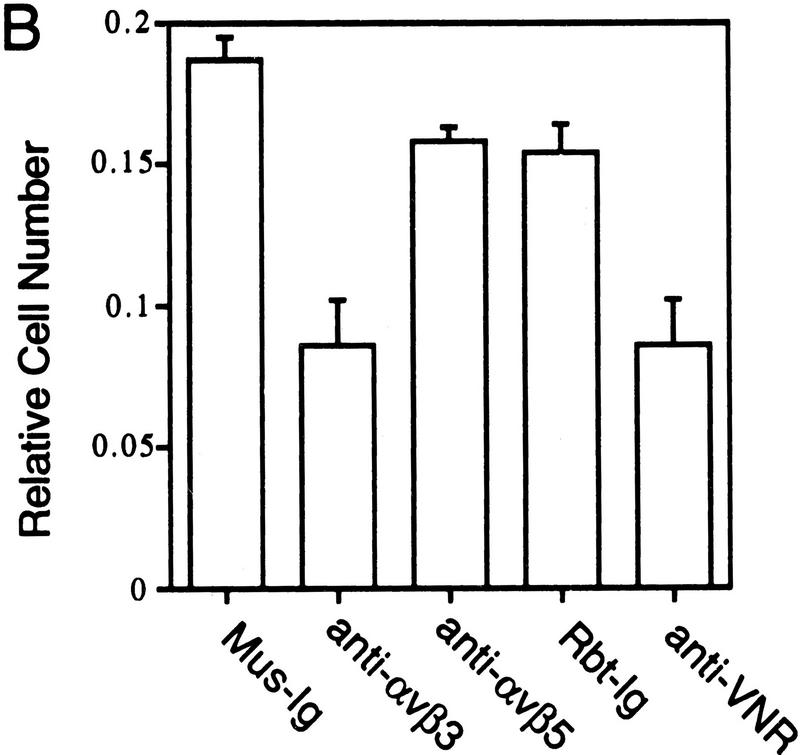
In these experiments the ability of Del1 to mediate the adhesion of HUVEC was evaluated, and various antibody and peptide antagonists employed to link this function to the αvβ3 cellular integrin receptor. When bacterial recombinant Del1 protein was adhered to dishes, a significant portion of HUVECs remained bound to the Del1-coated wells (Fig. 5A). RGD peptides reduced cell attachment to less than one-third, whereas negative control RGE peptides had no effect. A well-characterized blocking monoclonal antibody raised against the human αvβ3 receptor and a blocking polyclonal rabbit antiserum raised against the αvβ3 (vitronectin) receptor were able to inhibit more than half of the binding to the recombinant Del1 protein (Fig. 5B) (Brooks et al. 1994b). Control murine and rabbit immunoglobulin, as well as a blocking monoclonal antibody to the αvβ5 receptor did not inhibit HUVEC binding to the Del1 protein.
Figure 5.
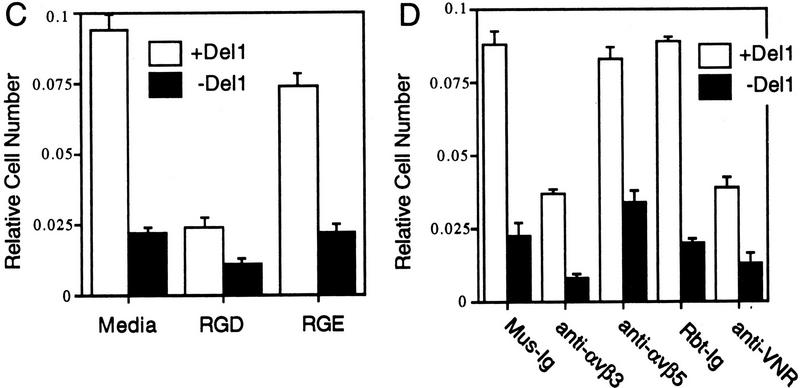
Del1 mediates adhesion of endothelial cells through specific binding to the αvβ3 integrin receptor. (A) HUVECs were allowed to bind to recombinant Del1 protein or to BSA. Binding was decreased over threefold by the addition of RGD-containing peptides; the negative control RGE peptides at the same concentration did not affect binding. (B) HUVEC binding to recombinant Del1 was inhibited by a monoclonal antibody specific for αvβ3, and by polyclonal rabbit antisera against αvβ3 (anti-VNR), but not by a monoclonal antibody to the αvβ5 receptor or control mouse (Mus-Ig) or rabbit immunoglobulins (Rbt-Ig). (C) HUVEC binding to extracellular matrix produced by yolk sac cells transfected with an expression vector containing a Del1 cDNA or an empty expression vector. Binding to the matrix produced by Del1-expressing cells is approximately fourfold greater and can be specifically competed by peptides encoding an RGD motif. (D) HUVEC binding to extracellular matrix produced by Del1-expressing cells is inhibited by a monoclonal antibody specific for αvβ3, and by polyclonal rabbit antisera against αvβ3 (anti-VNR), but not by a monoclonal antibody to the αvβ5 receptor or control mouse (Mus-Ig) or rabbit immunoglobulins (Rbt-Ig).
Similar experiments investigated the binding of HUVECs to extracellular matrix with or without Del1. For these experiments, YS-B yolk sac cells transfected with a Del1 expression vector, or yolk sac cells transfected with an empty expression vector, were employed to generate the Del1 and control matrices, respectively (Fig. 4B). Binding to the matrix containing Del1 was approximately fourfold greater than binding to the matrix without Del1 (Fig. 5C). Also, binding was decreased by fourfold when peptides containing the RGD motif were included in the binding reaction. The αvβ3 receptor antibodies inhibited binding to the Del1-containing matrix to less than half, whereas the αvβ5 antibody and the control immunoglobulins had no effect on binding (Fig. 5D). Taken together, these data suggest that Del1 in the extracellular matrix can mediate endothelial cell adhesion and that it does so at least in part through the cellular αvβ3 integrin receptor.
In vitro functional studies
To test the hypothesis that Del1 may function to regulate morphogenetic processes associated with the formation of vascular structures, experiments were conducted in vitro with transfected yolk sac cell lines expressing the recombinant major form of Del1 (Figs. 4B and 6A). The parental YS-B cells express a number of endothelial cell markers and form vascular-like structures when allowed to accumulate to high density or when plated on the membrane-like material Matrigel (Fig. 6B) (Corn et al. 1991; Wei et al. 1995; data not shown). YS-B cells transfected with the cDNA encoding the major form of Del1 were selected for varying levels of Del1 protein production (Fig. 4B). Cell lines transfected with the empty expression plasmid were selected to serve as negative controls.
Figure 6.
In vitro functional assays employing Del1 transfected yolk sac cells. (A) The parental yolk sac cell line YS-B under routine culture conditions. Phase contrast (original magnification, 100×). (B) YS-B cells after 24 hr on Matrigel, showing a pattern of vascular-like morphogenesis. Cells were stained with toluidine blue. Bright-field (40×). (C) Negative control transfectants form a vascular-like network on Matrigel after 24 hr. Light areas represent organized cells; photographed under dark-field illumination at 50×. (D) Transfectant clone L13, after 24 hr on Matrigel, shows some evidence of network formation, although less than YS-B cells not expressing Del1. (E) Transfectant clone L10, after 24 hr on Matrigel, reveals no evidence of network formation; cells instead produce numerous aggregates. Dark-field illumination (50×). (F) Native yolk sac YS-B cells grown on a matrix produced by negative control transfectants make a complex structural network. Light areas represent organized cells; photographed under dark-field illumination at 30×. (G) Native YS-B cells grown on a matrix produced by Del1 transfectants. Cells are forming a dense monolayer, with no evidence of organization. Photographed under dark-field illumination at 30×. (H) Aggregates of negative control transfected yolk sac cells were placed onto polymerized Matrigel. After 24 hr, cells showed sprouting angiogenesis. Photographed under phase contrast at 100×. (I) Aggregates of Del1-transfected yolk sac clone L10 were placed onto polymerized Matrigel as in H. Photographed after 24 hr (100×); these cells show no evidence of sprouting.
In the first experiments, the Del1-transfected yolk sac clones and yolk sac lines transfected with an empty expression plasmid were compared for their ability to form branching vascular-like structures on Matrigel. After 24 hr on Matrigel, the negative control transfectants had established an intricate network typical for these cells (Fig. 6C). Cells secreting high levels of Del1 protein, clone L10, showed a markedly different pattern, assembling into multiple well-spaced clusters (Fig. 6E). This abrogation of morphogenesis was directly related to the level of Del1 expression, as clones expressing low and moderate amounts of Del1, clones L13 and L14 (Fig. 4B), showed some degree of branching morphology (Fig. 6D; data not shown).
Next, we wanted to evaluate the ability of recombinant Del1 protein to regulate in vitro morphogenesis of the native YS-B yolk sac cell line. Because Del1 protein is deposited in the extracellular matrix, we employed the Del1 expressing clone L10 to generate a cell culture matrix containing Del1. Matrix generated by negative control clones should differ only by the absence of Del1. Transfected and control lines were cultured for 7 days and then removed from the culture dish by extensive washing with PBS plus 1 mm EDTA. Nontransfected yolk sac cells grown on the matrix produced by negative control transfectants assembled into a lace-like network (Fig. 6F). Nontransfected yolk sac cells grown on matrix containing Del1 revealed no evidence of morphogenesis; instead they formed a dense monolayer (Fig. 6G).
Finally, an in vitro angiogenesis sprouting assay was employed with the transfected yolk sac lines. This assay has been employed by a number of groups to evaluate angiogenic potential (Pepper et al. 1991). Transfected cells were allowed to stand overnight in a conical tube to allow them to aggregate, and the cell mass was then placed on Matrigel. The ability of the Del1-expressing cells to migrate onto the Matrigel and assemble into branching structures was compared to control cells. Within 24 hr the control cells formed a series of branching projections, whereas the cells expressing Del1 remained virtually confined to the cellular aggregate (Fig. 6H,I).
In vivo angiogenesis assays
To determine whether Del1 gene expression can alter in vivo angiogenesis, the chick chorioallantoic membrane (CAM) assay was employed. Embryonic yolk sac cells and 143B osteosarcoma cells stably transfected with a Del1 major expression construct were grown on coverslips that were inverted and placed on the CAM at 9 days of development. At 13 days, vascular development in the CAM under these cells was compared to that under cells transfected with a negative control construct. Del1 expression by both cell types correlated with dramatic changes in the vascular pattern of the CAM. There was an overall loss of the normal vasculature, with only the largest vessels remaining intact (Fig. 7). The regular branching pattern characteristic of normal vessel formation in the CAM was thus lost. There were two additional features characteristic of vessel development in the CAM exposed to Del1 protein. First, areas of pooled red blood cells could be seen outside of the vasculature in the mesodermal layer (Fig. 7B, arrowheads). These free red blood cells in the CAM most likely resulted from the breakdown of existing vessels. Second, the native vasculature was replaced with a disorganized array of capillary-size vessels extending under the majority of the area of the coverslip (Fig. 7, B, arrows, and D). The number of these capillary-like structures was much greater than the normal number of capillaries in the CAM, and the regular pattern characteristic of the CAM capillary structure was missing.
Figure 7.
In vivo angiogenesis assays. (A) CAM below YS-B cells transfected with a negative control construct (original magnification, 30×). Embryos were harvested, placed into dishes, and coverslips containing cultured cells were inverted on the CAM. (B) Chick CAM below YS-B cells expressing Del1. Arrowheads indicate regions of pooled red cells outside of the vasculature; arrows indicate a plexus of capillary-like vessels (30×). (C) Chick CAM below 143B osteosarcoma cells transfected with a negative control construct (75×). (D) Chick CAM below 143B cells expressing Del1. A dense array of capillary-like vessels is visible (75×).
Discussion
In the experiments reported here we have employed an enhancer trap event in a transgenic mouse to identify and characterize an embryonic endothelial cell protein. Expression of the reporter transgene in this particular line of mice was distinct from the other transgenic lines, suggesting that expression of the transgene was under control of potent enhancer elements flanking the site of insertion, and thus reflecting the transcriptional pattern of the gene encoded in this locus. Such enhancer trap events have been widely employed in a directed fashion in model systems such as Drosophila to identify genes that regulate developmental processes (Okane and Gehring 1987). This approach has been proposed as a methodology for the study of developmentally regulated murine genes, and reporter transgene integration has previously allowed the identification and cloning of new murine genes (Allen et al. 1988; Soinen et al. 1992).
The expression pattern of Del1 is unique and provides clues regarding its potential functional role in vascular development. Del1 is expressed initially in the endothelial progenitor cells of the extraembryonic mesoderm at ∼7 dpc, and in isolated mesodermal cells of the embryo that appear to be the mammalian equivalent of the angioblasts characterized in avians (Peault et al. 1983; Noden 1989; Pardanaud et al. 1989; Coffin and Poole 1991). Del1 is thus one of the earliest markers of the endothelial cell lineage, appearing simultaneously with flk-1 and CD31 (Millauer et al. 1993; Baldwin et al. 1994). However, a striking feature of Del1 expression is that it begins to decline after the endothelial cell contributes to vascular formation and disappears completely by birth. Early transient expression in endothelial cells suggests that Del1 has a regulatory role in endothelial cell differentiation or the process of vascular morphogenesis.
Del1 expression is identified in an interesting group of nonendothelial cells. Expression is seen in areas of endochondral bone formation shortly after condensation of the mesenchyme. By 13.5 dpc, hypertrophic chondrocytes express high levels of Del1, and this expression persists late into embryogenesis. Del1 in areas of cartilage formation could serve a supporting function for bone formation, with no link to its role in the vasculature. However, a more attractive hypothesis is that Del1 expression by the hypertrophic chondrocytes reflects a mechanism by which these cells regulate vascularization of bone-forming regions. Early in bone development, vascularization of the cartilage is actively inhibited (Hallmann et al. 1987). Overexpression of vascular endothelial cell growth factor (VEGF) in the embryo can induce vascularization of all organs, but inhibitory factors produced by the cartilage forming cells prevent blood vessel growth into the cartilage (Flamme et al. 1995). Although there is evidence that TGF-β may contribute to this activity, it is clear that other factors are involved (Pepper et al. 1991). After the basic bony pattern is established, hypertrophic chondrocytes produce factors that are stimulatory for endothelial cell growth and attract blood vessels from the developing periosteum. Del1 may have a role in this complex regulation of vascular growth. Also, in the eye, Del1 expression is noted in cells that are well known to produce factors that regulate vascular formation. The early retinal and mesenchymal cells synthesize the early vitreous, which is inhibitory to vascular formation. The lens is never vascularized throughout life, despite a growth factor-rich environment (Tripathi et al. 1991). The vitreous, lens, and cornea are avascular in the adult because aqueous humor that is produced by the ciliary body and bathes these tissues is inhibitory to angiogenesis (Caprioli 1992). Del1 is expressed in these eye tissues where angiogenesis is inhibited (Fig. 1K; data not shown).
As a first approach to investigating the function of Del1, we have employed an in vitro model of yolk sac development and an in vivo model of angiogenesis. In embryogenesis the yolk sac is a site of rapid morphogenesis, with unparalleled vascular development (Gilbert 1994). Del1 is expressed in endothelial precursors in the extraembryonic mesoderm prior to vascularization, but expression disappears as these cells form the vasculature. The yolk sac cells employed in these experiments correlate with this later stage of development. They spontaneously form vascular-like structures in vitro, and they do not express Del1. The gain-of-function experiments conducted here with stably transfected clones of yolk sac cells are strongly suggestive that the presence of Del1 inhibits the initiation of vessel formation in the yolk sac and that Del1 may serve a similar function in vivo in the extraembryonic mesoderm. The chick chorioallantoic membrane has been widely employed to identify and study agents that promote and inhibit angiogenesis (Wilting et al. 1991). Results of CAM assays reported here suggest that Del1 is capable of mediating the breakdown of existing vascular beds and directly or indirectly inducing restructuring of the CAM vasculature.
Given the functional actions of Del1 in these assays and the unique pattern of embryonic expression, it is most likely that this molecule is involved in the poorly understood process of vascular remodeling. During embryonic development, the vasculature must be broken down and reformed continuously to accommodate the changes in vascular patterning and the need to match the increasing size of the embryo. Vascular development in the embryo must thus be a balance between positive- and negative-acting forces. Del1 may be one of the factors that contributes to the breakdown of the remodeling vasculature. This type of action is critical for remodeling in early vessel formation and might—in combination with other factors—account for the absence of blood vessels in cartilage and other nonvascular areas.
Del1 has several potential functional domains, including an RGD motif in EGF-like repeat 2. By inference from the known solution structure of other EGF-like repeats, the RGD of Del1 would be positioned at the tip of loop B of EGF repeat 2, an advantageous configuration for interaction with integrin receptors (Appella et al. 1988; Rao et al. 1995). Binding studies employing HUVECs suggest that the RGD motif mediates binding to endothelial cells and that Del1 in the matrix is available for this interaction. The ability of Del1 to bind the αvβ3 integrin receptor suggests that Del1 may be involved in the angiogenesis-related functions that have been assigned to this receptor. When endothelial cells are stimulated to divide by angiogenic cytokines, signaling via the αvβ3 integrin receptor appears to promote entry into the cell cycle rather than programmed cell death (Brooks et al. 1994a,b). In addition to supporting cell division, this integrin receptor promotes tissue invasion by directly binding catalytically active matrix metalloproteinase MMP-2 (Brooks et al. 1997). Under conditions of cytokine-induced angiogenesis, ligand binding to the αvβ3 receptor is thus proangiogenic. If the overall actions of Del1 inhibit the endothelial cell from contributing to vascular formation, as suggested by the data presented here, it might function as a competitive antagonist, blocking access to ligands that support angiogenesis.
Another possibility is that the cellular program resulting from signaling through the αvβ3 receptor can be modified by additional signaling pathways. These additional signals could be provided by Del1 itself. Homology of the Del1 EGF repeats to those of the Notch receptor and its ligands suggests that Del1 may interact with other proteins containing EGF repeats (Rebay et al. 1991; Artavanis-Tsakonas et al. 1995; Rao et al. 1995). Homology to Notch and Pref-1 also suggests functional mechanisms of action (Fig. 2B). The Notch signaling pathway has been studied extensively in Drosophila and other developmental model systems and appears to maintain an undifferentiated state until the cell receives more specific developmental cues (Artavanis-Tsakonas et al. 1995). Pref-1 has been shown to inhibit adipocyte differentiation (Smas and Sul 1993). Del1 may thus inhibit vascular formation through an autocrine signaling pathway that blocks endothelial cell differentiation. This signaling could be mediated through a receptor interacting with the EGF repeats of Del1, either alone or in concert with signaling through the αvβ3 receptor. Interestingly, a fragment of EGF has been shown to inhibit endothelial cell motility and angiogenesis (Nelson et al. 1995).
In summary, we have investigated the cell- and developmental-specific pattern of expression of an endothelial cell marker, employing expression of a reporter transgene integrated into the locus, mRNA blot, in situ hybridization, and immunohistochemistry studies. The data suggest that Del1 is expressed in angioblasts and early endothelial cells that are specifying vascular development, and subsequently by a restricted group of nonendothelial cells that are known to regulate vascular growth and remodeling. We have employed genomic and cDNA cloning to characterize the complex transcription unit in this locus and describe homologies to other highly conserved genes that have fundamental roles in development. We have characterized the Del1 protein as a new ligand for the αvβ3 integrin receptor and have shown that it is primarily secreted into the extracellular matrix. Finally, in vitro and in vivo models of vascular formation suggest that Del1 regulates the process of primary morphogenesis or the process of vascular remodeling.
Materials and methods
Generation and analysis of the transgenic mouse
The plasmid construct for generating the transgenic line employed in these studies contained 2211 bp of the mouse SPARC 5′-flanking sequence, bacterial β-galactosidase gene, and the SV40 polyadenylation sequence. Early-stage mouse embryos were isolated, fixed, and stained as a whole mount in X-gal as described (Hogan et al. 1994). Late-stage embryos were partially dissected, and the eyes of adult mice were removed for fixation and staining. Embryos and tissues were examined and photographed as whole mounts, and subsequently dehydrated and embedded in paraffin for sectioning.
Genomic and cDNA cloning
High-molecular-weight genomic DNA prepared from a transgenic mouse was partially digested with Sau3a and cloned into Lambda FIX (Stratagene) to generate a library of ∼2 million clones. Screening this library with a transgene probe allowed cloning of flanking region sequence that was subsequently used to isolate overlapping clones from a wild-type 129/SvJ mouse genomic library representing 50 kb of the native locus (Ausubel et al. 1987). Genomic DNA fragments were used for exon trapping (Buckler et al. 1991). A 160-bp putative exon was employed as a probe to screen cDNA libraries constructed from 8.5- and 11.5-dpc mouse embryo RNA and human lung RNA. cDNAs were subcloned into plasmid for dideoxy chain termination sequencing.
Cultured cell lines
BAEC, Molt4, K562, Ltk, NIH-3T3, P19, MEL, F9, and HeLa cells were obtained from the ATCC and cultured under recommended conditions. HUVECs were supplied by Clonetics, Inc., and grown under recommended conditions. Yolk sac cells were derived and cultured as described previously (Corn et al. 1991; Wei et al. 1995). Mouse EOMA cells were cultured as described (Obeso et al. 1990).
RNA isolation and Northern blot analysis
Cultured cells, adult organs, 15.5-dpc whole embryos, and organs dissected from 15.5-dpc embryos were disrupted with a polytron, and RNA was isolated over a CsCl gradient as described (Ausubel et al. 1987). For Northern blot analysis, 20 μg of RNA was size fractionated on 1.3% agarose gels containing 2.2 m formaldehyde, transferred to nitrocellulose, and hybridized to a 713-bp PstI–NcoI fragment or a 644-bp SacI–HindIII Del1 cDNA fragment.
In situ hybridization
Slides for in situ hybridization were generated from paraformaldehyde-fixed, paraffin-embedded mouse embryos according to established methodology or were purchased from Novagen. A 713-bp PstI–NcoI Del1 cDNA fragment encoding the EGF-like repeats was cloned into pGEM5Zf(+) for in vitro RNA probe transcription. Both antisense and sense cRNA probes were labeled with [33P]UTP employing a MAXIscript RNA transcription kit (Ambion). Hybridization, washing, and probe detection were as described (Hogan et al. 1994).
Antibody generation and purification, Western blotting, and immunohistochemistry
A partial Del1 cDNA encoding amino acids 353–489 of the murine gene was cloned into pMALC2 (New England Biolabs) to generate a maltose-binding protein (MBP)–partial Del1 fusion protein. Recombinant fusion protein was expressed and affinity purified, and antisera generated according to established methodology (Harlow and Lane 1988).
Immune serum was purified over sequential total bacterial lysate, MBP, and MBP–Del1 Sepharose columns. The specificity of the antiserum was evaluated with Western blots containing protein from bacteria expressing the recombinant fusion protein before and after cleavage with factor Xa and MBP alone.
For Western blots of eukaryotic protein, cells were harvested by lysis in a standard lysate buffer containing NP-40 or Laemmli reducing SDS loading buffer (Ausubel et al. 1987). Extracellular matrix was harvested by first removing cells with 1 mm EDTA in PBS and then scraping the cell culture dish with a small volume of Laemmli buffer at 90°C.
Immunohistochemistry was performed on sections prepared from Bouin’s fixed, paraffin-embedded, staged mouse embryos according to well-established methodology (Hogan et al. 1994). The affinity-purified Del1 antiserum was employed at a dilution of 1:500 to 1:1000, and the specificity of staining verified by experiments with preimmune serum and competition with recombinant protein.
Yolk sac cell transfections
An expression vector containing the open reading frame of the major Del1 transcript in phbAPr-3-neo (kindly provided by Dr. L. Kedes, University of Southern California, Los Angeles) was transfected into yolk sac cells with Lipofectamine (GIBCO BRL), and clones selected in the presence of 1000 μg/ml of G418. Clones were evaluated for Del1 expression by Northern and Western blotting, and a group of clones with varying amounts of Del1 protein were selected for further study. Negative control clones were randomly selected from a transfection with the empty phbAPr-3–neo vector.
Binding studies with human umbilical vein endothelial cells
Recombinant Del1 protein was produced with Escherichia coli strain BL21 (DE) transformed with a murine major Del1 cDNA clone in plasmid pET28a (Novagen Inc., Madison, WI). Insoluble inclusion bodies were collected by centrifugation and dissolved in 8 m urea, 50 mm Tris-Cl (pH 8.0), 0.5 m EDTA, and 100 mm DTT for 2 hr at room temperature. Recombinant protein was refolded by diluting protein to a concentration of 0.01 mg/ml in 100 mm Tris-Cl (pH 8.0), 100 mm (NH4)2SO4, 100 μm Triton X-100, 2 mm reduced glutathione, and 0.4 mm oxidized glutathione, followed by incubation at 4°C for 5 days. Recombinant protein was purified with His-Bind resin (Novagen) according to established protocol and dialyzed into 100 mm Tris-Cl (pH 8.0), 100 mm (NH4)2SO4, and 100 μm Triton X-100.
Nontissue culture 96-well plates were coated with 1–20 μg of either Del1 protein or BSA diluted in calcium- and magnesium-free PBS and incubated for 24 hr at 4°C. The plates were washed with PBS and blocked for 30 min with a solution of heat-treated PBS containing 3% BSA. HUVECs were harvested by trypsinization and resuspended in an adhesion buffer [Hank’s balanced salt solution (pH 7.4) containing 10 mm HEPES, 2.2 mm MgCl2, 0.2 mm MnCl2, and 1% BSA). Cells (104/100 μl) were added to each well in the presence or absence of peptides or antibodies. Antibodies included the anti-human αvβ3 monoclonal antibody LM609 (Chemicon, Inc.) and a rabbit polyclonal antiserum against the human vitronectin receptor (anti-VNR, GIBCO, Inc., Gaithersburg, MD); both were employed at 10 μg/ml to provide blocking activity. Peptide antagonists included GRGDdSP and the control peptide GRGESP (all from GIBCO, Inc.), at a concentration of 500 μm. Cells were incubated at 37°C for 60–90 min, and wells were washed until no cells remained in the BSA control wells. Peptides and antibodies were preincubated with cells for 30 min before being placed in wells. To quantify adherent cells, 100 μl of media was added to each well, and relative cell number determined with the Cell Titer AQ reagent (Promega, Inc.).
For the assay evaluating binding to extracellular matrix with or without Del1, the matrix was generated by growing 5 × 105 transfected cells per well in 96-well plates for 48 hrs. Transfected cells were removed with 1 mm EDTA and extensive washing, until no cells remained in the BSA control wells.
In vitro assays
In vitro angiogenesis assays on Matrigel (Biocoat, Becton Dickinson) were conducted in 24-well plates coated with 50 μl of Matrigel. Transfectants were plated at a density of 5 × 104 cells/well (low density) or 2 × 105 cells/well (high density) and observed for several days. For the assay evaluating morphogenetic potential of wild type yolk sac cells on Del1-conditioned matrix, the matrix was generated as above and 106 wild-type yolk sac cells were plated on the matrix produced by the Del1 or the control transfectants. Cells were cultured and observed for several days. For the in vitro angiogenesis sprouting assay, Del1 and control transfectants were trypsinized and 106 cells cultured in 15-ml conical tubes for 48 hr followed by culture in bacterial petri dishes for 4–7 days. Resulting cell aggregates were collected for Del1 and control transfectants, and these were transferred to 24-well plates coated with Matrigel. Sprouting angiogenesis was evaluated at 24 and 48 hr.
In vivo angiogenesis assays
Embryos were harvested and assays performed as per well described methodology (Wilting et al. 1991). Yolk sac cells and 143B osteosarcoma cells stably transfected with a Del1 major expression construct were grown on coverslips that were inverted and placed on the CAM at 9 days of development. At 13 days, vascular development in the CAM under these cells was compared to that under cells transfected with a negative control construct.
Acknowledgments
This work was supported by grant RO1 HL52168 from the National Heart, Lung, and Blood Institute (T.Q.), an Established Investigator Award from the American Heart Association (T.Q.), and an Advanced Technology Grant from the National Institute of Standards and Technology (Progenitor, Inc.). B.L.M.H. is an Investigator of the Howard Hughes Medical Institute. GenBank accession numbers for the various forms of human and murine Del1 are AF031524, AF031525, U70312, and U70313.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL tomq1@leland.stanford.edu; FAX (650) 725-2178.
References
- Allen ND, Cran DG, Barton SC, Hettle S, Reik W, Surani MA. Transgenes as probes for active chromosomal domains in mouse development. Nature. 1988;333:852–855. doi: 10.1038/333852a0. [DOI] [PubMed] [Google Scholar]
- Alves F, Vogel W, Mossie K, Millauer B, Hofler H, Ullrich A. Distinct structural characteristics of dicoidin I subfamily receptor tyrosine kinases and complementary expression in human cancer. Oncogene. 1995;10:609–618. [PubMed] [Google Scholar]
- Appella E, Weber IT, Blasi F. Structure and function of epidermal growth factor-like regions in proteins. FEBS Lett. 1988;231:1–4. doi: 10.1016/0014-5793(88)80690-2. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Matsuno K, Fortini ME. Notch signalling. Science. 1995;268:225–232. doi: 10.1126/science.7716513. [DOI] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current protocols in molecular biology. New York, NY: Greene/Wiley; 1987. [Google Scholar]
- Baldwin HD, Shen HM, Yan H, DeLisser HM, Chung A, Mickanin C, Trask T, Kischbaum NE, Newman PJ, Albelda SM. Platelet endothelial cell adhesion molecule-1 (PECAM/CD31): Alternatively spliced, functionally distinct isoforms expressed during mammalian cardiovascular development. Development. 1994;120:2539–2552. doi: 10.1242/dev.120.9.2539. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin αvβ3 for angiogenesis. Science. 1994a;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994b;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMp-2 to the surface of invasive cells by interaction with integrin αvβ3. Cell. 1997;85:683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- Buckler AJ, Chang DD, Graw SL, Brook JD, Haber DA, Sharp PA, Housman DE. Exon amplification: A strategy to isolate mammalian genes based on RNA splicing. Proc Natl Acad Sci. 1991;88:4005–4009. doi: 10.1073/pnas.88.9.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli J. The ciliary epithelia and aqueous humor. In: Hart WM, editor. Adler’s physiology of the eye. St. Louis, MO: Mosby–Year Book Publishing; 1992. pp. 228–242. [Google Scholar]
- Coffin JD, Poole TJ. Endothelial cell origin and migration in embryonic heart and cranial blood vessel development. Anat Rec. 1991;231:383–395. doi: 10.1002/ar.1092310312. [DOI] [PubMed] [Google Scholar]
- Corn BJ, Reed MA, Dishong SL, Li Y, Wagner TE. Culture and successful transplantation of embryonic yolk-sac cells. Clin Biotechnol. 1991;3:15–19. [Google Scholar]
- Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radjziejewski C, Maisonpierre PC, Yancopoulos GD. Isolation of Angiopoietin-1, a ligand for the TIE-2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- del Amo FF, Gendron Maguire M, Swiatek PJ, Jenkins NA, Copeland NG, Gridley T. Cloning, analysis, and chromosomal localization of Notch-1, a mouse homolog of Drosophila Notch. Genomics. 1993;15:259–264. doi: 10.1006/geno.1993.1055. [DOI] [PubMed] [Google Scholar]
- Drake CJ, Davis LA, Little CD. Antibodies to β-1 integrins cause alterations of aortic vasculogenesis, in vivo. Dev Biol. 1992;193:83–91. doi: 10.1002/aja.1001930111. [DOI] [PubMed] [Google Scholar]
- Drake CJ, Cheresh DA, Little CD. An antagonist of integrin αvβ3 prevents maturation of blood vessels during embrionic neovascularization. J Cell Sci. 1995;108:2655–2661. doi: 10.1242/jcs.108.7.2655. [DOI] [PubMed] [Google Scholar]
- Dumont DJ, Yamaguchi TP, Conlon RA, Rossant J, Breitman ML. tek, a novel tyrosine kinase gene located on mouse chromosome 4, is expressed in endothelial cells and their presumptive precursors. Oncogene. 1992;7:1471–1480. [PubMed] [Google Scholar]
- Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman M. Dominant negative and targeted null mutation in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes & Dev. 1994;8:1897–1909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- Flamme I, von Reutern M, Drexler HCA, Syed-Ali S, Risau W. Overexpression of vascular endothelial growth factor in the avian embryo induces hypervascularization and increases vascular permeability without alterations of embryonic pattern formation. Dev Biol. 1995;171:399–414. doi: 10.1006/dbio.1995.1291. [DOI] [PubMed] [Google Scholar]
- Fong G, Rossant J, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- Gilbert SF. Developmental biology. Sunderland, MA: Sinauer Associates; 1994. [Google Scholar]
- Hallmann R, Feinberg RN, Latker CH, Sasse J, Risau W. Regression of blood vessels precedes cartilage differentiation during chick limb development. Differentiation. 1987;34:98–105. doi: 10.1111/j.1432-0436.1987.tb00055.x. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- Hogan BLM, Beddington R, Constantini F, Lacy E. Manipulating the mouse embryo. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- Holland PWH, Harper SJ, McVey JH, Hogan BLM. In vitro expression of mRNA for the calcium-binding protein SPARC (osteonectin) revealed by in situ hybridization. J Cell Biol. 1987;105:473–482. doi: 10.1083/jcb.105.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh DA, Andrews ME, Raff RA. A sea urchin gene encodes a polypeptide homologous to epidermal growth factor. Science. 1987;237:1487–1490. doi: 10.1126/science.3498216. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: Modulation, and signalling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jenny RJ, Pittman DD, Toole JJ, Kriz RW, Aldape RA, Hewich RM, Kaufman RJ, Mann KG. Complete cDNA and derived amino acid sequence of human factor V. Proc Natl Acad Sci. 1987;84:4846–4850. doi: 10.1073/pnas.84.14.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- Matthews W, Jordan CT, Gavin M, Jenkins NA, Copeland NG, Lemischka IR. A receptor tyrosine kinase cDNA isolated from a population of enriched primitive hematopoietic cells and exhibiting close linkage to c-kit. Proc Natl Acad Sci. 1991;88:9026–9030. doi: 10.1073/pnas.88.20.9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller NPH, Risau W, Ullrich A. High affinity VEGF binding and developmental expression suggest flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- Nelson J, Allen WE, Scott WN, Balie JR, Walker B, McFerran NV, Wilson DJ. Murine epidermal growth factor (EGF) fragment (33-42) inhibits both EGF- and laminin-dependent endothelial cell motility and angiogenesis. Cancer Res. 1995;55:3772–3776. [PubMed] [Google Scholar]
- Noden DM. Embryonic origins and assembly of blood vessels. Am Rev Respir Dis. 1989;140:1097–1103. doi: 10.1164/ajrccm/140.4.1097. [DOI] [PubMed] [Google Scholar]
- Obeso J, Weber J, Auerbach R. A hemangioendothelioma-derived cell line: Its use as a model for the study of endothelial cell biology. Lab Invest. 1990;63:259–269. [PubMed] [Google Scholar]
- Okane CJ, Gehring WJ. Detection in situ of genomic regulatory elements in Drosophila. Proc Natl Acad Sci. 1987;84:9123–9127. doi: 10.1073/pnas.84.24.9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly MS, Holmgren L, Chen C, Folkman J. Angiostatin induces and sustains dormancy of human primary tumors in mice. Nature Med. 1996;2:689–692. doi: 10.1038/nm0696-689. [DOI] [PubMed] [Google Scholar]
- Pardanaud L, Yassine F, Dieterlen Lievre F. Relationship between vasculogenesis, angiogenesis and haemopoiesis during avian ontogeny. Development. 1989;105:473–485. doi: 10.1242/dev.105.3.473. [DOI] [PubMed] [Google Scholar]
- Partanen J, Armstrong E, Makela TP, Korhonen J, Sandberg M, Penkonen R, Knuutila S, Huebner K, Alitalo K. A novel endothelial cell surface receptor tyrosine kinase with extracellular epidermal growth factor homology domains. Mol Cell Biol. 1992;12:1698–1707. doi: 10.1128/mcb.12.4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peault BM, Thiery JP, Le Douarin N. Surface marker for hemopoietic and endothelial cell lineages in quail that is defined by a monoclonal antibody. Proc Natl Acad Sci. 1983;80:2976–2980. doi: 10.1073/pnas.80.10.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper MS, Montesano R, Vassalli JD, Orci L. Chondrocytes inhibit endothelial sprout formation in vitro: Evidence for involvement of a transforming growth factor-β. J Cell Physiol. 1991;146:170–179. doi: 10.1002/jcp.1041460122. [DOI] [PubMed] [Google Scholar]
- Poole S, Firtel RA, Lamer E. Sequence and expression of the discoidin I gene family in Dictyostelium discoideum. J Mol Biol. 1981;153:273–289. doi: 10.1016/0022-2836(81)90278-3. [DOI] [PubMed] [Google Scholar]
- Rao Z, Handford P, Mayhew M, Knott V, Brownlee GG, Stuart D. The structure of a calcium binding epidermal growth factor-like domain: Its role in protein-protein interactions. Cell. 1995;82:131–141. doi: 10.1016/0092-8674(95)90059-4. [DOI] [PubMed] [Google Scholar]
- Rebay I, Fleming RJ, Fehon RG, Cherbas L, Cherbas P, Artavanis Tsakonas S. Specific EGF repeats of Notch mediate interactions with Delta and Serrate: Implications for Notch as a multifunctional receptor. Cell. 1991;67:687–699. doi: 10.1016/0092-8674(91)90064-6. [DOI] [PubMed] [Google Scholar]
- Sato T, Qin Y, Kozak CA, Audus KL. Tie-1 and tie-2 define another class of putative receptor tyrosine kinase genes expressed in early embryonic vascular system. Proc Natl Acad Sci. 1993;90:9355–9358. doi: 10.1073/pnas.90.20.9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu X, Breitman ML, Schuh A. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Smas CM, Sul H. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell. 1993;73:725–734. doi: 10.1016/0092-8674(93)90252-l. [DOI] [PubMed] [Google Scholar]
- Soinen R, Schoor M, Henseling U, Tepe C, Kisters-Woike B, Rossant J, Gossler A. The mouse enhancer trap locus (Etl-1): A novel mammalian gene related to Drosophila and yeast transcriptional regulator genes. Mech Dev. 1992;39:111–123. doi: 10.1016/0925-4773(92)90030-n. [DOI] [PubMed] [Google Scholar]
- Stubbs JD, Lekutis C, Singer KL, Bui A, Yuzuki D, Srinivasan U, Parry G. cDNA cloning of a mouse mammary epithelial cell surface protein reveals the existence of epidermal growth factor-like domains linked to factor VIII-like sequences. Proc Natl Acad Sci. 1990;87:8417–8421. doi: 10.1073/pnas.87.21.8417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U, Theres C, Knust E. crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell. 1990;61:787–799. doi: 10.1016/0092-8674(90)90189-l. [DOI] [PubMed] [Google Scholar]
- Toole JJ, Knopf JL, Wozney JM, Sultzman L, Buecker JL, Pittman DD, Kaufman RJ, Brown E, Shoemaker C, Orr EC, Amphlett GW, Foster WB, Coe ML, Knutson GJ, Fass DN, Hewick RM. Molecular cloning of a cDNA encoding human antihaemophilic factor. Nature. 1984;312:342–347. doi: 10.1038/312342a0. [DOI] [PubMed] [Google Scholar]
- Tripathi BJ, Tripathi RC, Livingston AM, Borisuth NS. The role of growth factors in the embryogenesis and differentiation of the eye. Am J Anat. 1991;192:442–471. doi: 10.1002/aja.1001920411. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences: The limits of variation. J Mol Biol. 1985;184:99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]
- Wei Y, Quertermous T, Wagner TE. Directed endothelial differentiation of cultured embryonic yolk sac cells in vivo provides a novel cell-based system for gene therapy. Stem Cell. 1995;13:541–547. doi: 10.1002/stem.5530130512. [DOI] [PubMed] [Google Scholar]
- Wilting J, Christ B, Bokeloh M. A modified chorioallantoic membrane (CAM) assay for qualitative and quantitative study of growth factors. Anat Embryol. 1991;183:259–271. doi: 10.1007/BF00192214. [DOI] [PubMed] [Google Scholar]