Abstract
The aim of this study was to determine the clinical features, and outcome of the patients with miliary tuberculosis (TB).
We retrospectively evaluated 263 patients (142 male, 121 female, mean age: 44 years, range: 16–89 years) with miliary TB. Criteria for the diagnosis of miliary TB were at least one of the followings in the presence of clinical presentation suggestive of miliary TB such as prolonged fever, night sweats, anorexia, weight loss: radiologic criterion and pathological criterion and/or microbiological criterion; pathological criterion and/or microbiological criterion.
The miliary pattern was seen in 88% of the patients. Predisposing factors were found in 41% of the patients. Most frequent clinical features and laboratory findings were fever (100%), fatigue (91%), anorexia (85%), weight loss (66%), hepatomegaly (20%), splenomegaly (19%), choroid tubercules (8%), anemia (86%), pancytopenia (12%), and accelerated erythrocyte sedimentation rate (89%). Tuberculin skin test was positive in 29% of cases. Fifty percent of the patients met the criteria for fever of unknown origin. Acid-fast bacilli were demonstrated in 41% of patients (81/195), and cultures for Mycobacterium tuberculosis were positive in 51% (148/292) of tested specimens (predominantly sputum, CSF, and bronchial lavage). Blood cultures were positive in 20% (19/97). Granulomas in tissue samples of liver, lung, and bone marrow were present in 100% (21/21), 95% (18/19), and 82% (23/28), respectively. A total of 223 patients (85%) were given a quadruple anti-TB treatment. Forty-four (17%) patients died within 1 year after diagnosis established. Age, serum albumin, presence of military pattern, presence of mental changes, and hemoglobin concentration were found as independent predictors of mortality. Fever resolved within first 21 days in the majority (90%) of the cases.
Miliary infiltrates on chest X-ray should raise the possibility of miliary TB especially in countries where TB is endemic. Although biopsy of the lungs and liver may have higher yield rate of organ involvement histopathologicaly, less invasive procedures including a bone marrow biopsy and blood cultures should be preferred owing to low complication rates.
Keywords: diagnosis, miliary tuberculosis, prognosis
1. Introduction
Although the global incidence of tuberculosis (TB) has been slowly decreasing, the worldwide disease burden remains a major health problem. One-third of the world population is estimated to be infected with Mycobacterium tuberculosis (latent TB infection) and 10% of these individuals will develop active TB in their lifetime.[1] In 2014, an estimated 9.6 million people developed TB and 1.5 million died from the disease.[2] It is also a low endemic disease in our country (annual incidence in 2014 = 18/100.000).[2] Miliary TB accounts for ∼1% of all TB cases and is a clinical picture resulting from massive lymphohematogenous dissemination of bacilli-laden focus.[3–10] Characteristic histopathological feature of miliary TB is a tubercle (granuloma) measuring ∼2 mm in ≥2 noncontiguous organs. Although clinical features of the disease are not diagnostic, some findings may imply it.[11–15] The definite diagnosis is established by radiological and histological, and microbiological findings. To the best of our knowledge, this study evaluated the largest number of cases with miliary TB to date. In this study, we aimed to evaluate clinical features, diagnosis, treatment, and prognosis of the patients with miliary TB followed during the last 34 years. Initial 38 cases (those detected before 1988) have been published previously.[11]
2. Patients and method
Medical records of 15 tertiary care centers in Turkey were searched retrospectively for adult miliary TB cases diagnosed between 1981 and 2015. Radiologic criterion for miliary TB was defined as miliary pattern on chest X-ray and/or on high-resolution computed tomography (HRCT).
Pathological criterion for miliary TB was defined as detection of miliary organ involvement of ≥2 separate organs by any biopsy or autopsy.
Microbiological criterion for miliary TB was defined as recovery of Mycobacterium tuberculosis from blood specimen.
Criteria for the diagnosis of miliary TB were at least one of the followings in the presence of clinical presentation suggestive of miliary TB such as prolonged fever, night sweats, anorexia, and weight loss:
-
(1)
Radiologic criterion and pathological criterion and/or microbiological criterion.
-
(2)
Pathological criterion and/or microbiological criterion.
Miliary infiltrates on chest X-ray were classified as typical (multiple 1–3-mm well-defined nodules throughout all lung fields) or atypical (predominant nodules that measured >3 mm or reticulonodular pattern) miliary pattern.[11]
Patients fulfilling the diagnostic criteria of fever of unknown origin (FUO) and paradoxical reaction (PR) were also determined.[16–18] Paradoxical reaction (PR) is defined as a transient expansion (worsening) of a pre-existing tuberculous lesion or the development of new lesions under appropriate anti-TB therapy.
Ehrlich-Ziehl-Neelsen (EZN) method was used to stain acid-fast bacilli (AFB) from clinical samples (fluid or solid tissue). M tuberculosis was cultured in Löwenstein–Jensen media. Blood obtained from 97 patients was cultured in nonradiometric automated TB blood culture system (BACTEC™ Myco/F Lytic culture medium). M tuberculosis DNA was investigated by polymerase chain reaction (PCR). IS 6110-specific primers were used to amplify M tuberculosis complex.[19] In addition, paraffin-embedded granulomatous tissue samples of 21 patients were available during this review and sections of these samples were stained by a pathologist by EZN to detect AFB. M tuberculosis DNA was studied by PCR in 15 of them.
Age, sex, previous history of TB, exposure to active TB, the predisposing factors to TB, and tuberculin skin test (TST) were noted. Induration at the TST site was measured in millimeters after 48 to 72 hours by experienced people. A positive TST was defined as an induration of ≥5 mm in immunosuppressive patients and ≥10 mm in immunocompetent patients.
Clinical and laboratory data and their prognosis were evaluated. Anti-TB drug combinations, their side effects, and complications of the disease were also noted.
The diagnosis of hepatotoxicity from anti-TB drugs was established by the presence of at least one of the followings:[20] increased Alanine transaminase (ALT) and/or Aspartate transaminase (AST) 5 times of upper limit of normal (40 IU/L), increased total bilirubin level of >1.5 mg/dL, presence of clinical signs and symptoms suggesting acute hepatitis such as anorexia, nausea, vomiting, and jaundice associated with increased ALT and/or AST 3 times of upper limit of normal.
Instutional ethics committee approval and informed consent are obligatory parts in all interventional studies (human or animal) without any doubt. However, recently published The National Code on Clinical Trials has declared that ethics approval is not necessary for real retrospective studies.[21]
Clinical and laboratory features of surviving and dead patients were compared by univariate analysis. Categorical and continuous variables are compared by using χ2 and Mann–Whitney U tests, respectively. A “P” value of <0.05 (2-sided test) was considered to indicate significance. To evaluate the prognostic value of significant variables, hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated by using Cox Regression Model.
3. Results
Our research yielded 263 cases of miliary TB (142 males), mean age 44 years (range: 16–89 years). Totally, 44 (17%) patients died within 1 year after diagnosis established.
Signs and symptoms of the patients are given in Table 1. Most frequent complaints were fever (100%), fatigue (91%), anorexia (85%), and weight loss (66%). The findings were fever (100%), lymphadenopathies (21.3%), hepatomegaly (20%), and splenomegaly (19%). Fundoscopic examination was performed in 159 patients; 20 (12%) showed choroid tubercles. Meningitis and acute respiratory distress syndrome (ARDS) developed in 17% (46/263) and 10% (26/263) of the patients, respectively.
Table 1.
Signs and symptoms of 263 patients with miliary TB.

Twenty-four (9%) patients described exposure to patients with active TB. Twenty-three (9%) patients described previous history of TB and all patients were given the treatment for the appropriate period. A predisposing condition for TB was described in 94 (41%) (Table 2). FUO criteria were fulfilled in 131 (50%) patients. Hematological and biochemical findings are given in Table 3.
Table 2.
Predisposing factors in 263 patients with miliary TB.
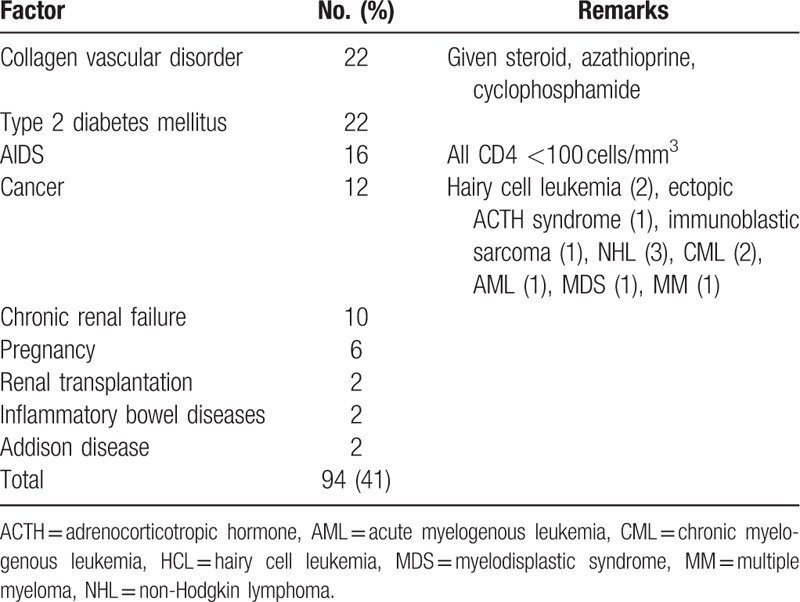
Table 3.
Hematological and biochemical findings of 263 patients with miliary TB.

Anemia, lymphopenia, and pancytopenia developed in nearly 86%, 48%, and 12% of the patients, respectively. Elevated transaminase levels (38%) are more frequently encountered than that of alkaline phosphatase (19%). Elevations in transaminase and bilirubin levels did not exceed 6 times of upper limit of normal. Erythrocyte sedimentation rate was usually accelerated (normal in 11%).
TST positivity was seen in 29% (61/210) of the cases.
A miliary pattern was seen on chest X-ray and/or HRCT in 232 (88%) patients. Miliary pattern developed approximately 50 days (range 21–150) after the emergence of fever.
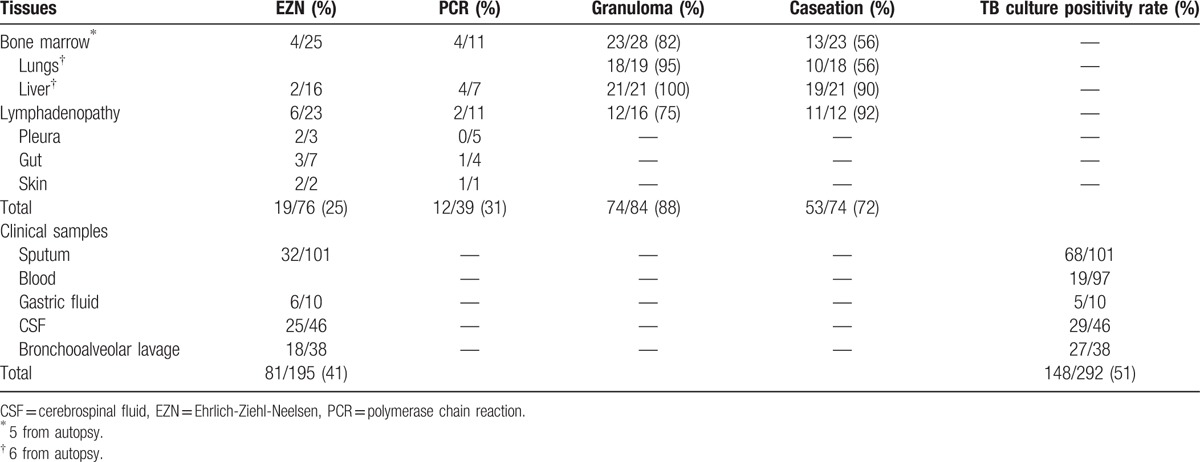
The results of microbiological studies (EZN, TB culture, and PCR) and histopathological results were given in Table 4. EZN staining was applied to 195 samples and 81 (41%) remained positive. Culture positivity was found in 148 of 292 (51%) samples. TB blood culture yielded M tuberculosis in a rate of 20% within 16 to 39 days. Eighty-nine tissue samples including mainly bone marrow samples were studied and granulomas were detected in 74 (88%). Caseating necrosis was noted in 53 (72%). AFB were seen in 25% in granulomatous tissues, whereas 7 of 39 (31%) were positive for M tuberculosis DNA by PCR.
Table 4.
Microbiological and histopathological findings of tissue and clinical samples from miliary tuberculosis.
The most frequently used drug combination was isoniasid (INH), rifampicin (RIF), pyrazinamide (PZA), ethambutol (EMB) or streptomycin (SM) (227 patients; 86%). Steroids in addition to anti-TB therapy were also given to 72 patients (46 with meningitis and 26 with ARDS). Fever resolved in a mean duration of 14 days (range: 2–90 days, interquartile range: 7–21 days), and radiological improvement was noted in mean duration of 3 months (range: 3 weeks–6 months). Fever resolved within first 21 days in the majority (90%) of the cases. Fever resolved in 6th month and radiological improvement was noted in 24th month in 1 patient. Surviving cases were followed-up for 1 year. Forty-four (17%) of the patients died: 8 before initiation of treatment and 36 during treatment. The diagnosis of miliary TB was confirmed with autopsy in 7 patients (Table 5).
Table 5.
Autopsy findings of 7 patients died of miliary TB who could not be diagnosed clinically.

Forty-two patients (16%) developed hepatotoxicity in days 3 to 10 and improved within 10 days (median 5–60 days) of discontinuation. Liver enzymes did not increase after initiating again. A total of 116 patients (44%) developed complications. The organ system involvements in decreasing order were meningitis (71 cases), cellulitis (3 cases), and 1 subcutaneous abscess, ARDS (26 cases), convulsions (3 cases), spontaneous pneumothorax (5 cases), small bowel perforation (5 cases), 6th cranial nerve palsy (1 case), and paradoxical expansion in the miliary lesions (1 case). Twelve (10%) of these complications were paradoxical reactions (5 spontaneous pneumothorax, 5 small bowel perforation, 1 subcutaneous abscess, 1 paradoxical expansion in the miliary lesions). A univariate analysis was used for the detection of the factors associated with mortality (Table 6). Multivariate Cox regression analysis revealed that older age (hazard ratio [HR] 1.019, confidence interval [CI] 1.002–1.036, P = 0.029), low albumin level (HR 0.440, CI 0.269–0.721, P = 0.001), hemoglobin concentration, (HR 0.825, CI 0.712–0.957, P = 0.011) existence of miliary pattern (HR 2.203, CI 1.060–4.579, P = 0.034) and mental change alterations (HR 2.734, CI 1.398–5.347, P = 0.003) at the diagnosis were independent clinical determinants of survival.
Table 6.
Comparison of continuous and categorical variables in patients surviving or died.

4. Discussion
Although the global incidence of TB has been slowly decreasing with globally conducted program, miliary TB incidence will relatively increase owing to widespread use of immunosuppressive drugs and HIV/AIDS pandemicity. Large amounts of bacillemia in miliary TB involve organs with high blood supply including lungs, liver, bone marrow, eyes, kidneys, and adrenals.[5,7,8] Miliary TB is a form of extrapulmonary TB and corresponds to ∼1% of all TB.[6]
Clinical findings (sign and symptoms) and routine laboratory data of the patients with miliaryTB may imply this diagnosis but are not diagnostic.[3,4,6,7,12–14] This disease has a wide range of clinical spectra from ARDS to FUO. The patients generally present with fever, malaise, night sweats, cough, dyspnea, poor appetite, and weight loss lasting for weeks (generally >3). Clinical signs and symptoms of our patients with miliary TB (except the one with TB cellulitis) are compatible with the results of the previous studies.[5,8,9,12,22–24] Among the findings from physical examination, choroidal tubercles in the eye are pathognomonic for miliary TB.[9] The rate of choroidal tubercles was reported between 2% and 21% in miliary TB series.[14,22,23,25] In our study, this figure was found to be 8%. Choroidal tubercles are reported to be less frequent in adults[26] than autopsy probably because of not performing a routine eye examination and not using midriatics during this examination.[8,13,22,23,25] In an autopsy series, choroidal tubercles are found in nearly 50% of the cases.[7] Therefore, a systematic ophthalmoscopic examination after mydriatic administration is recommended in all patients suspected of having miliary TB. Meningitis has been described in 10% to 30% of patients with miliary TB.[4,5,9,22,23,25] We found 18% rate of meningitis. Skin involvement, which is generally subcutaneous abscess, is seen in 1% to 9% of cases reported in the miliary TB series.[4,8,12,22,23,25] In our series, it was 1.5%. Although TB cellulitis is not reported in those series, we have seen 3 such cases. In miliaryTB series, pleurisy was seen in 5 to 38%, we found it in 18% of the cases.
The clinical significance and predicting role of hematological changes seen in miliary TB (anemia, leukopenia, leukocytosis, monocytosis, leukomoid reaction, thrombocytopenia, agranulocytosis, and pancytopenia) are controversial.[4,8,12–14,23,25,27] The most frequently encountered one is anemia of chronic illness. Pancytopenia is rare and leukopenia and thrombocytopenia are more frequent. Pancytopenia associated with FUO should raise the suspicion of miliaryTB. Although the correlation of hematological findings with prognosis could not be shown in previous case series, our cases were generally anemic (86%) and pancytopenia was seen in 12%.[4,22]
In nearly half of the cases, an elevation in alkaline phosphatase and/or transaminase level is seen.[8,12,23–25] Liver function tests are not correlated with the liver histology.[8,23] Transaminase and alkaline phosphatase levels were elevated in nearly one-third of our cases. ESR, an acute phase reactant, is accelerated in miliary TB, whereas level of albumin, a negative acute phase reactant, decreases. An accelerated ESR and hypoalbuminemia were seen in the majority of our cases (90% and 75% respectively).
Previous studies reported an underlying disorder making the host vulnerable to miliary TB (HIV infection, collagen-vascular disorder, diabetes mellitus, neoplasm, chronic renal failure, pregnancy, steroid use, and alcoholism) in nearly half (30%–66%) of the cases.[4,12,13,23,26] A predisposing factor was determined in nearly half of our cases as well, and these factors did not correlate with the mortality. Although TB is endemic in our country, HIV infection is rare. Among our series, miliary TB developed in 16 HIV-infected cases.
Anergy to TST is a more frequent event in miliary TB when compared to pulmonary or other forms of extrapulmonary TB cases.[3,9] This observation can be supported by the finding that it often turns positive following improvement during treatment. The rate of TST positivity decreased gradually from 80% to 32% during the last 30 years.[8,12–14,22,25] This decrease remains to be explained. A negative TST (owing to anergy) does not necessarily show a poor prognosis.[22,23] Nearly ∼two-third of our cases had negative TST results and did not correlate with the mortality. The test was repeated after improvement with the therapy and turned positive in nearly half of the cases.
Although miliary TB is known to cause FUO, except from the series of Proudfoot et al,[14] FUO was not discussed in miliary TB series.[4,8,22–25] Proudfoot et al reported that their 25% of the cases with miliary TB fulfilled the criteria of FUO. Nearly half of our cases had FUO.In FUO series, miliary or disseminated TB seems to be a major cause of prolonged fever.[28–36] Therefore, miliary TB should be considered in the differential diagnosis list in a patient with FUO in the countries where TB is endemic.
Miliary nodules are either micro (1–3 mm) or macronodules (3–10 mm) radiologically.[37] The classical miliary nodules are micronodules and are seen as typical miliary pattern on chest X-ray. Macronodules are seen as atypical miliary nodules. The clinical miliary TB form which does not associate miliary foci on chest X-ray is called cryptic miliary TB and seen in a rate of 30% in clinical series.[11,14] Before naming it as cryptic miliary TB, an HRCT should be obtained since the sensitivity of HRCT for micronodules is higher than chest X-ray.[37,38] Miliary nodules were seen on chest X-ray in rates of 40% to 100% in the previous studies.[8,12–14,24,27] Absence of the miliary infiltrations on admission cannot exclude the diagnosis.[8,12–14,24,28] For detecting the lesions radiologically, at least 2.5 weeks after the initiation of fever are required.[39] For this reason, if miliary TB is a concern, sequential chest X-ray studies should be obtained. The diagnosis was established by detection of miliary pattern on chest X-ray and/or HRCT in ∼90% of our cases. The diagnosis was established by culture positivity, laparotomy, and autopsy in patients without a miliary pattern on chest X-ray (cryptic miliary TB).
Yielding the causative agent is important in the exact diagnosis. Routine mycobacterial blood culture and/or bone marrow culture is recommended during evaluations for mycobacterial infections in miliary TB patients. Nonradiometric automated TB blood culture systems effectively yield Mycobacterium species. In Wang et al's miliary TB series, the sensitivity of blood and bone marrow mycobacterial culture is found to be 64.7% and 38.5%, respectively.[4] In the same study, combined results of bone marrow culture and histopathologic examination are much higher than that of only blood culture (93% vs. 65%). In addition, bone marrow biopsy results can be obtained within 1 week, allowing anti-TB therapy to be started early. The bone marrow biopsy procedure is safer than the others. We have obtained BACTEC™ Myco/F Lytic culture medium in 97 patients, 19 (20%) yielded M tuberculosis in days 16 to 39. Previous studies showed the rate of AFB in granulomas as 0% to 44%.[40–43] New molecular diagnostics such as PCR contributed much to the diagnosis of TB. This method detects M tuberculosis DNA in fluid and tissue samples in a sensitivity ranging from 37% to 100%.[3,19,44,45]
Detection of granulomas in several tissue samples including lungs, liver, bone marrow, and lymph node is important in the diagnosis of miliary TB. Three studies reported the rate of granulomas in transbronchial biopsy samples as 10%, 63%, and 75%.[4,12,23] We found this figure as 95% in tissue samples of lungs (6 from autopsy). In miliary TB series, hepatic granulomas were detected in a range of 67% to 100%.[4,12,14,23,41] Absence of hepatomegaly or normal liver function tests cannot rule out the presence of granulomas.[8,25] Although liver function tests were normal and hepatomegaly was not present in nearly one-third of the cases, liver biopsy showed granulomas in all of 21 patients experienced this procedure. Although liver biopsy has a high diagnostic rate, as its complications are more frequent than bone marrow biopsy, the latter is recommended to perform initially. In the studies, diagnostic rate of bone marrow biopsy is reported ∼50%.[4,8,12,14,19,40] We found this rate as 82% (5 from autopsy). A caseating granuloma should be remembered to be specific for TB.
Paradoxical reaction (PR) is not a rare event. A PR can be encountered at varying rates of 6% to 30% according to given clinical forms of TB.[18] This reaction may develop during 2nd week to 9th month (∼3rd month). When PR develops, the treatment is continued. Steroids may be added or surgical treatment can be applied when needed. PR was not mentioned in miliary TB series. We have noted PR in 2% of our cases on treatment (2 pneumothorax, 2 bowel perforation, 1 expansion of a preexisting lesion, 1 subcutaneous abscesses).Three of these cases have been published previously.[46–48]
Miliary TB cases die within 1 year if they remain untreated.[3,9] Anti-TB therapy is essential for survival. A consensus for optimum duration of therapy is lacking in the published miliary TB series. The duration is given as 24 months in one,[25] 6 and 9 months in the 2 others.[4,24] American Thoracic Society, Centers for Disease Control and Prevention, Infectious Diseases Society of America,[49] and British Thoracic Society[50] guidelines recommended 6 months’ therapy for miliary TB without meningitis. If meningitis associates, the duration should be 12 months. Radiological improvement generally takes 2.5 to 5 months.[14,23,24] Steroids are recommended to add when complications such as meningitis, pericarditis, ARDS, and adrenal insufficiency associate.[1,3,8,25] Main risk factors predicting the mortality are advanced age, dyspnea, mental changes, meningitis, and failure to initiate therapy quickly.[12,23,24] The main factor among these ones is failure to diagnose and treat quickly. The mortality rate is ∼25% (14%–30%), and seemed to remain unchanged during last 25 years.[3,4,8,9,12,14,23–25] According to Cox Regression analyses, age, albumin level, military pattern, presence of mental changes, and hemoglobin level were significantly associated with mortality with negative correlation.
Fever resolved in a mean duration of 24 days (range: 2–90 days, interquartile range:7–21 days) and radiological improvement was obtained within 3 months (3 weeks to 6 months) with anti-TB therapy in our patients. Fever disappeared within first 21 days in the majority (75%). Surviving cases were followed-up for a mean duration of 2.5 years (range: 1–10 years) and 1 recurred.
Our study has several limitations. The main limitation of our study is its retrospective design; however, miliary TB is very rare presentation of TB even in highly endemic countries. Therefore, it is time consuming and difficult to perform a prospective cohort study. Second limitation is that information on variables may potentially be incomplete as this was a retrospective chart review; however, statistical adjustment (data completion) could be done with appropriate techniques by competent statistician. Third limitation is the low rate of positive blood cultures. It is expected to have higher rates of mycobacterial growth in cultures obtained using procedures that are up to the standards.
In conclusion, prolonged fever (>21 days) and miliary infiltrates on chest X-ray and/or HRCT support the diagnosis of miliary TB. Although biopsies obtained from lungs and liver have a higher yield rate, owing to low complication rate, bone marrow biopsy can be performed initially. In patients with FUO, miliary TB should be considered in the differential diagnosis especially in countries where TB is endemic. As mortality is high in this disease, when suspected, after a quick diagnostic work-up, therapy should be administered immediately.
Acknowledgments
We are thankful to all participating university and education training hospitals and physicians who dedicated to war with tuberculosis in Turkey. This study has no financial support.
Footnotes
Abbreviations: AFB = acide fast bacillus, CSF = cerebrospinal fluid, EMB = ethambutol, EZN = Ehrlich-Ziehl-Neelsen, FUO = fever of unknown origin, HRCT = High resolution computed tomogram, INH = isoniasid, PR = paradoxical reaction, PZA = pyrazinamid, RIF = rifampicin, SM = streptomycin, TB = tuberculosis, TST = tuberculin skin test.
The authors report no conflicts of interest.
References
- [1].Dheda K, Barry CE, Maartens G. Tuberculosis. Lancet 2016;387:1211–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].World Health Organization. Global tuberculosis report 2015. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- [3].Ray S, Talukdar A, Kundu S, et al. Diagnosis and management of miliary tuberculosis: current state and future perspectives. Ther Clin Risk Manag 2013;9:9–26. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [4].Wang J-Y, Hsueh P-R, Wang S-K, et al. Disseminated tuberculosis: a 10-year experience in a medical center. Medicine (Baltimore) 2007;86:39–46. [DOI] [PubMed] [Google Scholar]
- [5].Prout S, Benatar SR. Disseminated tuberculosis. A study of 62 cases. South Afr Med J Suid-Afr Tydskr Vir Geneeskd 1980;58:835–42. [PubMed] [Google Scholar]
- [6].Rieder HL, Snider DE, Cauthen GM. Extrapulmonary tuberculosis in the United States. Am Rev Respir Dis 1990;141:347–51. [DOI] [PubMed] [Google Scholar]
- [7].Slavin RE, Walsh TJ, Pollack AD. Late generalized tuberculosis: a clinical pathologic analysis and comparison of 100 cases in the preantibiotic and antibiotic eras. Medicine (Baltimore) 1980;59:352–66. [PubMed] [Google Scholar]
- [8].Gelb AF, Leffler C, Brewin A, et al. Miliary tuberculosis. Am Rev Respir Dis 1973;108:1327–33. [DOI] [PubMed] [Google Scholar]
- [9].Sharma SK, Mohan A, Sharma A, et al. Miliary tuberculosis: new insights into an old disease. Lancet Infect Dis 2005;5:415–30. [DOI] [PubMed] [Google Scholar]
- [10].Enarson DA. Tuberculosis and Nontuberculous Mycobacterial Infections. Fourth Edition Edited by David Schlossberg. Am J Epidemiol 2000;152:294–6. [Google Scholar]
- [11].Mert A, Bilir M, Tabak F, et al. Miliary tuberculosis: clinical manifestations, diagnosis and outcome in 38 adults. Respirology 2001;6:217–24. [DOI] [PubMed] [Google Scholar]
- [12].Kim JH, Langston AA, Gallis HA. Miliary tuberculosis: epidemiology, clinical manifestations, diagnosis, and outcome. Rev Infect Dis 1990;12:583–90. [DOI] [PubMed] [Google Scholar]
- [13].Alvarez S, McCabe WR. Extrapulmonary tuberculosis revisited: a review of experience at Boston City and other hospitals. Medicine (Baltimore) 1984;63:25–55. [PubMed] [Google Scholar]
- [14].Proudfoot AT, Akhtar AJ, Douglas AC, et al. Miliary tuberculosis in adults. Br Med J 1969;2:273–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mert A, Ozaras R. Clinical importance of miliary pattern in the chest X-ray of a patient with fever of unknown origin. Intern Med Tokyo Jpn 2005;44:161. [DOI] [PubMed] [Google Scholar]
- [16].Petersdorf RG, Beeson PB. Fever of unexplained origin: report on 100 cases. Medicine (Baltimore) 1961;40:1–30. [DOI] [PubMed] [Google Scholar]
- [17].Cheng VCC, Ho PL, Lee RA, et al. Clinical spectrum of paradoxical deterioration during antituberculosis therapy in non-HIV-infected patients. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol 2002;21:803–9. [DOI] [PubMed] [Google Scholar]
- [18].Mert A, Ozaras R. Paradoxical reaction: can it be seen after completion of the anti-tuberculous therapy? Scand J Infect Dis 2004;36:78–9. [DOI] [PubMed] [Google Scholar]
- [19].Eisenach KD, Cave MD, Bates JH, et al. Polymerase chain reaction amplification of a repetitive DNA sequence specific for Mycobacterium tuberculosis. J Infect Dis 1990;161:977–81. [DOI] [PubMed] [Google Scholar]
- [20].Tahaoğlu K, Ataç G, Sevim T, et al. The management of anti-tuberculosis drug-induced hepatotoxicity. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis 2001;5:65–9. [PubMed] [Google Scholar]
- [21].Kıraç FS. Is ethics approval necessary for all trials? A clear but not certain process. Mol Imaging Radionucl Ther 2013;22:73–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Maartens G, Willcox PA, Benatar SR. Miliary tuberculosis: rapid diagnosis, hematologic abnormalities, and outcome in 109 treated adults. Am J Med 1990;89:291–6. [DOI] [PubMed] [Google Scholar]
- [23].Munt PW. Miliary tuberculosis in the chemotherapy era: with a clinical review in 69 American adults. Medicine (Baltimore) 1972;51:139–55. [DOI] [PubMed] [Google Scholar]
- [24].Hussain SF, Irfan M, Abbasi M, et al. Clinical characteristics of 110 miliary tuberculosis patients from a low HIV prevalence country. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis 2004;8:493–9. [PubMed] [Google Scholar]
- [25].Sharma SK, Mohan A, Pande JN, et al. Clinical profile, laboratory characteristics and outcome in miliary tuberculosis. QJM Mon J Assoc Physicians 1995;88:29–37. [PubMed] [Google Scholar]
- [26].Sahn SA, Neff TA. Miliary tuberculosis. Am J Med 1974;56:494–505. [DOI] [PubMed] [Google Scholar]
- [27].Debre R. Miliary tuberculosis in children. Lancet Lond Engl 1952;2:545–9. [DOI] [PubMed] [Google Scholar]
- [28].Howard P, Hahn HH, Palmer PL, et al. Fever of unknown origin: a prospective study of 100 patients. Tex Med 1977;73:56–9. [PubMed] [Google Scholar]
- [29].Larson EB, Featherstone HJ, Petersdorf RG. Fever of undetermined origin: diagnosis and follow-up of 105 cases, 1970-1980. Medicine (Baltimore) 1982;61:269–92. [PubMed] [Google Scholar]
- [30].Barbado FJ, Vázquez JJ, Peña JM, et al. Pyrexia of unknown origin: changing spectrum of diseases in two consecutive series. Postgrad Med J 1992;68:884–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Knockaert DC, Vanneste LJ, Vanneste SB, et al. Fever of unknown origin in the 1980 s. An update of the diagnostic spectrum. Arch Intern Med 1992;152:51–5. [PubMed] [Google Scholar]
- [32].Kazanjian PH. Fever of unknown origin: review of 86 patients treated in community hospitals. Clin Infect Dis Off Publ Infect Dis Soc Am 1992;15:968–73. [DOI] [PubMed] [Google Scholar]
- [33].Shoji S, Imamura A, Imai Y, et al. Fever of unknown origin: a review of 80 patients from the Shin’etsu area of Japan from 1986-1992. Intern Med Tokyo Jpn 1994;33:74–6. [DOI] [PubMed] [Google Scholar]
- [34].Iikuni Y, Okada J, Kondo H, et al. Current fever of unknown origin 1982-1992. Intern Med Tokyo Jpn 1994;33:67–73. [DOI] [PubMed] [Google Scholar]
- [35].Handa R, Singh S, Singh N, et al. Fever of unknown origin: a prospective study. Trop Doct 1996;26:169–70. [DOI] [PubMed] [Google Scholar]
- [36].de Kleijn EM, Vandenbroucke JP, van der Meer JW. Fever of unknown origin (FUO). I A. prospective multicenter study of 167 patients with FUO, using fixed epidemiologic entry criteria. The Netherlands FUO Study Group. Medicine (Baltimore) 1997;76:392–400. [DOI] [PubMed] [Google Scholar]
- [37].Andreu J, Mauleón S, Pallisa E, et al. Miliary lung disease revisited. Curr Probl Diagn Radiol 2002;31:189–97. [PubMed] [Google Scholar]
- [38].Collazos J, Guerra E, Mayo J, et al. Tuberculosis as a cause of recurrent fever of unknown origin. J Infect 2000;41:269–72. [DOI] [PubMed] [Google Scholar]
- [39].Bottiger LE, Nordenstam HH, Wester PO. Disseminated tuberculosis as a cause of fever of obscure origin. Lancet Lond Engl 1962;1:19–20. [DOI] [PubMed] [Google Scholar]
- [40].Grinsztejn B, Fandinho FC, Veloso VG, et al. Mycobacteremia in patients with the acquired immunodeficiency syndrome. Arch Intern Med 1997;157:2359–63. [PubMed] [Google Scholar]
- [41].Harrington PT, Gutiérrez JJ, Ramirez-Ronda CH, et al. Granulomatous hepatitis. Rev Infect Dis 1982;4:638–55. [DOI] [PubMed] [Google Scholar]
- [42].Mert A, Ozaras R, Bilir M, et al. Ziehl-Neelsen staining and polymerase chain reaction study of tissue from tuberculous granulomas. Respirol Carlton Vic 2003;8:548. [DOI] [PubMed] [Google Scholar]
- [43].Akcan Y, Tuncer S, Hayran M, et al. PCR on disseminated tuberculosis in bone marrow and liver biopsy specimens: correlation to histopathological and clinical diagnosis. Scand J Infect Dis 1997;29:271–4. [DOI] [PubMed] [Google Scholar]
- [44].Iles PB, Emerson PA. Tuberculous lymphadenitis. Br Med J 1974;1:143–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Forbes BA, Hicks KE. Direct detection of Mycobacterium tuberculosis in respiratory specimens in a clinical laboratory by polymerase chain reaction. J Clin Microbiol 1993;31:1688–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mert A, Bilir M, Akman C, et al. Spontaneous pneumothorax: a rare complication of miliary tuberculosis. Ann Thorac Cardiovasc Surg Off J Assoc Thorac Cardiovasc Surg Asia 2001;7:45–8. [PubMed] [Google Scholar]
- [47].Mert A, Bilir M, Ozaras R, et al. A rare complication of miliary tuberculosis: intestinal perforation. Acta Chir Belg 2001;101:42–5. [PubMed] [Google Scholar]
- [48].Mert A, Bilir M, Ozturk R, et al. Tuberculous subcutaneous abscesses developing during miliary tuberculosis therapy. Scand J Infect Dis 2000;32:37–40. [DOI] [PubMed] [Google Scholar]
- [49].Blumberg HM, Burman WJ, Chaisson RE, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med 2003;167:603–62. [DOI] [PubMed] [Google Scholar]
- [50].Chemotherapy, management of tuberculosis in the United Kingdom: recommendations 1998. Joint Tuberculosis Committee of the British Thoracic Society. Thorax 1998;53:536–48. [PMC free article] [PubMed] [Google Scholar]


