Abstract
Lower extremity peripheral artery disease (PAD) is one manifestation of atherosclerosis. Patients with PAD have an increased rate of mortality due to concurrent coronary artery disease and hypertension. Betablockers (BB) may, therefore, be prescribed, especially in case of heart failure. However, BB safety in PAD is controversial, because of presumed peripheral hemodynamic consequences of BB that could lead to worsening of symptoms in patients with PAD. In this context, we aimed to determine the impact of BB on all-cause and cardiovascular mortality and amputation rate at 1 year after hospitalization for PAD from the COPART Registry population. This is a prospective multicenter observational study collecting data from consecutive patients hospitalized for PAD in vascular medicine departments of 4 academic hospitals in France. Patients with, either claudication, critical limb ischemia or acute lower limb ischemia related to a documented PAD were included. We compared the outcomes of patients with BB versus those without BB in their prescription list at hospital discharge. The mean age of the study population was 70.9 years, predominantly composed of males (71%). Among the 1267 patients at admission, 28% were treated by BB for hypertension, prior myocardial infarction or heart failure. During their hospital stay, 40% underwent revascularization (including bypass surgery 29% and angioplasty 74%), 17% required an amputation, and 5% died. In a multivariate analysis, only prior myocardial infarction was found associated with BB prescription with an odds ratio (OR) of 3.11, P < 0.001. Conversely, chronic obstructive pulmonary disease or PAD with ulcer impeded BB prescription (OR: 0.57 and 0.64, P = 0.007; P = 0.001, respectively). One-year overall mortality of patients with BB did not differ from those without (23% vs. 23%, P = 0.95). The 1-year amputation rate did not differ either (4% vs. 6%, P = 0.14). Patients hospitalized for PAD with a BB in their prescription did not worsen their outcome at 1 year compared to patients without BB. Based on these safety data, prospective study could be conducted to assess the effect of BB on long-term mortality and amputation rate in patients with mild, moderate, and severe PAD.
Keywords: adult, amputation, betablockers, mortality, peripheral artery disease
1. Introduction
Lower extremity peripheral artery disease (PAD) is a common manifestation of atherosclerosis and is associated with an increased risk of coronary artery disease and cardiovascular death.[1–3] A substantial proportion of patients aged over 65 years develop PAD, around 2% if we consider intermittent claudication symptoms, and over 10%, when measuring a low ankle brachial index (ABI) (≤0.90). [4–6] More broadly, prevalence for PAD was 10.69% in a large sample of almost 12 million United States of America insured citizens, and its annual incidence was 2.35%.[7] PAD has a poor prognosis and represents a heavy burden in cardiovascular morbidity and mortality. A 5-year follow-up study found a 33.2% mortality rate in PAD patients and an even more higher rate within 10 years, with 62% of death in men and 33% in women.[8,9] The majority of patients with PAD not only combine multiple cardiovascular risk factors at the time of diagnosis but also present a polyvascular disease, which contribute to such a high risk of death. However, major cardiovascular events remain substantial among patients with isolated PAD. According to a large meta-analysis, the 10-year mortality rate in men with isolated low ABI was 18.7%, and 12.6% in women.[1] Globally, PAD is associated with virtually twice the 10-year total mortality, cardiovascular mortality, and major coronary event rates compared with those without PAD in each Framingham risk score category.[1]
While PAD patients often have concomitant diseases requiring betablockers (BB) (e.g., coronary artery disease, heart failure, or hypertension), their use is still controversial in this population, especially in case of critical limb ischemia (CLI), a more severe condition with a 1-year mortality rate over 20%.[10–12] In real life, Narins et al[13] noticed in a prospective cohort that following myocardial infarction, the added presence of intermittent claudication was associated with an underuse of BB therapy and was a strong independent predictor of recurrent cardiovascular events. The concept that beta-receptor antagonism may worsen limb symptoms in patients with PAD is based on several potential mechanisms, including reduction of cardiac output, induction of reflex sympathetic activity, and an imbalance of alpha and beta agonism in the peripheral vasculature, resulting in vasoconstriction and impaired peripheral perfusion. Concerns have therefore emerged regarding the potential for BB to worsen limb or general prognostic in PAD.[14] Moreover, evidence supporting or refuting the use of BB in PAD from meta-analyses remains elusive.[15–17] Radack et al concluded that BB could probably be used safely in PAD patients,[15] whereas meta-analysis from Miyajima et al showed that there was a significant worsening in maximal walking distance and initial claudication distance in patients receiving BB.[16] The latest Cochrane systematic review on this subject concludes with the absence of evidence supporting that BB adversely affect walking distance in people with intermittent claudication. However, the authors recommend that BB should be used with caution, in the case of CLI for which acute lowering of blood pressure is contraindicated. They underline that no large published trials are available and recommend high-quality, randomized trials to be conducted to evaluate the role of BB in patients with mild, moderate, and severe PAD.[17] In this context, we carried out a study, aimed at assessing BB prescription in patients hospitalized for PAD from the COPART Registry and analyzing their impact on 1-year morbidity and mortality.
2. Methods
The COPART Registry is a multicenter registry, collecting exhaustive data prospectively on consecutive patients hospitalized for PAD in 4 academic centers in France since May 17, 2004 to July 15, 2010 (Bordeaux, Limoges, Paris, and Toulouse). Details regarding the COPART Registry have already been published.[18] In each vascular center, care to patients was provided according to the usual practice without any change in the management strategy. Initial clinical history, disease characteristics, and therapeutic data were collected. The enrolled patients gave their informed consent to participate. This study was approved by the institutional review board of the Toulouse University Hospital.
2.1. Inclusion criteria
Each patient required the following criteria to be included: age ≥18 years; consent to participate to the registry and a first hospitalization specifically for clinical PAD of atherosclerotic origin. Clinical presentations could be severe claudication—associated with an ABI <0.90 or >1.30 or, a lower limb arterial stenosis >50% on duplex ultrasound, angiography or angiography computerized tomography in the case of normal ABI at rest—CLI with or without ulceration or gangrene and acute lower limb ischemia related to a documented PAD with significant arterial stenosis. Staging of patients’ PAD was established according to The Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC) guidelines.[10]
2.2. Exclusion criteria
Patients with arterial occlusive disease not related to atherosclerosis, acute lower limb ischemia of embolic origin and patients refusing to participate were excluded from the registry.
2.3. Follow-up
After the initial hospitalization, all patients were followed up for at least 12 months. For this purpose, mortality data at registrar's offices have been consulted, and nonfatal events were retrieved through the hospitals’ files, mailing, or phone contacts to the physicians or the patients if necessary. The primary outcome for this study was the overall mortality during the 1-year follow-up. Secondary outcomes were cardiovascular mortality and occurrence of amputation.
2.4. Statistical analysis
Discrete variables are presented as number and percentage, and continuous variables as mean and standard deviation. Comparisons were made using chi-square test (or Fisher exact tests, when appropriate) for discrete variables, and Student t test for continuous variables. Multivariate analysis was performed using linear logistic regression to calculate odds ratio (OR) and 95% confidence interval (95% CI) for outcome events: overall mortality, cardiovascular mortality, and amputation. All subsequent P values are reported for 2-tailed tests with a 5% threshold. All analyses were performed with SAS statistical software version 9.2 (SAS Inc., Cary, NC).
3. Results
Over a 6-year-period, 1267 patients were included in this study. Table 1 shows the study population characteristics.
Table 1.
Baseline characteristics of the study population at admittance.
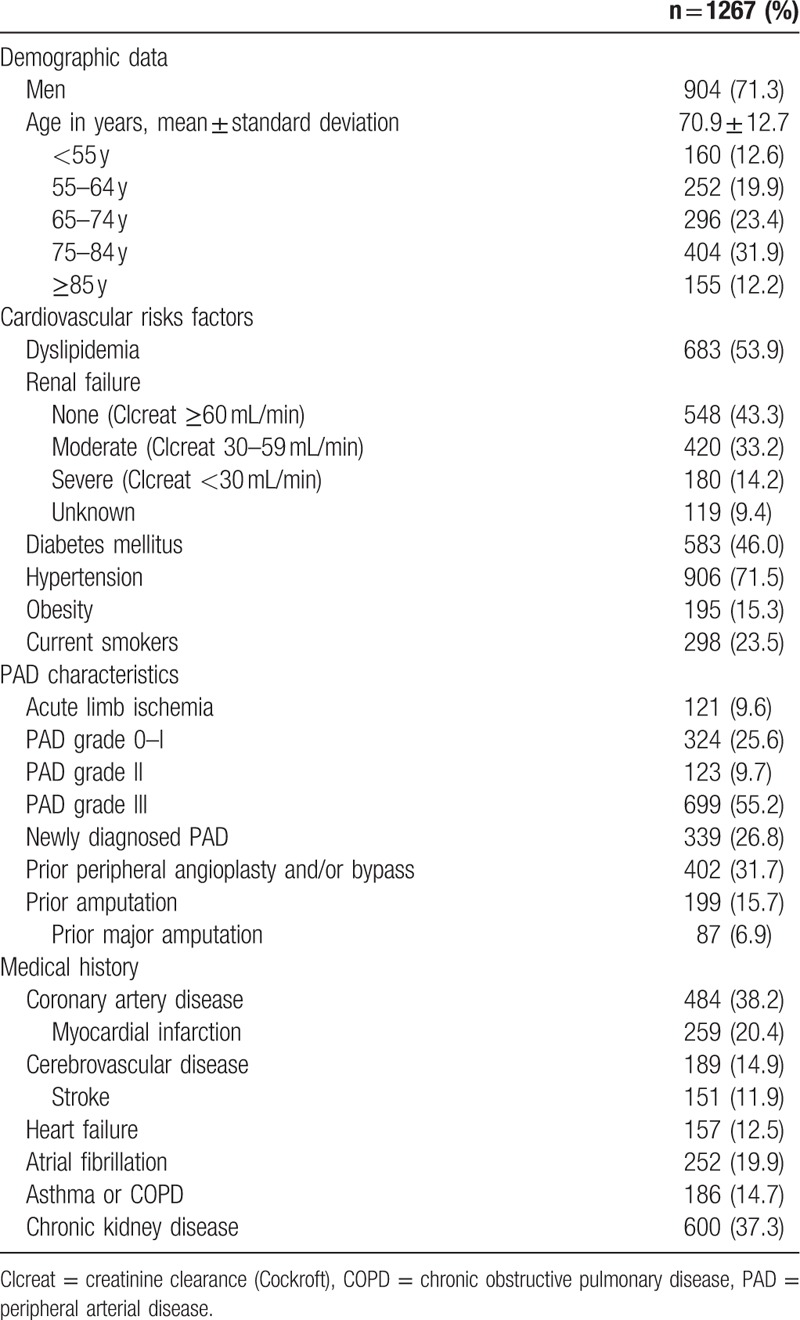
3.1. At admission
At entry, 339 (27%) patients were newly diagnosed with PAD. One quarter of the patients presented with claudication (PAD grade 0–I) and 2/3 with CLI (PAD grades II–III). BB were present for 1/4 of the patients at admittance (28%). History of hypertension, prior myocardial infarction or heart failure were associated with BB prescription: 85% versus 69%, 38% versus 17%, 17% versus 10%, as compared to those without this condition, P < 0.001, P < 0.001, P = 0.004, respectively. Proportion of patients with BB and PAD grade III was significantly lower than patients with any other PAD grade (P = 0.02).
In multivariate analysis, history of hypertension or prior myocardial infarction were independent factors for BB prescription with OR (95% CI) of 2.60 (1.75–3.86), P < 0.001 and 3.02 (2.15–4.25), P < 0.001, respectively. History of asthma or chronic obstructive pulmonary disease (COPD) and PAD grade III were associated with lower rates of BB prescription at admittance with OR (95% CI) of 0.57 (0.37–0.90), P = 0.02 and 0.55 (0.40–0.75), P < 0.001, respectively.
3.2. At discharge
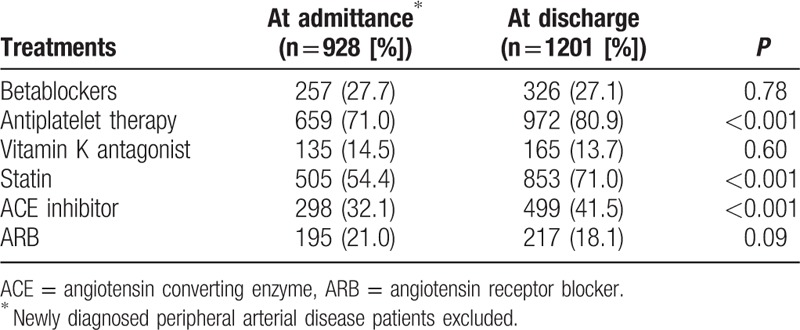
A revascularization (including bypass surgery 29% and angioplasty 74%) has been performed in 40% of the cases before discharge. Nonetheless, the proportion of patients who required amputation was 17%. During hospitalization, 66 (5%) subjects died. At discharge patients received a prescription with antiplatelet therapy in 81%, vitamin K antagonists in 14%, statins in 71%, angiotensin converting enzyme (ACE) inhibitors in 42%, angiotensin receptor blocker (ARB) in 18%, and BB in 27%. For consistent comparisons, we restrained the analysis of cardiovascular treatment changes during hospitalization to the 928 patients with already known PAD at entry and still alive at hospital discharge (Table 2). Antiplatelet therapy, statin, and ACE inhibitor prescriptions significantly increased from admittance to discharge (P < 0.001) but not the BB prescription.
Table 2.
Cardiovascular treatments at admittance and at discharge.
History of hypertension, prior myocardial infarction or coronary artery disease, were associated with BB prescription (81% vs 67%, 35% vs 15%, and 65% vs 28%, P < 0.001 respectively) (Table 3). Conversely, BB were less prescribed in the case of asthma or COPD (11% vs 16%, P = 0.03), PAD grade III (45% vs 57%, P = 0.003), or in elderly. There was no significant difference between patients with and those without BB for sex, ABI, renal failure, or diabetes. Neither other drugs prescription nor the revascularization procedure were influenced by the presence of BB, except for ARB (22% vs 17%, P = 0.04). Patients who underwent amputation during their hospitalization were less treated by BB (13% vs 19%, P = 0.02). In multivariate analysis (Fig. 1), prior myocardial infarction favored BB prescription with an OR (95% CI) of 3.11 (2.29–4.21), P < 0.001. History of asthma or COPD and PAD grade III had a negative relationship with OR (95% CI) of 0.57 (0.37–0.85), P = 0.007 and 0.64 (0.49–0.84), P = 0.01, respectively.
Table 3.
Characteristics of patients with or without BB at discharge.
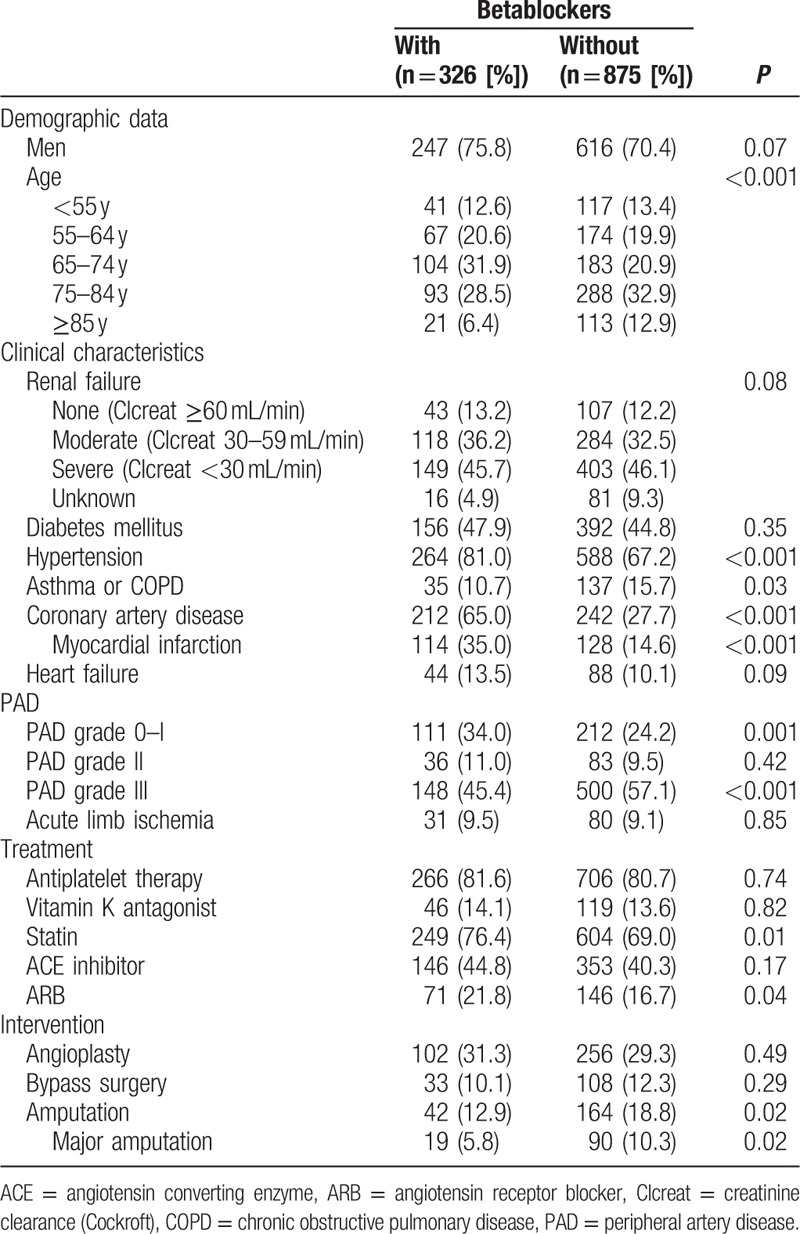
Figure 1.
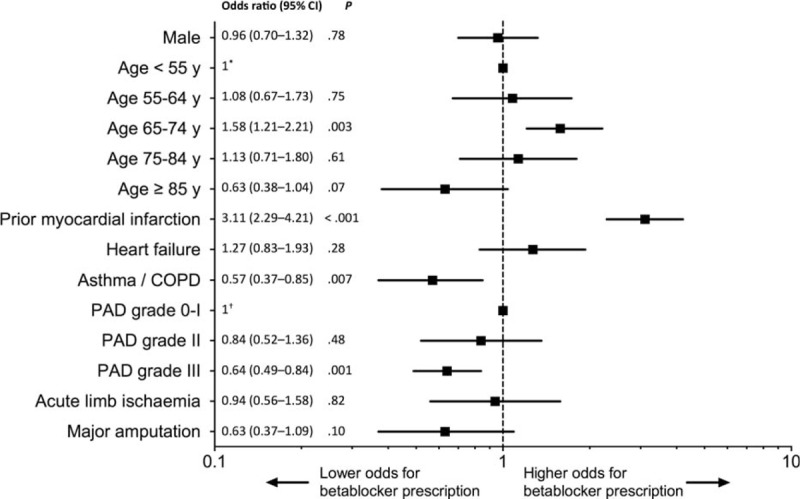
Factors associated with the prescription of betablockers at discharge. Multivariate analysis adjusted on sex, age, prior myocardial infarction, heart failure, asthma/COPD, PAD grade, and major amputation.∗Reference for age classes, †reference for PAD grades. PAD = peripheral artery disease, y = years, COPD = chronic obstructive pulmonary disease.
3.3. Follow-up at 1 year
At 1 year, 271 patients (23%) died, including 160 (13%) from cardiovascular origin (Table 4). Advanced age and prior myocardial infarction were independent factors of overall mortality (Fig. 2). Overall and cardiovascular mortalities did not differ according to BB status: OR (95% CI) 1.34 (0.92–1.97), P = 0.13 and 1.08 (0.77–1.50), P = 0.65, respectively.
Table 4.
Outcome at 1 year of patients with or without BB at discharge.
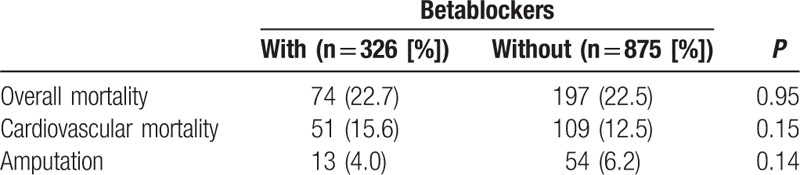
Figure 2.
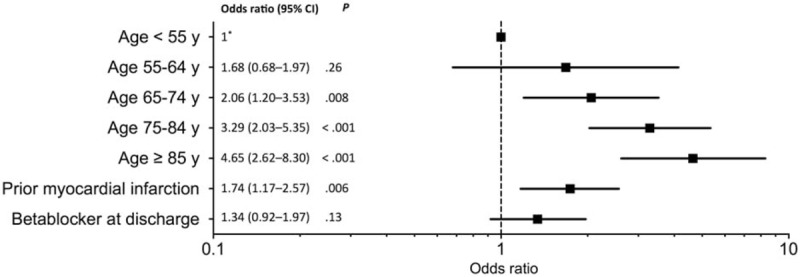
Factors associated with overall mortality at 1 year. Multivariate analysis adjusted on age, prior myocardial infarction, and betablockers.∗Reference for age classes.
At 1 year, 67 patients (6%) underwent amputation, with no difference between BB users and nonusers, respectively, 4% versus 6% (P = 0.14), OR (95% CI) 0.63 (0.33–1.19), P = 0.16.
4. Discussion
In this study, we found that patients hospitalized for PAD and treated by BB at hospital discharge did not worsen their outcome at 1 year compared to patients not treated by BB. Indeed, neither overall mortality, nor cardiovascular mortality, or amputation rates were impacted by the presence of BB in the prescription list. Different meta-analyses concluded with the absence of evidence supporting that BB adversely affect PAD patients with intermittent claudication.[15–17] But the selected studies only addressed the effect of BB on limb impairment and were restrained to patients with intermittent claudication.[19–28] Deleterious effects of BB are specifically suspected in CLI, which may explain the lower rate of BB prescription in PAD grade III patients in our study. Predictors of BB prescription were indeed essentially prior myocardial infarction, and in a lesser extent the history of hypertension or coronary artery disease. On the contrary, severe PAD (PAD grade III) and asthma/COPD impeded BB prescription.
However, restraints on BB prescription were not merely related to the fear of PAD worsening in the most compromised cases. Only 33% of the patients with heart failure history had BB in their prescription list. Similarly, only 47% of the patients with myocardial infarction in their medical history were treated by BB. Overall, BB were underused in this study population, according to the European guidelines.[29–31] As a reminder, without contraindication, BB are recommended for patients with a chronic heart failure and left ventricular ejection function (LVEF) <40%,[29] or a history of acute coronary syndrome without persistent ST-segment elevation and LVEF < 40%.[31] BB may also be the first line antiangina therapy in stable coronary artery disease.[30] This underuse may represent a bias on the outcome at 1 year in our study, but the phenomenon is commonly and broadly noticed in the literature.[32–34]
We noticed a trend for a lower amputation rate in patients treated by BB at 1 year, but the difference was not statistically significant. This is consistent with the results reported in a recent Danish study, where 16945 symptomatic PAD patients were included and treated either by primary vascular surgery or by endovascular reconstruction. Among them, the 7828 BB users presented a reduced risk of major amputation: hazard ratio (95% IC) 0.80 (0.70–0.93).[35]
In our multicenter cohort of hospitalized patients for PAD, demographic characteristics, cardiovascular risk factors, and medical history were comparable to others PAD cohort studies.[2,36–39] These cohorts reported between 286 and 8273 PAD patients based on ABI < 0.9 or history of amputation or peripheral revascularization. The mean age ranged from 69.2 to 73.9 years, men represented 46.0% to 81.2%, current smokers 15.9% to 39.3%, diabetes 25.9% to 44.2%, hypertension 63.4% to 81.0%, and hyperlipidemia 57.2% to 66.7%. Other vascular beds were affected in these PAD patients with cerebrovascular disease in 12.6% to 23.0% and coronary artery disease in 24.1% to 51.7%.[2,36–39] However, clinical presentation of our patients was more severe: only 1/4 presented with intermittent claudication, whereas the 2/3 had CLI and 10% an acute limb ischemia. This may explain that the 22% 1-year mortality rate in our study was higher than in other similar studies (4%–8%).[8,40,41] We found that presence of BB was not associated with an increased risk of mortality. In other words, it also means that BB did not decrease the mortality rate, whereas 2/3 of the deaths were of cardiovascular origin. In previous studies, including symptomatic PAD patients with prior myocardial infarction, BB were associated with a lower risk of recurrence of acute myocardial infarction.[13,42] In the Danish study, BB were not associated with a higher mortality rate, but were associated with an increased risk of acute myocardial infarction and/or stroke during the follow-up period.[35] In our study, we did not find any increase of cardiovascular fatal events associated with BB.
Our study has some limitations. This is an observational study, not a randomized trial. Findings should, therefore, be addressed with usual caution, although we made multivariate analysis to take into account factors affecting BB prescription. Details on what BB and what dosage are not provided in our study. We are aware that BB have different pharmacokinetic and pharmacodynamic properties, which introduce heterogeneity in the analysis. However, the data collection was not designed to collect prospectively these details. Moreover, we used patient's prescription list as a proxy for actual drug use, but we had no information regarding patient's compliance to treatment. While our registry is one of the largest ones for patients hospitalized for PAD, we were unable to perform subgroup analyses because of statistical power weakening in smaller groups. However, we are confident regarding exhaustiveness in the follow-up of death and amputation. Not only because the death criteria were easily retrieved, and the amputations were done in the participating hospitals, but also because the accuracy of the data sources in the COPART Registry was previously described as good.[18,43,44]
5. Conclusion
Patients hospitalized for PAD could safely carry on with their BB treatment with no increase in the 1-year overall mortality or the amputation rate. Based on these safety data, and aware of the high cardiac mortality rate in PAD, a high-quality, prospective randomized trial would be of interest to evaluate the role of BB in patients with mild, moderate, and severe PAD on mortality, regardless the coronary artery disease history. As witnessed in this study, where PAD grade III was an impediment to BB prescription, attention should be paid to CLI in further studies, in order to establish the safety of BB in this severe condition.
Footnotes
Abbreviations: 95% CI = 95% confidence interval, ABI = ankle brachial index, ACE = angiotensin converting enzyme, ARB = angiotensin receptor blocker, BB = betablockers, CLI = critical limb ischemia, COPD = chronic obstructive pulmonary disease, LVEF = left ventricular ejection function, OR = odds ratio, PAD = peripheral artery disease.
TM and AG contributed equally as co–first authors.
Funding/support: This study has been supported by the national program PHRC 2007 DIRC SOOM. The COPART Registry is supported by the French Vascular Medicine Society.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Fowkes FGR, Murray GD, Butcher I, et al. Ankle Brachial Index Collaboration. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA 2008;300:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Steg PG, Bhatt DL, Wilson PWF, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA 2007;297:1197–206. [DOI] [PubMed] [Google Scholar]
- [3].Bhatt DL, Eagle KA, Ohman EM, et al. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA 2010;304:1350–7. [DOI] [PubMed] [Google Scholar]
- [4].Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res 2015;116:1509–26. [DOI] [PubMed] [Google Scholar]
- [5].Fowkes FGR, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013;382:1329–40. [DOI] [PubMed] [Google Scholar]
- [6].Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001;286:1317–24. [DOI] [PubMed] [Google Scholar]
- [7].Nehler MR, Duval S, Diao L, et al. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J Vasc Surg 2014;60:686–95. [DOI] [PubMed] [Google Scholar]
- [8].Caro J, Migliaccio-Walle K, Ishak KJ, et al. The morbidity and mortality following a diagnosis of peripheral arterial disease: long-term follow-up of a large database. BMC Cardiovasc Disord 2005;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med 1992;326:381–6. [DOI] [PubMed] [Google Scholar]
- [10].Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 2007;45:S5–67. [DOI] [PubMed] [Google Scholar]
- [11].Westin GG, Armstrong EJ, Bang H, et al. Association between statin medications and mortality, major adverse cardiovascular event, and amputation-free survival in patients with critical limb ischemia. J Am Coll Cardiol 2014;63:682–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Varu VN, Hogg ME, Kibbe MR. Critical limb ischemia. J Vasc Surg 2010;51:230–41. [DOI] [PubMed] [Google Scholar]
- [13].Narins CR, Zareba W, Moss AJ, et al. Relationship between intermittent claudication, inflammation, thrombosis, and recurrent cardiac events among survivors of myocardial infarction. Arch Intern Med 2004;164:440–6. [DOI] [PubMed] [Google Scholar]
- [14].Frohlich ED, Tarazi RC, Dustan HP. Peripheral arterial insufficiency. A complication of beta-adrenergic blocking therapy. JAMA 1969;208:2471–2. [DOI] [PubMed] [Google Scholar]
- [15].Radack K, Deck C. Beta-adrenergic blocker therapy does not worsen intermittent claudication in subjects with peripheral arterial disease. A meta-analysis of randomized controlled trials. Arch Intern Med 1991;151:1769–76. [PubMed] [Google Scholar]
- [16].Miyajima R, Sano K, Yoshida H. Beta-adrenergic blocking agents and intermittent claudication: systematic review. Yakugaku Zasshi 2004;124:825–31. [DOI] [PubMed] [Google Scholar]
- [17].Paravastu SC, Mendonca DA, Da Silva A. Beta blockers for peripheral arterial disease. Cochrane Database Syst Rev 2013;9:CD005508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cambou JP, Aboyans V, Constans J, et al. Characteristics and outcome of patients hospitalised for lower extremity peripheral artery disease in France: the COPART Registry. Eur J Vasc Endovasc Surg 2010;39:577–85. [DOI] [PubMed] [Google Scholar]
- [19].Lepäntalo M. Chronic effects of labetalol, pindolol, and propranolol on calf blood flow in intermittent claudication. Clin Pharmacol Ther 1985;37:7–12. [DOI] [PubMed] [Google Scholar]
- [20].Lepäntalo M. Chronic effects of metoprolol and methyldopa on calf blood flow in intermittent claudication. Br J Clin Pharmacol 1984;18:90–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Solomon SA, Ramsay LE, Yeo WW, et al. Beta blockade and intermittent claudication: placebo controlled trial of atenolol and nifedipine and their combination. BMJ 1991;303:1100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hiatt WR, Stoll S, Nies AS. Effect of beta-adrenergic blockers on the peripheral circulation in patients with peripheral vascular disease. Circulation 1985;72:1226–31. [DOI] [PubMed] [Google Scholar]
- [23].Svendsen TL, Jelnes R, Tønnesen KH. The effects of acebutolol and metoprolol on walking distances and distal blood pressure in hypertensive patients with intermittent claudication. Acta Med Scand 1986;219:161–5. [DOI] [PubMed] [Google Scholar]
- [24].Reichert N, Shibolet S, Adar R, et al. Controlled trial of propranolol in intermittent claudication. Clin Pharmacol Ther 1975;17:612–5. [DOI] [PubMed] [Google Scholar]
- [25].Roberts DH, Tsao Y, McLoughlin GA, et al. Placebo-controlled comparison of captopril, atenolol, labetalol, and pindolol in hypertension complicated by intermittent claudication. Lancet 1987;2:650–3. [DOI] [PubMed] [Google Scholar]
- [26].Bogaert MG, Clement DL. Lack of influence of propranolol and metoprolol on walking distance in patients with chronic intermittent claudication. Eur Heart J 1983;4:203–4. [DOI] [PubMed] [Google Scholar]
- [27].Diehm C, Pittrow D, Lawall H. Effect of nebivolol vs. hydrochlorothiazide on the walking capacity in hypertensive patients with intermittent claudication. J Hypertens 2011;29:1448–56. [DOI] [PubMed] [Google Scholar]
- [28].Espinola-Klein C, Weisser G, Jagodzinski A, et al. β-Blockers in patients with intermittent claudication and arterial hypertension: results from the nebivolol or metoprolol in arterial occlusive disease trial. Hypertension 2011;58:148–54. [DOI] [PubMed] [Google Scholar]
- [29].McMurray JJV, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787–847. [DOI] [PubMed] [Google Scholar]
- [30].Montalescot G, Sechtem U, Achenbach S, et al. Task Force Members. 2013 ESC guidelines on the management of stable coronary artery disease: the task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- [31].Roffi M, Patrono C, Collet J-P, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- [32].Komajda M, Follath F, Swedberg K, et al. The EuroHeart Failure Survey programme—a survey on the quality of care among patients with heart failure in Europe. Part 2: Treatment. Eur Heart J 2003;24:464–74. [DOI] [PubMed] [Google Scholar]
- [33].Hasdai D, Behar S, Wallentin L, et al. A prospective survey of the characteristics, treatments and outcomes of patients with acute coronary syndromes in Europe and the Mediterranean basin; the Euro Heart Survey of Acute Coronary Syndromes (Euro Heart Survey ACS). Eur Heart J 2002;23:1190–201. [DOI] [PubMed] [Google Scholar]
- [34].Soumerai SB, McLaughlin TJ, Spiegelman D, et al. Adverse outcomes of underuse of beta-blockers in elderly survivors of acute myocardial infarction. JAMA 1997;277:115–21. [PubMed] [Google Scholar]
- [35].Høgh A, Lindholt JS, Nielsen H, et al. Beta-blocker use and clinical outcomes after primary vascular surgery: a nationwide propensity score-matched study. Eur J Vasc Endovasc Surg 2013;46:93–102. [DOI] [PubMed] [Google Scholar]
- [36].Blacher J, Cacoub P, Luizy F, et al. Peripheral arterial disease versus other localizations of vascular disease: the ATTEST study. J Vasc Surg 2006;44:314–8. [DOI] [PubMed] [Google Scholar]
- [37].Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA 2006;295:180–9. [DOI] [PubMed] [Google Scholar]
- [38].Diehm C, Lange S, Darius H, et al. Association of low ankle brachial index with high mortality in primary care. Eur Heart J 2006;27:1743–9. [DOI] [PubMed] [Google Scholar]
- [39].Alzamora MT, Forés R, Baena-Díez JM, et al. The peripheral arterial disease study (PERART/ARTPER): prevalence and risk factors in the general population. BMC Public Health 2010;10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dormandy JA, Murray GD. The fate of the claudicant—a prospective study of 1969 claudicants. Eur J Vasc Surg 1991;5:131–3. [DOI] [PubMed] [Google Scholar]
- [41].Rossi E, Biasucci LM, Citterio F, et al. Risk of myocardial infarction and angina in patients with severe peripheral vascular disease: predictive role of C-reactive protein. Circulation 2002;105:800–3. [DOI] [PubMed] [Google Scholar]
- [42].Aronow WS. Peripheral arterial disease of the lower extremities. Arch Med Sci 2012;8:375–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lacroix P, Aboyans V, Desormais I, et al. Chronic kidney disease and the short-term risk of mortality and amputation in patients hospitalized for peripheral artery disease. J Vasc Surg 2013;58:966–71. [DOI] [PubMed] [Google Scholar]
- [44].Desormais I, Aboyans V, Bura A, et al. Anemia, an independent predictive factor for amputation and mortality in patients hospitalized for peripheral artery disease. Eur J Vasc Endovasc Surg 2014;48:202–7. [DOI] [PubMed] [Google Scholar]


