Abstract
Gastric polyps are frequently reported in patients undergoing upper endoscopic procedures. In this retrospective study, the association between hyperplastic polyps and celiac disease in Northern Sardinia was estimated.
Age, gender, body mass index, and medications taken in the 2 preceding months, including proton-pump inhibitors (PPIs), H2 receptor blockers (anti-H2), Helicobacter pylori status, endoscopic findings, and histology from charts of patients undergoing esophago-gastro-duodenoscopy were reviewed. Polyps were classified as hyperplastic, fundic gland, inflammatory, and adenomatous.
3.7% (423/11379) patients had celiac disease. Prevalence of gastric polyps was 4.2% (3.8% among celiac vs 4.2% nonceliac patients). Inflammatory polyp was the most common histotype (55.8% and 56.2%) followed by fundic gland polyps (31.4% and 43.7%), hyperplastic (8.7% and 0%), and adenomas, in celiac and nonceliac patients, respectively. Fundic gland polyps were more common in PPI users (odds ratio: 4.06) than in nonusers (2.65, P = 0.001) among celiac and nonceliac patients. Age older than 50, female gender, esophago-gastro-duodenoscopy year, and PPI use were associated with the presence of polyps, whereas active H pylori infection was not.
Gastric polyps were common in Sardinian patients undergoing esophago-gastro-duodenoscopy. However, the previously reported association between hyperplastic polyps and celiac disease was not confirmed in our study.
Keywords: celiac disease, gastric hyperplastic polyps, gastric polyps
1. Introduction
The increasing use of upper endoscopic procedures in the general population has led to the frequent description of visible abnormalities of gastric mucosa. Among these, the presence of gastric polyps is a relatively common finding, even though these abnormalities are not always confined to the mucosal lining, and may also be submucosal or extrinsic.[1]
Gastric polyps are found in less than 10% of upper endoscopies. For example in the United States, it was reported a prevalence around 6%,[2,3] although in other countries such as Brazil and Greece lower rates have been observed.[4,5] Gastric polyps represent a heterogeneous group of lesions with variable aspect concerning histology, neoplastic potential, treatment, and follow-up.[2,6–8]
The most frequent polyp histotypes are hyperplastic polyps, fundic gland (or cystic) polyps, and gastric adenomas. Hyperplastic polyps are more frequent in regions were H pylori infection is more prevalent, accounting for 70% to 75% of all gastric polyps.[3] Response to a chronic inflammatory trigger such as chronic gastritis from H pylori infection or bile reflux in patients with gastroenterostomies may result in tissue hyperplasia and polyp development.[6–8] In contrast, in developed countries, where the prevalence of H pylori infection is lower and proton pump inhibitor (PPI) use is higher, the most commonly encountered polyps are fundic gland polyps.[8]
Fundic gland polyps are more common in patients using the PPI.[8] However, they may also be present in the stomach of patients with polyposis syndrome. They are in general multiple and asymptomatic, detected especially in the fundus and corpus of the stomach. Rarely, they can have a size able to cause obstruction of pylorus and vomiting.[8–10]
Gastric adenomas are typically isolated and located in the antrum and are associated with a low risk of carcinogenesis.[11] They may arise within a chronic atrophic gastritis. Usually they are asymptomatic and in few occasions may bleed. Sporadic gastric adenomas occur more frequently in the elderly.[4–6]
In 1990, Doris et al[12] reported for the first time fundic gland polyps regression, although incomplete, in a 50-year-old woman with celiac disease (CD) when on gluten-free diet. More recently, a case series from Mexico suggested that the association between CD and hyperplastic gastric polyps might not be casual.[13]
The purpose of the present study was to evaluate in a large series of subjects whether gastric hyperplastic polyps may be considered an additional manifestation of CD.
2. Methods
2.1. Study design
This was a retrospective single-center study. Charts from out- and in-patients undergoing upper endoscopy at an open access Digestive Endoscopy Service (Clinica Medica, University of Sassari, Italy), from January 1995 to December 2013 were reviewed. Patients were referred to the endoscopy by family physicians and/or specialists for any reason (dyspeptic or reflux symptoms, suspicious of CD, follow-up, and so on). All patients were white Caucasian from Northern Sardinia, a well-known and documented genetically homogeneous population. Information such as age, gender, body weight, and height were obtained, as well as use of medications taken in the 2 months preceding the esophago-gastro-duodenoscopy (EGD), including PPIs (without differentiating among the PPI molecules), H2 receptor blockers (Anti-H2). All patients were from the same geographic area (Northern Sardinia) and were evaluated at the time of EGD by a gastroenterologist by using a standard form.
For patients who underwent multiple EGDs within the given time period, only results from the first procedure were included.
2.2. Data analysis
Demographic data, endoscopic findings, and histology examinations were entered in a computerized system for analysis. Increased body weight was defined as a body mass index (BMI) greater than 25.0 (kg/m2). The number and the size of polyps in the stomach were not reported. Removed polyps as well as the gastric biopsies were analyzed for histology by the same pathologist expert in gastrointestinal diseases. Polyps were defined on the base of histotype as hyperplastic, fundic gland, adenomatous, and inflammatory. More specifically, polyps were classified as simply inflammatory polyps when the pathologist observed a prominent inflammatory component in hyperplastic polyps. Because of the retrospective nature of the study, a specific histotype polyp classification was not applied to our series; however, the histology of polyps was described by the pathologist according to Shaib et al.[3]
2.3. H pylori infection
It was defined by the presence of H pylori on histological examination of gastric biopsies and a positive rapid urease test or a[13] C-urea breath test (UBT), according to previously reported methods.[14] On the base of H pylori infection, patients were classified as having H pylori and active chronic gastritis or gastritis associated with metaplasia and/or atrophy. Patients without H pylori infection were classified as normal or having gastritis with metaplasia and/or atrophy.
2.4. Celiac disease
The diagnosis was made according to national and international guidelines as previously described.[15]
2.5. Ethical considerations
An Institutional Review Board approval was obtained from the ethics committee Comitato di Bioetica dell’Azienda Ospedaliero-Universitaria di Sassari (Prot No 2099/CE, 2014).
2.6. Statistical analysis
Patients were stratified into 2 groups based on the presence or the absence of polyps. The prevalence of polyps was calculated for each age decade and gender and expressed as absolute numbers and proportions. Unadjusted (crude) odds ratios (ORs) with their 95% confidence intervals (CIs) were calculated to estimate the strength of associations with a diagnosis of CD, age (greater or lower than 50 years), gender, body mass index, H pylori status, use of PPIs, and anti-H2. Thereafter, a logistic regression analysis was carried out to calculate adjusted ORs for all co-factors, by using a backward stepwise elimination procedure with a P value to exit set at < 0.10. In addition, ORs for polyps detection adjusted for age, gender, and BMI were calculated according to the PPI use for each polyp histotype in patients with and without CD. All the statistical analyses were conducted by using SPSS Statistical Package (version 16.0, Chicago, IL) and the results were considered significant when P values were less than 0.05.
3. Results
Overall a total of 11,379 charts were retrieved from January 1995 to December 2013. The proportion of women was 61.2% (i.e., 6962/11,379), mean age was 52.8 ± 17.2 years in men and 50.1 ± 17.5 years in women. The presence of polyps (any histotype) was found in 475 patients (4.2%). Their frequency, regardless of histotype, increased in patients older than 50 years. Prevalence of gastric polyps was significantly higher among women (Table 1). Distributions of gastric polyps according to age decades and gender, reported in Fig. 1, showed a steady increase of the risk with age, more evident in females. Representative polyps images observed during endoscopy are displayed in Fig. 2.
Table 1.
Crude odds ratio and 95% confidence interval for celiac disease and other study variables among patients with and without gastric polyps detected by upper endoscopy.
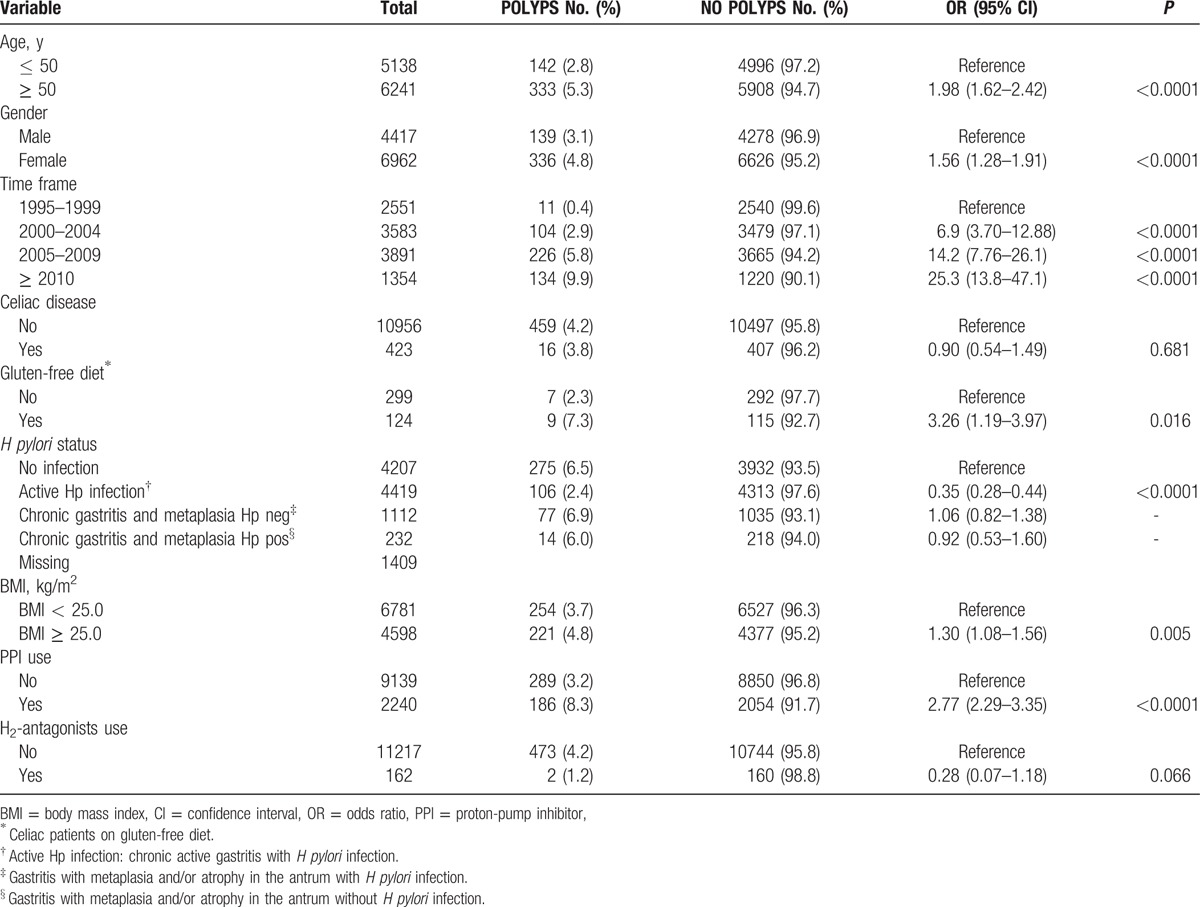
Figure 1.
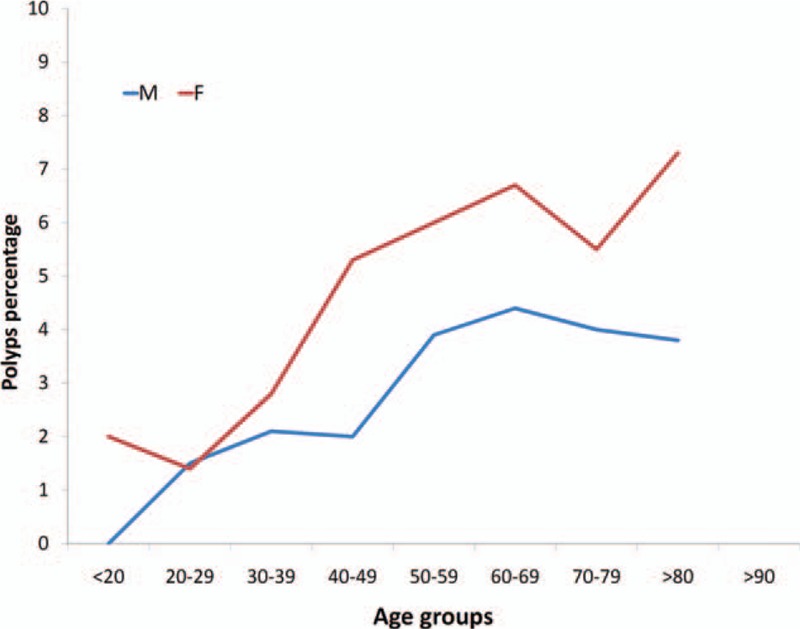
Prevalence of gastric polyps in patients who underwent to esophago-gastro-duodenoscopy according to gender and age decades.
Figure 2.
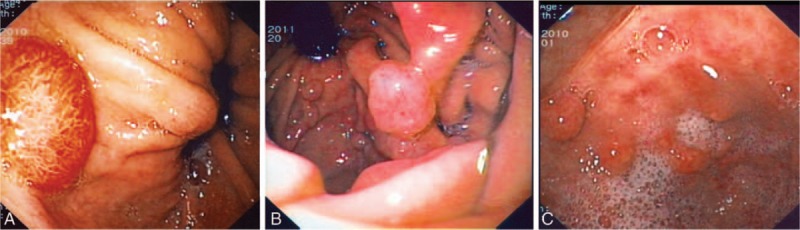
Representative images of hyperplastic (A), fundic gland (B), and inflammatory (C) polyps observed by esophago-gastro-duodenoscopy.
Moreover, a progressive rise of gastric polyps prevalence over the study period (from 1995 to 2013) was noticed (Table 1).
Patients with a diagnosis of CD were 3.7% (423/11,379), including 124 on gluten-free diet (previously diagnosed CD) and 299 on free diet (newly diagnosed CD) (Table 1). The prevalence of CD was significantly higher among women (4.7% vs 2.1%; P < 0.0001). Although distribution of polyps was not significantly different between celiac (16/423; 3.8%) and nonceliac (459/10,956; 4.2%) patients, interestingly CD patients on gluten-free diet had an increased risk to develop polyps (OR: 3.26; P = 0.016) compared to patients with a new diagnosis of CD (Table 1).
In patients with an active H pylori infection, a significantly reduced risk of having polyp lesions was observed (OR: 0.35; P < 0.0001). In contrast, patients with gastritis and metaplasia, regardless of H pylori status, did not differ in the risk to develop polyps from patients with neither gastritis nor infection. Overweight displayed a slight risk for the presence of polyp lesions. Use of PPIs was strongly associated with the presence of polyp lesions. Additional analysis showed that consumption of PPIs increased notably over time from 3.6%, 12.3%, 28.5% to 44.1% in 1995–1999, 2000–2004, 2005–2009, 2010–2013, respectively. We were not able to find any significant association between the use of anti-H2 and polyps (Table 1).
Table 2 shows the logistic regression analysis. Age older than 50 years, female gender, more recent EGD, and use of PPIs remained significantly associated with the presence of polyps. An active H pylori infection was inversely and significantly associated with gastric polyps. Body mass index and anti-H2 use were excluded by the stepwise regression method from the list of significantly associated co-factors.
Table 2.
Logistic regression analysis including variables potentially associated with the presence of gastric polyps.
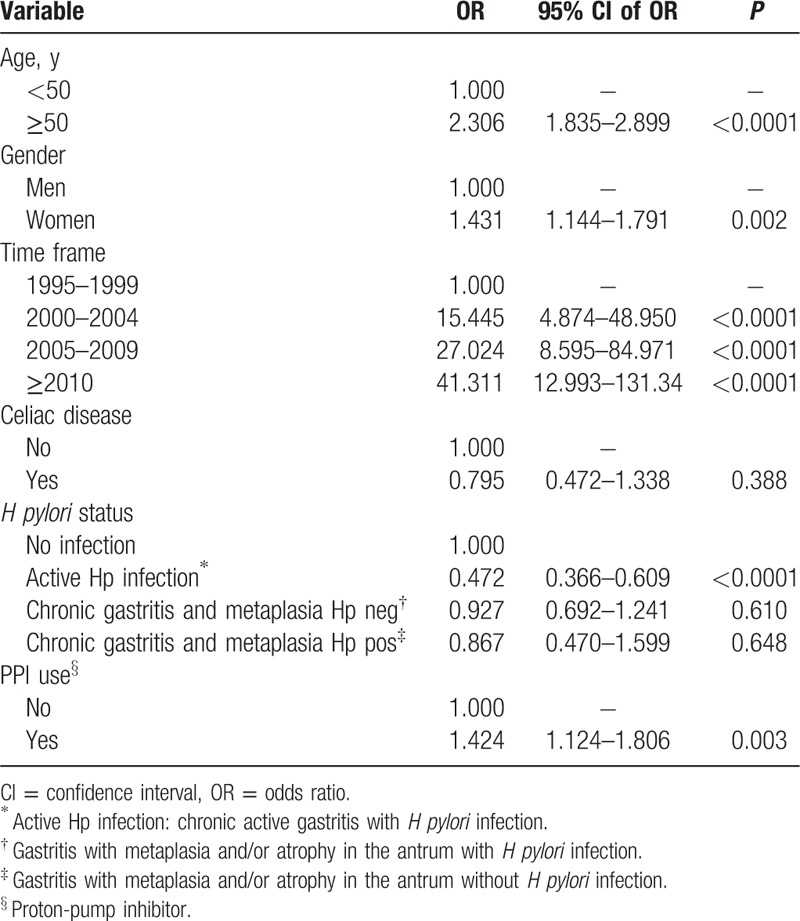
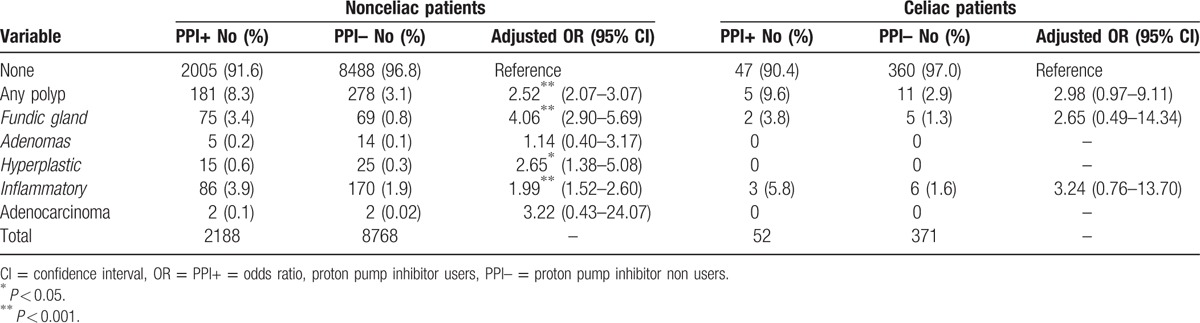
Histological features of polyps in celiac and nonceliac patients among PPI and no-PPI users are displayed in Table 3. Inflammatory polyps were the most common histotype (256/459; 55.8% and 9/16; 56.2%; P = 0.969) of all lesions, followed by fundic gland polyps (144/459; 31.4% and 7/16; 43.7%; P = 0.296), hyperplastic (40/459, 8.7% and 0; P = 0.217), and adenomas in celiac and nonceliac patients, respectively. The risk for having polyps, after adjusting for age, gender, and BMI, was higher in PPI users compared to nonusers both in celiac and nonceliac patients (Table 3). Hyperplastic polyps and adenomas were absent among CD patients and their frequency was also low in the control group compared to the other histotypes. Neoplastic polyp lesions (adenocarcinomas) were detected among 4 patients without CD and zero patients with CD.
Table 3.
Distribution of gastric polyps in celiac and nonceliac patients according to the use of proton pump inhibitors and to the histotype. Adjusted odd ratios were calculated by using logistic regression with age, gender, and body mass index as co-factors.
4. Discussion
Our study confirms that gastric polyps are relatively common in daily clinical practice, being found in 4.2% of subjects undergoing EGD for any reason, according to previous series. However, higher rates have been reported in United States.[3] Usually, in countries with a high prevalence of H pylori infection, hyperplastic polyps and adenomas are observed as the more prevalent histotype compared with fundic gland polyps.[3,7–8] In contrast, in countries where the use of PPIs is widespread and the prevalence of H pylori infection is low, the most common histotype incidentally detected is the fundic gland polyp.[3,7–8] Interestingly, in the cohort studied, inflammatory polyp was the most common histotype of all lesions, followed by fundic gland and hyperplastic polyps.
These differences in the histotype rate among polyps in Sardinian patients may be consistent with the presence of the major risk factors acting simultaneously. In a previous study, we observed a dramatic falling rate of acquisition of H pylori infections in Sardinia since at least 1950.[14] It was suggested that this change in H pylori prevalence reflected improvements in sanitary conditions and the introduction of public water systems associated with higher standards of living, in the second half of the twentieth century.[14] At the same time, the population experienced a dramatic increase of PPI use. Prevalence of PPI users in the population that underwent EGD from 1995 to 2013 rose from 3.6% to 44.1% respectively. From our data, we can suppose that there was an overlap of the major risk factors affecting the development of gastric polyps or even additional risk factors not yet identified.
Celiac disease is common in Sardinia[15] given the high frequency of haplotypes predisposing to the disease HLA DQ2 and DQ8.[16] For this reason, Sardinian physicians are focused in recognizing atypical signs and/or symptoms of celiac disease. Recently, a case series suggested an association between CD and hyperplastic gastric polyps.[12,13] Given the potential risk of malignancy and or bleeding of hyperplastic polyps, CD patients may need a closer endoscopic follow-up. Because of that in the present study, we wanted to test this hypothesis in a region with a high prevalence of CD. Our analysis was unable to find an association between CD and the presence of gastric polyps, and more specifically hyperplastic polyps. Although distribution of polyps was not significantly different between celiac and nonceliac patients, surprisingly, CD patients on gluten-free diet had an increased risk to develop polyps compared to patients with a newly diagnosed CD. However, the number of patients with CD and polyps was too small to allow further subanalysis between patient with CD on gluten-free diet or on free diet and polyp histotype. For these reasons it is difficult to draw defined conclusions about the higher prevalence of polyps in patients on gluten-free diet, even though results are statistically significant. Prevalence of gastric polyps in gluten-free diet CD patients was not greater than in patients taking PPIs (7.3% vs 8.3%). Adjusting for all confounding variables CD patients display a lower risk to develop hyperplastic polyps and adenomas. It may be supposed that previous reports showing an increased association[12,13] have been biased by a small patients’ sample, and that the suggested association occurred likely by chance.
Age older than 50 years, female gender, more recent EGD, and use of PPIs, were the major risk factors associated with the presence of polyps in the logistic regression analysis.
Association of gastric polyps with female gender and age has been reported in other studies, especially fundic gland polyps occur in females more often than in males.[17,18] In addition, according to previous results, the present study clearly showed an increased polyps prevalence with increasing age in both genders.
In a large screening cohort including 7,603 subjects undergoing a baseline and follow-up EGD and tested for H pylori, a positive association between the infection and hyperplastic polyps (OR 2.01; 95% CI 1.66–2.41) was found.[19] In addition, successful H pylori eradication markedly reduces the presence of hyperplastic polyps compared with the persistent H pylori positive group.[19,20]
In contrast, the present study showed a negative association between H pylori infection and presence of gastric polyps. Similar results were also found in Italy. The authors observed that smoking habit, but not H pylori infection, increases the risk for benign epithelial gastric polyps in patients with atrophic body gastritis.[21] In our series, the inverse effect of H pylori was prevalent in patients with an active gastritis compared to patients with gastritis associated with metaplasia and/or atrophy. Traditionally, gastric hyperplastic polyps were associated with mucosal atrophy, caused by H pylori infection or autoimmune gastritis, respectively.[22] However, in the most recent years, Carmack et al[2] described an increased proportion of hyperplastic polyps in patients with a normal or reactive gastric mucosa with no stigmata of H pylori infection. The findings by Carmack might have influenced our results too. More detailed analysis demonstrated that in patients negative for H pylori infections, detection of polyps was more frequent in PPI user compared to PPI no-user (12.2% vs 4.9%).
Where the use of PPI is common such as in industrialized countries, fundic gland polyps are the most frequent polyps observed in the stomach. Hypergastrinemia due to long-term therapy with PPIs or to Zollinger–Ellison syndrome or to the presence of gastrinoma may be associated with fundic gland polyps development.[23,24] Disappearance of fundic gland polyps following PPIs cessation is an additional evidence of this correlation.[25] However, there are investigations where polyps showed no such association, even after long-term (9 years) follow-up.[18,26–27] Although from our analysis emerged a high consumption of PPI among patients undergoing EGD, fundic gland polyps were not the most common histotype.
The higher prevalence of gastric polyps detected in patients with an increased body weight was not confirmed by the multivariate analysis. This is consistent with studies carried out in patients undergoing bariatric surgery where the incidence of gastric polyps found in these patients was not increased compared to the general population.[28,29]
In conclusion, according to literature gastric polyps are relatively common in subjects undergoing EGD even in Sardinia. Results of the present study showed that the detection rate of gastric polyps was significantly higher in females than in males and increased with age, especially after age 50 years, and in more recent years. The distribution of pathological types was slightly different compared to previous reports given the fact that inflammatory polyps were the most frequent histotype. The occurrence of gastric polyps was negatively associated with H pylori infection. More importantly, hyperplastic polyps were not confirmed as an additional manifestation of CD.
Footnotes
Abbreviations: Anti-H2 = H2 receptor blockers, BMI = body mass index, CI = confidence interval, EGD = esophago-gastro-duodenoscopy, OR = odds ratio, PPI = proton-pump inhibitor, UBT = 13C-urea breath test.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Hirota WK, Zuckerman MJ, Adler DG, et al. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc 2006;63:570–80. [DOI] [PubMed] [Google Scholar]
- [2].Carmack SW, Genta RM, Schuler CM, et al. The current spectrum of gastric polyps: a 1-year national study of over 120,000 patients. Am J Gastroenterol 2009;104:1524–32. [DOI] [PubMed] [Google Scholar]
- [3].Shaib YH, Rugge M, Graham DY, et al. Management of gastric polyps: an endoscopy-based approach. Clin Gastroenterol Hepatol 2013;11:1374–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dijkhuizen SM, Entius MM, Clement MJ, et al. Multiple hyperplastic polyps in the stomach: evidence for clonality and neoplastic potential. Gastroenterology 1997;112:561. [DOI] [PubMed] [Google Scholar]
- [5].Archimandritis A, Spiliadis C, Tzivras M, et al. Gastric epithelial polyps: a retrospective endoscopic study of 12974 symptomatic patients. Ital J Gastroenterol 1996;28:387–90. [PubMed] [Google Scholar]
- [6].Stolte M, Sticht T, Eidt S, et al. Frequency location and age and sex distribution of various types of gastric polyp. Endoscopy 1994;26:659–65. [DOI] [PubMed] [Google Scholar]
- [7].Elhanafi S, Saadi M, Lou W, et al. Gastric polyps: association with Helicobacter pylori status and the pathology of the surrounding mucosa, a cross sectional study. World J Gastrointest Endosc 2015;7:995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jalving M, Koornstra JJ, Wesseling J, JH, et al. Increased risk of fundic gland polyps during long-term proton pump inhibitor therapy. Aliment Pharmacol Ther 2006;24:1341–8. [DOI] [PubMed] [Google Scholar]
- [9].Stolte M, Bethke B, Seifert E, et al. Observation of gastric glandular cysts in the corpus mucosa of the stomach under omeprazole treatment. Z Gastroenterol 1995;33:146–9. [PubMed] [Google Scholar]
- [10].Choudhry U, Boyce HW, Jr, Coppola D. Proton pump inhibitor-associated gastric polyps: a retrospective analysis of their frequency, and endoscopic, histologic, and ultrastructural characteristics. Am J Clin Pathol 1998;110:615–21. [DOI] [PubMed] [Google Scholar]
- [11].Kolodziejczyk P, Yao T, Oya M, et al. Long-term follow-up study of patients with gastric adenomas with malignant transformation. An immunohistochemical and histochemical analysis. Cancer 1994;74:2896–907. [DOI] [PubMed] [Google Scholar]
- [12].Doris I, Qizilbash A, Meghji M. Fundic gland polyposis occurring with adult celiac disease. Can Assoc Radiol J 1990;41:149–50. [PubMed] [Google Scholar]
- [13].Grube-Pagola P, Remes-Troche JM. Celiac disease and gastric hyperplastic polyps: a case series of an uncommon association. Eur J Gastroenterol Hepatol 2014;26:807–11. [DOI] [PubMed] [Google Scholar]
- [14].Dore MP, Marras G, Rocchi C, et al. Changing prevalence of Helicobacter pylori infection and peptic ulcer among dyspeptic Sardinian patients. Intern Emerg Med 2015;10:787–94. [DOI] [PubMed] [Google Scholar]
- [15].Dore MP, Cuccu M, Pes GM, et al. Clinical pattern of celiac disease in a population residing in North Sardinia (Italy). Recenti Prog Med 2012;103:564–9. [DOI] [PubMed] [Google Scholar]
- [16].Catassi C, Doloretta Macis M, Rätsch IM, et al. The distribution of DQ genes in the Saharawi population provides only a partial explanation for the high celiac disease prevalence. Tissue Antigens 2001;58:402–6. [DOI] [PubMed] [Google Scholar]
- [17].Zheng E, Ni S, Yu Y, et al. Impact of gender and age on the occurrence of gastric polyps: data analysis of 69575 southeastern Chinese patients. Turk J Gastroenterol 2015;26:474–9. [DOI] [PubMed] [Google Scholar]
- [18].Zelter A, Fernández JL, Bilder C, et al. Fundic gland polyps and association with proton pump inhibitor intake: a prospective study in 1,780 endoscopies. Dig Dis Sci 2011;6:1743–8. [DOI] [PubMed] [Google Scholar]
- [19].Nam SY, Park BJ, Ryu KH, et al. Effect of Helicobacter pylori infection and its eradication on the fate of gastric polyps. Eur J Gastroenterol Hepatol 2016;28:449–54. [DOI] [PubMed] [Google Scholar]
- [20].Cao H, Wang B, Zhang Z, et al. Distribution trends of gastric polyps: an endoscopy database analysis of 24,121 northern Chinese patients. J Gastroenterol Hepatol 2012;27:1175–80. [DOI] [PubMed] [Google Scholar]
- [21].Dirschmid K, Platz-Baudin C, Stolte M. Why is the hyperplastic polyp a marker for the precancerous condition of the gastric mucosa? Virchows Arch 2006;448:80–4. [DOI] [PubMed] [Google Scholar]
- [22].Di Giulio E, Lahner E, Micheletti A, et al. Occurrence and risk factors for benign epithelial gastric polyps in atrophic body gastritis on diagnosis and follow-up. Aliment Pharmacol Ther 2005;21:567–74. [DOI] [PubMed] [Google Scholar]
- [23].el-Zimaity HM, Jackson FW, Graham DY. Fundic gland polyps developing during omeprazole therapy. Am J Gastroenterol 1997;92:1858–60. [PubMed] [Google Scholar]
- [24].Modlin IM, Gilligan CJ, Lawton GP, et al. Gastric carcinoids. The Yale Experience. Arch Surg 1995;130:250–5. [DOI] [PubMed] [Google Scholar]
- [25].Kim JS, Chae HS, Kim HK, et al. Spontaneous resolution of multiple fundic gland polyps after cessation of treatment with omeprazole. Korean J Gastroenterol 2008;51:305–8. [PubMed] [Google Scholar]
- [26].Declich P, Tavani E, Ferrara A, et al. Sporadic fundic gland polyps: clinico-pathologic features and associated diseases. Pol J Pathol 2005;56:131–7. [PubMed] [Google Scholar]
- [27].Huang CZ, Lai RX, Mai L, et al. Relative risk factors associated with the development of fundic gland polyps. Eur J Gastroenterol Hepatol 2014;26:1217–21. [DOI] [PubMed] [Google Scholar]
- [28].Raghavendra RS, Kini D. Benign, premalignant, and malignant lesions encountered in bariatric surgery. JSLS 2012;16:360–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Almazeedi S, Al-Sabah S, Al-Mulla A, et al. Gastric histopathologies in patients undergoing laparoscopic sleeve gastrectomies. Obes Surg 2013;23:314–9. [DOI] [PubMed] [Google Scholar]


